Abstract
We report a facile and efficient method for the covalent 2D‐patterning of monolayer graphene via laser irradiation. We utilized the photo‐cleavage of dibenzoylperoxide (DBPO) and optimized the subsequent radical additions to non‐activated graphene up to that level where controlled covalent 2D‐patterning of graphene initiated by spatially resolved laser writing is possible. The covalent 2D‐functionalization of graphene, which is monitored by scanning Raman microscopy (SRM) is completely reversible. This new concept enables write/read/erase control over the covalent chemical information stored on the graphene surface.
Keywords: covalent functionalization, covalent patterning, graphene, Raman spectroscopy, write/read/erase
A write/read/erase concept for spatially controlled patterning utilizing covalent functionalization of monolayer graphene on a Si/SiO2 substrate is made possible by laser decomposition of benzoyl peroxide. The introduced chemical information and its reversible binding were investigated by Raman spectroscopy. This method allows the production of a wide array of highly specialized graphene surfaces.

Introduction
Research into the 2D carbon allotrope graphene has been a challenging topic spanning physics, chemistry and materials science since its discovery in 2004.[ 1 , 2 , 3 ] The chemical functionalization of graphene plays a fundamental role in modifying the outstanding properties of this atomically thin all carbon architecture. For this purpose, first prototype concepts of both covalent and non‐covalent functionalization of graphene have been developed in recent years.[ 4 , 5 ] The covalent modification is accompanied by the introduction of sp3 centers carrying the chemically bound addends. In this regard, additions of in situ generated radicals has been demonstrated to proceed rather facile. [6] Prominent examples are radical additions initiated by the reductive decomposition of diazonium salts,[ 7 , 8 ] iodonium salts,[ 9 , 10 , 11 , 12 ] aryl‐, and alkylhalides.[ 13 , 14 , 15 ] This reaction type can be further facilitated by reductive alkali metal activation of graphite (metal intercalation) or of graphene supported on substrates.[ 12 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ] The first steps towards this endeavor constituted a random and locally uncontrolled functionalization.[ 22 , 23 ] The next exciting challenge is the specific tailoring of covalent functionalization to confined graphene regions leading to controlled graphene patterning with covalently bound addends (2D chemistry of graphene). The spatial controlled patterning of functional moieties onto monolayer graphene presents the possibility of combining several unique properties, like region specific hydrophilicity‐hydrophobicity, catalytically or optically active domains, anchoring group reservoirs for molecular recognition and patterned electron conductivity. This allows for the generation of materials, surfaces and devices engineered for specific demands. So far, some first approaches towards 2D‐patterning of graphene relied on the implementation of masks consisting of poly(methyl methacrylate) (PMMA) and subsequent lithography to access graphene for covalent functionalization in predefined regions. For the corresponding covalent functionalization Diels–Alder reactions, [24] hydrogenations and reactions with diazonium ions have been applied. [25] The drawback of the initial studies is the comparatively low degree of functionalization as indicated by low ID/IG ratios in the Raman spectra. We have recently considerably improved the field of covalent 2D‐patterning of graphene by a) combining the mask assisted 2D‐patterning with the reductive activation of graphene prior to the covalent addend [26] and b) by establishing a spatially resolved bottom side fluorination of graphene. [27] Although these improvements are very important, alternative concepts avoiding the implication of mask‐assisted or other types of pre‐patterning methods are highly welcome. The photochemical reactivity of graphene towards peroxides opening the opportunity of laser writing represents an attractive approach for this task. [28]
Herein, we report a facile and efficient method for the covalent 2D‐patterning of monolayer graphene via laser irradiation affording high degrees of functionalization. We utilized the photo‐cleavage of dibenzoylperoxide (DBPO) and optimized the subsequent radical additions to non‐activated graphene up to that level where controlled covalent 2D‐patterning of graphene initiated by spatially resolved laser writing is possible. Furthermore, the covalent 2D‐functionalization of graphene is completely reversible, enabling a write/read/erase control over the covalent chemical information stored on the graphene surface.
