Abstract
Aim
To estimate the cost‐effectiveness of dapagliflozin added to standard therapy, vs. standard therapy only, in patients with heart failure (HF) with reduced ejection fraction (HFrEF), from the perspective of UK, German, and Spanish payers.
Methods and results
A lifetime Markov model was built to estimate outcomes in patients with HFrEF. Health states were defined by Kansas City Cardiomyopathy Questionnaire total symptom score, type 2 diabetes and worsening HF events. The incidence of worsening HF and all‐cause mortality was estimated using negative binomial regression models and parametric survival analysis, respectively. Direct healthcare costs (2019 British pounds/Euro) and patient‐reported outcomes (EQ‐5D) were sourced from the existing literature and the Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF), respectively; the median duration of follow‐up in DAPA‐HF was 18.2 months (range: 0–27.8). Future costs and effects were discounted at 3.0% for the Spanish and German analyses and 3.5% for the UK analysis. In the UK setting, treatment with dapagliflozin was estimated to increase life‐years and quality‐adjusted life‐years (QALYs) from 5.62 to 6.20 (+0.58) and 4.13 to 4.61 (+0.48), respectively, and reduce lifetime hospitalizations for HF (925 and 820 events per 1000 patients for placebo and dapagliflozin, respectively). Similar results were obtained for Germany and Spain. The incremental cost‐effectiveness ratios were £5822, €5379 and €9406/QALY in the UK, Germany and Spain, respectively. In probabilistic sensitivity analyses, more than 90% of simulations were cost‐effective at a willingness‐to‐pay threshold of £20 000/QALY in UK and €20 000/QALY in Germany and Spain.
Conclusion
Dapagliflozin is likely to be a cost‐effective treatment for HFrEF in the UK, German and Spanish healthcare systems.
Keywords: Heart failure, Sodium–glucose co‐transporter 2, Cost‐effectiveness, Hospitalization, Survival, Quality of life
Introduction
Heart failure (HF) represents a major public health challenge; it is a severely symptomatic syndrome, greatly reducing quality of life, and is a leading cause of hospitalization and death. 1 Global HF prevalence is estimated at 23 million, including 16 million people in Europe. 2 , 3 HF is the leading cause of hospital admission in people over the age of 65 years, 4 accounting for 5% of all acute hospital admissions in Europe. 5 HF represents an expensive and growing public health problem. 6 , 7 , 8 It has been estimated that 1‐year costs related to HF in the European Union are around €29 billion, 9 with frequent, prolonged and repeat hospitalizations accounting for the majority of these costs. 10 The economic burden of HF is likely to increase substantially over the next decade, driven by the ageing of the population and an estimated 46% increase in HF prevalence by 2030. 11 , 12
The primary goals of HF treatment are to improve symptoms and quality of life, prevent hospital admission and reduce mortality. At present, evidence‐based therapy is only available for HF patients with reduced ejection fraction (HFrEF), who represent at least half of all cases of this syndrome. Current treatment of HFrEF consists of pharmacological therapy and devices, in selected patients. Optimum pharmacological treatment includes a renin‐angiotensin system blocker (ideally coupled with a neprilysin inhibitor), a beta‐blocker and mineralocorticoid receptor antagonist, usually given with a diuretic to control fluid retention. 13 However, as identified in international guidelines, there is need for more therapies to improve morbidity and mortality in HF patients. The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure trial (DAPA‐HF) demonstrated that dapagliflozin (10 mg once daily), added to standard HFrEF therapy, reduced the risk of hospitalization for HF (HHF) (by 30%) and cardiovascular death (by 18%), when compared with placebo. 14
A key consideration for healthcare decision‐makers involves reconciling additional, short‐term, expenditure on disease management with delivering long‐term projected health outcome benefits. Cost‐effectiveness evaluations play an important role in supporting decision‐makers seeking to understand the value of competing health technologies and management strategies. Thus, the objective of this study is to assess the cost‐effectiveness of dapagliflozin added to standard therapy vs. standard therapy alone from a multinational European payer perspective.
Methods
DAPA‐HF (NCT03036124) was a randomized, double‐blind, placebo‐controlled, event‐driven, trial in patients with HFrEF. 14 , 15 The efficacy and safety of dapagliflozin 10 mg once daily, added to standard care, was compared with matching placebo. The design, baseline characteristics, and results of the trial have been published. The Ethics Committee of each of the 410 participating institutions (in 20 countries) approved the protocol, and all patients gave written informed consent.
Trial design and patient population
Men and women aged ≥18 years with HF were eligible for DAPA‐HF if they were in New York Heart Association (NYHA) functional class II to IV, had a left ventricular ejection fraction ≤40%, and were optimally treated with pharmacological and device therapy. Patients were excluded if they had any symptoms of hypotension, a systolic blood pressure >95 mmHg, estimated glomerular filtration rate <30 mL/min/1.73 m2 (or rapidly declining renal function), or type 1 diabetes mellitus. A full list of inclusion and exclusion criteria is provided in the design paper. 14 Patients enrolled in DAPA‐HF were randomized to receive dapagliflozin or placebo in addition to standard therapy, stratified based on diagnosis of type 2 diabetes mellitus (T2DM) at screening. Following randomization, follow‐up visits occurred at 14, 60, 120, 240, 360 days and every 4 months thereafter. The median duration of follow‐up was 18.2 months (range: 0–27.8 months). The primary outcome was the composite of an episode of worsening HF (HHF or an urgent visit because of worsening HF requiring intravenous therapy) or cardiovascular death, whichever occurred first. The secondary endpoints were: the occurrence of HHF or cardiovascular death, HHF (first and recurrent) and cardiovascular death, change from baseline to 8 months in the total symptom score of the Kansas City Cardiomyopathy Questionnaire (KCCQ‐TSS), 12 the incidence of a composite worsening renal function outcome and death from any cause.
