Abstract
Background
Coronavirus disease 2019 (COVID‐19) convalescent plasma (CCP) collection began in two Brazilian hospitals for treatment of severe/critical patients.
Methods and Materials
Mild/moderate COVID‐19 convalescents were selected as CCP donors after reverse transcription polymerase chain reaction (RT‐PCR) confirmed severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and absence of symptoms for ≥14 days plus (a) age (18‐60 years), body weight greater than 55 kg; (b) immunohematological studies; (c) no infectious markers of hepatitis B virus, hepatitis C virus, human immunodeficiency virus, human T‐lymphotropic virus‐1/2, Chagas and syphilis infection; (d) no HLA antibodies (multiparous); (e) second RT‐PCR (nasopharyngeal swab and/or blood) negativity; (f) virus neutralization test (cytopathic effect–based virus neutralization test neutralizing antibody) and anti–nucleocapsid protein SARS‐CoV‐2 IgM, IgG, and IgA enzyme‐linked immunosorbent assays.
Results
Among 271 donors (41 females, 230 males), 250 presented with neutralizing antibodies. Final RT‐PCR was negative on swab (77.0%) or blood (88.4%; P = .46). Final definition of RT‐PCR was only defined at more than 28 days after full recovery in 59 of 174 (33.9%) RT‐PCR –ve, and 25/69 RT‐PCR +ve (36.2%; 13 between 35 and 48 days). Neutralizing antibody titers of 160 or greater were found in 63.6%. Correlation between IgG signal/cutoff of 5.0 or greater and neutralizing antibody of 160 or greater was 82.4%. Combination of final RT‐PCR –ve with neutralizing antibody ≥160 was 41.3% (112/271). Serial plasma collection showed decline in neutralizing antibody titers and IgA levels (P < .05), probably denoting a “golden period” for CCP collection (≤28 days after joining the program); IgA might have an important role as neutralizing antibody. Donor's weight, days between disease onset and serial plasma collection, and IgG and IgM levels are important predictors for neutralizing antibody titer.
Conclusions
RT‐PCR +ve cases are still detected in 36.2% within 28 to 48 days after recovery. High anti–nucleocapsid protein IgG levels may be used as a surrogate marker to neutralizing antibody.
Keywords: convalescent plasma therapy, COVID‐19, passive immune therapy, SARS‐COV‐2; coronavirus
Abbreviations
- CCP
coronavirus disease 2019 convalescent plasma
- COVID‐19
coronavirus disease 2019
- CPE
cytopathic effect
- ELISA
enzyme‐linked immunosorbent assay
- ln
natural logarithm
- NF
nasopharyngeal
- NP
nucleocapsid protein
- PB
peripheral blood
- PBST
phosphate‐buffered saline with Tween
- RT‐PCR
reverse transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- S/CO
signal/cutoff
- VNT
virus neutralization test
- WB
whole blood
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is an enveloped nonsegmented positive‐sense RNA β‐coronavirus, from the Coronaviridae family, 1 first diagnosed in patients with atypical pneumonia of unknown origin in Wuhan, China, in December 2019, 2 causing a new disease, named coronavirus disease 2019 (COVID‐19), with high infectious potential. 3 Most patients are asymptomatic or develop mild symptoms.2. Others, recognized as high‐risk groups (elderly, diabetes, hypertension, cardiopathy, pulmonary diseases, cancer, or body mass index [BMI] >30) might develop severe manifestations with multiple organ failure and high mortality. 4 From China, it spread globally, affecting more than 16.1 million people and causing more than 647 000 deaths (July 26, 2020). Brazil had the second largest number of cases in the world (2 394 000), with 86 400 deaths (https://coronavirus.jhu.edu/map.html).
No specific single therapeutic measure has been proven efficient for COVID‐19 treatment. Clinical trials study a number of specific approaches. 5 Passive immunotherapy through transfusion of coronavirus disease 2019 convalescent plasma (CCP) has been investigated by several protocols. 6 Convalescent plasma has been used historically to treat epidemic diseases such as influenza in 1918, 7 and more recently for severe acute respiratory syndrome), 8 , 9 Ebola, 10 , 11 Middle East respiratory syndrome (MERS), 12 and other diseases. 13 , 14 , 15 , 16 , 17 Passive immunotherapy studies have shown in vivo a higher viral clearance and changes in patients' immune response, particularly in early stages. 18
Use of CCP for treating COVID‐19 was proposed since the early Chinese cases. 19 , 20 , 21 , 22 Rationale for using CCP relies on the assumption that neutralizing antibodies produced by convalescents might suppress severe viremia in a patient. 6 , 23 , 24 Because of limited data available to document the efficacy of CCP therapy, clinical trials 25 , 26 , 27 , 28 , 29 , 30 have targeted severe cases, with the premise of rapid immunity transfer to patients, bridging the time between infection and seroconversion (before own immune response development). Suppression of the initial virus load by CCP might be followed by modification in the recipient's immune response, particularly at early stages, avoiding severe viral effects in several organs.
The safety and efficacy of CCP collected from patients who have recovered from COVID‐19 to mitigate the development of symptoms after infection should be studied under controlled trials. 31 , 32 It is important to characterize the CCP product and convalescent donors after resolution of infection, 33 since it is a challenge to identify who are the best neutralizing antibody producers and the kinetics of neutralizing antibody.
This study describes the data obtained from CCP donors from two Brazilian hospitals, evaluating their humoral immune response, together with kinetics of anti‐nucleocapsid protein (NP), IgM, IgG, IgA, and neutralizing antibody.
2. MATERIAL AND METHODS
2.1. Institutional review board approval
The study was approved by both hospitals institutional review boards and the Brazilian Commission on Ethics and Research under request CAAE: 30259220.4.2001.5461 (approval number 3.977.618).
2.2. CCP donor recruitment
Convalescent donors with mild symptoms were recruited by media and pamphlets or referenced by hospital doctors, under a standard procedure for donor preselection: age 18 to 60 years, body weight greater than 55 kg, previous positive SARS‐CoV‐2 nasopharyngeal swab reverse transcription polymerase chain reaction (RT‐PCR), and full clinical recovery for 14 days or more, based on national guidelines. 34
2.3. Selection criteria
Donors fulfilling these requirements underwent a medical interview, with routine donor medical examination, and explained about the study's objectives and how to donate plasma via whole blood (WB) or plasmapheresis. Data captured inquiries about symptoms, onset of disease, duration and type of symptoms (fever, dry cough, myalgia, runny nose, dyspnea, diarrhea, anosmia, and headache). Furthermore, we evaluated the interval between the onset of full recovery (absence of symptoms referred by donors) and the first medical interview; the interval spanning up to two consecutive plasma collections and full recovery; interval between the first nasopharyngeal swab (RT‐PCR1) and medical interview, and intervals between the successive RT‐PCR tests (see Figure 1).
FIGURE 1.
