Abstract
Background
The uptake rate of non–vitamin K oral anticoagulants (NOAC) for the treatment of non‐valvular atrial fibrillation (AF) was far lower in the Netherlands (NL) compared to Belgium (BE). Also, patients on VKA in NL were treated with a higher target international normalized ratio (INR) range of 2.5 to 3.5.
Objectives
To explore the effect of these differences on thromboembolism (TE) and bleeding.
Methods
Data from the GARFIELD‐AF registry was used. Patients with new‐onset AF and ≥1 investigator‐determined risk factor for stroke were included between 2010 and 2016. Event rates from 2 years of follow‐up were used.
Results
In NL and BE, 1186 and 1705 patients were included, respectively. Female sex (42.3% vs 42.2%), mean age (70.7 vs 71.3 years), CHA2DS2‐VASc (3.1 vs 3.1), and HAS‐BLED score (1.4 vs 1.5) were comparable between NL and BE. At diagnosis in NL vs BE, 72.1% vs 14.6% received vitamin K antagonists (VKA) and 17.8% vs 65.5% NOACs, varying greatly across cohorts. Mean INR was 2.9 (±1.0) and 2.4 (±1.0) in NL and BE, respectively. Event rates per 100 patient‐years in NL and BE, respectively, of all‐cause mortality (3.38 vs 3.90; hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.65‐1.15), ischemic stroke/TE (0.82 vs 0.72; HR 1.14, 95% CI 0.62‐2.11), and major bleeding (2.06 vs 1.54; HR 1.33, 95% CI 0.89‐1.99) did not differ significantly.
Conclusions
In GARFIELD‐AF, despite similar characteristics, patients on anticoagulants were treated differently in NL and BE. Although the rate of major bleeding was 33% higher in NL, variations in bleeding, mortality, and TE rates were not statistically significant.
Keywords: anticoagulants, hemorrhage, international normalized ratio, registries, stroke
Essentials.
The impact of different anticoagulation strategies in atrial fibrillation (AF) in a real‐world setting is unknown.
Dutch and Belgian patients from the GARFIELD‐AF registry were analyzed.
Characteristics were similar, but the type and intensity of anticoagulation treatment differed.
Variations in bleeding, mortality, and thromboembolism rates were not statistically significant.
1. INTRODUCTION
In the neighboring countries the Netherlands (NL) and Belgium (BE), oral anticoagulation (OAC) treatment strategies in atrial fibrillation (AF) have been noticeably different. In these countries, non–vitamin K oral anticoagulants (NOAC) were approved for AF in 2011 and 2012, respectively. In 2012, more than 50% of patients with newly diagnosed AF were treated with NOACs in BE, compared to approximately 3% in NL. 1 , 2 One of the reasons for the lower uptake rate in NL was an advisory report from the Health Council of the Netherlands warranting a careful introduction of NOACs, given the uncertainties of the safety and efficacy of these drugs in a real‐world setting, and a lack of systematic monitoring hereon. 3 Also, more experience with anticoagulant management by physicians in BE in comparison to NL could have influenced NOAC uptake rates, as vitamin K antagonist (VKA) care in BE is organized by general physicians (GP), but in NL is organized by specialized anticoagulation clinics. Moreover, before 2012, cardiologists in BE already had experience with NOACs due to the availability of dabigatran through compassionate use programs. 4
A second difference in OAC treatment strategy between these countries was that before 2016, the majority of AF patients on VKA were treated with a target international normalized ratio (INR) range of 2.5 to 3.5 in NL (therapeutic INR range: 2.0‐3.5), compared to the internationally used range of 2.0 to 3.0 in BE. It was hypothesized that aiming for a higher target INR range would give a higher net clinical benefit of VKA treatment. As of 2016, however, target INR range in NL lowered to correspond with international guidelines.
It is important to research how these differences in treatment strategy relate to thromboembolism and bleeding in AF. Because the populations in these countries are quite similar, a comparative analysis can provide us with some unique insights. In this article, we will explore differences in patient characteristics, treatment strategies, and outcomes in newly diagnosed AF patients between NL and BE. For these analyses, data from the worldwide GARFIELD‐AF registry was used, comprising the largest Dutch and Belgian AF cohort to date.
2. METHODS
GARFIELD‐AF is the largest, prospective, worldwide registry of patients with a new diagnosis of atrial fibrillation. Patients were enrolled in five independent, consecutive cohorts: (a) 2009 to 2011, (b) 2011 to 2012, (c) 2013 to 2014, (d) 2014 to 2015, and (e) 2015 to 2016. In NL and BE, patients were included as of November 2010 and May 2012, respectively. Patients aged ≥18 years were eligible for inclusion if they were diagnosed with non‐valvular AF within the previous 6 weeks, and had ≥1 investigator‐determined risk factor for stroke. Patients with transient AF due to a reversible cause were excluded. Follow‐up data was collected every 4 months for 2 years. During follow‐up, data on mortality, ischemic stroke, thromboembolism (TE), and major or clinically relevant non‐major bleeding (CRNMB) were registered. Major bleeding and CRNMB were both defined according to International Society on Thrombosis and Haemostasis (ISTH) criteria. 5 , 6 Chronic kidney disease (CKD) was defined according to the guidelines of the National Kidney Foundation (NKF). 7 The study sponsor and coordinating centre is the Thrombosis Research Institute (TRI) based in London, United Kingdom. The study methods have been described elsewhere. 8 The study was approved by the ethical committees of all participating centres and is registered at ClinicalTrials.gov (NCT01090362).