Results and Discussion
Our approach towards a mask‐less locally controlled functionalization utilizes the generation of reactive aryl radicals generated from DBPO deposited onto the graphene monolayer. The oxygen‐oxygen bond in DBPO is rather weak and therefore can undergo homolysis resulting in the formation of two benzoyloxyl radicals. Subsequent decarboxylation of the benzoyloxyl radicals leads to the generation of two phenyl radicals.[ 29 , 30 ] The decomposition of DBPO is initiated by either photolytic or thermolytic cleavage of the peroxide bond. For our 2D‐patterning we induced the homolytic cleavage of the DBPO by irradiation with a 532 nm laser (Scheme 1 b). [28] The created phenyl radicals undergo covalent topside binding to graphene. Solely supratopic binding, however, would create a huge amount of strain energy and unrealistic bending of the graphene lattice. [22] In our cases this is compensated by antaratopic back bonding of substrate functionalities quenching the initially formed radical centers on graphene and allowing for the establishment of strain free environments.[ 22 , 27 , 31 , 32 ] Here, Si/SiO2 as a substrate plays an important role as it allows for the antaratopic backside bonding through its functional groups, relieving strain.
Scheme 1.

a) Mechanism of the laser induced functionalization of graphene with DBPO, which involves antaratopic back bonding of the Si/SiO2 substrate. b) Laser patterning of graphene on Si/SiO2 by spatially controlled cleavage of DBPO: A) Drop casting of DBPO dissolved in diethyl ether leading to a DBPO coating on top of the graphene monolayer; B) Laser irradiation with a 532 nm laser of locally predefined areas and subsequent functionalization of the monolayer graphene by the laser‐generated reactive radical intermediates; C) Removal of the residual crystalline DBPO coating by washing with acetone.
For the laser induced binding of phenyl radicals to graphene DBPO was dissolved in diethyl ether and one drop of the 10−3 M solution was placed on top of monolayer graphene deposited on a Si/SiO2 substrate (for experimental details see electronic supplementary information ESI). After evaporation of the diethyl ether at room temperature, a thin, crystalline film of DBPO formed on the graphene surface (Figure S1). This DBPO film is the fundamental starting point for our locally controlled functionalization of the graphene layer beneath. By laser irradiation, the peroxide bond is cleaved homolytically and after extrusion of CO2 reactive phenyl radicals are formed. The radicals are only formed in those areas where the laser hits the DBPO film resulting in a locally controlled addend binding accompanied by the generation of sp3 carbon defects. In accordance to the work of Brus et al. [28] we have chosen a green laser (532 nm) for the photoexcitation of the peroxide film. As can be seen in Figure 1, a 60 second (0.88 mW, laser spot size ≈1 μm2) irradiation of the film‐covered graphene sample clearly results in a significant increase of the D‐band intensity, indicative for the covalent binding of photo‐generated phenyl radicals.
Figure 1.

Raman spectrum of the pristine monolayer graphene on Si/SiO2 (black) and Raman spectrum after illumination of the DBPO film for 60 seconds (red). λexec.=532 nm, 0.88 mW.
In principle, the laser based covalent “writing” of functional entities on graphene cannot fully be decoupled from an additional radical generation/graphene functionalization attributed to a laser based Raman read‐out of the “written” information. Therefore, we have investigated the contribution to the respective ID/IG ratios of our chosen standard “reading” conditions (0,88 mW, integration time: 10 s) to the initial ID/IG ratio of the writing process (Figure 2).
Figure 2.

Black: Raman measurement of DBPO coated graphene after laser irradiation of 70 s. Red: Raman measurement of the same spot with an additional 10 s laser irradiation showing the ID/IG increase due to the read‐out measurements.
Here, the peroxide covered sample was irradiated for 70 seconds at the same laser spot, resulting in an ID/IG ratio of the writing process of 0.84. Subsequently, the same spot was irradiated for another 10 seconds to simulate the standard Raman reading conditions. As can be seen the additionally applied laser time leads to a subsequent increase of the initial ID/IG ratio of about 0.24. It has to be kept in mind, that this additional functionalization becomes less dominant the longer the initial laser writing time and therefore the achieved degree of functionalization has been chosen. Furthermore, a continues laser irradiation of the sample over a certain time span yields a very similar ID/IG ratio as can be achieved for iterative sample irradiations summing up to the same time span (Figure S2). These results nicely contribute to our picture that a laser based covalent writing of functional moieties on graphene is in an easy and highly efficient way possible by our chosen approach.
