Abstract
Aim of the study
To evaluate differences in the cellular expression of DNA damage/repair and reactive oxygen/nitrogen species between human periodontitis and peri‐implantitis lesions.
Material and methods
40 patients presenting with generalized severe periodontitis and 40 patients with severe peri‐implantitis were included. Soft tissue biopsies were collected from diseased sites in conjunction with surgical therapy and prepared for histological analysis. Four regions of interest were identified: the pocket epithelium (PE), the infiltrated connective tissue (ICT), which was divided into one inner area facing the PE (ICT‐1) and one outer area (ICT‐2). A non‐infiltrated connective tissue area (NCT) lateral of the ICT was also selected.
Results
It was demonstrated that the ICT of peri‐implantitis specimens was considerably larger and contained significantly larger area proportions and densities of CD68‐, MPO‐ and iNOS‐positive cells than that of periodontitis samples. Cellular densities were overall higher in the inner ICT zone lateral of the PE (ICT‐1) than in the outer ICT compartment (ICT‐2). While the NCT area lateral of the ICT comprised significantly larger proportions and densities of y‐H2AX‐, iNOS‐, NOX2‐, MPO‐ and PAD4/MPO‐positive cells in peri‐implantitis than in periodontitis sites, a reverse difference was noted for the area proportion and density of 8‐OHdG‐positive cells in the PE.
Conclusions
It is suggested that peri‐implantitis lesions are associated with an enhanced and upregulated host response and contain larger numbers of neutrophils, macrophages and iNOS‐positive cells than periodontitis lesions.
Keywords: biopsy, cell density, histology, immunohistochemistry, inflammation, peri‐implant disease, periodontal disease
Clinical relevance.
Scientific rationale: Peri‐implantitis lesions were found to be considerably larger and contained greater densities of plasma cells, neutrophils and macrophages than periodontitis lesions. The numerous functional mechanisms among neutrophils and other front‐line cells should be considered when evaluating histopathological differences between the two lesions.
Principle findings: The present study demonstrated that the ICT of peri‐implantitis specimens contained significantly larger densities of CD68‐, MPO‐ and iNOS‐positive cells than that of periodontitis samples. Cellular densities were overall higher in the inner ICT zone lateral of the pocket epithelium than in the outer ICT compartment. While the non‐infiltrated connective tissue area comprised significantly larger densities of y‐H2AX‐, iNOS‐, NOX2‐, MPO‐ and PAD4/MPO‐positive cells in peri‐implantitis than in periodontitis sites, a reverse difference was noted for the density of 8‐OHdG‐positive cells in the pocket epithelium.
Practical implications: It is suggested that peri‐implantitis lesions are associated with an enhanced and upregulated host response. Moreover, as the NCT area was located outside the ICT and at a further distance from the pocket area than the ICT, the observation that the majority of the functional cell markers occurred in larger quantities in peri‐implantitis than in periodontitis specimens is remarkable.
1. INTRODUCTION
Disease definitions for periodontitis and peri‐implantitis were presented at the 2017 World Workshop on Classification of Periodontal and Peri‐Implant Diseases and Conditions. Thus, periodontitis is a chronic inflammatory disease associated with dysbiotic dental plaque biofilms and characterized by progressive destruction of the tooth‐supporting apparatus, including loss of clinical attachment and alveolar bone (Papapanou et al., 2018; Sanz et al., 2020). Peri‐implantitis is a plaque‐associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri‐implant mucosa and progressive loss of the supporting bone (Berglundh et al., 2018; Schwarz et al., 2018). While the two diseases have several clinical features in common, for example clinical signs of inflammation, bleeding on probing, increased probing depth, in addition to loss of supporting tissues, peri‐implantitis seems to progress in a non‐linear and accelerating pattern and in a rate that is faster than that of periodontitis (Berglundh et al., 2018; Derks et al., 2016; Fransson et al., 2010; Schwarz et al., 2018).
Analysis of samples obtained from pre‐clinical in vivo experiments revealed that peri‐implantitis lesions were larger and contained higher numbers of neutrophils and osteoclasts than periodontitis lesions. In addition, peri‐implantitis sites were poorly encapsulated, extended to the crestal bone and lacked an epithelial lining between the lesion and the biofilm in the apical portion of the pocket (Carcuac et al., 2013; Lindhe et al., 1992). While interpretation of data obtained from pre‐clinical studies is restricted to the design of the experimental models, analysis of human biopsy material from periodontitis and peri‐implantitis sites provides unique and accurate assessments of the lesion characteristics. Such specimens, however, are for obvious ethical reasons limited to the soft tissue components of the diseased site (Berglundh et al., 2011; Schwarz et al., 2018). Early reports on analysis of human peri‐implantitis lesions were based on small patient samples, and results were presented in descriptive terms of high densities of plasma cells, lymphocytes, neutrophils and macrophages (Berglundh et al., 2004; Gualini & Berglundh, 2003; Piattelli et al., 1998; Sanz et al., 1991). In studies comparing periodontitis and peri‐implantitis lesions in humans (Becker et al., 2014; Bullon et al., 2004; Carcuac & Berglundh, 2014; Fretwurst et al., 2020; Galindo‐Moreno et al., 2017; Ghighi et al., 2018; Karatas et al., 2019; Kasnak et al., 2018; Konttinen et al., 2006), peri‐implantitis lesions were found to be considerably larger and contained greater densities of plasma cells, neutrophils and macrophages than periodontitis lesions.
The observation of enhanced numbers and densities of neutrophils in peri‐implantitis lesions underlines the severity of the disease. As neutrophils represent the front‐line of host response against pathogens, the majority of this cell group is expected to reside in pocket‐associated connective tissue compartments in both periodontitis and peri‐implantitis lesions. Previous reports on human peri‐implantitis samples, however, have indicated that neutrophils also occur in high numbers in central portions of the lesion (Berglundh et al., 2004; Gualini & Berglundh, 2003). In addition to the observed variation in density and location of neutrophils between periodontitis and peri‐implantitis samples, differences in functional aspects of the cells may also exist. Thus, neutrophils operate with several functions to eliminate microorganisms, including the “respiratory burst” which leads to the production of superoxide (O2 −) and other reactive oxygen species (ROS) (El‐Benna et al., 2016). Other mechanisms, for example NADPH oxidases (NOX) and PAD4‐mediated histone citrullination, are involved in the neutrophil extracellular trap (NET) release (Rohrbach et al., 2012; White et al., 2016), while nitric oxide synthases (NOS) contribute to the production of nitric oxide (NO) (Saini & Singh, 2019).