2.1. Statistical analysis
Continuous variables are expressed as means with standard deviation, and categorical variables as frequencies with percentages. Data from patients with missing values were removed from the respective analyses. For statistical comparison, a t‐test was used for continuous variables and a chi‐squared test for categorical variables. Time in therapeutic range (TTR) was calculated using the Roosendaal method. 9 For BE, an INR range of 2.0 to 3.0 was applied in the calculations. For NL, TTR was calculated using two definitions. The first was applying the range of 2.0 to 3.0 and the second an INR range of 2.0 to 3.5 for INR values before 1 January 2016, and 2.0 to 3.0 hereafter. Only the first occurrence of each adverse event within the first 2 years of follow‐up was analyzed. Events are described as number of events per 100 patient‐years. A Cox proportional hazards model was used for comparison of time‐to‐event, described as unadjusted hazard ratios (HR) with 95% confidence intervals (CI). A density plot was made for a comparison of INR and TTR measurements, with a histogram and an illustration of the density curve applying a kernel smoothing function to the INR and TTR data. A two‐tailed P‐value of <.05 was considered significant. Data analysis was performed with SAS Enterprise Guide, version 7.1 (SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
In NL and BE, 1186 and 1705 patients were included in GARFIELD‐AF, respectively. Mean follow‐up was 1.9 years in both countries. Mean age (70.7 vs 71.3 years), female sex (42.3% vs 42.2%), CHA2DS2‐VASc (3.1 vs 3.1) and HAS‐BLED score (1.4 vs 1.5) were comparable between NL and BE, respectively. 10 , 11 Congestive heart failure (15.4% vs 9.3%) and CKD (13.3% vs 10.0%) were more common in BE, compared to NL. Diabetes mellitus (20.1% vs 16.4%) and acute coronary syndrome (14.7% vs 9.6%) were more common in NL, compared to BE (Table 1).
Table 1.
Baseline characteristics by country
| Characteristic | The Netherlands | Belgium | P‐value a |
|---|---|---|---|
| N = 1186 | N = 1705 | ||
| Female sex | 502 (42.3) | 720 (42.2) | .96 |
| Age | 70.7 ± 10.0 | 71.3 ± 10.8 | .14 |
| BMI | 28.5 ± 5.3 | 28.8 ± 5.7 | .27 |
| Care setting specialty | |||
| Cardiology | 1094 (92.2) | 1484 (87.0) | <.0001 |
| Other hospital departments | 30 (2.5) | 90 (5.3) | |
| General practice | 62 (5.2) | 131 (7.7) | |
| CHF | 110 (9.3) | 263 (15.4) | <.0001 |
| Hypertension | 775 (65.5) | 1160 (68.2) | .14 |
| Diabetes Mellitus | 238 (20.1) | 279 (16.4) | .01 |
| Stroke/TIA | 134 (11.3) | 169 (9.9) | .22 |
| PE or DVT | 22 (1.9) | 41 (2.4) | .33 |
| CAD | 221 (18.6) | 289 (17.0) | .24 |
| PVD | 86 (7.3) | 135 (8.0) | .51 |
| ACS | 174 (14.7) | 164 (9.6) | <.0001 |
| CKD, moderate or severe | 118 (10.0) | 224 (13.3) | .01 |
| Previous bleeding | 25 (2.1) | 46 (2.7) | .31 |
| Risk scores | |||
| CHA2DS2‐VASc | 3.1 ± 1.5 | 3.1 ± 1.6 | .22 |
| HAS‐BLED | 1.4 ± 0.9 | 1.5 ± 0.9 | .25 |
| Antithrombotic treatment | |||
| NOAC ± AP | 209 (17.8) | 1110 (65.5) | <.0001 |
| DTI ± AP | 66/209 (31.6) | 267/1110 (24.1) | |
| FXa ± AP | 143/209 (68.4) | 843/1110 (75.9) | |
| VKA ± AP | 847 (72.1) | 247 (14.6) | |
| Acenocoumarol ± AP | 744/847 (87.8) | 54/247 (21.9) | |
| Phenprocoumon ± AP | 99/847 (11.7) | 36/247 (14.6) | |
| Warfarin ± AP | 1/847 (0.1) | 155/247 (62.8) | |
| Other or unknown ± AP | 3/847 (0.4) | 2/247 (0.8) | |
| AP monotherapy | 56 (4.8) | 179 (10.6) | |
| None | 63 (5.4) | 158 (9.3) | |
The aggregated data of all cohorts are displayed. Categorical data is presented in n (% of total) and continuous data in mean ± standard deviation, unless stated otherwise.
