Abstract
The effects of glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) on renal outcomes in patients with type 2 diabetes at high cardiovascular risk are modest or neutral. However, GLP‐1RAs may confer clinical benefits in those at high risk of progressive renal function loss. We examined the effects of once‐weekly exenatide (EQW) on estimated glomerular filtration rate (eGFR) slope and urinary albumin:creatinine ratio (UACR) as a function of baseline UACR in 3503 EXSCEL participants (23.7%) with eGFR data available and 2828 participants (19.2%) with UACR change data available. EQW improved eGFR slope assessed via mixed model repeated measures, compared with placebo, in participants with baseline UACR >100 mg/g (0.79 mL/min/1.73 m2/year [95% confidence interval {CI} 0.24–1.34]) and UACR >200 mg/g (1.32 mL/min/1.73 m2/year [95% CI 0.57–2.06]), but not at lower UACR thresholds. EQW reduced UACR, compared with placebo, assessed via analysis of covariance, consistently across subgroups with baseline UACR >30 mg/g (28.2% reduction), baseline UACR >100 mg (22.5% reduction) and baseline UACR >200 mg (34.5% reduction). This post hoc EXSCEL analysis suggests that EQW reduces UACR, with improvement in eGFR slope specifically in participants with elevated baseline UACR.
Keywords: diabetic kidney disease, exenatide, GLP‐1RA, glucagon‐like peptide‐1 receptor agonists, pooled analysis
1. INTRODUCTION
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) reduced the incidence of composite renal outcomes in several cardiovascular outcome trials. 1 , 2 , 3 , 4 , 5 However, the magnitude of this effect was modest, and driven mainly by reductions in albuminuria. Interestingly, the effects of GLP‐1RAs on renal outcomes seem to be greater in patients with established chronic kidney disease (CKD). Specifically, in a subgroup analysis of the LEADER trial, the effect of liraglutide compared with placebo on estimated glomerular filtration rate (eGFR) appeared to be greater in participants with macroalbuminuria or in those with eGFR between 30 and 60 mL/min/1.73 m. 2 , 6 In addition, in the AWARD‐7 trial, the effect of dulaglutide on clinical kidney outcomes and rate of eGFR decline was more pronounced in participants with macroalbuminuria at baseline. 7 Whether this effect represents a class effect, and which specific subpopulation of patients may derive renal benefit from GLP‐1RAs, remains incompletely understood.
The current established renal endpoints for clinical trials in patients with type 2 diabetes and CKD are composites including doubling of serum creatinine and end‐stage renal disease. These are late manifestations in the progression of CKD. Therefore, large trials of long duration are required to establish drug efficacy using these outcomes. eGFR decline (eGFR slope) is proposed as a valid surrogate for major clinical kidney endpoints. 8 A number of studies have shown a strong and consistent relationship between eGFR slope and risk of adverse kidney outcomes, cardiovascular disease and all‐cause mortality. Importantly, treatment effects on eGFR slope are strongly associated with treatment effects on renal outcomes. 9 , 10 , 11 These data support use of eGFR slope as a valid endpoint for future trials in diabetes and CKD. In this context, it is relevant to obtain further insight into the effects of exenatide on eGFR slope in patients with type 2 diabetes at low, moderate and high risk of CKD progression. Therefore, we examined the effects of once‐weekly exenatide (EQW) on eGFR slope and change in urinary albumin:creatinine ratio (UACR), as a function of baseline UACR and other risk markers of CKD progression, in the subset of EXSCEL participants with UACR measured at baseline.
2. METHODS
The EXSCEL trial (NCT01144338) assessed the effect of EQW on cardiovascular outcomes in a broad population of participants with type 2 diabetes mellitus, with or without known cardiovascular disease. The study design, baseline characteristics and primary results have been published previously. 12 , 13 EXSCEL was conducted as a pragmatic clinical trial; apart from calcitonin, which was assayed centrally, only local laboratory values were collected, and measurement of UACR was not mandated. As such, baseline UACR measurements are only available for a subset of participants.
