Abstract
Background
Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial (CABOSUN) was a randomized, open‐label, phase 2 trial evaluating first‐line cabozantinib versus sunitinib in patients with advanced renal cell carcinoma (aRCC). This post hoc analysis evaluated quality‐adjusted survival using Quality‐adjusted Time Without Symptoms of disease or Toxicity of treatment (Q‐TWiST).
Methods
Survival plots for cabozantinib and sunitinib (650‐day follow‐up) were partitioned into 3 health states: time spent before disease progression without toxicity (TWiST; toxicity based on National Cancer Institute Common Terminology Criteria for Adverse Events [version 4.0] grade 3/4 adverse events), time spent before disease progression with toxicity (TOX; durations of adverse events based on published literature), and time after disease recurrence (relapse) or progression to death (REL). Q‐TWiST was the sum of the mean time spent in each state, with each state weighted to reflect patient preferences (from 0 [worst] to 1 [best]) using utility scores. TWiST was always weighted as 1. Overall survival and time to disease progression were based on all randomized patients (157 patients); TOX was based on all randomized and treated patients (150 patients).
Results
Across all utility combinations tested, Q‐TWiST was found to be longer with cabozantinib versus sunitinib (range of differences, +24 days to +137 days). Q‐TWiST differences that were found to be statistically significant (+92 days [95% confidence interval, 5‐178 days] to +137 days [95% confidence interval, 60‐214 days]) were of a clinically meaningful effect size (≥80 days), and were based on utility values that included those considered relevant for patients with aRCC (REL utility weight of 0.355, TOX utility weight of 0‐1, and TWiST utility weight of 1).
Conclusions
In patients with aRCC, first‐line cabozantinib was found to provide longer quality‐adjusted survival compared with sunitinib. These findings may help to inform clinical decision making.
Lay Summary
Cabozantinib and sunitinib are drugs that are used to treat patients with advanced kidney cancer. Clinical trials have shown that cabozantinib offers benefits over sunitinib, giving patients more time before their cancer progresses.
It is important that this additional time before disease progression does not come at the expense of patients' quality of life, which can be affected by treatment side effects and/or ongoing cancer symptoms. Both quantity and quality of life are central to optimal treatment.
In the current analysis of patients with advanced kidney cancer who were initiating treatment for the first time, cabozantinib provided more quality time before cancer progression compared with sunitinib.
Keywords: carcinoma, protein tyrosine kinases, quality of life, renal cell
Short abstract
The current post hoc analysis of the Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial (CABOSUN) finds that cabozantinib offers statistically and clinically significant improvements in quality‐adjusted survival compared with sunitinib in treatment‐naive patients with advanced renal cell carcinoma (aRCC). This finding may help to inform clinical decision making in the first‐line treatment of patients with aRCC.
Introduction
Advanced renal cell carcinoma (aRCC) imposes a substantial burden on patients in terms of mortality, morbidity, and impaired health‐related quality of life (HRQOL). HRQOL is adversely affected in patients with aRCC by disease‐related symptoms such as fatigue, weakness, pain, constipation, diarrhea, or shortness of breath, and treatment‐related adverse events (AEs) frequently contribute to impaired HRQOL. 1 , 2 , 3 In the management of patients with aRCC, HRQOL therefore is an important factor to consider in addition to survival outcomes and should be included in the clinical decision‐making process.
Tyrosine kinase inhibitors (TKIs) are targeted agents that are widely used for the treatment of aRCC. 4 , 5 Cabozantinib is a TKI directed against receptors for vascular endothelial growth factor (VEGF), MET, and AXL, and initially was approved in the United States in April 2016 for the treatment of patients with aRCC who have received prior antiangiogenic therapy. 6 On the strength of the results of the Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial (CABOSUN), 7 , 8 the US licence for cabozantinib was extended in December 2017 to include the first‐line treatment of patients with aRCC. In Europe, cabozantinib is approved for the treatment of adults with aRCC after receipt of prior VEGF‐targeted therapy or for the first‐line treatment of adults with intermediate‐risk or poor‐risk disease. 10
The efficacy and safety of cabozantinib as first‐line therapy for aRCC were established in the randomized, phase 2 CABOSUN trial of treatment‐naive patients with aRCC of intermediate or poor risk. 7 , 8 The CABOSUN trial demonstrated significantly improved progression‐free survival (PFS; by independent review committee) with cabozantinib compared with sunitinib (8.6 months vs 5.3 months; hazard ratio, 0.48 [P = .0008]). 8 After a median follow‐up of 34.5 months, the median overall survival (OS) was 26.6 months with cabozantinib and 21.2 months with sunitinib (hazard ratio, 0.80; 95% confidence interval [95% CI], 0.53‐1.21). 8 Grade 3/4 AEs occurred in approximately 68% of patients who were treated with cabozantinib compared with 65% of those treated with sunitinib. The most common grade 3/4 AEs with cabozantinib and sunitinib, respectively, were hypertension (28% vs 21%), diarrhea (10% vs 11%), fatigue (6% vs 17%), and a decreased platelet count (1% vs 11%). 8 Another trial, A Study of Cabozantinib (XL184) vs Everolimus in Subjects With Metastatic Renal Cell Carcinoma (METEOR), which evaluated the efficacy and tolerability of cabozantinib compared with everolimus after disease progression while receiving VEGF‐targeted therapy, demonstrated similar safety results with cabozantinib, with 68% of patients having grade 3/4 AEs, most commonly hypertension (15%), diarrhea (11%), and fatigue (9%). 11 , 12
The significant symptom burden of patients with aRCC who are receiving TKI therapy underscores the importance of clinical trial reporting to inform patients and physicians about both the quantity of life and QOL associated with treatment. Quality‐adjusted Time Without Symptoms of disease or Toxicity of treatment (Q‐TWiST) is a measure that integrates both the length and quality of survival into a single index by considering OS time, time to disease progression without toxicity, and time to disease progression with toxicity, together with patient preferences for each of these 3 states. 13 , 14 , 15 All of these aspects are directly relevant to patients with aRCC; indeed, the Q‐TWiST composite endpoint uniquely allows individual patients to factor in their preferences for the different health states to determine the magnitude of benefit of specific treatments in a clinical trial. Q‐TWiST is related to quality‐adjusted life‐years, but is based on discrete health states specific for oncology and is commonly used to evaluate the benefits and risks of treatment in oncology, including aRCC. 15 , 16 , 17 , 18
Because the CABOSUN trial did not include a prospective HRQOL analysis, 7 the primary goal of this post hoc analysis was to evaluate Q‐TWiST as a means of assessing the respective impact of cabozantinib and sunitinib on HRQOL in treatment‐naive patients with aRCC of intermediate or poor risk.