The next step was the determination of the most suitable parameters for the photo‐generation of the intermediate aryl‐radicals and their subsequent efficient binding to the graphene lattice. For this purpose, we carried out a systematic study, analyzing the influence of the laser intensity and irradiation time of a 532 nm laser on the degree of functionalization. In a first experiment, we selected a specific irradiation spot on the graphene surface and varied the irradiation time from 30 seconds to 16 minutes (30 s, 60 s, 120 s,…). It has to be kept in mind that for the read‐out of the written information additional Raman measurements of 10 second laser irradiation has been carried out for each data point, extending the over‐all irradiation time of the sample to 40 s, 80 s, 150 s … (Figure 3, individual spectral information presented in Figure S3). As discussed before, for the read‐out process an additional contribution of about 0.24 to the respective ID/IG ratio has to be expected—affecting to a higher degree the data points obtained for shorter irradiation time spans.
Figure 3.
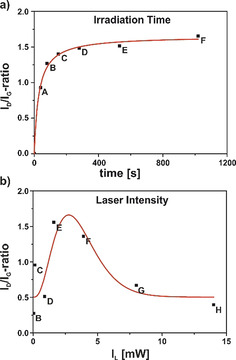
Evolution of the ID/IG ratios for the a) irradiation‐time study ranging from 30 seconds up to 16 minutes and the b) laser‐intensity study with laser powers of 0.017 mW to 14 mW—the individual Raman spectra are depicted in Figure S3 and S4 in the Supporting Information.
The time study revealed that already irradiating for one minute leads to a significant increase of the D‐band intensity, which correlates with an increase of addend carrying sp3 centers. After two minutes, the D‐band did not increase any further indicating that all of the DBPO present at the irradiated area decayed to the aryl radicals resulting in a saturation of addend binding (Figure 3 a).
In a second experiment we have varied the laser intensity from 0.017 mW to 14 mW applied for a fixed time span of one minute. In contrast to the experiment described above, we have here changed the measuring spots on the sample after each individual measurement. As the analysis of the obtained spectral information shows (Figure 3 b), the most intense D‐band emerges after an irradiation with 1.6 mW laser intensity. At higher intensities of 4 mW and above a decrease in D‐band intensity can be detected (respective Raman spectra are depicted in Figure S4) due to the fact that the energy introduced to the system at these intensities already leads to a defunctionalization, similar to the already reported laser‐induced defunctionalization of carbon nanotubes. [12] Here, the higher laser intensity and therefore a locally increased temperature results in the cleavage of the newly formed C−C bonds, reverting the sp3 centers in the graphene lattice back to sp2 configuration. A laser‐induced defunctionalization is even more likely to occur in graphene due to the fact that the defunctionalization leads to a decrease of strain energy in the whole system. The concept of detaching functional moieties through laser or thermal treatment is widely utilized in the concept of the TG‐MS characterization of covalently functionalized graphene samples [33] and in the annealing of graphene oxide [34] yielding a higher quality material with less defects.
In order to investigate the thermal stability of the graphene patterning, we carried out a temperature dependent Raman investigation to access insights into the reversibility of the covalent addend binding. Since the peroxide bond of DBPO can also be cleaved thermally upon heating, generating phenyl radicals, it has to be expected that a thermal heating of the wafer leads also to a parallel graphene functionalization up to a certain temperature threshold. [32] We started the measurements with a moderate temperature ramp of 5 °C per minute from 0 °C up to 125 °C (Figure 4 a). Due to the low heating rate, it was possible to follow the temperature‐based functionalization process by Raman spectroscopy. As illustrated by the respective optical images of the wafer (Figure S5) the DBPO film has vanished when a temperature of 100 °C was reached. For the investigation of the thermally‐induced addend defunctionalization processes we increased the temperature ramp at 125 °C to 50 °C per minute, up to a final temperature of 450 °C.
Figure 4.

a) Temperature‐dependent Raman measurement of monolayer Graphene coated with DBPO. b) Plot of D‐band intensity evolution during functionalization (blue area) and defunctionalization (red area) over the temperature range from 0 °C to 450 °C, measured with a 532 nm laser, an integration time of 10 seconds, and an intensity of 0.88 mW.
