Abstract
Adaptogens comprise a category of herbal medicinal and nutritional products promoting adaptability, resilience, and survival of living organisms in stress. The aim of this review was to summarize the growing knowledge about common adaptogenic plants used in various traditional medical systems (TMS) and conventional medicine and to provide a modern rationale for their use in the treatment of stress‐induced and aging‐related disorders. Adaptogens have pharmacologically pleiotropic effects on the neuroendocrine‐immune system, which explain their traditional use for the treatment of a wide range of conditions. They exhibit a biphasic dose‐effect response: at low doses they function as mild stress‐mimetics, which activate the adaptive stress‐response signaling pathways to cope with severe stress. That is in line with their traditional use for preventing premature aging and to maintain good health and vitality. However, the potential of adaptogens remains poorly explored. Treatment of stress and aging‐related diseases require novel approaches. Some combinations of adaptogenic plants provide unique effects due to their synergistic interactions in organisms not obtainable by any ingredient independently. Further progress in this field needs to focus on discovering new combinations of adaptogens based on traditional medical concepts. Robust and rigorous approaches including network pharmacology and systems pharmacology could help in analyzing potential synergistic effects and, more broadly, future uses of adaptogens. In conclusion, the evolution of the adaptogenic concept has led back to basics of TMS and a new level of understanding of holistic approach. It provides a rationale for their use in stress‐induced and aging‐related diseases.
Keywords: adaptogen, aging, ethnopharmacology, network pharmacology, stress
1. INTRODUCTION
Numerous systematic reviews, meta‐analyses of preclinical and clinical studies, and comprehensive assessment reports 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 on the efficacy and safety of adaptogenic plants have been published in the last several decades. The aim of this review is to summarize our knowledge about common concept relating to adaptogenic plants used as officinal medical preparations in the USSR/Russian and in traditional Chinese medicine (TCM), Ayurveda, Kampo, and other traditional medical systems (TMS) and alternative medical systems, and to analyze how such preparations have been studied scientifically. This provides a basis for assessing the use of adaptogens in the treatment of stress‐induced and aging‐related disorders.
Adaptogens must be innocuous and cause minimal disorder in the physiological functions of an organism, and have nonspecific actions, that is, increase resistance to adverse influences of a wide range of factors with physical, chemical, and biological properties. In addition, they typically possess normalizing actions irrespective of the direction of the foregoing pathologic changes.
1.1. Evolution of the adaptogenic concept: From postulates to evidence‐based statements
The term adaptogens is currently widely used in alternative and complementary medicine, as well as in pharmacognosy, phytomedicine, and phytotherapy research. 5 It was implemented in scientific lexicon in the middle of the 20th century in the Soviet Union with the aim of characterizing the physiological mechanisms of action of compounds and some medicinal plants that presumably increased the nonspecific resilience of organisms to harmful challenges. The definition of adaptogens is continuously updated (Table 1), incorporating the increasing body of scientific evidence related to understanding their pharmacological and molecular mechanisms of action.
Table 1.
Definitions of adaptogens
|
|
|
|
|
|
|
|
|
|
|
|
Importantly, the term adaptogen is related to a physiological process—adaptation to environmental challenges, which is a multistep process including diverse mechanisms of extracellular and intracellular interactions. The renewed definition of adaptogens 16 , 17 is supported by the results of recent studies on the molecular mechanisms of action of adaptogens in a variety of regulatory systems from the cellular to entire organism levels. 11 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63
Similar to antioxidants and vitamins, adaptogens constitute a category of nutritional and herbal medicinal products essential for good health, adaptability, resilience, survival, and healthy aging. Regardless of the nature of the stimulus (stressor), an adaptogen increases adaptability, resilience, and survival by activating adaptive signaling pathways of cellular and organismal defence systems (stress system e.g., neuroendocrine‐immune complex). Furthermore, adaptogens trigger the generation of hormones (cortisol, corticotropin‐releasing hormone [CRH] and gonadotropin‐releasing hormones, urocortin, neuropeptide Y), playing key roles in metabolic regulation and homeostasis. Meanwhile, multitarget mechanisms of action and a wide range of pharmacological effects indicate their nonspecific pharmacological activity.
Therefore, adaptogens are most likely effective for the prevention and treatment of stress‐induced and adult‐onset disorders such as chronic fatigue, memory impairment, depression, anxiety, sleep disturbance, diabetes, heart disease and high blood pressure, chronic inflammation and autoimmune diseases, cold and flu, infections, skin diseases, liver diseases, and cancer. This can be achieved due to their ability to activate the innate defence system, increase resistance to stress, adapt organisms to stress, increase recovery of stress‐induced damages, provide energy to fight fatigue, reduce aging‐associated decline of the neuroendocrine‐immune system. Table 2 provides a summary of the general characteristics of adaptogens, which comprise a category of nutritional and herbal medicinal products.
Table 2.
Summary of characteristics of adaptogens
| Definition: Adaptogens are natural compounds or plant extracts that increase adaptability, resilience, and survival of organisms to stress. |
| Chemical class: Various, predominantly tetracyclic triterpenes, phenethyl‐and phenylpropanoids glycosides, stilbenes, lignans, etc. |
| Pharmacological activity/health claims: adaptogenic |
| Mechanism of action: Multitarget effects on neuroendocrine‐immune system including: |
| (i) Triggering of intracellular and extracellular adaptive signaling pathways that promote cell survival and organismal resilience in stress |
| (ii) Regulation of metabolism and homeostasis via effects on expression of stress hormones (corticotropin and gonadotropin‐releasing hormones, urocortin, cortisol, neuropeptide Y, heat shock proteins Hsp70) and their receptors. |
| Indications for use: Stress‐induced fatigue, mental and behavioral disorders, aging‐associated diseases. |
2. BACKGROUND OF THE ADAPTOGENIC CONCEPT
2.1. Origin of the adaptogenic concept and use in officinal medicine of the USSR
The term adaptogen was introduced in 1958 by the Soviet toxicologist Lazarev, who applied it to the synthetic stimulant dibazol (2‐phenyl‐imidazol) assuming that adaptogens increase the nonspecific resistance of organisms under conditions of stress resulting in increased endurance, stamina, and performance. 6 This assumption was based on the results of intensive studies of Schisandra chinensis in the USSR during World War II, 64 , 65 , 66 with the goal of finding an alternative to stimulants used by the German and U.K. army to increase the attention and endurance of pilots. 67 The aim was also to supply the Soviet Armed Forces and Military Industry (soldiers, pilots, sailors, and civilians engaged in the production of weapons and war materials) with easily available natural stimulants, presumably extracts from S. chinensis berry or seeds. 68
The interest in S. chinensis (known as limonnik = лимонник in Russian) arose from ethnopharmacological investigations by. Komarov (1895) and Arsenyev (1903–1907) in far eastern Siberia and northern Manchuria. The berries and seeds were determined to have been used by Nanai hunters (natives of far eastern Siberia and Chinese Manchuria, also known as Goldis or Samagir) as a tonic to reduce thirst, hunger, and exhaustion and to improve night‐time vision. 69
The first studies on the stimulating and tonic effects on S. chinensis were published in World War II‐era military journals. 64 , 65 , 66 During the 1960s and 1970s, other Soviet scientists extended the research of adaptogens to “rejuvenating and invigorating” medicinal plants traditionally used in China, Korea, Japan, Siberia, and the far east of the USSR for a variety of pathological conditions including diseases and their symptoms such as hypodynamia, asthenia, shortness of breath, palpitation, insomnia, hemorrhage, impotence, and diabetes. 70 , 71 , 72
The authors screened many plants assuming that “adaptogens must be safe and normalize body functions irrespective of the nature of stressors” and in 1967, some were incorporated into official medical practice in the USSR as central nervous system (CNS)‐stimulating medicinal products and as tonics to fight fatigue and general weakness during convalescence for infectious diseases, chemotherapy and psychiatric disorders, after surgery, poisoning, heart attacks, ischemia, chemotherapy, and psychiatric disorders (Table 3). Rhodiola rosea extract (Rhodiolae roseae rhizomatum et radicum extractum liquidum) is an example of an adaptogenic medicinal product used since 1975 in officinal medicine in the USSR/Russia. It is indicated for “decreased mental and physical capacities such as weakness, exhaustion, tiredness and loss of concentration, as well as during convalescence.” The extent of adaptogen research conducted in the USSR was enormous with more than 1000 pharmacological and clinical studies published in Russia until 1982.
Table 3.
Adaptogenic plants used in officinal medicine in the USSR/Russiaa
| Name of plant | Products | Pharmacopoeia monograph |
|---|---|---|
| Aralia elata (Miq.) Seem (A. mandshurica Rupr. et maxim.) | Radices | FS.2.5.0058.18 |
| Tincture | FS 42‐1647‐93 | |
| Dry extract in tablets | FS 42‐1755‐81 | |
| Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. | Radices and rhizomes, | FS.2.5.0053.15 |
| Liquid extract | FS.3.4.0009.18 | |
| Oplopanax elatus (Nakai) Nakai (Echinopanax elatum Nakai) | Radices and rhizomes, | FS 42‐314‐72 |
| Tincture | FS 42‐1887‐82 | |
| Panax ginseng C.A. Meyer | Radices | FS.2.5.0013.15 |
| Tincture | FS 42‐1886‐82 | |
| Rhaponticum carthamoides (Willd).Iljin | Radices and rhizomes, | FS.2.5.0091.18 |
| Liquid extract | FS 42‐1995‐99 | |
| Rhodiola rosea L. (a synonym of Sedum roseum (L.) Scop.) | Radices and rhizomes, | FS.2.5.0036.15 |
| Liquid extract | FS.3.4.0008.18 | |
| Schisandra chinensis (Turcz.) Bail. | Fruits | FS.2.5.0081.18 |
| Seeds | FS.2.5.0082.18 | |
| Tincture from seeds, | FS 42‐1822‐90 | |
| Tincture from fruits, | VFS 42‐117‐72 | |
| Oil from seeds in capsules | VFS 42‐3423‐99 |
The State Pharmacopoeia of the Russian Federation, 2018. http://femb.ru/femb/pharmacopea.php (Accessed date: March 15, 2020).
Most common extracts or compounds isolated from Siberian Ginseng (Eleutherococcus senticosus), Schisandra (S. chinensis), Ginseng (Panax ginseng), and Golden Root (R. rosea) have been studied. All adaptogenic plants and preparations from them have been clinically tested and approved before incorporation into official medical practice. The list of clinically approved true adaptogenic plants with related pharmacopeial monographs is presented in Table 3.
Regardless of the formal indication for use in officinal medicine as tonics, adaptogens were widely used in:
sports medicine to promote quicker recovery after heavy exercise and overstraining,
occupational medicine for protection against negative environmental factors, and
geriatric medicine with the aim of promoting health by preventing and treating diseases and disabilities in older adults.
