Abstract
The aim of this study was to evaluate the presence and characterization of chemotherapy‐induced neuropathy (CIPN) and neuropathic pain 5 years after adjuvant chemotherapy with docetaxel or oxaliplatin. Patients from an ongoing prospective study, who had received adjuvant chemotherapy with docetaxel or oxaliplatin in 2011 to 2012 were invited to participate. The patients underwent a thorough examination with interview, neurological examination, questionnaires, assessment tools, nerve conduction studies (NCS), quantitative sensory testing, MScan motor unit number estimation (MUNE), and corneal confocal microscopy (CCM). Patients were divided into no, possible, probable, and confirmed CIPN. Out of the 132 eligible patients, 63 agreed to participate: 28 had received docetaxel and 35 had received oxaliplatin. Forty‐one percent had confirmed CIPN, 34% possible or probable CIPN, and 22% did not have CIPN. The CIPN was characterized mainly by sensory nerve fiber loss, with a more pronounced large fiber than small fiber loss but also some motor fiber loss identified on NCS and MUNE. In general, patients had mild neuropathy with relatively low scores on assessment tools and no association with mood and quality of life. CCM was not useful as a diagnostic tool. Of the patients with probable or confirmed CIPN, 30% experienced pain, which was most often mild, but still interfered moderately with daily life in 20% to 25% and was associated with lower quality of life. In conclusion CIPN was confirmed in 41% 5 years after chemotherapy. The neuropathy was generally mild, but in patients with neuropathic pain it was associated with lower quality of life.
Keywords: assessment tools, chemotherapy, corneal confocal microscopy, large fiber neuropathy, motor unit number estimation, nerve conduction study, neuropathic pain, neuropathy, pain, small fiber neuropathy
1. INTRODUCTION
Chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most disabling long‐term consequences of taxanes and platinum compounds. CIPN is a distal, symmetric, mainly sensory neuropathy, with a stocking‐and‐glove‐distribution. 1 The incidence of CIPN increases with cumulative dosing and often results in a reduction or termination of the chemotherapy dose. 2 , 3
The pathophysiology of chronic CIPN is complex and not well understood. It predominantly affects sensory function, but little is known about the relationship between loss and gain of large and small sensory fiber function and whether motor fibers are affected. 4 , 5 , 6 , 7 Scales used for CIPN focus on a clinical grading of the severity of patient symptoms. 8 , 9 , 10 A detailed and well‐validated scale is the total neuropathy score (TNS) that combines the patient's subjective symptoms with the clinical signs. 11 , 12 , 13 The Common Terminology Criteria for Adverse Events (CTCAE) screening tool, 14 is commonly used, but it is a crude grading system. 15 , 16 There is an increased awareness of the need for multimodal assessment tools to accurately diagnose and characterize CIPN. 4 , 17
No screening tools have been developed for detecting the presence or absence of CIPN. Despite the similarity between CIPN and other polyneuropathies, such as painful diabetic polyneuropathy, 18 the screening tools commonly used for diabetes, such as the grading system proposed by the Toronto Diabetic Neuropathy Expert group, 19 the Michigan Neuropathy Screening Instrument (MNSI), 20 and the Toronto Clinical Scoring System (TCSS), 21 are not used for diagnosing CIPN.
Several techniques are available to classify and characterize peripheral neuropathy. These include clinical assessment and structural and electrophysiology tests such as skin biopsy for intraepidermal nerve fiber density calculation, 22 corneal confocal microscopy (CCM), 23 nerve conduction studies (NCS), 4 , 24 and the newer MScanfit motor unit number estimation (MUNE) method. 25 , 26
There is a need for a detailed assessment of large and small nerve fiber function as well as a quantification of motor function after chemotherapy. 17 We therefore conducted a deep‐profile study of patients 5 years after receiving chemotherapy with docetaxel or oxaliplatin using a wide scale of assessment tools to determine the pattern of their neuropathy and neuropathic pain and the effect of quality of life and psychological functioning. The study is part of a 5‐year follow‐up of a prospective questionnaire study. 27 , 28
2. METHODS AND MATERIALS
Patients were recruited from a prospective questionnaire study that included patients scheduled for adjuvant docetaxel for high‐risk breast cancer (n = 100) or adjuvant oxaliplatin for high‐risk colorectal cancer (n = 74) at Aarhus University Hospital, Denmark from 2011 to 2012. 27 At the 5‐year follow‐up, 80 of 94 eligible patients (85%) treated with docetaxel and 52 of 57 eligible patients (91%) treated with oxaliplatin answered a questionnaire about symptoms of neuropathy, these results are published elsewhere. 28 These patients were invited to participate in this study, which was carried out from 2016 to 2017. The control study participants used in this study were local age‐matched individuals recruited from other outgoing studies at the center. One hundred study participants without chronic pain, neurological disorders, or signs of neuropathy (mean age 61.2 years, SD 10.9) served as controls for the CCM) and 32 healthy subjects (mean age 63.4 years, SD 8.3) as controls for the MScan MUNE.
2.1. Interview and neurological examination
An interview was done with questions focusing on sensory and motor symptoms of polyneuropathy, pain, other diseases, and medicine consumption. A comprehensive structured upper and lower limb neurological examination was performed. 29 Pain and sensory abnormalities to pinprick (Owen Mumford Neuropen with sterile neurotips and Semmes‐Weinstein monofilament No. 5.88, Stoelting, Wood Dale, IL), light touch (SENSELab Brush‐05; Somedic AB, Sweden), and warm (38°C) and cold (25°C) thermal rollers (Somedic Rolltemp, Somedic AB, Sweden) were mapped on a body chart of upper and lower extremities.
2.2. Questionnaires
In this study, we focused on the questionnaires regarding pain, neuropathy, mental health, and quality of life. Patients completed the Douleur Neuropathique 4 Questions questionnaire (DN4q). 30 Pain descriptors were assessed using the Neuropathic Pain Symptom Inventory (NPSI). 31 Pain interference was assessed using the Patient‐Reported Outcomes Measurement Information System (PROMIS) Pain Interference. 32 The scores were converted into T‐scores, which are standardized relative to an American/US reference population 32 , 33 and categorized according to the severity thresholds in cancer. 32 , 33 , 34 Sleep disturbance and symptoms of fatigue, anxiety, and depression were assessed using the PROMIS Short Form 6a. 32 Quality of life (QoL) score was assessed with the EQ‐5D (EuroQol Group 1995).