Significantly, the D‐band intensity at ≈1350 cm−1 increased with increasing temperature to an ID/IG ratio of 2.0 at 50 °C. After 50 °C the D‐band starts to decrease until 80 °C and increases again at 85 °C just to decrease once more at around 125 °C (Figure 4).
The behavior of the D‐band is similar to the observations made by Cançado et al. [35] where upon reaching a high functionalization degree the ID/IG ratio starts to decrease though further functionalization is occurring. This is attributed to the fact that the defect distance decreases below 3 nm, as disorder in the sp2 system progresses. If two defects are closer than the average distance an electron‐hole pair travels before scattering with a phonon, the contribution to the D‐band will no longer add up independently, so the intensity of the D‐band will decrease with respect to the G‐band, although further functionalization is occurring. The increase of ID/IG ratio at 85 °C indicates an increase in inter defect distance, resulting in a return to the low functionalization regime. During this process, the system eventually reaches the low functionalization regime where upon further defunctionalization the D‐band intensity decreases down to the values of intact graphene.
This behavior can also be nicely followed by the development of the 2D‐band and D′‐band intensities. The 2D‐band development starts with a high I2D/IG ratio expected for monolayer graphene and constantly decreases during the functionalization process up to 85 °C due to the dampening effect of the functionalization. During the defunctionalization of the graphene monolayer, the I2D/IG ratio goes back almost to the initial value (Figure S6). For the D′‐band an inverse trend is observed. It increases during the functionalization period and starts to decrease when approaching the defunctionalization regime (Figure S7). These studies show that thermal treatment of the 2D graphene architectures allows for controlling the degree of functionalization and can even lead to a restauration of an intact graphene lattice by erasing the chemical information stored during the preceding writing process (Scheme 2).
Scheme 2.

Schematic illustration of the write/read/erase concept.
We now describe the imaging process of the laser induced 2D‐functionalization of graphene. The laser writing as well as the read out of the stored 2D information is based on screening studies pointed out above. Therefore, a Raman map was designed, using the shape of “FAU”. Each mapping point was irradiated with a 532 nm laser for 30 seconds applying a laser intensity of 1.6 mW. The successful patterning of chemical information concludes the “writing process”. For the visualization of the imprinted pattern, as seen in Scheme 2, a “reading process” was established were a Raman map of the functionalized area is performed with a 633 nm laser—lower ID/IG contribution in the reading process (see Figure S8). The ID/IG ratios depicted and spatially resolved in Figure 5 provide excellent imaging of the chemical information of the 2D‐patterned graphene. An average ID/IG ratio of 1.5 for the predefined functionalization spots is displayed, whilst the unfunctionalized background and intact graphene region yields an ID/IG ratio between 0.4 and 0.9. By displaying the difference in ID/IG ratios between background and patterned area (Figure 5 b), Raman measurements allow for visualization of the spatially resolved functionalization areas. One can observe a local introduction of sp3 carbon centers at those points where irradiation by the 532 nm laser occurred. The resolution for differentiating between two patterning spots is approximately 2 μm and is limited by the spot size of the laser and the mesh of the “reading” map. Defined boundaries indicate the areas between the intact graphene lattice and the sp3 regimes. With these “writing” and “reading” procedures we have realized 2D graphene‐patterning by generating addend regions introducing areas of high functionalization. These take the shape of a desired pattern and an area of unfunctionalized graphene is left in the background. By thermal treatment, the chemical information stored on the graphene can be erased as shown by our temperature dependent Raman study (Figure 4 a). This allows for the precise adjustment of the degree of functionalization, complete restauration of the graphene, and reusability for further storage of new chemical information.
Figure 5.

a) Raman map of 6912 measure points in a 186×144 μm area obtained by a 633 nm laser with an intensity of 1.6 mW (integration time: 8 s), showing the ID/IG ratios of the locally functionalized graphene, visualizing the predefined pattern. b) Raman point spectra obtained by the Raman map, of the patterned area I and the background area II normalized on the G‐band.