These areas of practical use of adaptogens were of socioeconomic importance in the USSR, a superpower where great achievements in space, military power, and sports have been the subjects of pride and special attention. Indeed, adaptogens were used in space medicine by Soviet cosmonauts during long missions on the MIR station, 73 , 74 as well as by sailors aboard ships; on submarines during long Arctic, Antarctic, or tropical expeditions; and by pilots and sportsmen in multiple stressful conditions such as hypoxia, irradiation, cold, and physical and mental overload. In addition, adaptogens termed “Kremlin Magic Pills” and “Elixir of Youth” that increase strength, stamina, and longevity were popular among elite elderly leaders of Communistic Party of the USSR, which governed the country for many years.
In conclusion, the concept of adaptogens can be traced back to their first definitions provided by the Soviet scientists Lazarev and Brekhman, and the introduction of herbal medicinal products as official medicaments and in the State pharmacopoeia of the USSR.
2.2. Ethnopharmacological background
Key points of the adaptogenic concept defined by Brekhman and Dardymov in 1969 are in line with basic principles of the TMS of China, Korea, Japan, India (Ayurveda), and Middle Asia (Yunani).
For instance, an assumption is that some adaptogens used in TCM, Kampo, and Ayurveda medicine (e.g., Ginseng, Ashwagandha, Andrographis, Bryony) must have normalizing effects, irrespective of the nature of the disease. Herbalists refer to adaptogens as restoratives, qi‐tonics, rasayanas, or rejuvenating herbs. Tonic herbs are classified as the highest and most sought‐after herbal remedies in many traditional systems of healing such as TCM and Ayurveda. Both traditional systems are based on holistic approaches to patients and treatment, suggesting that the patient is an individual and not a disease. Holistic medicine strives to consider the whole person, suggesting that one can only achieve optimal health by complex treatment of all imbalances (physical, emotional, or spiritual) induced by environmental factors. Consequently, multitarget therapy by herbal preparations have polyvalent actions on various mediators, effectors, and regulatory systems, presumably making it the most effective approach for the treatment of complex diseases.
Both TMS have a similar notion of “life vital energy” and activating the body and mind: the qi in TCM and the prana in Ayurveda. Similar notions exist in various cultures including the Greek pneuma, the Armenian zorutyun (զորություն), the Polynesian mana, the German od, and the Hebrew ruah. Prana is also referred to as life force, subtle, or bioplasmic energy. Below are brief descriptions of the ethnopharmacological roots of the adaptogenic concept.
2.2.1. Traditional Chinese, Korean, and Japanese medicines
TCM is about 5000 years old, so billions of people in China (the world's biggest population with ~1.4 billion) have been treated with these herbal medicines/botanicals for centuries.
The core of the TCM concept is the yin‐yang theory consisting of two natural, complementary, and contradictory forces of opposite polarity that interact to form a dynamic system in which the entire is dual and better/superior than the collected parts. According to this philosophy, everything has both yin and yang features (for instance, shadow cannot exist without light), which are in dynamic equilibrium (balance); yin is negative/passive/dark/female/water, while yang is positive/active/bright/male/fire. Although yin is stronger, they are always in balance.
We can find many relevant examples of the yin‐yang balance when this concept is applied to the regulation of cellular and organismal homeostasis 75 (e.g., cyclic adenosine monophosphate [cAMP] and cyclic guanosine monophosphate [c‐GMP], prostacyclin and thromboxane, sympathetic and parasympathetic nervous systems, testosterone, cortisol). For example, the testosterone/cortisol ratio is associated with stress‐related disorder symptoms such as fatigue, decreased performance, and impaired recovery from overtraining syndrome in sports medicine. 76 The major symptoms and signs of overtraining were categorized 77 as:
physiological (chronic fatigue, decreased performance and muscular strength, muscle soreness, extended recovery time, increased oxygen uptake at physical loads, loss of appetite, and decreased body fat).
psychological (difficulty concentrating, emotional instability characterized as restlessness and excitation followed by apathy and depression),
immunological (immunosuppression characterized as decreased blood immunoglobulins and lymphocyte count, decreased chemotaxis of neutrophils, increased susceptibility to infection),
biochemical (decreased free testosterone and raised cortisol levels, elevated lactate, and reduced hemoglobin levels in blood).
All of these symptoms of overtraining healthy subjects in stress as well as their overall health status are in line with a subpar health status 78 known in TCM as “shanghuo” or “re‐qi” (upper fever, pathology fire, internal heat, or excessive energy associated with energy metabolism), which is characterized by a general decline in health, cut of energy, weakness, impaired physiological functions and adaptability (presumably Xie‐Huo in TCM), leading to the onset and progression of diseases. 79
In other words, “shanghuo” 79 is a state of decreased resistance (or increased susceptibility) leading to stress and progression of diseases. That is similar to low‐grade inflammation, 80 resulting in and involving whole‐body systems such as the neuroendocrine‐immune (stress‐system), cardiovascular, and other systems.
According to TCM, the onset of disease is due to both external (wind, cold, heat, dampness, dryness, fire) and internal causes—excessive emotional activity induces the yin‐yang imbalance of the following seven emotions: joy, anger, anxiety, concentration, grief, fear, and fright. Bacteria, viruses, and chemicals are not considered to be causes. Most people whose health is not affected by external factors, but in whom excessive emotional activity causes a severe yin‐yang imbalance, experience blockage of qi and impairment of vital organ function. According to TCM theory, “shanghuo” caused by emotional stress can induce insomnia, depression, increase susceptibility to infectious diseases, and promote cardiovascular disease and tumor progression. Therefore, unsurprisingly the idea to prevent and treat stress‐induced disorders caused by a yin‐yang imbalance with prophylactic treatment using medicinal plants trace back to centuries (e.g, Weibing in China, Mibyeong in Korea, 81 and Mibyou in Japan. 82 Subsequently, the concepts underlying preventive treatment for subhealth by adaptogens (presumably “fu zheng” in TCM for strengthening body resistance or strengthening vital qi) were implemented in USSR under the names Medical Fitness, Farmacosanacia, and Valeology. 83
According to TCM, the treatment of diseases must rectify harmony, and restore qi and the yin‐yang balance. It is the quality, quantity, and balance of qi that determine the state of health and lifespan. Food and air affect health; therefore, diet and breathing exercises are of primary importance. According to The Divine Husbandman's Classic of the Materia Medica, the earliest existing monograph of TCM prepared 4000 years ago, P. ginseng tonifies the primal qi and qi of all organs, particularly those of the lungs and spleen. Therefore, it has been indicated for deficiency of qi in patients with shallow breathing, shortness of breath, coldness of limbs, profuse sweating, or weakness and has been used to reduce the symptoms of stress and inflammation and delay aging. 84
Medicinal plants are considered for the treatment of diseases and recovery of vital energy, which is believed to gradually dissipate throughout life. So, it is important to conserve it using diet, kung fu, breathing exercises, and herbal medicines. As an example, fatigue is due to qi deficiency, and P. ginseng (tonic herb) activates qi and therefore has nourishing effects in fatigue. 47 , 85 , 86 , 87 , 88
In TCM, all known medicinal plants are divided into three categories: inferior, middle, and superior. The highest forms of medicine revered in China are the superior herbs (tonic herbs), which help everything to heal and nurtures life itself. Superior herbs are thought to possess restorative properties and are used as general tonics for the treatment of disease and in convalescence. The most well‐known broad action medicinal plant in TCM is ginseng. 89 , 90
The pharmacological activity of ginseng was first described in the 1st century by an unknown author. According to his records, ginseng improves mental activity and visual acuity, dispels pathogenic factors, enhances longevity with long‐term intake tonifying five vital organs of the body (spleen, lung, heart, kidney, and liver). According to other ancient regards written by Hongjing Tao (AD 456–536), ginseng can be used to enhance cognitive function; improve blood circulation; relieve thirst and feelings of solidity; and cure internal coldness, pain in the chest or abdomen, vomiting, and diarrhea. These and other beneficial effects of ginseng have also been described in other more complete and comprehensive medical textbooks including treatment for general weakness and fatigue.
“Kampo” (Traditional Japanese Academic Medicine) developed on the Japanese Islands from ca. 500 AD based on Ancient Chinese Medicine (ACM)—the common ancestor system of Japanese Kampo, Korean Medicine (KM), and Traditional Chinese Medicine (TCM). Subsequent independent developments and European influence in the 16th century resulted in a divergent cultural evolution establishing Kampo as an independent TMS distinct from other systems. Over the past centuries, fundamental philosophical differences have developed. 91 Kampo is mostly based on the systematic collection of case histories—empirical knowledge of the effect of Kampo preparation. As Kampo is regulated by the Japanese government, Kampo prescriptions (as finished pharmaceutical products) are included in the Japanese Pharmacopoeia (JP) and covered by the national health insurance. Every Kampo formula is indicated for individuals with the same “symptom patterns” (sho), based on a pathological status of an individual. 91
A special class of Kampo prescriptions with close similarity to the adaptogenic concept are the so‐called “support preparations” or Hozai. The term hozai is used to describe preparations that are applied to stop or partially reverse the symptoms of physical weakness and degenerative diseases. Hozai can be used in cases of typically geriatric ailments but also in any other case of physical decay. 92 , 93
The traditionally accepted explanation for the activity of Kampo medicines ‐ including Hozai —was summarized in the 18th century CE by the philosopher Yoshimasu Todo (1702–1773), who stated that curative and toxic effects are two phases of the same process; since diseases are triggered by uncontrolled poisoning, the patient has to be healed by a positive, challenging poisoning. This controlled poisoning initiates a regeneration reaction that removes toxicity from the body, thus restoring the patient's health. 94 In this context, hozai and adaptogens are similar since adaptogens are eustressors (i.e., good stressors) acting as mild stress mimetics or stress‐vaccines that induce a stress‐protective response, 12 , 14 , 27 , 60 , 95 which is in line with the basics of Kampo medicine. 91 The relationship of the two concepts is illustrated by P. ginseng root—one of the classical USSR Adaptogens. 8 This is an essential component drugs of most Hozai preparations (Table 4). 96 The two major prescriptions of the hozai category are Juzentaihoto 97 and Hochuekkito 98 (Table 4).
Table 4.
Crude drugs and their respective daily dosages (g) in the two traditional Kampo Hozai prescriptionsa
| Juzentaihoto (Japanese name) 十全大補湯 | Hochuekkito (Japanese name) 補中益気湯 |
| Shi‐Quan‐Da‐Bu‐Tang (Chinese name) | Bu‐Zong‐Yi‐Qi‐Tang (Chinese name) |
| Sipjeondaebotang (Korean name) | Bojungikgitang (Korean name) |
| Tonifying the Middle and Augment the Qi Decoction | Tonifying Decoction |
| https://kampo.ca/herbs-formulas/formulas/juzentaihoto/ | https://kampo.ca/herbs-formulas/formulas/hochuekkito/ |
| Ginseng Radix—3 | Ginseng Radix—4 |
| Astragali Radix—3 | Astragali Radix—4 |
| Glycyrrhizae Radix—1.5 | Glycyrrhizae Radix—1.5 |
| Angelicae sinensis Radix—3 | Angelicae sinensis Radix—3 |
| Atractylodis macrocephalae Rhizoma—3 | Atractylodis macrocephalae Rhizoma—4 |
| Paeoniae Radix—3 | Bupleuri Radix—2 |
| Cinnamomi Cortex—3 | Jujubae Fructus—2 |
| Ligusticum Rhizoma—3 | Zingiberis Rhizoma—0.5 |
| Sclerotium Poriae Cocos—3 | Cimicifugae Rhizoma—1 |
| Rehmanniae Radix preparata—3 | Citri reticulatae Pericarpium—2 |
Corresponding daily dose is 7.5 g of dried extracts in representative finished pharmaceutical products (JP: The Japanese Pharmacopoeia). Both formulations are regarded as effective by the Japanese regulatory authorities and are available as finished pharmaceutical products of equal quality to traditional herbal medicinal products registered in the EU under coverage of the Japanese National Health Insurance.