Pre‐specified safety analyses included any serious adverse event (AE), AEs leading to discontinuation of trial treatment, AEs of interest (volume depletion, worsening renal function, major hypoglycaemic episodes, fracture, diabetic ketoacidosis, amputation) and any diagnosis of Fournier's gangrene, as well as laboratory findings of note. The EuroQol five‐dimensional five‐level questionnaire (EQ‐5D‐5L), a patient self‐reported questionnaire, commonly used to derive a standardized measure of health status, also referred to as a utility score, was prospectively collected to support health economic evaluations of DAPA‐HF. Patients completed both EQ‐5D‐5L and KCCQ at baseline, 4, 8, 12 months, and every 12 months thereafter until the end of the study.
Economic model
Decision problem and model
We developed a Markov state‐transition cohort model designed to assess the cost‐effectiveness of dapagliflozin, used in addition to regional standard therapy, in comparison with standard therapy alone, in the UK, Germany and Spain. The model employed a lifetime perspective to accommodate the chronic and progressive nature of HF, with a monthly cycle length, consistent with previous HF economic models. 16 , 17 , 18 The primary model outcome was the incremental cost‐effectiveness ratio (ICER), expressed as the cost per quality‐adjusted life‐year (QALY) gained. The future value of both costs and effects were discounted using established country‐specific discounting rates: 3.5% per annum for the UK and 3.0% per annum for Germany and Spain. 19
Analysis
Base‐case analysis reflected the overall DAPA‐HF population, with additional analyses of clinically relevant pre‐specified subgroups: age (≤65 vs. >65 years); HF duration (≤2 years vs. >2 years); prior HHF (yes/no), T2DM (yes/no), ischaemic aetiology (yes/no); and the following stratified by ≤ median vs. > median – left ventricular ejection fraction, N‐terminal pro B‐type natriuretic peptide (NT‐proBNP), body mass index, creatinine and KCCQ‐TSS. Deterministic sensitivity analysis was used to explore the impact of varying input variables on cost‐effectiveness and probabilistic sensitivity analysis was used to quantify overall variable uncertainty. 14
Disease progression
Disease progression within the model was captured using transitions between discrete health states, characterized by KCCQ‐TSS quartiles as an effective measure of patient symptoms and a proxy for disease severity and in post‐hoc analysis was found to be a strong predictor of patient outcomes with an approximate 2.5‐fold increase in risk of all‐cause mortality (online supplementary Table S1 ) for patients in the lowest quartile of KCCQ‐TSS in comparison with the highest quartile. Quartiles were chosen to ensure adequate patient numbers in each subgroup to permit statistically robust analysis, while retaining sufficient granularity in predicting patient outcomes; however, previously published analysis has shown a consistent effect when stratifying patients by KCCQ‐TSS tertiles. 20 Model health states were further stratified by baseline T2DM status in order to capture increased risk, reduced quality of life and increased background management costs for patients with comorbid T2DM (Figure 1 ). Transition probabilities between health states defined by KCCQ‐TSS quartiles were derived using monthly transition count data, assuming that the last observation was carried forward. Independent transition matrices were derived based on the first 4 months of follow‐up in DAPA‐HF, after which an inflection point was observed, and a second transition matrix was applied from month 5 onwards. 20 Transition counts had a multinomial likelihood, which was combined with a flat Dirichlet prior distribution using Gibbs sampling to obtain the posterior probability distribution of the KCCQ‐TSS transition matrix (online supplementary Table S2 ). 21 KCCQ‐TSS transition probabilities were assumed to be equivalent for patients with and without baseline T2DM, and new cases of T2DM were not modelled.
Figure 1.
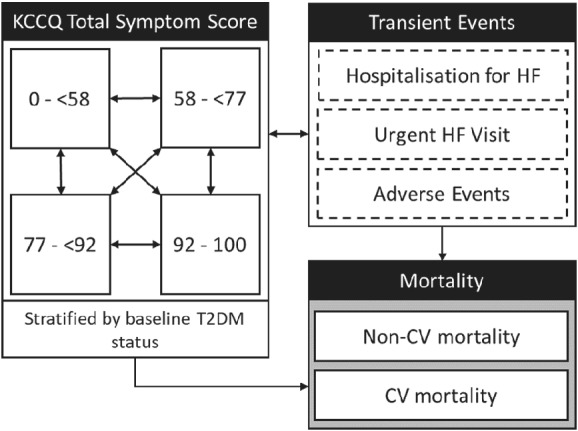
Model schematic. CV, cardiovascular; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; T2DM, type 2 diabetes mellitus.
Mortality, hospitalization for heart failure and adverse events
The model captured the incidence of first and recurrent HHF and urgent HF visits as discrete clinical events, as well as capturing the occurrence of relevant AEs, cardiovascular and all‐cause mortality. Parametric multivariable survival analysis was used to model all‐cause mortality and cardiovascular mortality over time, adjusted for time‐updated KCCQ‐TSS, baseline patient characteristics including T2DM and study arm, as such, patients with lower KCCQ‐TSS or comorbid T2DM were at increased risk of mortality and HHF events. Analysis was conducted from an intention‐to‐treat perspective using the complete DAPA‐HF dataset. 14 The approach to statistical model selection was consistent with guidelines for the analysis of survival data alongside clinical trials, with goodness‐of‐fit assessed through minimizing Akaike Information Criterion. 22 , 23 Mortality was assumed to follow a Weibull distribution in the base‐case analysis, following survival extrapolations compared to previously published long‐term projections to ensure plausible extrapolations. 24 , 25 , 26 Sensitivity of the results of the model to the choice of survival distribution was assessed using deterministic sensitivity analysis. The proportion of deaths attributable to non‐cardiovascular causes was calculated using country‐specific life‐tables, adjusted to remove cardiovascular mortality to avoid double‐counting. 27 , 28 , 29 , 30 Negative binominal generalized estimating multivariable regression models were developed to estimate the incidence of HHF and urgent HF visits in order to capture both first and subsequent episodes of worsening HF. As only 33 first urgent HF visits were observed in DAPA‐HF, the incidence of urgent HF visits was applied using a constant, treatment arm specific incidence rate. Details of the regression models developed for mortality and HHF are provided in online supplementary Table S3 .