This analysis included all EXSCEL participants with eGFR recorded at baseline and at least one post‐baseline eGFR measurement (to permit eGFR slope determination) or UACR recorded at baseline and at least one post‐baseline UACR measurement (to permit percentage change in UACR determination). The main endpoints in the present analysis were annual rate of eGFR decline (eGFR slope) and UACR change. The effects of exenatide on these outcomes were determined as a function of baseline UACR, other baseline demographics and clinical chemistry variables.
The effect of EQW on eGFR slope was estimated using a mixed model repeated measures analysis, with eGFR as the dependent variable, time and baseline eGFR as linear covariates, treatment arm and treatment‐by‐time interaction as fixed effects, and participant as a random effect. eGFR was calculated from site‐reported serum creatinine values using the Modification of Diet in Renal Disease equation. 14 Percentage change in UACR with EQW treatment was calculated by analysis of covariance using log‐transformed UACR values, with baseline log‐transformed UACR as a covariate. Participants with baseline UACR values recorded as zero were excluded before log‐transformation. All analyses were performed in the intention‐to‐treat population. EXSCEL was not designed or powered to examine eGFR slope or UACR. As such, this post hoc analysis is exploratory, and all reported P values are nominal with values <0.05 considered nominally significant. Data were prepared in sas 9.4, and all analyses were performed in R version 3.4.1.
3. RESULTS
Of 14 752 EXSCEL participants, eGFR slope data were available for 13 826 (93.7%), and baseline UACR measurements were available for 3503 (23.7%). Data regarding percentage UACR change from baseline to first post‐baseline measurement were available for 2828 participants (19.2%). Baseline characteristics of participants with baseline UACR measurements were generally similar to the overall EXSCEL population, and balanced between treatment groups (Supplementary Table S1).
3.1. Effects of EQW on eGFR slope
The median (interquartile range) follow‐up for eGFR in participants with UACR measured at baseline was 3.3 (2.1, 4.3) years, and with an average of 6.8 eGFR measurements per participant. In EXSCEL participants with UACR measured at baseline, the mean (SE) eGFR decline was −0.87 (0.07) mL/min/1.73 m2/year in the placebo arm and −0.85 (0.07) mL/min/1.73 m2/year in the EQW arm, resulting in a neutral estimated EQW treatment effect on eGFR slope (0.02 mL/min/1.73 m2/year [95% CI −0.18 to 0.22]; Supplementary Table S2). In the subgroup of participants with baseline UACR >100 mg/g, the mean (SE) eGFR slope was −2.85 (0.20) mL/min/1.73 m2/year in the placebo group and −2.07 (0.20) mL/min/1.73 m2/year in the EQW group, leading to a between‐group difference of 0.79 mL/min/1.73 m2/year (95% CI 0.24–1.34) in favour of EQW (Figure 1A). Among participants with baseline UACR >200 mg/g, the mean (SE) eGFR slope was −3.73 (0.26) mL/min/1.73 m2/year in the placebo group and −2.43 (0.27) mL/min/1.73 m2/year in the EQW group, leading to a between‐group difference of 1.32 mL/min/1.73 m2/year (95% CI 0.57–2.06). In participant subgroups with baseline UACR values <30 mg/g, or with a baseline UACR cut‐off >30 mg/g, no EQW effect on eGFR slope was observed. The effect of EQW compared with placebo on eGFR slope was consistent regardless of baseline eGFR, cardiovascular disease history, renin‐angiotensin‐aldosterone system (RAAS) inhibitor use, and systolic blood pressure, with a small difference observed in subgroups defined by baseline body mass index (Figure 2A).
FIGURE 1.