Materials and Methods
CABOSUN Study Design
Detailed methods of the CABOSUN study (Alliance for Clinical Trials in Oncology A031203; ClinicalTrials.gov identifier NCT01835158) have been published previously. 7 , 8 Briefly, CABOSUN was a randomized, open‐label, phase 2 trial in adults with aRCC of intermediate or poor risk who had received no previous systemic treatment. At baseline, the Eastern Cooperative Oncology Group performance status score was required to be 0 to 2. Patients were randomized 1:1 to cabozantinib at a dose of 60 mg/day (daily) or to sunitinib at a dose of 50 mg/day (4 weeks on/2 weeks off). Study treatment was continued until disease progression, intolerance to therapy, withdrawal of consent, or death (if earlier). The primary endpoint was PFS, with disease progression defined as first radiographic progression noted by computed tomography or magnetic resonance imaging scans using Response Evaluation Criteria in Solid Tumors (RECIST) (version 1.1) based on retrospective analysis by an independent review committee (using censoring rules as per the US Food and Drug Administration guidance on oncology endpoints [see Supporting Materials]). 8 Routine safety evaluations were performed, and AE severity grades were assessed by the investigator using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). 7 , 8
Q‐TWiST Analysis
The Q‐TWiST analysis integrates time before disease progression with and without toxicity, time after disease progression, OS time, and patient preferences for each health state to generate a single index for quality‐adjusted life‐years. To generate Q‐TWiST, the duration of survival is partitioned into 3 mutually exclusive health states (see Supporting Fig. 1): 1) time without disease progression and without any toxicity (Time without Symptoms of disease or Toxicity [TWiST]); 2) time before disease progression but with grade 3/4 toxicity (Toxicity [TOX]); and 3) time after disease progression until death (recurrence [Relapse; REL]). The mean time spent in each of these health states is calculated for each treatment arm. To calculate Q‐TWiST (ie, the quality‐adjusted time spent in each health state), the TWiST, TOX, and REL health states are weighted by a utility score reflective of the value of the health state (see below), and the mean times spent in each of the weighted health states are added.
Definition of Toxicity
Toxicity was defined as the presence of grade 3/4 AEs with treatment with cabozantinib and sunitinib, as reported in the CABOSUN trial. 7 , 8 In the case of multiple grade 3/4 AEs occurring on the same day, only the AE with the longest duration was counted; in the case of death (from any cause) occurring before disease progression, the day of death was counted as 1 day spent in TOX. Because the duration of AEs was not reported in the CABOSUN trial, data from other studies were used to estimate the median durations of AEs. For the cabozantinib group, the median durations of the grade 3/4 AEs of diarrhea (7 days), fatigue (14 days), and hypertension (15 days) were based on data from the METEOR trial 12 ; for all other grade 3/4 AEs, a mean value of 11 days was used based on the weighted durations of diarrhea, fatigue, and hypertension and considering the number of events in the METEOR trial. For the sunitinib group, a systematic literature review was performed to identify relevant data in patients with aRCC. Only 1 report was identified from a phase 3 trial comparing sunitinib with interferon‐α, 19 which reported median durations for the most common grade 3/4 treatment‐related AEs with sunitinib (17 days for fatigue, 22 days for hypertension, and 12 days for diarrhea); for all other grade 3/4 AEs, a mean value of 16 days was used based on the weighted durations of diarrhea, fatigue, and hypertension and considering the number of events reported in the study by Patil et al. 19 A sensitivity analysis was performed using the same AE durations for sunitinib as those described above for cabozantinib to account for potential between‐group differences in AE duration that may have occurred for reasons unrelated to the study drugs.