Both formulations are mainly used in cases of geriatric ailments and physical decline. 93 Juzentaihoto is also used for decubitus ulcers, radiation sickness, rheumatoid arthritis, supportive therapy in cancer, and to reduce adverse effects from surgical treatment and chemotherapy. The indications given by the Japanese national health insurance for Hochuekkito are related to general vigor, anorexia, myasthenia gravis, chronic gastritis, and atopic dermatitis. 99 , 100
The Western indications, for which hozai are most often used in Japan, are related to cachexia, 101 , 102 a loss of skeletal muscle mass that differs from weight loss due to malnutrition, anorexia nervosa, or anorexia due to depression or sarcopenia (aging‐related muscle loss).
In conclusion, shanghuo, a state of decreased resistance to stress can be treated with what—first in the Soviet/Russian literature—has been labeled adaptogenic plants. These will and increase the nonspecific resistance to stress; the yin‐yang balance, a synonym of homeostasis (see the next section of this chapter); and vital energy or qi, which has a similar meaning as adaptability or a state of nonspecific resistance. The concept of hozai is very similar to the adaptogenic concept, particularly in the context of their modes of action as eustressors (i.e., good stressors), and as mild stress mimetics or stress‐vaccines that induce a stress‐protective response; its systematic use in gerontology might be very beneficial, as has already been demonstrated in Japan.
The multipurpose use of adaptogens (ginseng) in numerous conditions suggests their nonspecific and normalizing effects in organisms. The traditional use of ginseng in billions of people for centuries is one important argument in favor of it being nontoxic, innocuous, and not influencing normal bodily functions more than necessary.
2.2.2. Ayurveda
Ayurveda is a conventional medicinal system with varied treatments, which originated over 3 millennia ago in South Asia. 103 In Ayurvedic philosophy, the central concept is the Tridosha theory suggesting that good health occurs when there is a dynamic balance between three fundamental dynamic forces or dosh as called Vata, Pitta, and Kapha.
-
o
Vata is the combination of air and water, which is associated with the function of the nervous system. An imbalance leads to pain, sleeplessness, and inability to concentrate and stay on task.
-
o
Pitta is the combination of fire and water, and is associated with bile, digestion, and metabolism.
-
o
Kapha is the combination of water and earth, and is associated with mucous, lubrication, and transporting nutrients into the arterial system.
According to Ayurvedic theory, the life vital energy, Prana, comes from the air into the brain via respiration. Prana is settled in the brain and governs emotions, memory, and other functions of the mind. It also rules the functioning of the heart and enters the bloodstream to control all vital organs.
In Ayurveda, the plants known as rasayana are used as rejuvenating and for improving the overall health of anyone undergoing this treatment. The word rasayana literally means the path that rasa takes (rasa: the primordial tissue or plasma; ayana: path). According to Ayurveda, the qualities of rasa‐dhatu influence the health of other dhatus (tissues) of the body, as it is the most primary in function and works as the basic unit. Hence any medicinal plant or formulation that improves the quality of rasa (rasayanas), strengthen or promotes the health of all tissues of the body. Apart from promoting good health, increasing the ability to concentrate, improving memory and mood, an important effect of rasayana therapy is increasing resistance to diseases. 104 The rasayana effect is not a specific pharmacological action, but rather a complex response operating through a comprehensive holistic mechanism of regulation of homeostasis.
Species most commonly used in Ayurveda as rejuvenating include:
-
o
Ashwagandha—Withania somnifera (L.) Dunal
-
o
Kalmegh—Andrographis paniculata (Burm. F.) Wall. Ex. Nees.
-
o
Yasthimadhu (Licorice)—Glycyrrhiza glabra L.
-
o
Satavari—Asparagus racemosus Willd
-
o
Tulsi (Holy basil)—Ocimum tenuiflorum L. (syn.: Ocimum sanctum L.)
-
o
Pipul (Pepper)—Piper longum L.
-
o
Guduchi—Tinospora cordifolia Miers
-
o
Amla—Emblica officinalis Gaertn
-
o
Haritaki—Terminalia chebula Retz.
W. somnifera is used in Ayurveda toward promoting health and longevity, slowing the aging process, revitalizing the body, reducing anxiety, and creating a general sense of well‐being. These traditional applications of W. somnifera are due to a wide range of pharmacological effects observed in recent preclinical studies in animals and clinical trials in humans including anxiolytic, sedative, anti‐inflammatory, analgesic, immunomodulatory, antioxidant effects, cardiopulmonary, and hypotensive effects. 105
A. paniculata, “the king of bitters,” is used in Ayurvedic and other traditional health care systems of India, China, and other Asian countries for numerous medicinal purposes, for example as an effective antipyretic treatment against a variety of infectious diseases including bronchitis, tonsillitis, tuberculosis, malarial and intermittent fever, urinary infection with difficult painful urination, dysentery, bacillary dysentery, colitis, dyspepsia, hepatitis, mouth ulcers, colic, otitis, vaginitis, pelvic inflammatory disease, chickenpox, carbuncles, sores, and eczema. The plant is effective for venomous snake bites, burns, and traumatic infection. Efficacy for prophylaxis and symptomatic treatment of upper respiratory infections such as the common cold, bronchitis uncomplicated sinusitis and pharyngotonsillitis, urinary tract infections, and acute diarrhea has been supported by clinical studies. 4
The root of the liquorice plant (Glycyrrhiza sp.) is also oa well‐known rasayana drug in Ayurveda mainly due to anti‐inflammatory, antiviral, and antimicrobial activities.
In Ayurveda, A. racemosus is used as rasayana medicine and is acknowledged for promoting physical and mental health. Its wide range of therapeutic effects such as antitussive, antiplasmodial, anti‐leishmanial, antibacterial, hepatoprotective, diuretic, antiulcer, antidiarrheal, antenatal tonic, cardioprotective, anticancerous, antiepileptic, and antidepressant are likely associated with its immunomodulatory and adaptogenic activities. 106 , 107 However, many of these therapeutic claims go well beyond preventive medical concepts.
In Ayurveda, P. longum is used in hepatosplenomegaly, respiratory disorders including asthma, chronic cough, tuberculosis, skin disorders, piles, diabetes, and anemia. It is also beneficial in fever and infection including typhoid and has analgesic effects in dyspepsia, worm infestation, and abdominal pain. It is also reported to have aphrodisiac properties. P. longum, P. nigrum, and Zingiber officinalis are combined in the Ayurvedic formulation Trikatu, which is effective in several ailments. It increases the action of other drugs by increasing the bioavailability, as piperine is the main biomarker compound. 108
In Ayurveda, Guduchi (T. cordifolia) is effective against various infections to boost immunity, especially in the convalescent period, as it has antipyretic, analgesic, and anti‐inflammatory properties. It is also useful for dyspepsia, anorexia, liver disorders, dysentery, and worms, and is prescribed for anemia, diabetes mellitus, gout, and rheumatoid arthritis.
In Ayurveda, E. officinalis is used for the treatment of peptic ulcer, dyspepsia, altered gastrointestinal motility (diarrhea, constipation, vomiting), and symptoms from pancreatitis, piles, liver disorders, diabetes, tuberculosis, and other lung infections. It has anti‐inflammatory and antistress effects. Regular intake of E. officinalis fruit has been advised for the general maintenance of health and preventive healthcare. External application is prescribed for alopecia or baldness, toothache, and ophthalmic conditions. 109
T. chebula is considered as digestive and gives strength to tissues, particularly the sense organs. It purifies blood and has laxative and antipyretic actions. It is prescribed for dyspepsia, piles, hepatosplenomegaly, irritable bowel syndrome, and cardiac dysfunction. Triphala, a formulation containing equal parts of E. officinalis, T. chebula, and T. bellerica, is used as a laxative and general well‐being as it maintains the balance of Vata, Pitta, and Kapha.
Modern practices derived from Ayurveda are now classified as a type of complementary or alternative medicine, especially in the Global North.
In conclusion, the fundamental philosophy of Ayurvedic medicine, particularly in the context of homeostasis regulation of the stress‐system (neuroendocrine‐immune complex, see below), nonspecific resistance (vital life energy = prana), pharmacologically pleiotropic or polyvalent effects, and the antiaging effects of adaptogens is very similar to the concept of adaptogens.
2.2.3. Impact of ancient Greece, Rome, and medieval TMS of middle Asia
Yunani or Unani is the term for Parsi‐Arabic traditional medicine as practiced in the Indian subcontinent, and in Muslim culture in central and southern Asia. The term is derived from Arabic Greek and has Hellenistic origin based on teachings of the Greek physicians Hippocrates, Dioscórides, and Galen Unani. It was further developed and enriched by Abu‐Ali Ibn Sina (Avicenna), Amirdovlat, and other medieval physicians and philosophers. 110
For instance, Amirdovlat devoted considerable attention to those medicinal plants, which had antitoxic (lavender, marigold, ironwort) and tonic properties (birthwort, bryony). Amirdovlat used bryony, the sacred medicinal plant, as a panacea for all diseases to prevent premature aging and maintain good health and vitality. 111 , 112
In pre‐Christian times, the root of Bryonia alba L. was an occult object in Armenia (Loshtak in Armenian), where it was used as a drug for all diseases. 113 , 114 It has been referenced by the scientists of ancient Greece (Dioscórides, Hippocrates, Theophrastus), Rome (Celsius, Columella, Galen, Plinius), and Asia (Amirdovlat, Avicenna), and was studied in Jensen's 1914 thesis. 114 , 115 The Bryonia root has been used to treat a wide range of conditions and disorders including fatigue, gout, arthritis, rheumatism, neuralgia, pain. psoriasis, abscesses, allergies, leprosy, edema, bronchitis, pleurisy, asthma, tuberculosis, tonsillitis, lung inflammation, cough, influenza, fever, sciatica, ulcers, gastrointestinal diseases, liver diseases, cancer, hypertension, cardiovascular diseases, epilepsy, lockjaw, paralysis, hysteria, madness, sleeplessness, and impotence. It has also been used as a laxative, cathartic, lactogenic, anthelmintic, diuretic, expectorant, and to induce abortion, as well as a cosmetic to remove spots, pimples, warts, blackheads and bruises; to prevent allergic reactions and for the prevention of hair loss. 110 , 111 , 114
Bryonia extract was integrated in official medicine as a tonic and adaptogenic drug in Armenia, Russia, Ukraine, and Belorussia in the 1990s of the XX century and the first decade of the XXI century. 114 Preparations from Bryonia alba L. root extract (“Loshtak” tablets) were registered as medicines by the Russian Federation in 2002, Belarus in 2003, Ukraine in 2007, and Armenia in 1992 and 2003 as an adaptogen and tonic in asthenia; agent for decreased resistance to infections; maintenance of working capacity, coordination, and mental activity; and prevention of stress, radiation‐ and chemotherapy‐induced toxicity and disorders, and so forth.