The following specific AEs were modelled: volume depletion, worsening renal function, episodes of major hypoglycaemia, fracture, diabetic ketoacidosis, amputation, urinary tract infection and genital infection. The incidence of genital infection was not routinely collected in DAPA‐HF, as such modelled incidence rates are based on the dapagliflozin and placebo arms of DECLARE‐TIMI 58. 31 Serious AEs were not modelled in their entirety due to the overall favourable tolerability profile associated with dapagliflozin, with 35.7% of patients treated with dapagliflozin experiencing a serious AE in comparison with 40.2% in the placebo arm of DAPA‐HF. Treatment specific AEs were modelled assuming a constant hazard; these are reported in online supplementary Table S4 .
Health‐related quality of life
Utility estimates were derived from a pooled analysis of individual patient‐level EQ‐5D‐5L data from DAPA‐HF. 14 EQ‐5D‐5L responses were first mapped to EQ‐5D‐3L applying the mapping function developed by van Hout et al. 32 Responses were then converted to utility index scores using published UK utility values for EQ‐5D health states, derived using the time trade‐off method described in Dolan. 33 Linear mixed effects regression models were fitted to patient reported utility values adjusting for KCCQ‐TSS, T2DM status, age and sex in addition to the incidence of discrete clinical events. Stable estimates of disutility associated with major hypoglycaemia, diabetic ketoacidosis and amputation could not be derived from DAPA‐HF due to their low incidence; relevant disutility values were sourced from published literature. The full DAPA‐HF utility analysis is provided in online supplementary Table S5 . Derived health state utility values associated with KCCQ‐TSS quartiles and the utility decrement associated with comorbid T2DM and the incidence of events are shown in Table 1 . 34 , 35 , 36 , 37 , 38 Consequently, the estimation of QALYs was determined by KCCQ‐TSS state specific occupancy over time, the proportion of the cohort with comorbid T2DM, and the incidence of discrete clinical events such as worsening HF events and AEs (applied during the month of incidence only).
Table 1.
Utility inputs
| Health state | Mean | SE | Source |
|---|---|---|---|
| KCCQ‐TSS: 1–<58 | 0.600 | 0.016 | DAPA‐HF |
| KCCQ‐TSS: 58–<77 | 0.705 | 0.016 | DAPA‐HF |
| KCCQ‐TSS: 77–<92 | 0.773 | 0.016 | DAPA‐HF |
| KCCQ‐TSS: 92–100 | 0.833 | 0.016 | DAPA‐HF |
| Baseline comorbidities | |||
| T2DM a | −0.017 | 0.003 | DAPA‐HF |
| HF events | |||
| HHF | −0.321 | 0.020 | DAPA‐HF c |
| Urgent HF visit | −0.036 | 0.011 | DAPA‐HF |
| Adverse events | |||
| Volume depletion | −0.051 | 0.012 | DAPA‐HF |
| Renal dysfunction | −0.076 | 0.014 | DAPA‐HF |
| Major hypoglycaemia | −0.014 | 0.001 b | Currie et al., 34 Beaudet et al. 35 |
| Fracture | −0.149 | 0.033 | DAPA‐HF |
| Diabetic ketoacidosis | −0.009 | 0.010 | Peasgood et al. 36 |
| Amputation | −0.280 | 0.053 | UKPDS 62 37 , Beaudet et al. 35 |
| Genital infection | −0.003 | 0.001 | Barry et al. 38 |
| Urinary tract infection | −0.003 | 0.001 | Barry et al. 38 |
HF, heart failure; HHF, hospitalization for heart failure; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; SE, standard error; T2DM, type 2 diabetes mellitus.
Applied to the proportion of DAPA‐HF cohort with T2DM at study entry.
Assumed 10% of mean value.
Input has been scaled to represent estimated quality‐adjusted life‐years lost due to event incidence and is applied for 1 month only.
Resource use
Each of the modelled health states (including the transient health states describing the incidence of events) were assigned a relevant health state cost [2019 British pound (GBP)/Euro]. The proportion of the cohort residing within each health state informed the accrual of costs over time. Costs describing background resource use associated with HFrEF were applied to all patients and included contact with primary care, cardiologist visits and A&E referrals with additional costs included for patients with comorbid T2DM. Event‐specific costs were applied as a one‐off cost in the model with the occurrence of an event. Patient time on treatment was informed by the annual probability of premature discontinuation derived from DAPA‐HF (7% per annum). Dapagliflozin treatment costs were applied while patients remained on therapy, with only the costs of standard therapy applied in patients discontinued from dapagliflozin; additional monitoring related costs (e.g. patient review and checking blood chemistry), specifically two outpatient visits, were applied to those receiving dapagliflozin within the first year. The country specific cost inputs are reported in online supplementary Table S6 .
Results
The characteristics of patients at baseline are reported in Table 2 . The utility values applied in the model are shown in Table 1 . The country specific costs utilized in the analyses are shown in online supplementary Table S6 .
Table 2.
