Abstract
Background
The cause of amyotrophic lateral sclerosis (ALS) is unknown, but occupations have been explored as a potential proxy measure of risk. There is a substantial body of literature connecting military service to ALS. We aimed to summarize and assess the quality of this evidence.
Methods
Systematic review of the literature, including observational studies which explored one of the following exposures: general military service (army, air force, marines, or navy); or specific exposures associated with military service measured among military personnel. The outcome of interest was ALS incidence, which could include onset, diagnosis, or death from ALS.
Results
A total of 2642 articles were screened. Following exclusion, 19 articles remained for inclusion in the systematic review, including 1 meta‐analysis and 18 original observational studies. Most studies were of moderate quality. In general, the relationship between military service was suggestive of an increased risk, particularly among Gulf War and WWII veterans. Exposure to pesticides (including Agent Orange) certain chemicals (exhaust, burning agents), heavy metals, and head trauma appeared to increase the risk of ALS among military personnel.
Conclusions
There is a possible association between military service and the subsequent development of ALS; however, the evidence was limited. Studies were generally hindered by small sample sizes and inadequate follow‐up time. Future studies should endeavor to objectively measure specific exposures, or combinations thereof, associated with military service, as this will be of vital importance in implementing preventative strategies into military organizations.
Keywords: amyotrophic lateral sclerosis, military service, risk factors
1. INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive, degenerative neurological disease. 1 It is the most common form of motor neuron disease (MND), but still considered rare, with a global incidence of approximately 2/100,000 persons. 2 ALS onset typically occurs in later life, peaking between 50 and 75 years of age, affecting persons of both sexes and all ethnicities. 1 The consequences of ALS can be devastating for those affected and their families. Disease progression is rapid, with only half of patients living longer than three years following diagnosis. 3
The cause of ALS is unknown; however, men, people of European descent, and those a family history are more likely to develop the disease. 4 In recent years, occupations have been explored as a potential proxy measure for ALS risk. In two reports from the United States, it was found that a disproportionately large fraction of men affected by ALS have a history of military service. 4 , 5 Military personnel are subjected to intense physical exertion and are more likely than their civilian counterparts to be exposed to certain agents, including lead, pesticides, smoking, and trauma, thought to be linked to ALS. 6
In 2017, a meta‐analysis concluded that there is indeed an increased risk of ALS associated with service in the military. 7 Since this publication, more studies have explored this relationship. As well, researchers have begun to explore the effect of specific military‐related exposures which might contribute to this elevated risk of ALS. 6 We aimed to not only update the findings from the meta‐analysis, but also highlight these putative military‐related risk factors for ALS. To our knowledge, this is the first study to summarize and assess the quality of the evidence through a systematic review of the literature concerning military service and military‐related exposures and ALS.
2. METHODS
2.1. Eligibility criteria
Eligibility criteria were developed a priori with no deviations during implementation. We included observational study designs appropriate for the study of etiology: case–control, cohort, or meta‐analyses. Studies must have explored one of the following exposures: general military service (army, air force, marines, or navy); or specific exposures associated with military service measured among military personnel. The outcome of interest was ALS incidence, which could include onset, diagnosis, or death due to ALS. Typically, death is not considered a measure of incidence; however, in the context of ALS, it has been used as such due to the rapidly fatal nature of the disease. 8 Studies were included if they reported a risk estimate (odds ratio, rate ratio, risk ratio, hazard ratio, and standardized morbidity ratio); included a minimum of 5 cases of ALS; and were published in English. Case reports, intervention, descriptive studies of incidence/prevalence and natural history studies of ALS were excluded. No restrictions were imposed in terms of age, sex, ethnicity, or publication date. If an original study was included in a meta‐analysis, we reported only the results of the meta‐analysis.
2.2. Search strategy
We performed a comprehensive literature search using a strategy developed by persons with expertise in neuroepidemiology and systematic review methodology, with the aid of two research librarians at the Karolinska University Library. Medline, Embase, and Web of Science were searched for articles published up to June 25, 2019. The detailed search strategy for all three databases is available in the Appendix. Finally, reference lists within articles were screened, and experts were consulted to identify papers which may have been missed.
2.3. Data extraction
Articles were stored in EndNote where duplicates were deleted. Articles were screened at three levels: title, abstract, and full paper based on the inclusion criteria. Titles and abstracts were screened by one reviewer (KAM), and full papers were reviewed by three independent reviewers (KAM, KAS, and LMS), with disagreements resolved by consensus.
2.4. Quality assessment
The AMSTAR (A MeaSurement Tool to Assess systematic Reviews) 2 9 was employed for the quality assessment of meta‐analyses, and the Newcastle‐Ottawa Scale (NOS) 10 was used to evaluate the quality of observational studies. The AMSTAR 2 does not generate a quality score, but aids in the identification of high‐quality meta‐analyses. 9 The NOS has 3 subscales, selection, comparability, and exposure/outcome, with points awarded on each subscale for a maximum of 9 points. 10 Two reviewers (KAM and KAS) independently scored each original observational study using the NOS, with discrepancies resolved by consensus. All studies were retained, regardless of quality score.
3. RESULTS
A total of 2642 articles were screened, 2 of which were identified from reference lists of relevant articles. Of these, 102 were reviewed at the full‐paper level. Following exclusion, 19 articles remained, including 1 meta‐analysis and 18 original observational studies. Reasons for exclusion included, but were not limited to: lack of military personnel (n = 51), review paper (n = 11), and absence of a risk estimate (n = 10). For the full screening results, see the PRISMA flowchart in Figure 1.
Figure 1.