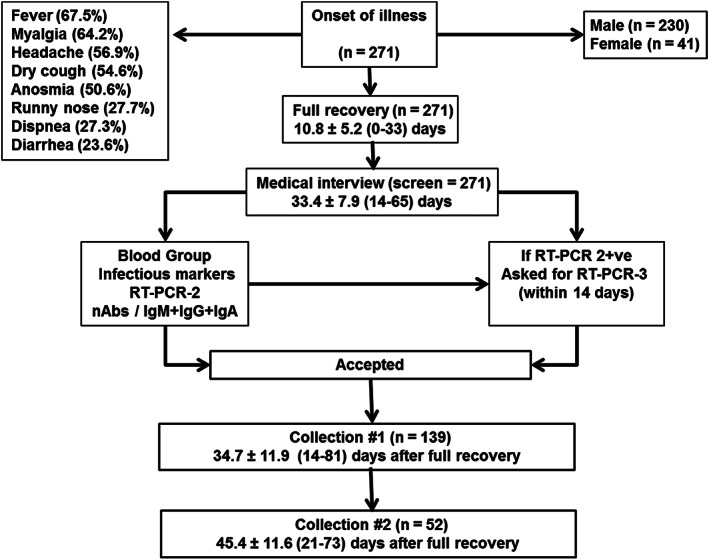
CCP program stages. All donors with a previous RT‐PCR1 positive had their first medical interview on the average 33.4 ± 7.9 (range, 14‐65) days after onset of symptoms, which lasted 10.8 ± 5.2 (range, 0‐33) days. Main symptoms are shown in upper left square.). Only one donor was asymptomatic (RT‐PCR1 +ve), due to close contact with a confirmed patient. If RT‐PCR2 was positive, donors were asked to collect another RT‐PCR within 14 days. If RT‐PCR3 negative, they were accepted in the program; otherwise, they were discarded. Accepted donors with multiple collection (apheresis) are displayed, based on the mean ± SD (range) of days after full recovery
2.4. CCP screening
Donors approved by the medical examination had also to sign an informed consent form and undergo a series of tests:
Routine blood donor screening tests. Donors were tested for: ABO and D, irregular antibodies to red blood cell antigens (immunohematologic tests), and infectious diseases according to the Brazilian legislation. 35 Only nonreactive donors were accepted. Multiparous female donors were tested for the presence of anti‐HLA antibodies (Lifecodes LifeScreen Deluxe, Immucor, Waukesha WI).
SARS‐CoV‐2 RT‐PCR. All donors had a previously positive RT‐PCR (swab), undergoing a second test (RT‐PCR2), either by swab or peripheral blood (PB), according to Corman et al 36 (targeting the E and RdRp genes), with sensitivity of five copies/reaction. A cycle threshold value less than 40 was interpreted as positive. Donors with nonreactive RT‐PCR2 (swab or PB) were accepted; those who remained reactive by the swab RT‐PCR2 were asked to perform a third test after 14 days (RT‐PCR3). If the RT‐PCR3 test was nonreactive, the donor was accepted; otherwise, the donor was rejected.
Neutralizing antibodies. A cytopathic effect (CPE)‐based virus neutralization test (VNT) was carried out with SARS‐CoV‐2 (GenBank: MT MT350282) in 96‐well plates containing 5 × 104 cells/mL of Vero cells (ATCC CCL‐81). 37 Serum samples were initially inactivated for 30 minutes at 56°C. We used eight dilutions (1:20 to 1:10240). Subsequently, sera were mixed vol/vol with the virus (100 tissue culture infectious doses, 50% endpoint per well) and preincubated at 37°C for 1 hour for neutralization. The serum plus virus mixture was transferred onto the confluent cell monolayer and incubated for 3 days at 37°C (5% CO2). After 72 hours, plates were analyzed with light microscopy. Gross CPE was observed on Vero cells, distinguishing the presence/absence of CPE‐VNT. Neutralizing antibody titer is described as the highest serum dilution neutralizing virus growth. For double check, plates were fixed and stained with amido black (0.1% amido black solution [w/w] with 5.4% acetic acid, 0.7% sodium acetate) for 30 minutes. A strong internal positive control serum (RT‐qPCR positive + plaque reduction neutralization test >640) was used in each run. The method was adapted from Nurtop et al, 38 and used for SARS‐CoV studies. 39 , 40 , 41 , 42 , 43 , 44 All CPE‐VNT procedures were performed in a Biosafety Level 3 laboratory, following World Health Organization recommendations. 45 Neutralizing antibody titers were transformed in natural logarithm (ln) for normal distribution.
Immunoglobulins (IgA, IgM, and IgG) nucleocapsid protein–based SARS‐CoV‐2 enzyme‐linked immunosorbent assays. The method was adapted from Oliveira et al 46 Ninety‐six‐well high‐binding polystyrene plates (Corning, New York) were coated with the new coronavirurs protein spike antigen 7 (nCoV‐PS‐Ag7) nucleoprotein antigen (Fapon Biotech Inc., Dongguan, China), at 0.2 μg/mL in sodium carbonate‐sodium bicarbonate buffer and incubated at 37°C for 1 hour. Unspecific binding of the antibodies was avoided by blocking with bovine serum (BS) (Advagen Biotech ltda, Itu, Brazil) at 37°C. After 3× washing with phosphate‐buffered saline with Tween (PBST), 100 μL of appropriately diluted (1:50 for IgA and IgM; 1:100 for IgG) serum sample in PBST was added and incubated for 1 hour at 37°C. After washing 3× with PBST, bound antibodies were detected with the secondary antibodies conjugated with horseradish peroxidase of goat anti‐human IgA [1:3000], IgM [1:3000] and IgG [1:4000] (Sigma‐Aldrich Co., Deisenhofen, Germany). After incubation for 1 hour at 37°C and three PBST washes, 100 μL of 3,3′,5,5′‐ tetramethylbenzidine (Sigma‐Aldrich Co., Deisenhofen, Germany) was added to each well and the mixture was incubated for 10 minutes at room temperature. The reaction was stopped by adding 0.2 N sulfuric acid to the mixture, and the optical density at 450 nm was measured (BMG Labtech, Ortenberg, Germany), and transformed into signal/cutoff (S/CO) ratio. Details on the reproducibility of neutralizing antibody and ELISA assays are discussed in the supplement.
Plasma collection. CCP was collected via WB (150 mL plasma/ collection) or plasmapheresis (600‐mL plasma collection; Trima Accel version 6, Terumo BCT, Lakewood, CO ‐). Apheresis donation was allowed up to four times in a 1‐month period, with 42% of apheresis collection pathogen‐inactivated using amotosalen/ultraviolet A illumination (INTERCEPT, Cerus Corporation, Concord, CA).
Statistical analysis. The distribution patterns from variables were checked by the Kolmogorov‐Smirnov and Shapiro‐Wilk tests. We used the t test, Fisher exact test, or analysis of variance (parametric data), and the two‐tailed Mann‐Whitney U test, two‐tailed Spearman's correlation, Wilcoxon matched‐pairs signed‐rank or Kruskall‐Wallis test (nonparametric variables). Bonferroni adjustment was employed whenever possible. Since there were signs of dependence between neutralizing antibodies and several variables, and testing for neutralizing antibodies is not available for most transfusion services, 33 we developed by stepwise multiple regression a surrogate model to replace the use of neutralizing antibodies as a key requirement for CCP procurement. All statistical analyses used computer software (STATA version 15, (StataCorp, College Station, Texas), with 5% significance level accepted.
3. RESULTS
3.1. Donor demographics
There was a total of 271 COVID‐19 convalescent volunteers—41 women (15.1%) and 230 men (84.9%), as shown in Figure 1 and Tables S1 and S2. The mean duration of symptoms was 10.8 ± 5.2 (range, 0‐33) days until complete recovery (absence of any symptom).
There were 41 female donors, 32 nulliparous and 9 multiparous, who were screened for HLA antibodies; none were reactive.