Characteristics of patients at baseline
| Characteristic | Dapagliflozin + standard therapy | Standard therapy |
|---|---|---|
| Age (years) | 66.2 (11) | 66.5 (10.8) |
| Female sex (%) | 23.8 | 23.0 |
| Body mass index (kg/m2) | 28.2 (6) | 28.1 (5.9) |
| Race (%) | ||
| White | 70.0 | 70.5 |
| Black | 5.1 | 4.4 |
| Asian | 23.3 | 23.8 |
| Other | 1.6 | 1.3 |
| Region (%) | ||
| North America | 14.1 | 14.4 |
| South America | 16.9 | 17.5 |
| Europe | 46.1 | 44.7 |
| Asia‐Pacific | 22.9 | 23.3 |
| NYHA functional class (%) | ||
| II | 67.7 | 67.4 |
| III | 31.5 | 31.7 |
| IV | 0.8 | 1.0 |
| Heart rate (bpm) | 71.5 (11.6) | 71.5 (11.8) |
| Systolic blood pressure (mmHg) | 122 (16.3) | 121.6 (16.3) |
| Left ventricular ejection fraction (%) | 31.2 (6.7) | 30.9 (6.9) |
| Median NT‐proBNP [IQR] (pg/mL) | 1428 [857–2655] | 1446 [857–2641] |
| Principal cause of HF (%) | ||
| Ischaemic | 55.5 | 57.3 |
| Non‐ischaemic | 36.1 | 35.0 |
| Unknown | 8.4 | 7.7 |
| Medical history (%) | ||
| HHF | 47.4 | 47.5 |
| Atrial fibrillation | 38.6 | 38.0 |
| Diabetes mellitus | 41.8 | 41.8 |
| Estimated GFR | ||
| Mean (mL/min/1.73 m2) | 66 (19.6) | 65.5 (19.3) |
| <60 mL/min/1.73 m2 (%) | 40.6 | 40.7 |
| Device therapy (%) | ||
| Implantable cardioverter‐defibrillator | 26.2 | 26.1 |
| Cardiac resynchronization therapy | 8.0 | 6.9 |
| HF medication (%) | ||
| Diuretic | 93.4 | 93.5 |
| ACE inhibitor | 56.1 | 56.1 |
| ARB | 28.4 | 26.7 |
| Sacubitril/valsartan | 10.5 | 10.9 |
| Beta‐blocker | 96.0 | 96.2 |
| Mineralocorticoid receptor antagonist | 71.5 | 70.6 |
| Digitalis | 18.8 | 18.6 |
Numbers in brackets are standard deviations.
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; GFR, glomerular filtration rate; HF, heart failure; HHF, hospitalization for heart failure; IQR, interquartile range; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
The modelled results indicate that dapagliflozin in addition to standard therapy was cost‐effective for the treatment of HFrEF in all three country settings (Table 3 ). Treatment with dapagliflozin was associated with QALY gains of 0.48, 0.50 and 0.50, and an incremental cost per QALY gained of £5822, €5379 and €9406/QALY in the UK, Germany and Spain, respectively. QALY gains in all three countries were driven largely by improved life expectancy for patients treated with dapagliflozin, with 14 fewer deaths after 1 year, and 57 fewer deaths after 5 years per 1000 treated patients. Variations in predicted life‐years and QALYs reported for UK, Germany and Spain reflect the use of country specific life tables and discount rates (Table 3 ). Differences in cost per QALY were driven primarily by drug acquisition costs. HHF, urgent HF visits and cardiovascular deaths avoided provided important cost offsets to expenditure on dapagliflozin (Table 3 ). Over a lifetime horizon, treatment with dapagliflozin was estimated to be associated with 105 fewer HHF events and 22 fewer urgent HF visits per 1000 treated patients. The resultant cost offsets in the UK, Germany and Spain were £378, €655 and €520, respectively, reflecting differences in healthcare costs in each country.
Table 3.
Base‐case results
| Dapagliflozin + standard therapy | Standard therapy | Incremental | |
|---|---|---|---|
| UK | |||
| Total costs | £16 408 | £13 628 | £2780 |
| Treatment, monitoring and adverse events | £4287 | £1917 | £2370 |
| Worsening HF events and CV death | £3851 | £4229 | – £378 |
| Background resource use | £8270 | £7482 | £788 |
| Total LYs | 6.20 | 5.62 | 0.58 |
| Total QALYs | 4.61 | 4.13 | 0.48 |
| ICER | – | – | £5822/QALY |
| Germany | |||
| Total costs | €25 328 | €22 647 | €2681 |
| Treatment, monitoring and adverse events | €7637 | €5059 | €2578 |
| Worsening HF events and CV death | €9944 | €10 598 | – €655 |
| Background resource use | €7747 | €6990 | €757 |
| Total LYs | 6.35 | 5.74 | 0.61 |
| Total QALYs | 4.72 | 4.22 | 0.50 |
| ICER | – | – | €5379/QALY |
| Spain | |||
| Total costs | €24 330 | €19 642 | €4688 |
| Treatment, monitoring and adverse events | €10 139 | €5785 | €4354 |
| Worsening HF events and CV death | €5425 | €5945 | – €520 |
| Background resource use | €8766 | €7912 | €854 |
| Total LYs | 6.35 | 5.74 | 0.61 |
| Total QALYs | 4.72 | 4.22 | 0.50 |
| ICER | – | – | €9406/QALY |
| Clinical events a | |||
| HHF (per 1000 treated patients) | 820 | 925 | −105 |
| Urgent HF visit (per 1000 treated patients) | 32 | 54 | −22 |
| 1‐year survival | 91.8% | 90.3% | 1.6% |
| 2‐year survival | 82.7% | 79.6% | 3.1% |
| 5‐year survival | 56.6% | 50.9% | 5.7% |
CV, cardiovascular; HF, heart failure; HHF, hospitalization for heart failure; ICER, incremental cost‐effectiveness ratio; LY, life‐year; QALY, quality‐adjusted life‐years.
Clinical events reported relate to output from the UK model.
