Abstract
Melanoma is known to show considerable variation in its histopathological presentation. In exceptional cases, heterologous or divergent differentiation (metaplastic melanoma) can be observed. We report a case of a 69‐year‐old man who was diagnosed with nodular melanoma on the right upper leg. One year later, the patient presented with an inguinal lymph node metastasis and a lymph node dissection was carried out. In two out of five positive lymph nodes, an angiosarcomatous component was found next to a conventional melanoma component. Shortly after, the patient developed two in‐transit metastases in which again an angiosarcomatous component was seen. The vascular component stained positive for ERG and CD31 and negative for melanocytic markers (Mart‐1, S100, SOX‐10), while the conventional melanoma had an opposite staining pattern. Molecular analysis on both components showed an identical mutation in the NRAS gene, which in our opinion proves the divergent differentiation. To the best of our knowledge, this is the first case report describing angiosarcomatous transdifferentiation of melanoma.
Keywords: angiosarcoma, angiosarcomatous transdifferentiation, divergent differentiation, heterologous differentiation, melanoma, transdifferentiation
1. INTRODUCTION
Melanoma is known for its variability in morphology, which in some cases complicates recognition. Besides a variable morphology, divergent (or heterologous) differentiation in malignant melanoma is another pitfall that adds difficulty to the diagnosis. Different types of divergent differentiation have been described including fibroblastic/myofibroblastic, schwannian and perineurial, smooth muscle, rhadbomyosarcomatous, osteocartilaginous, ganglionic and ganglioneuroblastic, neuroendocrine, and epithelial. 1 , 2 Malignant melanomas mimicking angiosarcoma have been described, but to our knowledge true divergent differentiation into angiosarcoma has never been reported in the literature. 3 In this paper, we present such a case.
2. CASE REPORT
A 69‐year‐old man was diagnosed with a cutaneous nodular melanoma with a Breslow thickness of 13.0 mm in the right upper leg in 2017 without any signs of heterologous differentiation (Figure 1). Subsequently, a therapeutic re‐excision and a sentinel node procedure from the right groin were performed. The sentinel node showed metastatic melanoma (diameter 2.3 mm) and routine follow up was performed. One year later, the patient presented with a new enlarged lymph node in the right groin. A biopsy showed metastatic melanoma and an inguinal lymph node dissection was carried out.
FIGURE 1.
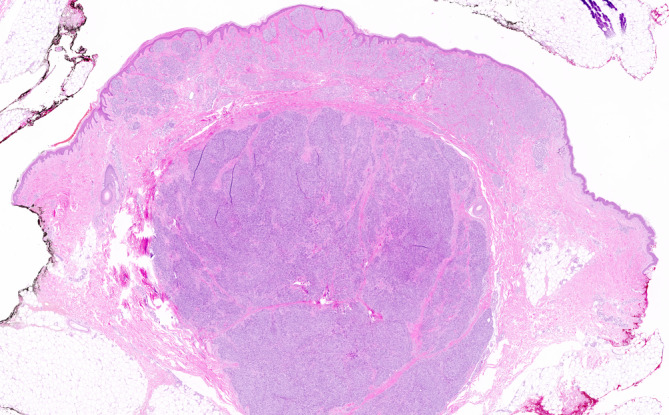
Skin excision of the right upper leg showing nodular melanoma (hematoxylin and eosin ×10)
Fourteen lymph nodes were harvested, with five lymph nodes showing metastatic melanoma. In the two largest lymph node metastases, conventional melanoma was seen next to a vascular component (Figure 2). The vascular spaces were lined by highly atypical endothelial cells with vesicular and hyperchromatic nuclei and eosinophilic cytoplasm. Mitotic figures were present. The vascular spaces contained erythrocytes (Figures 3 and 4).
FIGURE 2.