3.2. Specific SARS‐CoV‐2 tests
3.2.1. RT‐PCR
From the initial 271 potential donors, 11 refused to undergo an additional RT‐PCR and were being discarded. From the available 260 donors, a second RT‐PCR was done via PB only (n = 14), both swab and PB (n = 107), or swab only (n = 139). Figure 2 depicts the pattern of all RT‐PCR results (swab or PB). There was also a lower sensitivity and agreement for PB RT‐PCR vs swab, with only 66 of 107 (61.7%; 95% confidence interval [CI], 51.8%‐70.9%) donors with complete agreement between both tests (kappa index = 0.077; P = .46). For PB and swab, the cycle threshold ( (mean ± SD) was, respectively, 37 ± 1.3 × 36 ± 1.8 (P = .34, Wilcoxon test).
FIGURE 2.
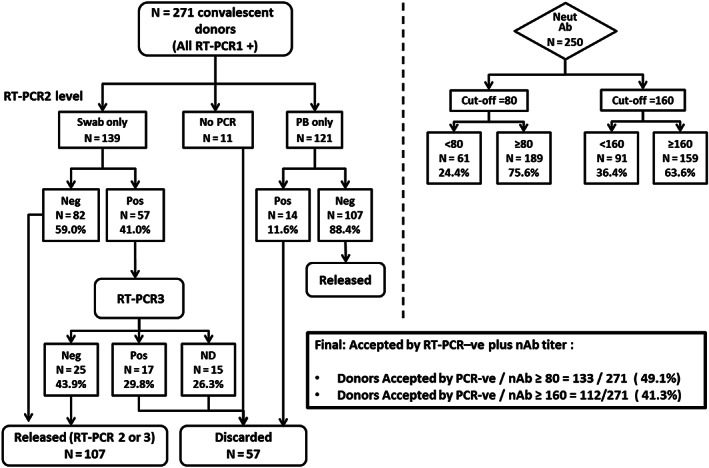
Distribution of 271 convalescent donors, based on nasopharyngeal swab (NF swab, n = 139) or peripheral blood RT‐PCR (PB, n = 121), and neutralizing antibody tests (n = 250). Eleven (11) donors did not collect RT‐PCR2. There were 107 donors who were tested by both PB and swab RT‐PCR2, whose cycle threshold (mean ± SD) was, respectively, 37 ± 1.3 × 36 ± 1.8 (P = .34, Wilcoxon test). The final percentage of accepted donors according to the combination of RT‐PCR2 or 3 and neutralizing antibody titer of 80 or higher or 160 or higher is 49.1% (95% CI, 43.0%‐55.2%) or 41.3% (95% CI, 35.4%‐47.4%), respectively (middle box)
For donors who had a full RT‐PCR and days after full recovery assessment (n = 243), there were 174 RT‐PCR –ve (71.6%; 95% CI, 65.5%‐77.2%) and 69 RT‐PCR +ve (28.5%; 95% CI, 22.8%‐34.5%). The mean time (SD and range) for achieving a negative RT‐PCR by either nylon‐flocked (NF) swab (n = 136), PB RT‐PCR2 (n = 107), or both is shown in Figure 3. There were 59 of 174 (33.9%; 95% CI, 26.9%‐41.5%) and 25 of 69 (36.2%; 95% CI, 25.0%‐48.7%) donors whose final PCR status were negative or positive, respectively, who had their final RT‐PCR definition 28 days or more after recovery. From the latter group, 52% remained RT‐PCR +ve between 35‐48 days (13/25; 95% CI, 31.3%‐72.2%). We have broken the long persistent RT‐PCR +ve donors total data according to NF swab (n = 32) or PB RT‐PCR (n = 37), showing a striking difference between both methods, where 62.5% (20/32) vs 13.5% (5/37) donors tested by NF swab or PB only, respectively, remained reactive after more than 28 days (P < .01; Fisher exact test).
FIGURE 3.
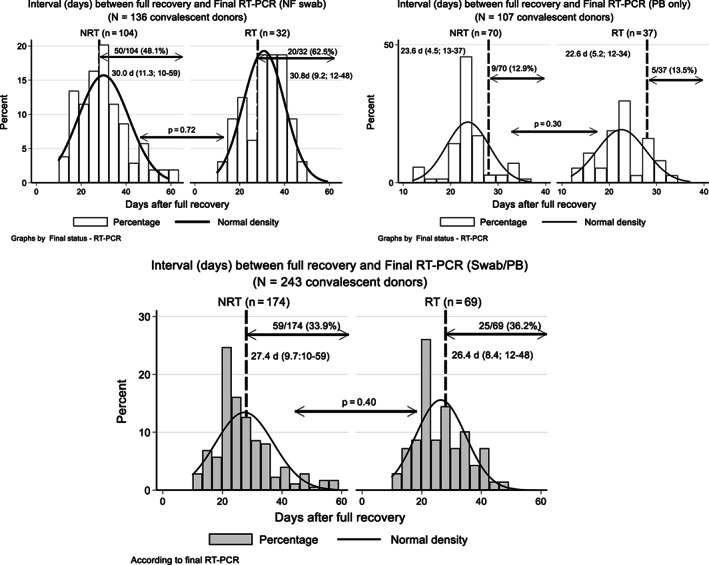
Length of days after full recovery (absence of any symptoms referred by the donors) from 243 CCP donors who had a full definition of final RT‐PCR status (RT‐PCR2 or 3), given as mean (±SD; range). Long dashed vertical line marks the 28‐day period after full recovery, where several regulatory agencies consider safe to donate blood once COVID‐19 symptoms have vanished. 35 , 47 , 48 Upper left and right figures show donors tested only by nasopharyngeal swab (n = 136) or peripheral blood (n = 107) RT‐PCR, respectively. The combination of both methods is shown in the lower figure, with a total of 33.9% (n = 59/174) and 36.2% (25/69) donors, respectively, for RT‐PCR –ve and RT‐PCR +ve, who had their final PCR status defined after the 28‐day period after full recovery
3.2.2. Neutralizing antibodies
We were able to test neutralizing antibodies in 250 donors. The combination of the final RT‐PCR –ve results with neutralizing antibodies of 80 or greater or 160 or greater was 133 (49.1%; 95% CI, 43.0%‐55.2%) or 112 (41.3%; 95% CI, 35.4%‐47.4%), respectively (Figures 2, 4 and S1).
FIGURE 4.
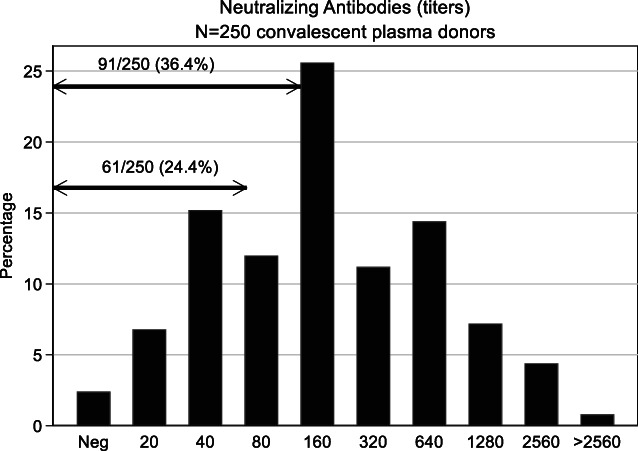
Neutralizing antibody titers from 250 donors (male = 218; female = 32). Titers <20 denote a negative reaction (absence of nAb). Lower and upper arrow indicates number (percentage) of donors with titers <80 and <160, respectively, which were not accepted as CCP donors, based on the chosen cutoff
3.2.3. Neutralizing antibody titer and RT‐PCR
Given that a positive RT‐PCR is a consequence of viral RNA in the CCP donor (true infectious state still uncertain), we evaluated the neutralizing antibody titer with the RT‐PCR pattern in 226 donors being 172 RT‐PCR –ve and 54 RT‐PCR +ve (ln titer = 5.2 ± 1.4; 95% CI, 5.0‐5.4 × 5.1 ± 1.3; 95% CI, 4.8‐5.5), with no statistical difference (P = .39), including for sex (male = 5.2 ± 1.4; 95% CI, 5.0‐5.4 × female 4.9 ± 1.3; 95% CI, 4.4‐5.3; P = .26).