Conclusion
We have introduced a very facile and efficient method for the covalent 2D‐patterning of monolayer graphene via laser irradiation. We utilized the photo‐cleavage of dibenzoylperoxide (DBPO) and optimized the subsequent radical additions to non‐activated graphene up to a level where controlled covalent 2D‐patterning of graphene initiated by spatially resolved laser writing is possible. Efficient covalent binding takes place exclusively on the laser illuminated areas. The visualization of the covalent addend patterning has been accomplished by scanning with a 633 nm laser and analyzing the corresponding ID/IG ratios in the spatially resolved Raman spectra. The covalent 2D‐functionalization of graphene, which is monitored by scanning Raman microscopy (SRM) is completely reversible. This can be seen, for example, from stepwise heating causing controlled defunctionalization and eventually the complete restauration of the graphene sp2 lattice. This new concept enables write/read/erase control over the covalent chemical information stored on the graphene surface. Moreover, by systematically varying the nature of covalent functionalities many types of programmable surface architectures can be generated opening a wide field of sophisticated applications. Investigations along these lines are currently under way in our laboratories examining other potential radical starters like AIBN and heteroatom marked DBPO as well as further 2D materials like MoS2.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project‐ID 182849149—SFB 953. Open access funding enabled and organized by Projekt DEAL.
K. F. Edelthalhammer, D. Dasler, L. Jurkiewicz, T. Nagel, S. Al-Fogra, F. Hauke, A. Hirsch, Angew. Chem. Int. Ed. 2020, 59, 23329.
References
- 1. Castro Neto A. H., Guinea F., Peres N. M. R., Novoselov K. S., Geim A. K., Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar]
- 2. Mak K. F., Ju L., Wang F., Heinz T. F., Solid State Commun. 2012, 152, 1341–1349. [Google Scholar]
- 3. Novoselov K. S., Geim A. K., Morozov S. V., Jiang D., Zhang Y., Dubonos S. V., Grigorieva I. V., Firsov A. A., Science 2004, 306, 666–669. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch A., Englert J. M., Hauke F., Acc. Chem. Res. 2013, 46, 87–96. [DOI] [PubMed] [Google Scholar]
- 5. Malig J., Stephenson A. W. I., Wagner P., Wallace G. G., Officer D. L., Guldi D. M., Chem. Commun. 2012, 48, 8745–8747. [DOI] [PubMed] [Google Scholar]
- 6. Park J., Yan M., Acc. Chem. Res. 2013, 46, 181–189. [DOI] [PubMed] [Google Scholar]
- 7. Bahr J. L., Tour J. M., Chem. Mater. 2001, 13, 3823–3824. [Google Scholar]
- 8. Bahr J. L., Yang J., Kosynkin D. V., Bronikowski M. J., Smalley R. E., Tour J. M., J. Am. Chem. Soc. 2001, 123, 6536–6542. [DOI] [PubMed] [Google Scholar]
- 9. Merritt E. A., Olofsson B., Angew. Chem. Int. Ed. 2009, 48, 9052–9070; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 9214–9234. [Google Scholar]
- 10. Malmgren J., Santoro S., Jalalian N., Himo F., Olofsson B., Chem. Eur. J. 2013, 19, 10334–10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan C. K., Beechem T. E., Ohta T., Brumbach M. T., Wheeler D. R., Stevenson K. J., J. Phys. Chem. C 2013, 117, 12038–12044. [Google Scholar]
- 12. Hof F., Schäfer R. A., Weiss C., Hauke F., Hirsch A., Chem. Eur. J. 2014, 20, 16644–16651. [DOI] [PubMed] [Google Scholar]