Even though neutrophils provide a host protective role and their antimicrobial products are physiologically balanced by antioxidant systems (Kanzaki et al., 2017; Wang et al., 2017), the host response may also generate cytotoxic and genotoxic side effects (Hajishengallis, 2020). Thus, DNA damage in cells may occur either by the formation of 8‐oxo‐guanine (8‐OHdG) (Salehi et al., 2018) or phosphorylation of the H2A histone variant γ‐H2AX (Löbrich et al., 2010). As a consequence, there is also a cellular response to DNA damage including the activation of the checkpoint kinase 2 (Chk2) (Zannini et al., 2014). Taken together, the numerous functional mechanisms among neutrophils and other front‐line cells should be considered when evaluating histopathological differences between periodontitis and peri‐implantitis. In a continuing evaluation of human samples representing severe periodontitis and advanced peri‐implantitis, initially reported by Carcuac and Berglundh (2014), the aim of the present investigation was to analyse differences in the cellular expression of DNA damage/repair and reactive oxygen/nitrogen species (RONS) between human periodontitis and peri‐implantitis lesions.
2. MATERIAL AND METHODS
Forty patients (24 women, eleven smokers, mean age 64 ± 11.45 years, range 40‐89) with generalized severe chronic periodontitis and 40 patients (23 women, eleven smokers, mean age 70 ± 10.41 years, range 46‐93) with severe peri‐implantitis were recruited from the Clinics of Periodontics in Gothenburg and Mölndal, Public Dental Services, Region Västra Götaland, Sweden. The study protocol was approved by the local Human Review Board of Gothenburg (Dnr 245‐10). Details on the recruitment of patients and biopsy sampling procedures were previously described by Carcuac and Berglundh (2014). In brief, before enrolment, all subjects received information about the study and signed an informed consent. Patients were excluded if they had undergone periodontal or peri‐implant therapy during the last 6 months and if they presented any systemic disease that could have affected the periodontal and peri‐implant tissue conditions. All patients received a detailed and individualized case presentation, oral hygiene instructions and a professional supra‐gingival cleaning prior to the study. Soft tissue biopsies were collected from diseased sites in patients with periodontitis [bone loss ≥50% and probing pocket depth (PPD) ≥7 mm with bleeding on probing (BoP)] and peri‐implantitis [peri‐implant bone loss ≥3 mm and PPD ≥7 mm, with BoP and/or suppuration at ≥1 implant ‐ function time for implants 2‐10 years]. The biopsies were obtained in conjunction with periodontal/peri‐implantitis surgery or tooth/implant removal, mounted in a plastic cassette (Tissue‐Tek Paraform Sectionable Cassette System; Sakura Finetek Europe, Netherlands) and placed in 4% buffered formalin for 48 hr.
2.1. Immunochemistry
The tissue samples were stored in 70% ethanol, kept at 4°C, subsequently dehydrated and embedded in paraffin. From each tissue portion, 5‐µm‐thick sections were produced in a microtome, dewaxed and incubated in DIVA antigen‐retrieval solution (Biocare Medical, Histolab, Concord, CA, USA) at 60°C overnight. The sections were incubated with a primary antibody for 30 min followed by incubation with Envision horseradish peroxidase (HRP)‐labelled polymer (Agilent, Santa Clara, CA, USA) for 30 min. Positive cells were detected using DAB substrate (Agilent). The antibodies used for the immunohistochemical preparations and their dilutions are presented in Table 1. The γ‐H2AX marker (EMD Millipore Corporation) was used to identify DNA‐damaged sites caused by double‐stranded breaks (DSB), while the 8‐OHdG marker (Abcam) was used to estimate the DNA damage caused by oxidative stress induced by oxygen species (ROS). The Chk2 marker (Abcam) indicated DNA damage response (DDS) involved in the repair of DNA damage. Neutrophils/polymorphonuclear cells were identified by monoclonal rabbit anti‐human antibody myeloperoxidase (MPO) (Agilent), while the CD68 monoclonal antibody (Agilent) was used to detect macrophages. The iNOS marker (Abcam) was used to identify the secretion of antimicrobial NO and the NOX2 marker (Abcam) indicated the production of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. Finally, the formation of NETs was identified by double staining using a combination of MPO and PAD4 markers (Abcam). Counterstaining was performed with haematoxylin. Finally, the sections were mounted and coverslipped. Human oral mucosa tissue sections were used as positive controls, while negative controls were produced by substituting the primary antibody with non‐immune serum.
Table 1.
Description of the antibodies used for the immunohistochemical analysis
| Antibody | Type | Dilution | Target | Isotype | Company |
|---|---|---|---|---|---|
| γ‐H2AX | Rabbit monoclonal | 1:300 | DNA double‐strand breaks | 9F3 | EMD Millipore Corporation, Temecula, CA USA |
| 8‐OHdG | Mouse monoclonal | 1:8,000 | ROS oxidative stress | IgG2b | GeneTex, Irvine, CA, USA |
| Chk2 | Rabbit polyclonal | 1:400 | DNA repair | Poly IgG | Abcam, Cambridge, UK |
| MPO | Rabbit polyclonal | 1:1,500 | Polymorphonuclear cells | Poly IgG | Agilent, Santa Clara, CA, USA |
| CD68 | Mouse monoclonal | 1:200 | Macrophages | IgG3 | Agilent, Santa Clara, CA, USA |
| iNOS | Rabbit polyclonal | 1:50 | Antimicrobial NO | Poly IgG | Abcam, Cambridge, UK |
| NOX2 | Mouse monoclonal | 1:500 | Antimicrobial NADPH | IgG1 | Abcam, Cambridge, UK |
| PAD4/MPO | Rabbit polyclonal | 1:100/1:1,500 | Neutrophils NETs | Poly IgG | Abcam, Cambridge, UK; Agilent, Santa Clara, CA, USA |
Abbreviations: γ‐H2AX, phosphorylated histone family member X; 8‐OHdG, 8‐hydroxyguanosine; Chk2, checkpoint kinase 2; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2; PAD4, peptidylarginine deiminase 4; ROS, reactive oxygen species; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; NETs, neutrophil extracellular traps.