In conclusion, experiences in ancient Greece, Rome, and Medieval TMS of Middle Asia, particularly regarding the multitasking effects of medicinal plants as a panacea for all diseases can be expressed using the modern concept of adaptogens, and their benefits at low doses to prevent premature aging and maintain good health and vitality.
2.2.4. European traditions and core rational elements of homeopathy
The basic idea of homeopathy assumes that a substance at a high dose causes the symptom of disease in healthy subjects, while curing similar symptoms in illness if applied at a low dose.
Homeopathic preparations are made from ingredients which, in undiluted form, cause symptoms similar to the disease they aim to treat. These ingredients are repeatedly diluted, with shaking at each stage (Table 5). Homeopaths consider that this technique prevents side effects, enhances the ability of preparations to amplify a response, and generates curative properties, even for ingredients that are chemically inactive or so significantly diluted that none of the original material remains. While high‐potency preparations (i.e., highly diluted ones) clearly cannot be evaluated using bioscientific concepts and methods, lower potency ones may well exert relevant pharmacological and toxicological effects.
Table 5.
Dilution scales and homeopathic potency
| Dilution scales | |
|---|---|
| Mother tincture | Homeopathic potency |
| 1 vol of tincture + 9 vol solvent = D1 | 1X |
| 1 vol of D1 + 9 vol solvent = D2 | 2X |
| 1 vol of D2 + 9 vol solvent = D3 | 3X |
| 1 vol of tincture + 99 vol solvent = C1 | 1H |
| 1 vol of C1 + 99 vol solvent = C1 | 2H |
Note: Preparations obtained by dilution of 1 M solution (6.02 x 1023 molecules per L) in potencies higher of D24 do not actually contain a single active molecule.
Homeopathic preparations are generally not tested and regulated under the same laws as conventional drugs. Usage varies from only 2% of people in Britain and the United States using homeopathy in any 1 year, to 15% in India, where homeopathy is now considered part of its traditional medicine. Homeopathic medicines are generally considered safe, with rare exceptions.
However, homeopaths have been criticized for putting patients at risk by advising them to avoid conventional medical treatments.
According to homeopathic theory, the efficacy and safety of the same plant significantly depends on when and where it was collected, and how it was processed. For example, freshly collected summer roots of Bryonia are used in the homeopathic tincture Acofit and is indicated in lumbago, neuromyelitis, and radiculomyositis, whereas 20% of ethanolic extract and dried powder of the roots are recognized as a treatment for bronchitis, pleurisy, asthma, whooping cough, and other inflammatory disorders. 114 , 115 , 116 , 117 , 118 Homeopathic tablets and pellets are used in the United States, England, France, Germany, and Russia for the treatment of rheumatic pain and headache; acute inflammation of the pleura and abdomen; and fever and viral infections (mainly in combination with Aconitum, i.e. Bryaconel Heel, 1994). Various preparations of Bryonia roots are used to relieve muscle pain and diminish the symptoms of asthma and epilepsy. 116 , 119
In addition to homeopathy, other traditions also pay close attention to self‐healing and coping with adverse situations. Anthroposophical medicine is a complementary medical tradition founded in the 1920s by Rudolf Steiner, 120 who advocated for the use of Viscum album L. (the European white‐berry mistletoe) in cancer. 121 It is a holistic approach to medicine focusing on ensuring that the conditions for health are present in a person.
Anthroposophical therapies are intended to enhance an organism's ability to heal in line with the adaptability concept and the concept of adaptive homeostasis, as explained below.
V. album L., an obligate hemiparasite plant growing on apple, pear, plum, hawthorn, beech, willow, poplar, maple, sweetgum, oak, almond, elm, pine, spruce, juniper, and eucalyptus, exhibits immunostimulatory, anti‐inflammatory, analgesic, antioxidant, antiglycemic, antihypertensive, and neuroprotective properties. 122 In allopathic doses, mistletoe preparations (fresh juice, tinctures, and decoctions of various parts) are used in various countries (Armenia, Russia, Ukraine, Bulgaria, the Czech Republic) to treat cough, broken bones, diarrhea, rheumatism, gout, inflammation of lymphatic glands, wounds, and ulcers, as well as hypotensive, antiatherosclerotic, antiosteoarthritis, analgesic, sedative, and antiepileptic remedies. 123 It is worth noting that mistletoe growing on different trees are used for different purposes. Thus, mistletoe growing on the willow is mainly used as a sedative, whereas mistletoe growing on the pear is used in cardiovascular medicine, and the one growing on the hawthorn is used as a hypotensive drug. 123
In homeostatic doses, the mistletoe preparations Iscador, Eurixor, Helixor, Abnoba‐viscum, and Isorel standardized for the content of mistletoe lectin 1 (1 ng/kg) are widely used in Europe as alternative adjuvant therapies against colon, oral, lung, pancreatic, and breast cancers. 124 The mistletoe extracts boost immunity, delay tumor progression, improve the quality of life, and increase survival and lifespan of cancer patients by helping with coping, fatigue, sleep, exhaustion, energy, nausea, vomiting, appetite, depression, anxiety, the ability to work, and emotional and functional well‐being. 125 , 126 , 127 , 128 Mistletoe treatment also alleviates the adverse effects from chemotherapies. 129
In conclusion, the same substance can have dose‐dependent reversal effects. 130 In small doses, it can activate defence systems and exhibit beneficial/curative effects, while in high doses, it can inhibit the defence system and be harmful for the organism. The “bell shape” dose‐effect relationship is common for adaptogens, which have high therapeutic indices (effective dose: toxic dose ratio). In addition, toxic medicinal plants in small doses activate the body's defence systems, particularly the immune system, to cope with cancer and other diseases associated with suppressed immunity. Adaptogens similarly activate the body's defence systems, but at doses not toxic for humans.
2.3. Physiological background on the adaptogenic concept
The concept of adaptogens is based on Hans Selye's theory of stress and homeostasis. The word “stress” is commonly used in numerous conditions and has quite different meanings in daily life. In this review, we used commonly accepted definitions of stress, homeostasis, adaptive stress response, and adaptive homeostasis 131 (Table 6). Repeated mild exposure or low doses of stress induce the increased resistance of cells and organisms to subsequent stress exposure, resulting in an adaptation favouring survival. This phenomenon of adaptation to repetitive low‐level stress was first described by Hans Selye in 1936.
Table 6.
Definitions of stress, stress system, homeostasis, adaptation, adaptedness, adaptability, resilience, adaptive homeostasis, adaptive stress response (hormesis), adaptive stress system, and adaptive signaling pathways
|
|
|
|
|
|
|
|
|
Survival of organisms and resistance to stress depends on adaptability, and adaptive homeostasis is the threshold that determines an organism's innate tolerance to a given level of stress (Figure 1).
Figure 1.
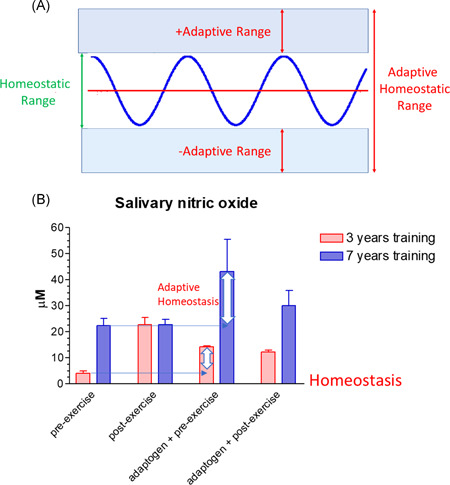
(A) Adaptive homeostasis was defined as the transient reversible adjustments of the homeostatic range in response to exposure to signaling molecules or events. Any biological function or measurement oscillate around a mean or median, within a homeostatic range that is considered a “normal” or physiological, upgraded from Reference [131]. (B) Adaptogens and physical exercise adjust the homeostatic range of salivary nitric oxide. Effects of physical exercise and androgens on the nitric oxide level in saliva of athletes regularly trained for 3 and 7 years 141 [Color figure can be viewed at wileyonlinelibrary.com]
In recent years our understanding of mechanisms underlying the health benefits of natural dietary compounds has improved considerably. Based on modern concepts, plants synthesize in their most susceptible parts (flowers, roots, and leaves) special secondary metabolites for self‐protection against microorganisms, insects, and other pests, as well as to mitigate harmful environmental conditions. 142 , 143 , 144 In animals that use plants as their primary nutrition multiple mechanisms to counteract the potentially poisonous effects of phytotoxins have evolved. These natural compounds are not noxious in humans at lower doses but are able to induce mild cellular stress responses. 145 The ability of plant secondary metabolites to activate the adaptive cellular stress response pathway in human body is one of their essential mechanisms of action. 142 , 144
This phenomenon has been categorized as hormesis or as adaptive stress response, pre‐conditioning. 146 , 147 The multiple mediators of the stress signaling system (the neuroendocrine–immune complex) including different growth factors, antioxidants, and stress‐resistant proteins such as heat shock proteins (Hsps) are involved in stress‐induced responses of the innate and adaptive defence systems. 17 , 148 , 149 We suggest that adaptogens are the first line of plant secondary metabolites activating adaptive stress response pathways 17 (Figure 2).
Figure 2.
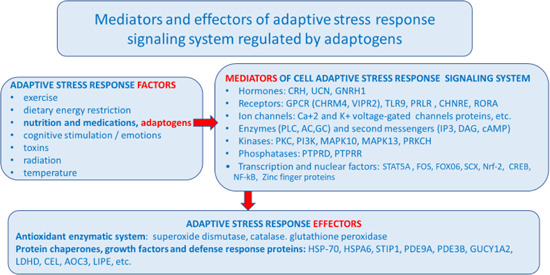
Adaptive stress response factors, mediators, and effectors (updated and adapted from Reference [143] and authors’ drawings. 17 Adaptive stress response involves activation of intracellular and extracellular signaling pathways and increased expression of antiapoptotic proteins, neuropeptides, antioxidant enzymes, and defense response of an organism resulting in increased survival. One basic mechanism of action of adaptogens, that is, that they activate adaptive cellular stress response pathways in humans’ brain cells [Color figure can be viewed at wileyonlinelibrary.com]
Adaptive stress response is important in cell maturation, with initiation by mild stress of mechanisms of repair and maintenance to protect cells against subsequent stresses, while chronic stress induce progressive failure of these mechanisms, leading to cellular senescence, aging, and death. 150 With cellular maintenance on overdrive, the organism can continue to protect himself from chronic inflammation, which causes a range of serious illnesses, particularly aging‐related diseases.