3.2.4. Anti‐NP (IgM, IgG, and IgA) and RT‐PCR
There was no difference on IgM, IgG, or IgA S/CO based on sex for 250 CCP donors (IgM, P = .14; IgG, P = .43; IgA, P = .56, Mann‐Whitney). IgM, IgG, and IgA had a statistically significant correlation with donor's weight, and among each other (all P < .01; Table S3 and Figure S2). Only IgM correlated moderately with the interval between onset of symptoms and the medical interview, but it dropped very fast; IgM was detected in only 4.4% of CCP donors more than 40 days after the onset of disease. There were 226 CCP donors whose final PCR status could be jointly studied with IgM, IgG, and IgA. Figure S3 shows no statistical difference observed for each class between the final RT‐PCR status.
3.2.5. Immunoglobulin levels (S/CO) and neutralizing antibody titers
There was a moderate correlation between the neutralizing antibody titers and the S/CO levels for all three anti‐NP classes (all P < .01). Figure 5 shows the linear correlation for anti‐NP IgM, IgG, and IgA according to neutralizing antibody titers in 226 donors. We also evaluated what would be the agreement for IgG S/CO 5 or greater and neutralizing antibody titers of 160 or greater (n = 159 donors). Using this proposed cutoff; a total of 131 of 159 donors (82.4%; 95% CI, 75.6%‐88.0%) are classified as bearing simultaneously high IgG S/CO (≥5) and high neutralizing antibody titer (≥160), being a possible surrogate alternative for places where no neutralizing antibody tests are available.
FIGURE 5.
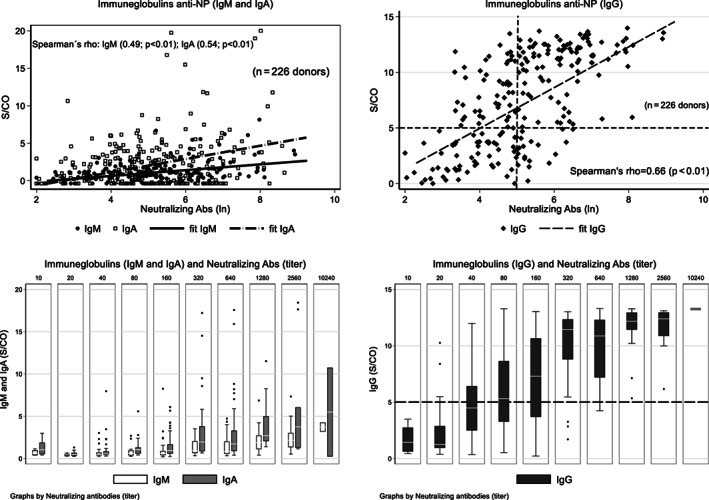
Immunoglobulin anti‐NP (IgM, and IgA, left; IgG right) S/CO according to ln nAb titers from 226 convalescent plasma with final RT‐PCR status. The horizontal and vertical long‐dashed lines on the right upper quadrant (IgG) denotes a proposed limit for both high IgG S/CO level (≥5.0; above the horizontal dashed line) and neutralizing antibody titer ≥160 (right of vertical dash line). Agreement between IgG S/CO ≥5.0 and neutralizing antibody ≥160 was 82.4%. There is a strong correlation between immunoglobulin levels (S/CO) and neutralizing antibody titers (ln), as measured by the Spearman's rho correlation coefficient (0.49, 0.66, and 0.54 for IgM, IgG, and IgA, respectively; all P < .01)
3.2.6. Full recovery and neutralizing antibody titers
We found no statistical correlation using both the interval between the onset of disease or period after full recovery with neutralizing antibody titers from 250 donors (P = .60), even if separated by the final RT‐PCR +ve (n = 55; P = .28) or RT‐PCR –ve (n = 171; P = .46).
3.2.7. Correlation between neutralizing antibody and immunoglobulins after multiple collections
We studied the neutralizing antibody titer or IgM, IgG, and IgM S/CO in 52 donors undergoing serial plasmapheresis and compared their levels with the initial screening sample. We were concerned about a potential depletion due to a serial short time collection or by natural drop after interruption of immunological stimulation (or both). The median interval between the medical interview (screen) and the time of the first and second plasmapheresis was 15 days (range, 1‐48d) and 8 days (range, 4‐25d), respectively. Data are shown in Figure 6, with a statistically significant decline for neutralizing antibody titer and IgA, with no changes for IgM and IgG (not shown). Collections from 25 donors were canceled due to declining neutralizing antibodies.
FIGURE 6.
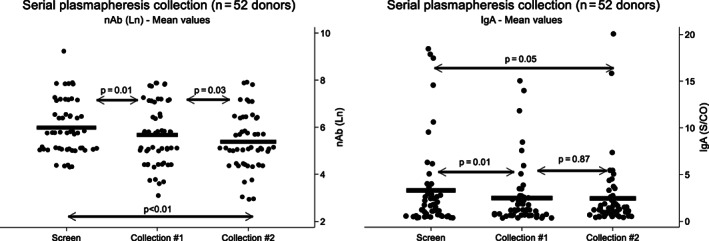
Decline in neutralizing antibody titers (ln) and IgA (S/CO) from 52 CCP donors who donated at least twice by plasmapheresis. Horizontal line shows the mean for each marker. There is a median interval of 14 days between collection of screen sample and Collection 1, and additional 8 days (median) between Collections 1 and 2. Left: neutralizing antibody titers (ln); right: IgA (S/CO). There is a statistically significant decline for nAb for all periods (screen, Collections 1 and 2; all P < .05). For IgA, statistical difference was seen for screen sample × Collection 1 (P = .01) and screen sample × Collection 2 (P = .05). There was no statistically significant decline for IgM and IgG. All results obtained by Wilcoxon test
3.2.8. A model for studied variables and neutralizing antibody titers
The proposed model, derived from 131 donors, defined weight, IgG, and IgM (S/CO) and the interval (days) between the onset of illness and plasma collection as the most important covariables, with a fair adjusted R2 = 0.5017 (P < .01). We have also evaluated all covariables by the added variable plot, which shows the relationship between neutralizing antibody (y‐axis) and each independent variable (x‐axis), adjusting for the effects of other remaining independent variables, 47 as shown in Figure 7. Both the model and the basis for added variable plot are further discussed in the supplement.
FIGURE 7.