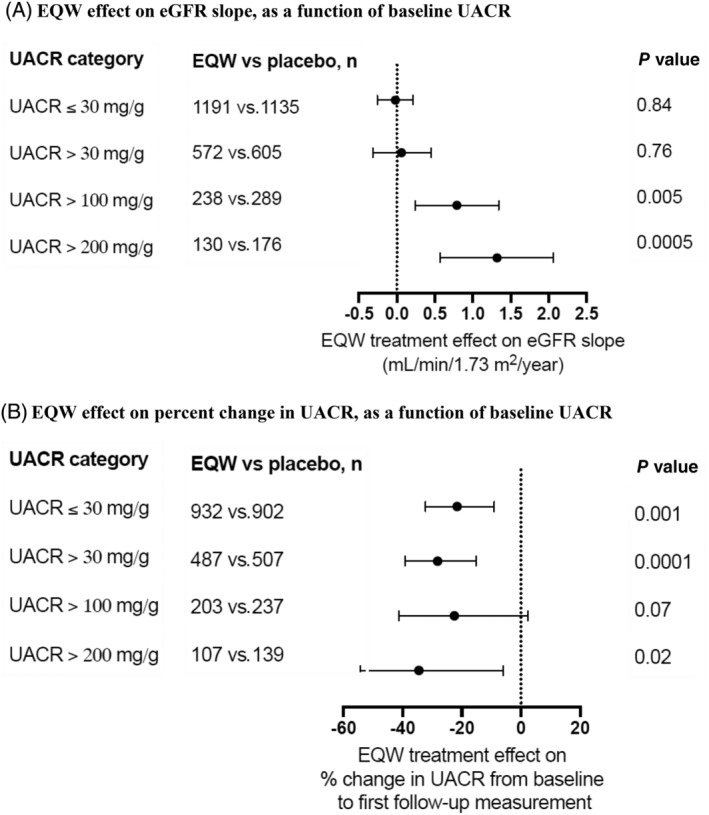
Once‐weekly exenatide (EQW) effects on renal disease progression, as a function of baseline urinary albumin:creatinine ratio (UACR). A, EQW effect on estimated glomerular filtration rate slope, as a function of baseline UACR. B, EQW effect on percent change in UACR, as a function of baseline UACR
FIGURE 2.
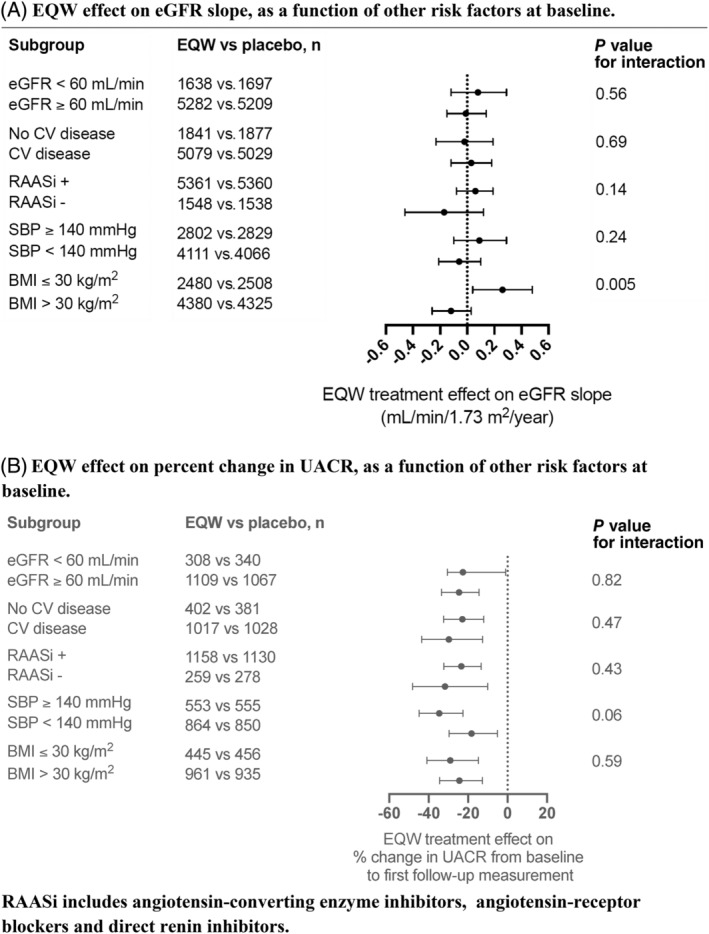
Once‐weekly exenatide (EQW) effects on renal disease progression, as a function of other risk factors. A, EQW effect on estimated glomerular filtration rate (eGFR) slope, as a function of other risk factors at baseline. B, EQW effect on percent change in urinary albumin:creatinine ratio (UACR), as a function of other risk factors at baseline. Renin‐angiotensin‐aldosterone system inhibitors (RAASi) include angiotensin‐converting enzyme inhibitors, angiotensin‐receptor blockers and direct renin inhibitors. BMI, body mass index; CV, cardiovascular; SBP, systolic blood pressure
3.2. Effects of EQW on UACR
The median (interquartile range) time until first follow‐up UACR measurement in participants with baseline and at least one follow‐up UACR measurement was 8.9 (5.7, 13.7) months, and the EQW effect on UACR change was −25.0% (95% CI −33.0 to 16.0; from 15.0 to 13.0 mg/g and 15.9 to 17.4 mg/g in the EQW and placebo groups, respectively; Supplementary Tables S3 and S4). The EQW effect on UACR change, compared with placebo, was similar across the UACR subgroups examined, with a nominally significant improvement in the UACR ≤30 mg/g, UACR >30 mg/g and UACR >200 mg/g groups (change −21.6% [95% CI −32.2 to −9.1], −28.2% [95% CI −39.2 to −15.2] and −34.5% [95% CI −54.4 to −6.0], respectively [Figure 1B, Supplementary Table S3]). The effect of EQW on UACR was also consistent across subgroups defined by baseline eGFR, cardiovascular disease history, RAAS inhibitor use, systolic blood pressure and body mass index (Figure 2B).
4. DISCUSSION
In this EXSCEL post hoc sub‐analysis, we assessed the effects of EQW on eGFR slope and albuminuria, as a function of renal and cardiovascular risk markers in participants with type 2 diabetes, with or without known cardiovascular disease. EQW treatment slowed the progression of eGFR decline in participants with elevated baseline albuminuria (>100 mg/g) but not among those with lesser degrees of albuminuria. The