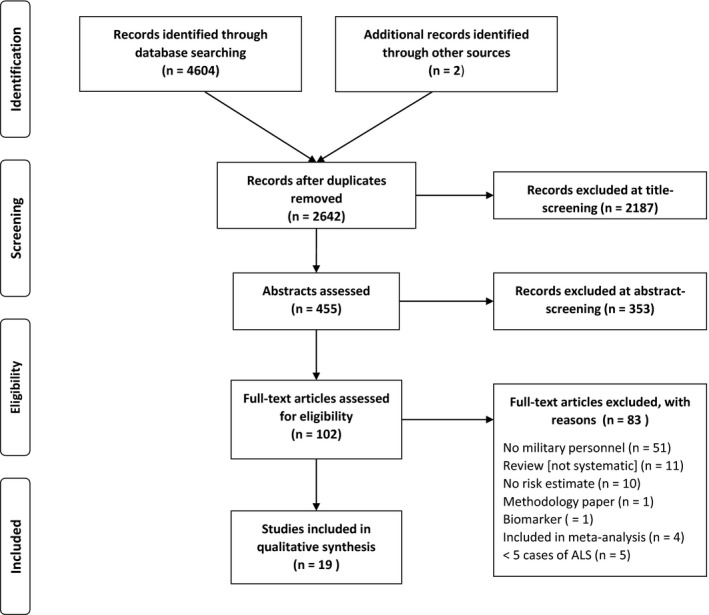
PRISMA Flowchart of study selection
Most studies were of moderate quality, with a median score of 7 of a possible 9 (range: 4 to 8). Funding for the included studies came primarily from government agencies (n = 16) and non‐profit foundations (n = 3). Studies were classified into three groups: those which explored general military service; military service during specific wars; and exposures associated with military service. Details of the 18 original articles are available in Table 1.
Table 1.
Description of included studies.
| Citation | Author, year | Study design | Region | Sample | Source of cases | Source of controls | Exposure | Main findings | QA score (0–9) |
|---|---|---|---|---|---|---|---|---|---|
| General military service | |||||||||
| 11 | Andrew, 2017 | Case–control | USA | 295 ALS cases (79 served in the military)/224 controls (42 served in the military) | Patients seen in one of two neurology clinics with a probable or definite ALS diagnosis | Patients with other neurological disease from the same neurology clinics as cases | Self‐reported military service/deployment | Neither military service (OR: 1.09; 95%CI: 0.67–1.78) nor deployment (OR: 0.73; 95%CI: 0.41–1.31) were associated with ALS risk | 5 |
| 12 | Dickerson, 2018 | Nested case–control | Denmark |
1079 male ALS cases (102 served in the military)/107 900 male controls (8833 served in the military). 747 female ALS cases (10 served in the military)/ 74 700 female controls (782 served in the military) |
National Patient Register (≥1 ICD code for ALS) | General Danish population | Military service for ≥1 year as recorded in the Danish Pension Fund | No altered risk of ALS associated with military service in men (OR: 1.15; 95%CI: 0.92–1.43) nor women (OR 1.11 95%CI: 0.55–2.25) | 8 |
| 13 | Peters, 2017 | Nested case–control | Sweden | 4567 ALS cases (31 served in the armed forces)/22 402 controls (195 served in the armed forces) | National Patient Register (≥1 ICD code for ALS) | General Swedish population | Occupation in the armed forces as reported in the census from 1970, 1980, & 1990 | No altered risk of ALS associated with employment in the armed forces (OR: 0.77; 95% CI: 0.52 to 1.14) | 7 |
| Military service during specific war periods | |||||||||
| Gulf War | |||||||||
| 14 | Smith, 2000 | Cohort | USA | 551,841 Gulf War veterans (6 ALS cases)/1 478704 non‐deployed service personnel (12 ALS cases) | Defense Manpower Data Center & Department of Defense hospitalization records (≥1 ICD code for ALS) | N/A | Deployment to the Gulf War | The risk of ALS was not different between those deployed to the Gulf War and those not deployed (risk ratio 0.94; 95%CI: 0.65–1.35) | 7 |
| 15 | Kang, 2001 | Cohort (Follow‐up study) | USA |
621,902 Gulf War veterans/746 248 non‐deployed service personnel Number of cases of ALS was not provided. |
Defense Manpower Data Center & Veterans Affairs death certificates | N/A | Deployment to the Gulf War | The risk of ALS was not different between those deployed to the Gulf War and those not deployed (rate ratio: 0.59; 95%CI: 0.21–1.66) | 7 |
| 16 | Barth, 2009 | Cohort | USA | 621,902 (23 ALS cases) Gulf War veterans/746 284 (38 ALS cases) non‐deployed military personnel | Defense Manpower Data Center & Veterans Affairs death certificates | N/A | Deployment to the Gulf War | The risk of death due to ALS was not different between Gulf War veterans and non‐deployed veterans (rate ratio = 0.96, 95% CI: 0.56, 1.62) | 7 |
| 17 | Haley, 2003 | Cohort | USA | 695 000 (20 ALS cases) | Veterans support groups | N/A | Deployment to the Gulf War | The observed incidence of ALS in Gulf War veterans under age 45 exceeded the expected incidence between 1995 and 1998 (standardized morbidity ratio: 2.27, p = 0.006) | 7 |
| 18 | Horner, 2003 | Cohort | USA | 696,118 (40 ALS cases) Gulf War veterans/1786 215 (67 ALS cases) non‐deployed military personnel | Publicized phone number and Internet ads, mass mailings to neurologists. Veterans Affairs and Department of Defense inpatient, outpatient, and pharmacy medical databases & benefit files, TriCare (health insurance plan) | N/A | Deployment to the Gulf War | The risk of ALS was elevated among Gulf War veterans (relative risk: 1.92; 95% CI: 1.29–2.84) | 8 |
| 19 | Coffmann, 2005 | Cohort | USA | 696,118 (42 ALS cases) Gulf War veterans/1786 215 (76 ALS cases) non‐deployed military personnel | Publicized phone number and Internet ads, mass mailings to neurologists. Veterans Affairs and Department of Defense inpatient, outpatient, and pharmacy medical databases & benefit files, TriCare (health insurance plan) | N/A | Deployment to the Gulf War | The risk of ALS was elevated among Gulf War veterans (relative risk: 1.77; 95%CI lower bound: 1.21) | 7 |