2.3. Quantitative sensory testing
Sensory testing was performed unilaterally on the dorsum of the foot using the German Research Network on Neuropathic Pain (DFNS) QST protocol. 35 Standardized equipment and verbal introduction were used and our center is certified to do the procedures of the DFNS protocol. Twelve out of 13 different parameters were assessed; only wind‐up ratio (WUR) was not examined and the stimulus/response function was shortened from 50 to 20 stimuli.
2.4. Neurophysiological examinations
NCS were recorded from the median sensory and motor nerves, the peroneal motor nerve and the tibial motor nerve, and bilaterally on the sural sensory nerve including the distal part of the nerve (dorsal sural nerve) with the Keypoint.net EMG machine. If a median entrapment neuropathy was identified the ulnar nerve was examined. For estimation of abnormalities in the dorsal sural sensory nerve, recordings were compared to normative control data collected at Aarhus University Hospital, Denmark. 36 Other NCS measures were compared to those of laboratory controls using z‐scores, taking into account height and age. Skin temperature had to be above 32°C. One abnormal parameter in at least two nerves, one being the sural nerve, had to be abnormal for the minimum case definition of a length‐dependent large fiber neuropathy. 37 The predetermined protocol of DOLORisk protocol did not include examination of the dorsal sural nerve, but was included here because studies have found it to be an early biomarker to identify CIPN. 3 , 38
To examine early signs of motor nerve damage MScanFit MUNE (MScan), a detailed stimulus response curve was performed on the median motor nerve at the wrist using a computerized program (TRONDNF, Institute of Neurology, London, UK). 25
2.5. Corneal confocal microscopy
CCM was used to analyze the density of the small fibers in the cornea. A trained investigator scanned both eyes with the Heidelberg Retina Tomograph III laser‐scanning confocal microscope (Heidelberg Engineering GmbH, Heidelberg, Germany). Fibers were counted automatically and compared to an age and gender matched control group. 23
2.6. Definition of polyneuropathy
The predefined case definition of polyneuropathy was the definition proposed by the Toronto Diabetic Neuropathy Expert Group of “possible”, “probable”, and “confirmed” polyneuropathy (Table S1). 19 , 39 Patients with an abnormal NCS or thermal detection threshold on QST without any symptoms and signs were diagnosed with subclinical neuropathy.
2.7. Neuropathy assessment tools
Patients completed the Michigan Neuropathy Scoring Instrument questionnaire part (MNSIq). A cut‐off ≥4/13 abnormal responses has been suggested as the cut‐off to define polyneuropathy. 20 The Toronto Clinical Scoring System (TCSS) 21 and the TNS clinical version (TNSc) 13 and reduced version (TNSr) 12 were used to grade the severity of neuropathy. A sensory version of the CTCAE v5 14 was performed by the primary investigator.
2.8. Grading neuropathic pain
Neuropathic pain was graded as “possible”, “probable”, or “definite” in accordance with the NeuPSIG grading system. 40
2.9. Standard protocol approvals, registrations, and patient consents
The study was approved by the Danish Data Protection Agency (No. 1‐16‐02‐89‐16), the Central Denmark Region Committees on Health Research Ethics (No. 1‐10‐72‐359‐15), and the Danish Health and Medicines Authority (No. 3‐3013‐605/1/). The study was registered in clinicaltrials.gov (NCT02654691). All participants provided written informed consent. The study is part of the EU project DOLORisk. 29
2.10. Statistical analysis
As we expected the relation between symptoms and signs and nerve function to be similar across the two patient groups, our primary analyses are for the two patient groups combined. Statistical analysis was performed with STATA version 14.2. Means were presented with SD (SD) and medians with a 25% to 75% percentile (IQR). Normally distributed data were analyzed with student's t test and otherwise with Mann–Whitney or Kruskal–Wallis (rank sum) tests. Binary data were analyzed with Fisher's exact test. Analysis of variance (ANOVA) between more than two groups was tested using one‐way ANOVA. Test for trends were done with linear regression and adjusted for possible confounders. Data from the QST were analyzed with the standardized program Equista, version 1.3.5. A z‐score was calculated as described in earlier studies compared to a normal control group and adjusted for test site, age, and gender. 35 , 41 Correlation was tested with Spearman's rho. Missing data was not replaced.
3. RESULTS
3.1. Study participants
Of the 132 eligible patients, 63 (47.7%) accepted to participate in the clinical study; 28 patients had received docetaxel and 35 oxaliplatin. Demographics are presented in Table 1. There were no statistical significant differences in clinical characteristics and the percentage reporting symptoms of neuropathy in the questionnaire study, 28 between those who participated in the clinical study and those who did not, except for the number of patients with diabetes in patients treated with docetaxel (3/28 participating and 0/52 not participating) (Table S2).
TABLE 1.
Patient characteristics
| Docetaxel (breast cancer) (n = 28) | Oxaliplatin (colorectal cancer) (n = 35) | P value | |
|---|---|---|---|
| Age at follow‐up, years, mean (SD) | 57.0 (7.2) | 68.9 (6.6) | < .001 |
| Age at first cycle of chemotherapy, years, mean (SD) | 52.2 (7.2) | 63.9 (6.6) | < .001 |
| Mean time since first treatment, years, mean (SD) | 4.8 (0.4) | 5.1 (0.4) | .01 |
| Females, No. (%) | 28 (100.0) | 12 (34.3) | < .001 |
| Height, cm, mean (SD) | 167.4 (5.1) | 173.7 (8.0) | < .001 |
| Weight, kg, mean (SD) | 78.8 (17.1) | 79.2 (12.4) | .91 |
| Body mass index (kg/m2), mean (SD) | 28.0 (5.6) | 26.3 (4.0) | .15 |
| Diabetes, No. (%) | 3 (10.7) | 3 (8.6) | 1.00 |
| Cumulative dose (mg), mean (SD) | 580.3 (186.3) | 1150.7 (435.3) | < .001 |
| Cumulative dose/body area (mg/m2), mean (SD) | 306.6 (89.5) | 608.1 (227.5) | < .001 |
| Endocrine therapy, No. (%) | 19 (67.9) | ||
| Smoking, No. (%) | 1.00 | ||
| Yes, No. (%) | 3 (10.7) | 3 (8.8) | |
| Never, No. (%) | 12 (42.9) | 14 (41.2) | |
| Former smoker, No. (%) | 13 (46.4) | 17 (50.0) | |
| Alcohol above >7/14 units for women/men, No. (%) | 4 (15.4) | 5 (15.6) | 1.00 |
3.2. Grading of CIPN
We found that 26 (41.3%) patients had confirmed CIPN (62.8% of oxaliplatin and 14.3% of docetaxel treated patients), 15 (23.8%) probable CIPN, 6 (9.5%) possible CIPN, 14 (22.2%) no CIPN, and 2 patients had subclinical PNP (Table S3). Of the 26 patients with confirmed CIPN, 6 (23%) had mixed small and large fiber neuropathy based on NCS including the dorsal sural nerve and thermal detection thresholds, 15 (58%) had pure large fiber neuropathy and 5 (8%) had pure small fiber neuropathy (Table S3). Of the 21 patients with abnormal NCS, 9 had abnormal NCS results only when the dorsal sural nerve assessment was included, of which 4 had abnormal cold or warm sensation (Table S3). When including the dorsal sural nerve only 5/63 (7.9%) had small fiber loss only, compared to 9/63 (14.3%) when grading without the dorsal sural nerve. Table S4 shows the mean values of the amplitude and velocity in the four groups.
All patients with symptoms of polyneuropathy had onset during chemotherapy treatment or within months after receiving chemotherapy, including the four with diabetes. Seven patients had comorbidities that could give symptoms in the feet: four had a history with a lumbar disc prolapse or sciatica, one a brain hemorrhage, one sarcoidosis, and one a deep vein thrombosis. Five of these patients had symptoms and signs of polyneuropathy that were consistent with CIPN on clinical examination and confirmed with NCS in four patients. One patient with confirmed neuropathy had relapse and re‐induction of chemotherapy, and one patient with probable CIPN, had recently deceived 1 cycle of oxaliplatin due to a second cancer. No other patients had metastases (including spinal metastases) to our knowledge. Symptoms in the form of tingling or numbness in the feet at baseline was reported by eight patients, seven of which had confirmed polyneuropathy (Table S3).
3.3. Assessment tools
All of the patients with polyneuropathy had grade 1 to 2 sensory symptoms on the CTCAE v5. 14 The results for the MNSIq, TCSS, TNSc, and TNSr are presented in Figure 1 and Table S3. In general, patients had mild neuropathy with relatively low scores on all tools (Figure 1). For all of the four assessment tools, there was a significant correlation between the certainty of neuropathy and the score, but many patients with confirmed CIPN had scores below the cut‐off for neuropathy on the MNSIq and the TCSS (Figure 1). The highest observed correlations were for the TCSS and the TNSr (Figure 1). There was no difference in MNSIq, TCSS, TNSc, or TNSr scores in patients who had received docetaxel compared to oxaliplatin P > .06.
FIGURE 1.
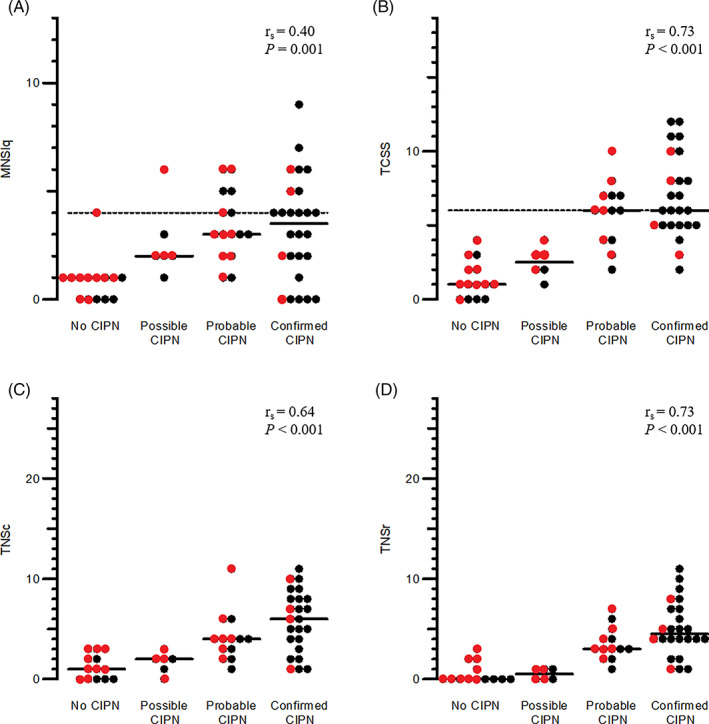
Total scores of the questionnaire Michigan Neuropathy Screening Instrument questionnaire (MNSIq), the Toronto Clinical Scoring System (TCSS), and the total neuropathy score (TNS)Filled line median score, dashes lines cut‐point between no CIPN and mild CIPN.Red dots: docetaxel; black dots: oxaliplatin rs, Spearman's Rho
3.4. Bedside and quantitative sensory testing
The CIPN was characterized mainly by sensory loss, in particular to large fiber functions. Compared with patients without CIPN, patients with confirmed CIPN had increased cold (ie, decreased cold detection temperature), warm, mechanical detection and vibration detection thresholds and more often paradoxical heat sensations, whereas gain of function was present in only a few patients (Figures 2A and 3 and Figure S1). This was consistent with the bedside sensory testing, where none of the patients had pinprick hyperalgesia or dynamic mechanical, cold or warm allodynia on the feet, legs, arms, or hands.
FIGURE 2.
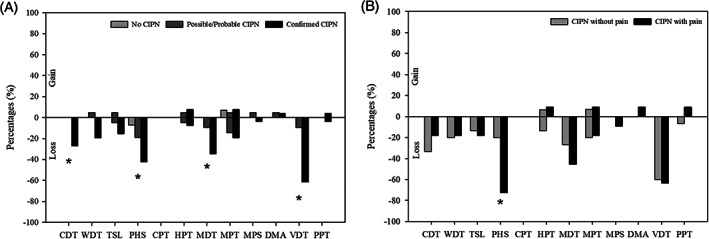
Quantitative sensory testing showing loss or gain of function (z‐scores outside the 95% confidence interval of the DFNS reference database) on the 12 parameters. The comparisons were done between the groups with A, no CIPN, possible/probable CIPN, and confirmed CIPN and B, confirmed CIPN with and without neuropathic pain. Patients with subclinical neuropathy were excluded. * P < .05. CDT, cold detection threshold; CIPN, chemotherapy‐induced peripheral neuropathy; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PPT, pain pressure threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold
FIGURE 3.