The adaptive stress response is a survival mechanism. All functions of the body systems (e.g., cardiovascular, immune, nervous, endocrine, gastrointestinal digestive) are regulated by about 30,000 genes and fragments of DNA, which are located in the nucleus of every single cell. The activity of genes depends on the signals/stimuli received from numerous receptors and various proteins located on the outside surface of the cell membrane. The receptors specifically trigger signals from extracellular molecules—stressors (Figure 3) and transfer the signals to genes via many signaling cascades (adaptive signaling pathways), which can interact and influence each other in a complex molecular network (Figure 4). Collectively, this stimulus‐response system is known as the adaptive stress response system of the body responding to environmental stress. 16 , 58 , 143 , 148 , 149 , 151
Figure 3.
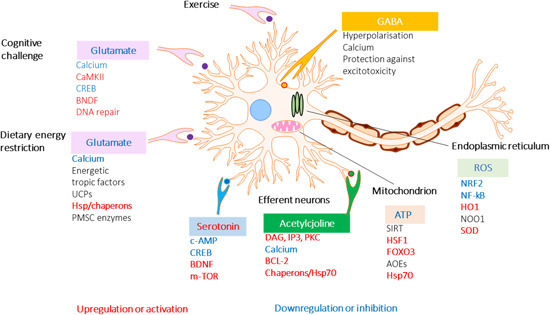
Effects of adaptogens on adaptive stress response signaling pathways that promote synaptic plasticity and protect neurons against degeneration. Illustration of a glutamatergic neuron receiving excitatory signals from neurons activated in response to intellectual tasks, exercise, and dietary energy restriction. Postsynaptic receptors for glutamate, acetylcholine, and serotonin, are activated to trigger intracellular signaling pathways and transcription factors that activate the expression of neuroprotective proteins including antiapoptotic proteins, brain‐mitochondrial uncoupling proteins (UCPs), and derived neurotrophic factor (BDNF). BDNF activates neuronal growth by stimulating the mammalian target of rapamycin (mTOR). Mild cellular stress resulting from dietary energy restriction and oxidative stress (ROS) activates adaptive stress response pathways including those that upregulate antioxidant enzymes (AOEs) and protein chaperones. CREB, cyclic AMP response element‐binding protein; CaMKII, calcium/calmodulin kinase II; DAG, diacylglycerol; FOXO3, forkhead box protein O3; HO1, heme oxygenase 1; HSF1, heat shock factor 1; IP3 PKC, inositol trisphosphate 3 protein kinase C; NF‐B, nuclear factor B; NRF2, nuclear regulatory factor 2 NQO1, NAD(P)H‐quinone oxidoreductase 1 (updated and adapted from Reference [59] and from authors’ drawings 16 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
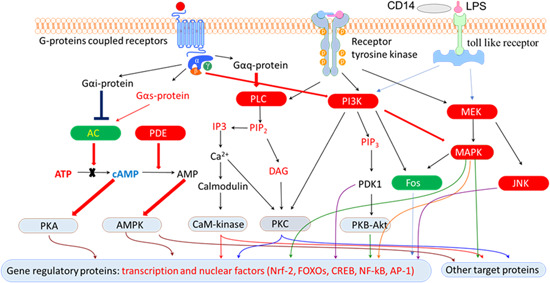
Effects of adaptogens on adaptive stress response intracellular signaling pathways (updated from authors’ drawings 17 ). Activation of the PI3K/AKT/mTOR signaling pathway positively regulates cell cycle, proliferation, neural long‐term potentiation (memory cognitive functions and longevity. AC, adenylate cyclase; AMPK, 5' AMP‐activated protein kinase; AP‐1, activator protein 1 transcription factor; CREB, cyclic AMP response element‐binding protein; DAG, diacylglycerol; Fos, Fos proto‐oncogene, AP‐1 transcription factor subunit; FOXOs, forkhead box proteins; IP3, inositol 1,4,5‐trisphosphate; JNK, c‐Jun N‐terminal kinases; MaM‐kinase, Ca2+/calmodulin‐dependent protein kinase II; MAPK–MEK (MAPK/ERK), mitogen‐activated protein kinases; NF‐κB, nuclear factor kappa‐light‐chain‐enhancer of activated B cells; NRF2, nuclear regulatory factor 2; PDE, 3',5'‐cyclic‐AMP phosphodiesterase; PI3K, phosphoinositide 3‐kinase; PIP3, phosphatidylinositol (3,4,5)‐trisphosphate; PIP2, phosphatidylinositol (4,5)‐bisphosphate; PKA, cAMP‐dependent protein kinase; PKB‐Akt, serine/threonine‐specific protein kinase; PKC, protein kinase C; PLC, phospholipase C [Color figure can be viewed at wileyonlinelibrary.com]
In conclusion, the adaptive stress response is a survival mechanism that includes the genetic response to environmental mild stressors. The mild stressors include exercise, calorie restriction, and adaptogens, which activate adaptive signaling pathways of the adaptive stress system to boost the body's cellular maintenance functions into high gear with cells having a more efficient response. Adaptogens trigger the adaptive stress response to reduce chronic inflammation (inflammaging) and promote healthy aging.
3. ADAPTOGENIC PLANTS AND THEIR ACTIVE COMPOUNDS
The principal active constituents of adaptogenic plants (as investigated to date, Table 7) can be divided into three main chemical groups 16 :
-
o
compounds with a tetracyclic skeleton like cortisol and testosterone—terpenoids ginsenosides, sitoindosides, cucurbitacines, and withanolides,
-
o
structural analogues of catecholamines or tyrosine—lignans (schizandrin B from S. chinensis, eleutheroside E from E. senticosus), phenylpropane derivatives (rosavin from R. rosea and syringin from E. senticosus), phenylethane derivatives (tyrosol and salidroside from R. rosea),
-
o
structural analogues of resolvins 152 —oxylipins (polyhydroxylated polyunsaturated fatty acids 16 ).
Table 7.
List of the plants reported to have antistress (adaptogenic) activity and used in traditional medicinal systems as rejuvenating medicinal plants, qi tonics, rasayanas, or restoratives
| Plants reported in Literature as adaptogens (1)* and for antistressa | References supporting such use in specific medical systems | |||
|---|---|---|---|---|
| Referenceb | TCM—qi tonifying | Ayurveda | ||
| 1 | Aegle marmelos (L.) Corrêa | [153] | [154] | |
| 2 | Ajuga turkestanica (Regel) Briq. | [155] | ||
| 3 | Albizia julibrissin Durazz. | [156] | ||
| 4 | Alstonia scholaris (L.) R. Br. | [157] | [158] | |
| 5 | Allium sativum L. | [159] | ||
| 6 | Andrographis paniculata (Burm.f.) Nees | [160, 161] | [162] | |
| 7 | Annona muricata L. | [163] | ||
| 8 | Aralia elata (Miq) Seem. | [164] | ||
| 9 | Aralia elata var. mandshurica (Rupr. & Maxim.) J.Wen (syn. Aralia mandshurica Rupr. & Maxim) | [165, 166, 167] | ||
| 10 | Aralia cordata var. sachalinensis (Regel) Nakai (syn.Aralia schmidtiiPojark.) | [165] | ||
| 11 | Argyreia nervosa (Burm. f.) Bojer (syn. Argyreia speciosa (L. f.) Sweet) | [168] | [168] | |
| 12 | Asparagus racemosus Willd. | [169] | [170, 171] | |
| 13 | Azadirachta indica A. Juss. | [172] | [173] | |
| 14 | Bacopa monnieri (L.) Wettst. | [174] | [175, 176] | |
| 15 | Bergenia crassifolia (L.) Fritsch | [177, 178, 179] | ||
| 16 | Boerhaavia diffusa Brandegee | [180] | [181] | |
| 17 | Bryonia alba L. | [12, 114] | ||
| 18 | Butea monosperma (Lam.) Taub. | [182] | [183] | |
| 19 | Caesalpinia bonduc (L.) Roxb. | [184] | [185] | |
| 20 | Cannabis sativa L. | [186] | [187] | |
| 21 | Carum carvi L. | [188] | [188] | |
| 22 | Centella asiatica (L.) Urb. | [189] | [190] | |
| 23 | Chlorophytum borivilianum Santapau & R.R.Fern. | [191, 192] | [193, 194] | |
| 24 | Chrysactinia mexicana A. Gray | [195] | ||
| 25 | Cicer arietinum L. | [196] | [170] | |
| 26 | Clematis alpina subsp. sibirica (L.) Kuntze (syn. Atragene sibirica L.) | [197] | ||
| 27 | Cnestis ferruginea Vahl ex DC. | [198] | ||
| 28 | Codonopsis pilosula (Franch.) Nannf. | [10] | [199, 200] | |
| 29 | Convolvulus pluricaulis Chois | [201] | [190] | |
| 30 | Curculigo orchioides Gaertn. | [202] | [203, 204] | [204] |
| 31 | Curcumin from Turmeric (Curcuma longa) | [205] | [204] | |
| 32 | Dioscorea deltoidea Wall. ex Griseb. | [206] | ||
| 33 | Diospyros malabarica (Desr.) Kostel. (Syn. Diospyros peregrina (Gaertn.) Gürke) | [207] | ||
| 34 | Elaeagnus rhamnoides (L.) A.Nelson. (Syn.Hippophae rhamnoides L.) | [208, 209] | [210] | |
| 35 | Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. | [8, 165, 166] | ||
| 36 | Eleutherococcus sessiliflorus (Rupr. & Maxim.) S.Y. Hu (syn Acanthopanax sessiliflorus (Rupr. & Maxim.) Seem.) | [8, 166] | [211, 212] | |
| 37 | Emblica officinalis Gaetrn. | [170] | [213, 214] | |
| 38 | Eucommia ulmoides Oliv. | [215] | [216, 217] | |
| 39 | Evolvulus alsinoides (L.) L. | [218, 219, 220] | [218, 219] | |
| 40 | Fagopyrum esculentum Moench | [221] | ||
| 41 | Firmiana simplex (L.) W. Wight (Syn Sterculia plantanifolia L.) | [222] | ||
| 42 | Gentiana pedicellata (D.Don) Wall | [223] | ||
| 43 | Ginkgo biloba L. | [224] | ||
| 44 | Glycyrrhiza glabra L. | [193, 225] | [226] | [193, 227] |
| 45 | Hebanthe eriantha (Poir.) Pedersen (Syn.Pfaffia paniculata (Mart.) Kuntze) | [228] | ||
| 46 | Heteropterys aphrodisiaca Machado | [229] | ||
| 47 | Heteropterys tomentosa A.Juss. | [230] | ||
| 48 | Hibiscus cannabinus L. | [231] | ||
| 49 | Holoptelea integrifolia Planch | [232] | [233] | |
| 50 | Hoppea dichotoma Willd. | [234] | ||
| 51 | Hypericum perforatum L. | [235] | ||
| 52 | Justicia diffusa Willd. (Syn Rostellularia diffusa (Willd.) Nees.) | [236] | ||
| 53 | Lagenaria siceraria (Molina) Standl. | [237] | ||
| 54 | Lepidium meyenii Walp. (Syn. Lepidium peruvianum G.Chacón) | [238] | ||
| 55 | Marantodes pumilum (Blume) Kuntze. (Syn.Labisia pumila (Blume) Mez) | [239] | ||