Subgroup analysis and parameter uncertainty
Dapagliflozin remained cost‐effective with only modest changes from the results of the base‐case analysis across patient subgroups and the countries of interest (Figure 2 and online supplementary Tables S7–S9 ). The subgroup that exhibited the largest change from base‐case cost‐effectiveness was that defined by median NT‐proBNP concentration. In patients with an NT‐proBNP at or below the median value, estimated QALY gains increased compared to the base‐case results, as did total costs (driven by improved life expectancy). This resulted in modest increases in the estimated ICERs to £6671, €6125 and €10 706 for the UK, Germany and Spain, respectively. In general, dapagliflozin was more cost‐effective in more high‐risk patient subgroups such as those with lower ejection fraction, higher NT‐proBNP level, prior HHF, lower KCCQ‐TSS, and longer duration of HF, as a result of the higher incidence of clinical events, and lower incremental costs due to shorter life expectancy, compared to lower risk patient subgroups. A noteworthy exception to this overall finding were patients without T2DM, where dapagliflozin was more cost‐effective than in those with T2DM. This finding reflected the higher annual costs associated with comorbid T2DM. Consequently, increased life expectancy with dapagliflozin resulted in an increase in incremental costs. There was also a reduction in incremental health gains as patients with T2DM at baseline had poorer health‐related quality of life, thereby resulting in fewer QALYs gained per life‐year gained. Cost‐effectiveness was also robust to the choice of survival distribution, with all survival distributions resulting in ICERs less than £20 000 or €20 000/QALY (online supplementary Tables S10–S12 ).
Figure 2.
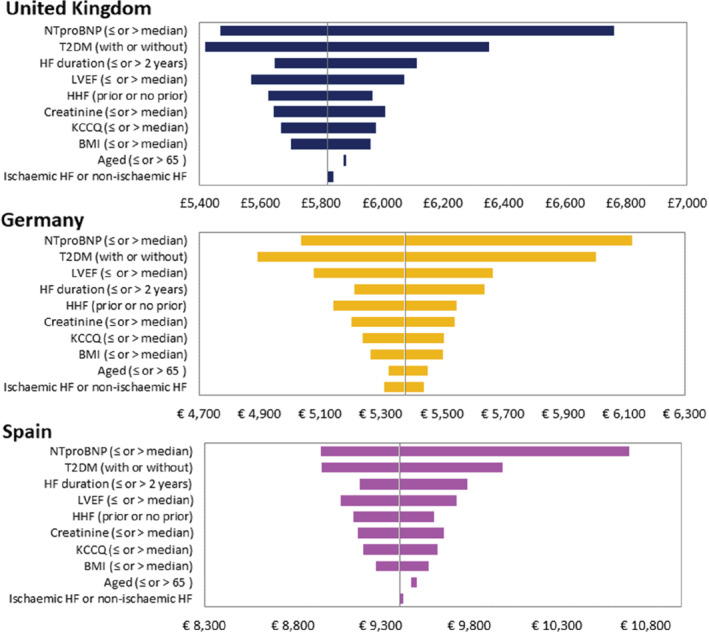
Results of subgroup analysis. BMI, body mass index; HF, heart failure; HHF, hospitalization for heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; NTproBNP, N‐terminal pro‐B‐type natriuretic peptide; T2DM, type 2 diabetes mellitus.
In probabilistic sensitivity analysis, 96% of simulations were cost‐effective at a willingness‐to‐pay threshold of £20 000/QALY for the UK, and 97% and 91% of simulations were cost‐effective at a willingness‐to‐pay threshold of €20 000/QALY for Germany and Spain, respectively (Figure 3 ).
Figure 3.
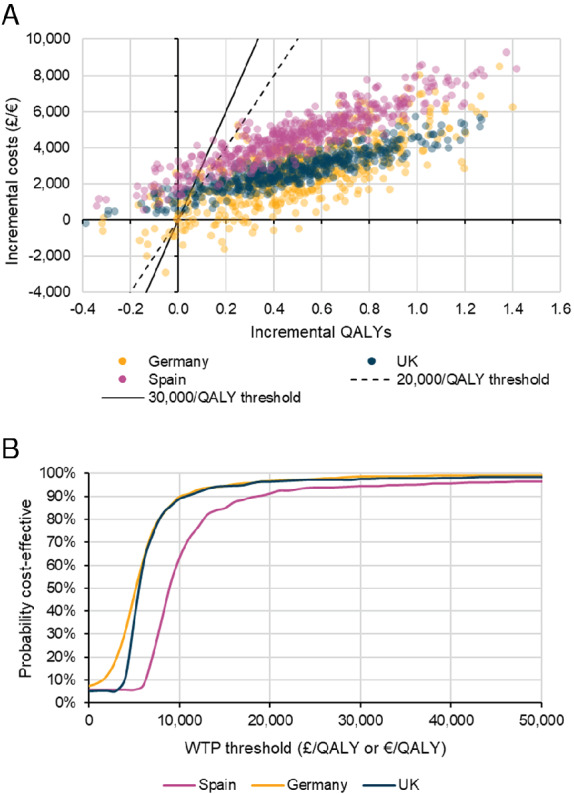
Probabilistic model results: (A) incremental costs and benefits, (B) cost‐effectiveness acceptability curves. QALY, quality‐adjusted life‐year; WTP, willingness‐to‐pay.
Discussion
The base‐case analyses for the UK, Germany and Spain indicate that dapagliflozin, in addition to standard therapy, is cost‐effective for the treatment of HFrEF, compared with standard therapy alone, at established willingness‐to‐pay thresholds. These results were principally driven by reductions in cardiovascular and all‐cause mortality, resulting in significant life‐year and QALY gains for those treated with dapagliflozin. The avoidance of HHF was associated with more modest QALY gains but contributed to important cost‐savings, which partially offset the additional cost of dapagliflozin.