2.2. Histological analysis
Qualitative and quantitative histological examinations of the inflammatory cell infiltrate were performed using a microscope (Leitz DM‐RBE microscope, Leica, Wetzlar, Germany). Each section was captured with a Glissando Desktop Scanner (Objective Imaging Inc, Kansasville, WI, USA) and transferred to a computer equipped with the computerized image analysis software Image‐Pro Premier (IPP, version 10; Media Cybernetics Inc., Rockville, MD, USA). The examinations were performed by one trained investigator (C.D) who was blinded to the origin of the samples. A mouse cursor was used to outline the different regions of interest (ROIs) (Figure 1). Thus, the entire area of the infiltrated connective tissue (ICT) lateral of the pocket epithelium (PE) was depicted and divided into two equivalent ROIs: one inner area, facing the PE (ICT‐1), and one outer area (ICT‐2). The pocket epithelium (PE) was also selected as an ROI. In addition, a non‐infiltrated connective tissue area (NCT) of about 0.10–0.50 mm2 lateral of the ICT was selected and designated as ROI in each specimen. The smart segmentation tool of the IPP software was used to identify each cell marker, using a differential method analysis of colour, intensity, morphology and size. Thus, the total area occupied by positive cells was assessed for each marker and its percentage area relative to the area of the ROI was calculated. Lastly, the average cell size for each cell marker category was assessed from 10 randomly selected sections. The number of positive cells in the different ROIs was computed using the data from the ROIs total area, the average cell size and the total area occupied by the positive cells. Cell numbers were expressed as total number and density of cells (number of cells/mm2) within the ROI.
Figure 1.
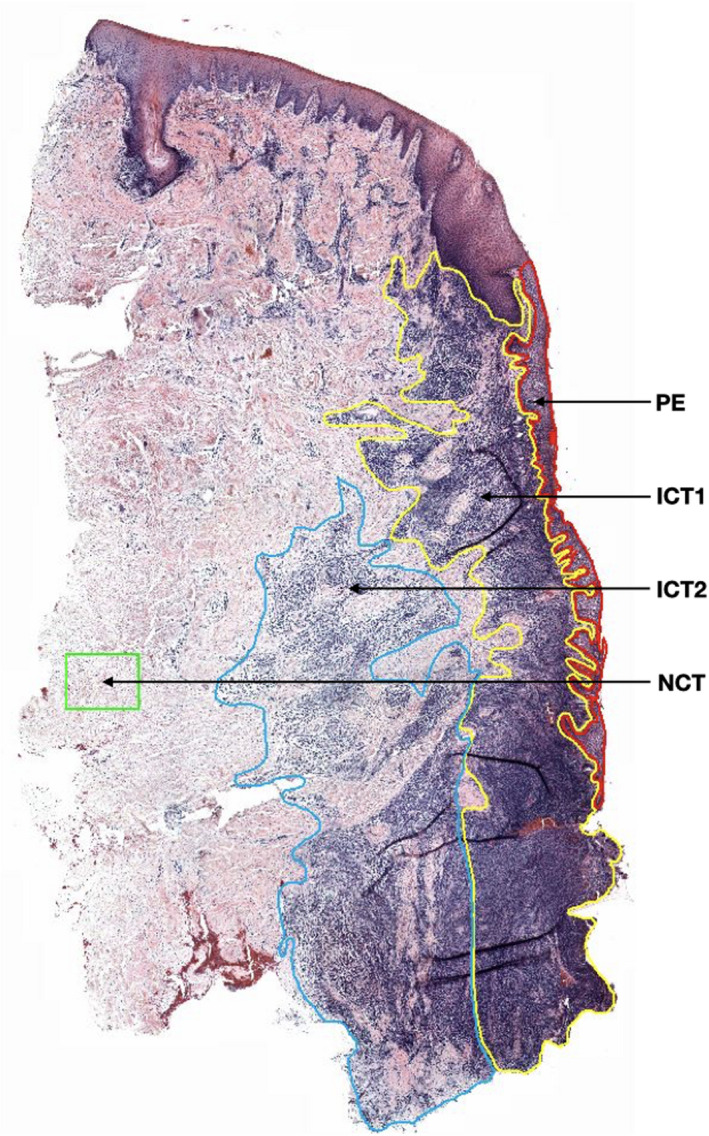
Histological section showing the different regions of interest (ROIs). PE (pocket epithelium—in red), ICT‐1 (inner portion of the infiltrated connective tissue—in yellow), ICT‐2 (outer portion of the infiltrated connective tissue—in blue), NCT (non‐infiltrated connective tissue—in green). Haematoxylin and eosin marker
2.3. Data analysis
Mean values and standard deviations were calculated for each variable and patient. The Mann–Whitney U test for independent variables was used for comparisons between patient groups (peri‐implantitis vs periodontitis), while differences in densities between ROIs within the same disease category were analysed using the non‐parametric Wilcoxon signed‐rank test for dependent variables. The possible confounding effects of smoking on the results were analysed using the Kruskal–Wallis test for independent variables. The null hypothesis was rejected at p < 0.05. As previously reported by Carcuac and Berglundh (2014), a difference in area proportions of cells of 3% between peri‐implantitis and periodontitis lesions required a sample size of 30 subjects in each group, based on an α of 0.05, a standard deviation of 1.1%–2.5% and a power of 80%.
3. RESULTS
There were no statistically significant differences regarding age and gender distribution between the 2 patient groups. The number of smokers was equally distributed between the periodontitis and the peri‐implantitis groups. There were no significant differences between smokers and non‐smokers for any of the tested biomarkers (significance values adjusted by the Bonferroni correction for multiple tests >0.92).
Micrographs of periodontitis and peri‐implantitis specimens representing each cell marker are illustrated in Figure 2. The results from the analysis of the total area of ROIs and area proportions of positive cells are presented in Table 2. The areas of the ICT and PE were larger in peri‐implantitis than in periodontitis sections (4.23 ± 0.19 mm2 vs. 2.44 ± 0.31 mm2 and 0.77 ± 0.20 mm2 vs 0.64 ± 0.20 mm2, respectively). These differences were statistically significant. Chk2‐positive cells dominated among positive cells and occupied about 15%–16% of the ICT and PE areas in both periodontitis and peri‐implantitis lesions. The area proportions of the ICT occupied by CD68‐, MPO‐ and iNOS‐positive cells were significantly greater in peri‐implantitis than in periodontitis lesions, while a reverse difference between the two types of tissue samples was observed for the area proportions of the 8‐OHdG‐positive cells in PE. The area proportions of the reference‐NCT area occupied by γ‐H2AX‐, iNOS‐, NOX2‐, MPO‐ and PAD4/MPO‐positive cells were significantly greater in peri‐implantitis than in periodontitis sites.