| World War II, Korean and Vietnam Wars | |||||||||
| 20 | Beard, 2016 | Case–control | USA | 621/958 | US Department of Veteran's Affairs National Registry of Veterans with ALS | Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis (GENEVA) study | Deployment to WWII, Korean, Vietnam, or Gulf War | The odds of ALS were increased among persons who served in WWII (OR: 4.79; 95%CI: 1.48–15.49) or the Korean War (OR: 3.52; 95%CI: 1.07–11.54), but not the Vietnam or Gulf War | 6 |
| 21 | Weisskopf, 2005 | Cohort | USA | 281,874 male military veterans (217 ALS cases) and 126,414 non‐veterans (63 ALS cases) | Cancer Prevention Study II & National Death Index | N/A |
Self‐reported period of service coinciding with: WWII Korean War Vietnam War |
Men who served during WWII had an elevated risk of ALS (relative risk: 1.60; 95%CI: 1.12–2.30), but there was no altered risk of ALS among those who served during the Korean or Vietnam Wars | 7 |
| 22 | Weisskopf, 2015 | Cohort | USA | 267,817 male military veterans (221 ALS cases) and 428,926 non‐veterans (154 ALS cases) | National Longitudinal Mortality Study & National Death Index | N/A |
Self‐reported period of service coinciding with: WWII Korean War Vietnam War |
Men who served during WWII had an elevated risk of ALS (HR: 1.47; 95%CI: 1.13, 1.91), but there was no altered risk of ALS among those who served during the Korean or Vietnam Wars | 7 |
| 23 | Cragg, 2017 | Cohort (Follow‐up to study 21) | USA | 331,001 male military veterans (358 ALS cases) and 676,912 non‐veterans (285 ALS cases) | National Longitudinal Mortality Study & National Death Index | N/A |
Self‐reported period of service coinciding with: WWII Korean War Vietnam War |
Men who served during WWII had an elevated risk of ALS (HR: 1.3; 95%CI: 1.1–1.6), but there was no altered risk of ALS among those who served during the Korean or Vietnam Wars | 7 |
| Specific exposures related to military service | |||||||||
| 20 | Beard, 2016 | Case–control | USA | 621/958 | US Department of Veteran's Affairs National Registry of Veterans with ALS | Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis (GENEVA) study | Military‐related factors | Herbicide exposure (OR: 2.52; 95%CI: 1.05–6.05), pyridostigmine bromide use (OR: 2.70; 95%CI: 1.05–6.96), exhaust from heaters or generators (OR: 1.75; 95%CI: 1.05–2.92), explosions within one mile of the person (OR: 1.87; 95%CI: 1.11–3.14), exposure to burning agents in the field (OR: 7.75; 95%CI: 1.92–31.24) and Agent Orange (OR: 2.80; 95%CI: 1.44–5.44) all increased the risk of ALS. | 6 |
| 24 | Miranda, 2008 | Cohort | USA | 41 ALS cases | Defense Manpower Data Center, VA, DoD |
Defense Manpower Data Center, Veteran's Administration, DoD inpatient, outpatient, and pharmacy databases |
Service in specific locations during the Gulf War | Deployment to the region near the site of the Khamisiyah munitions explosion was associated a heightened risk of ALS (relative risk: 1.7; 95%CI: 0.7–3.7) | 6 |
| 25 | Yi, 2013 | Cohort | South Korea | 114 562 Korean veterans of the Vietnam War (1865 ALS cases) | Survey, Ministry of National Defense and Ministry of Government Administration and Home Affairs | N/A | Agent Orange | Veterans who self‐reported high exposure to Agent Orange during the Vietnam war were more likely to report having ALS (OR: 2.23; 95%CI: 2.02–2.47) | 4 |
| 26 | Schmidt, 2010 | Case–control | USA | 241/597 | US Department of Veteran's Affairs National Registry of Veterans with ALS | GENEVA study |
Cigarette smoking Head injury |
Having a head injury within 15 years of the reference date (date of ALS diagnosis or corresponding date for controls) was associated with increased odds of ALS (2.33; 95%CI: 1.18–4.61). There was no association between smoking and ALS | 7 |
| 27 | Fang, 2010 | Case–control | USA | 200/229 | US Department of Veteran's Affairs National Registry of Veterans with ALS | GENEVA Study | Blood lead levels | A doubling of blood lead was associated with a 1.9‐fold increased risk of ALS (95% CI: 1.3, 2.7) | 8 |
| 28 | Peters, 2016 | Case–control | USA | 163/229 | US Department of Veteran's Affairs National Registry of Veterans with ALS | GENEVA study | Blood selenium, zinc, copper and manganese levels |
ALS was inversely associated with selenium (OR: 0.4; 95% CI: 0.2–0.8) and zinc (OR: 0.4; 95% CI: 0.2–0.8), and positively associated with copper (OR: 3.4; 95% CI: 1.5–7.9). No linear trend was found for manganese (OR: 0.9; 95% CI: 0.6–1.3) |
7 |
Abbreviations: ALS, Amyotrophic lateral sclerosis; CI, confidence interval; HR, hazard ratio; ICD, International classification of disease; N/A, not applicable; OR, odds ratio; QA, Quality assessment, measured according to the Newcastle‐Ottawa Scale.