Utility Scores
Utility scores, representing the patient preference associated with TWiST, TOX, and REL, were used to weight the 3 health states, and ranged from 0 (equivalent to death) to 1 (perfect health). The TWiST period (ie, the time before disease progression and without any toxicity) is the optimal health state and always was weighted as 1. For TOX and REL, different utility weightings (in increments of 0.25) were assessed, reflecting the different possible patient preferences associated with these health states. We also tested utility weightings that were identified in a study assessing UK societal preferences for health states in patients with aRCC who were receiving first‐line therapies. 19
Statistical Analyses
Time‐to‐event analyses, defined as the time from randomization to the event of interest, were performed for each treatment arm using Kaplan‐Meier analysis. Event and censoring times for OS and PFS followed definitions described previously. 8 Analyses of efficacy endpoints (OS and PFS) were based on all randomized patients in the CABOSUN trial (intent‐to‐treat population). Safety outcomes (presence of AEs) were based on all randomized patients who received at least 1 dose of the study treatment in the CABOSUN trial (safety population). The data cutoff date for PFS and safety outcomes (presence of AEs) was September 2016, and the data cutoff date for OS was July 2017. The follow‐up period in the Q‐TWiST analyses involved the first 650 days in the CABOSUN trial (21.4 months, corresponding to the median OS in the CABOSUN trial 7 ). The mean time spent in TWiST, TOX, and REL was calculated by the area under the curve of the partitioned survival plots. Q‐TWiST was calculated by multiplying the mean time spent in each health state by the corresponding utility value (u), and summing the weighted time spent in each health state (Q‐TWiST = [uTOX × TOX] + [uTWiST × TWiST] + [uREL × REL]); a longer Q‐TWiST time represents an improvement in HRQOL. The difference in the mean Q‐TWiST between treatments was calculated and presented with 95% CIs.
Results
In the CABOSUN trial, a total of 157 patients were randomized (79 to cabozantinib and 78 to sunitinib) and 150 patients were treated (78 with cabozantinib and 72 with sunitinib). 7 , 8 The treatment groups were balanced with respect to baseline demographic and disease characteristics. The mean age of the patients was 63 years and approximately 78% of patients were male; the majority of patients had an Eastern Cooperative Oncology Group performance status score of 0 (45.9%) or 1 (41.4%); approximately 81% of patients had intermediate‐risk disease and 19% had poor‐risk disease, and 36% had bone metastases. 7 , 8
The survival plots for cabozantinib and sunitinib (with up to 650 days of follow‐up), partitioned into the 3 health states of TWiST, TOX, and REL (prior to utility weighting), are shown in Figure 1. 11 , 19 The mean durations spent in each of the 3 health states, represented by the areas under the curves, are shown in Table 1. 11 , 19 Using patient preference (utility) weightings of 1 for the TWiST health state and 0 for the TOX and REL health states, patients receiving cabozantinib spent significantly more time in TWiST (ie, before disease progression and without toxicities) than those being treated with sunitinib (317 days vs 180 days; difference: 137 days [95% CI, 60‐214 days]). The mean time spent in TOX was not significantly different between patients treated with cabozantinib versus sunitinib (31 days vs 39 days; difference: −8 days [95% CI, −25 to 9 days]). The mean time spent in REL was shorter with cabozantinib than with sunitinib (154 days vs 259 days; difference: −105 days [95% CI, −206 to −5 days]) (Table 2). 11 , 19 , 20
FIGURE 1.
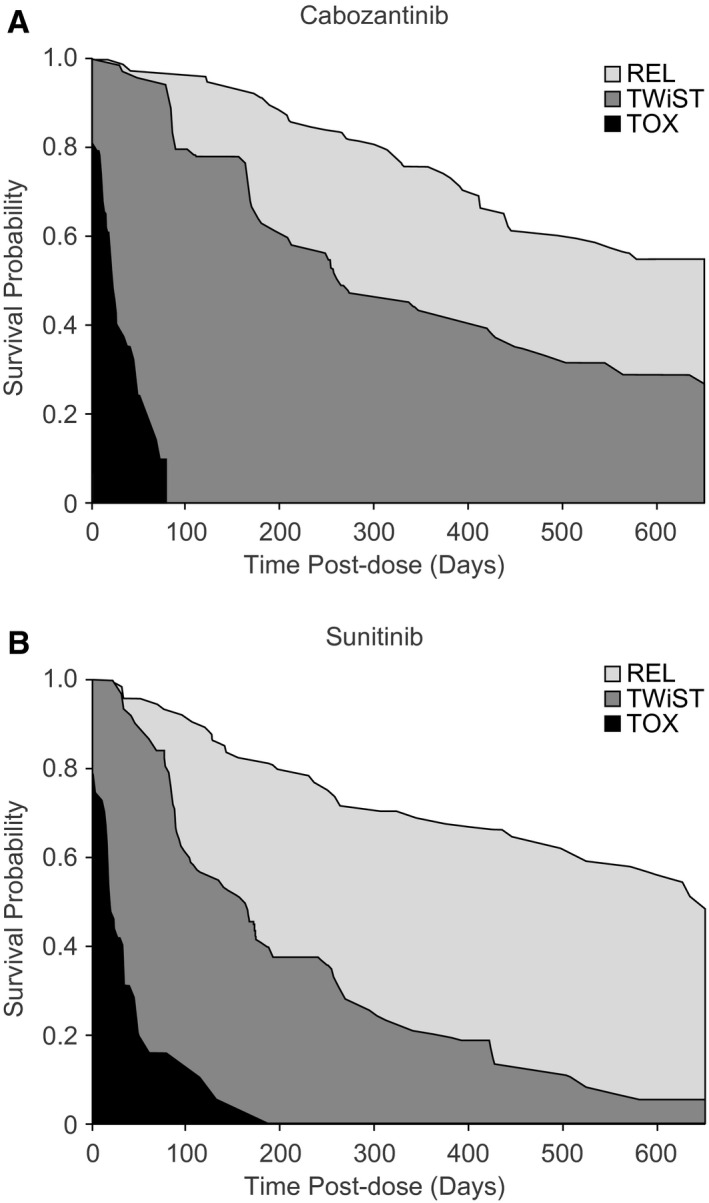
Partitioned Kaplan‐Meier survival curves representing the time spent in TWiST, TOX, and REL with (A) cabozantinib and (B) sunitinib. The areas between the curves correspond to the mean time spent in each health state (prior to utility weighting). Durations of adverse events are based on the A Study of Cabozantinib (XL184) vs Everolimus in Subjects With Metastatic Renal Cell Carcinoma (METEOR) trial 11 for patients treated with cabozantinib or the study by Patil et al 19 for the sunitinib group. REL indicates time after disease recurrence (relapse) or progression to death; TOX, time with toxicity before disease progression; TWiST, time without symptoms of disease and without toxicity.