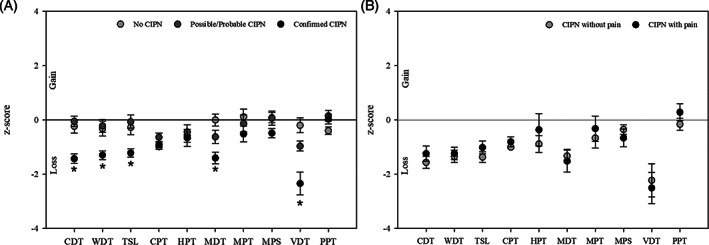
Quantitative sensory testing showing z‐scores indicating loss or gain in function on the 10 parameters. The comparisons were done between the groups with A, no CIPN, possible/probable CIPN, and confirmed CIPN and B, Confirmed CIPN with and without neuropathic pain. Patients with subclinical neuropathy are excluded (n = 2). CDT, cold detection threshold; CIPN, chemotherapy‐induced peripheral neuropathy; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; PHS, paradoxical heat sensation; PPT, pain pressure threshold; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold. * P value < .05
The quantitative sensory testing (QST)‐based vibration detection thresholds, which can be used bedside, were abnormal in 72.7% of the patients with an abnormal NCS including the dorsal sural nerve (Table S3). The vibration test had a sensitivity of 72.7% (49.8%‐89.3%), specificity of 95.0% (83.1%‐99.4%), positive predictive value of 88.9% (65.3%‐98.6%), and negative predictive value of 86.4% (72.6%‐94.8%) for identifying large fiber polyneuropathy confirmed with NCS including the dorsal sural nerve. There was a poor agreement between abnormal thermal detection and mechanical pain thresholds (Table S3).
3.5. MScan MUNE
Despite a similar size of the CMAP amplitude (P > .34) (Figure 4A), the motor unit counts were lower (Figure 4B) and the motor units larger (Figure 4C) in patients with abnormal NCS than in healthy controls, indicating nerve damage with subsequent compensation in the form of nerve collateral sprouting. There were, however, no significant differences between patients with large fiber neuropathy and those without, also not when dividing them into types of chemotherapy. There was no correlation between severity (TNS) and MUNE when using Spearman's rho. Data from each patient are shown in Table S3. We excluded five patients who had received oxaliplatin and two who had received docetaxel due to carpal tunnel syndrome. The patients with subclinical PNP were also excluded, and seven patients did not which to participate due to discomfort.
FIGURE 4.
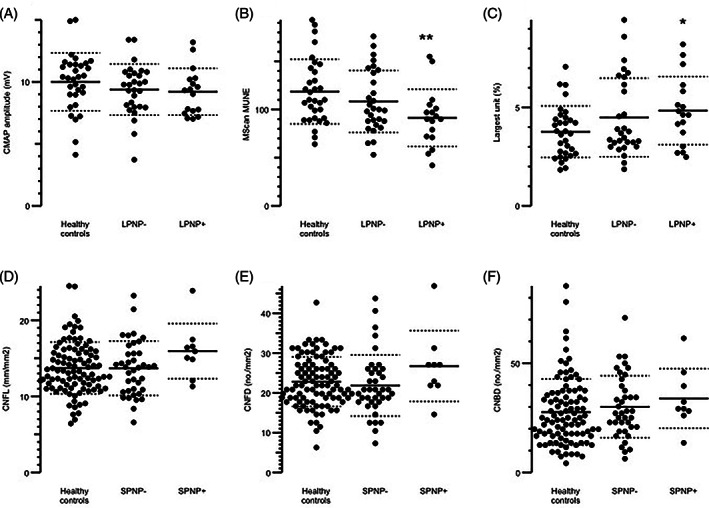
Mscan parameters A‐C, and corneal confocal microscopy (CCM) parameters D‐F. MScan parameters were compared between patients with large fiber polyneuropathy (LPNP+), patients without large fiber polyneuropathy (LPNP‐), and local age‐matched healthy controls. A, The compound muscle action potential (CMAP) amplitude, B, Motor unit number estimations (MScan MUNE), C, The Largest unit (%). CCM parameters were compared between patients with no small fiber loss (SPNP‐), with small fiber loss (SPNP+), and local age‐matched controls in D, Cornea nerve fiber length (CNFL), E, Cornea nerve branch density (CNBD), and F, Cornea nerve fiber density (CNFD. Patients were excluded from the MScan evaluation if they had carpal tunnel syndrome (n = 7) or did not wish to participate (n = 9). CCM was performed on 53 of the participants; 10 did not wish to participate, and two patients had a subclinical CIPN and were not included here. Horizontal lines indicate means, and dashed lines SDs. * P = .018 between healthy controls and LPNP+, ** P = .006 between healthy controls and LPNP+
3.6. Corneal confocal microscopy
There were no significant differences in CNFL, CNFD, or CNBD between the four polyneuropathy groups (P > .16) and also no differences between those with and without small fiber polyneuropathy (defined as increased CDT or WDT) (P > .15) and the results were within the normal range of a local reference group (Figure 4D‐F). There were no significant difference in CNFL, CNFD, or CNBD between oxaliplatin and docetaxel (P > .34). Also, there were no correlations between any of the CCM parameters and the CDT or WDT with Spearman's rho ranging from −0.14 to 0.24.
3.7. Mental health and QoL
There were no significant differences in sleep impairment, symptoms of anxiety, depression, and sleep, or QoL between the four groups when adjusting for age, gender, and type of chemotherapy (P > .18) (Table 2). All of the T‐score means were below the thresholds for mild symptoms or impairment. 32 , 33
TABLE 2.