3.1. General military service
The relationship between general military service and ALS risk has been explored extensively. Our results included one meta‐analysis from 2017, which encompassed 9 studies, 7 and 3 original studies. 11 , 12 , 13 While the meta‐analysis suggested an increased risk, the three subsequent studies reported no relationship between military service and ALS risk. 11 , 12 , 13
The meta‐analysis by Tai et al 7 summarized evidence between 1981 and 2016 regarding the risk of ALS associated with general military service. The analysis included 6 case–control and 3 cohort studies, with moderate heterogeneity between studies (I 2 = 55%). Among the 9 studies, the pooled odds ratio (OR) was 1.29 (95% confidence interval [CI]: 1.08–1.54), suggesting a significant increased risk of ALS among military personnel. Sensitivity analyses revealed that studies of higher quality were more likely to yield a positive association between military service and ALS risk than studies of moderate quality. The quality of the review itself was high, based on the AMSTAR criteria; however, there were noted limitations to the included studies, such as small sample sizes, biased control selection, and potentially imprecise case selection. 7
A case–control study in New England, USA compared newly‐diagnosed ALS patients to controls seen for other neurological conditions in two neurology clinics. 11 Between 2009 and 2015, 295 cases of ALS and 224 controls were enrolled. More ALS cases had served in the military (28% of cases versus 20% of controls), but the risk estimate did not reach statistical significance (OR: 1.09; 95%CI: 0.67–1.78 adjusted for age and gender). 11
The incidence of ALS was measured among all men and women who had served in the military for a minimum of one year in Denmark 12 using a population‐based nested case–control design. ALS cases were identified using International Classification of Disease [ICD] codes for ALS from hospitalization discharge reports and matched 10:1 to non‐ALS controls randomly selected from the general population. A total of 102 (9.45%) men and 10 (1.34%) women with ALS had been employed in the military, compared to 8833 (8.19%) men and 782 (1.05%) women without ALS. Following adjustment for socioeconomic status, residential location, and marital status, there was no risk of ALS among men (OR: 1.15; 95%CI: 0.92 to 1.43) nor women (OR: 1.11; 95%CI: 0.55–2.25). 12
Similarly, linked registries in Sweden were accessed to explore the relationship between occupation and risk of ALS, defined using ICD codes from hospital and physician billings. 13 Among 4567 ALS cases, 31(0.68%) had stated in the 1990 census that they were employed in the armed forces, compared to 195/22,402 controls (0.87%). Following adjustment for age, sex, and education level, there was no risk of ALS among armed forces personnel (OR: 0.77; 95%CI: 0.52 to 1.14). 13
3.2. Military service during specific war periods
Six cohort studies 14 , 15 , 16 , 17 , 18 , 19 and one case–control study 20 investigated the risk of ALS among American Gulf War veterans. Three prospective studies and one retrospective study explored the risk of ALS related to serving during World War II, the Korean, and Vietnam Wars, all based in the United States. 20 , 21 , 22 , 23
3.2.1. Gulf War (1990‐1991)
Of the 6 cohort studies published, each employed different methods to identify persons with ALS from the same source population of American veterans. Three reported no association, 14 , 15 , 16 while three reported a significantly increased risk of ALS among those deployed to the Gulf War. 17 , 18 , 19
The first cohort study to report on the risk of ALS associated with the Gulf War was published 9 years following the end of the war. 14 The primary outcome was hospitalization for ALS (based on ICD codes) within all Department of Defense hospitals, with those deployed to the Gulf War (n = 551,841) compared to active service persons who were not deployed during the same period (n = 1,478,704). Between 1991 and 1997, 6 persons who had served in the Gulf War were hospitalized for ALS, compared to 12 persons who had not served (age‐adjusted risk ratio: 1.66; 95% CI: 0.62–4.44). 14
The same cohort was subsequently studied using death records to identify cases of ALS. 15 Information on cause of death was gathered from Veterans Affairs death certificates or the National Death Index. Gulf War veterans (n = 621,902) were compared to active service personnel who were not deployed (n = 746,248). There was no increased risk of ALS among the Gulf War veterans (rate ratio: 0.59; 95%CI: 0.21–1.66). The number of deaths in each cohort was not provided. A subsequent study followed this cohort for an additional 6 years. 16 Of the Gulf War veterans, 23 had died due to ALS, compared to 38 persons within the non‐deployed cohort. The rate ratio remained statistically insignificant at 0.96 (95% CI: 0.56–1.62), adjusting for gender, race, branch of service, type of unit, age, and marital status. 16
Cases of ALS diagnosed between 1991 and 1998 were counted from a pool of 695,000 Gulf War veterans and compared to expected rates of ALS from the US male population. 17 Cases were identified through veteran's meetings, ALS support groups, Department of Defense records, and medical record review. Among 89 identified cases, 20 met criteria for a confirmed diagnosis of ALS. Most cases (17) were diagnosed prior to 45 years of age, and subsequent analyses focused only on these “young‐onset” cases. Immediately following the Gulf War (between 1991 and 1994), there were 4 cases of ALS observed, compared to an expected number of 4.25 (standardized morbidity ratio [SMR]: 0.94; 95% CI: 0.26–2.41). Between 1995 and 1998, there were 13 confirmed cases relative to an expected number of 5.72, resulting in a significantly elevated SMR of 2.27 (95% CI, 1.27 to 3.88). 17
Similarly, broad case ascertainment methods were used in another cohort study from 2003. 18 Researchers passively recruited ALS cases from military personnel through the Internet and mass mailings of information to neurologists across the United States. 18 Active ascertainment included the linkage of multiple Department of Defense registries. All subjects enrolled through these means had their medical records reviewed to confirm the diagnosis of ALS. Over a ten‐year period, 40 cases of ALS were found from the cohort of persons who were deployed to the Gulf War (n = 696,118) compared to 72 cases among those who served in the military during the same time period, but whom were not deployed (n = 1,786,215). This resulted in an age‐adjusted risk ratio of 1.92 (95% CI: 1.29–2.84), suggesting an elevated risk of ALS among the Gulf War veterans. 18 A subsequent study re‐analyzed the same cohort to measure the effect of potential under‐ascertainment of cases among non‐deployed veterans. Following correction for this potential bias, the risk ratio remained significantly elevated for the deployed cohort (RR: 1.77; 95% CI lower bound: 1.21). 19