Abbreviations: ACS, acute coronary syndrome; AP, antiplatelet agents; BMI, body mass index (kg/m2); CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DTI, direct thrombin Inhibitor; DVT, deep venous thrombosis; FXa, direct factor Xa inhibitor; NOAC, non–vitamin K antagonist oral anticoagulant; PE, pulmonary embolism; PVD, peripheral vascular disease; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
P‐values calculated using chi‐square tests for categorical variables and t‐tests for continuous variables.
3.2. Differences in antithrombotic treatment
Overall, at diagnosis in NL vs BE 72.1% vs 14.6% received VKA and 17.8% vs 65.5% NOAC, which varied significantly across time (Figure 1). At diagnosis in the most recent cohort in NL (N = 158) and BE (N = 406), 33.5% vs 7.7% were treated with VKA, 62.0% vs 76.9% with NOAC, 2.5% vs 6.5% with antiplatelet monotherapy, and 1.9% vs 9.0% with no antithrombotic therapy. Overall in NL and BE, antiplatelet therapy was used on top of OAC in 13.4% vs 14.8% of patients, respectively.
FIGURE 1.
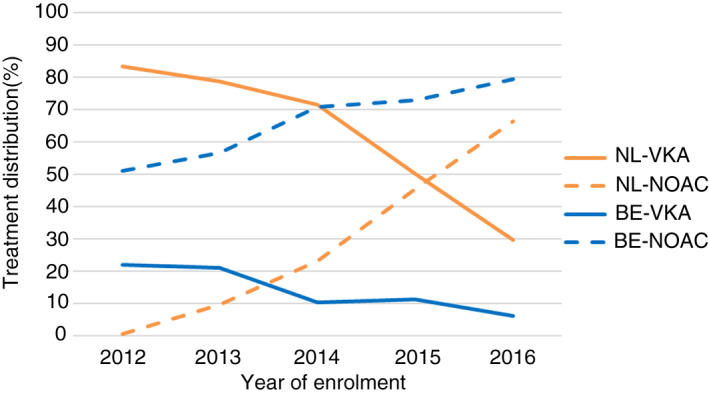
Non–vitamin K antagonist oral anticoagulant and vitamin K antagonist treatment distribution by year of enrolment and country
During the first 2 years of follow‐up from all cohorts, mean INR was significantly higher in NL (2.9 ± 1.0 vs 2.4 ± 1.0) compared to BE. Of all INR values recorded in NL and BE, 35.0% vs 19.7% were above 3.0, 51.9% vs 48.2% between 2.0 to 3.0, and 13.1% vs 32.1% below 2.0 (Figure 2 and Table 2). Mean TTR in NL (range 2.0‐3.5 before 2016 and 2.0‐3.0 as of 2016) and BE (range 2.0‐3.0) was 75.5 ± 14.9 and 48.7 ± 23.8, respectively (Table 2). The proportion of patients with a TTR ≥ 65% was 79.4% and 28.9% in NL and BE, respectively. Density plots of TTR for NL and BE are displayed in Figures 3 and 4.
FIGURE 2.
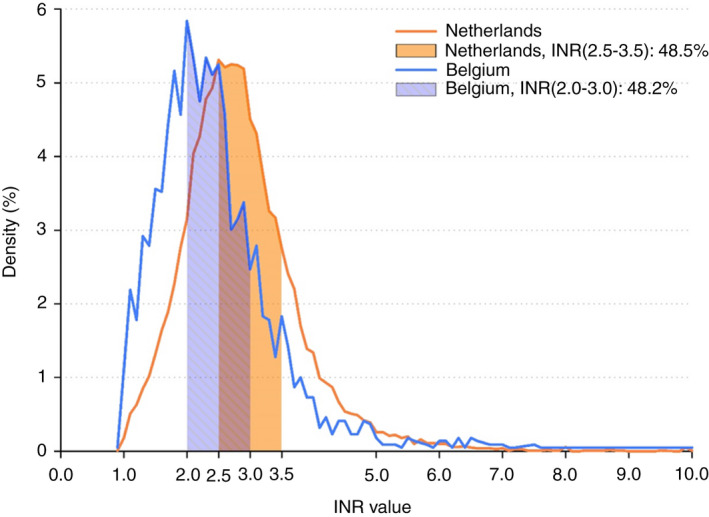
The distribution of all international normalized ratio (INR) values for the Netherlands and Belgium. Percentage of INR values in their respective target range are displayed by country
Table 2.