Characteristics of Mental Health Between the Different CIPN Groups
| No CIPN (n = 16) | Possible CIPN (n = 6) | Probable CIPN (n = 15) | Confirmed CIPN (n = 26) | P value | ||
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Docetaxel, No. (%) | 11 (39.3) | 4 (14.3) | 9 (32.1) | 4 (14.3) | ||
| Oxaliplatin, No. (%) | 5 (14.3) | 2 (5.7) | 6 (17.1) | 22 (62.9) | .23 | |
| PROMIS depression, T‐score, mean (SD) | 47.5 (11.1) | 44.5 (7.9) | 45.4 (8.5) | 46.7 (9.1) | .38 | |
| docetaxel, T‐score, mean (SD) | 48.2 (11.8) | 45.8 (9.5) | 48.7 (9.1) | 58.0 (11.4) | .22 | |
| oxaliplatin, T‐score, mean (SD) | 46.1 (10.6) | 41.8 (4.8) | 40.4 (4.9) | 44.6 (7.1) | .63 | |
| Symptoms of depression, mild–severe, No. (%) | 5 (31.2) | 1 (16.7) | 3 (20.0) | 3 (11.5) | ||
| PROMIS anxiety, T‐score, mean (SD) | 48.4 (11.6) | 43.6 (8.2) | 49.4 (6.9) | 48.3 (8.9) | .76 | |
| docetaxel, T‐score, mean (SD) | 48.4 (11.6) | 45.9 (9.6) | 48.4 (7.6) | 55.0 (11.4) | .51 | |
| oxaliplatin, T‐score, mean (SD) | 48.3 (12.9) | 39.1 (0.0) | 50.9 (5.8) | 47.0 (8.1) | .84 | |
| Symptoms of anxiety, mild–severe, No. (%) | 4 (25.0) | 1 (16.7) | 2 (13.3) | 5 (19.2) | ||
| PROMIS sleep, T‐score, mean (SD) | 50.1 (6.4) | 44.8 (12.3) | 51.4 (5.0) | 48.4 (7.3) | .65 | |
| docetaxel, T‐score, mean (SD) | 51.2 (6.8) | 44.9 (11.9) | 52.5 (4.8) | 51.7 (8.2) | .57 | |
| oxaliplatin, T‐score, mean (SD) | 47.6 (4.8) | 44.5 (18.1) | 49.8 (5.1) | 47.8 (7.1) | .80 | |
| Sleep impairment, mild–severe, No. (%) | 4 (25.0) | 2 (33.3) | 3 (20.0) | 7 (26.9) | ||
| PROMIS fatigue, T‐score, mean (SD) | 48.5 (9.3) | 47.0 (9.7) | 52.4 (8.3) | 48.0 (9.1) | .18 | |
| docetaxel, T‐score, mean (SD) | 50.8 (8.3) | 48.0 (11.4) | 52.2 (6.2) | 57.2 (13.7) | .22 | |
| oxaliplatin, T‐score, mean (SD) | 43.6 (10.4) | 45.0 (8.3) | 52.8 (11.5) | 46.4 (7.3) | .58 | |
| Symptoms of fatigue, mild–severe, No. (%) | 6 (37.5) | 2 (33.3) | 8 (53.3) | 9 (34.6) | ||
| Quality of life EQ5D VAS, median (IQR) | 80.0 (79.2; 90.0) | 89.0 (85.0; 94.0) | 77.0 (60.0; 85.0) | 87.0 (69.0; 90.0) | .18 | |
| docetaxel, median (IQR) | 80.0 (79.0; 90.0) | 85.0 (85.0; 89.0) | 79.0 (62.5; 82.5) | 68.0 (38.5; 89.2) | .22 | |
| oxaliplatin, median (IQR) | 80.0 (77.5; 97.5) | 94.0 (86.5; 99.0) | 73.0 (54.5; 90.0) | 88.0 (69.0; 90.0) | .58 | |
| Confirmed CIPN without pain(n = 15) | Confirmed CIPN with pain(n = 11) | P value | ||||
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Docetaxel, No. (%) | 1 | 3 | ||||
| Oxaliplatin, No. (%) | 14 | 8 | .055 | |||
| PROMIS depression, T‐score, mean (SD) | 44.4 (7.3) | 49.8 (10.6) | .44 | |||
| Symptoms of depression, mild–severe, No. (%) | 1 (6.7) | 2 (18.2) | ||||
| PROMIS anxiety, T‐score, mean (SD) | 45.9 (7.8) | 51.4 (9.6) | .35 | |||
| Symptoms of anxiety, mild–severe, No. (%) | 1 (6.7) | 4 (36.4) | ||||
| PROMIS sleep, T‐score, mean (SD) | 46.0 (6.3) | 51.6 (7.4) | .16 | |||
| Sleep impairment, mild–severe, No. (%) | 3 (20.0) | 4 (36.4) | ||||
| PROMIS fatigue, T‐score, mean (SD) | 43.7 (6.9) | 53.9 (8.6) | .002 | |||
| Symptoms of fatigue, mild–severe, No. (%) | 2 (13.3) | 4 (63.6) | ||||
| Quality of life EQ5DVAS, median (IQR) | 90.0 (80.0; 90.0) | 69.0 (49.0; 87.0) | .005 | |||
Note: P value adjusted for age, gender, and if relevant type of chemotherapy.
Abbreviations: PROMIS, Patient‐Reported Outcome Measurement Information System; IQR, interquartile range.
3.8. Neuropathic pain
Of the 26 patients with confirmed CIPN, 42.3% (11/26) had painful CIPN and 53.3% (8/15) of patients with probable CIPN had painful CIPN. Among patients treated with oxaliplatin, 8/22 (36%) with definite CIPN had neuropathic pain and 3/6 (50%) with probable CIPN had neuropathic pain. Among patients treated with docetaxel, 3/4 (75%) with definite CIPN had neuropathic pain and 6/9 (67%) with probable CIPN had neuropathic pain (Table S3). The mean pain intensity was mild to moderate, and in most patients the pain interfered only mildly with their daily life (Table 3). However, 25% of patients with probable neuropathic pain and 18% of patients with definite neuropathic pain had at least moderate interference and patients with painful confirmed CIPN had a significantly lower QoL score and felt more fatigue than patients with confirmed CIPN without pain (P = .002 and P = .005) (Table 2). We also saw no differences between neuropathy groups, when looking separately in the two types of chemotherapy (Table 2). There was no difference in the QST profile among patients with and without pain except that the painful CIPN group had more paradoxical heat sensation than the pain‐free CIPN group (Figure 2B).
TABLE 3.
Pain Characteristics
| Grade of CIPN: | Probable | Confirmed |
|---|---|---|
| Patients with pain: | (n = 8) | (n = 11) |
| NPSI | ||
| Burning pain (0‐10), mean (SD) | 1.4 (2.2) | 3.8 (3.7) |
| Pressing pain (0‐10), mean (SD) | 2.1 (3.2) | 2.8 (3.0) |
| Paroxysmal pain (0‐10), mean (SD) | 0.9 (1.4) | 2.4 (2.9) |
| Evoked pain (0‐10), mean (SD) | 0.8 (0.9) | 2.5 (2.6) |
| provoked by brushing (0‐10), mean (SD) | 0.2 (0.7) | 2.2 (2.8) |
| provoked by pressure (0‐10), mean (SD) | 0.9 (1.1) | 3.2 (3.5) |
| provoked by contact with cold (0‐10), mean (SD) | 1.2 (2.1) | 2.1 (3.3) |
| Pins and needles/tingling, mean (SD) | 3.4 (3.3) | 3.2 (2.7) |
| Total sum of NPSI (0‐100), mean (SD) | 16.6 (17.3) | 28.1 (22.7) |
| PROMIS Pain, T‐score, mean (SD) | 54.4 (6.3) | 55.3 (6.3) |
| Pain interference, mild, No. (%) | 5 (62.5) | 8 (72.7) |
| Pain interference, moderate, No. (%) | 2 (25.0) | 2 (18.2) |
| Average pain intensity in the last 24 hours, mean (SD) | 2.2 (2.0) | 4.1 (3.0) |
| Average pain intensity in the last 7 days, mean (SD) | 2.5 (2.2) | 4.5 (2.7) |
| Analgesics, No. (%) | 0 (0.0) | 3 (27.3) |
Note: P values were calculated with student's t test.