Figure 2.
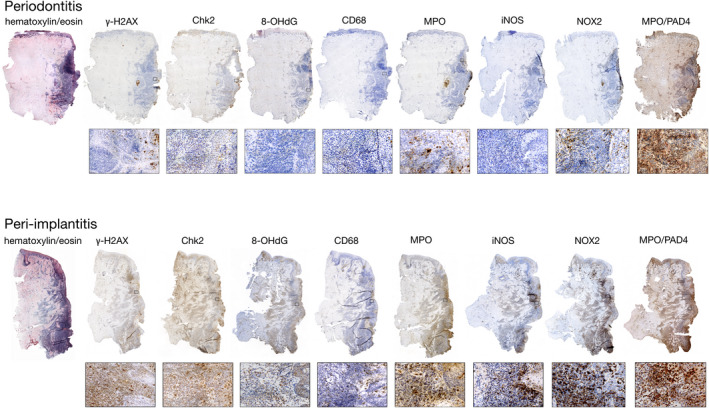
Histological sections prepared from periodontitis and peri‐implantitis sites. Pocket area located to the right. Markers identified in the text. Magnifications ×20 and ×400
Table 2.
ROI total area (mm2) and area proportions of positive cells (%) in periodontitis (n = 40) and peri‐implantitis (n = 40) sites
| ROI | Lesion | Total area (mm2) | Positive area (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γ‐H2AX | 8‐OHdG | Chk2 | MPO | CD68 | iNOS | NOX2 | MPO/PAD4 | |||
| ICT | Periodontitis | 2.44 ± 0.31 | 3.01 ± 2.78 | 3.09 ± 3.89 | 15.10 ± 9.76 | 3.21 ± 3.03 | 1.32 ± 1.71 | 4.95 ± 4.32 | 5.56 ± 4.98 | 0.14 ± 0.31 |
| Peri‐implantitis | 4.23 ± 0.19* | 3.37 ± 2.39 | 1.81 ± 3.04 | 15.81 ± 12.49 | 8.95 ± 8.56* | 5.12 ± 2.77* | 7.56 ± 5.32* | 7.02 ± 7.80 | 0.17 ± 0.25 | |
| PE | Periodontitis | 0.64 ± 0.20 | 3.79 ± 5.19 | 4.235 ± 4.99 | 16.97 ± 11.65 | 4.42 ± 4.89 | 0.59 ± 0.93 | 7.33 ± 7.41 | 2.97 ± 3.53 | 0.20 ± 0.33 |
| Peri‐implantitis | 0.77 ± 0.20* | 5.28 ± 6.17 | 2.92 ± 5.86* | 18.20 ± 15.21 | 7.06 ± 6.86 | 0.64 ± 0.96 | 8.89 ± 7.30 | 4.95 ± 6.43 | 0.45 ± 0.76 | |
| NCT | Periodontitis | 0.40 ± 0.04 | 0.27 ± 0.28 | 0.43 ± 0.46 | 3.63 ± 3.31 | 0.35 ± 1.31 | 0.17 ± 0.18 | 0.74 ± 9.63 | 0.10 ± 0.16 | 0.00 ± 0.01 |
| Peri‐implantitis | 0.35 ± 0.04 | 0.59 ± 0.44* | 0.42 ± 0.77 | 4.10 ± 4.67 | 0.42 ± 0.91* | 0.29 ± 0.30 | 1.19 ± 0.90* | 0.63 ± 1.76* | 0.01 ± 0.02* | |
Values in mean ± SD.
Abbreviations: ICT, infiltrated connective tissue; PE, pocket epithelium; NCT, non‐infiltrated connective tissue; γ‐H2AX, phosphorylated histone family member X; 8‐OHdG, 8 hydroxyguanosine; Chk2, checkpoint kinase 2; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2; PAD4, peptidylarginine deiminase 4.
p < 0.05 (Mann–Whitney U test).
The results from the assessment of the total number of positive cells within the ROIs are depicted in Table 3. The numbers of γ‐H2AX‐, iNOS‐, MPO‐, PAD‐4/MPO‐ and CD68‐positive cells in the ICT were significantly higher in peri‐implantitis than in periodontitis specimens. The PE in peri‐implantitis sites contained significantly greater numbers of MPO‐positive cells and smaller numbers of 8‐OHdG‐positive cells than that in periodontitis sites. The NCT exhibited a significantly larger number of γ‐H2AX‐, MPO‐, PAD4/MPO‐ and CD68‐positive cells in peri‐implantitis than in periodontitis sites.
Table 3.