- 13. Barthos R., Méhn D., Demortier A., Pierard N., Morciaux Y., Demortier G., Fonseca A., Nagy J. B., Carbon 2005, 43, 321–325. [Google Scholar]
- 14. Liang F., Sadana A. K., Peera A., Chattopadhyay J., Gu Z., Hauge R. H., Billups W. E., Nano Lett. 2004, 4, 1257–1260. [Google Scholar]
- 15. Vecera P., Edelthalhammer K. F., Hauke F., Hirsch A., Phys. Status Solidi B 2014, 251, 2536–2540. [Google Scholar]
- 16. Criado A., Melchionna M., Marchesan S., Prato M., Angew. Chem. Int. Ed. 2015, 54, 10734–10750; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 10882–10900. [Google Scholar]
- 17. Paulus G. L. C., Wang Q. H., Strano M. S., Acc. Chem. Res. 2013, 46, 160–170. [DOI] [PubMed] [Google Scholar]
- 18. Englert J. M., Knirsch K. C., Dotzer C., Butz B., Hauke F., Spiecker E., Hirsch A., Chem. Commun. 2012, 48, 5025–5027. [DOI] [PubMed] [Google Scholar]
- 19. Dresselhaus M. S., Dresselhaus G., Adv. Phys. 1981, 30, 139–326. [Google Scholar]
- 20. Enoki T., Suzuki M., Endo M., in Graphite intercalation compounds and applications, Oxford University Press, New York, 2003, pp. 9–10. [Google Scholar]
- 21. Chacón-Torres J. C., Ganin A. Y., Rosseinsky M. J., Pichler T., Phys. Rev. B 2012, 86, 0754061–0754066. [Google Scholar]
- 22. Amsharov K., Sharapa D. I., Vasilyev O. A., Oliver M., Hauke F., Goerling A., Soni H., Hirsch A., Carbon 2020, 158, 435–448. [Google Scholar]
- 23. Holzwarth J., Amsharov K. Y., Sharapa D. I., Reger D., Roshchyna K., Lungerich D., Jux N., Hauke F., Clark T., Hirsch A., Angew. Chem. Int. Ed. 2017, 56, 12184–12190; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 12352–12358. [Google Scholar]
- 24. Li J., Li M., Zhou L.-L., Lang S.-Y., Lu H.-Y., Wang D., Chen C.-F., Wan L.-J., J. Am. Chem. Soc. 2016, 138, 7448–7451. [DOI] [PubMed] [Google Scholar]
- 25. Sun Z., Pint C. L., Marcano D. C., Zhang C., Yao J., Ruan G., Yan Z., Zhu Y., Hauge R. H., Tour J. M., Nat. Commun. 2011, 2, 559. [DOI] [PubMed] [Google Scholar]
- 26. Wei T., Kohring M., Chen M., Yang S., Weber H. B., Hauke F., Hirsch A., Angew. Chem. Int. Ed. 2020, 59, 5602–5606; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 5651–5655. [Google Scholar]
- 27. Bao L., Zhao B., Lloret V., Halik M., Hauke F., Hirsch A., Angew. Chem. Int. Ed. 2020, 59, 6700–6705; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 6766–6771. [Google Scholar]
- 28. Liu H., Ryu S., Chen Z., Steigerwald M. L., Nuckolls C., Brus L. E., J. Am. Chem. Soc. 2009, 131, 17099–17101. [DOI] [PubMed] [Google Scholar]
- 29. den Hollander J. A., van der Ploeg J. P. M., Tetrahedron 1976, 32, 2433–2436. [Google Scholar]
- 30. Akihide K., Hirochika S., Masayuki Y., Katsumi T., Bull. Chem. Soc. Jpn. 1980, 53, 1393–1398. [Google Scholar]
- 31. Knirsch K. C., Schäfer R. A., Hauke F., Hirsch A., Angew. Chem. 2016, 128, 5956–5960. [Google Scholar]
- 32. Gao G., Liu D., Tang S., Huang C., He M., Guo Y., Sun X., Gao B., Sci. Rep. 2016, 6, 20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schäfer R. A., Dasler D., Mundloch U., Hauke F., Hirsch A., J. Am. Chem. Soc. 2016, 138, 1647–1652. [DOI] [PubMed] [Google Scholar]
- 34. Chen C.-M., Huang J.-Q., Zhang Q., Gong W.-Z., Yang Q.-H., Wang M.-Z., Yang Y.-G., Carbon 2012, 50, 659–667. [Google Scholar]
- 35. Cançado L. G., Jorio A., Ferreira E. H. M., Stavale F., Achete C. A., Capaz R. B., Moutinho M. V. O., Lombardo A., Kulmala T. S., Ferrari A. C., Nano Lett. 2011, 11, 3190–3196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