Our results are consistent with other contemporary cost‐effectiveness analyses in patients with HFrEF. In PARADIGM‐HF, the hazard ratio for the primary endpoint (cardiovascular mortality or HHF) was 0.80 for sacubitril/valsartan compared with enalapril, with QALY gains of 0.42 to 0.52 and ICERs ranging from £17 100 to €26 278 in the UK and Germany, respectively. 24 , 39 In DAPA‐HF the hazard ratio for a similar primary outcome was 0.74, with QALY gains of 0.48 to 0.50. The more favourable ICERs in DAPA‐HF reflect the lower incremental cost of dapagliflozin, compared with that of sacubitril/valsartan. Other recent relevant cost‐effectiveness analyses include those for ivabradine, cardiac resynchronization therapy (CRT) and cardiac contractility modulation (CCM). 25 , 26 , 40 , 41 , 42 These studies reported QALY gains of 0.18 to 0.28 (ivabradine); 0.77 (CRT) and 0.68 and 1.03 (CCM), resulting in an ICER of £13 764 and €17 488 for ivabradine in the UK and Spain, respectively; €21 500/QALY for CRT in Spain; and £22 988/QALY for CCM in the UK. Importantly, some patients in DAPA‐HF were treated with sacubitril/valsartan, ivabradine and CRT at baseline and the benefits of dapagliflozin in these subgroups were consistent with the overall benefit in the trial. 14
Cost‐effectiveness analysis provides a formal mechanism to establish whether the incremental cost of a new technology is justified by its health gains and associated cost offsets; however, this approach does not necessarily capture the full value to a healthcare system. For example, while we have applied appropriate unit costs for HHF, for healthcare systems running at, or close to, capacity the value of even modest reductions in hospital admissions can have a meaningful impact on service delivery. This is of particular relevance to HF given the expected increase in its prevalence and where the majority of costs are incurred within secondary care. It should be noted that we did not consider healthcare costs unrelated to HF. This approach is consistent with both guidelines and previously published cost‐effectiveness analyses in patients with HFrEF. 43 However, as the gains in quality‐adjusted life expectancy reported in this study largely reflect an increase in life expectancy, patients treated with dapagliflozin may incur additional healthcare expenditure unrelated to HF. Whether unrelated healthcare costs should feature within cost‐effectiveness analyses is the subject of ongoing debate. 44 , 45
Our analysis is the first we know of using a cost‐effectiveness model capturing disease progression in HF patients using transitions between discrete health states characterized by the KCCQ‐TSS, rather than using NYHA functional class. KCCQ is an established measure of health status in HF and, unlike NYHA class, is a patient‐ rather than physician‐reported outcome. Patients provide a quantitative, continuous, assessment of their symptoms, with a score of up to 100 points (compared with four categories with NYHA class). A recent systematic literature review of cost‐effectiveness models used to evaluate pharmacologic intervention in adults with HF identified 64 publications; the majority employed Markov (n = 28) or trial‐based analytical approaches. 18 A substantial proportion (n = 40) used NYHA disease classification to categorize disease severity; however, the review questioned the appropriateness of this instrument “given limitations around reproducibility, reliability, and clinician interpretation of what is a ‘normal’ functional capacity in the typically older individuals who develop HF”. 46 , 47 , 48 Indeed, NYHA functional classification is widely considered to provide a subjective, arbitrary, and non‐patient‐centric assessment of HF symptoms. 20 An assessment of cardiologists evaluating the symptoms of HF using the NYHA classification identified no consistent method of applying NYHA class, reflecting its subjectivity. 48 Given the requirement for homogeneous health states within Markov model frameworks, this additional source of heterogeneity within NYHA classification represents a limitation. A second issue with NYHA class relates to modelling disease progression. It is conventional to model time‐dependent NYHA class membership using clinical trial data to estimate transition probabilities. For example, King et al. 49 assessed the cost‐effectiveness of sacubitril/valsartan combination therapy compared with enalapril using NYHA transitions derived from PARADIGM‐HF. These transitions resulted in HF severity generally improving over time. Given HF is a chronic and progressive condition, this observation lacks clinical face validity. Examination of NYHA transitions within DAPA‐HF gave similar findings, with more patients treated with dapagliflozin migrating to NYHA classes II and I within the trial (data not shown). The requirement for HF cost‐effectiveness models to evaluate costs and outcomes over a lifetime therefore presents a challenge, as extrapolating the observed within‐trial NYHA transition over a longer time period is likely to provide overly optimistic results. Importantly, time‐dependent disease severity, captured by KCCQ quartiles, results in the opposite pattern to that seen with NYHA, i.e. a progressive deterioration over the long term. Thus, as well as its other advantages described above, the KCCQ may result in more realistic estimates of long‐term evolution of health status in HF.
As with any study of this type, there are some limitations. In general, the requirement to extrapolate beyond the median follow‐up time of approximately 18 months in DAPA‐HF introduced uncertainty, as in all health economic analyses based on trials. 14 However, it is reassuring that sensitivity analysis showed that, although incremental benefits associated with dapagliflozin were sensitive to the choice of survival distribution, cost‐effectiveness remained robust. A further limitation of DAPA‐HF, like most other trials, is the modest number of patients enrolled in any single country, which prevents robust evaluation within individual countries. 14 Our analysis indicated that cost‐effectiveness is maintained in both lower and higher risk subgroups suggesting the results were not overly sensitive to underlying event rates. Furthermore, the results were not sensitive to different country‐specific cost inputs, providing some reassurance as to the potential generalizability of these findings.
In conclusion, our results show that the benefits of dapagliflozin, added to standard therapy, are achieved at an acceptable cost in patients with HFrEF. Dapagliflozin is likely to be a cost‐effective treatment for HFrEF in the UK, German and Spanish healthcare systems.
Supporting information
Appendix S1. Supporting Information
Table S1. Parameterisations for the adjusted mortality equations.
Table S2. KCCQ‐TSS transition probabilities.
Table S3. Regression models developed for hospitalization for heart failure and urgent heart failure visits.
Table S4. Annual probability of adverse events.
Table S5. Summary of mixed effects model used to derive patient utility (fixed effects only).
Table S6. Costs inputs for UK, Germany and Spain.
Table S7. Results of subgroup analysis (UK).