Most recently, 621 prevalent ALS cases and 958 frequency‐matched controls from the Veterans Affairs registry were questioned regarding their previous military deployments. 20 There was no difference in the proportion of persons deployed to the Gulf War among cases and controls (2% for each; OR: 0.52; 95% CI: 0.17–1.63, adjusted for age, use of the Veterans Affairs healthcare system, sex, race/ethnicity, and military branch). 20
3.2.2. World War II (1939–1945), Korean War (1950–1953), and Vietnam War (1955–1975)
Three cohort studies and a single case–control study reported on the risk of ALS associated with World War II, the Korean War, and the Vietnam War. Consistent results were found for an increased risk of ALS after serving during WWII, but not the Korean nor Vietnam Wars. 20 , 21 , 22 , 23
Over 500,000 men from across the United States were followed from 1989 to 1998 as part of the Cancer Prevention Study II. 21 Information on military service was collected via questionnaire, in which participants were asked to note their years of service. The outcome, death due to ALS, was based ICD codes in the National Death Index. From the full cohort, 116 men had served during the period of WWII and later died due to ALS, which resulted in a relative risk of 1.60 (95%CI: 1.12–2.30) adjusted for age, smoking status, education, alcohol intake, pesticide/herbicide exposure, and occupation. Military service during the Korean and Vietnam wars was also associated with an elevated risk of death due to ALS; however, neither met statistical significance, with few deaths in either group (n = 36 and 4, respectively). 21
A decade later, the same authors utilized census information relating to period of service in the US military. 22 Death due to ALS between 1979 and 2002 was collected from the National Longitudinal Mortality Study and verified in the National Death Index. Among 696,743 men and 392,571 women, there were 375 and 96 deaths due to ALS, respectively. Service during the WWII time period was associated with an increased risk of death due to ALS among men (hazard ratio: 1.47; 95%CI: 1.13, 1.91), but none of the other periods were associated with risk of ALS death. There were fewer than five women who had served during any of the other war periods and died of ALS. 22
As an extension, the same research group followed this cohort for an additional 9 years. 23 Due to the small number of women who had served during any of the war periods, the analysis was restricted to men. Between 1979 and 2011, there were 1,007,913 who had responded to the census, of whom, 185 reported serving during WWII, and had died of ALS, resulting in a hazard ratio of 1.3 (95%CI: 1.1–1.6). No other war periods were associated with ALS risk. 23
Among 621 prevalent ALS cases and 958 frequency‐matched controls from the Veterans Affairs Registry who were questioned regarding their previous deployments, the odds of ALS were increased among persons who served in World War II or the Korean War (OR: 4.79; 95%CI: 1.48–15.49 and OR: 3.52; 95%CI: 1.07–11.54, respectively) relative to non‐deployment. 20 There was no altered risk of ALS associated with serving in either the Vietnam war, or any other wars (including Panama and Iraq/Persian Gulf region).
3.3. Exposures related to military service
Six studies reported on ALS risk factors which resulted from specific exposures experienced by military personnel, five based in the United States and one in South Korea. 20 , 24 , 25 , 26 , 27 , 28 Exposures included chemical warfare agents, herbicides, smoking, head injury, and heavy metals.
A spatial analysis of troop movements during the Gulf War sought to determine whether specific geographic locations were associated with an increased risk of ALS. 24 Immediately following the war, the United States Army conducted demolition operations of the munitions facilities in Khamisiyah, Iraq, which contained chemical warfare agents, and may have exposed soldiers nearby. Based on 41 cases of ALS with known locations during the war, researchers concluded that persons who had served near Khamisiyah, Iraq had the highest risk of ALS (RR: 1.7; 95%CI: 0.7–3.7, adjusted for branch of service) compared to those who were deployed outside of this region. Though the difference did not reach statistical significance, the posterior probability that the location was associated with ALS was over 89%. 24
In 2004, a mail survey was sent out to South Korean veterans of the Vietnam war. 25 Of 164,208 eligible subjects, 114,562 (69.8%) responded. Participants were asked to self‐report their Agent Orange exposure during the Vietnam War and whether they had ALS. There were 537 veterans who reported having ALS and low exposure to Agent Orange, and 1328 who reported a high exposure. Following adjustment for age, military rank, smoking, drinking, physical activity, education, household income, herbicide experience in Korea, and body mass index, this resulted in an OR of 2.23 (95%CI: 2.02–2.47) for the high exposure group. However, when geographical proximity to Agent Orange spraying based on known battalion/company location was explored as the exposure, the authors reported no relationship with ALS (OR: 0.95; 95%CI: 0.86–1.06). 25
Four studies 20 , 26 , 27 , 28 of ALS risk have been published using the Genes and Environmental Exposures in Veterans with ALS (GENEVA) 29 study, a case–control study based in the United States with the aim of identifying risk factors for ALS. Participants included persons who had ever been members of the U.S. Army, Air Force, Navy, Marine Corps, Coast Guard, activated Reserves, or National Guard. Cases and controls were recruited from Veterans Affairs, using active and passive methods. Diagnoses of ALS were confirmed by medical record review by a neurologist. Controls were randomly sampled from the same source population and frequency‐matched to cases by age, gender, race, and use of Veterans Administration health care. All participants completed a telephone survey which asked them about a series of environmental exposures specific to the military.
