Abstract
All the cells in our bodies are derived from the germ cells of our parents, just as our own germ cells become the bodies of our children. The integrity of the genetic information inherited from these germ cells is of paramount importance in establishing the health of each generation and perpetuating our species into the future. There is a large and growing body of evidence strongly suggesting the existence of substances that may threaten this integrity by acting as human germ cell mutagens. However, there generally are no absolute regulatory requirements to test agents for germ cell effects. In addition, the current regulatory testing paradigms do not evaluate the impacts of epigenetically mediated intergenerational effects, and there is no regulatory framework to apply new and emerging tests in regulatory decision making. At the 50th annual meeting of the Environmental Mutagenesis and Genomics Society held in Washington, DC, in September 2019, a workshop took place that examined the heritable effects of hazardous exposures to germ cells, using tobacco smoke as the example hazard. This synopsis provides a summary of areas of concern regarding heritable hazards from tobacco smoke exposures identified at the workshop and the value of the Clean Sheet framework in organizing information to address knowledge and testing gaps.
Keywords: epigenomics, genetic toxicology, germ cells, heritable effects, risk assessment, tobacco smoke
1. INTRODUCTION
Genotoxicity testing over the past five decades has focused primarily on somatic cells and the potential carcinogenic risk if any genomic damage was found. However, this was not always the case. Concern that some drugs and chemicals being introduced into the environment might cause germ cell mutation was the primary motivating factor in the 1960s for the establishment of mutagenicity testing and formation of the Environmental Mutagen Society, now the Environmental Mutagenesis and Genomics Society (EMGS) (DeMarini 2020). However, this focus shifted by the mid‐1970s to a concern for somatic cell mutagenesis and cancer due to the difficulty and expense of germ cell mutation assays and the development of the Salmonella (Ames) mutagenicity assay (Claxton et al. 2010; DeMarini 2020). Although no human germ cell mutagen has yet been declared by any national or international agency, data have accumulated that suggest that several environmental exposures, especially tobacco smoke, are likely human germ cell mutagens (DeMarini 2012; Beal et al. 2017; Marchetti et al. 2020).
A report from a meeting of the International Workshop on Genotoxicity Testing (IWGT) detailed heritable outcomes that need to be considered when asking what risk management questions must be addressed and what testing strategies are needed for risk assessment (Yauk et al. 2015). However, most regulatory bodies across the world do not have an absolute requirement to test drugs and environmental chemicals prior to approval for their ability to cause heritable damage or to assess the ramifications that such damage might have on human health (Cimino 2006). Furthermore, regulatory attention on genotoxicity has been based on a standard, one‐size‐fits‐all testing approach that does not incorporate risk specific to germ cells very well, as described further below.
Recently, an approach referred to as the “Clean Sheet” was proposed to encourage the examination of a substance's potential adverse effects based on its projected mode of action and anticipated human exposure versus relying exclusively on a predetermined set of standard assays (Dearfield et al. 2017; Luijten et al. 2020). This strategy emphasizes flexible and innovative exposure‐based approaches that analyze endpoints most relevant to human health risks. Broader application of the Clean Sheet approach can be facilitated by using case studies to explore this novel regulatory framework in different decision‐making contexts.
On September 19, 2019, a workshop entitled Heritable Hazards of Smoking: Applying the “Clean Sheet” Framework to Further Science and Policy was held in advance of the 50th Annual EMGS Meeting in Washington, DC. There is mounting evidence suggesting tobacco smoke exposure imparts heritable effects on germ cells with negative health implications for the resulting offspring (Beal et al. 2017). Given the historical prevalence of tobacco smoking, it is also a substance with a legacy of exposure that is carried among us today and will continue to be passed along to the next generations. Also, the availability of alternatives to traditional tobacco products (e.g., e‐cigarettes), which are increasingly being used by young people, means that exposure to tobacco and related products will likely remain a public health concern over the long term.
The workshop assembled researchers to present evidence regarding the genetic and epigenetic toxicity of tobacco smoke and related substances to germ cells, as well as the health effects identified in progeny born of those cells. In addition to exploring the utility of the Clean Sheet framework, the focus of this workshop was to heighten the awareness and urgency of heritable hazards via germ cell exposures and to encourage regulatory bodies to consider equally the health impacts to germ cells and somatic cells from exposures to environmental chemicals and pharmaceuticals.
2. APPLYING THE CLEAN SHEET FRAMEWORK TO THE CASE OF GERM CELL TOBACCO SMOKE EXPOSURE
The purpose of the Clean Sheet framework is to provide a systematic but flexible approach to risk assessment aimed at producing the most relevant and informative data for decision making (Dearfield et al. 2017). It details a series of steps that begins with identifying what the problem is and what risk management questions are most relevant to address (planning and scoping). During this step, the exposed populations and anticipated exposure levels are projected to initiate an exposure analysis. Next, the effort assembles what data, evidence, and information are already extant regarding the problem (build the knowledge base). After the knowledge base is assembled, the flexibility of the approach is brought into play with development of hypotheses about how the specific genetic endpoint being analyzed will lead to the adverse outcome of interest (create a rational biological argument). This step provides the basis for determining what genetic testing is most relevant to perform for risk assessment purposes (select assays, perform them, and review the results steps). The resulting data are then used to quantify risk, that is, dose–response analysis and selection of a point of departure (PoD) on the dose–response curve for extrapolation to human exposure levels. Once risk is characterized by combining the relevant testing data and exposure analyses, risk managers can use this information to develop actions to prevent or mitigate the adverse outcome.
In following the Clean Sheet framework, the workshop coordinators first undertook problem formulation and agreed that the current risk assessment paradigms fail to fully address the heritable consequences of environmental exposures (Table 1). The planning and scoping involved establishing the specific endpoints of concern: germ cell and heritable mutations, intergenerational phenotypic changes, and epigenetic effects transmitted across generations. Following this, a decision was made to use tobacco smoke as the case study for the problem, given the widespread population‐level exposure and extensive data. An expert group was then assembled to present the evidence and information associated with this exposure.
TABLE 1.
Application of the Clean Sheet framework at the Heritable Hazards of Smoking Workshop
| “Clean Sheet” approach steps | Workshop information/evidence |
|---|---|
| Planning and scoping (including anticipated exposure) |
|
| Build knowledge base |
|
| Create rational biological argument |
|
| Select assays and perform them |
|
| Review results | Evidence strongly suggests that exposure to tobacco smoke can result in genomic and epigenomic changes to germ cells that are inherited and produce negative health effects in the resulting offspring |
| Select appropriate point of departure (for quantitative analysis) |
|
| Determine expected exposure | The workshop focus did not include an exposure assessment, so quantitation of exposure was not discussed |
| Estimate acceptable levels for endpoints of human relevance | As there were no quantitative data and an exposure assessment, estimated levels could not be derived |
| Risk characterization |
|
At the workshop, experts first reviewed current regulatory processes for germ cell mutagenicity, considered the unique features of germ cell biology and heritable effects, and reviewed evidence from recent key studies supporting the potential for heritable genetic and epigenetic effects of tobacco products. A directed discussion with audience participation focused on key questions developed during the problem formulation. This discussion involved evaluating the weight of evidence, identifying data gaps and uncertainties, and interpreting the implications for risk assessment. Finally, ethical considerations and advocacy needs were examined in the context of risk assessment and regulatory action. Ultimately, the Clean Sheet framework was applied to the heritable risk of tobacco smoking with the goal of providing regulatory agencies with information on which to base future actions.
3. STATE OF REGULATION OF GERM CELL MUTAGENS
Given the far‐reaching health implications of germ cell mutations and other genomic changes (Stenson et al. 2017), identifying chemical agents that may produce such effects should be of high importance to regulatory agencies. However, standard toxicity testing paradigms are generally poorly designed to fully capture perturbations to germ cells that could result in heritable effects (Marchetti et al. 2020). This is in large part due to the limited window of development to which germ cells are typically exposed during testing, in addition to the fact that many assays expose only male germ cells, thus ignoring female germ cell susceptibility altogether. For instance, the in vivo rodent dominant lethal test (OECD 2016a) exposes adult male parents to a toxicant and measures embryonic lethality of their offspring halfway through gestation. Although this test would identify chemicals that cause severe genomic abnormalities during adult spermatogenesis that result in fertilization failures or embryonic death (Marchetti and Wyrobek 2005), it would not identify toxicants that cause non‐lethal mutations or epimutations. Furthermore, because the males are exposed only as adults, the test would not identify effects that result specifically from exposure to primordial germ cells (PGCs) or other early stages of germ cell development.
The mammalian spermatogonial chromosomal aberration test (OECD 2016b) likewise examines effects only during the mitotic proliferation of spermatogonia in adult animals, and there is no equivalent standard test for female germ cells. Similarly, the transgenic rodent gene mutation assay (OECD 2013) can be applied to identify chemicals that are mutagenic in male germ cells (Marchetti et al. 2018), but it is not applicable to female germ cells because not enough of them can be collected to conduct the assay. The lack of practical methods to assess mutagenicity in female germ cells was acknowledged as a critical research gap in the IWGT report (Yauk et al. 2015).
Under the global harmonized system, a substance may be classified as a Category 1A germ cell mutagen only if there is positive evidence from human epidemiological studies (UN 2017). However, these data are inherently difficult to produce given the implausibility of obtaining human populations exposed to a single toxicant, the invasive procedures required to obtain female germ cells or developing embryonic germ cells, and the technical limitations in identifying small genomic changes out of the 3 billion base pairs of the human genome. Consequently, no substances to date have been designated as Category 1A germ cell mutagens. However, this result is more likely a reflection of regulatory testing shortcomings than of biological reality.
At present, there is no standardized test guideline adequately designed to identify heritable effects from germ cell exposures, particularly those mediated through epigenetic changes. This omission means that there is essentially no place in the risk assessment process to account for such effects. Therefore, it is necessary for risk assessment paradigms to shift in ways that are better able to protect the health of present and future generations. This is where the Clean Sheet approach is most applicable because the hazards specific to germ cell biology necessitate different testing design from the standard genetic toxicity testing battery.
4. UNIQUE GERM CELL BIOLOGY
To design relevant testing for germ cell damage, it is critical to understand the underlying biology of germ cell development (crucial for building the knowledge base). Using the knowledge of spermatogenesis and oogenesis can help researchers make better decisions regarding what testing assays to apply in determining when and how genomic damage to germ cells can occur (Figure 1). It also furthers the understanding of what chemical insults to germ cells mean in terms of adverse outcomes in exposed individuals. These are the types of data that are most useful for regulatory bodies to consider because they are most relevant to human heritable risk.
FIGURE 1.
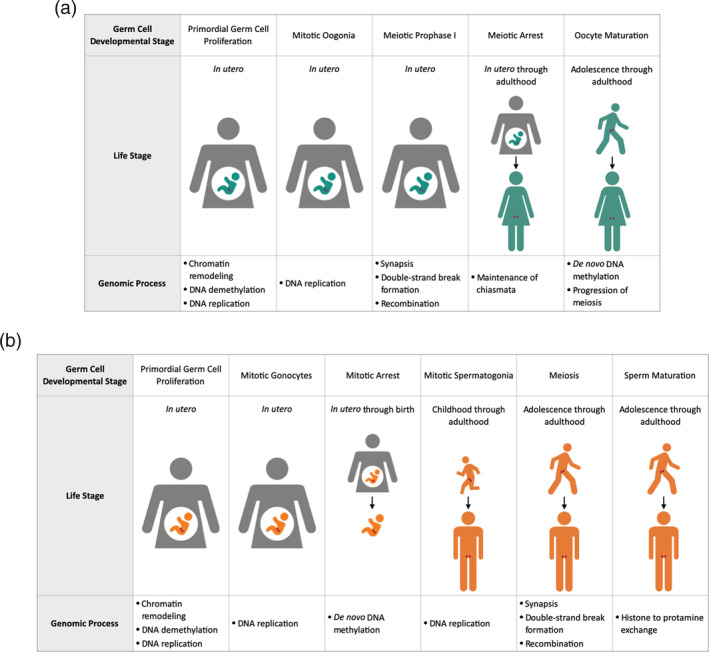
Overview of select developmental stages and genomic processes subject to environmental insults in (a) female and (b) male germ cells
In humans, the earliest germ cells, PGCs, are first formed in the embryo approximately 2–3 weeks after fertilization (De Felici 2013). In both XX and XY embryos, the initial PGCs migrate to the genital ridge by the sixth week and continue mitotic proliferation through the 10th week post fertilization (Tang et al. 2016). After this time, germ cell development progresses in a sex‐specific manner.
In females, immature oogonia first enter meiosis at Embryonic Week 10 (Kurimoto and Saitou 2019). From this time until Embryonic Week 20, oogonia both proliferate via mitosis and progress through the diplotene stage of the first meiotic prophase before arresting in the dictyate stage (Sánchez and Smitz 2012; De Felici 2013). Now primary oocytes, these cells remain in the dictyate stage until puberty, after which time one primary oocyte per month resumes meiosis through the second meiotic prophase (Winship et al. 2018). The oocyte arrests again in this phase and completes meiosis only upon fertilization (Jones et al. 2013).
In males, the immature gonocytes continue mitotic proliferation until entering G0 mitotic arrest during the second trimester (Phillips et al. 2010; Kurimoto and Saitou 2019). Shortly after birth, gonocytes differentiate into primary A spermatogonia, which resume mitosis at age 5–7 years (Neto et al. 2016). Male germ cells first enter meiosis at puberty, when a subset of differentiated B spermatogonia give rise to spermatocytes that undergo meiosis and eventually produce mature spermatozoa (Neto et al. 2016). Throughout the rest of adulthood, male germ cells continue to undergo both mitosis to renew spermatogonial stem cells (SSCs) and proliferate spermatogonia, as well as meiosis to produce mature sperm.
As in somatic cells, mitotic germ cells are subject to DNA replication errors and chromosome missegregation, although they occur at relatively low rates under ambient conditions. Meiosis introduces a further level of risk for loss of genomic integrity due to the physiological induction of DNA double‐strand breaks (DSB) (Lindsey et al. 2013). DSBs allow for crossover events that facilitate genetic recombination and serve to tether homologous chromosomes (Winship et al. 2018). Genetic errors can be introduced during DSB repair or if repair fails to occur. Activation of cell‐cycle checkpoints can facilitate repair of DNA damage or trigger apoptosis if damage is too severe (Winship et al. 2018).
The ability of germ cells to cope with DNA damage is dependent upon both sex and developmental stage. For instance, fetal primary oocytes likely possess efficient DNA repair capacity but also appear to have a highly sensitive apoptotic response that correlates with the high rate of germ cell apoptosis that occurs prior to birth (Winship et al. 2018). During the protracted postnatal period of meiotic arrest, primary oocytes may be particularly sensitive to DNA damage but exhibit a decreasing ability to repair the damage with age (Myers and Hutt 2013). Oocytes also exhibit a relatively high incidence of aneuploidy (Jones et al. 2013) that substantially increases with increased maternal age at meiotic completion (Stuppia 2013).
Similar to fetal oocytes, postnatal gonocytes and spermatogonia appear to possess robust DNA repair capabilities (García‐Rodríguez et al. 2019). Apoptosis also serves as a quality‐control measure, with approximately 60% of differentiating B spermatogonia being eliminated in this way (Di Persio et al. 2017; García‐Rodríguez et al. 2019). However, mature spermatozoa have a limited capacity to perform DNA repair and are unable to complete apoptosis, which can result in the retention of spermatozoa with damaged and/or fragmented DNA (García‐Rodríguez et al. 2019). Additionally, the proportion of spermatozoa with DNA damage increases with age, which corresponds with a decrease in the rate of apoptosis (Singh et al. 2003). The DNA‐repair deficient phase of spermatogenesis represents a sensitive window for the accumulation of DNA damage that can persist and be transmitted by the sperm to the fertilized egg and result in genetic abnormalities impacting proper embryonic development (Marchetti and Wyrobek 2005).
In addition to DNA damage, the processes involved in germ cell specification and differentiation introduce periods of susceptibility to aberrant reprogramming of the epigenome. PGC specification is driven by expression of BLIMP1 and SOX17, which suppress somatic gene expression and promote germ cell gene expression, respectively (Tang et al. 2015). These transcription factors further lead to repression of the maintenance DNA methyltransferase DNMT1 and the de novo DNA methyltransferases DNMT3A and DNMT3B, as well as upregulation of TET1, which converts 5‐methylcytosine to 5‐hydroxymethylcytosine. As a result, global CpG methylation levels fall from approximately 80% at the time of PGC specification to approximately 5% in week 9 fetal PGCs (Tang et al. 2015). This demethylation facilitates parental imprint erasure as well as X chromosome reactivation.
Despite global demethylation, certain regions of the PGC genome are more resistant and retain their DNA methylation (Tang et al. 2015). In particular, evolutionarily young retrotransposons retain higher levels of methylation, which maintain their repression. In addition, approximately 6% of regions escaping DNA demethylation are depleted of repetitive elements and occur in genes with enhanced expression in the brain (Tang et al. 2015). These demethylation‐resistant genes, which are associated with diseases such as metabolic disorders, schizophrenia, and multiple sclerosis, may be important for epigenetic inheritance.
Although hypomethylation of gene promoter regions is generally associated with derepression of the gene, promoter methylation is decoupled from gene expression in PGCs, with only about 12% of hypomethylated genes exhibiting upregulation (Tang et al. 2015). These upregulated genes include those in the piRNA pathway as well as Krüppel‐associated box zinc finger genes that recruit repressive complexes to specific sequences, resulting in heterochromatin formation. Changes to posttranslational histone modifications during PGC development also serve to alter chromatin configuration (Tang et al. 2015). Therefore, chromatin reorganization adds an additional layer of transcriptional control during germ cell reprogramming.
Male fetal germ cells begin to regain DNA methylation in the second trimester after entering mitotic quiescence (Tang et al. 2015). De novo methylation appears to be largely completed within a few days after birth and, thus, prior to any meiotic activity (Wermann et al. 2010). Because de novo methylation occurs mostly in utero, male germ cell methylation marks may be highly influenced by the maternal environment. Conclusive studies on de novo DNA methylation of female germ cells have not been completed in humans (Yu et al. 2017). However, limited human data indicate that female germ cells remain hypomethylated until at least puberty (Wermann et al. 2010) and, similar to mice, de novo DNA methylation may coincide with the onset of oocyte growth (Sasaki and Matsui 2008).
In males, an additional epigenetic reprogramming event is the replacement of most histones with protamines during sperm maturation, which facilitates greater compaction of chromatin and results in a smaller nucleus. However, approximately 5–10% of histones are retained in mature sperm under physiological conditions (Lempradl 2020). Retained histones generally occur at the promoters of developmental genes as well as in areas of heterochromatin marked with H3K9 histone methylation (Lempradl 2020).
Germ cells undergo specific genomic and epigenomic processes during development that are distinct from somatic cells. Appropriately accounting for these differences is important for designing studies that are capable of determining windows of susceptibility when toxicant exposures are most likely to cause effects to germ cells that may impair fertility and/or be inherited by individuals descended from those cells.
5. EVIDENCE PRESENTED AT THE WORKSHOP
The biological knowledge described above set the stage for the interpretation of results from different studies, and the Clean Sheet provided a framework for considering them regardless of whether they were conducted following established test guidelines or not. The data from such studies can help to characterize the mode of action(s) of potential germ cell mutagens and, thus, provide insights on how to prevent damaging exposures (Yauk et al. 2013; Sasaki et al. 2020). It should be noted that the evidence summarized below should not be construed as a comprehensive review of the existing literature. Rather, presenters described empirical data from key studies in both animal models and humans (epidemiological studies) on the effects of tobacco smoking directly on germ cells or on the offspring of smokers. In pregnant females who smoke, both the somatic and germ cells of the F1 generation are exposed, with potential heritable effects manifesting in the F2 generation. In adult female smokers who cease smoking prior to conception, impacts on their own oocytes manifest in the F1 generation. In the case of paternal smoking, germline‐mediated effects are evident in the F1 generation. The presenters cautioned that these studies presume germ cell exposure through the smoker, not secondhand or sidestream smoke.
5.1. Effects of smoking on the germ cell genome
The available evidence on the genomic impact of tobacco smoking in human germ cells was reviewed recently by Beal et al. (2017); here, we briefly summarize some of the main findings presented in that comprehensive review. In humans, tobacco smoking was associated with (a) an increase by 1.2‐ to 5.0‐fold in the incidence of DNA breaks in sperm as measured by the TUNEL and comet assays; (b) an increase in the incidence of sperm aneuploidies for all chromosomes that have been investigated, with effect sizes ranging from 1.6 to 3.0; and (c) an increase by 1.2‐ to 7.6‐fold in the formation of sperm DNA adducts caused by a metabolite of benzo[a]pyrene (B[a]P), a known human carcinogen and main component of tobacco smoke. In mice, cigarette smoke exposure has been found to significantly increase the frequency of tandem‐repeat mutations in SSCs. This effect was found with both mainstream and sidestream smoke exposure. When exposed to B[a]P in utero, F1 mice were found to exhibit an increased mutation frequency in their sperm (Meier et al. 2017).
Less is known about direct effects of tobacco smoke on female germ cells. However, according to a review by Zenzes (2000), smoking may decrease the quality and quantity of oocytes. For example, smoking was associated with an 8–17% reduction in oocytes retrieved during in vitro fertilization, with higher smoking rates corresponding with a greater reduction. The effect of smoking upon oocyte number was further compounded by increasing age. Conversely, a dose‐dependent relationship was found between increased smoking and earlier age of menopause, which is caused in part by oocyte depletion. A dose‐dependent relationship has also been found between maternal smoking and incidence of spontaneous abortion, further suggesting smoking may cause genomic aberrations in female germ cells.
5.2. Effects of smoking on the germ cell epigenome
The effects of smoking on the germ cell epigenome have been evaluated by examining changes in DNA methylation levels across the genome. Based on one of the largest studies of DNA methylation patterns in the sperm of adult males by Jenkins et al. (2017), smoking did not appear to correlate with global changes in methylation levels; however, it did appear to be associated with an increase in the variability of methylation levels. Differential methylation appeared to be enriched at sites reported to escape protamine replacement during spermatogenesis, suggesting that DNA with retained histones may be more sensitive to environmental insults. This study also demonstrated that CpG sites that are resistant to demethylation during germ cell reprogramming appeared to be overrepresented in the differentially methylated sites.
In laboratory studies on mice, nicotine alone has also been found to induce DNA methylation changes in sperm. According to a study by McCarthy et al. (2018), the sperm of mice exposed to nicotine via drinking water exhibited a global increase in DNA methylation levels. However, DNA methylation levels specifically in the promoter region of the D2 dopamine receptor were decreased. This finding corresponded with a significant decrease in D2 receptor mRNA expression in the striatum of male F1 mice descended from nicotine‐exposed fathers. Although there were tissue‐ and sex‐specific differences in the effects observed in the F1 generation, these data suggest that nicotine may be capable of inducing epimutations in sperm that affect the phenotype of the resulting offspring.
5.3. Germ cell‐mediated effects of smoking on offspring genome
A few human studies provide evidence supporting an impact of paternal tobacco smoking on the offspring genome. One small human study by Linschooten et al. (2013) indicated that paternal smoking within 6 months of conception correlated with a significant increase in tandem repeat minisatellite mutations in their offspring. Furthermore, the effect appeared to be dose dependent, with 5.3, 19, and 33% of children from nonsmoker, irregular smoker, and daily smoker fathers, respectively, exhibiting mutations. An additional human study by Laubenthal et al. (2012) found that paternal preconception smoking was positively associated with single‐ and double‐strand DNA breaks in the cord blood of their offspring. These human findings are complemented by a recent mouse study by Beal et al. (2019) showing that paternal exposure to B[a]P significantly increased copy number duplications and de novo mutations in the offspring. Thus, there is growing evidence that paternal tobacco smoking and exposure to the components of tobacco smoke lead to an increase in the number of mutations that offspring inherit.
5.4. Germ cell‐mediated effects of smoking on offspring epigenome
The impact of tobacco smoking on the offspring epigenome is less established. However, recent studies provide support for epigenomic alterations. In a human study by Knudsen et al. (2019), whole blood samples were collected from adolescent and adult F1 offspring to examine the effects of paternal (F0) smoking on DNA methylation. This study identified differentially methylated regions associated with paternal smoking in genes related to innate immune system pathways as well as lipid metabolism and fatty acid biosynthesis. The study was unable to conclusively determine if the observed effects were due to altered methylation in the fathers' sperm because it is possible that secondhand smoke during gestation and/or childhood could have contributed to these changes. However, it was notable that persistent DNA methylation changes were discernible in adult F1 offspring up to 54 years of age.
Based upon a separate study by Joubert et al. (2016) of cord blood collected from 6,685 newborns, maternal smoking was associated with significant differences in DNA methylation of the aryl hydrocarbon receptor repressor gene. However, because this study included women who continued to smoke during pregnancy and, thus, during somatic development of the newborns, it cannot be determined how many of these methylation changes, if any, were attributable specifically to changes induced in the maternal germ cells.
5.5. Germ cell‐mediated effects of smoking on offspring phenotype
Evidence is accumulating that preconception tobacco smoking can result in a variety of phenotypic outcomes in the offspring. For instance, paternal cigarette smoking appears to impair the fertility of their male offspring. Specifically, Axelsson et al. (2018) found that paternal smoking near the time of conception correlated with a 41% decrease in sperm concentration and a 51% decrease in sperm count in the adult sons of smoking fathers. A more recent study by Haervig et al. (2020) with a larger cohort using data from the Danish National Birth Cohort produced similar findings.
Two types of childhood cancers appear to exhibit elevated risk from germ cell exposures to tobacco smoke. Pang et al. (2003) reported that leukemia risk was elevated in children of fathers who smoked prior to conception. They also found that hepatoblastoma risk was elevated with both paternal and maternal preconception smoking, and this risk was compounded further when both parents smoked prior to conception. These and other supporting data were considered sufficient by the International Agency for Research on Cancer to conclude that paternal tobacco smoking is a causative factor for an increased risk of childhood cancer (IARC 2012).
Asthma is another disease linked to germline exposure to tobacco smoke. One large cohort study by Accordini et al. (2018) utilized data collected from 2,233 mothers and 1,964 fathers to examine the associations between parental and grandparental smoking and asthma risk in offspring. In the case of offspring (F2) whose mothers (F1) were exposed to maternal (F0) smoking in utero, there was a nonsignificant relative risk ratio (RRR) of 1.3 for asthma. The RRR was lower for offspring (F2) of fathers (F1) exposed to maternal (F0) smoking in utero. However, a significant increase in asthma was found for offspring (F2) of fathers (F1) who started smoking prior to the age of 15 years, with an RRR of 1.43. This elevated risk was eliminated if the father began smoking after 15 years of age. A previous study by Svanes et al. (2017) had found similar results, with significant increases in risk for childhood asthma associated with grandmaternal smoking as well as with paternal smoking starting before the age of 15 years.
An earlier, smaller cohort study by Li et al. (2005) found a higher risk of asthma associated with grandmaternal smoking compared to the Accordini et al. study. In offspring (F2) whose grandmothers (F0) smoked while pregnant with their mothers (F1), there was an odds ratio (OR) of 2.1 for developing childhood asthma. No elevated risk of asthma was found for offspring (F2) whose mothers (F1) quit smoking prior to pregnancy.
Cohort studies have indicated that grandparental smoking also elevates the risk of autism, autism traits, and attention‐deficit/hyperactivity disorder (ADHD). A study by Golding et al. (2017) using data from the Avon Longitudinal Study of Parents and Children cohort showed significantly elevated odds for autism traits and diagnosed autism in grandoffspring (F2) of pregnant smokers (F0). A separate study by Yim (2019) using data from the Nurses' Health Study II found that grandmaternal (F0) smoking was associated with an adjusted OR of 1.18 (95% CI, 1.11, 1.25) for ADHD in the F2 generation.
Animal studies indicate an elevated risk for asthma from germ cell exposure to nicotine alone. In a multigenerational study by Rehan et al. (2012), the F2 offspring of F1 rats exposed to nicotine both in utero and for 21 days postnatally exhibited significant decreases in pulmonary function, indicative of an asthma‐like phenotype. Because the F2 generation was exposed to nicotine via the germ cells developing in the F1 generation, the phenotypic response in the F2 generation was mediated likely by germ cell changes. In a follow‐up study (Rehan et al. 2012), this asthma‐like phenotype was found to persist into the F3 generation, further supporting the possibility of germline inheritance.
Mouse studies indicate that both grandmaternal and paternal exposure to nicotine alone can also increase ADHD‐like phenotypes in offspring (Zhu et al. 2014; McCarthy et al. 2018). In the former case, Zhu et al. (2014) found that the F1 offspring of female (F0) mice exposed to nicotine for 3 weeks prior to mating and for the duration of pregnancy exhibited ADHD‐like behaviors in both sexes. However, only F2 mice descended from F1 females continued to exhibit these behaviors. The behaviors were also found in F3 mice descended from F2 females. Similarly, a study by McCarthy et al. (2018) from the same lab found that when adult male (F0) mice were exposed to nicotine, both male and female F1 mice exhibited ADHD‐like behaviors.
Overall, the evidence presented at the workshop strongly suggests that exposure to tobacco smoke can result in genomic and epigenomic changes to germ cells that are inherited and produce negative health effects in the resulting offspring. However, despite the breadth of information provided, the workshop attendees agreed that additional pieces of information are required to further the risk assessment of tobacco smoke exposure to germ cells.
6. DATA GAPS IDENTIFIED AT THE WORKSHOP
To perform a quantitation of effects for germ cells, a dose–response analysis of the relevant test results is needed. However, one of the greatest omissions in most studies examining effects of tobacco smoke on germ cells is demonstration of a clear dose–response relationship. Most epidemiological studies class people either as never or ever smokers with little differentiation by duration or intensity of smoking. In animal studies, only one dosing condition is often tested. Because toxicology is classically founded in the principle that “the dose makes the poison” (although nonmonotonic responses should not be precluded and in the context of germ cells, the developmental window of exposure is likely an important determinant of effect), evidence of a clear dose response would strengthen the evidence of heritable germ cell impacts of smoking. In addition, such an analysis would provide the basis for a quantitation of risk.
Similarly, further studies identifying the specific cellular and molecular mechanisms by which heritable effects are manifested would bolster the evidence and aid in creating a rational biological argument for heritable risk. However, a major complicating factor with evaluating risk from tobacco smoke is that it is a complex and variable mixture with over 4,000 individual compounds. Any number of these compounds could lead to various molecular initiating events that could synergize, antagonize, or otherwise interact with one another. Furthermore, products such as e‐cigarettes are increasingly being used as alternatives to tobacco cigarettes, particularly among young people (Jaspers 2019). These products present their own unique combinations of active and inactive ingredients that require specific attention in the risk assessment process.
Beyond mixture toxicity, more concerted study of mechanisms based upon germ cell biology could better inform which stages of germ cell development are most sensitive to tobacco smoke exposure. Studies specifically examining the link between genomic and/or epigenomic changes in parental germ cells to phenotypes observed in offspring and grandoffspring are lacking. Although many of the phenotypic outcomes reported in offspring are likely due to epigenomic changes in parental germ cells, this link has not been explicitly examined in a meaningful way. Information regarding epigenomic changes in germ cells that may be inherited in offspring are especially needed. The question of how many generations through which such epigenetic changes may persist also needs to be addressed systematically.
7. IMPLICATIONS FOR RISK ASSESSMENT
Risk assessment should be focused on addressing the most appropriate risk management questions relevant to human health outcomes (Dearfield et al. 2017). The subject workshop sought to address the question of the heritable risk posed to germ cells by exposure to tobacco products (see Table 1), which is of high importance for population health but has not been accounted for specifically in standard risk assessment processes. Given that 1.1 billion people smoke cigarettes worldwide (WHO 2019a), current human exposure to tobacco smoke is extensive. Furthermore, this number does not include individuals who may be exposed currently to secondhand/sidestream smoke or those who were exposed ancestrally, either from parental or grandparental smoking going back several decades. Therefore, the vast scale of human exposure, including germ cell exposure during various potentially susceptible windows of development, supports the need for further assessment.
As summarized in Table 1, the workshop made substantial progress in assessing the heritable risk of tobacco product exposure to germ cells using the Clean Sheet framework. As was shown during the workshop, there is a wealth of qualitative information supporting the biological argument that tobacco smoke can alter the genome and epigenome of both nascent and adult germ cells. However, the available data for tobacco smoke exposure are not sufficient for quantitating the risk of adverse health effects in the offspring of smokers, although certain components of tobacco smoke may provide some useful information. Based on the evidence presented and the data gaps identified above, the key information needed now is (a) human data directly linking parental tobacco smoking status, germ cell damage, and offspring phenotype in a dose‐responsive manner (e.g., number of cigarettes, years of smoking) and (b) mechanistic data to better define the adverse outcome pathway (AOP) by which such heritable effects occur.
Conducting a large, multigenerational cohort study designed specifically toward these ends would be one of the most valuable steps in advancing research on the heritable effects from exposure to tobacco smoke. Several large cohort studies already exist in multiple countries, and the available data could be readily leveraged for this purpose. Specifically, a human study investigating the F2 health effects from F0 grandmaternal smoking where the F0 smoking dose is known would improve our understanding of heritable effects established during PGC development (Escher and Robotti 2019). Multiple phenotypic endpoints in the F2 generation should be evaluated, including those related to cardiopulmonary, allergy/asthma, metabolic, and neurodevelopmental disorders and diseases. To assist in mechanistic understanding, genomic and epigenomic data from the F0, F1, and F2 generations should also be collected to the extent feasible. Multigenerational animal studies could assist in obtaining additional molecular data, although differences in gametogenesis and development must be thoughtfully accounted for in study design.
Because the available evidence indicates that sex and developmental differences in germ cells may also alter their susceptibility to genomic damage, it would be reasonable to conduct additional studies of paternal smoking with similar endpoints analyzed. In particular, F0 paternal smoking before the age of 15 appears to increase F1 asthma risk (Svanes et al. 2017). Thus, further studies to examine this time as a particularly sensitive window for male germ cell exposure to tobacco smoke are warranted.
Information from such studies could then be used to determine a PoD for germ cell genomic damage from tobacco smoke; see Johnson et al. (2014) for PoD determination. Combined with existing exposure data, the PoD data would allow for development of risk estimations for use in regulatory decision making (Dearfield et al. 2017) in those jurisdictions where it is appropriate to do so. We note that the Clean Sheet framework can be applied to any relevant human exposure (e.g., air pollution) to ensure that regulatory action adequately accounts for the risk specific to heritable germ cell effects. Until a formal accounting of the risk is completed and regulatory action is implemented, as appropriate, potentially heritable germ cell damage from relevant human exposures may continue to be inflicted today and experienced by multiple generations in the future.
8. ETHICAL CONSIDERATIONS AND ADVOCACY NEEDS
Ethical considerations, advocacy, and communication needs were major topics of discussion at the workshop after the scientific evidence for heritable risk from tobacco smoke was presented. Germline effects of tobacco exposure raise novel, intergenerational ethical issues (le Goff 2019). Not only may individuals whose germ cells are exposed to genotoxic agents suffer reproductive health consequences, but also individuals conceived from these germ cells may bear severe developmental and health consequences. The deferred nature of effects inherited from germ cells deeply challenges our framework for moral accountability because future generations consisting of people yet to be conceived do not have rights in the same way existing humans do. Although causation between present health outcomes and ancestral exposure is extremely difficult to establish, when causation can be demonstrated, it creates a responsibility in the present for effects that can be expected to occur in future generations.
The body of evidence presented at the workshop unequivocally shows that the risk that tobacco smoke poses to germ cells is substantial, even if not yet precisely quantified. From a bioethics standpoint, knowledge of a significant risk that contemporary actions impose onto future generations is sufficient to entail responsibility on the part of tobacco corporations, regulatory bodies, and individual smokers (le Goff 2019). Steps to assume this responsibility include (a) the study and incorporation of germ cell effects, in addition to individual or fetal health effects, into the assessment of tobacco smoke toxicity; (b) the regulatory limitation of availability and access of tobacco products; and (c) legal and social efforts to hold corporations accountable.
Beyond efforts to contribute to the improvement of regulation, researchers need to communicate their science in a clear and meaningful way to the general public and decision makers alike. Although most people are aware that tobacco smoke is bad for their own health, and some may perceive fetuses as additionally subject to harm, it is much less likely that people would consider impacts to their germ cells in particular and the potential consequences for future generations. Confining this knowledge to the scientific community without a concerted effort to share it more widely with the general public does a disservice to society and could be construed as morally irresponsible.
9. WORKSHOP CONSENSUS
The Heritable Hazards of Smoking workshop was convened on the premise that current risk assessment paradigms are unable to account for heritable effects from chemical exposures of germ cells and, therefore, cannot be relied upon to guide appropriate regulatory decisions. The Clean Sheet framework was employed as a tool to better articulate the problem and provide direction for future actions in the interest of improving public health.
During the workshop, a wide range of evidence was presented indicating that tobacco smoke can cause genomic and epigenomic alterations to germ cells, resulting in impaired fertility as well as negative health effects in subsequent generations. However, it was widely acknowledged that additional studies that demonstrate a dose response and provide more information on molecular mechanisms are necessary to aid in the development of an AOP and provide a more compelling argument for regulatory action. Therefore, future research should prioritize addressing these gaps through use of large cohort data, modern omics technologies, and targeted animal studies.
In addition to advancing the science, researchers should improve their communication of the science to the public. Raising awareness about concerns for heritable germ cell effects among those beyond the research community would help to both inform individual decision making and build a larger base of support for regulatory change.
Although the data are insufficient for quantitative risk purposes at the present time, based on the evidence presented, there are many other actions regulatory agencies can take to protect individuals from tobacco‐related harm and subsequent health risks; see Codex Alimentarius (2015) for information on risk management options from risk assessment outcomes. An important action already taken is the labeling of tobacco products that warn of health risks, although the messaging specific to heritable effects should be more prominent.
Guidance to particularly susceptible populations to avoid exposure would also be extremely useful. Young people as a susceptible population were discussed at length during the workshop. Based on the biology of oogenesis and spermatogenesis, there are a multitude of opportunities for deleterious genetic and epigenetic effects on the germ cells of exposed humans. Exposures to young people, while their germ cells are still developing, can be particularly deleterious. Effective measures to minimize the risk posed to their genetic material are needed from regulatory agencies.
Although tobacco smoke was the primary focus of the workshop, the workshop acknowledged that there are emerging concerns about germ cell harms from exposures to related substances such as e‐cigarettes, smokeless tobacco, and cannabis. Although the general health concerns surrounding cigarette smoking have been promoted widely and internalized by the public, these alternative products may be seen as relatively harmless, particularly among young people (Jaspers 2019). Thus, as the public health ramifications of cigarette smoking are abating with reduced usage (WHO 2019b), a new wave of current and future health impacts may be rising with increased usage of these alternative products. In response, regulatory agencies should take a more proactive and comprehensive approach to mitigating the potential risk to avoid a new health crisis.
Regardless of the substance, hazards to germ cells and the progeny to which they give rise must be accounted for explicitly in chemical risk assessment and regulatory decision making. Insults to the genomic integrity of germ cells introduce a significant risk to the welfare and health of people who inherit these genomic changes. If we continue to ignore these risks, we may be jeopardizing irreversibly human health and wellbeing for generations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
All authors contributed to the organization of workshop, drafted the manuscript, provided critical revisions, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to thank all the workshop presenters and attendees for their invaluable contributions to this work. The authors also thank Environmental and Molecular Mutagenesis and the Escher Family Fund at Silicon Valley Community Foundation for providing funding for the workshop. The work was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health (T32ES015457) (A. B.). Abigail Bline received a stipend from the NIEHS T32 grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Accepted by: B. Gollapudi
Workshop Presenters: Pradeep Bhide, Florida State University, Tallahassee, FL. Chris Bostic, Action on Smoking and Health, Washington, DC. John W. Holloway, University of Southampton, Southampton, UK. Ilona Jaspers, UNC Gillings School of Global Public Health, Chapel Hill, NC. Anne Le Goff, UCLA Institute for Society & Genetics, Los Angeles, CA. Cecilie Svanes, University of Bergen, Bergen, Norway. Jacquetta Trasler, McGill University and Research Institute‐McGill University Health Centre, Montreal, QC, Canada. Gyeyoon Yim, Harvard T.H. Chan School of Public Health, Boston, MA.
Funding information Environmental and Molecular Mutagenesis; Escher Family Fund at Silicon Valley Community Foundation; National Institute of Environmental Health Sciences of the National Institutes of Health, Grant/Award Number: T32ES015457
REFERENCES
- Accordini, S. , Calciano, L. , Johannessen, A. , Portas, L. , Benediktsdóttir, B. , Bertelsen, R. , Bråbäck, L. , Carsin, A. , Dharmage, S. , Dratva, J. , Forsberg, B. , Real, F. , Heinrich, J. , Holloway, J. , Holm, M. , Janson, C. , Jögi, R. , Leynaert, B. , Malinovschi, A. , Marcon, A. , Rovira, J. , Raherison, C. , Sánchez‐Ramos, J. , Schlünssen, V. , Bono, R. , Corsico, A. , Demoly, P. , Arenas, S. , Nowak, D. , Pin, I. , Weyler, J. , Jarvis, D. and Svanes, C. (2018) A three‐generation study on the association of tobacco smoking with asthma. International Journal of Epidemiology, 47(4), 1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson, J. , Sabra, S. , Rylander, L. , Rignell‐Hydbom, A. , Lindh, C. and Giwercman, A. (2018) Association between paternal smoking at the time of pregnancy and the semen quality in sons. PLoS One, 13(11), e0207221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal, M. , Yauk, C. and Marchetti, F. (2017) From sperm to offspring: assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutation Research, 773, 26–50. [DOI] [PubMed] [Google Scholar]
- Beal, M.A. , Meier, M.J. , Williams, A. , Rowan‐Carroll, A. , Gagné, R. , Lindsay, S.J. , Fitzgerald, T. , Hurles, M.E. , Marchetti, F. and Yauk, C.L. (2019) Paternal exposure to benzo(a)pyrene induces genome‐wide mutations in mouse offspring. Communications Biology, 2, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino, M.C. (2006) Comparative overview of current international strategies and guidelines for genetic toxicology testing for regulatory purposes. Environmental and Molecular Mutagenesis, 47, 362–390. [DOI] [PubMed] [Google Scholar]
- Claxton, L.D. , Umbuzeiro Gde, A. and DeMarini, D.M. (2010) The salmonella mutagenicity assay: the stethoscope of genetic toxicology for the 21st century. Environmental Health Perspectives, 118, 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codex Alimentarius . (2015). Guidance for risk management options in light of different risk assessment outcomes. Available at: http://www.fao.org/fileadmin/user_upload/codexalimentarius/committee/docs/INF_CCCF_e.pdf
- De Felici, M. (2013) Origin, migration, and proliferation of human primordial germ cells In: Coticchio G., Albertini D. and de Santis L. (Eds.) Oogenesis. London, England: Springer‐Verlag, pp. 19–37. [Google Scholar]
- Dearfield, K.L. , Gollapudi, B.B. , Bemis, J.C. , Benz, R.D. , Douglas, G.R. , Elespuru, R.K. , Johnson, G.E. , Kirkland, D.J. , LeBaron, M.J. , Li, A.P. , Marchetti, F. , Pottenger, L.H. , Rorije, E. , Tanir, J.Y. , Thybaud, V. , van Benthem, J. , Yauk, C.L. , Zeiger, E. and Luijten, M. (2017) Next generation testing strategy for assessment of genomic damage: a conceptual framework and considerations. Environmental and Molecular Mutagenesis, 58, 264–283. [DOI] [PubMed] [Google Scholar]
- DeMarini, D.M. (2012) Declaring the existence of human germ‐cell mutagens. Environmental and Molecular Mutagenesis, 53, 166–172. [DOI] [PubMed] [Google Scholar]
- DeMarini, D.M. (2020) The mutagenesis moonshot: the propitious beginnings of the environmental mutagenesis and genomics society. Environmental and Molecular Mutagenesis, 61, 8–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Persio, S. , Saracino, R. , Fera, S. , Muciaccia, B. , Esposito, V. , Boitani, C. , Berloco, B. , Nudo, F. , Spadetta, G. , Stefanini, M. , de Rooij, D. and Vicini, E. (2017) Spermatogonial kinetics in humans. Development, 144, 3440–3439. [DOI] [PubMed] [Google Scholar]
- Escher, J. and Robotti, S. (2019) Pregnancy drugs, fetal germline epigenome, and risks for next‐generation pathology: a call to action. Environmental and Molecular Mutagenesis, 60, 445–454. [DOI] [PubMed] [Google Scholar]
- García‐Rodríguez, A. , Gosálvez, J. , Agarwal, A. , Roy, R. and Johnston, S. (2019) DNA damage and repair in human reproductive cells. International Journal of Molecular Sciences, 20(31), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding, J. , Ellis, G. , Gregory, S. , Birmingham, K. , Iles‐Caven, Y. , Rai, D. and Pembrey, M. (2017) Grand‐maternal smoking in pregnancy and grandchild's autistic traits and diagnosed autism. Scientific Reports, 7, 46179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haervig, K.K. , Høyer, B.B. , Giwercman, A. , Hougaard, K.S. , Ramlau‐Hansen, C.H. , Specht, I.O. , Toft, G. , Bonde, J.P. and Søgaard Tøttenborg, S. (2020) Fetal exposure to paternal smoking and semen quality in the adult son. Andrology, 8, 1117–1125. [DOI] [PubMed] [Google Scholar]
- IARC . (2012) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Personal Habits and Indoor Combustion, Vol. 100E Lyon, France: IARC, p. 575. [PMC free article] [PubMed] [Google Scholar]
- Jaspers, I. (2019). Toxicology of e‐cigarettes. Heritable hazards of smoking: applying the "clean sheet" framework to further science and policy. Workshop Conducted at the 50th Annual Meeting of the Environmental Mutagenesis and Genomics Society, Washington, DC. [no abstract available].
- Jenkins, T. , James, E. , Alonso, D. , Hoidal, J. , Murphy, P. , Hotaling, J. , Cairns, B. , Carrell, D. and Aston, K. (2017) Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology, 5, 1089–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, G.E. , Soeteman‐Hernandez, L.G. , Gollapudi, B.B. , Bodger, O.G. , Dearfield, K.L. , Heflich, R.H. , Hixon, J.G. , Lovell, D.P. , MacGregor, J.T. , Pottenger, L.H. , Thompson, C.M. , Abraham, L. , Thybaud, V. , Tanir, J.Y. , Zeiger, E. , van Benthem, J. and White, P.A. (2014) Derivation of point of departure (PoD) estimates in genetic toxicology studies and their potential applications in risk assessment. Environmental and Molecular Mutagenesis, 55, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, K. , Lane, S. and Holt, J. (2013) Start and stop signals of oocyte meiotic maturation In: Coticchio G., Albertini D. and de Santis L. (Eds.) Oogenesis. London, England: Springer‐Verlag, pp. 183–193. [Google Scholar]
- Joubert, B. , Felix, J. , Yousefi, P. , Bakulski, K. , Just, A. , Breton, C. , Reese, S. , Markunas, C. , Richmond, R. , Xu, C. , Küpers, L. , Oh, S. , Hoyo, C. , Gruzieva, O. , Söderhäll, C. , Salas, L. , Baïz, N. , Zhang, H. , Lepeule, J. , Ruiz, C. , Ligthart, S. , Wang, T. , Taylor, J. , Duijts, L. , Sharp, G. , Jankipersadsing, S. , Nilsen, R. , Vaez, A. , Fallin, M. , Hu, D. , Litonjua, A. , Fuemmeler, B. , Huen, K. , Kere, J. , Kull, I. , Muthe‐Kaas, M. , Gehring, U. , Bustamante, M. , Saurel‐Coubizolles, M. , Quraishi, B. , Ren, J. , Tost, J. , Gonzalez, J. , Peters, M. , Håberg, S. , Xu, Z. , van Meurs, J. , Gaunt, T. , Kerkhof, M. , Corpeleijn, E. , Feinberg, A. and Eng, C. (2016) DNA methylation in newborns and maternal smoking in pregnancy: genome‐wide consortium meta‐analysis. The American Journal of Human Genetics, 98, 680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen, G. , Rezwan, F. , Johannessen, A. , Skulstad, S. , Bertelsen, R. , Real, F. , Krauss‐Etschmann, S. , Patil, V. , Jarvis, D. , Arshad, S. , Holloway, J. and Svanes, C. (2019) Epigenome‐wide association of father's smoking with offspring DNA methylation: a hypothesis‐generating study. Environmental Epigenetics, 5(4), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurimoto, K. and Saitou, M. (2019) Germ cll reprogramming. Current Topics in Developmental Biology, 135, 91–125. [DOI] [PubMed] [Google Scholar]
- Laubenthal, J. , Zlobinskaya, O. , Poterlowicz, K. , Baumgartner, A. , Gdula, M. , Fthenou, E. , Kermarou, M. , Hepworth, S. , Kleinjans, C. , van Schooten, F. , Brunbog, G. , Godschalk, R. , Schmid, T. and Anderson, D. (2012) Cigarette smoke‐induced transgenerational alterations in genome stability in cord blood of human F1 offspring. The FASEB Journal, 26(10), 3946–3956. [DOI] [PubMed] [Google Scholar]
- le Goff, A. (2019). Implications for bioethics. Heritable hazards of smoking: applying the "clean sheet" framework to further science and policy. Workshop Conducted at the 50th Annual Meeting of the Environmental Mutagenesis and Genomics Society, Washington, DC. [no abstract available].
- Lempradl, A. (2020) Germ cell‐mediated mechanisms of epigenetic inheritance. Seminars in Cell & Developmental Biology, 97, 116–122. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Langholz, B. , Salam, M. and Gilliland, F. (2005) Maternal and grandmaternal smoking patterns are associated with early childhood asthma. CHEST, 127(4), 1232–1241. [DOI] [PubMed] [Google Scholar]
- Lindsey, S. , Byrnes, D. , Eller, M. , Rosa, A. , Dabas, N. , Escandon, J. and Grichnik, J. (2013) Potential role of meiosis proteins in melanoma chromosomal instability. Journal of Skin Cancer, 190109, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linschooten, J. , Verhofstad, N. , Gutzkow, K. , Olsen, A. , Yauk, C. , Oligschläger, Y. , Brunborg, G. , van Schooten, F. and Godschalk, R. (2013) Paternal lifestyle as a potential source of germline mutations transmitted to offspring. The FASEB Journal, 27, 2873–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten, M. , Ball, N.S. , Dearfield, K.L. , Gollapudi, B.B. , Johnson, G.E. , Madia, F. , Peel, L. , Pfuhler, S. , Settivari, R.S. , ter Burg, W. , White, P.A. and van Benthem, J. (2020) Utility of a next generation framework for assessment of genomic damage: a case study using the industrial chemical benzene. Environmental and Molecular Mutagenesis, 61, 94–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, F. , Aardema, M.J. , Beevers, C. , van Benthem, J. , Godschalk, R. , Williams, A. , Yauk, C.L. , Young, R. and Douglas, G.R. (2018) Identifying germ cell mutagens using OECD test guideline 488 (transgenic rodent somatic and germ cell gene mutation assays) and integration with somatic cell testing. Mutation Research/Genetic Toxicology Environmental Mutagenesis, 832‐833, 7–18. [DOI] [PubMed] [Google Scholar]
- Marchetti, F. , Douglas, G.R. and Yauk, C.L. (2020) A return to the origin of the EMGS: rejuvenating the quest for human germ cell mutagens and determining the risk to future generations. Environmental and Molecular Mutagenesis, 61(1), 42–54. [DOI] [PubMed] [Google Scholar]
- Marchetti, F. and Wyrobek, A.J. (2005) Mechanisms and consequences of paternally‐transmitted chromosomal abnormalities. Birth Defects Research, Part C, Embryo Today: Reviews, 75(2), 112–129. [DOI] [PubMed] [Google Scholar]
- McCarthy, D. , Morgan, T. , Lowe, S. , Williamson, M. , Spencer, T. , Biederman, J. and Bhide, P. (2018) Nicotine exposure of male mice produces behavioral impairment in multiple generations of descendants. PLoS Biology, 16(10), e2006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, M. , O'Brien, J. , Beal, M. , Allan, B. , Yauk, C. and Marchetti, F. (2017) In utero exposure to benzo[a]pyrene increases mutation burden in the soma and sperm of adult mice. Environmental Health Perspectives, 125(1), 82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, M. and Hutt, K. (2013) Damage control in the female germline: protecting primordial follicles In: Coticchio G., Albertini D. and de Santis L. (Eds.) Oogenesis. London, England: Spring‐Verlag, pp. 39–47. [Google Scholar]
- Neto, F. , Bach, P. , Najari, B. , Li, P. and Goldstein, M. (2016) Spermatogenesis in humans and its affecting factors. Seminars in Cell & Developmental Biology, 59, 10–26. [DOI] [PubMed] [Google Scholar]
- OECD . (2013) Test 488: Transgenic Rodent Somatic and Germ Cells Gene Mutation Assays. Paris, France: OECD Publishing. [Google Scholar]
- OECD . (2016a) Test 478: Rodent Dominant Lethal Test. OECD Guidelines for the Testing of Chemicals. Paris, France: OECD Publishing; 10.1787/9789264264823-en. [DOI] [Google Scholar]
- OECD . (2016b) Test 483: Mammalian Spermatogonial Chromosomal Aberration Test. Paris, France: OECD Publishing. [Google Scholar]
- Pang, D. , McNally, R. and Birch, J. (2003) Parental smoking and childhood cancer: results from the United Kingdom Childhood Cancer Study. British Journal of Cancer, 88, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, B. , Gassei, K. and Orwig, K. (2010) Spermatogonial stem cell regulation and spermatogenesis. Philosophical Transactions of the Royal Society B, 365, 1663–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehan, V. , Liu, J. , Naeem, E. , Tian, J. , Sakuri, R. , Kwong, K. , Akbari, O. and Torday, J. (2012) Perinatal nicotine exposure induces asthma in second generation offspring. BMC Medicine, 10, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez, F. and Smitz, J. (2012) Molecular control of oogenesis. Biochimica et Biophysica Acta, 1822, 1896–1912. [DOI] [PubMed] [Google Scholar]
- Sasaki, H. and Matsui, Y. (2008) Epigenetic events in mammalian germ‐cell development: reprogramming and beyond. Nature Reviews Genetics, 9, 129–140. [DOI] [PubMed] [Google Scholar]
- Sasaki, J.C. , Allemang, A. , Bryce, S.M. , Custer, L. , Dearfield, K.L. , Dietz, Y. , Elhajouji, A. , Escobar, P.A. , Fornace, A.J., Jr. , Froetschl, R. , Galloway, S. , Hemmann, U. , Hendriks, G. , Li, H.‐H. , Luijten, M. , Ouedraogo, G. , Peel, L. , Pfuhler, S. , Roberts, D.J. , Thybaud, V. , van Benthem, J. , Yauk, C.L. and Schuler, M. (2020) Application of the adverse outcome pathway framework to genotoxic modes of action. Environmental and Molecular Mutagenesis, 61, 114–134. [DOI] [PubMed] [Google Scholar]
- Singh, N. , Muller, C. and Berger, R. (2003) Effects of age on DNA double‐strand breaks and apoptosis in human sperm. Fertility and Sterility, 80(6), 1420–1430. [DOI] [PubMed] [Google Scholar]
- Stenson, P.D. , Mort, M. , Ball, E.V. , Evans, K. , Hayden, M. , Heywood, S. , Hussain, M. , Phillips, A.D. and Cooper, D.N. (2017) The human gene mutation database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next‐generation sequencing studies. Human Genetics, 136, 665–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuppia, L. (2013) Origins of oocyte aneuploidy In: Coticchio G., Albertini D. and de Santis L. (Eds.) Oogenesis. London, England: Springer‐Verlag, pp. 219–227. [Google Scholar]
- Svanes, C. , Koplin, J. , Skulstad, S. , Johannessen, A. , Bertelsen, R. , Benediktsdottir Bråbäck, L. , Carsin, A. , Dharmage, S. , Dratva, J. , Forsberg, B. , Gislason, T. , Heinrich, J. , Holm, M. , Janson, C. , Jarvis, D. , Jögi, R. , Krauss‐Etschmann, S. , Lindberg, E. , Macsali, F. , Malinovschi, A. , Modig, L. , Norbäck, D. , Omenaas, E. , Saure, E. , Sigsgaard, T. , Skorge, T. , Svanes, Ø. , Torén, K. , Torres, C. , Schlünssen, V. and Real, F. (2017) Father's environment before conception and asthma risk in his children: a multi‐generation analysis of the Respiratory Health in Northern Europe study. International Journal of Epidemiology, 46(1), 235–245. [DOI] [PubMed] [Google Scholar]
- Tang, W. , Dietmann, S. , Irie, N. , Leitch, H. , Floros, V. , Bradshaw, C. , Hackett, J. , Chinnery, P. and Surani, M. (2015) A unique gene regulatory network resets the human germline epigenome for development. Cell, 161, 1453–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, W. , Kobayashi, T. , Irie, N. , Dietmann, S. and Surani, M. (2016) Specification and epigenetic programming of the human germ line. Nature Reviews Genetics, 17, 585–600. [DOI] [PubMed] [Google Scholar]
- UN . (2017) Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 7th edition. New York, NY: United Nations. [Google Scholar]
- Wermann, H. , Stoop, H. , Gillis, A.J.M. , Honecker, F. , van Gurp, R. , Ammerpohl, O. , Richter, J. , Oosterhuis, J.W. , Bokemeyer, C. and Looijenga, L.H.J. (2010) Global DNA methylation in fetal human germ cells and germ cell tumours: association with differentiation and cisplatin resistance. The Journal of Pathology, 221(4), 433–442. [DOI] [PubMed] [Google Scholar]
- WHO . (2019a) Tobacco Fact Sheet. Geneva, Switzerland: World Health Organization; Available at: https://www.who.int/news-room/fact-sheets/detail/tobacco. [Google Scholar]
- WHO . (2019b) WHO Global Report on Trends in Prevalence of Tobacco Use 2000–2025, 3rd edition. World Health Organization; Available at: https://www.who.int/publications/i/item/who‐global‐report‐on‐trends‐in‐prevalence‐of‐tobacco‐use‐2000‐2025‐third‐edition. [Google Scholar]
- Winship, A. , Stringer, J. , Liew, S. and Hutt, K. (2018) The importance of DNA repair for maintaining oocyte quality in response to anti‐cancer treatments, environmental toxins and maternal ageing. Human Reproduction Update, 24(2), 119–134. [DOI] [PubMed] [Google Scholar]
- Yauk, C.L. , Aardema, M. , van Benthem, J. , Bishop, J. , Dearfield, K.L. , DeMarini, D.M. , Dubrova, Y.E. , Honma, M. , Lupski, J.R. , Marchetti, F. , Meistrich, M.L. , Pacchierotti, P. , Stewart, J. , Waters, M.D. and Douglas, G.R. (2015) Approaches for identifying germ cell mutagens: report of the 2013 IWGT workshop on germ cell assays. Mutation Research, 783, 36–54. [DOI] [PubMed] [Google Scholar]
- Yauk, C.L. , Bishop, J. , Dearfield, K.L. , Douglas, G.R. , Hales, B.F. , Luijten, M. , O'Brien, J.M. , Robaire, B. , Sram, R. , van Benthem, J. , Wade, M.G. , White, P.A. and Marchetti, F. (2013) The development of adverse outcome pathways for mutagenic effects for the Organization for Economic Co‐operation and Development. Environmental and Molecular Mutagenesis, 54, 79–81. [DOI] [PubMed] [Google Scholar]
- Yim, G. (2019). Grandmaternal smoking and risk for ADHD. Heritable hazards of smoking: applying the "clean sheet" framework to further science and policy. Workshop Conducted at the 50th Annual Meeting of the Environmental Mutagenesis and Genomics Society, Washington, DC. [no abstract available]
- Yu, B. , Dong, X. , Gravina, S. , Kartal, Ö. , Schimmel, T. , Cohen, J. , Tortoriello, D. , Zody, R. , Hawkins, R.D. and Vijg, J. (2017) Genome‐wide single‐cell DNA methylomics reveals increased non‐CpG methylation during human oocyte maturation. Stem Cell Reports., 9, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenzes, M. (2000) Smoking and reproduction: gene damage to human gametes and embryos. Human Reproduction Update, 6(2), 122–131. [DOI] [PubMed] [Google Scholar]
- Zhu, J. , Lee, K. , Spencer, T. , Biederman, J. and Bhide, P. (2014) Transgenerational transmission of hyperactivity in a mouse model of ADHD. The Journal of Neuroscience, 34(8), 2768–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]