effect of EQW on eGFR slope was consistent regardless of the presence or absence of other risk markers of CKD progression. In contrast to the effect of EQW on eGFR, which was seen only in subgroups with higher degrees of baseline albuminuria, the effect of EQW on albuminuria was observed in all subgroups, independent of baseline albuminuria or other risk markers of CKD progression.
Glucagon‐like peptide‐1 receptor agonists have beneficial effects on traditional risk factors for CKD such as hyperglycaemia, hypertension, obesity and dyslipidaemia. However, data from preclinical studies suggest that GLP‐1RAs also exert direct effects on the kidney. GLP‐1RAs may induce natriuresis and diuresis by inhibiting NHE3‐dependent sodium reabsorption in the proximal tubule. Other effects that may contribute to the renal benefits of GLP‐1RAs include reduction of renal RAAS activation, reduction of renal hypoxia, and inhibition of pro‐inflammatory pathways. 15 , 16
The observed effect of EQW on albuminuria in the present analysis is consistent with previous observations for exenatide, lixisenatide, liraglutide and semaglutide, where reductions in albuminuria and/or new persistent macroalbuminuria were observed. 1 , 4 , 6 , 17 The effect of GLP‐1RAs on eGFR slope is less well characterized. However, our observation that EQW reduces eGFR slope specifically in participants with higher albuminuria is consistent with data from other studies in which GLP‐1RAs slowed progression of eGFR decline in participants with moderate‐to‐severe CKD. 3 , 7
Albuminuria is a strong predictor of declining renal function and end‐stage renal disease in patients with type 2 diabetes. In line with previous studies, the rate of eGFR decline was higher in subgroups with higher degrees of baseline albuminuria in EXSCEL participants. The greater effects of EQW on eGFR slope in participants with higher degrees of baseline albuminuria, who are at higher risk of end‐stage renal disease, suggest that future randomized controlled clinical trials assessing the effects on renal outcomes of EQW could consider focusing on those with higher degrees of albuminuria. The greater relative effects of EQW in participants at high risk of CKD progression also suggest greater absolute benefits, leading to lower numbers‐needed‐to‐treat and potentially higher cost‐effectiveness.