INR and TTR distribution by country
| The Netherlands | Belgium | |
|---|---|---|
| N = 705 | N = 121 | |
| TTR INR method 1 a | 75.5 ± 14.9 | 48.7 ± 23.8 |
| ≥65 | 79.4% | 28.9% |
| TTR INR method 2 b | 55.4 ± 16.9 | 48.7 ± 23.8 |
| ≥65 | 28.2% | 28.9% |
| INR | 2.9 ± 1.0 | 2.4 ± 1.0 |
| <2.0 | 13.1% | 32.1% |
| <2.5 | 34.3% | 58.5% |
| 2.0‐3.0 | 51.9% | 48.2% |
| 2.5‐3.5 | 48.0% | 31.3% |
| 2.0‐3.5 | 69.2% | 57.7% |
| >3.0 | 35.0% | 19.7% |
| >3.5 | 17.7% | 10.2% |
Only cases with at least one INR measurement were analyzed. All INR measurements were treated independently. Categorical data is presented in % and continuous data in mean ± standard deviation.
Abbreviations: BE, Belgium; INR, international normalized ratio; NL, the Netherlands; TTR, time in therapeutic range.
Method 1: For BE TTR was calculated using an INR range of 2.0‐3.0 and for NL TTR was calculated using INR range of 2.0‐3.5 for INR values before 1 January 2016, and 2.0‐3.0 thereafter.
Method 2: For both countries TTR was calculated using INR range of 2.0‐3.0.
FIGURE 3.
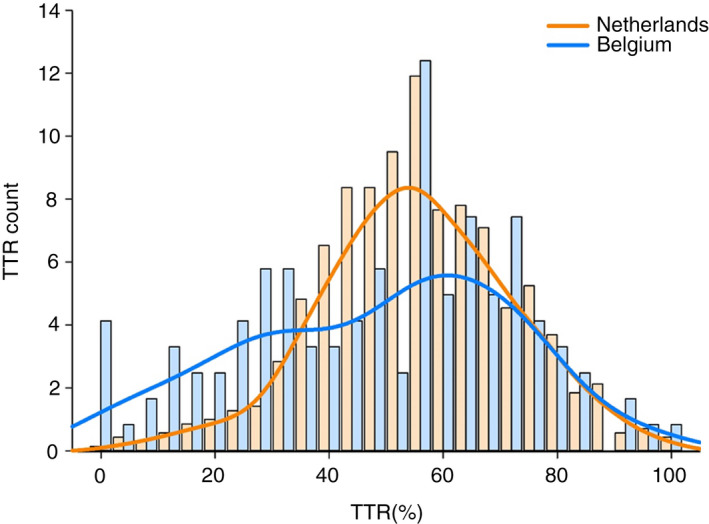
Kernel‐smoothed density of time in therapeutic range (international normalized ratio range 2.0‐3.0) by country
FIGURE 4.
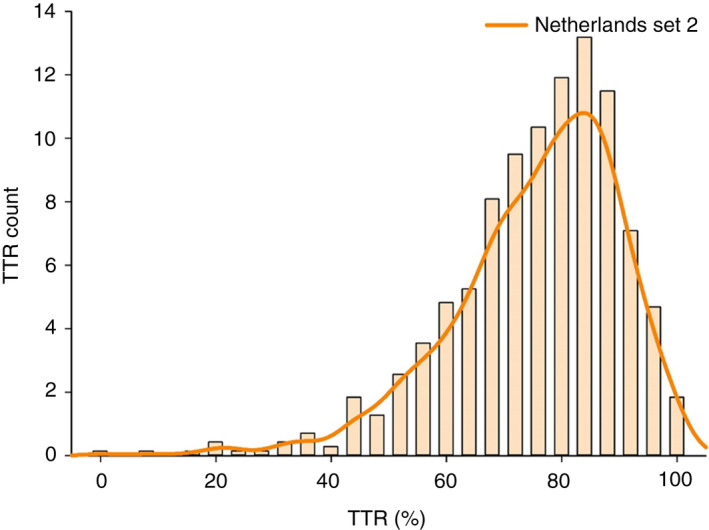
Kernel‐smoothed density of time in therapeutic range (international normalized ratio range 2.0‐3.5) for the Netherlands
3.3. Outcomes
Overall, event rates per 100 patient‐years in NL vs BE of all‐cause mortality (3.38 vs 3.90; HR 0.86, 95% CI 0.65‐1.15), ischemic stroke/TE (0.82 vs 0.72; HR 1.14, 95% CI 0.62‐2.11), and major bleeding (2.06 vs 1.54; HR 1.33, 95% CI 0.89‐1.99) were not significantly different (Table 3). Moreover, there were no statistically significant differences between NL and BE in the rates of cardiovascular mortality (0.95 vs 1.05; HR 0.89, 95% CI 0.52‐1.54), non‐cardiovascular mortality (1.53 vs 2.17; HR 0.71, 95% CI 0.47‐1.06), or CRNMB (2.13 vs 1.80; HR 1.18, 95% CI 0.80‐1.74). In NOACs vs VKAs, the rates of major bleeding (1.31; 95% CI 0.93‐1.85 vs 2.10; 95% CI 1.56‐2.85) and CRNMB (1.68; 95% CI 1.24‐2.27 vs 2.38; 95% CI 1.79‐3.17) were non‐significantly lower with NOACs in comparison to VKAs, respectively (Tables S1 and S2 in supporting information).