Cell size (µm2), total estimated number of positive cells and density of positive cells (cells/mm2) in periodontitis (n = 40) and peri‐implantitis (n = 40) sites
| γ‐H2AX | 8‐OHdG | Chk2 | MPO | CD68 | iNOS | NOX2 | MPO/PAD4 | |
|---|---|---|---|---|---|---|---|---|
| Cell size (µm2) | 34.97 | 47.86 | 29.73 | 47.05 | 86.10 | 45.49 | 72.12 | 41.61 |
| ICT | ||||||||
| Total no. of cells | ||||||||
| Periodontitis | 2314 ± 5519 | 1439 ± 3081 | 12033 ± 14059 | 1182 ± 2722 | 262 ± 622 | 2330 ± 2347 | 1918 ± 2053 | 74 ± 133 |
| Peri‐implantitis | 4178 ± 4929* | 1735 ± 3952 | 25171 ± 29969 | 10324 ± 18080* | 2746 ± 2667* | 7558 ± 8317* | 4841 ± 7343 | 178 ± 320* |
| No. of cells/mm2 | ||||||||
| Periodontitis | 860 ± 795 | 645 ± 812 | 5079 ± 3282 | 682 ± 644 | 154 ± 199 | 1087 ± 950 | 771 ± 691 | 33 ± 74 |
| Peri‐implantitis | 962 ± 683 | 378 ± 636 | 5317 ± 4203 | 1901 ± 1818* | 594 ± 322* | 1663 ± 1169* | 974 ± 1081 | 41 ± 59 |
| PE | ||||||||
| Total no. of cells | ||||||||
| Periodontitis | 973 ± 1741 | 586 ± 729 | 4632 ± 4910 | 270 ± 440 | 20 ± 34 | 1314 ± 1948 | 386 ± 609 | 43 ± 78 |
| Peri‐implantitis | 863 ± 845 | 405 ± 1162* | 5048 ± 5799 | 574 ± 899* | 29 ± 48 | 1668 ± 1777 | 387 ± 411 | 83 ± 133 |
| No. of cells/mm2 | ||||||||
| Periodontitis | 1085 ± 1485 | 888 ± 1043 | 5707 ± 3917 | 941 ± 1040 | 69 ± 108 | 1611 ± 1629 | 412 ± 490 | 49 ± 80 |
| Peri‐implantitis | 1509 ± 1765 | 610 ± 1224* | 6122 ± 5117 | 1499 ± 1459 | 74 ± 111 | 1955 ± 1606 | 686 ± 892 | 107 ± 184 |
| NCT | ||||||||
| Total no. of cells | ||||||||
| Periodontitis | 32 ± 31 | 29 ± 31 | 439 ± 428 | 9 ± 31 | 6 ± 7 | 67 ± 53 | 5 ± 7 | 0.38 ± 0.88 |
| Peri‐implantitis | 67 ± 57* | 26 ± 44 | 419 ± 440 | 26 ± 55* | 12 ± 15* | 103 ± 89 | 22 ± 75 | 0.57 ± 1.03* |
| No. of cells/mm2 | ||||||||
| Periodontitis | 78 ± 79 | 89 ± 97 | 1222 ± 1114 | 74 ± 279 | 19 ± 21 | 163 ± 139 | 13 ± 22 | 1 ± 2 |
| Peri‐implantitis | 168 ± 127* | 88 ± 162 | 1379 ± 1572 | 88 ± 193* | 33 ± 35 | 261 ± 198* | 87 ± 244* | 2 ± 4* |
Values in mean ± SD.
Abbreviations: ICT, infiltrated connective tissue; PE, pocket epithelium; NCT, non‐infiltrated connective tissue; γȀH2AX, phosphorylated histone family member X; 8‐OHdG, 8 hydroxyguanosine; Chk2, checkpoint kinase 2; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2; PAD4, peptidylarginine deiminase 4.
p < 0.05 (Mann–Whitney U test).
The results from the analysis of cellular densities within the different ROIs are reported in Table 3. The ICT contained significantly higher densities of iNOS‐, MPO‐ and CD68‐positive cells in peri‐implantitis than in periodontitis lesions. The density of MPO‐positive cells in the ICT of peri‐implantitis specimens was about 3 times larger than that in periodontitis lesions. Within the PE, 8‐OHdG‐positive cells presented with a significantly lower density in peri‐implantitis than in periodontitis sites. The NCT in peri‐implantitis samples exhibited significantly higher densities of γ‐H2AX‐, iNOS‐, NOX2‐, MPO‐ and PAD4/MPO‐positive cells than the NCT in periodontitis specimens. The density of NOX2‐positive cells was almost 6 times larger than the corresponding density in periodontitis lesions. There were marked differences in distribution of cellular densities between ICT‐1 and ICT‐2 of both periodontitis and peri‐implantitis sites (Table 4). Thus, ICT‐1 exhibited overall about 1.5–3 times higher cellular densities than ICT‐2. These differences were statistically significant for each cell marker.
Table 4.
Relative density (cells/mm2) of positive cells in ICT‐1 and ICT‐2 of periodontitis (n = 40) and peri‐implantitis (n = 40) sites
| Lesion | ROI | Density of positive cells (cells/mm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| γ‐H2AX | 8‐OHdG | Chk2 | MPO | CD68 | iNOS | NOX2 | MPO/PAD4 | ||
| Periodontitis | ICT1 | 1160 ± 1033 | 834 ± 961 | 6430 ± 4075 | 1019 ± 1001 | 193 ± 242 | 1340 ± 1185 | 1190 ± 1091 | 61 ± 193 |
| ICT2 | 613 ± 737* | 471 ± 709* | 4017 ± 3279* | 360 ± 431* | 116 ± 170* | 869 ± 864* | 467 ± 480* | 17 ± 25* | |
| Peri‐implantitis | ICT1 | 1243 ± 1098 | 445 ± 621 | 6086 ± 4780 | 2417 ± 2218 | 626 ± 365 | 2090 ± 1497 | 1280 ± 1323 | 63 ± 98 |
| ICT2 | 728 ± 613* | 316 ± 675* | 4599 ± 382* | 1344 ± 1488* | 560 ± 289* | 1245 ± 950* | 686 ± 910* | 22 ± 28* | |
Values in mean ± SD.
Abbreviations: ICT‐1, inner portion of infiltrated connective tissue; ICT‐2, outer portion of infiltrated connective tissue; γ‐H2AX, phosphorylated histone family member X; 8‐OHdG, 8 hydroxyguanosine; Chk2, checkpoint kinase 2; MPO, myeloperoxidase; iNOS, inducible nitric oxide synthase; NOX2, NADPH oxidase 2; PAD4, peptidylarginine deiminase 4.
p < 0.05 (Wilcoxon signed‐rank test).
4. DISCUSSION
The present study evaluated differences in the cellular expression of DNA damage/repair and RONS between human periodontitis and peri‐implantitis lesions. It was demonstrated that the ICT of peri‐implantitis specimens was considerably larger and contained significantly larger area proportions and densities of CD68‐, MPO‐ and iNOS‐positive cells than that of periodontitis samples. Cellular densities were overall higher in the inner ICT zone lateral of the PE (ICT‐1) than in the outer, deeply located ICT compartment (ICT‐2). While the NCT area lateral of the ICT comprised significantly larger proportions and densities of γ‐H2AX‐, iNOS‐, NOX2‐, MPO‐ and PAD4/MPO‐positive cells in peri‐implantitis than in periodontitis sites, a reverse difference was noted for the area proportion and density of 8‐OHdG‐positive cells in the PE. It is suggested that peri‐implantitis lesions are larger and present with an enhanced and upregulated local host response in comparison with periodontitis lesions.
Histological findings on phenotype markers in the human specimens of the present study were previously presented by Carcuac and Berglundh (2014). Thus, the observation on differences in size of the ICT together with numbers and densities of MPO‐ and CD68‐positive cells in the present study is in agreement with data previously reported. In this context, it should be emphasized that different histological methods for the cell counting procedures were applied. While Carcuac and Berglundh (2014) used a well‐established point‐counting procedure to assess the percentage of positive cell markers within the ICT (e.g. Liljenberg et al., 1994; Zitzmann et al., 2001), a new quantitative method that included analysis of colour, intensity, morphology and size of cells was employed in the current investigation. The high consistency of results regarding numbers and densities of MPO‐ and CD68‐positive cells between the present study and Carcuac and Berglundh (2014) validates the accuracy of the methods. Other studies have also reported on differences between human peri‐implantitis and periodontitis lesions. Galindo‐Moreno et al. (2017) analysed soft tissue biopsies harvested from 15 subjects with peri‐implantitis and 15 with periodontitis. The histological analysis revealed that the inflammatory infiltrate in peri‐implantitis samples was more severe and contained higher proportions of plasma cells. Ghighi et al. (2018) analysed soft tissue biopsies obtained from sites with persisting disease following initial mechanical therapy in 11 patients with peri‐implantitis and 10 patients with periodontitis. It was reported that peri‐implantitis samples exhibited greater numbers of CD3‐, CD20‐ and CD68‐positive cells and an increased expression of tissue inhibitor of matrix metalloproteinase‐2 when compared to periodontitis and healthy sites. The findings presented by Galindo‐Moreno et al. (2017) and Ghighi et al. (2018) are in agreement with data obtained from the present material and underline the consistency of differences between peri‐implantitis and periodontitis lesions.
One of the main differences between peri‐implantitis and periodontitis lesions observed in the present study was the cellular expression of iNOS. Not only the ICT area but also the NCT compartment of peri‐implantitis specimens contained larger proportions and densities of iNOS‐positive cells than the corresponding regions in periodontitis samples. Similar findings were presented by Fretwurst et al. (2020) who analysed iNOS‐positive cells in a study on macrophage M1/M2 polarization in peri‐implantitis and periodontitis lesions in 14 patients. The analysis revealed that the number of cells positive for the double staining of CD68 and iNOS (M1 polarization) was higher in peri‐implantitis than in periodontitis lesions. On the other hand, Karatas et al. (2019) in a histological study on gingival and peri‐implant soft tissue biopsies collected from 60 patients reported that the expression of iNOS was lower in peri‐implant mucositis than in periodontitis and peri‐implantitis specimens, while no difference was observed between periodontitis and peri‐implantitis sites. Nevertheless, the observed higher number and densities of macrophages (CD68), neutrophils (MPO) and iNOS‐positive cells in peri‐implantitis lesions in the present study indicate an enhanced local host response, which is line with observations on a more pronounced rate of disease progression in peri‐implantitis as opposed to periodontitis (Berglundh et al., 2018; Derks & Tomasi, 2015).
The dominating cell marker in both types of specimens in the present material was the Chk2, which indicates expression of DNA repair mechanisms (Jackson & Bartek, 2009). The density of this marker was considerably higher than other markers but did not differ between peri‐implantitis and periodontitis samples in any of the ROIs. While the expression of DNA repair was higher in the ICT and PE compartments than in the NCT region of both tissue types, the cellular density of this marker in the NCT was about 10 times higher than that of the other markers. Thus, DNA repair appears to be a common feature of cells in normal tissues but becomes upregulated in inflamed areas. In this context, it is notable that the number and density of cells positive for the 8‐OHdG marker indicating ROS‐induced DNA damage within the PE were higher in periodontitis than in peri‐implantitis specimens. A similar trend was also observed in the ICT area. These findings are partly in contrast with data presented by Kasnak et al. (2018). They evaluated the expression of 8‐OHdG in the sulcular/PE of soft tissue biopsies obtained from patients with peri‐implantitis, periodontitis and periodontally healthy tissues. It was reported that the expression of 8‐OHdG was higher in peri‐implantitis and periodontitis specimens than in healthy tissue samples, while no differences were observed between peri‐implantitis and periodontitis. Based on the results presented by Kasnak et al. (2018) and the data from the present study showing considerably lower densities in the NCT than in the PE and ICT, it is evident that the 8‐OHdG is a biomarker for inflammation. Similar results were reported from a meta‐analysis of data on salivary samples from patients with periodontitis and periodontally healthy individuals (Paredes‐Sánchez et al., 2018). The differences in number and density of cells positive for the 8‐OHdG marker in PE between periodontitis and peri‐implantitis sites in the present study should also be viewed in the context of variations in dimensions of soft tissue components and histopathological characteristics between peri‐implantitis and periodontitis sites as described in previous reports (Berglundh et al., 2011, 2018; Carcuac & Berglundh, 2014; Schwarz et al., 2018). Thus, the PE in peri‐implantitis sites does not cover the entire pocket area, which results in an uncovered apical 3rd of an inflamed connective tissue facing the biofilm in the pocket. In addition, due to the lack of a periodontal ligament, the ICT of peri‐implantitis spreads to the crestal bone. Taken together, the ICT in peri‐implantitis, as opposed to the ICT in periodontitis sites, is larger and extends apical of the PE (Berglundh et al., 2019). This feature should also be addressed regarding the finding of higher densities and numbers of iNOS‐positive cells in peri‐implantitis than in periodontitis, which indicates an enhanced secretion of the antimicrobial NO in the conspicuous exposure of pathogens to the uncovered apical part of the ICT of peri‐implantitis pocket area.
The present study also detected differences in cell numbers and densities in the NCT area between the two types of tissue samples. The NCT area was selected as a reference region that was clearly separated from the ICT. Although cell densities and numbers were overall considerably smaller in the NCT than in the ICT and PE areas in both tissue categories, the NCT in peri‐implantitis specimens showed 2–6 times higher levels of MPO‐, iNOS‐, NOX2‐ and MPO/PAD4‐positive cells and a higher expression of γ‐H2AX than the NCT of periodontitis sites. The importance of this observation is unclear as the expression of the present cell markers is a typical characteristic for inflammation. Thus, NOX are involved in host defence mechanisms including the formation of superoxide ions and release of NET (Bedard & Krause, 2007; White et al., 2016), while PAD4‐mediated histone citrullination promotes the release of antimicrobial molecules outside the cellular compartment (Rohrbach et al., 2012). The NCT is by its definition a NCT area consisting of normal connective tissue constituents. As the NCT area was located outside the ICT and at a further distance from the pocket area than the ICT, the observation that the majority of the functional cell markers occurred in larger quantities in peri‐implantitis than in periodontitis specimens is remarkable.