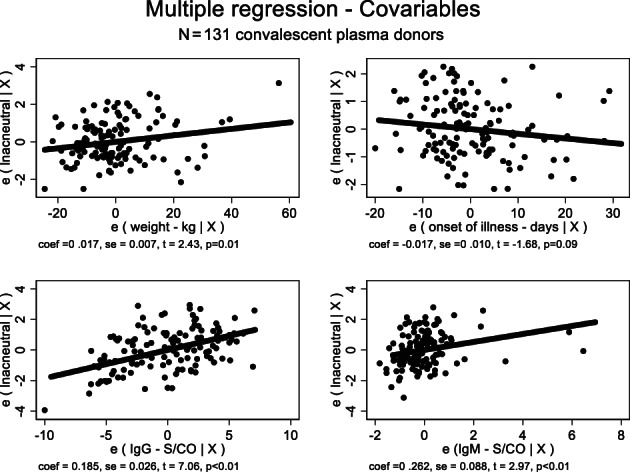
Added variable plot derived from a stepwise multiple regression between neutralizing antibody titers (ln) and the key variables found to exert main dependence on neutralizing antibody: Weight (kg); time since onset of illness (days); IgG and IgM (S/CO). The y‐axis ‐ e (lnacneutral | X) ‐ shows the relationship between neutralizing antibody and each independent variable (x‐axis), adjusting for the effects of other remaining independent variables. 47 There is a mild, negative effect just for the onset of illness in days (P = .09). All the remaining independent variables are statistically significant. The model follows the equation: Y = 2.585 + 0.017b ‐ 0.017c + 0.185d + 0.262e, where Y = neutralizing antibody titer (ln); b = weight (kg); c = onset of illness (days); d = IgG S/CO; e = IgM S/CO
3.2.9. CCP units
From 15 April to 31 May 2020, there were a total of 354 CCP donations, rendering 1081 units of plasma (154 WB and 927 apheresis). There were 60 collected units (5.3%) that were segregated from the inventory, given that the neutralizing antibody titer from the collected bag was less than 80 and therefore considered inadequate by the protocol.
3.2.10. Adverse effects on donors
From 354 plasma donations up to 31 May, there was a loss of venipuncture in 6 cases (1.7%), paresthesia in 3 (0.9%) and vasovagal reaction in 4 (1.1%). One donor had phlebitis 5 days after his third collection. He was properly treated and left the program.
3.2.11. Infectious markers
There were only two donors (0.74%; 95% CI, 0.09%‐2.64%) rejected due to infectious markers: anti–hepatitis B core antigen and syphilis.
4. DISCUSSION
CCP seems to be the simplest immunologic therapy for the COVID‐19 pandemic. 48 Countries with relatively well organized transfusion services are able to implement a CCP program, following basic recommendations. Neutralizing antibodies have the ability to bind and interfere in the viral replication independently of the host immune cellular defense; in addition, neutralizing antibodies inhibit viral growth in laboratory culture, whereas binding antibodies may not prevent viral cell infection or block viral growth in culture. Our strategy of screening high‐titer neutralizing antibody CCP donors might contribute to the capacity of blocking viral infection 49 , 50 , 51 through (a) preventing virus binding to angiotensin‐converting enzyme 2 via receptor‐binding domain within the S1 unit spike (S) protein, whose affinity is 10‐ to 20‐fold higher than SARS‐CoV 52 ; (b) aggregation of virus particles; and (c) lysis through activation of the complement system. 53 Recognition by CD8+ T cells of viral infected and apoptotic cells leads to phagocytosis, contributing to less lung damage due to the cytokine storm and faster patient recovery. 54 SARS‐CoV‐2 neutralizing antibodies are found 2 to 3 weeks after infection, being unable to cross‐react with SARS‐CoV, 55 suggesting different epitope(s) from S protein. It is not clear what would be the minimum protective neutralizing antibody titer, though most centers use a range between 80 and 160. 33 , 56 Initially, we used a cutoff of 80 or greater, upgrading to 160 or greater due to titer fluctuations in serial collections.
Our cohort has an average of 33.4 ± 7.9, 22.7 ± 7.6, and 34.1 ± 10.9 days between onset of symptoms and first medical interview, full recovery and medical interview, and full recovery and first plasma collection, respectively. It shows differences from Robbiani's cohort, 57 with patients not enrolled for a CCP transfusional program, a longer period after onset of disease, older age, and close contact with people diagnosed with SARS‐CoV‐2 infection.
We raise a concern about the length of days that donors remained RT‐PCR +ve, due to the current recommendation to donate after 28 days or more after full recovery of symptoms. 32 , 33 , 34 , 57 , 58 To et al 59 detected viral RNA after more than 20 days in one third of patients, but that may not associate with persistent infectivity after seroconversion. 60 , 61 We observed few RT‐PCR +ve patients with virus isolation more than 28 days after recovery (Durigon EL, manuscript in preparation). Although rare, there is already a case reporting a longer viral shedding. 62 It is known that the amount of viral RNA is highly reduced over time (>14 days), and most likely asymptomatic CCP donors with prolonged RT‐PCR +ve and high neutralizing antibody titers might actually have noninfectious coated‐antibody virions. 63 , 64 , 65 However, we consider appropriate to be cautious at this moment and avoid definitive assumptions until stronger evidence is published, either supporting or refuting this point. Since approximately 35% of our donor cohort remains RT‐PCR +ve for longer period (>28 days, with one positive case until 48 days), perhaps regulatory authorities could review the consequences of extending the current 28‐day period to a 56‐day period as a temporary WB donor rejection policy after SARS‐CoV‐2 infection, if donors are donating for reasons other than CCP donation (balancing temporary shortages in highly affected regions). It might also be appropriate to review or implement pathogen reduction methods in convalescent donors whose units are intended for general use other than passive immunotherapy.
Specific IgG antibodies are found earlier in the course of the infection, with progressive increase and permanent levels after infection has been cleared. 66 It is also possible that detected neutralizing antibodies are targeted against both S1 and S2 units, 55 similarly to SARS CoV‐1, 67 suggesting possible alternative mechanisms for viral clearance. In addition, there is no proof that in vitro tests correlate to in vivo clinical response for SARS‐CoV‐2. 68
We have found 9.2% of donors with titers of 20 or less (n = 23); Robiani et al 57 found 18% of donors with no neutralizing antibodies. SARS shows neutralizing antibody persistence for at least 2 years before decline 49 , 69 ; however, neutralizing antibody anti‐SARS‐CoV‐2 might not develop in 5% of patients, and only 70% had titers greater than 500, with higher titers in elderly/middle‐aged patients. 55 Our cohort has mainly young adults (36.2 ± 8.5; range, 19‐60 years), with 36.4% having neutralizing antibody titers less than 160. Despite the fact that we saw no decline in neutralizing antibody titer based on days after the onset of disease, we were concerned how titers dropped quickly after serial plasmapheresis, not observed for severe acute respiratory syndrome. 26 A longitudinal study is under way to better understand the neutralizing antibody kinetics.
CD4+ T cells play a major role in the elaboration of protective response, where several class II CD4+ T epitopes are under study as vaccine candidates. Grifoni et al 51 demonstrated that spike‐specific CD4+ T responses correlated well with the level of anti‐spike receptor‐binding domain IgG and IgA titers. Since IgA may be particularly important in mucosal viral infections, 70 , 71 , 72 our findings between neutralizing antibodies decline with decreasing levels of IgA antibodies might indicate some connection, 73 possibly a key neutralizing role of IgA class, but pending further studies. There is likely a “golden period” of approximately 21 to 28 days for plasma collection once it has started, as there is a declining trend for neutralizing antibody titers with time. It is also possible that protective antibodies other than neutralizing antibodies are directed not only against the spike region, once nearly one‐third of reported CD4+ T‐cell reactivity for SARS‐CoV‐2 and other coronaviruses is accounted for nonspike regions (M, nsp6, ORF3a, and N). 51 CCP therapy might provide to recipients additional immunoglobulins or other molecules not well defined yet by the current screening protocols. We do not know whether the CD4+ T‐cell and antibody responses in severe and critical acute cases (the intention to treat patients) are the same as in patients with mild or moderate symptoms. Comparing the immunologic responses in different subsets of patients might probably reveal important features about the multiple pattern of COVID‐19.
Our initial goal was to accept a neutralizing antibody titer of 80 or greater (75.6% of donors; 95% CI, 69.8%‐80.80%); however, this level was unstable in subsequent donations, so we changed the cutoff to 160 or greater (63.6% of donors; 95% CI, 57.3%‐69.5%). Neutralizing antibodies are difficult to perform, requiring a Biosafety Level 3, not regularly available; suitable alternatives must be proposed on a global scale. We established a good correlation between high IgG S/CO and high neutralizing antibody titers. The proposed model seems to be important particularly for low‐ and middle‐income countries. 33 This was an observational study, with unknown previous variable influence on the model. However, an R2 = 0.5017 is not a disincentive for surrogate models, showing a moderate/strong correlation between neutralizing antibody titers with the proposed variables. To et al 59 also found a strong correlation between neutralizing antibodies and IgG levels.
An interesting finding was the relation between weight and neutralizing antibody titers. High body mass index (≥30) has been associated with worse prognosis, 74 mainly if less than 60 years of age. 75 Overweight donors might have a higher capacity to produce neutralizing antibodies; however, the relatively younger age of our CCP donors might have a protective clinical effect over the severity in some donors.
Despite the fact that all CCP donors were first‐time donors, the rejection rate was not different from our normal 2.01% rejection rate (P = .13) for regular donors. However, the small number of this cohort underpowers any comparison with our volunteer WB donation rate.
Though one hospital in this consortium adopted pathogen reduction in all collected plasma, we acknowledge that most centers involved in CCP collection have not implemented pathogen reduction yet. 58 There are evidences that SARS‐CoV‐2 might not be transfused by blood components. 76 However, pathogen reduction should not be neglected in case it is already available, particularly due to its capacity of coronavirus inactivation. 77 , 78 The possibility of having hundreds or thousands of donors participating in CCP programs (intended for older, immunosuppressed patients or patients with different comorbidities), harboring detectable RNA for many days after recovery (especially after the standard 28‐day temporary deferral period), some with no molecular tests as screening procedures and no PR methods applicable, should be regarded with caution.
This project was devised in the beginning of the COVID‐19 pandemic in Brazil, without knowledge of which resources should be applicable for the expected growing number of cases and previous understanding about the neutralizing antibody pattern in CCP donors, complicated by rejections due to a long, persistent RT‐PCR +ve, leading us to compare the RT‐PCR2 by swab and PB, confirming the lower sensitivity of PB RT‐PCR against nasopharyngeal swab. A rate of 11.6% PB RT‐PCR +ve was observed, which is different from other studies, 2 , 79 , 80 perhaps because of different methodologies, pooling or earlier collection in acute patients.
We recognize some weaknesses, such as missing data in some CCP donors and our small number (n = 271). Nevertheless, this is also a strength for Brazil, which faces several limitations and is in the midst of a major outbreak (probably with greater consequences than Zika or dengue).
We understand that this sort of therapy might not be available to all patients in vast countries like Brazil, unless units are collected, frozen, and sent regularly to requesting places, under a centralized distribution system. On the other hand, we consider that the experience gained from this first pandemic wave would leave us more confident for the future, whenever additional waves reappear.
A main strength of this paper is that quality over quantity matters. 33 Having a CCP program based on CCP donations with no adequate specific testing procedures might not be a good strategy, since no more than 50% of CCP donors are acceptable based on our protocol. Not all CCP donors will be high neutralizing antibody producers, though some might be excellent ones. 57 , 81 In case neutralizing antibody is definitely considered as the key principle for passive therapy, then random CCP with no stringent collection protocols might jeopardize the clinical outcome, which shall be evaluated by controlled clinical studies. 82
CONFLICT OF INTEREST
C.P.S. is funded by Grant 2018/23680‐0 (Fundação de. Amparo à Pesquisa do Estado de São Paulo); D.B.A. by Grant 88 887.131387/2016‐00 (Coordenação de Aperfeiçoamento de Pessoal de. Nível Superior ‐ CAPES), R.R.G.M. by Grant 2017/24769‐2 (Fundação de. Amparo à Pesquisa do Estado de São Paulo) and E.L.D. by Grants 2016/20045‐7 and 2020/06409‐1 (Fundação de Amparo à Pesquisa do Estado de São Paulo). All other authors have no conflict of interest.
AUTHOR CONTRIBUTION
Conceptualization: S.W., J.M.K., N.H., L.V.R., and L.F.R.; investigation: S.W., J.M.K., R.R.G.M., R.F.W., C.B.B., R.M.F., A.P.Y., G.C., A.S., R.A., P.S., D.B.A., C.P.S., E.L.D., M.D.B.C., and E.K.; formal analysis: S.W. and J.M.K.; resources: L.F.L.R. and L.V.R.; writing: S.W., J.M.K., R.F.W., and R.M.F.; project administration: N.H. and A.A.C.; funding acquisition, L.F.R. and L.V.R. This project was partially supported by the initiative “Todos Pela Saúde”.
Supporting information
Table S1 Demographics and interval between onset of symptoms, full recovery, medical interview (days), and serial RT‐PCR tests from 271 convalescent plasma donors. *Female donors donated only once in the program. Males were heavier than females (85.5 ± 14.0 × 69.6 ± 12.0 kg; P < .01), but also had a shorter time of symptoms (10.5 ± 5.3 × 12.0 ± 4.4 days; P = .01). RT‐PCR1, = first RT‐PCR test (diagnosis during symptoms present); RT‐PCR2, second RT‐PCR test, performed right after the medical interview (swab or peripheral blood); RT‐PCR3, third RT‐PCR (swab only), performed on donors whose RT‐PCR2 was positive.
Table S2 ‐ Clinical symptoms in 271 convalescent donors. Except for myalgia in males, all clinical symptoms were nonstatistically different by sex
Table S3 – Spearman's correlation between neutralizing antibodies (ln), weight (kg), IgM, IgG, and IgA. All showed statistically significant results at 0.01 level (Bonferroni adjusted)
Figure S1 ‐ Sex distribution of neutralizing antibodies (ln), female (n = 32), left; male (n = 218, middle). There was no difference in titers according to sex (P = . 47). Vertical long‐dashed line represents the cutoff titer ≥160 (63.6% of all donors).
Figure S2 ‐ Correlation between IgM, IgG, and IgA with onset of symptoms (A, C, E, left) and weight (B, D, F, right). Only IgM correlated moderately with onset of symptoms. Weight correlated with all SARS‐CoV‐2 antibody classes (all P < .01). IgM was still detected in 4.4% of CCP donors >40 days after onset of symptoms (lower quadrant).
Figure S3 ‐ Immunoglobulin anti‐NP (IgM, IgG, and IgA) S/CO from 226 donors, according to the CCP donor final RT‐PCR status. No statistical difference (Mann‐Whitney) was seen between both groups (PCR‐RT –ve and RT‐PCR +ve) for each immunoglobulin class. (RT‐PCR –ve × RT‐PCR +ve; P = .51; .92; .74, for IgM, IgG, and IgA, respectively.)
ACKNOWLEDGMENTS
We thank Marion Lanteri for reading the initial drafts of this paper and providing important inputs and suggestions. Also, we must recognize the value and contribution of all volunteer, nonremunerated convalescent donors who have willingly supported this program, even after a traumatic period of being infected by this mysterious, multifaceted, and relatively unknown virus.
Wendel S, Kutner JM, Machado R, et al. Screening for SARS‐CoV‐2 antibodies in convalescent plasma in Brazil: Preliminary lessons from a voluntary convalescent donor program. Transfusion. 2020;60:2938–2951. 10.1111/trf.16065
REFERENCES
- 1. World Health Organization Naming the coronavirus disease (COVID‐19) and the virus that causes it. Available at https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/naming‐the‐coronavirus‐disease‐(covid‐2019)‐and‐the‐virus‐that‐causes‐it. Accessed June 2, 2020.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guo YR, Cao QD. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak – an update on the status. Mil Med Res. 2020;7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO) Clinical management of COVID‐19. Interim guidance 27 May, 2020 – WHO/2019‐nCoV/clinical/2020.5. https://www.who.int/publications-detail/clinical-management-of-covid-19. Accessed August 2, 2020.
- 6. Valk SJ, Piechotta V, Chai KL, et al. Convalescent plasma or Hyperimmune immunoglobulin for people with COVID‐19: a rapid review. Cochrane Database Syst Rev. 2020;5:CD013600 10.1002/14651858.CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGuire L, Redden W. Treatment of influenzal pneumonia by the use of convalescent human serum. JAMA. 1919;72:709–713. [Google Scholar]
- 8. Cheng Y, Wong R, Soo YOY, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang JS, Chen JT, Liu YX, et al. A serological survey on neutralizing antibody titer of SARS convalescent sera. J Med Virol. 2005;77:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood. J Infect Dis. 1999;179(Suppl 1):S18–S23. 10.1086/514298. [DOI] [PubMed] [Google Scholar]
- 11. Sahr F, Ansuman R, Massaquoi TA, et al. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Infect. 2017;74:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS‐CoV infection. Saudi Arabia. Emerg Infect Dis. 2016;22(9):1554–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park WH, Freeman RG. The prophylactic use of measles convalescent serum. JAMA. 1926;87(8):556–558. [Google Scholar]
- 14. Park WH. Therapeutic use of antipoliomyelitits serum in preparalytic cases of poliomyelitis. JAMA. 1932;99:1050–1053. [Google Scholar]
- 15. Rambar AC. Mumps; use of convalescent serum in the treatment and prophylaxis of orchitis. Am J Dis Child. 1946;71:1–13. [PubMed] [Google Scholar]
- 16. Stinebaugh BJ, Schloeder FX, Johnson KM, Mackenzie RB, Entwisle G, de Alba E. Bolivian hemorrhagic fever. A report of four cases. Am J Med. 1966;40:217–230. [DOI] [PubMed] [Google Scholar]
- 17. Luke TC, Casadevall A, Watowich SJ, Hoffman SL, Beigel JH, Burgess TH. Hark back: Passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38(4 suppl):e66–e73. [DOI] [PubMed] [Google Scholar]
- 18. Mair‐Jenkins J, Saavedra‐Campos M, Baillie JK, et al. Convalescent plasma study Group.Version 2. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta‐analysis. J Infect Dis. 2015. Jan 1;211:80–90. 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. J Am Med Assoc. 2020;323:1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roback JD, Guarner J. Convalescent plasma to treat COVID‐19. Possibilities and challenges. JAMA. 2020;323:1561–1562. 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 22. Casadevall A, Pirofski L. The convalescent sera options for containing COVID‐19. J Clin Invest. 2020;130:1545–1548. 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barone P, DeSimone RA. Convalescent plasma to treat coronavirus disease 2019 (COVID‐19): Considerations for clinical trial design. Transfusion. 2020;60:1123–1127. 10.1111/trf.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knudson CM, Jackson JB. COVID‐19 convalescent plasma: phase 2. Transfusion. 2020;60:1332–1333. 10.1111/trf.15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA. 2020;324:460–470. 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu, STH , Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID‐19: A matched 1 control study. MedRxiv. 10.1101/2020.05.20.20102236 [DOI]
- 28. Dzik S. COVID‐19 convalescent plasma: Now is the time for better science. Transfus Med Rev. 2020;34:141–144. 10.1016/j.tmrv.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Casadevall A, Joyner MJ, Pirofski LA. Randomized trial of convalescent plasma for COVID‐19—Potentially hopeful signals. JAMA. 2020;324:455–457. 10.1001/jama.2020.10218. [DOI] [PubMed] [Google Scholar]
- 30. Joyner MJ, Wright RS, Fairweather DL, et al. Early safety indicators of COVID‐19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;11:140200 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong HK, Lee CK. Pivotal role of convalescent plasma in managing emerging infectious diseases. Vox Sang. 2020. 10.1111/vox.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Epstein J, Burnouf T. Points to consider in the preparation and transfusion of COVID‐19 convalescent plasma. Vox Sang. 2020. 10.1111/vox.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Epstein J, Smid M, Wendel S, et al. Use of COVID‐19 convalescent plasma in low‐ and middle‐income countries: A call for ethical principles and the assurance of quality and safety. Vox Sang. 2020. 10.1111/vox.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brazilian Ministry of Health ‐ Nota Técnica N° 19/2020/Sei/Gstco/Dire1/Anvisa. Processo n° 25351.912548/2020–05. Aspectos regulatórios do uso de plasma de doador convalescente para tratamento da Covid‐19. http://portal.anvisa.gov.br/documents/219201/4340788/Nota+Te%C2%B4cnica+Anvisa+Uso+Plasma+Convalescente+COVID+19.pdf/2d0db2be-482a-47e3-91c4-0b835e86eabb. Accessed June 4, 2020.
- 35.Brazilian Ministry of Health – Act 158, February 4, 2016. https://bvsms.saude.gov.br/bvs/saudelegis/gm/2016/prt0158_04_02_2016.html. Accessed June 5, 2020.
- 36. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):2000045 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Araujo DB, Machado RRG, Amgarten DE, et al. SARS‐CoV‐2 isolation from the first reported patients in Brazil and establishment of a coordinated task network. Mem Inst Oswaldo Cruz. 10.1590/0074-02760200342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nurtop E, Villarroel PMS, Pastorino B, et al. Combination of ELISA screening and seroneutralisation tests to expedite Zika virus seroprevalence studies. Virol J. 2018;15(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almeida FJ, Olmos RD, Oliveira DBL, et al. Hematuria associated with SARS‐CoV‐2 infection in a child. Pediatr Infect Dis J. 2020;39(7):e161. [DOI] [PubMed] [Google Scholar]
- 40. Tan CW, Chia WN, Chen MI‐C, et al. A SARS‐CoV‐2 surrogate virus neutralization test (sVNT) based on antibody‐mediated blockage of ACE2‐spike (RBD) protein‐protein interaction. Nat Res. 2020. https://www.researchsquare.com/article/rs-24574/v1. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Q, Zhang H, Huang K, et al. SARS‐CoV‐2 neutralizing serum antibodies in cats: A serological investigation. bioRxiv. 2020.
- 42. Tu C, Crameri G, Kong X, et al. Antibodies to SARS coronavirus in civets. Emerg Infect Dis. 2004;10(12):2244–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao S, Li W, Schuurman N, van Kuppeveld F, Bosch BJ, Egberink H. Serological screening for coronavirus infections in cats. Viruses. 2019;11(8):743 10.3390/v11080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meyer B, Drosten C, Müller MA. Serological assays for emerging coronaviruses: Challenges and pitfalls. Virus Res. 2020;194(January):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization (WHO). Laboratory biosafety guidance related to the novel coronavirus (2019‐nCoV). 2020;(February):1–12. https://www.who.int/docs/default-source/coronaviruse/laboratory-biosafety-novel-coronavirus-version-1-1.pdf?sfvrsn=912a9847_2.