| 56 | Melilotus officinalis (L.) Pall. | [240] | ||
| 57 | Mitragyna inermis (Willd.) Kuntze (Syn Mitragyna africana (Willd.) Korth.) | [241] | ||
| 58 | Momordica charantia L. | [242] | ||
| 59 | Morus alba L. | [243] | ||
| 60 | Mucuna pruriens (L.) DC. | [244] | [190] | |
| 61 | Murraya koenigii (L.) Spreng. | [245] | ||
| 62 | Mussaenda frondosa L. | [246] | ||
| 63 | Nelumbo nucifera Gaertn. | [247] | [248] | |
| 64 | Nigella sativa L. | [249] | ||
| 65 | Ocimum tenuiflorum L. (Syn.Ocimum sanctum L.) | [250, 251, 252] | ||
| 66 | Oplopanax elatus (Nakai) Nakai (Syn. Echinopanax elatum Nakai) | [165, 166, 253] | [165] | |
| 67 | Panax ginseng C.A.Mey. | [8, 165, 187, 224] | [84, 85, 254] | |
| 68 | Panax notoginseng (Burk.) FH Chen | [107] | ||
| 69 | Panax pseudoginseng Wall. | [255] | [256] | |
| 70 | Pandanus odorifer (Forssk.) Kuntze (Syn.Pandanus odoratissimus L.f.) | [257] | ||
| 71 | Paullinia cupana Kunth | [258] | ||
| 72 | Putranjiva roxburghii Wall. (Syn. Drypetes roxburghii (Wall.) Hurus.) | [259] | ||
| 73 | Piper longum L. | [260, 261] | [170, 261] | |
| 74 | Polyalthia cerasoides (Roxb.) Bedd. | [163, 262] | ||
| 75 | Polyscias filicifolia (C.Moore ex E.Fourn.) L.H.Bailey | [263] | ||
| 76 | Potentilla alba L. | [264] | ||
| 77 | Prunella vulgaris L. | [265] | ||
| 78 | Psidium guajava L. | [266] | ||
| 79 | Ptychopetalum olacoides Benth. | [267] | ||
| 80 | Pueraria tuberosa (Roxb. ex Willd.) DC. | [268] | ||
| 81 | Rhaponticum carthamoides (Willd.)Iljin (Syn. Leuzea carthamoides (Willd.) DC.) | [8, 269] | ||
| 82 | Rhodiola crenulata (Hook.f. & Thomson) H.Ohba | [270, 271] | ||
| 83 | Rhodiola heterodonta (Hook. f. & Thomson) Boriss. | [272, 273] | ||
| 84 | Rhodiola imbricata Edgew. | [271, 274] | ||
| 85 | Rhodiola rosea L. [today classed as Sedum roseum (L.) Scop.] | [8, 21, 66, 72, 275, 276] | [277] | [278] |
| 86 | Rubia cordifolia L. | [279] | . | [280] |
| 87 | Salvia miltiorrhiza Bunge | [281] | [282] | |
| 88 | Schisandra chinensis (Turcz.) Baill. | [18, 64, 69, 165, 283] | [284, 285] | |
| 89 | Scutellaria baicalensis Georgi | [286] | [287] | |
| 90 | Serratula tinctoria L. (Syn.Serratula inermis Poir.) | [288] | ||
| 91 | Sida cordifolia L. | [289] | [289] | |
| 92 | Silene italica (L.) Pers. | [290] | ||
| 93 | Sinomenium acutum (Thunb.) Rehder & E.H.Wilson | [291] | ||
| 94 | Solanum torvum SW. | [292] | ||
| 95 | Serratula coronate L. | [293] | ||
| 96 | Sutherlandia frutescens (L.) R.Br. | [294] | ||
| 97 | Syzygium aromaticum (L.) Merr. & L.M.Perry. (Syn. Eugenia caryophyllus (Spreng.) Bullock & S.G.Harrison) | [295] | [185] | |
| 98 | Terminalia chebula Retz. | [296] | [297] | [170, 193] |
| 99 | Tinospora sinensis (Lour.) Merr. (Syn.Tinospora cordifolia (Willd.) Miers, Syn Tinospora malabarica (Lam.) Hook. f. & Thomson) | [298, 299] | [170, 300] | |
| 100 | Tribulus terrestris L. | [301] | [301] | |
| 101 | Trichilia catigua A.Juss. | [229] | ||
| 102 | Trichopodium zeylanicum (Gaertn.) Thwaites (Syn.Trichopus zeylanicus Gaertn.) | [302, 303] | [302, 303] | |
| 103 | Trigonella foenum‐graecum L. | [304, 305, 306] | [307] | |
| 104 | Tylophora indica (Burm. f.) Merr. | [308] | ||
| 105 | Turnera diffusa Willd. ex Schult. | [229] | ||
| 106 | Uncaria tomentosa (Willd. ex Schult.) DC. | [309] | ||
| 107 | Vitis vinifera L. | [310] | [310] | |
| 108 | Withania somnifera (L.) Dunal | [232, 311, 312, 313] | [170, 314, 315, 316, 317, 318] | |
| 109 | Zingiber officinale Roscoe | [319] | ||
The number of plants reported as being adaptogenic has increased exponentially during the past decades. However, it should be emphasized that only a few comply with the most important criterium—exhibiting multitarget effects on the neuroendocrine‐immune system. These effects include triggering intracellular and extracellular adaptive signaling pathways that promote cell survival and organismal resilience in stress; and regulating metabolism and homeostasis via effects on the expression of stress hormones (corticotropin‐ and gonadotropin‐releasing hormones, urocortin, cortisol, melatonin, Hsp70, and neuropeptide Y) and their receptors. 16 , 17 , 18 , 19 , 20 , 28
Various adaptogens and their active principles—for example, salidroside, 320 , 321 , 322 , 323 , 324 , 325 , 326 schisandrin A, 327 schisandrin B, 328 withaferin A, 329 , 330 , 331 , 332 , 333 , 334 Ginsenoside 20(S)‑Rg3, 335 Ginsenoside 20(S)‐Rh2, 336 compound K, 337 , 338 and 20(S)‐25‐methoxy‐protopanaxatriol 339 , 340 —exhibit anticancer effects in various in vitro and in vivo models of breast, colorectal, prostate, hepatic, and intestinal cancers, and so forth by interacting with multiple intracellular signaling pathways, including the inhibition of proinflammatory pathways, such as the ERK/MAPK 341 and STAT3 signaling pathways. 320 , 321 , 322 , 323 , 324 , 325 , 326 , 327 , 328 , 329 , 330 , 331 , 332 , 333 , 334 , 335 , 336 , 337 , 338 , 339 , 340
It was found that compound K, an intestinal microbiome metabolite of ginsenoside Rb1, 342 one of the major ginsenosides of Panax ginseng, has much stronger cancer chemopreventive activity than its precursor (Rb1 in HCT‐116 and HT‐19 human colorectal cancer cell lines), suggesting that Rb1 may have potential clinical significance in the prevention of inflammatory‐associated colorectal cancer 343 because of the regulation of the microbiome balance and compound K. 343 , 344
R. rosea extracts and the active compound salidroside decrease the growth of bladder cancer cell lines via the inhibition of the mTOR pathway and induction of autophagy. 345 Salidroside was shown to exhibit antioxidant, anti‐inflammatory, and anticancer effects in human breast cancer in vitro and in vivo experimental models. 346 Salidroside treatment significantly inhibits MCF‐7 breast cancer cell proliferation, colony formation, migration, invasion, apoptosis, and cell‐cycle arrest at the G0/G1 phase in vitro and significantly suppressed tumor growth in vivo. 346
In vitro and in vivo experiments demonstrated that salidroside enhances the chemotherapeutic effect of apatinib in gastric cancer. 347 Ginseng potentiates the effects of chemotherapeutic agents via synergistic activities, supported by cell‐cycle evaluations, apoptotic observations, and computer‐based docking analysis. 348
Finally, the results of many studies suggest that adaptogens might be useful for the prevention of liver cancer because of the upregulation of Nrf2 signaling, followed by the induction of the antioxidant and phase II detoxifying engines, for example, induction of the phase II detoxification enzyme NQO1 in hepatocarcinoma cells by lignans of S. chinensis, tigloylgomisin H (TGH), and angeloylgomisin H (AGH), which have exhibited a relatively high chemoprevention index (10.80 and 4.59, respectively). 349
4. CURRENT AND PROSPECTIVE USE OF ADAPTOGENS IN STRESS‐INDUCED AND AGING‐RELATED DISEASES
Stress‐protective and stimulating effects are characteristic and common pharmacological effects of adaptogens, 73 , 350 , 351 which have been observed in many animals and humans’ studies. The effects of adaptogens on cognitive functions and physical endurance in stress are summarized in several reviews. 10 , 22 , 26 , 27 , 350 , 352
The main difference between adaptogens and conventional stimulants such as caffeine and amphetamine is that after prolonged use, the latter can cause the user to develop both tolerance and addiction (Table 8). 27 , 352
Table 8.
The differences in properties between adaptogens and other stimulants
| Stimulants | Adaptogens | |
|---|---|---|
| Stress protective (neuro‐, hepato‐, cardio‐protective) | No | High |
| Recovery process after exhaustive physical load | Low | High |
| Energy depletion | Yes | No |
| Performance in stress | – | Increased |
| Survival in stress | – | Increased |
| Quality of arousal | Poor | Good |
| Addiction potential | Yes | No |
| Side effects | Yes | Rare |
| DNA/proteins synthesis | Decreased | Increased |
| NPY mediated activation of Hsp70 | – | Increased |
Primarily, adaptogens have potential benefits in cases of behavior‐related disorders, mental illness, stress‐induced fatigue (Figure 5), and cognitive function. 11 , 14 , 15 , 26 , 27 , 48 , 74 , 75 , 216 , 275 , 350 , 353 , 354 , 355 , 356 , 357 , 358 , 359 , 360 , 361 , 362 , 363 , 364 , 365 , 366 , 367 In a number of clinical studies, the beneficial effects of adaptogens have been demonstrated on healthy subjects in stress conditions. 26 , 27 , 48 , 74 , 75 , 324 , 353 , 356 , 357 , 359 , 362 This is especially true of the mental and physical performance of fatigue and mental strain. Furthermore, the efficacy of adaptogens in mild and moderate depression has been demonstrated. 275 , 355 , 358 , 360 , 363 , 366
Figure 5.