GENEVA participants were asked about their smoking status and history of head injuries. 26 Persons were categorized as smokers if they self‐reported smoking at least one cigarette per day for 6 months or longer. Among 241 incident cases and 597 controls, there was no association of ALS with cigarette smoking. Head injuries were associated with an increased risk of ALS, only among veterans who were >30 years of age (OR 1.88, 95% CI 1.15–3.08) and veterans who had a head injury <=15 years prior to the reference date (ALS diagnosis date or corresponding date for controls) (OR: 2.33; 95%CI: 1.18–4.61). 26
Blood lead levels were compared among 184 ALS cases from the National Registry of Veterans and 194 controls from the GENEVA cohort. 27 A doubling of blood lead levels was associated with a statistically significantly increased odds of ALS (OR: 2.6; 95% CI: 1.9–3.7) after adjustment for age. 27 Using the same study design, researchers examined blood levels of selenium, zinc, copper, and manganese among 163 ALS cases and 229 controls. 28 A doubling of copper levels was associated with increased odds of having ALS (OR: 3.4; 95% CI: 1.5–7.9), while a doubling of selenium or zinc was associated with reduced odds of ALS (OR: 0.4; 95% CI: 0.2–0.8 and OR: 0.4; 95% CI: 0.2–0.8, respectively). The relationship between manganese levels and ALS was more complex as it was non‐linear. The strongest association was found when the mid‐level amount of manganese was compared to the lowest 20% (OR: 2.3; 95% CI: 1.2–4.3). 28
Most recently, information on 39 specific military exposures was collected via standardized telephone interview from 619 ALS cases and 956 frequency‐matched controls in the GENEVA cohort. 20 Six questions related to general military exposures, 15 related to WWII, the Korean, Vietnam, or Gulf War, and the remaining questions specifically asked about each war. The number of respondents to these last questions was smaller than the full cohort size, ranging from 16 to 231 cases, depending on the war. Among the general questions, herbicide exposure for military purposes (OR: 2.52; 95% CI: 1.05–6.05) and pyridostigmine bromide (pre‐treatment pills to protect against nerve agents) use (OR: 2.70; 95% CI: 1.05–6.96) were both found to increase the risk of ALS. Of the exposures which potentially occurred during all four wars, two were found to increase the risk of ALS: exhaust from heaters or generators (OR: 1.75; 95%CI: 1.05–2.92), and explosions in the air or ground within one mile of the person (OR: 1.87; 95%CI: 1.11–3.14). Among those deployed to WWII or the Korean or Vietnams Wars, those who were exposed to burning agents in the field had an increased risk of ALS (OR: 7.75; 95%CI: 1.92–31.24). Among Vietnam War veterans, those exposed to Agent Orange had an increased risk of ALS (OR: 2.80; 95%CI: 1.44–5.44). None of the other exposures significantly altered the risk of ALS. 20
4. DISCUSSION
We systematically reviewed the literature on the risk factors for the development of ALS associated with military service. Between 2000 and 2019, 18 original studies and 1 meta‐analysis were published, which met criteria for inclusion. The majority of studies reported on the risk of ALS associated with military service in general (12 studies, including original studies captured in the meta‐analysis), followed by studies which reported on the risk associated with deployment to the Gulf War (7 studies).
In 2006, the USA Department of Veterans Affairs commissioned a report which concluded that “there is limited and suggestive evidence of an association between military service and later development of ALS”. 30 Eleven years later, a high‐quality meta‐analysis concluded that there is indeed an increased risk; albeit a moderate one. 7 Since the publication of this meta‐analysis, we found an additional three studies that found no altered risk of ALS associated with military service. 11 , 12 , 13 There were common limitations to studies which explored this relationship. First, death due to ALS was used as an outcome in one‐third of studies. 7 While death has been shown to be an appropriate surrogate for ALS incidence, 8 it may be confounded by military service, which can be related to survival. 31 Further, most studies were completed on American veterans. Historically, veterans have had greater access to healthcare than the general American population, which may have also contributed to an ascertainment bias, in which military personnel were over‐represented.
Generally, studies of higher quality tended to report a positive association between military service and ALS. For instance, one of the largest studies, a population‐representative study from Denmark, indicated that military service was associated with a 1.3‐fold increased risk of ALS. 32 This study also revealed a gradient in which a ten‐year increase in years employed by the military was associated with increased odds of developing ALS. 32
Five years prior to the report on military service and ALS, the USA Veterans Affairs made a policy decision to provide disability compensation to Gulf War veterans who developed ALS. 30 Subsequent to this decision, more evidence has emerged to support an association between deployment to the Gulf War and ALS. Three cohort studies reported a significantly increased risk of ALS among persons deployed to the Gulf War, 17 , 18 , 19 while three reported no association. 14 , 15 , 16 It should be noted that the number of ALS cases from the six cohort studies was small, ranging from 6 14 to 40. 18 Further, all studies drew from the same population of American veterans, but each used different methods of case ascertainment, making it unclear how much repetition in cases occurred between studies. The highest quality study, containing the largest number of cases, found an almost two‐times increased risk of ALS associated with deployment. 18 The three earlier studies that reported no altered risk (one of which was a follow‐up of the same cohort) had fewer than 25 cases. It is certainly possible that they were under‐powered to detect a difference. Furthermore, all studies were completed in the decade following the end of the war, which may not provide adequate follow‐up time given that 85% of the persons deployed to the Gulf War were under the age of 35 in 1990, 18 and the onset of ALS peaks between 50 and 75 years of age. Again, the evidence varies, but the higher quality studies point to an elevated risk of ALS associated with Gulf War service. As the number of cases increases, future studies will be necessary to confirm or refute this relationship.
Of the other major 20th century wars, only military service during WWII was consistently associated with the subsequent development of ALS. Three cohort studies explored these relationships, one of which was a follow‐up to an earlier study using the same sources. 21 , 22 , 23 All were limited to the study of men, used self‐report to define the exposure and death records as the outcome. None had information on deployment status; therefore, if the risk factor for ALS was an exposure that occurred during deployment, then the true risk may be even higher. Reasons for an increased risk in WWII are not known. One specific factor to this period—ionizing radiation—was not found to increase the risk of ALS in Japan. 33 It is possible that these findings are related, in part, to a lack of follow‐up time and power, as those who served in more recent years have not yet met the typical risk period for ALS onset.