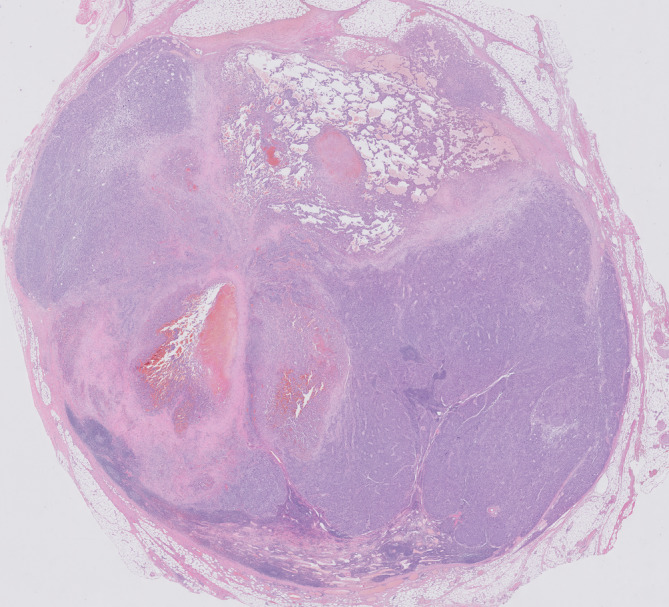
Lymph node with metastatic melanoma. The angiosarcomatous component is visible in the central and upper part of the lymph node (hematoxylin and eosin ×5)
FIGURE 3.
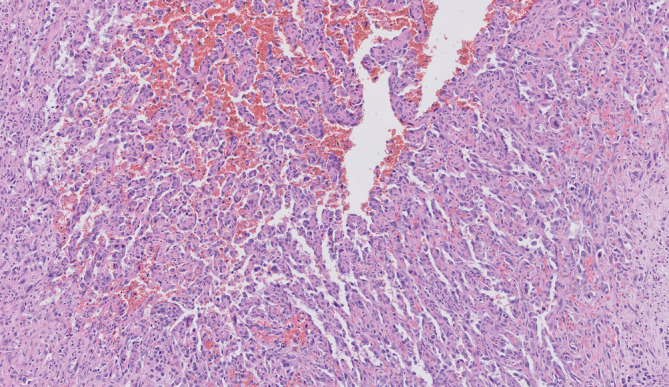
A close‐up of the angiosarcomatous component shows vascular channels filled with erythrocytes (hematoxylin and eosin ×100)
FIGURE 4.
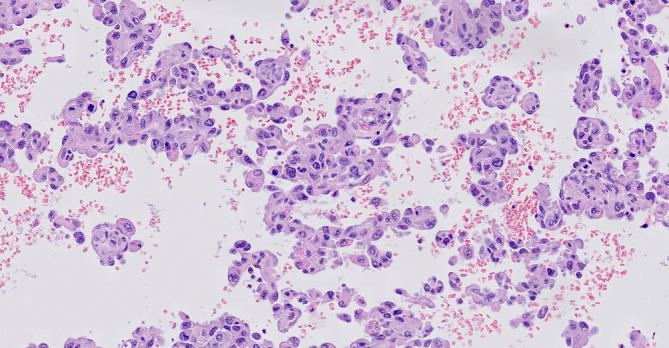
Detail of the angiosarcomatous component. Highly atypical endothelial cells can be seen and mitotic figures are present. The vascular spaces contain erythrocytes (hematoxylin and eosin ×200)
Melanocytic markers (MART‐1, S100, SOX10) were positive only in the solid sheets of melanocytes, while the vascular component was negative. In contrast, ERG and CD31 were strongly expressed in the vascular component while the solid component was entirely negative (Figure 5A, B, C, D). This, combined with the endothelial atypia, was identified as metastatic angiosarcoma.
FIGURE 5.