TABLE 1.
Mean Times Spent in TWiST, TOX, and REL With Cabozantinib and Sunitinib
| Treatment | No. | Mean (SE), Days | Difference Between Cabozantinib Versus Sunitinib | ||
|---|---|---|---|---|---|
| Mean (SE), Days | 95% CI | ||||
| TWiST | Cabozantinib | 79 | 317 (29) | 137 (39) | 60 to 214 |
| Sunitinib | 78 | 180 (26) | |||
| TOX | Cabozantinib | 79 | 31 (4) | −8 (9) | −25 to 9 |
| Sunitinib | 78 | 39 (8) | |||
| REL | Cabozantinib | 79 | 154 (36) | −105 (51) | −206 to −5 |
| Sunitinib | 78 | 259 (36) | |||
Abbreviations: 95% CI, 95% confidence interval; REL, time after disease recurrence (relapse) or progression to death; SE, standard error; TOX, time with toxicity before disease progression; TWiST, time without symptoms of disease and without toxicity.
Mean times spent in TWiST, TOX, and REL states (prior to utility weighting) were estimated using partitioned Kaplan‐Meier survival curves. Durations of adverse events were based on the A Study of Cabozantinib (XL184) vs Everolimus in Subjects With Metastatic Renal Cell Carcinoma (METEOR) trial 11 for cabozantinib or the study by Patil et al 19 for the sunitinib group.
TABLE 2.
Difference in Q‐TWiST Between Cabozantinib and Sunitinib Using Different Combinations of Utilities for TOX and REL and a Utility of 1 for TWiST
| TOX Weighting | REL Weighting | TWIST Weighting | Q‐TWiST Difference: Cabozantinib Versus Sunitinib | |
|---|---|---|---|---|
| Mean (SE), Days | 95% CI | |||
| 0.00 | 0.00 | 1.00 | 137 (39) a | 60 to 214 |
| 0.25 | 0.00 | 1.00 | 135 (39) a | 57 to 212 |
| 0.50 | 0.00 | 1.00 | 133 (40) a | 55 to 211 |
| 0.75 | 0.00 | 1.00 | 131 (40) a | 53 to 209 |
| 1.00 | 0.00 | 1.00 | 129 (40) a | 50 to 208 |
| 0.00 | 0.25 | 1.00 | 110 (41) a | 29 to 192 |
| 0.25 | 0.25 | 1.00 | 109 (41) a | 27 to 190 |
| 0.50 | 0.25 | 1.00 | 107 (42) a | 25 to 188 |
| 0.75 | 0.25 | 1.00 | 105 (42) a | 22 to 187 |
| 1.00 | 0.25 | 1.00 | 103 (42) a | 20 to 185 |
| 0.00 b | 0.355 b | 1.00 | 99 (43) a | 14 to 184 |
| 1.00 b | 0.355 b | 1.00 | 92 (44) a | 5 to 178 |
| 0.00 | 0.50 | 1.00 | 84 (47) | −8 to 176 |
| 0.25 | 0.50 | 1.00 | 82 (47) | −10 to 174 |
| 0.50 | 0.50 | 1.00 | 80 (47) | −12 to 173 |
| 0.75 | 0.50 | 1.00 | 78 (47) | −15 to 171 |
| 1.00 | 0.50 | 1.00 | 76 (48) | −17 to 170 |
| 0.00 | 0.75 | 1.00 | 58 (55) | −50 to 166 |
| 0.25 | 0.75 | 1.00 | 56 (55) | −52 to 164 |
| 0.50 | 0.75 | 1.00 | 54 (55) | −54 to 162 |
| 0.75 | 0.75 | 1.00 | 52 (55) | −57 to 160 |
| 1.00 | 0.75 | 1.00 | 50 (56) | −59 to 159 |
| 0.00 | 1.00 | 1.00 | 32 (65) | −95 to 158 |
| 0.25 | 1.00 | 1.00 | 30 (65) | −97 to 156 |
| 0.50 | 1.00 | 1.00 | 28 (65) | −99 to 154 |
| 0.75 | 1.00 | 1.00 | 26 (65) | −102 to 153 |
| 1.00 | 1.00 | 1.00 | 24 (65) | −104 to 151 |
Abbreviations: 95% CI, 95% confidence interval; Q‐TWiST, quality‐adjusted time without symptoms of disease or toxicity of treatment; REL, time after disease recurrence (relapse) or progression to death; SE, standard; error TOX, time with toxicity before disease progression; TWiST, time without symptoms of disease and without toxicity.