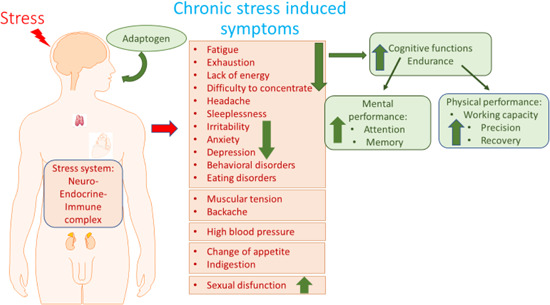
Chronic stress‐induced symptoms and effect of adaptogens, updated from authors’ drawings 14 [Color figure can be viewed at wileyonlinelibrary.com]
The prophylactic use of adaptogens seems to be justified in healthy subjects for preventing aging‐related diseases, and to attenuate stress‐induced harmful effects. 26 , 27 , 95 , 317 , 368 , 369 , 370 , 371
Several systematic reviews and assessment reports have been conducted on the clinical efficacy and safety of ginseng, 2 , 372 , 373 , 374 Eleutherococcus, 375 Rhodiola, 376 , 377 , 378 , 379 , 380 , 381 , 382 Withania, 383 , 384 , 385 , 386 , 387 , 388 and other adaptogens on several indications such as cognitive function, 33 , 72 cardiovascular diseases, 389 chronic pulmonary disease, 390 prevention of common cold, 391 and erectile dysfunction. 392 The clinical evidence of the benefits of W. somnifera in male infertility is also promising but very limited to provide sufficiently robust evidence because of the small number of eligible studies and available data. 393 The results suggest the potential role of W. somnifera in managing diabetes mellitus, but evidence is not robust because of insufficient available clinical data. Furthermore, well‐designed randomized controlled trials (RCTs) with a larger sample size and longer duration are warranted to evaluate its effect primarily on blood glucose, HbA1c, and insulin. 386 In five studies conducted in patients with anxiety and stress, significant (in most cases) improvements were observed with Withania intervention as compared with placebo, but cases of potential bias were identified. 383 There is some evidence from randomized, placebo‐controlled, double‐blind trials regarding the benefits of W. somnifera on cognitive function, such as improved performance on cognitive tasks, attention, and reaction time. 385 However, the study population was heterogeneous, including older adults with mild cognitive impairment and adults with schizophrenia, schizoaffective disorder, or bipolar disorder.
In most of the early clinical studies on Eleutherococcus preparations conducted in the USSR in the 1960s and 1970s, positive results were commonly reported. 394 However, most of these trials lacked good methodology (e.g., lack of randomization, proper control, blinding, statistical tools, description of inclusion and exclusion criteria, description of the medication, diagnosis, study design, and small sample size). In 2009, Li et al. assessed the efficacy and safety of Eleutherococcus in patients with acute ischemic stroke in a Cochrane systematic review. The authors included 13 RCTs (962 participants). The primary outcome measure in all included trials was the improvement of the neurological deficit after treatment. Eleutherococcus was found to significantly increase the number of participants with improvement in neurological impairment. However, because the risk of bias in all of the included trials was high, the authors concluded that much larger trials of greater methodological quality are required. 375 In the EMEA assessment report dated March 25, 2014, the authors concluded that despite the large number of studies on the topic, Eleutherococcus root preparations do not reach the level of “well‐established use” scientific evidence sufficient to grant a marketing authorization, although in total, the data available are sufficient to justify further research on the concept of adaptogens. 3
Similar decisions were made in 2011 and 2012 regarding Rhodiola 1 and ginseng. 2 The beneficial effects of ginseng on cognitive function have been demonstrated in several studies, but the evidence was not sufficient to achieve the designation of well‐established use in 2012 because of the heterogeneity of the investigated preparations, limited numbers of participants, differences in study design, and methodological quality. 2 Because the number of clinical trials on the clinical efficacy of R. rosea was limited, we could not conclude that there was sufficient evidence for well‐established use in the treatment of fatigue or mental weakness. However, the data support the plausibility of the use of the traditional herbal medicinal products of R. rosea as adaptogens. 1
In Sweden, Norway, and Denmark, Rhodiola traditional herbal medicinal product is indicated as an adaptogen in situations of decreased performance such as fatigue and sensation of weakness.
In a systematic review and meta‐analysis of 11 RCTs of R. rosea, Hung et al. 381 concluded that “the methodological quality of most trials was moderate or good. Five of the 11 RCTs reached more than 3 points on the Jadad score (i.e., good quality). R. rosea may have beneficial effects on physical performance, mental performance, and certain mental health conditions. Only a few mild adverse events were reported. There is, however, a lack of independent replications of the single different studies”.
Extracts of Red Korean Ginseng have been tested extensively in mice and isolated cells infected with influenza virus. The antiviral protective effects were observed regardless of influenza virus strains, including various subtypes of H1N1, H3N2, H5N1, and H7N9. Mice inoculated with a lethal dose of virus and ginseng preparations were protected against weight loss with 100% survival rates during primary infection, and they developed immunity against secondary viral infection. 395 , 396 The use of various ginseng extracts to treat mice infected with influenza virus decreased the interleukin (IL)‐6 and IL‐8 cytokines and increased antiviral cytokine interferon (IFN) upon influenza virus infection. 397 , 398 , 399 , 400 It was demonstrated that ginsenosides, particularly Rb1, interact with viral hemagglutinin proteins, preventing the virus from binding to host cells and viral entry into the cytoplasm. 401 Meanwhile, ginseng polysaccharide fraction exhibits a strong antiviral effect in mice infected with influenza A virus, predominantly by reducing the accumulation of tumor necrosis factor α (TNF‐α)/inducible nitric oxide synthase (iNOS)‐producing dendritic cells (tipDCs) in mouse lungs. 402 Clinical trials suggest that ginseng is an effective prophylactic agent for respiratory infections, reducing the risk and duration of colds and flu and providing symptom relief. 403 , 404 , 405
The efficacy and safety of Andrographis‐containing preparations were studied in patients with common cold in Scandinavia, South America, and India. 406 , 407 , 408 , 409 , 410 , 411 Evidence from a meta‐analysis of the results of 33 RCTs showed that Andrographis relieves inflammatory symptoms and shortens the duration of cough, sore throat, and sick leave/time to resolution when compared with usual care. 411
Several epidemiological studies conducted in the USSR during the 1970s appeared to establish that Eleutherococcus root extract, given prophylactically, can reduce morbidity rates during an influenza virus epidemic as well as typical complications of influenza infection, such as bronchitis, pneumonia, and otitis. 3 Eleutherococcus is an effective antiviral agent that induces IFN‐γ production 412 , 413 , 414 , 415 , 416 and increases leukocyte, cytotoxic T‐cell, T‐helper, and B‐ and T‐lymphocyte counts in peripheral blood. 412 , 417 , 418 , 419 , 420 The efficacy of adaptogens in the treatment of acute respiratory tract diseases is possibly also partially associated with the downregulation of proinflammatory NF‐kB signaling in various cells and tissues involved in the acute inflammatory response.
The fixed combination (Kan Jang) of Andrographis and Eleutherococcus has been used since 1979 in Sweden as an herbal medicine (“naturmedel”), with well‐established use (“naturläkemedel”) in Denmark since 1997 for reducing the severity and duration of symptoms of common cold. 3 This combination was tested in controlled clinical trials for the treatment of common cold and influenza‐associated uncomplicated upper respiratory infections as well as for the prevention of common colds. 421 , 422 , 423 , 424 The studies confirmed the safety and superior efficacy of this combination regimen as compared with monodrug therapy, 425 presumably because of its antiviral effects, 426 , 427 , 428 , 429 , 430 , 431 , 432 effects on innate and adaptive immunity, 433 , 434 , 435 , 436 , 437 and anti‐inflammatory, antioxidant, and detoxifying effects 438 , 439 , 440 , 441 of both adaptogenic plants as well as due to their synery. 25 It should be noted that the postmarketing pharmacovigilance assessment of Kan Jang showed a high benefit–risk ratio: one adverse event in about 100,000 patients was recorded for the 23‐year period from the adverse event reports (concerning mainly allergic reactions) to the Swedish and Danish medical product agencies. Further studies are needed to evaluate the efficacy of these plants in patients with COVID‐19 and other viral respiratory invidious diseases.
One more possible benefit of adaptogens in respiratory tract infectious diseases might be their beneficial effect during patient convalescence. Adjuvant therapy with Chisan/ADAPT‐232, a fixed combination of Eleutherococcus, R. rosea, and S. chinensis, in pneumonia has a positive effect on patient recovery by decreasing the duration of the acute phase of the illness, increasing patient mental performance during the rehabilitation period, and improving patient quality of life (QOL). 354 Both the clinical and laboratory results of the present study suggest that Chisan (ADAPT‐232) can be recommended in the standard treatment of patients with acute nonspecific pneumonia as an adjuvant to increase patient QOL and to expedite their recovery.
Dietary supplements containing Rhodiola, Withania, Ginseng, Eleutherococcus, Schisandra, and other adaptogenic plant extracts are widely used all over the world, 21 , 69 , 87 , 160 , 161 , 261 , 318 , 442 , 443 , 444 , 445 , 446 while in China, Korea, Japan, Russia, and some neighbor countries various pharmaceutical forms of adaptogenic plants form a part of official medicine. 447 , 448 , 449 Overall, it is well documented now that adaptogens act polyvalently with positive effects on aging‐related disorders including atherosclerosis and other chronic inflammatory diseases, metabolic diseases, neurodegenerative cognitive impairment as well as cancer. 1 , 2 , 3 , 4 , 10 , 13 , 15 , 17 , 21 , 44 , 57 , 69 , 278 , 444 For example, numerous in vivo and in vitro studies on P. ginseng have shown its beneficial effects in aging, CNS disorders, and neurodegenerative and cardiovascular diseases, cancer, immune deficiency, and hepatotoxicity. Clinical trials have been conducted on the effects of ginseng preparations on cognitive function, lipid and glucose metabolism, cardiovascular function, erectile dysfunction, quality of life, improvement of the immune system, and chronic respiratory diseases. 57 All of them are associated with the metabolic regulation of homeostasis and threatened adaptability of the stress system. Adaptogenic plants possess compounds that exhibit anticancer activity and potentiate the effects of antitumor drugs, suggesting that they can be used alone or as adjuvants to conventional chemotherapy to improve their efficacy or reduce radiotherapy‐ or chemotherapy‐induced toxicity, 348 for example, nausea and vomiting. 114 , 450 Supplementation with adaptogens is also considered a promising therapy for cancer‐related fatigue, a debilitating syndrome that persists for years in many cancer survivors. 88
More evidence from controlled clinical studies supporting health claims and indications for use in diseases are required.
5. CORE RATIONAL OF THE ADAPTOGENIC CONCEPT
5.1. Mechanisms of adaptogenic and stress‐protective actions
The pathogenesis of complex diseases as well as the adaptive stress response, inflammation, and senescence are multistep processes which involve extracellular and intracellular communications at differing stages of stress regulation and cannot be limited to the few biochemical interactions that occur in the brain or other tissues. Clearly, for the description of the mechanism of action of adaptogens the reductionist model that assumes a single drug—single receptor interaction is insufficient and not valid. Adaptogens have many molecular targets 16 , 17 involved in the metabolic regulation of homeostasis at both the cellular and systemic levels and play a role of stress response modifiers. 11 , 16 , 17 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 49 , 56 , 60
Network pharmacology with the use of the systems biology offers exciting new opportunities for understanding such complex systems. 16 During the past several decades, many molecules, signaling pathways, and networks targeted by adaptogens have been identified. 11 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 49 , 56 , 60 They include stress hormones and some other important mediators of homeostasis regulation such as the molecular chaperons Hsp70, neuropeptide Y, G protein‐coupled receptors (GPCRs), dopamine‐cAMP‐PKA‐CERT, IP3, PLC, DAG, phosphoinositide 3‐kinase (PI3K), nuclear factor kappa‐light‐chain‐enhancer of activated B cells (NF‐κB)‐mediated signaling pathways, stress‐activated kinase c‐Jun N‐terminal kinase (JNK), forkhead box protein O3 (FOXO3), cortisol, estrogens, and nitric oxide (NO). 11 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 49 , 56 , 60 The mechanisms of action of adaptogens are mainly associated with metabolic regulation via extracellular communication of hypothalamic–pituitary–adrenal (HPA)‐axis hormones and activation of intracellular adaptive stress response signaling pathways. 16
5.1.1. Effect of adaptogens on extracellular communications within the neuroendocrine‐immune system
The hypothetical mechanism of adaptogens’ action on HPA‐axis hormones in stress is presented in Figure 6. The HPA axis plays a pivotal role in regulating the majority of endocrine hormones associated with the CNS. Stress hormones regulate growth, appetite, blood pressure, emotion, sexual function, body temperature, sleep, biorhythms, and hydration. They are produced by the endocrine system, are secreted into the bloodstream, and target other tissues to regulate physiological functions. The main function of stress hormones is to maintain homeostasis to counteract stress
Figure 6.