Table S8. Results of subgroup analysis (Spain).
Table S9. Results of subgroup analysis (Germany).
Table S10. Results of deterministic scenario analysis (UK).
Table S11. Results of deterministic scenario analysis (Spain).
Table S12. Results of deterministic scenario analysis (Germany)
Acknowledgements
Medical writing and editorial support were provided by Vanessa Gross of Health Economics and Outcomes Research Ltd. JJVMcM is supported by a British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217.
Funding
This work was supported by AstraZeneca who provided support for the analysis and medical writing for this study.
Conflict of interest: P.M. and O.D. are employees of Health Economics and Outcomes Research Ltd., Cardiff, UK, who received fees from AstraZeneca in relation to this study.
J.J.V.M. reports non‐financial support and other from AstraZeneca, during the conduct of the study; non‐financial support and other from Cardiorentis, Amgen, Oxford University/Bayer, Theracos, Abbvie, other from DalCor, Pfizer, Novartis, Glaxo Smith Kline, Vifor‐Fresenius, Kidney Research UK; and other from Bayer, Merck, Bristol‐Myers Squibb (BMS), outside the submitted work. P.S.J. reports other from AstraZeneca, during the conduct of the study; and personal fees from Novartis, Cytokinetics, other from Novartis, grants from Boehringer Ingelheim, outside the submitted work; and is Director of Global Clinical Trial Partners (GCTP) Ltd. K.F. D. reports other from AstraZeneca, during the conduct of the study; and personal fees from Eli Lilly, outside the submitted work. M.B. reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Servier, Medtronic, Vifor, Novartis, outside the submitted work. M.C.P. reports other from AstraZeneca, during the conduct of the study; and personal fees from AstraZeneca, Alnylam, Napp, Takeda, Novo Nordisk, Corvia, grants and personal fees from Novartis, Boehringer Ingelheim, grants from Bayer, SQ Pharmaceuticals, outside the submitted work. K.B. and L.Q. are employees of AstraZeneca.
References
- 1. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K; ESC Committee for Practice Guidelines (CPG) . ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008;29:2388–2442. [DOI] [PubMed] [Google Scholar]
- 3. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 4. Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation 2012;126:501–506. [DOI] [PubMed] [Google Scholar]
- 5. Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace 2011;13:Suppl 2:ii13–17. [DOI] [PubMed] [Google Scholar]
- 6. Azad N, Lemay G. Management of chronic heart failure in the older population. J Geriatr Cardiol 2014;11:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. National Institute for Health and Care Excellence . Acute heart failure: diagnosis and management Clinical guideline [CG187]. 2014. https://www.nice.org.uk/guidance/cg187 (9 March 2020). [PubMed]
- 8. Rodríguez‐Artalejo F, Banegas Banegas JR, Guallar‐Castillón P. Epidemiology of heart failure. Rev Esp Cardiol 2004;57:163–170. [PubMed] [Google Scholar]
- 9. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014;171:368–376. [DOI] [PubMed] [Google Scholar]
- 10. Gheorghiade M, Vaduganathan M, Fonarow GC, Bonow RO. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013;61:391–403. [DOI] [PubMed] [Google Scholar]
- 11. Lawson CA, Zaccardi F, Squire I, Ling S, Davies MJ, Lam CSP, Mamas MA, Khunti K, Kadam UT. 20‐year trends in cause‐specific heart failure outcomes by sex, socioeconomic status, and place of diagnosis: a population‐based study. Lancet Public Health 2019;4:e406–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cowie MR, Anker SD, Cleland JG, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López‐Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail 2014;1:110–145. [DOI] [PubMed] [Google Scholar]
- 13. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JG, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJ. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019;21:1169–1186. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets D, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, DeMets DL, Inzucchi SE, Køber L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjöstrand M, Solomon SD; DAPA‐HF Committees and Investigators . A trial to evaluate the effect of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA‐HF). Eur J Heart Fail 2019;21:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence . Technology appraisal guidance [TA388]: Sacubitril valsartan for treating symptomatic chronic heart failure with reduced ejection fraction. 27 April 2016. https://www.nice.org.uk/guidance/ta388 (7 August 2020).
- 17. National Institute for Health and Care Excellence . Technology appraisal guidance [TA267]: Ivabradine for treating chronic heart failure. 28 November 2012. https://www.nice.org.uk/guidance/ta267 (7 August 2020).
- 18. Di Tanna GL, Bychenkova A, O'Neill F, Wirtz HS, Miller P, Hartaigh BÓ, Globe G. Evaluating cost‐effectiveness models for pharmacologic interventions in adults with heart failure: a systematic literature review. Pharmacoeconomics 2019;37:359–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Attema AE, Brouwer WB, Claxton K. Discounting in economic evaluations. Pharmacoeconomics 2018;36:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJ. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020;141:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Welton N, Sutton A, Cooper N, Abrams KR, Ades AE. Evidence Synthesis for Decision Making in Healthcare. Chichester: John Wiley & Sons, Ltd; 2012. [Google Scholar]
- 22. Latimer N. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations alongside Clinical Trials ‐ Extrapolation with Patient‐Level Data 2013. http://nicedsu.org.uk/wp‐content/uploads/2016/03/NICE‐DSU‐TSD‐Survival‐analysis.updated‐March‐2013.v2.pdf (13 March 2020). [PubMed]
- 23. Bagust A, Beale S. Survival analysis and extrapolation modeling of time‐to‐event clinical trial data for economic evaluation an alternative approach. Med Decis Making 2014;34:343–351. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, Mumby‐Croft J, Woodcock F, Lacey M, Haroun R, Deschaseaux C. Cost‐effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart 2018;104:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Almenar L, Diaz B, Quesada A, Crespo C, Martí B, Mealing S, Linde C, Daubert C. Cost‐effectiveness analysis of cardiac resynchronization therapy in patients with NYHA I and NYHA II heart failure in Spain. Int J Technol Assess Health Care 2013;29:140–146. [DOI] [PubMed] [Google Scholar]
- 26. Griffiths A, Paracha N, Davies A, Branscombe N, Cowie MR, Sculpher M. The cost effectiveness of ivabradine in the treatment of chronic heart failure from the U.K. National Health Service perspective. Heart 2014;100:1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Office for National Statistics . National life tables: UK. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesunitedkingdomreferencetables (13 March 2020).