With regard to the design of the present study of using data obtained from analysis of cell markers in histological specimens, limitations concerning interpretation of results should be considered. Thus, cellular expression of various markers in a biopsy material represents a cross‐sectional approach and may not necessarily reveal an ongoing process or molecular production. This limitation is not unique for the present study as it refers to investigations overall using histological preparations of biopsy material. It is, however, important to point out that several of the markers used in the present protocol indicate functional features and results should be interpreted accordingly.
In conclusion, the destructive nature of peri‐implantitis lesions is associated with an enhanced and upregulated host response including larger numbers of neutrophils, macrophages and iNOS‐positive cells than periodontitis lesions. While structural differences between periodontal and peri‐implant tissues may explain variations in the spread of the lesions, other potential factors produced by the local environment of the titanium device should be considered.
REFERENCES
- Becker, S. T. , Beck‐Broichsitter, B. E. , Graetz, C. , Dörfer, C. E. , Wiltfang, J. , & Häsler, R. (2014). Peri‐implantitis versus periodontitis: Functional differences indicated by transcriptome profiling. Clinical Implant Dentistry and Related Research, 16(3), 401‐411. [DOI] [PubMed] [Google Scholar]
- Bedard, K. , & Krause, K. H. (2007). The NOX family of ROS‐generating NADPH oxidases: Physiology and pathophysiology. Physiological Reviews, 87(1), 245‐313. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Armitage, G. , Araujo, M. G. , Avila‐Ortiz, G. , Blanco, J. , Camargo, P. M. , Chen, S. , Cochran, D. , Derks, J. , Figuero, E. , Hämmerle, C. H. F. , Heitz‐Mayfield, L. J. A. , Huynh‐Ba, G. , Iacono, V. , Koo, K.‐T. , Lambert, F. , McCauley, L. , Quirynen, M. , Renvert, S. , … Zitzmann, N. (2018). Peri‐implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Clinical Periodontology, 45(Suppl 20), S286‐S291. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Gislason, O. , Lekholm, U. , Sennerby, L. , & Lindhe, J. (2004). Histopathological observations of human periimplantitis lesions. Journal of Clinical Periodontology, 31(5), 341‐347. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Jepsen, S. , Stadlinger, B. , & Terheyden, H. (2019). Peri‐implantitis and its prevention. Clinical Oral Implants Research, 30(2), 150‐155. [DOI] [PubMed] [Google Scholar]
- Berglundh, T. , Zitzmann, N. U. , & Donati, M. (2011). Are peri‐implantitis lesions different from periodontitis lesions? Journal of Clinical Periodontology, 38(Suppl 11), 188‐202. [DOI] [PubMed] [Google Scholar]
- Bullon, P. , Fioroni, M. , Goteri, G. , Rubini, C. , & Battino, M. (2004). Immunohistochemical analysis of soft tissues in implants with healthy and peri‐implantitis condition, and aggressive periodontitis. Clinical Oral Implants Research, 15(5), 553‐559. [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , Abrahamsson, I. , Albouy, J. P. , Linder, E. , Larsson, L. , & Berglundh, T. (2013). Experimental periodontitis and peri‐implantitis in dogs. Clinical Oral Implants Research, 24(4), 363‐371. [DOI] [PubMed] [Google Scholar]
- Carcuac, O. , & Berglundh, T. (2014). Composition of human peri‐implantitis and periodontitis lesions. Journal of Dental Research, 93(11), 1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks, J. , Schaller, D. , Håkansson, J. , Wennström, J. L. , Tomasi, C. , & Berglundh, T. (2016). Peri‐implantitis – Onset and pattern of progression. Journal of Clinical Periodontology, 43(4), 383‐388. [DOI] [PubMed] [Google Scholar]
- Derks, J. , & Tomasi, C. (2015). Peri‐implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology, 42(Suppl 16), S158‐S171. [DOI] [PubMed] [Google Scholar]
- El‐Benna, J. , Hurtado‐Nedelec, M. , Marzaioli, V. , Marie, J. C. , Gougerot‐Pocidalo, M. A. , & Dang, P. M. (2016). Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunological Reviews, 273(1), 180‐193. [DOI] [PubMed] [Google Scholar]
- Fransson, C. , Tomasi, C. , Pikner, S. S. , Gröndahl, K. , Wennström, J. L. , Leyland, A. H. , & Berglundh, T. (2010). Severity and pattern of peri‐implantitis‐associated bone loss. Journal of Clinical Periodontology, 37(5), 442‐448. [DOI] [PubMed] [Google Scholar]
- Fretwurst, T. , Garaicoa‐Pazmino, C. , Nelson, K. , Giannobile, W. V. , Squarize, C. H. , Larsson, L. , & Castilho, R. M. (2020). Characterization of macrophages infiltrating peri‐implantitis lesions. Clinical Oral Implants Research, 31(3), 274–281. 10.1111/clr.13568 [DOI] [PubMed] [Google Scholar]
- Galindo‐Moreno, P. , López‐Martínez, J. , Caba‐Molina, M. et al (2017). Morphological and immunophenotypical differences between chronic periodontitis and peri‐implantitis – A cross‐sectional study. European Journal of Oral Implantology, 10(4), 453‐463. [PubMed] [Google Scholar]
- Ghighi, M. , Llorens, A. , Baroukh, B. , Chaussain, C. , Bouchard, P. , & Gosset, M. (2018). Differences between inflammatory and catabolic mediators of peri‐implantitis and periodontitis lesions following initial mechanical therapy: An exploratory study. Journal of Periodontal Research, 53(1), 29‐39. [DOI] [PubMed] [Google Scholar]
- Gualini, F. , & Berglundh, T. (2003). Immunohistochemical characteristics of inflammatory lesions at implants. Journal of Clinical Periodontology, 30(1), 14‐18. [DOI] [PubMed] [Google Scholar]