Durations of adverse events were based on the A Study of Cabozantinib (XL184) vs Everolimus in Subjects With Metastatic Renal Cell Carcinoma (METEOR) trial 11 for cabozantinib or the study by Patil et al 19 for the sunitinib group.
The difference in Q‐TWiST (cabozantinib minus sunitinib) was statistically significant (95% CI did not include zero).
Utility values were reported to be relevant to patients with advanced renal cell carcinoma who were receiving first‐line therapy. 20
The difference in Q‐TWiST (the difference in the sum of the time spent in the TWiST, TOX, and REL health states for cabozantinib minus the sum of the time spent in these health states for sunitinib) is shown in Table 2, 11 , 19 , 20 for a range of utility weighting (ie, patient preference) scenarios for each health state (TWiST, TOX, and REL). The difference in Q‐TWiST represents an overall composite difference for cabozantinib compared with sunitinib from the CABOSUN trial that included OS, disease control, and toxicity and was specific to a patient's preference for each health state. Different combinations of utility weightings were used for TOX and REL, representing different possible patient preferences for these states, with TWiST always set at 1. Across all TOX and REL utility weighting combinations, cabozantinib was associated with a longer mean time spent in Q‐TWiST compared with sunitinib; the difference in Q‐TWiST with cabozantinib versus sunitinib ranged from +137 days (assuming TOX and REL were both utility weighted as 0) to +24 days (assuming TOX and REL were both utility weighted as 1). The prolongation in Q‐TWiST with cabozantinib versus sunitinib was statistically significant (95% CI did not include zero) when assuming a utility weighting of 0 or 0.25 for REL, and any utility weighting for TOX (difference ranging from +103 days to +137 days). Q‐TWiST also was found to be statistically significantly longer with cabozantinib compared with sunitinib when using utility weightings for REL and TOX that were established to be relevant for patients with aRCC who were receiving first‐line therapy (0.355 for progressive disease; utility weighting range, 0.47 [hand‐foot syndrome]‐0.75 [grade 1/2 fatigue] for treatment‐related toxicities 20 ). Thus, assuming a utility weighting of 0.355 for REL and a utility weighting of 1 for TWiST, the benefit in Q‐TWiST with cabozantinib versus sunitinib in the CABOSUN trial was statistically significant across all possible utility weightings for TOX, ranging from +92 days (TOX utility weighting of 1) to +99 days (TOX utility weighting of 0) (Table 2). 11 , 19 , 20
Given that the duration of AEs was not recorded in the CABOSUN trial, we performed a sensitivity analysis in which the AE duration was considered equal for cabozantinib and sunitinib (see Supporting Table 1). Similar results were observed in this sensitivity analysis as in the base case. Patients receiving cabozantinib spent more time in TWiST compared with those receiving sunitinib (difference: 125 days; 95% CI, 49‐202 days) and less time in REL (difference: −105 days; 95% CI, −206 to −5 days), whereas time spent in TOX was similar with both treatments (difference: 4 days; 95% CI, −9 to 17 days). Furthermore, as in the base case analysis, the mean time spent in Q‐TWiST was found to be longer with cabozantinib compared with sunitinib across all TOX and REL utility weighting combinations, with statistically significant differences noted when REL was weighted at 0 or 0.25 and TOX had any weighting (see Supporting Table 2).
Discussion
The results of the current Q‐TWiST analysis supplement and complement the original safety and efficacy results from the CABOSUN trial, providing novel insights into the comparative effect of first‐line cabozantinib and sunitinib on the QOL of patients with aRCC. The CABOSUN trial demonstrated significantly improved PFS with cabozantinib compared with sunitinib and similar rates of grade 3/4 AEs were reported to occur (68% vs 65%, respectively). 8 The current HRQOL analysis of the CABOSUN trial further demonstrated that patients receiving cabozantinib had longer quality‐adjusted time without disease progression and treatment toxicity, as measured by Q‐TWiST, compared with those receiving sunitinib. The longer duration of Q‐TWiST with cabozantinib compared with sunitinib primarily was due to a longer mean time spent without disease progression and without grade 3/4 toxicities with cabozantinib compared with sunitinib (TWiST, +137 days).
The results of the current analysis offer patients and their physicians a more comprehensive and personalized understanding of the impact of treatment with cabozantinib compared with sunitinib among patients with aRCC because it integrated OS, disease control, and toxicity together with an individual patient's preference for each of these health states. These findings also may offer additional insights to payers seeking to estimate the overall cost impact and management implications of treatment regimens when implemented in routine care.
In the Q‐TWiST model, TWiST, the state prior to disease progression and without significant toxicity, was considered to be the preferred health state for all patients in a particular population receiving treatment, thus resulting in a constant utility weighting of 1 (although it may be slightly lower than this absolute value of 1 in patients with aRCC). Health states in which the patient is experiencing grade 3/4 toxicity (TOX) or disease progression (REL) are expected to have utility weighting scores <1, but the exact value attributed to these states may vary according to the importance an individual patient places on avoiding TOX or REL. For example, a patient who wishes to pursue aggressive treatment to minimize the risk of disease progression would be represented by a high utility weighting score for TOX (eg, 0.75); in contrast, for a patient who wishes to minimize treatment‐related toxicity even at the risk of earlier disease progression, a lower utility weighting for TOX (eg, 0.25) may be more representative.
The current analysis assessed a wide range of possible utility weighting combinations for each of the health states. Q‐TWiST was found to be longer with cabozantinib compared with sunitinib across all utility weighting combinations tested, ranging from +24 days (assuming a TOX utility weighting of 1 and a REL utility weighting of 1) to +137 days (assuming a TOX utility weighting of 0 and a REL utility weighting of 0). The difference in the Q‐TWiST benefit with cabozantinib over sunitinib was statistically significant when assuming any utility weighting for TOX and a utility weighting of 0 or 0.25 for REL (Q‐TWiST difference ranging from +103 days to +137 days), and borderline statistically significant with a utility weighting of 0.50 for REL (Q‐TWiST difference ranging from +76 days to +84 days). It is important to note that a clinically meaningful effect size in Q‐TWiST previously has been defined as a threshold of ≥10% of a trial's median OS time. 21 Given that the median OS with cabozantinib in the CABOSUN trial was 800 days (26.6 months), 8 a difference in Q‐TWiST of ≥80 days between the 2 arms is considered a clinically meaningful benefit. In the current study, all Q‐TWiST differences between cabozantinib and sunitinib that were found to be statistically significant (+103 days to +137 days; assuming a utility weighting for REL of up to 0.25 and any utility weighting for TOX) also exceeded the clinically meaningful threshold. This finding can be interpreted to indicate that a patient who places maximal importance on avoiding disease recurrence (ie, REL utility weighting of 0) without regard for toxicity (ie, TOX utility weighting of 0‐1) can expect to spend a mean of up to 137 additional quality‐adjusted days (Q‐TWiST) with first‐line cabozantinib compared with sunitinib. In patients with aRCC who are receiving first‐line therapies, utility weightings have been reported to be 0.355 for progressive disease and 0.47 to 0.75 for treatment‐related toxicities. 20 Using these weightings (REL utility weighting of 0.355, TOX utility weighting of 0‐1, and TWiST utility weighting of 1), the Q‐TWiST benefit with cabozantinib over sunitinib also was statistically significant and clinically meaningful, with Q‐TWiST differences ranging from +92 days to +99 days.
The impact on Q‐TWiST of first‐line TKI treatment of aRCC also has been assessed in a study comparing pazopanib with sunitinib. Differences in Q‐TWiST between pazopanib and sunitinib ranged from −11 days (TOX utility weighting of 1 and REL utility weighting of 0) to +43 days (TOX utility weighting of 0 and REL utility weighting of 1) in favor of pazopanib. 18 However, the differences in Q‐TWiST between pazopanib and sunitinib always were smaller than the clinically important difference in Q‐TWiST for this study (ie, <10% of 28 months of OS). 18
To our knowledge to date, there has been limited evaluation of recent immunotherapy options for the first‐line treatment of patients with aRCC with respect to Q‐TWiST. However, the Nivolumab Combined With Ipilimumab Versus Sunitinib in Previously Untreated Advanced or Metastatic Renal Cell Carcinoma (CheckMate 214) trial found a clinically important and significant mean Q‐TWiST improvement of 3.5 months (95% CI, 2.0‐4.9 months) with nivolumab plus ipilimumab compared with sunitinib at 36 months in previously untreated patients with aRCC with intermediate‐risk or poor‐risk disease. 22 In light of the growing recognition of Q‐TWiST as a means of HRQOL assessment in oncology populations, integration of Q‐TWiST into the design of future randomized clinical trials (either as a composite endpoint or as disaggregated components) should be considered to enable the standardized assessment of the HRQOL impact of emerging aRCC options. In this way, clinical trial case report forms also could be developed to incorporate relevant data fields, including (for example) the start and end dates of toxicity‐related treatment interruptions for use as a proxy for AE duration for the TOX definition.
A few limitations should be considered when interpreting the results of the analyses in the current study. The AE durations for the TOX health state were estimates based on the published literature for cabozantinib and sunitinib; AE durations were not assessed in the CABOSUN trial. Because the literature‐based estimates suggested a longer duration of AEs with sunitinib compared with cabozantinib, a sensitivity analysis was performed assuming equivalence of AE duration for both treatments. The results of the sensitivity analysis were consistent and confirmatory with those of the primary analysis. Another limitation is that, consistent with existing methodology, 15 , 16 , 17 , 18 only the more severe toxicities were considered for TOX because these are likely to have the greatest effect on patients' lives. However, lower grade (1‐2) toxicities could have had an impact on HRQOL. Finally, some clinically meaningful findings did not reach statistical significance, potentially due to the small sample size of the trial. For example, although any Q‐TWiST difference of ≥65 days would be considered clinically meaningful, some of these differences were only of borderline statistical significance under certain utility weighting combinations (eg, with a REL utility weighting of 0.50 and a TOX utility weighting ranging from 0‐1).