While studies of military service and deployment are informative, neither can point to a specific etiological agent. For this reason, we also explored the literature on risk factors for ALS experienced by military personnel. Six studies explored risk factors among military personnel and varied in terms of exposure, case ascertainment, and quality. Briefly, exposure to pesticides (Agent Orange), chemicals (exhaust, burning agents), heavy metals, and head trauma, appeared to increase the risk of ALS among military personnel. Two studies reported a more than doubling of risk associated with Agent Orange exposure. 20 , 25 This finding is in concordance with results from a 2012 meta‐analysis which reported a significantly increased risk of ALS associated with pesticide exposure. 34 However, both studies were based on self‐reported exposures and when the South Korean study attempted to use a more objective measure (known battalion/company location), the association disappeared. 25 Proximity to explosions also appeared to increase ALS risk, as noted by two studies. 20 , 24 The first likely related to the release of nerve agents into the atmosphere following the explosion of a munitions factory. 24 The second was based on 151 ALS cases who self‐reported being within one mile of an explosion. 20 It is unclear how an explosion would subsequently lead to ALS, but it may be related to traumatic brain injury or perhaps trauma, both of which have been associated with an increased risk of ALS. 35 , 36 A second study provides further support for a role of traumatic brain injury among military personnel and ALS risk. Self‐reported head trauma was associated with an increased risk of ALS. 26 Cigarette smoking was not found to increase ALS risk among military personnel, contrary to findings from the general population where smoking is considered an established risk factor for ALS. 37 Each of these self‐reported exposures is susceptible to recall bias or reporting error. For instance, a study published after the final search for this review used a more objective measure of traumatic brain injury (US Department of Defense registry data) and found no increased risk of ALS among 139 Veterans with definite ALS. 38 Further, one study reported an association between pyridostigmine bromide (prophylactic pills used in the Gulf War) use and ALS, but found that when soldiers who had implausible values for these exposures (persons who served before the Gulf War) were removed, the association disappeared. 20
Last, heavy metal levels in blood were explored in military personnel and findings were suggestive of an increased risk of ALS associated with higher levels of lead and copper and lower levels of selenium and zinc. 27 , 28 The roles of these metals in mitigating or aggravating oxidative stress may explain these findings. 39 Copper and lead are the most common heavy metals in ammunition fumes. It is possible that these metals serve as a proxy for a wider range of exposures (i.e., various particulate matter and gases from ammunition fumes) and that the combined exposure is a risk factor for ALS. However, these levels were measured on prevalent cases of ALS; therefore, it is impossible to determine whether they represent a cause or consequence of the disease.
All in all, the evidence regarding specific risk factors for ALS was scattered. Most evidence was based on self‐reported exposures and is likely susceptible to reporting errors. None of the factors identified had sufficient evidence to characterize as true etiological factors.
4.1. Strengths and limitations
Strengths of this review include its comprehensive methodology, including a thorough search of three large databases. Articles were screened by multiple trained reviewers and disagreements resolved by consensus, but it is still possible that some studies were missed. Publication bias may have influenced results; however, 6 of the 18 original studies reported null findings, suggesting that this body of literature may be less susceptible to this form of bias. Ultimately, the quality of a review hinges on the quality of the original research. While the average quality score of the included studies was high, there were some common limitations which should be noted. Many of the studies included a small number of ALS cases. This is an inherent challenge to studying a rare disease, but it may have resulted in a lack of statistical power to detect differences between groups. Most studies reported on both sexes; however, overall there were very few female cases of ALS included. There is a clear preponderance of men with ALS, by a factor of 1.2–1.5. 3 The military is also a heavily male‐dominated field; between 1973 and 2010, women never represented more than 14% of active‐duty military personnel in the United States. 40 It is possible that men bear a greater biological risk than women, or they may be more likely to be exposed to environmental risk factors, such as those common among military personnel. Most studies adequately controlled for the sex disparity through matching or adjustment of their models, but the findings of this systematic review pertain mostly to men due to the overwhelming proportion of men in each cohort. The studies were also predominantly of Americans of European descent, meaning that the generalizability of these findings to other groups is not known. None of the studies explored a cumulative risk score, and only a single study investigated the effect modification of multiple variables. 26 The case–control studies of prevalent ALS cases may have been subject to survivor bias, in that only persons who survive long enough to participate in a study would be included. Cohort studies were largely limited by a lack of adequate follow‐up time given that persons typically serve in the military as young adults, while ALS affects older adults.
4.2. Future directions
In their profession, military personnel are exposed to strenuous physical activity, trauma, and contaminants. 6 Although many questions remain unanswered, there appears to be an increased risk of ALS among military personnel, particularly among individuals who were deployed to WWII or the Gulf War. Understanding specific exposures, or combinations thereof, associated with ALS is of vital importance in order to implement preventative strategies into military organizations. Future studies should endeavor to access large source populations and report their power calculations to ensure that null results are not misinterpreted; consider duration of exposures; and include adequate follow‐up time to ensure that they are capturing a true “at‐risk” population. Standardization of study design and exposure definitions may be required to better understand these relationships through international efforts.
CONFLICT OF INTEREST
KA McKay, KA Smith, L Smertinaite, F Fang, C Ingre, and F Taube report no disclosures.
STUDY SPONSORSHIP
This research was funded with a research grant from the Swedish Armed Forces.
Supporting information
Appendix
ACKNOWLEDGEMENTS
Special thank you to Sabina Gillsund and Magdalena Svanberg, librarians with the Karolinska University Library, for their fundamental work in helping to develop and implement the literature search strategy.
FUNDING INFORMATION
KA McKay receives postdoctoral research support from the Canadian Institutes of Health Research (CIHR) and Forte, and was funded for this project by the Swedish Armed Forces.