- Hajishengallis, G. (2000). New developments in neutrophil biology and periodontitis. Periodontology 2000, 82(1), 78‐92. [DOI] [PubMed] [Google Scholar]
- Jackson, S. P. , & Bartek, J. (2009). The DNA‐damage response in human biology and disease. Nature, 461(7267), 1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki, H. , Wada, S. , Narimiya, T. , Yamaguchi, Y. , Katsumata, Y. , Itohiya, K. , Fukaya, S. , Miyamoto, Y. , & Nakamura, Y. (2017). Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Frontiers in Physiology, 8, 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatas, O. , Balci Yuce, H. , Taskan, M. M. , Gevrek, F. , Lafci, E. , & Kasap, H. (2019). Histological evaluation of peri‐implant mucosal and gingival tissues in peri‐implantitis, peri‐implant mucositis and periodontitis patients: A cross‐sectional clinical study. Acta Odontologica Scandinavica, 78, 241–249. [DOI] [PubMed] [Google Scholar]
- Kasnak, G. , Firatli, E. , Könönen, E. , Olgac, V. , Zeidán‐Chuliá, F. , & Gursoy, U. K. (2018). Elevated levels of 8‐OHdG and PARK7/DJ‐1 in peri‐implantitis mucosa. Clinical Implant Dentistry and Related Research, 20(4), 574‐582. [DOI] [PubMed] [Google Scholar]
- Konttinen, Y. T. , Lappalainen, R. , Laine, P. , Kitti, U. , Santavirta, S. , & Teronen, O. (2006). Immunohistochemical evaluation of inflammatory mediators in failing implants. International Journal of Periodontics & Restorative Dentistry, 26(2), 135‐141. [PubMed] [Google Scholar]
- Liljenberg, B. , Lindhe, J. , Berglundh, T. , Dahlén, G. , & Jonsson, R. (1994). Some microbiological, histopathological and immunohistochemical characteristics of progressive periodontal disease. Journal of Clinical Periodontology, 21(10), 720‐727. [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , Berglundh, T. , Ericsson, I. , Liljenberg, B. , & Marinello, C. (1992). Experimental breakdown of peri‐implant and periodontal tissues. A study in the beagle dog. Clinical Oral Implants Research, 3(1), 9‐16. [DOI] [PubMed] [Google Scholar]
- Löbrich, M. , Shibata, A. , Beucher, A. , Fisher, A. , Ensminger, M. , Goodarzi, A. A. , Barton, O. , Jeggo, P. A. (2010). gammaH2AX foci analysis for monitoring DNA double‐strand break repair: Strengths, limitations and optimization. Cell Cycle, 9(4), 662‐669. [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , Sanz, M. , Buduneli, N. , Dietrich, T. , Feres, M. , Fine, D. H. , Flemmig, T. F. , Garcia, R. , Giannobile, W. V. , Graziani, F. , Greenwell, H. , Herrera, D. , Kao, R. T. , Kebschull, M. , Kinane, D. F. , Kirkwood, K. L. , Kocher, T. , Kornman, K. S. , Kumar, P. S. , … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Periodontology, 89(Suppl 1), S173‐S182. [DOI] [PubMed] [Google Scholar]
- Paredes‐Sánchez, E. , Montiel‐Company, J. M. , Iranzo‐Cortés, J. E. , Almerich‐Torres, T. , Bellot‐Arcís, C. , & Almerich‐Silla, J. M. (2018). Meta‐analysis of the use of 8‐OHdG in saliva as a marker of periodontal disease. Disease Markers, 2018, 7916578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattelli, A. , Scarano, A. , & Piattelli, M. (1998). Histologic observations on 230 retrieved dental implants: 8 years' experience (1989–1996). Journal of Periodontology, 69(2), 178‐184. [DOI] [PubMed] [Google Scholar]
- Rohrbach, A. S. , Slade, D. J. , Thompson, P. R. , & Mowen, K. A. (2012). Activation of PAD4 in NET formation. Frontiers in Immunology, 3, 360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini, R. , & Singh, S. (2019). Inducible nitric oxide synthase: An asset to neutrophils. Journal of Leukocyte Biology, 105(1), 49‐61. [DOI] [PubMed] [Google Scholar]
- Salehi, F. , Behboudi, H. , Kavoosi, G. , & Ardestani, S. K. (2018). Oxidative DNA damage induced by ROS‐modulating agents with the ability to target DNA: A comparison of the biological characteristics of citrus pectin and apple pectin. Scientific Reports, 8(1), 13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, M. , Alandez, J. , Lazaro, P. , Calvo, J. L. , Quirynen, M. , & van Steenberghe, D. (1991). Histo‐pathologic characteristics of peri‐implant soft tissues in Brånemark implants with 2 distinct clinical and radiological patterns. Clinical Oral Implants Research, 2(3), 128‐134. [DOI] [PubMed] [Google Scholar]
- Sanz, M. , Herrera, D. , Kebschull, M. , Chapple, I. , Jepsen, S. , Beglundh, T. , Sculean, A. , Tonetti, M. S. ; EFP Workshop Participants and Methodological Consultants . (2020). Treatment of stage I‐III periodontitis ‐ The EFP S3 level clinical practice guideline. Journal of Clinical Periodontology, 47, 4–60. 10.1111/jcpe.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, F. , Derks, J. , Monje, A. , & Wang, H. L. (2018). Peri‐implantitis. Journal of Periodontology, 89(Suppl 1), S267‐S290. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Andrukhov, O. , & Rausch‐Fan, X. (2017). Oxidative stress and antioxidant system in periodontitis. Frontiers in Physiology, 8, 910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, P. C. , Chicca, I. J. , Cooper, P. R. , Milward, M. R. , & Chapple, I. L. (2016). Neutrophil extracellular traps in periodontitis: A web of intrigue. Journal of Dental Research, 95(1), 26‐34. [DOI] [PubMed] [Google Scholar]
- Zannini, L. , Delia, D. , & Buscemi, G. (2014). CHK2 kinase in the DNA damage response and beyond. Journal of Molecular Cell Biology, 6(6), 442‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzmann, N. U. , Berglundh, T. , Marinello, C. P. , & Lindhe, J. (2001). Experimental peri‐implant mucositis in man. Journal of Clinical Periodontology, 28(6), 517‐523. [DOI] [PubMed] [Google Scholar]