Conclusions
In this post hoc analysis of the CABOSUN trial, patients with aRCC who were receiving first‐line treatment with cabozantinib were found to have longer Q‐TWiST than those receiving sunitinib. Differences in Q‐TWiST between cabozantinib and sunitinib that were statistically significant were of a clinically important magnitude when using utility weightings that were relevant for patients with aRCC. The longer duration of Q‐TWiST with cabozantinib compared with sunitinib is related primarily to a longer time spent without disease progression and without grade 3/4 toxicities (TWiST). Taken together, the findings of the current study, which integrated treatment efficacy, toxicity, and patient preference, have indicated that the overall benefit of quality‐adjusted survival is longer with cabozantinib than with sunitinib and may help to inform clinical decision making in the management of patients with aRCC.
Funding Support
Sponsored by funds from Ipsen. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers U10CA180821, U10CA180882, and UG1CA189823 to the Alliance for Clinical Trials in Oncology. Work performed as part of the study was supported by the National Institutes for Health P30 Cancer Center Support Grant, award number P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest Disclosures
Ronald C. Chen has acted as a paid consultant for Accuray Inc, AbbVie, and Bayer for work performed outside of the current study. Toni K. Choueiri has received grants, personal fees, nonfinancial support, and other support from Bristol‐Myers Squibb, Exelixis Inc, and Pfizer for work performed as part of the current study and has received personal fees for advisory boards, consultation, article preparation, and travel/lodging and clinical trial grants from AstraZeneca, Bristol‐Myers Squibb, Eisai, EMD Serono Exelixis Inc, GlaxoSmithKline, Lilly, Merck, Novartis, and Pfizer for work performed outside of the current study; holds stock in Pionyr and Tempest; and sits on the National Comprehensive Cancer Network kidney panel. In addition, his institution (Dana‐Farber Cancer Institute) may have received additional independent funding from drug companies or/and royalties potentially involved in research around the subject matter. Marion Feuilly and Florence Marteau are employees of Ipsen, which was a sponsor of the current study. Jie Meng and Johanna Lister are employees of Analytica Laser, a Certara company that has received consulting fees from Ipsen Pharma SAS. Michael J. Morris has acted as a member of the advisory boards for Advanced Accelerator Applications, Astellas Pharma, Bayer, Johnson and Johnson and Progenics, for which he has received no compensation; has received research support from Progenics; has received personal fees from Curium and ORIC Pharmaceuticals; and has received research support from Endocyte for work performed outside of the current study. Aaron D. Falchook made no disclosures. Daniel J. George has received grants from Acerta Pharmaceuticals; has acted as a senior editor for the American Association for Cancer Research; has received grants from and acted as a paid consultant for Astellas Pharma; has acted as a paid consultant for AstraZeneca; has received personal fees from Axess Oncology; has received grants, personal fees, and nonfinancial support from Bayer H/C Pharmaceuticals; has received grants and personal fees from Bristol‐Myers Squibb; has received grants from Calithera; has received grants from Capio Biosciences; has received personal fees from EMD Serono; has received grants, personal fees, and nonfinancial support from Exelixis Inc; has acted as a paid consultant for Flatiron, Merck Sharp & Dohme, Michael J Hennessey Associates, Myovant Sciences Inc, Physician Education Resource, Vizuri Health Sciences, and Platform Q; has received personal fees from Ipsen; has received grants and personal fees from Janssen Pharmaceuticals and Novartis and Pfizer; has received personal fees from Millennium Medical Publishing, Modra Pharmaceuticals B.V., NCI Genitourinary, and Nektar Therapeutics; has received grants, personal fees, and nonfinancial support from Sanofi; has received personal fees from UroGPO; has received personal fees and nonfinancial support from UroToday; has received personal fees from Acceleron Pharma Inc, BioPharm, Celgene, Dendreon, Genentech, GlaxoSmithKline, Innocrin Pharma, Medivation, Merck, and Genzyme; and has received research funding from Dendreon, Genentech, Innocrin Pharma, and Millennium for work performed outside of the current study. Darren R. Feldman has received NIH P30 Cancer Center Support Grant P30 CA008748 for work performed as part of the current study and has received research funding from Astellas, Novartis, Seattle Genetics, and Decibel and royalties from UpToDate for work performed outside of the current study. Aaron D. Falchook made no disclosures.
Author Contributions
Ronald C. Chen: Visualization and writing–review and editing. Toni K. Choueiri: Visualization and writing–review and editing. Marion Feuilly: Conceptualization, methodology, visualization, and writing–review and editing. Jie Meng: Formal analysis, investigation, visualization, and writing–review and editing. Johanna Lister: Formal analysis, investigation, visualization, and writing–review and editing. Florence Marteau: Supervision, conceptualization, methodology, visualization, and writing–review and editing. Aaron D. Falchook: Visualization and writing–review and editing. Michael J. Morris: Visualization and writing–review and editing. Daniel J. George: Visualization and writing–review and editing. Darren R. Feldman: Visualization and writing–review and editing.
Supporting information
Supplementary Material
Supplementary Material
Chen RC, Choueiri TK, Feuilly M, Meng J, Lister J, Marteau F, Falchook AD, Morris MJ, George DJ, Feldman DR. Quality‐adjusted survival with first‐line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer. 2020:126:5311‐5318. 10.1002/cncr.33169
We thank all the patients involved in the current study as well as their caregivers and the investigators and research staff at the participating institutions. We thank Isabelle Kaufmann, PhD, of Oxford PharmaGenesis (Oxford, UK) for providing medical writing support, which was sponsored by Ipsen in accordance with Good Publication Practice guidelines.