Table 3.
Unadjusted event rates per 100 person‐years by country
| Outcome | The Netherlands | Belgium | Hazard ratio |
|---|---|---|---|
| N = 1186 | N = 1705 | (95% CI) | |
| All‐cause mortality | 3.38 (2.70‐4.24) | 3.90 (3.28‐4.65) | 0.86 (0.65‐1.15) |
| Cardiovascular | 0.95 (0.62‐1.45) | 1.05 (0.75‐1.47) | 0.89 (0.52‐1.54) |
| Non‐cardiovascular | 1.53 (1.10‐2.15) | 2.17 (1.71‐2.74) | 0.71 (0.47‐1.06) |
| Undetermined | 0.90 (0.58‐1.40) | 0.68 (0.45‐1.03) | 1.33 (0.72‐2.43) |
| Ischemic stroke/TE | 0.82 (0.51‐1.30) | 0.72 (0.48‐1.08) | 1.14 (0.62‐2.11) |
| Major bleeding | 2.06 (1.54‐2.76) | 1.54 (1.16‐2.04) | 1.33 (0.89‐1.99) |
| Intracranial bleeding | 0.41 (0.21‐0.78) | 0.25 (0.12‐0.50) | — |
| CRNMB | 2.13 (1.59‐2.84) | 1.80 (1.39‐2.33) | 1.18 (0.80‐1.74) |
Data are displayed as event rates per 100 person‐years and unadjusted hazard ratios with 95% confidence intervals. No hazard ratio for intracranial bleeding was calculated due to low number of events.
Abbreviations: CRNMB, clinically relevant non‐major bleeding; TE, thromboembolism.
4. DISCUSSION
The GARFIELD‐AF registry is the largest, prospective registry of patients with newly diagnosed AF in NL and BE to date, which included 1186 and 1705 patients, respectively. This report provides a unique comparison between outcome rates in AF, because AF patient characteristics between the Netherlands and Belgium are quite similar, while OAC management strategy in terms of target INR range and OAC preference differed greatly. Despite the above‐mentioned differences in treatment strategy, rates of all‐cause mortality (HR 0.86; 95% CI 0.65‐1.15), stroke/TE (HR 1.14; 95% CI 0.62‐2.11), and CRNMB (HR 1.18; 95% CI 0.80‐1.74) did not differ significantly between NL and BE. Although the rate of major bleeding was 33% higher in the Netherlands (HR 1.33; 95% CI 0.89‐1.99), the difference was not statistically significant, albeit the number of events were low.
In this study, the rates of major bleeding and stroke/TE were comparable to previous nationwide AF studies, although mortality rates vary. The XANTUS registry, a prospective registry of rivaroxaban in AF, enrolled 899 patients between 2012 and 2013 in NL. 12 Event rates per 100 patient‐years of major bleeding and thromboembolism were 2.4 (95% CI 1.4‐3.7) and 1.6 (95% CI 0.9‐2.8), respectively. The rate of all‐cause mortality was lower in XANTUS (1.0; 95% CI 0.4‐2.0), which is likely due to a younger population with fewer comorbidities in XANTUS. A Dutch study which compared dabigatran with acenocoumarol included 920 AF patients between 2010 and 2013. 13 This study reported event rates of dabigatran vs acenocoumarol for major bleeding of 2.1%/year (95% CI 1.0‐3.8) vs 4.3%/year (95% CI 2.9‐6.2), for stroke/TE 0.8%/year (95% CI 0.2‐2.1) vs 1.0%/year (95% CI 0.4‐2.1) and for all‐cause mortality 2.0%/year vs 1.6%/year. A prospective registry in older patients from GP offices in NL reported on 2068 AF‐patients on OAC (97% VKA, 3% dabigatran) between 2013 and 2014. 14 Event rates per 100 patient‐years of mortality was higher (6.7), while stroke (1.7), major bleeding (1.7), and CRNMB (2.7) seemed similar, although no CIs were reported. Stroke and bleeding rates from The Belgian Improvement Study on OAC Therapy were higher (4.9 and 5.9, respectively). However, patients for any OAC indication were enrolled and the study dates back to 2005. 15
Patients in NL and BE had overall relatively similar characteristics, with a similar predicted stroke and bleeding risk (Table 1). In NL, patients on VKA in GARFIELD‐AF were treated using target INR range 2.5 to 3.5 until January 2016 and 2.0 to 3.0 hereafter, the latter being equivalent to practice in Belgium and worldwide. This difference in practice is reflected by a significantly higher mean INR (2.9 ± 1.0 vs 2.4 ± 1.0) in NL in this study. It was Dutch practice for years to target a higher INR range, which was hypothesized to provide a net clinical benefit because the rate of ischemic stroke increases sharply when INR drops below 2.0, while (intracranial) bleeding risk seems to remain quite similar with INR 3.0 to 3.5 vs 2.0 to 3.0. 16 , 17 , 18 However, randomized study data hereon has always been lacking. Indeed, in this study the proportion of INR measurements below 2.0 is far lower (13.1% vs 32.1%) in NL vs BE, with the countereffect of more INR measurements above 3.0 (35.0% vs 19.7%) and 3.5 (17.7% vs 10.2%; Table 2). Despite this difference in VKA intensity, no significant difference in rates of ischemic stroke/TE, bleeding, and mortality were observed between BE and NL. These results should be interpreted with caution, as differences in the proportion of NOAC vs VKA users, but also differences in VKAs used between countries, could influence results. Given the low proportion of VKA use in Belgium, there were too few Belgian VKA patients with an adverse event to be able to adjust for confounders for this comparison.
As reflected in Figure 1, the proportion of patients on NOAC therapy was much higher in BE, but the difference diminished significantly as the years progressed. In the most recent cohort in NL and BE, 33.5% vs 7.7% were treated with VKA and 62.0% vs 76.9% with NOAC, respectively. When the NOACs were introduced in NL, discussion arose around the safety of these agents for usage in daily practice. 3 , 19 One of the concerns was a lack of monitoring for therapy adherence or side effects with NOACs, especially given the high mean TTR as an indicator for therapy adherence and low bleeding rates already being achieved by the specialized Dutch anticoagulation clinics. 20 This, combined with a lack of real‐world data, resulted in a careful introduction of NOACs in NL, as seen in this study. Moreover, until 2016 NOACs could only be prescribed by cardiologists and the drugs were only reimbursed with a physician's statement form. As of 2016, Dutch GPs were allowed to prescribe NOACs, and as of 2018, all NOACs were reimbursed without the need of a physician's statement form. In BE, patients on VKA are treated and monitored mainly by GPs and NOACs were adopted very early. BE entered the GARFIELD‐AF registry from cohort 2, which coincided with reimbursement of the first available NOAC, dabigatran. Furthermore, NOACs were made available to cardiologists (who included most GARFIELD‐AF patients) the year before by means of so‐called “compassionate use and medical need” programs. 4 These programs allow the use of drugs with an approved European indication before the drug is commercially available. So, Belgian physicians were already familiar with the use of these drugs.
However, since then there is robust evidence showing the safety of these agents in the real world, although issues such as medication adherence and off‐label dosing persist. 21 Also, NOACs have proven to be a cost‐effective alternative to VKAs. 22 Because NOACs reduce ischemic stroke rate by 20% and intracranial bleeding rate by 50% in comparison to warfarin, one could hypothesize that a faster NOAC uptake could have prevented more adverse events. 23 When comparing patients on NOAC vs VKA in the combined NL–BE cohort, the rate of major bleeding per 100 patient‐years (1.3; 95% CI 0.9‐1.9 vs 2.1; 95% CI 1.6‐2.9) and CRNMB (1.7; 95% CI 1.2‐2.3 vs 2.4; 95% CI 1.8‐3.2) were lower with NOACs, although non‐significant, respectively (Tables S1 and S2). This could be an explanation for the non‐significantly 33% higher major bleeding rate in NE, although event rates were too low for a reliable adjustment for possible confounders.
4.1. Strengths and limitations
A strength of this study is that all patients were newly diagnosed with AF, so differences in patient experience with OAC use were minimal. Moreover, we compared the largest NL and BE AF cohorts to date. However, the comparison was underpowered to detect small differences in absolute adverse event rates. Also, confounding could have played an important role concerning event rates, although no event rates were significantly different when comparing NL to BE.
5. CONCLUSION
In GARFIELD‐AF, despite similar characteristics, patients were treated differently in NL and BE with predominantly VKA vs NOAC and a higher target INR range in NL, respectively. Although the rate of major bleeding was 33% higher in NL, variations in bleeding, mortality, and stroke/TE rates were not statistically significant.
CONFLICTS OF INTEREST
J. Seelig is supported by an unrestricted research grant from the Dutch Federation of Anticoagulation clinics (“FNT”). F. W. A. Verheugt has received honoraria for consulting and presentations from Bayer HealthCare, Boehringer‐Ingelheim, BMS/Pfizer, and Daiichi‐Sankyo. F. Cools reports speaker fees from Boehringer‐Ingelheim Pharma, Bayer AG, Pfizer, and speaker fees and a modest research grant from Daiichi‐Sankyo Europe. F. Cools is national coordinator for Garfield‐AF in Belgium. H. ten Cate received compensation for presentations from Boehringer, Bayer, Leo, Daiichi; is advisor for Alveron; and receives research support from Bayer and Pfizer. He is an unpaid chairman of the board of the Dutch Federation of Anticoagulation Clinics and national coordinator for Garfield‐AF in the Netherlands. H. ten Cate is adjunct professor at the Center for Thrombosis and Haemostasis, Gutenberg Medical Center, Mainz, Germany. The other authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
J. Seelig wrote the manuscript with support from M. E. W. Hemels, F. Cools, and H. ten Cate. H. ten Cate and F. Cools were the national study coordinators of the Netherlands and Belgium, respectively. S. Virdone led the data analysis. All authors contributed to and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was reviewed and approved by the ethics committees of each participating center.
Supporting information
Table S1‐S2
ACKNOWLEDGMENTS
We thank the physicians, nurses, and patients involved in the GARFIELD‐AF registry. We also thank Madhusudana Rao (TRI) and Saverio Virdone (TRI) for providing data analyses. The authors of this manuscript extend their gratitude to the 15 Dutch and 25 Belgian medical centers whose participation in GARFIELD‐AF has made the results presented in this paper possible (listed in the Appendix).
Appendix 1.
Dutch and Belgian GARFIELD‐AF sites and investigators that contributed to the results in this paper: Dr P. A. M. Hoogslag, Diaconessenhuis Meppel, Meppel, the Netherlands; Dr B. E. Groenemeijer, Gelre Ziekenhuizen, Apeldoorn, the Netherlands; Dr P. Buiks, Huisartsenpraktijk Ewijk, Ewijk, the Netherlands; Dr A. Lucassen, St. Jans Gasthuis, Weert, the Netherlands; Dr J. P. R. Herrman, Onze Lieve Vrouwe Gasthuis, Amsterdam, the Netherlands; Dr P. R. Nierop, St. Franciscus Gasthuis, Rotterdam, the Netherlands; Dr W. Terpstra, Slingeland Ziekenhuis, Doetinchem, the Netherlands; Prof. Dr L. V. A. Boersma, St. Antonius Ziekenhuis, Nieuwegein, the Netherlands; Dr W. Hermans, Elisabeth‐TweeSteden Ziekenhuis, Tilburg, the Netherlands; Dr M. G. C. Pieterse, Stichting Cardiologie Amsterdam, Amsterdam, the Netherlands; Prof. Dr Hugo ten Cate, Stichting Trombosedienst Maastricht, Maastricht, the Netherlands; Dr H. J. Adriaansen, Trombosedienst Apeldoorn‐Zutphen, Apeldoorn, the Netherlands; Dr M. C. M. Bongaerts, Trombosedienst Rivierenland, Tiel, the Netherlands; Dr C. Guldener, WECOR, Etten‐Leur, the Netherlands; Dr S. H. K. The, Ziekenhuis Bethesda, Hoogeveen, the Netherlands; Dr O. Xhaët, CHU UCL Namur, Yvoir, Belgium; Dr A. de Wolf, RZ Heilig Hart, Tienen, Belgium; Dr A. Heyse, AZ Glorieux, Ronse, Belgium; Dr J. Voet, AZ Nikolaas, Sint‐Niklaas, Belgium; Dr G. Vervoort, AZ Sint‐Maarten, Mechelen, Belgium; Dr B. Wollaert, ZNA Stuivenberg, Antwerpen, Belgium; Dr K. Hermans, AZ Sint‐Lucas, Gent, Belgium; Dr S. Verstraete, AZ Zeno, Knokke‐Heist, Belgium; Dr G. H. Mairesse, Cliniques du Sud‐Luxembourg, Arlon, Belgium; Dr D. Faes, Mariaziekenhuis Noord‐Limburg, Overpelt, Belgium; Dr L. Capiau, Huisartsenpraktijk BVBA, Wetteren, Belgium; Dr F. Cools, AZ Klina, Brasschaat, Antwerpen, Belgium; Dr G. Hollanders, Private Practice Cardiology, De Pinte, Belgium; Dr J. Vercammen, VZW Regionaal Ziekenhuis Jan Yperman, Ieper, Belgium; Dr Y. Balthazar, MG Balthazar‐Ballard, Natoye, Belgium; Dr M. Delforge, Centre Hospitalier Hutois, Huy, Belgium; Dr H. Striekwold, Heilig Hart Ziekenhuis, Mol, Belgium; Dr W. Anné, AZ Delta, Roeselare, Belgium; Dr I. Blankoff, CHU de Charleroi–Hôpital André Vésale, Montigny‐le‐Tilleul, Belgium; Dr M. Beutels, Huisartsen Het Laar, Merksem, Belgium; Dr A. Postolache, Clinique Saint‐Pierre, Ottignies‐Louvain‐la‐Neuve, Belgium; Dr P. Vandergoten, Europa Ziekenhuizen, Mechelen, Belgium; Dr P. Purnode, Kliniek Sint‐Jan, Sint‐Joost‐ten‐Node, Belgium; Dr P. Godart, CHU Ambroise Paré, Mons, Belgium; Dr T. Boussy, AZ Groeninge, Kortrijk, Belgium. The contributors listed outside of the author list declare to have no relevant conflicts of interest.