- 28. World Health Organization; Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000‐2016. https://www.who.int/healthinfo/global_burden_disease/estimates/en (7 August 2020). [Google Scholar]
- 29. Instituto Nacional de Estadistica . National results, by Autonomous Communities and by provinces. https://www.ine.es/dyngs/INEbase/en/operacion.htm?c=Estadistica_C&cid=1254736177004&menu=resultados&secc=1254736195384&idp=1254735573002 (7 August 2020).
- 30. Federal Statistical Office of Germany (Statistisches Bundesamt) . Destatis. Sterbefalle und Lebenserwartung (German death and life expectancy tables). https://www.destatis.de/DE/Themen/Gesellschaft‐Umwelt/Bevoelkerung/Sterbefaelle‐Lebenserwartung/_inhalt.html;jsessionid=F08FF169AAAC3CE37E8B7489C2CC1B9C.internet712 (7 August 2020).
- 31. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire D, Wilding JP, Ruff CT, Gause‐Nilsson IA, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE‐TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018;380:347–357. [DOI] [PubMed] [Google Scholar]
- 32. van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, Lloyd A, Scalone L, Kind P, Pickard AS. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L value sets. Value Health 2012;15:708–715. [DOI] [PubMed] [Google Scholar]
- 33. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–1108. [DOI] [PubMed] [Google Scholar]
- 34. Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health‐related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523–1534. [DOI] [PubMed] [Google Scholar]
- 35. Beaudet A, Clegg J, Thuresson P‐O, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health 2014;17:462–470. [DOI] [PubMed] [Google Scholar]
- 36. Peasgood T, Brennan A, Mansell P, Elliott J, Basarir H, Kruger J. The impact of diabetes‐related complications on preference‐based measures of health‐related quality of life in adults with type I diabetes. Med Decis Making 2016;36:1020–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ‐5D (UKPDS 62). Med Decis Making 2002;22:340–349. [DOI] [PubMed] [Google Scholar]
- 38. Barry HC, Ebell MH, Hickner J. Evaluation of suspected urinary tract infection in ambulatory women: a cost‐utility analysis of office‐based strategies. J Fam Pract 1997;44:49–60. [PubMed] [Google Scholar]
- 39. Gandjour A, Ostwald DA. Sacubitril/valsartan (LCZ696): a novel treatment for heart failure and its estimated cost effectiveness, budget impact, and disease burden reduction in Germany. Pharmacoeconomics 2018;36:1285–1296. [DOI] [PubMed] [Google Scholar]
- 40. Witte K, Hasenfuss G, Kloppe A, Burkhoff D, Green M, Moss J, Peel A, Mealing S, Durand Zaleski I, Cowie MR. Cost‐effectiveness of a cardiac contractility modulation device in heart failure with normal QRS duration. ESC Heart Fail 2019;6:1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krittayaphong R, Yadee J, Permsuwan U. Cost‐effectiveness analysis of the adjunctive therapy of ivabradine for the treatment of heart failure with reduced ejection fraction. Clinicoecon Outcomes Res 2019;11:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fernandez de Bobadilla J, Gonzalez Fernandez O, Lopez de Sa E, Lopez‐Sendon JL. Cost‐effectiveness analysis of ivabradine in heart failure with reduced left ventricular ejection fraction in Spain. Value Health 2014;17:A486. [DOI] [PubMed] [Google Scholar]
- 43. National Institute for Health and Care Excellence (NICE) . Process and methods [PMG9]: Guide to the methods of technology appraisal 2013. 4 April 2013. http://nice.org.uk/process/pmg9 (7 August 2020). [PubMed]
- 44. Morton A, Adler AI, Bell D, Briggs A, Brouwer W, Claxton K, Craig N, Fischer A, McGregor P, van Baal P. Unrelated future costs and unrelated future benefits: reflections on NICE guide to the methods of technology appraisal. Health Econ 2016;25:933–938. [DOI] [PubMed] [Google Scholar]
- 45. van Baal P, Morton A, Meltzer D, Brouwer W. Future unrelated medical costs need to be considered in cost effectiveness analysis. Eur J Health Econ 2019;20:1–5. [DOI] [PubMed] [Google Scholar]
- 46. Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung 2002;31:262–270. [DOI] [PubMed] [Google Scholar]
- 47. Gibelin P. An evaluation of symptom classification systems used for the assessment of patients with heart failure in France. Eur J Heart Fail 2001;3:739–746. [DOI] [PubMed] [Google Scholar]
- 48. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, Mayet J, Francis DP. Limitations of the New York Heart Association functional classification system and self‐reported walking distances in chronic heart failure. Heart 2007;93:476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost‐effectiveness of sacubitril‐valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC Heart Fail 2016;4:392–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Table S1. Parameterisations for the adjusted mortality equations.
Table S2. KCCQ‐TSS transition probabilities.
Table S3. Regression models developed for hospitalization for heart failure and urgent heart failure visits.
Table S4. Annual probability of adverse events.
Table S5. Summary of mixed effects model used to derive patient utility (fixed effects only).
Table S6. Costs inputs for UK, Germany and Spain.
Table S7. Results of subgroup analysis (UK).
Table S8. Results of subgroup analysis (Spain).
Table S9. Results of subgroup analysis (Germany).
Table S10. Results of deterministic scenario analysis (UK).
Table S11. Results of deterministic scenario analysis (Spain).
Table S12. Results of deterministic scenario analysis (Germany)