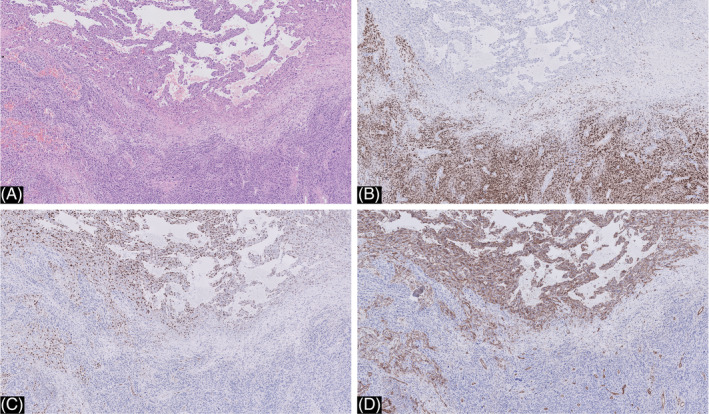
A, close‐up of the melanoma metastasis in the inguinal lymph node showing both components (hematoxylin and eosin ×20). B, immunohistochemistry for SOX‐10 was positive in the conventional melanoma and completely negative in the vascular component (SOX10 ×20). C and D, immunohistochemistry for Erg (C) and CD 31 (D) was positive in the vascular component and completely negative in the conventional melanoma (ERG ×20)
Because the angiosarcomatous component was present in two different lymph nodes next to the conventional melanoma, and because the patient had no previous history of angiosarcoma, divergent differentiation was suspected. Clinically, there was no suspicion of a second tumor. A positron emission tomography‐computed tomography (PET‐CT) scan was performed and no other tumor localization was detected.
To prove the divergent differentiation, molecular analysis was performed on both components. DNA was extracted from the areas that were indicated by a pathologist (GD) using the Cobas FFPE DNA extraction kit (Roche). Mutation analysis was performed using a custom‐designed Ion Torrent panel for next‐generation sequencing analysis (ThermoFisher) under NEN‐EN‐ISO‐15189 accreditation (registration number M257). With the pan‐tumor panel, mutations in hotspots of 24 genes can be analyzed, including AKT1, BRAF, GNAQ, GNA11, KIT, HRAS, MAP2K1, NRAS and PIK3CA, with a validated detection limit of 5% mutant alleles. Tumor cell content was estimated at 100% for both areas. The conventional melanoma and the angiosarcomatous component had an identical mutation in NRAS: c.181_182delinsAG p.(Gln61Arg) (Figure 6), with comparably high variant allele frequencies (VAF): 61% and 74%, respectively. The high VAFs in both components makes cross‐contamination during macrodissection highly unlikely. Other mutations with >5% VAF were not detected.
FIGURE 6.
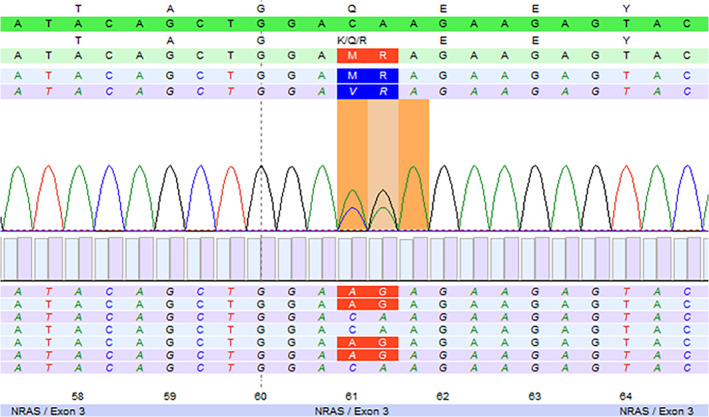
Next‐generation sequencing result showing the NRAS: c.181_182delinsAG p.(Gln61Arg) mutation. Both the conventional melanoma and the angiosarcomatous component had this identical mutation (molecular analyses performed on the lymph node metastasis and subsequent cutaneous in‐transit metastasis)
Three months later, the patient presented with two cutaneous in‐transit metastases in the upper leg which showed the same pattern of conventional melanoma next to an angiosarcomatous component. Molecular analysis of the angiosarcomatous component was repeated and revealed the previously observed mutational profile: NRAS c.181_182delinsAG p.(Gln61Arg) without other mutations (>5% VAF) in the hotspots of the genes in the panel. In our opinion, this proves the divergent differentiation of melanoma into angiosarcoma.
Shortly after, the patient developed another regional recurrence. A PET‐CT scan showed suspected supraclavicular (left), paraesophageal, para‐aortic, iliac (right), and inguinal (left and right) lymph node metastases. The patient was eligible for palliative immunotherapy. He developed a complete response on nivolumab the year after and he is still alive 3 years after the initial diagnosis.