In addition to GLP‐1RAs, sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors have also been shown to slow eGFR decline in post hoc analyses from cardiovascular safety trials. However, in contrast to GLP‐1RAs, the benefits of SGLT2 inhibitors on eGFR slope appear to be independent of baseline albuminuria, as shown, for example, by the effects of dapagliflozin on eGFR in the DECLARE TIMI 58 trial. 18 Similar findings were observed in the CANVAS programme, where canagliflozin slowed the loss of kidney function in all subgroups, including participants with normoalbuminuria. 19 The benefits of SGLT2 inhibitors extend to patients with high albuminuria and impaired eGFR. 20 Taken together, the available data suggest that SGLT2 inhibitors exert kidney protection across the spectrum of albuminuria whereas GLP‐1RA benefits are mainly restricted to individuals at higher risk of CKD progression.
This analysis has several limitations. Firstly, this was a post hoc analysis in a subgroup of participants and therefore the results can only be considered as hypothesis‐generating and should be confirmed in prospective clinical trials. Secondly, UACR collection was not mandated in the EXSCEL protocol, introducing a potential for bias in the population with albuminuria measurements available. In addition, albuminuria was measured in a single spot urine sample, which may have introduced random measurement variability. However, despite possible random error in albuminuria measurements, strong and consistent effects of EQW were observed.
In conclusion, the results of this analysis, combined with the existing literature, support the hypothesis for a GLP‐1RA class effect in improving albuminuria in a broad population, and slowing eGFR decline specifically in patients with elevated albuminuria. Prospective clinical trials are needed to confirm the benefits of exenatide in slowing progression of CKD in patients with type 2 diabetes and elevated albuminuria.
CONFLICTS OF INTEREST
A.B.v.d.A.‐v.d.B. has nothing to disclose. L.E.C., R.C.P., D.W.B. and C.D.S. are employees and/or shareholders of AstraZeneca. D.W.B. is a stockholder of Bristol‐Myers Squibb Company. R.J.M. has received research support and honoraria from AstraZeneca, GlaxoSmithKline plc., and Merck & Co., Inc. R.R.H. reports research support from AstraZeneca, Bayer and Merck Sharp & Dohme, and personal fees from Bayer, Intarcia, Merck Sharp & Dohme, Novartis and Novo Nordisk. H.J.L.H. receives research support from Abbvie, AstraZeneca, Boehringer Ingelheim and Janssen, and has consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Gilead, Janssen, Merck, MundiPharma, Mitsubishi Tanabe and Retrophin.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14175.
Supporting information
Supplementary Table S1 Baseline characteristics of subjects in EXSCEL with post‐baseline eGFR measurements, and those with baseline UACR measurements. Data are given as mean (SE) for continuous metrics and number (percent) for categorical characteristics. UACR is presented as median (interquartile range) due to a non‐normal distribution.
SupplementaryTable S2. Details of eGFR slope analysis.
Supplementary Table S3. Details of percent change in UACR analysis.
Supplementary Table S4. Details of absolute change in UACR.