Abbreviations: CIPN, chemotherapy‐induced peripheral neuropathy; NPSI, Neuropathic Pain Symptom Inventory.
4. DISCUSSION
We conducted detailed phenotyping of consecutive patients participating in a prospective study 27 who had received adjuvant docetaxel or oxaliplatin 4 to 5 years before. Of the 63 participating patients, 22% (14/63) did not have any signs or symptoms of neuropathy, 10% (6/63) had possible, 24% (15/63) probable, and 41% (26/63) had confirmed CIPN with abnormal NCS or cold or warm detection thresholds.
Patients generally had mild to moderate neuropathy. The median TCSS score was 6 for confirmed CIPN and the median score were 6 for TNSc and 4.5 for the TNSr. The neuropathy was characterized by sensory loss. Of the 26 patients with confirmed CIPN 81% had a large sensory fiber loss, but 29% had small fiber loss as identified with decreased sensation to cold and warm on QST. Additionally, 27% had abnormal paradoxical heat sensations, which are suggested to be an early sign of small fiber loss. 42 , 43 The neuropathy was classified as sensory or mixed sensory and motor neuropathy by the NCS‐recordings. Using the new method of Mscan MUNE, we found some signs of motor unit loss with subsequent nerve reinnervation in the upper extremities despite a lack of motor symptoms. Consistent with other studies, 44 , 45 there were no clear associations between QoL, symptoms of depression, anxiety, and fatigue and grading of neuropathy.
Many more patients had confirmed CIPN in the oxaliplatin group than in the docetaxel group. Coasting has been shown to worsen the sensory neuropathic symptoms of oxaliplatin after ended treatment, 46 this phenomenon is not described in docetaxel treated patients, but the few studies looking at the development of symptoms of polyneuropathy after docetaxel have shown that the symptoms persist in 32% to 42% of the patients. 28 , 47 , 48
Of the 41 patients with probable or confirmed CIPN, 30% had neuropathic pain in the feet (painful CIPN). In general, the pain intensity was mild with only mild interference with daily life, but 20% to 25% reported that the pain moderately interfered with their daily lives. The patients with painful confirmed CIPN also felt more fatigue and had a lower quality of life than those without pain. Clinical and quantitative sensory testing showed sensory loss and no evoked pain but some did report some evoked pain on the NPSI. Patients with confirmed CIPN and pain had similar sensory profiles as patients without pain except for a higher prevalence of paradoxical heat sensations, which suggests a more severe loss of small‐fiber function. 42 , 43
Symptom and sign scores using the different scales (MNSIq, TCSS, TNSc, and TNSr) increased with increasing grading of neuropathy, but the MNSIq and TCSS only had a score suggestive of neuropathy in around half of patients with confirmed neuropathy. Our findings emphasize the need for better screening and assessment tools for CIPN. 15 We found that more patients were diagnosed with confirmed CIPN when using the dorsal sural nerve than standard NCS alone. This is in agreement with previous studies suggesting that including assessment of the dorsal sural nerve improves the sensitivity of neurophysiological examination of peripheral neuropathy, which affects the most distal sensory fibers first. 38 , 49
In this study, we did not take a skin biopsy to estimate the intraepidermal nerve fiber density considered the gold standard for assessing small fiber neuropathy, but we used cold and warm detection thresholds assessed during QST. The CCM method is a suggested method to assess small fiber function in patients with diabetic neuropathy, 50 but its usefulness has been questioned. 51 , 52 We did not find that CCM measure was useful in diagnosing CIPN. This is in contrast to another small study on oxaliplatin, where there was a decrease in number of fibers and length density after four cycles. 53 For bedside testing, vibration detection threshold might be a useful screening tool, 54 which supports the use of vibration sense as a bedside test and early predictor of CIPN. 3 , 17 , 55 There was a poor agreement between abnormal thermal detection and mechanical pain thresholds, and more studies are needed to identify reliable bedside tests to assess small fiber function.
The strength of this study was that all patients from a prospective study of consecutive patients were invited to participate, the long‐term follow‐up, and that those participating were similar with respect to demographics and symptoms of neuropathy from the questionnaires study as those who did not. Also, the examination was thorough including a wide range of techniques assessing symptoms and signs and function of small and large sensory and motor fibers. It is a limitation that we did not do a clinical examination and NCS at baseline. The fact that 7 out of 26 patients with confirmed neuropathy had symptoms of numbness or tingling in the feet at baseline despite reporting that the symptoms of neuropathy started after starting chemotherapy suggests that a subgroup may have had worsening of a pre‐existing neuropathy. Also, we did not excluded if patients had developed polyneuropathy because of other causes than chemotherapy, such of hypothyroidism, vitamin B12 vitamin deficiency, or amyloidosis. Also, the small number of subjects is a limitation, particular when separating the patients depending on type of chemotherapy. However, despite a difference in symptoms in the acute phase, the symptoms and signs of chronic neuropathy are similar between docetaxel and oxaliplatin, supporting the decision to merge the data from patients, who had received docetaxel and oxaliplatin. 28 , 42
Using a multimodal testing in a representative group of consecutive patients treated with docetaxel and oxaliplatin, we provide a detailed characterization of long‐term CIPN. Confirmed CIPN was present in 41%, of these 42% had neuropathic pain. The neuropathy was generally mild to moderate and affected sensory, particular large fibers, and to a minor degree motor nerve fibers. The study emphasizes the need for better bedside screening and assessment tools to determine the presence of neuropathy.
Supporting information
FIGURE S1 Quantitative sensory testing showing z‐scores indicating loss or gain in function on the 10 parameters. The comparisons were done between confirmed CIPN in patients who had received A) docetaxel and B) oxaliplatin. . Patients with subclinical neuropathy are excluded (n = 2).