Seelig J, Hemels MEW, Xhaët O, et al; for the GARFIELD‐AF investigators . Impact of different anticoagulation management strategies on outcomes in atrial fibrillation: Dutch and Belgian results from the GARFIELD‐AF registry. J Thromb Haemost. 2020;18:3280–3288. 10.1111/jth.15081
Frank Cools and Hugo ten Cate are joint last authors.
Manuscript handled by: Sabine Eichinger
Final decision: Sabine Eichinger, 25 August 2020
Funding information
The Global Anticoagulant Registry in the FIELD–Atrial Fibrillation was sponsored by the Thrombosis Research Institute, London, United Kingdom. Funding of the registry was provided through an unrestricted research grant from Bayer Pharma AG, Berlin, Germany.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Cools F, Wollaert B, Vervoort G, et al. Treatment patterns in anticoagulant therapy in patients with newly diagnosed atrial fibrillation in Belgium: results from the GARFIELD‐AF registry. Acta Cardiol. 2019;74:309‐318. [DOI] [PubMed] [Google Scholar]
- 2. Seelig J, Verheugt FWA, Hemels MEW, et al. Changes in anticoagulant prescription in Dutch patients with recent‐onset atrial fibrillation: observations from the GARFIELD‐AF registry. Thromb J. 2020;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. New Anticoagulants ‐ A well‐dosed introduction: Health Council of the Netherlands. 2012. [27‐jan‐2020]. https://www.healthcouncil.nl/documents/advisory‐reports/2012/05/15/new‐anticoagulants‐a‐well‐dosed‐introduction
- 4. Compassionate use ‐ Medical need: FAMHP. [22‐apr‐2020]. https://www.famhp.be/en/human_use/medicines/medicines/research_development/compassionate_use_medical_need
- 5. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis .Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692‐694. [DOI] [PubMed] [Google Scholar]
- 6. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119‐2126. [DOI] [PubMed] [Google Scholar]
- 7. International Society of Nephrology . Chapter 2: definition, identification, and prediction of CKD progression. Kidney Int Suppl. 2013;3:63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kakkar AK, Mueller I, Bassand JP, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: Global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J. 2012;163:13‐19 e11. [DOI] [PubMed] [Google Scholar]
- 9. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236‐239. [PubMed] [Google Scholar]
- 10. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263‐272. [DOI] [PubMed] [Google Scholar]
- 11. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093‐1100. [DOI] [PubMed] [Google Scholar]
- 12. Pisters R, van Vugt SPG, Brouwer MA, et al. Real‐life use of Rivaroxaban in the Netherlands: data from the Xarelto for Prevention of Stroke in Patients with Atrial Fibrillation (XANTUS) registry. Neth Heart J. 2017;25:551‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korenstra J, Wijtvliet EP, Veeger NJ, et al. Effectiveness and safety of dabigatran versus acenocoumarol in 'real‐world' patients with atrial fibrillation. Europace. 2016;18:1319‐1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Doorn S, Tavenier A, Rutten FH, Hoes AW, Moons KGM, Geersing GJ. Risk of cardiac and non‐cardiac adverse events in community‐dwelling older patients with atrial fibrillation: a prospective cohort study in the Netherlands. BMJ Open. 2018;8:e021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Claes N, Buntinx F, Vijgen J, et al. The Belgian improvement study on oral anticoagulation therapy: a randomized clinical trial. Eur Heart J. 2005;26:2159‐2165. [DOI] [PubMed] [Google Scholar]
- 16. Hylek EM, Skates SJ, Sheehan MA, Singer DE. An analysis of the lowest effective intensity of prophylactic anticoagulation for patients with nonrheumatic atrial fibrillation. N Engl J Med. 1996;335:540‐546. [DOI] [PubMed] [Google Scholar]
- 17. Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJ, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11‐17. [DOI] [PubMed] [Google Scholar]
- 19. van Beek H. Nooit meer bloed prikken. Trouw. 5 October 2012;Sect. Front page.
- 20. Jaarverslagen: FNT; [12‐03‐2020]. https://www.fnt.nl/algemeen/jaarverslagen
- 21. Coleman CI, Briere JB, Fauchier L, et al. Meta‐analysis of real‐world evidence comparing non‐vitamin K antagonist oral anticoagulants with vitamin K antagonists for the treatment of patients with non‐valvular atrial fibrillation. J Mark Access Health Policy. 2019;7:1574541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, Bodalia PN, Bryden PA, et al. Oral anticoagulants for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of stroke in atrial fibrillation: systematic review, network meta‐analysis and cost‐effectiveness analysis. Health Technol Assess. 2017;21:1‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet. 2014;383:955‐962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