- 46. Oliveira DB, Almeida FJ, Durigon EL, et al. Prolonged shedding of Zika virus associated with congenital infection. N Engl J Med. 2016;375(12):1202–1204. 10.1056/NEJMc1607583. [DOI] [PubMed] [Google Scholar]
- 47. Gallup JL. Added‐variable plots with confidence intervals. Added‐variable plots with confidence intervals. Stata J. 2019;19(3):598–614. [Google Scholar]
- 48. Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS‐CoV‐2 and other human coronaviruses. Trends Immunol. 2020;41(5):355–359. 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taisheng L, Jing X, Yuxian H, et al. Long‐term persistence of robust antibody and cytotoxic T cell responses in recovered patients infected with SARS coronavirus. PLoS One. 2006;1(1):e24 10.1371/journal.pone.0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020;ciaa344 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS‐CoV‐2 coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell. 2020. 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the Prefusion conformation. Science. 2020;367:1260–1263. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou GY, Zhao Q. Perspectives on therapeutic neutralizing antibodies against the novel coronavirus SARS‐CoV‐2. Int J Biol Sci. 2020;16:1718–1723. 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tay MZ, Poh CM, Rénia L, et al. The trinity of COVID‐19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;28:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. medRxiv 2020. 10.1101/2020.03.30.20047365. [DOI]
- 56.Recommendations for investigational COVID‐19 convalescent plasma [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; April 13, 2020. https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ideprocess-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed April 13, 2020.
- 57. Robbiani DF, Gaebler C, Muecksch F, et al. Convergent antibody responses to SARS‐CoV‐2 infection in convalescent individuals – bioRxiv. 10.1101/2020.05.13.092619. [DOI] [PMC free article] [PubMed]
- 58. European Commission Directorate‐General For Health And Food Safety . An EU programme of COVID‐19 convalescent plasma collection and transfusion. Guidance on collection, testing, processing, storage, distribution and monitored use. https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/guidance_plasma_covid19_en.pdf. Accessed June 4, 2020.
- 59. To KK , Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang JR, Deng DT, Wu N, Yang B, Li HJ, Pan XB. Persistent viral RNA positivity during recovery period of a patient with SARS‐CoV‐2 infection. J Med Virol. 2020. 10.1002/jmv.25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li N, Wang X, Lv TF. Prolonged SARS‐CoV‐2 RNA shedding: Not a rare phenomenon. J Med Virol. 2020. 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu WD, Chang SY, Wang JT, et al. Prolonged virus shedding even after seroconversion in a patient with COVID‐19. J Infect. 2020;81:318–356. 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.WHO ‐ Criteria for releasing COVID‐19 patients from isolation. Scientific brief. https://www.who.int/news-room/commentaries/detail/criteria-for-releasing-covid-19-patients-from-isolation. Accessed July 25, 2020.
- 64. Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Long QX, Tang XJ, Shi QL, Li Q. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26:1200–1204. 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 66. Yujiao J, Miaochan W, Zhongbao Z, et al. Diagnostic value and dynamic variance of serum antibody in coronavirus disease 2019. Int J Infect Dis. 2020;94:49–52. 10.1016/j.ijid.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elshabrawy HA, Coughlin MM, Baker SC, Prabhakar BS. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS‐CoV spike protein are more broadly neutralizing. PLoS One. 2012;7:e50366 10.1371/journal.pone.0050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gniadek TJ, Donnersberger D. COVID‐19 convalescent plasma donor recruitment: Beware the Faustian bargains. Transfusion. 2020;60:1643–1644. 10.1111/trf.15871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 70. Yan H, Lamm ME, Björling E, et al. Multiple functions of immunoglobulin a in mucosal defense against viruses: An in vitro measles virus model. J Virol. 2002;76:10972–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Blutt SE, Miller AD, Salmon SL, Metzger DW, Conner ME. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012;5:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walsh EE, Falsey AR. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J Infect Dis. 2004;190:373–378. [DOI] [PubMed] [Google Scholar]
- 73. Cervia C, Nilsson J, Zurbuchen Y, et al. Systemic and mucosal antibody secretion specific to SARS‐CoV‐2 during mild versus severe COVID‐19. 10.1101/2020.05.21.108308. [DOI] [PMC free article] [PubMed]
- 74. Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID‐19 disease to younger ages. Lancet. 2020;395:1544–1545. 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Caussy C, Pattou F, Wallet F, et al. Prevalence of obesity among adult inpatients with COVID‐19 in France. Lancet Diabetes Endocrinol. 2020;8:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kwon SY, Kim EJ, Jung YS, Jang JS, Cho NS. Post‐donation COVID‐19 identification in blood donors. Vox Sang. 2020. 10.1111/vox.12925. [DOI] [PubMed] [Google Scholar]
- 77. Lanteri MC, Santa‐Maria F, Laughhunn A, et al. Inactivation of a broad Spectrum of viruses and parasites by photochemical treatment of plasma and platelets using Amotosalen and ultraviolet a light. Transfusion. 2020;60:1319–1331. 10.1111/trf.15807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Keil SD, Ragan I, Yonemura S, Hartson L, Dart NK, Bowen R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light‐based photochemical treatment. Vox Sang. 2020. 10.1111/vox.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26:1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luchsinger L, Ransegnola B, Jin D, et al. Serological Analysis of New York City COVID19 Convalescent Plasma Donor. 2020. 10.1101/2020.06.08.20124792. [DOI]
- 82. Bloch EM, Goel G, Wendel S, et al. on behalf of the ISBT Convalescent Plasma Working Group. Guidance for the Procurement of COVID‐19 Convalescent Plasma: Differences between High and Low‐Middle Income Countries. Vox Sang. 2020. First published June 13, 2020. 10.1111/vox.12970 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographics and interval between onset of symptoms, full recovery, medical interview (days), and serial RT‐PCR tests from 271 convalescent plasma donors. *Female donors donated only once in the program. Males were heavier than females (85.5 ± 14.0 × 69.6 ± 12.0 kg; P < .01), but also had a shorter time of symptoms (10.5 ± 5.3 × 12.0 ± 4.4 days; P = .01). RT‐PCR1, = first RT‐PCR test (diagnosis during symptoms present); RT‐PCR2, second RT‐PCR test, performed right after the medical interview (swab or peripheral blood); RT‐PCR3, third RT‐PCR (swab only), performed on donors whose RT‐PCR2 was positive.
Table S2 ‐ Clinical symptoms in 271 convalescent donors. Except for myalgia in males, all clinical symptoms were nonstatistically different by sex
Table S3 – Spearman's correlation between neutralizing antibodies (ln), weight (kg), IgM, IgG, and IgA. All showed statistically significant results at 0.01 level (Bonferroni adjusted)
Figure S1 ‐ Sex distribution of neutralizing antibodies (ln), female (n = 32), left; male (n = 218, middle). There was no difference in titers according to sex (P = . 47). Vertical long‐dashed line represents the cutoff titer ≥160 (63.6% of all donors).
Figure S2 ‐ Correlation between IgM, IgG, and IgA with onset of symptoms (A, C, E, left) and weight (B, D, F, right). Only IgM correlated moderately with onset of symptoms. Weight correlated with all SARS‐CoV‐2 antibody classes (all P < .01). IgM was still detected in 4.4% of CCP donors >40 days after onset of symptoms (lower quadrant).
Figure S3 ‐ Immunoglobulin anti‐NP (IgM, IgG, and IgA) S/CO from 226 donors, according to the CCP donor final RT‐PCR status. No statistical difference (Mann‐Whitney) was seen between both groups (PCR‐RT –ve and RT‐PCR +ve) for each immunoglobulin class. (RT‐PCR –ve × RT‐PCR +ve; P = .51; .92; .74, for IgM, IgG, and IgA, respectively.)