Abbreviations: CIPN, chemotherapy‐induced peripheral neuropathy; CDT, cold detection threshold; WDT, warm detection threshold; TSL, thermal sensory limen; threshold; MDT, mechanical detection threshold; MPT, mechanical pain threshold; MPS, mechanical pain sensitivity; VDT, vibration detection threshold; PPT, * P value <0.05
Supplemental Table S1. Case definition of polyneuropathy
Supplemental Table S2 Patient Characteristics and Questionnaire Results of Participants and Non‐Participants
Supplemental Table S3 Grading of Chemotherapy‐Induced Peripheral Neuropathy (CIPN) and Scores from Assessment Tools
Supplemental Table S4 Mean values of amplitude and velocity in measured nerves divided into groups of polyneuropathy
ACKNOWLEDGEMENT
The authors would like to thank Helle O. Andersen for secretarial and language assistance and Bente M. Christensen and Rud B. Andersen for help with clinical examinations. Also thanks to all the patients who took the time to answer the questionnaire and participate in the clinical examination.
Bennedsgaard K, Ventzel L, Andersen NT, et al. Oxaliplatin‐ and docetaxel‐induced polyneuropathy: clinical and neurophysiological characteristics. J Peripher Nerv Syst. 2020;25:377–387. 10.1111/jns.12413
Funding information Aarhus Universitet; Horizon 2020 Framework Programme, Grant/Award Number: 633491; Innovative Medicines Initiative Joint Undertaking, Grant/Award Number: 116007
REFERENCES
- 1. Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy‐induced peripheral neuropathy. Semin Oncol. 2006;33:15‐49. [DOI] [PubMed] [Google Scholar]
- 2. Andre T, Vernerey D, Mineur L, et al. 3 versus 6 months of oxaliplatin‐based adjuvant chemotherapy for patients with stage III colon cancer: disease‐free survival results from a randomized, open‐label, international duration evaluation of adjuvant (IDEA) France, phase III trial. J Clin Oncol. 2018;36:1469‐1477. [DOI] [PubMed] [Google Scholar]
- 3. Velasco R, Bruna J, Briani C, et al. Early predictors of oxaliplatin‐induced cumulative neuropathy in colorectal cancer patients. J Neurol Neurosurg Psychiatry. 2014;85:392‐398. [DOI] [PubMed] [Google Scholar]
- 4. Kandula T, Farrar MA, Kiernan MC, et al. Neurophysiological and clinical outcomes in chemotherapy‐induced neuropathy in cancer. Clin Neurophysiol. 2017;128:1166‐1175. [DOI] [PubMed] [Google Scholar]
- 5. Griffith KA, Zhu S, Johantgen M, et al. Oxaliplatin‐induced peripheral neuropathy and identification of unique severity groups in colorectal cancer. J Pain Symptom Manage. 2017;54:701‐706.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyette‐Davis JA, Hou S, Abdi S, Dougherty PM. An updated understanding of the mechanisms involved in chemotherapy‐induced neuropathy. Pain Manag. 2018;8:363‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Carvalho Barbosa M, Kosturakis AK, Eng C, et al. A quantitative sensory analysis of peripheral neuropathy in colorectal cancer and its exacerbation by oxaliplatin chemotherapy. Cancer Res. 2014;74:5955‐5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy‐induced peripheral neuropathy: the QLQ‐CIPN20. Eur J Cancer. 2005;41:1135‐1139. [DOI] [PubMed] [Google Scholar]
- 9. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the functional assessment of cancer therapy/Gynecologic oncology group ‐ neurotoxicity (fact/GOG‐Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer. 2003;13:741‐748. [DOI] [PubMed] [Google Scholar]
- 10. Leonard GD, Wright MA, Quinn MG, et al. Survey of oxaliplatin‐associated neurotoxicity using an interview‐based questionnaire in patients with metastatic colorectal cancer. BMC Cancer. 2005;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660‐1664. [DOI] [PubMed] [Google Scholar]
- 12. Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy‐induced peripheral neurotoxicity using the Total neuropathy scale. Neurology. 2003;61:1297‐1300. [DOI] [PubMed] [Google Scholar]
- 13. Cavaletti G, Jann S, Pace A, et al. Multi‐center assessment of the Total neuropathy score for chemotherapy‐induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11:135‐141. [DOI] [PubMed] [Google Scholar]
- 14. National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- 15. Cavaletti G, Frigeni B, Lanzani F, et al. Chemotherapy‐induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer. 2010;46:479‐494. [DOI] [PubMed] [Google Scholar]
- 16. Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy‐induced peripheral neuropathy. Ann Oncol. 1998;9:739‐744. [DOI] [PubMed] [Google Scholar]
- 17. Argyriou AA, Park SB, Islam B, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy‐induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019;90(12):1361‐1369. [DOI] [PubMed] [Google Scholar]
- 18. Gewandter JS, Burke L, Cavaletti G, et al. Content validity of symptom‐based measures for diabetic, chemotherapy, and HIV peripheral neuropathy. Muscle Nerve. 2017;55:366‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herman WH, Pop‐Busui R, Braffett BH, et al. Use of the Michigan neuropathy screening instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications. Diabet Med. 2012;29:937‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bril V, Perkins BA. Validation of the Toronto clinical scoring system for diabetic polyneuropathy. Diabetes Care. 2002;25:2048‐2052. [DOI] [PubMed] [Google Scholar]
- 22. Lauria G, Merkies IS, Faber CG. Small fibre neuropathy. Curr Opin Neurol. 2012;25:542‐549. [DOI] [PubMed] [Google Scholar]
- 23. Tavakoli M, Marshall A, Pitceathly R, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223:245‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fuglsang‐Frederiksen A, Pugdahl K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin Neurophysiol. 2011;122:440‐455. [DOI] [PubMed] [Google Scholar]
- 25. Bostock H. Estimating motor unit numbers from a CMAP scan. Muscle Nerve. 2016;53:889‐896. [DOI] [PubMed] [Google Scholar]
- 26. Kristensen AG, Bostock H, Finnerup NB, et al. Detection of early motor involvement in diabetic polyneuropathy using a novel MUNE method – MScanFit MUNE. Clin Neurophysiol. 2019;130:1981‐1987. [DOI] [PubMed] [Google Scholar]
- 27. Ventzel L, Jensen AB, Jensen AR, Jensen TS, Finnerup NB. Chemotherapy‐induced pain and neuropathy: a prospective study in patients treated with adjuvant oxaliplatin or docetaxel. Pain. 2016;157:560‐568. [DOI] [PubMed] [Google Scholar]
- 28. Bennedsgaard K, Ventzel L, Themistocleous AC, et al. Long‐term symptoms of polyneuropathy in breast and colorectal cancer patients treated with and without adjuvant chemotherapy. Cancer Med. 2020;9:5114‐5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pascal M, Themistocleous A, Baron R, et al. DOLORisk: study protocol for a multi‐centre observational study to understand the risk factors and determinants of neuropathic pain [version 2; peer review: 2 approved]. Wellcome Open Res. 2019;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29‐36. [DOI] [PubMed] [Google Scholar]
- 31. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the neuropathic pain symptom inventory. Pain. 2004;108:248‐257. [DOI] [PubMed] [Google Scholar]
- 32. Cella D, Riley W, Stone A, et al. The patient‐reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self‐reported health outcome item banks: 2005‐2008. J Clin Epidemiol. 2010;63:1179‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothrock NE, Hays RD, Spritzer K, Yount SE, Riley W, Cella D. Relative to the general US population, chronic diseases are associated with poorer health‐related quality of life as measured by the patient‐reported outcomes measurement information system (PROMIS). J Clin Epidemiol. 2010;63:1195‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cella D, Choi S, Garcia S, et al. Setting standards for severity of common symptoms in oncology using the PROMIS item banks and expert judgment. Qual Life Res. 2014;23:2651‐2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. 2006;123:231‐243. [DOI] [PubMed] [Google Scholar]
- 36. Kural MA, Karlsson P, Pugdahl K, Isak B, Fuglsang‐Frederiksen A, Tankisi H. Diagnostic utility of distal nerve conduction studies and sural near‐nerve needle recording in polyneuropathy. Clin Neurophysiol. 2017;128:1590‐1595. [DOI] [PubMed] [Google Scholar]
- 37. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27:620‐628. [DOI] [PubMed] [Google Scholar]
- 38. Frigeni B, Cacciavillani M, Ermani M, et al. Neurophysiological examination of dorsal sural nerve. Muscle Nerve. 2012;46(895–8):891‐894. [DOI] [PubMed] [Google Scholar]
- 39. Pascal M, Themistocleous A, Baron R, et al. DOLORisk: study protocol for a multi‐centre observational study to understand the risk factors and determinants of neuropathic pain [version 1; referees: 1 approved with reservations]. Wellcome Open Res. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maier C, Baron R, Tolle TR, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439‐450. [DOI] [PubMed] [Google Scholar]
- 42. Ventzel L, Madsen CS, Karlsson P, et al. Chronic pain and neuropathy following adjuvant chemotherapy. Pain Med. 2018;19:1813‐1824. [DOI] [PubMed] [Google Scholar]
- 43. Schaldemose EL, Horjales‐Araujo E, Svensson P, Finnerup NB. Altered thermal grill response and paradoxical heat sensations after topical capsaicin application. Pain. 2015;156:1101‐1111. [DOI] [PubMed] [Google Scholar]
- 44. Mols F, Beijers T, Vreugdenhil G, van de Poll‐Franse L. Chemotherapy‐induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22:2261‐2269. [DOI] [PubMed] [Google Scholar]
- 45. Tofthagen C, Donovan KA, Morgan MA, Shibata D, Yeh Y. Oxaliplatin‐induced peripheral neuropathy's effects on health‐related quality of life of colorectal cancer survivors. Support Care Cancer. 2013;21:3307‐3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grothey A. Clinical management of oxaliplatin‐associated neurotoxicity. Clin Colorectal Cancer. 2005;5(Suppl 1):S38‐S46. [DOI] [PubMed] [Google Scholar]
- 47. Andersen KG, Duriaud HM, Jensen HE, Kroman N, Kehlet H. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413‐2422. [DOI] [PubMed] [Google Scholar]
- 48. Eckhoff L, Knoop A, Jensen MB, Ewertz M. Persistence of docetaxel‐induced neuropathy and impact on quality of life among breast cancer survivors. Eur J Cancer. 2015;51:292‐300. [DOI] [PubMed] [Google Scholar]
- 49. Dalla Torre C, Zambello R, Cacciavillani M, et al. Lenalidomide long‐term neurotoxicity: clinical and neurophysiologic prospective study. Neurology. 2016;87:1161‐1166. [DOI] [PubMed] [Google Scholar]
- 50. Petropoulos IN, Alam U, Fadavi H, et al. Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care. 2013;36:3646‐3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andersen ST, Grosen K, Tankisi H, et al. Corneal confocal microscopy as a tool for detecting diabetic polyneuropathy in a cohort with screen‐detected type 2 diabetes: ADDITION‐Denmark. J Diabetes Complications. 2018;32:1153‐1159. [DOI] [PubMed] [Google Scholar]
- 52. Ferdousi M, Azmi S, Petropoulos IN, et al. Corneal confocal microscopy detects small fibre neuropathy in patients with upper gastrointestinal cancer and nerve regeneration in chemotherapy induced peripheral neuropathy. PLoS One. 2015;10:e0139394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campagnolo M, Lazzarini D, Fregona I, et al. Corneal confocal microscopy in patients with oxaliplatin‐induced peripheral neuropathy. J Peripher Nerv Syst. 2013;18:269‐271. [DOI] [PubMed] [Google Scholar]
- 54. Griffith KA, Dorsey SG, Renn CL, et al. Correspondence between neurophysiological and clinical measurements of chemotherapy‐induced peripheral neuropathy: secondary analysis of data from the CI‐PeriNomS study. J Peripher Nerv Syst. 2014;19:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gewandter JS, Gibbons CH, Campagnolo M, et al. Clinician‐rated measures for distal symmetrical axonal polyneuropathy: ACTTION systematic review. Neurology. 2019;93:346‐360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Quantitative sensory testing showing z‐scores indicating loss or gain in function on the 10 parameters. The comparisons were done between confirmed CIPN in patients who had received A) docetaxel and B) oxaliplatin. . Patients with subclinical neuropathy are excluded (n = 2).
Abbreviations: CIPN, chemotherapy‐induced peripheral neuropathy; CDT, cold detection threshold; WDT, warm detection threshold; TSL, thermal sensory limen; threshold; MDT, mechanical detection threshold; MPT, mechanical pain threshold; MPS, mechanical pain sensitivity; VDT, vibration detection threshold; PPT, * P value <0.05
Supplemental Table S1. Case definition of polyneuropathy
Supplemental Table S2 Patient Characteristics and Questionnaire Results of Participants and Non‐Participants
Supplemental Table S3 Grading of Chemotherapy‐Induced Peripheral Neuropathy (CIPN) and Scores from Assessment Tools
Supplemental Table S4 Mean values of amplitude and velocity in measured nerves divided into groups of polyneuropathy