REFERENCES
- 1. Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–649. [DOI] [PubMed] [Google Scholar]
- 2. Chio A, Logroscino G, Traynor BJ, et al. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41(2):118–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al‐Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–628. [DOI] [PubMed] [Google Scholar]
- 4. Mehta P, Kaye W, Raymond J, et al. Prevalence of Amyotrophic Lateral Sclerosis ‐ United States, 2014. MMWR ‐ Morb Mortal Wkly Rep. 2018;67(7):216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson LM, Topol B, Kaye W, et al. Estimation of the Prevalence of Amyotrophic Lateral Sclerosis in the United States Using National Administrative Healthcare Data from 2002 to 2004 and Capture‐Recapture Methodology. Neuroepidemiology. 2018;51(3–4):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology and survival. Epidemiol Rev. 2015;37:55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tai H, Cui L, Shen D, Li D, Cui B, Fang J. Military service and the risk of amyotrophic lateral sclerosis: A meta‐analysis. J Clin Neurosci. 2017;45:337–342. [DOI] [PubMed] [Google Scholar]
- 8. Marin B, Couratier P, Preux PM, Logroscino G. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology. 2011;36(1):29–38. [DOI] [PubMed] [Google Scholar]
- 9. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ (Research Methods Reporting). 2017;358:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta‐Analyses. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 11. Andrew AS, Caller TA, Tandan R, et al. Environmental and occupational exposures and amyotrophic lateral sclerosis in New England. Neurodegener Dis. 2017;17(2–3):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dickerson AS, Hansen J, Kioumourtzoglou MA, Specht AJ, Gredal O, Weisskopf MG. Study of occupation and amyotrophic lateral sclerosis in a Danish cohort. Occup Environ Med. 2018;75(9):630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters TL, Kamel F, Lundholm C, et al. Occupational exposures and the risk of amyotrophic lateral sclerosis. Occup Environ Med. 2017;74:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith TC, Gray GC, Knoke JD. Is systemic lupus erythematosus, amyotrophic lateral sclerosis, or fibromyalgia associated with Persian Gulf War service? An examination of Department of Defense hospitalization data. Am J Epidemiol. 2000;151(11):1053–1059. [DOI] [PubMed] [Google Scholar]
- 15. Kang HK, Bullman TA. Mortality among US veterans of the Persian Gulf War: 7‐year follow‐up. Am J Epidemiol. 2001;154(5):399–405. [DOI] [PubMed] [Google Scholar]
- 16. Barth SK, Kang HK, Bullman TA, Wallin MT. Neurological mortality among U.S. veterans of the Persian Gulf War: 13‐year follow‐up. Am J Ind Med. 2009;52(9):663–670. [DOI] [PubMed] [Google Scholar]
- 17. Haley RW. Excess incidence of ALS in young Gulf War veterans. Neurology. 2003;61(6):750–756. [DOI] [PubMed] [Google Scholar]
- 18. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742–749. [DOI] [PubMed] [Google Scholar]
- 19. Coffman CJ, Horner RD, Grambow SC, Lindquist J, Project VACSP . Estimating the occurrence of amyotrophic lateral sclerosis among Gulf War (1990–1991) veterans using capture‐recapture methods. Neuroepidemiology. 2005;24(3):141–150. [DOI] [PubMed] [Google Scholar]
- 20. Beard JD, Engel LS, Richardson DB, et al. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis etiology. Environ Int. 2016;91:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weisskopf MG, O’Reilly EJ, McCullough ML, et al. Prospective study of military service and mortality from ALS. Neurology. 2005;64(1):32–37. [DOI] [PubMed] [Google Scholar]
- 22. Weisskopf MG, Cudkowicz ME, Johnson N. Military Service and Amyotrophic Lateral Sclerosis in a Population‐based Cohort. Epidemiology. 2015;26(6):831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cragg JJ, Johnson NJ, Weisskopf MG. Military service and amyotrophic lateral sclerosis in a population‐based cohort: extended follow‐up 1979–2011. Epidemiology. 2017;28(2):e15–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Miranda ML, Alicia Overstreet Galeano M, Tassone E, Allen KD, Horner RD. Spatial analysis of the etiology of amyotrophic lateral sclerosis among 1991 Gulf War veterans. Neurotoxicology. 2008;29(6):964–970. [DOI] [PubMed] [Google Scholar]
- 25. Yi SW, Ohrr H, Hong JS, Yi JJ. Agent orange exposure and prevalence of self‐reported diseases in Korean Vietnam veterans. J Prev Med Public Heal. 2013;46(5):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J Neurol Sci. 2010;291(1–2):22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fang F, Kwee LC, Allen KD, et al. Association between blood lead and the risk of amyotrophic lateral sclerosis. Am J Epidemiol. 2010;171(10):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters TL, Beard JD, Umbach DM, et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology. 2016;54:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmidt S, Allen KD, Loiacono VT, et al. Genes and Environmental Exposures in Veterans with Amyotrophic Lateral Sclerosis: the GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30(3):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Department of Veterans A . Presumption of service connection for amyotrophic lateral sclerosis. Final rule. Fed Regist 2009;74(212):57072–57074. [PubMed] [Google Scholar]
- 31. McLaughlin R, Nielsen L, Waller M. An evaluation of the effect of military service on mortality: quantifying the healthy soldier effect. Ann Epidemiol. 2008;18(12):928–936. [DOI] [PubMed] [Google Scholar]
- 32. Seals RM, Kioumourtzoglou MA, Hansen J, Gredal O, Weisskopf MG. Amyotrophic lateral sclerosis and the military: a population‐based study in the Danish registries. Epidemiology. 2016;27(2):188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kondo K, Tsubaki T. Case‐Control studies of motor neuron Disease: Association with mechanical injuries. Arch Neurol. 1981;38(4):220–226. [DOI] [PubMed] [Google Scholar]
- 34. Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta‐analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012;117:112–119. [DOI] [PubMed] [Google Scholar]
- 35. Chen H, Richard M, Sandler DP, Umbach DM, Kamel F. Head injury and amyotrophic lateral sclerosis. Am J Epidemiol. 2007;166(7):810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pupillo E, Messina P, Logroscino G, et al. Trauma and amyotrophic lateral sclerosis: A case‐control study from a population‐based registry. Eur J Neurol. 2012;19(12):1509–1517. [DOI] [PubMed] [Google Scholar]
- 37. Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;73:1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sagiraju HKR, Zivković S, VanCott AC, et al. Amyotrophic Lateral Sclerosis among Veterans deployed in support of post‐9/11 U.S. conflicts. Mil Med. 2020;185(3–4):e501–e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jellinger KA. The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. Int Rev Neurobiol. 2013;110:1–47. [DOI] [PubMed] [Google Scholar]
- 40. Patten E, Parker K. Women in the U.S. Military: Growing Share, Distinctive Profile. Pew Social & Demographic Trends; 2010. https://www.pewsocialtrends.org/2011/12/22/women‐in‐the‐u‐s‐military‐growing‐share‐distinctive‐profile/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix


