Abstract
Direct oral anticoagulants (DOACs) are increasingly used for treatment and prevention of thromboembolic diseases, used in fixed dose regimens. Although their safety and efficacy profiles are considered optimal, clinical events still occur. Given that anticoagulation treatment is a delicate balance between clotting and bleeding, it is possible that an optimal target spot exists where the effect of anticoagulation achieves both the lowest possible risk of bleeding and thrombosis. Other currently available anticoagulants (ie, vitamin K antagonists and heparins) provide important clues for this. If such a target spot exists, tailored DOAC therapy may further benefit patients. This opinion article summarizes the current available evidence that suggests that such a tailored strategy could work. It also describes research suggestions for conducting studies in patient populations such as patients with extremes of body weight or impaired kidney function to evaluate whether tailored treatment with DOACs could lead to better patient outcomes.
Keywords: direct oral anticoagulants, atrial fibrillation, venous thromboembolism, pharmacokinetics, pharmacodynamics, epidemiology
1. INTRODUCTION
Since their introduction nearly a decade ago, the use of direct oral anticoagulants (DOACs) is rapidly increasing.[1] In large randomized clinical trials, DOACs were found to be noninferior to vitamin K antagonists (VKAs) for the prevention of arterial thromboembolism in patients with atrial fibrillation and for the management of venous thromboembolism.[2] Having fewer interactions with comedication and diet and a more stable pharmacokinetic profile than VKA, DOACs currently do not require routine laboratory monitoring. For these reasons, DOACs have replaced VKA in international guidelines as first choice of anticoagulant for management and prevention of thrombotic diseases.[3] Unfortunately, complications during management with DOACs still occur. In clinical trials, the risk for thromboembolic events in patients randomized to DOAC is 1% to 2% per year and although the risk of intracranial hemorrhage is lower with DOACs when compared with VKA, the overall major bleeding risk for DOAC use in observational studies remains 1% to 3% per year because of a large proportion of major extracranial bleeds.[3, 4, 5, 6] This is reflected in the large number of emergency rooms visits for adverse drug events, which is higher for anticoagulants than for any other drug class.[7] In addition, the total number of oral anticoagulant users has increased in the past 5 years, which is related to the ageing population and changing guidelines.[8]
Because both bleeding complications and recurrent thromboembolic events still occur during management of DOACs, there is room for improvement in the management of these patients. In this Forum Article, we will argue that it may be worthwhile to study the relationship between DOAC concentrations and patient‐important outcomes. First, we will discuss the balance between thrombosis and bleeding; second, the rationale for individual dosing of anticoagulants; and, last, we will appraise the current situation and discuss several research opportunities to further improve care in patients receiving anticoagulants.
2. BALANCING THROMBOSIS AND BLEEDING
In the field of thrombosis and hemostasis, a pair of scales is typically used to illustrate the delicate balance between clotting and bleeding. In the normal situation, both bleeding and clotting are balanced by a continuous equilibrium between procoagulant and anticoagulant processes.[9] If under pathological circumstances, the scale is tipped toward the procoagulant side, thrombosis can occur. If this is the case, we aim to rebalance the scale with anticoagulant therapy to find a new equilibrium where the risk of bleeding and risk of recurrence are weighed. Ideally, the anticoagulants’ effect reaches a so called “target” in the middle, where the effect of the anticoagulant achieves both the lowest possible risk of bleeding and thrombosis (Figure 1). The desired effect of anticoagulant therapy is similar to many other cardiovascular therapies (eg, treatments for hypertension, dyslipidemia, diabetes) for which a balance is maintained by measuring the effect of treatment (eg, blood pressure, cholesterol levels, blood glucose). Direct oral anticoagulants have a direct effect on the hemostatic system in a bidirectional manner as has been shown for dabigatran and edoxaban (ie, too much effect results in bleeding, too little effect in thromboembolism).[10, 11] For rivaroxaban and apixaban, this is less well known because of an absence of studies that have been conducted on this issue, although preliminary (small‐scale) studies suggest a similar bidirectional effect for rivaroxaban and apixaban as well.[12, 13] Given that the pharmacodynamics of all DOACs are similar as they selectively and specifically inhibit coagulation serine proteases,[14] it is likely that an optimal dose‐benefit relation exists for all DOACs. Here, two main questions need to be answered. First, do DOAC (level or activity) measurements correspond with the risk of clinical outcomes, and does a target spot (or therapeutic window) exist? Second, is this target range similar for specific patient populations, are perhaps different based on clinical characteristics, such as extremes of body weight and renal function?
Figure 1.
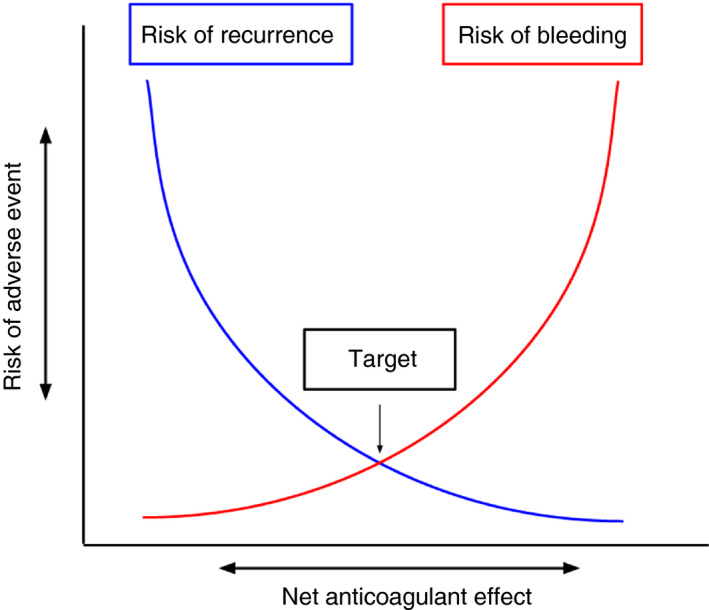
Simplified illustration of the theoretical optimal “target” between risk of adverse events and net anticoagulant effect
3. CLUES FOR A “TARGET SPOT” IN OTHER ANTICOAGULANTS
The existence of an optimal individual dose for DOACs might be supported by the fact that various other (similar acting) anticoagulants have an individual “target spot” or therapeutic range. For VKAs, it is well established that adequate regulation of the international normalized ratio optimizes the risk‐benefit ratio between bleeding and thromboembolic complications.[15, 16] For heparins, anti‐Xa level monitoring is suggested in patients with heparin resistance, a prolonged baseline activated partial thromboplastin time (APTT) or altered heparin responsiveness.[17] Unfractionated heparin is routinely monitored and adjusted based on the measured APTT, where an APTT of 1.5 to 2.5, that of baseline, is associated with the lowest bleeding and recurrence risk.[3, 18, 19] Low molecular weight heparin (LMWH) is dosed based on body weight and renal function.[20, 21, 22, 23] Subsequently, measurement of anti‐Xa levels are suggested to evaluate the safety of LMWH dosing in special patient populations (renal impairment, during pregnancy, critically ill patients).[3] These examples provide clues for the existence of a “target spot” with different classes of anticoagulants. It therefore may be worthwhile to at least investigate whether this optimal point between thrombosis and bleeding exists for DOACs as well.[24]
4. DOACS, CURRENT SITUATION, AND EVIDENCE FOR INDIVIDUAL BASED DOSING
4.1. Special situations or populations
Given the properties of DOACs (fixed‐dose regimen) and the evidence on anticoagulants that have similar mechanisms of effect, it seems reasonable to further investigate whether individually dosed regimens may improve patient important outcomes. This might especially be true for special patient populations or during specific situations.
4.1.1. Extremes in body weight
Currently, DOACs do not require dose adjustments for extremes in body weight or body mass index (BMI). Because patients at extremes of body weight were underrepresented in DOAC clinical trials (<20% weighed > 90 to 100 kg and < 15% weighed < 50 kg),[4, 5, 25] and randomized trials of DOACs for these specific patient groups are currently unavailable, the current recommendation of DOAC use in patients weighing > 120 kg or with a BMI > 40 kg/m2 is restricted to situations where VKAs cannot be used.[26, 27] For patients weighing < 50 kg, no recommendations are available. Interestingly, available pharmacokinetic studies provide insights into the relationship between body weight and drug levels. For dabigatran, a subgroup analysis within the Randomized Evaluation of Long‐Term Anticoagulation Therapy trial showed plasma trough concentrations 21% lower for high body weight (>100 kg) than for lower body weight (50‐100 kg).[10] For apixaban, a pharmacokinetic study found 31% lower peak concentration in the high body weight (>120 kg and BMI > 30 kg/m2) when compared with the reference group (65‐85 kg).[28] On the other hand, a recent study used data from 913 patients using rivaroxaban, including patients at extremes of body weight (>120 kg and < 50 kg), to develop a pharmacokinetic model. In this study, renal function was the best predictor of rivaroxaban exposure and addition of body weight to the model did not show a significant reduction in drug exposure. The authors concluded that rivaroxaban can be used at extremes of body weight.[29] The clinical implications of these pharmacokinetic studies are unknown. Pharmacokinetic studies that include clinical endpoints are therefore much needed. Given the possible differences in drug exposure at the extremes of body weight, it seems worthwhile to study whether these patients could benefit from assessing drug‐specific peak and through levels and dose adjustment, similar to weight‐based dose adjustments for LMWH.
4.1.2. Impaired kidney function
Because all DOACs are excreted by the kidneys to some extent, they require regular control of kidney function.[30] Because patients with a creatinine clearance (CrCl) less than 25 to 30 mL/min were excluded from the clinical trials,[4, 5, 25] data regarding the effectiveness and safety of DOACs in those with CrCl less than 25 mL/min are only available through nonrandomized small‐scale studies of which statistical type I/type II errors could play a role.[31, 32] Despite this knowledge gap, many DOACs are licensed and marketed for use up to a CrCl as low as 15 mL/min. Dose adjustments for patients with moderately impaired kidney function (CrCl 30‐50 mL/min) are advised although the recommended adjustments in licensed doses of DOACs for different stages of renal impairment differ between European and North American guidelines.[33] Current knowledge of DOAC pharmacokinetics in patients with advanced renal failure is limited. A review of pharmacokinetic studies showed increased peak level exposure in patients with impaired kidney function (CrCl 15‐30 mL/min) using DOACs.[34] These studies did not take clinical endpoints into account. For patients with advanced kidney failure, studying the benefits of tailored anticoagulant treatment might especially be interesting.
4.1.3. Prior gastrointestinal surgery
Because of a change in absorptive surface after gastrointestinal surgery, drug absorption of DOACs is expected to be altered in these patients. Anticoagulant drug levels are therefore prone to large inter‐individual variability. Available evidence for DOAC use in this patient population is very limited. Bariatric surgery or extensive bowel surgery may alter anticoagulant drug levels which can result in large inter‐individual variability.[35, 36]
4.1.4. Specific situations
The measurement of drug levels may also be useful in a number of other specific clinical situations such as the initiation of interacting comedication or the development of a recurrent event thrombosis despite use of anticoagulant therapy.[37] Also, in case of suspected therapy nonadherence or restarting anticoagulation after a major bleeding, DOAC trough concentrations might be of interest. Defining target levels to ensure the optimal balance between risk of thrombosis and bleeding could also provide guidance in these clinical scenarios. In addition, in the acute onset of thrombotic disease, risk of progress of thrombosis is highest. Therefore, one may need to shift the balance of anticoagulant treatment more toward “bleeding” to obtain a stronger anticoagulant effect (eg, achieved with initial higher doses of DOAC as is currently common practice in venous thrombosis management). In a recent cohort study, DOAC level measurements in acute clinical situations (ie, acute ischemic stroke, bleeding, and perioperative evaluation) affected clinical management in 77% of 234 patients.[38]
4.2. Inter‐individual differences and evidence for individual based dosing
In most recent studies on DOAC testing, DOAC concentration ranges (on‐therapy ranges) derived from the landmark randomized clinical trials are used as an indication for clinically relevant ranges.[39] However,, routine DOAC level assessments are not advised.[2] Yet, substantial inter‐individual variation in plasma drug levels of DOACs has been described in both observational studies as in clinical trials.[12, 40, 41] Given this variability, it is possible that specific patients groups are exposed to drug levels that are too high or too low. In another recent study on DOAC plasma levels among patients with nonvalvular atrial fibrillation using rivaroxaban or dabigatran, substantial between‐individual variation (different levels among different persons) in plasma levels was found; however, within‐individual DOAC levels (levels among the same individual at different time points) remained stable within or outside the on‐therapy range.[42] These data suggest that performing a DOAC measurement at the start of therapy provides an accurate estimate of future measurements and that repeated (costly and time‐consuming) measurements would not be necessary in a stable clinical situation. However, if the patient’s clinical characteristics evolve (eg, change in kidney function, body weight, comedication, comorbidities), a repeated measurement might be indicated. A clinical consequence could be (both in special patient populations as in patients without additional risk factors) that if this initial measurement is out of range, a different dose or different anticoagulant could be considered. As mentioned, studies evaluating the clinical benefit of such a proposed a strategy are still to be performed.
Whether measurement of certain pharmacokinetic parameters of DOACs and subsequent adjusted dosing schemes are superior in terms of patient important outcomes and cost‐effectiveness compared with the fixed‐dose regimen needs to be evaluated in future large observational studies or trials. At this moment, such studies are scarce; however, several available studies provide clues that a dose‐response relation between DOAC concentration and bleeding/thromboembolism exists. Data from the Randomized Evaluation of Long‐Term Anticoagulation Therapy trial, in which warfarin was compared to dabigatran in patients with atrial fibrillation, showed with a multivariate logistic regression model that the risk of ischemic events was inversely related to trough dabigatran concentrations (c‐statistic 0.66, 95% confidence interval 0.61‐0.71). It also showed major bleeding risk increased with dabigatran exposure (c‐statistic 0.72, 95% confidence interval 0.69‐0.74). In other words, higher dabigatran levels were associated with a lower risk of thromboembolism, yet a higher risk of major bleeding.[10] In a post hoc analysis of the ENGAGE‐AF trial [43] in which warfarin was compared with standard dose edoxaban (60 mg once daily) or low dose edoxaban (30 mg once daily) in patients with atrial fibrillation, low edoxaban plasma through concentrations were associated with higher risks of stroke and systemic embolism and high through values were associated with a higher risk of major bleeding.[11]
In a recent observational study in which DOAC concentrations were measured in 565 patients with atrial fibrillation from the START laboratory registry, thrombotic complications mainly occurred in patients with the lowest trough levels, in combination with a high CHA2DS2‐VASc score.[12] In another study in which the quality of anticoagulants was evaluated in patients (n = 460 patients, 51% on DOAC) with an acute stroke, low DOAC plasma levels (<50 ng/mL) were an independent predictor of higher stroke severity and large vessel occlusion (odds ratio 3.84, 95% confidence interval 1.80‐8.20) when compared with intermediate or high DOAC plasma levels. These studies do not however yet provide definitive evidence that monitoring anticoagulation levels will improve clinical outcomes, as they are limited in event rates and study design.
5. WHAT THE FUTURE MIGHT BRING
As discussed in previous sections, several clues supporting the possibility of an optimal dose‐benefit relation for DOACs are available. However, as only limited data are available, it is currently not known whether level‐dosed DOAC strategies are superior to fixed‐dosed DOAC strategies. These clues do, however, provide us with a number of research opportunities.
First, we need to know if patients on DOAC are willing to be monitored and tested on their DOAC level. Previous studies have shown that most patients on DOAC are satisfied with their current treatment and feel safe when using DOAC without regular thrombin inhibitor or anti‐Xa measurements.[44, 45] Because it is assumed that DOACs have a broad therapeutic window (ie, work the same for almost everybody; one size fits all approach) and that for efficacy and safety the actual DOAC plasma concentration is of less importance in a patient who takes this drug, gives credence to this patient strategy. Given that the overall bleeding and thromboembolism rates are similar as when using VKA, further supports that the fixed dose strategy is safe and effective for the majority of patients. Yet, according to a survey in patients who formerly used VKA and who were switched to DOAC up to 70% of them were willing to undergo some form of blood testing at least once a year if that could further increase the safety and efficacy of the DOAC they were using.[45]
Second, to find the optimal dose‐effect for DOACs, we need to further optimize and standardize laboratory tests. These DOAC‐specific drug level tests (ie, thrombin generation assays, ROTEM, and anti‐Xa assays for the inhibitors and diluted thrombin time for dabigatran) are becoming increasingly available in hospitals.[39] Currently available tests are mostly performed in plasma or whole blood. New point‐of‐care tests that are being developed for DOAC level or activity measurements may be useful [46] but there are still many uncertainties about the associations of these tests with drug intake and if blood and urine levels correlate.
Third, we need to know if risk models for thromboembolism and major bleeding, such as HAS‐BLED and CHA2DS2‐VASc, can be further improved by adding anticoagulation levels to the prediction scores and by predicting the risk not only in a landmark analysis (ie, around the time of diagnosis of atrial fibrillation or venous thromboembolism) but also through time (ie, dynamic prediction). Studies that can investigate whether DOAC‐specific measurements, either taken once, or over time correspond with risk of bleeding or thromboembolism could be performed by conducting large prospective studies or use available data from existing data (such as trials and registry data where patients on DOACs have been tested on DOAC plasma levels). In case there is an optimal target and subsequent dose for DOAC in patient populations or subgroups the final step would be to set up endpoint studies to guide dose regimen selection/DOAC titration in clinical trials, for example in special patient populations mentioned previously.
6. CONCLUSION
Even though fixed‐dose DOAC regimens have been shown to have several clinical important advantages and noninferior efficacy when compared with VKA, patients who use DOACs still have a yearly risk of 1% to 3% to develop major bleeding. It is likely that patients could further benefit from tailored DOAC therapy in regard to clinical outcomes. This might in particular be the case for special populations (eg, extremely high or low body weight, impaired kidney function, prior intestinal surgery) and in specific clinical situations (eg, patients that restart anticoagulation after a major bleeding or patients that experience a thrombotic event while on DOAC therapy).
CONFLICT OF INTEREST
None related to this work.
AUTHOR CONTRIBUTIONS
Myrthe M. A. Toorop, Willem M. Lijfering, and Luuk J. J. Scheres were the main investigators of the manuscript. Myrthe M. A. Toorop wrote the first draft of the manuscript and the final version. Willem M. Lijfering and Luuk J. J. Scheres were responsible for review of the manuscript.
ACKNOWLEDGMENTS
None.
Toorop MMA, Lijfering WM, Scheres LJJ. The relationship between DOAC levels and clinical outcomes: The measures tell the tale. J Thromb Haemost. 2020;18:3163–3168. 10.1111/jth.15104
Manuscript handled by: Claire McLintock
Final decision: Claire McLintock and 14‐ Sep‐ 2020
REFERENCES
- 1. Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968‐1975. [DOI] [PubMed] [Google Scholar]
- 3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest. 2016;149:315‐352. [DOI] [PubMed] [Google Scholar]
- 4. Schulman SKC, Kakkar AK, Mismetti P, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342‐2352. [DOI] [PubMed] [Google Scholar]
- 5. EINSTEIN Investigators BR . Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499‐2510. [DOI] [PubMed] [Google Scholar]
- 6. Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs warfarin: clinical experience. Am J Med. 2016;129:S33‐S40. [DOI] [PubMed] [Google Scholar]
- 7. Shehab N, Lovegrove MC, Geller AI, Rose KO, Weidle NJ, Budnitz DS. US emergency department visits for outpatient adverse drug events, 2013–2014. JAMA. 2016;316:2115‐2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Halvorsen S, Ghanima W, Fride Tvete I, et al. A nationwide registry study to compare bleeding rates in patients with atrial fibrillation being prescribed oral anticoagulants. Eur Heart J Cardiovasc Pharmacother. 2017;3:28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Astrup T. New aspects in blood coagulation and fibrinolysis and their relations to coronary thrombosis and coronary sclerosis. Wiener Zeitschrift fur innere Medizin und ihre Grenzgebiete. 1958;39:373‐386. [PubMed] [Google Scholar]
- 10. Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulation Therapy). J Am Coll Cardiol. 2014;63:321‐328. [DOI] [PubMed] [Google Scholar]
- 11. Ruff CT, Giugliano RP, Braunwald E, et al. Association between edoxaban dose, concentration, anti‐Factor Xa activity, and outcomes: an analysis of data from the randomised, double‐blind ENGAGE AF‐TIMI 48 trial. The Lancet. 2015;385:2288‐2295. [DOI] [PubMed] [Google Scholar]
- 12. Testa S, Paoletti O, Legnani C, et al. Low drug levels and thrombotic complications in high‐risk atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2018;16:842‐848. [DOI] [PubMed] [Google Scholar]
- 13. Testa S, Legnani C, Antonucci E, et al. Coordinator of SR. Drug levels and bleeding complications in atrial fibrillation patients treated with direct oral anticoagulants. J Thromb Haemost. 2019;17:1064‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baglin T. The role of the laboratory in treatment with new oral anticoagulants. J Thromb Haemost. 2013;11:122‐128. [DOI] [PubMed] [Google Scholar]
- 15. Cannegieter SC, Rosendaal FR, Wintzen AR, van der Meer FJM, Vandenbroucke JP, Briet E. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11‐17. [DOI] [PubMed] [Google Scholar]
- 16. Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A Method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69:236‐239. [PubMed] [Google Scholar]
- 17. Smythe MA, Priziola J, Dobesh PP, Wirth D, Cuker A, Wittkowsky AK. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koopman MMW, Prandoni P, Piovella F, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low‐molecular‐weight heparin administered at home. N Engl J Med. 1996;334:682‐687. [DOI] [PubMed] [Google Scholar]
- 19. Hirsh J. Heparin. N Engl J Med. 1991;324:1565‐1574. [DOI] [PubMed] [Google Scholar]
- 20. Summary of Product Characteristics. Nadroparin calcium Aspen 15,200 I.U. Available from: https://mricts‐mrpeu/human/downloads/DE_H_4763_003_FinalPI_6of8pdf (accessed July 2020).
- 21. Summary of product characteristics. Tinzaparin sodium 10,000 anti‐factor xa IU/ml. Available from: https://wwwmedicinesorguk/emc/product/3632/smpc (Accessed July 2020)
- 22. Summary of product characteristics. Fragmin® 10,000 IU/0.4ml solution for injection. Available from: https://wwwmedicinesorguk/emc/product/4245/smpc (Accessed July 2020)
- 23. Summary of product characteristics. Clexane® forte syringes 12,000 IU (120 mg)/0.8 ml solution for injection in pre‐filled syringes / Clexane® Forte Syringes 15,000 IU (150 mg)/1 ml solution for injection in pre‐filled syringes. Available from: https://wwwmedicinesorguk/emc/product/1695/smpc (Accessed July 2020)
- 24. ten Cate H. New oral anticoagulants: discussion on monitoring and adherence should start now! Thromb J. 2013;11:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799‐808. [DOI] [PubMed] [Google Scholar]
- 26. Martin K, Beyer‐Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J. Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis. 2016;41:206‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Upreti . Effect of extremes of body weight on pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Speed V, Green B, Roberts LN, et al. Fixed dose rivaroxaban can be used in extremes of bodyweight: a population pharmacokinetic analysis [published online ahead of print June 8, 2020]. J Thromb Haemost. [DOI] [PubMed] [Google Scholar]
- 30. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330‐1393. [DOI] [PubMed] [Google Scholar]
- 31. Weber J, Olyaei A, Shatzel J. The efficacy and safety of direct oral anticoagulants in patients with chronic renal insufficiency: a review of the literature. Eur J Haematol. 2019;102:312‐318. [DOI] [PubMed] [Google Scholar]
- 32. Derebail VK, Rheault MN, Kerlin BA. Role of direct oral anticoagulants in patients with kidney disease. Kidney Int. 2020;97:664‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parker K, Thachil J. The use of direct oral anticoagulants in chronic kidney disease. Br J Haematol. 2018;183:170‐184. [DOI] [PubMed] [Google Scholar]
- 34. Padrini R. Clinical pharmacokinetics and pharmacodynamics of direct oral anticoagulants in patients with renal failure. Eur J Drug Metab Pharmacokinet. 2018;44:1‐12. [DOI] [PubMed] [Google Scholar]
- 35. Martin KA, Lee CR, Farrell TM, Moll S. Oral anticoagulant use after bariatric surgery: a literature review and clinical guidance. Am J Med. 2017;130:517‐524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheung YW, Barco S, Mathot RAA, et al. Pharmacokinetics of dabigatran etexilate and rivaroxaban in patients with short bowel syndrome requiring parenteral nutrition: the PDER PAN study. Thromb Res. 2017;160:76‐82. [DOI] [PubMed] [Google Scholar]
- 37. Patel JP, Byrne RA, Patel RK, Arya R. Progress in the monitoring of direct oral anticoagulant therapy. Br J Haematol. 2019;184:912‐924. [DOI] [PubMed] [Google Scholar]
- 38. Winther‐Larsen A, Hvas AM. Clinical impact of direct oral anticoagulant measuring in a real‐life setting. Thromb Res. 2019;175:40‐45. [DOI] [PubMed] [Google Scholar]
- 39. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209‐219. [DOI] [PubMed] [Google Scholar]
- 40. Testa S, Tripodi A, Legnani C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: results observed in four anticoagulation clinics. Thromb Res. 2016;137:178‐183. [DOI] [PubMed] [Google Scholar]
- 41. Gulilat M, Tang A, Gryn SE, et al. Interpatient variation in rivaroxaban and apixaban plasma concentrations in routine care. Can J Cardiol. 2017;33:1036‐1043. [DOI] [PubMed] [Google Scholar]
- 42. Gulpen AJW, Ten Cate H, Henskens YMC, et al. The daily practice of direct oral anticoagulant use in patients with atrial fibrillation; an observational cohort study. PLoS One. 2019;14:e0217302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093‐2104. [DOI] [PubMed] [Google Scholar]
- 44. Prins MH, Bamber L, Cano SJ, et al. Patient‐reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135:281‐288. [DOI] [PubMed] [Google Scholar]
- 45. Toorop MMA, van Rein N, Nierman MC, et al. Switching from vitamin K antagonists to direct oral anticoagulants: treatment satisfaction and patient concerns. J Thromb Haemost. 2020;18:1390‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Harenberg J, Du S, Wehling M, et al. Measurement of dabigatran, rivaroxaban and apixaban in samples of plasma, serum and urine, under real life conditions. An international study. Clin Chem Lab Med. 2016;54:275‐283. [DOI] [PubMed] [Google Scholar]