ACKNOWLEDGMENTS
The EXSCEL trial was conducted jointly by the Duke Clinical Research Institute and the University of Oxford Diabetes Trials Unit, in collaboration with the sponsor Amylin Pharmaceuticals, a wholly owned subsidiary of AstraZeneca. R.R.H. is an emeritus UK National Institute for Health Research Senior Investigator.
van der Aart‐van der Beek AB, Clegg LE, Penland RC, et al. Effect of once‐weekly exenatide on estimated glomerular filtration rate slope depends on baseline renal risk: A post hoc analysis of the EXSCEL trial. Diabetes Obes Metab. 2020;22:2493–2498. 10.1111/dom.14175
A.B.v.d.A.‐v.d.B. and L.E.C. contributed equally to the work.
Funding information AstraZeneca
REFERENCES
- 1. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247‐2257. 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 2. Bethel MA, Mentz RJ, Merrill P, et al. Microvascular and cardiovascular outcomes according to renal function in patients treated with once‐weekly Exenatide: insights from the EXSCEL trial. Diabetes Care. 2019;43(2):446‐452. 10.2337/dc19-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311‐322. 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834‐1844. 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 5. Idzerda NMA, Clegg LE, Hernandez AF, et al. Prediction and validation of exenatide risk marker effects on progression of renal disease: insights from EXSCEL. Diabetes Obes Metab. 2020;22(5):798‐806. 10.1111/dom.13958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mann JFE, Ørsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839‐848. 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 7. Tuttle KR, Lakshmanan MC, Rayner B, et al. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate‐to‐severe chronic kidney disease (AWARD‐7): a multicentre, open‐label, randomised trial. Lancet Diabetes Endocrinol. 2018;6(8):605‐617. 10.1016/S2213-8587(18)30104-9. [DOI] [PubMed] [Google Scholar]
- 8. Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European medicines agency. Am J Kidney Dis Off J Natl Kidney Found. 2020;75(1):84‐104. 10.1053/j.ajkd.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 9. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA. 2014;311(24):2518‐2531. 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inker LA, Heerspink HJL, Tighiouart H, et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta‐analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30(9):1735‐1745. 10.1681/ASN.2019010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grams ME, Sang Y, Ballew SH, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta‐analysis of observational data. J Am Soc Nephrol. 2019;30(9):1746‐1755. 10.1681/ASN.2019010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide study of cardiovascular event lowering (EXSCEL) trial. Am Heart J. 2016;174:103‐110. 10.1016/j.ahj.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 13. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once‐weekly Exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228‐1239. 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461‐470. 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15. Muskiet MHA, Tonneijck L, Smits MM, et al. GLP‐1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13(10):605‐628. 10.1038/nrneph.2017.123. [DOI] [PubMed] [Google Scholar]
- 16. Thomas MC. The potential and pitfalls of GLP‐1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(Suppl 1):2S20‐2S27. 10.1016/S1262-3636(17)30069-1. [DOI] [PubMed] [Google Scholar]
- 17. Heerspink HJL, van der Aart‐van der Beek AB, Guja C, van Raalte DH, Suchower LJ, Hardy E, Sjöström CD. Exenatide once weekly decreases uACR over 26/28 weeks in patients with diabetes and elevated albuminuria: A pooled analysis. https://www.easd.org/virtualmeeting/home.html#!resources/exenatide-once-weekly-decreases-uacr-over-26-28-weeks-in-patients-with-diabetes-and-elevated-albuminuria-a-pooled-analysis-588a943f-79bc-41df-9487-d242a82de86b [DOI] [PMC free article] [PubMed]
- 18. Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE–TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7(8):606‐617. 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 19. Neuen BL, Ohkuma T, Neal B, et al. Effect of Canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol. 2019;30(11):2229‐2242. 10.1681/ASN.2019010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295‐2306. 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1 Baseline characteristics of subjects in EXSCEL with post‐baseline eGFR measurements, and those with baseline UACR measurements. Data are given as mean (SE) for continuous metrics and number (percent) for categorical characteristics. UACR is presented as median (interquartile range) due to a non‐normal distribution.
SupplementaryTable S2. Details of eGFR slope analysis.
Supplementary Table S3. Details of percent change in UACR analysis.
Supplementary Table S4. Details of absolute change in UACR.