3. DISCUSSION
We report on the first case of true angiosarcomatous transdifferentiation of melanoma. This was proven by immunohistochemistry and molecular analyses. Apparently vascular differentiation has been described earlier in melanoma as the angiomatoid subtype. 4 , 5 , 6 , 7 , 8 , 9 , 10 The difference with our case is that this represents a “pseudovascular” differentiation based on a lining with neoplastic cells that stain positive for melanocytic markers (such as S100, HMB‐45) and negative for vascular markers. In our case, however, the neoplastic cells lining the vascular spaces stain strongly positive with the ERG and CD31 stains, confirming its endothelial lineage. Absence of positivity for melanocytic markers further confirmed the true vascular nature.
The differential diagnosis includes a second or collision tumor. Clinically, there were no signs of another malignancy, which was confirmed by a PET‐CT scan. To prove divergent differentiation, molecular analysis was performed on both components of the lymph node metastasis. An identical mutational profile with the NRAS p.(Gln61Arg) mutation as a common oncogenic driver was found in both components, demonstrating divergence of melanoma to angiosarcoma. Molecular analysis of the subsequent in‐transit metastasis confirmed our previous findings.
In summary, we report the first case of true angiosarcomatous transdifferentiation of melanoma. Recognition of divergent differentiation in melanoma is important to prevent misdiagnosis.
Kilsdonk MJ, Romeijn TR, Kelder W, van Kempen LC, Diercks GF. Angiosarcomatous transdifferentiation of metastatic melanoma. J Cutan Pathol. 2020;47(12):1211–1214. 10.1111/cup.13857
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52(2):119‐129. doi: HIS2823 [pii]. [DOI] [PubMed] [Google Scholar]
- 2. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36(5):387‐402. doi: his894 [pii]. [DOI] [PubMed] [Google Scholar]
- 3. Ramos‐Rodriguez G, Ortiz‐Hidalgo C. Primary angiomatoid melanoma as an exceptional morphologic pattern in cutaneous melanoma. A case report and review of the literature. Actas Dermosifiliogr. 2015;106(3):13 10.1016/j.ad.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 4. Adler MJ, Beckstead J, White CR. Angiomatoid melanoma: a case of metastatic melanoma mimicking a vascular malignancy. Am J Dermatopathol. 1997;19(6):606‐609. 10.1097/00000372-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 5. Baron JA, Monzon F, Galaria N, Murphy GF. Angiomatoid melanoma: a novel pattern of differentiation in invasive periocular desmoplastic malignant melanoma. Hum Pathol. 2000;31(12):1520‐1522. doi: S0046‐8177(00)60798‐0 [pii]. [DOI] [PubMed] [Google Scholar]
- 6. Fabrizi G, Massi G. Angiomatoid Spitz naevus: a close simulator of regressing malignant melanoma. Br J Dermatol. 2001;145(5):845‐846. doi: 4421 [pii]. [DOI] [PubMed] [Google Scholar]
- 7. Fonda‐Pascual P, Moreno‐Arrones OM, Alegre‐Sanchez A, Garcia‐Del Real CM, Miguel‐Gomez L, Martin‐Gonzalez M. Primary cutaneous angiomatoid melanoma. J Dtsch Dermatol Ges. 2018;16(3):345‐347. 10.1111/ddg.13437. [DOI] [PubMed] [Google Scholar]
- 8. Paolino G, Muscardin LM, Buccini P, et al. Angiomatoid melanoma: a dermoscopic and pathologic challenge. G Ital Dermatol Venereol. 2019;154(6):718‐719. 10.23736/S0392-0488.18.05828-5. [DOI] [PubMed] [Google Scholar]
- 9. Zelger BG, Zelger B. Angiomatoid metastatic melanoma. Dermatol Surg. 2004;30(2 Pt 2):336‐340. doi: 30082 [pii]. [DOI] [PubMed] [Google Scholar]
- 10. Rawson R, Robbins E, McCarthy SW, Scolyer RA. Angiomatoid Spitz naevus: novel observations and clues to diagnosis of a rare variant. Pathology. 2016;48(7):739‐742. doi: S0031‐3025(16)40288‐6 [pii]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