See referenced original article on pages 5210‐2, this issue.
Data Availability
In cases in which patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.
References
- 1. Cella D, Cappelleri JC, Bushmakin A, et al. Quality of life predicts progression‐free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract. 2009;5:66‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Groot S, Redekop WK, Versteegh MM, et al. Health‐related quality of life and its determinants in patients with metastatic renal cell carcinoma. Qual Life Res. 2018;27:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palapattu GS, Kristo B, Rajfer J. Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol. 2002;4:163‐170. [PMC free article] [PubMed] [Google Scholar]
- 4. Motzer RJ, Jonasch E, Agarwal N, et al. NCCN Guidelines Kidney Cancer, Version 2. 2020. Published August 5, 2019. Accessed December 5, 2019. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- 5. Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2019;30:706‐720. [DOI] [PubMed] [Google Scholar]
- 6. US Food and Drug Administration . Cabozantinib (CABOMETYX). Published April 25, 2016. Accessed May 29, 2020. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/cabozantinib‐cabometyx
- 7. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN Trial [published correction appears in J Clin Oncol. 2017;35:3736] [published correction appears in J Clin Oncol. 2018;36:521]. J Clin Oncol. 2017;35:591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choueiri TK, Hessel C, Halabi S, et al. Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): progression‐free survival by independent review and overall survival update [published correction appears in Eur J Cancer. 2018;103:287]. Eur J Cancer. 2018;94:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Exelixis Inc. Prescribing Information: CABOMETYX® (cabozantinib) tablets, for oral use . Approval: 2012. 2017. Accessed May 29, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208692s002lbl.pdf
- 10. Ipsen . Summary of product characteristics: OPDIVO 10 mg/mL concentrate for solution for infusion. Accessed May 29, 2020. https://www.ema.europa.eu/en/documents/product‐information/opdivo‐epar‐product‐information_en.pdf
- 11. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373:1814‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open‐label, phase 3 trial. Lancet Oncol. 2016;17:917‐927. [DOI] [PubMed] [Google Scholar]
- 13. Solem CT, Kwon Y, Shah RM, Aly A, Botteman MF. Systematic review and benchmarking of Quality‐Adjusted Time Without Symptoms or Toxicity (Q‐TWiST) in oncology. Expert Rev Pharmacoecon Outcomes Res. 2018;18:245‐253. [DOI] [PubMed] [Google Scholar]
- 14. Friedlander M, Gebski V, Gibbs E, et al. Health‐related quality of life and patient‐centred outcomes with olaparib maintenance after chemotherapy in patients with platinum‐sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT Ov‐21): a placebo‐controlled, phase 3 randomised trial. Lancet Oncol. 2018;19:1126‐1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patil S, Figlin RA, Hutson TE, et al. Q‐TWiST analysis to estimate overall benefit for patients with metastatic renal cell carcinoma treated in a phase III trial of sunitinib vs interferon‐α. Br J Cancer. 2012;106:1587‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gelber RD, Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol. 1986;4:1772‐1779. [DOI] [PubMed] [Google Scholar]
- 17. Goldhirsch A, Gelber RD, Simes RJ, Glasziou P, Coates AS. Costs and benefits of adjuvant therapy in breast cancer: a quality‐adjusted survival analysis. J Clin Oncol. 1989;7:36‐44. [DOI] [PubMed] [Google Scholar]
- 18. Beaumont JL, Salsman JM, Diaz J, et al. Quality‐adjusted time without symptoms or toxicity analysis of pazopanib versus sunitinib in patients with renal cell carcinoma. Cancer. 2016;122:1108‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patil S, Figlin RA, Hutson TE, et al. TWiST analysis to estimate overall benefit for metastatic renal cell carcinoma (mRCC) patients (pts) treated in a phase III trial of sunitinib versus interferon‐alfa (IFN‐α) (Abstract 4594). Paper presented at: American Society of Clinical Oncology 2010 Annual Meeting; June 4‐8, 2010; Chicago, IL. [Google Scholar]
- 20. Swinburn P, Lloyd A, Nathan P, Choueiri TK, Cella D, Neary MP. Elicitation of health state utilities in metastatic renal cell carcinoma. Curr Med Res Opin. 2010;26:1091‐1096. [DOI] [PubMed] [Google Scholar]
- 21. Revicki DA, Feeny D, Hunt TL, Cole BF. Analyzing oncology clinical trial data using the Q‐TWiST method: clinical importance and sources for health state preference data. Qual Life Res. 2006;15:411‐423. [DOI] [PubMed] [Google Scholar]
- 22. Shah R, Botteman M, Kwon Y, Gooden KM, Cella D, Motzer R. Quality adjusted time without symptoms of disease progression or toxicity (Q‐TWiST) of nivolumab plus ipilimumab (NIVO+IPI) vs sunitinib (SUN) among untreated advanced renal cell carcinoma (ARCC) patients (patients) with intermediate or poor prognostic risk [abstract]. Value Health. 2019;22(suppl 2):S106. Abstract PCN264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
In cases in which patient data can be anonymized, Ipsen will share all individual participant data that underlie the results reported in this article with qualified researchers who provide a valid research question. Study documents, such as the study protocol and clinical study report, are not always available. Proposals should be submitted to DataSharing@Ipsen.com and will be assessed by a scientific review board. Data are available beginning 6 months and ending 5 years after publication; after this time, only raw data may be available.