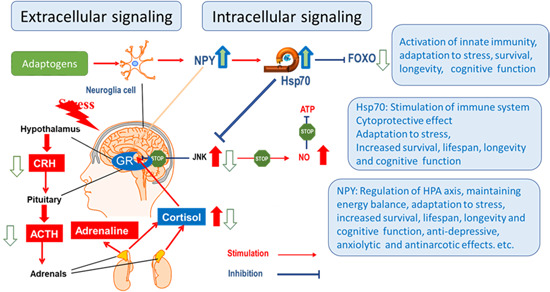
Hypothetical mechanism of action underlying the effects of adaptogens on the adaptive stress response in the hypothalamic–pituitary–adrenal axis: forkhead box O, neuropeptide‐Y (NPY), and Hsp70 signaling. Persistent chronic stress induces and blockage of negative feedback regulation of cortisol and disruption of ATP synthesis. During stress, corticotropin‐releasing hormone (CRH) is released from the hypothalamus, followed by the release of adrenocorticotropic hormone (ACTH) from the pituitary, which stimulates the release of adrenal hormones and NPY. Feedback regulation of overreaction is triggered by cortisol release from the adrenal cortex, followed by binding to glucocorticoid receptors (GRs) in the brain, which halts the further release of brain hormones, resulting in decreases of cortisol to normal levels. Although mild stress (eustress) is a vital part of life, chronic and severe stress can cause depression associated with the production of active oxygen‐containing molecules including nitric oxide, which inhibits ATP formation. The stress‐induced signaling protein c‐Jun N‐terminal kinase (JNK) inhibits GRs. Subsequently, this feedback control is inhibited and the cortisol content in the bloodstream of depressed patients is permanently high, which is associated with impaired memory, decreased ability to concentrate, fatigue, among others. Adaptogens normalize increased cortisol/corticosterone levels in the bloodstream and saliva of humans or animals 12 , 357 presumably due to direct interaction with GRs. Adaptogens also attenuate elevated JNK and cortisol levels during stress and activate the generation of Hsp70, which inhibits JNK. Therefore, the nitric oxide level no longer rises, and ATP production is not inhibited (adapted from authors’ drawings 26 ) [Color figure can be viewed at wileyonlinelibrary.com]
Ginsenoside Rg1 directly interacts with glucocorticoid receptor (GR) ligand‐binding sites and behaves as a partial agonist of GR. Ginsenoside Rb1 is a functional ligand of the estrogen receptor (ER).
Along with CRH, another primary upstream mediator of extracellular communications stimulated by adaptogens is the stress hormone neuropeptide‐Y (NPY). 23 , 28 Stimulation and release of NPY into the blood circulatory system are innate defence responses to mild stressors (adaptogens), which increase resistance to stress. This leads to stress‐protective and adaptive effects via various elements of the endocrine, immune, central nervous, sympathetic, cardiovascular, and gastrointestinal systems. Both Hsp72 and NPY play essential roles in stress, and pathogenesis of aging‐related diseases. The antinarcotic effects of adaptogens are mediated by NPY, which is an important intermediate involved in morphine tolerance and opioid dependence.
5.1.2. Molecular mechanisms of action—Effects on intracellular signaling pathways
Gene expression analysis has helped to gain an improved understanding of the molecular mechanisms of action of adaptogenic plants and elucidation of adaptive stress response signaling. 17 , 23 , 24 , 25 , 451 , 452 One recent study in which the gene expression profiles of isolated brain cells were exposed to adaptogens, showed that at least 88 of the 3516 genes regulated by adaptogens modulate many signaling pathways involved in the adaptive stress response. 17 Genes encoding neurohormones, transmembrane channels, and receptors, transcription regulators and ligand‐dependent nuclear receptors, protein kinases phosphatases, peptidases, metabolic enzymes, chaperones and other intermediates of intra‐ and extracellular communications (Table 9) are key elements in several canonical pathways involved in defence response, survival, longevity, and in maintaining of cellular and organismal homeostasis.
Table 9.
Genes regulated by adaptogens
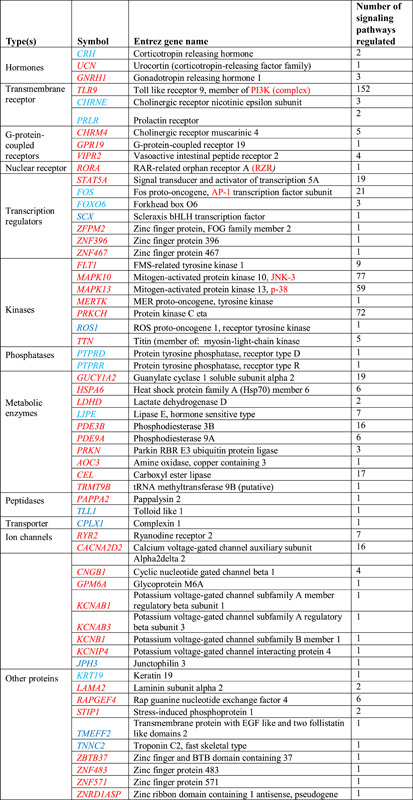
|
Note: Upregulated genes are in red color while downregulated genes are in blue color text.
Some of these proteins play key roles in regulating numerous processes. As an example, all adaptogens upregulate TLR9, a member of the PI3K (complex) gene‐encoding transmembrane receptor that plays key roles in regulating 152 signaling pathways including glucocorticoid receptor signaling, interleukins IL‐2, IL‐3, IL‐4, IL‐6, IL‐7, IL‐8, IL‐9, ILK, IL‐12, IL‐15, IL‐17A, IL‐17 signaling, B‐ and T‐cell receptor signaling, leukocyte extravasation, extracellular signal‐regulated protein kinase (ERK)/MAPK, PI3K/AKT signaling in the pathogenesis of influenza, lipopolysaccharide‐stimulated MAPK signaling, p53 and JNK signaling, production of NO and reactive oxygen species (ROS) in macrophages signaling, eNOS signaling NO signaling in the cardiovascular system, leptin signaling in obesity, type II diabetes mellitus signaling, IGF‐1 and insulin receptor signaling, prolactin signaling, AMPK signaling, and so forth. 17
All adaptogens upregulate protein kinase C (PKC) eta, an enzyme encoded by the PRKCH gene that plays key roles in the regulation of 72 signaling pathways including CRH signaling, androgen signaling, prolactin signaling, growth hormone signaling, melatonin signaling, Gαq signaling, feedback in cAMP signaling, Nrf2‐mediated oxidative stress response, production of NO and ROS in macrophages, mTOR signaling, NF‐κB activation by viruses, calcium‐induced T lymphocyte apoptosis, protein kinase A signaling, phospholipase C signaling, eNOS signaling, opioid signaling pathway, neuropathic pain signaling in dorsal horn neurons, axonal guidance signaling, CREB signaling in neurons, dopamine‐DARPP32 endothelin‐1 signaling, α‐adrenergic signaling, nNOS signaling in neurons, synaptic long‐term potentiation and synaptic long‐term depression signaling pathways. 17
All adaptogens upregulate mitogen‐activated protein kinases MAPK10 and MAPK13 which correspondingly involved in the regulation of 77 and 58 signaling pathways, including adaptive stress response signaling survival and longevity. These findings support the use of adaptogenic plants in TMS as a panacea for the treatment of numerous diseases.
All adaptogens tested (R. rosea L., E. senticosus, W. somnifera, R. carthamoides, and B. alba) activate the melatonin signaling pathway by acting through two GPCRs MT1 and MT2, and upregulating the ligand‐specific nuclear receptor RORA, which plays a role in different common aging diseases such as neurological disorders, hypertension, dyslipidemia, intellectual disability, retinopathy, and cancer. Furthermore, melatonin activates adaptive signaling pathways and upregulates the expression of UCN, GNRH1, TLR9, GP1BA, PLXNA4, CHRM4, GPR19, VIPR2, RORA, STAT5A, ZFPM2, ZNF396, FLT1, MAPK10, MERTK, PRKCH, and TTN, which are commonly regulated by all adaptogens tested. 17
The common features of recently tested extracts (B. alba L., Boswellia serrata Roxb. ex Colebr., Curcuma longa L., E. senticosus (Rupr. & Maxim.) Maxim, Rhaponticum carthamoides (Willd.) Iljin, R. rosea L., and W. somnifera (L.) Dunal) are related to the downregulation of ALOX12, which is also associated with the neuroprotective action of these medicinal plants as well as their potential benefits in neurodegenerative diseases. 452
R. rosea, W. somnifera, and E. senticosus downregulate the expression of key genes (ALOX5AP, DPEP2, LTC4S) involved in the biosynthesis of leukotrienes A, B, C, D, and E, resulting in inhibition of the leukotriene signaling pathway suggesting their potential benefits in Alzheimer's disease (Figure 7). 452
Figure 7.
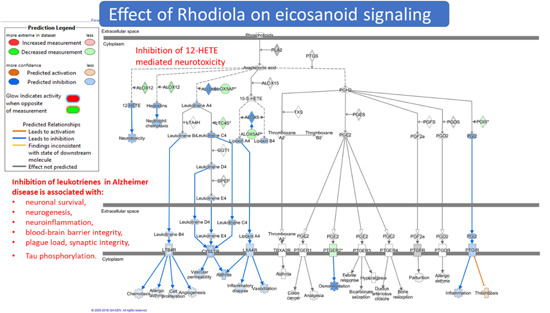
Effect of Rhodiola extract on the eicosanoids signaling pathway. Upregulated genes are shown in red color, while downregulated genes—in green color 452 [Color figure can be viewed at wileyonlinelibrary.com]
Adaptogens exhibit multitarget node of action targeting several receptors including receptors for corticosteroid, mineralocorticoid, progestin, estrogen, serotonin, NMDA, nicotinic acetylcholine, receptor tyrosine kinases, and many GPCRs. 11 , 16 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 44 , 49 , 56 , 60 , 453 , 454 , 455 , 456 , 457 , 458 , 459 , 460 , 461 , 462 , 463 , 464 , 465 , 466 , 467 , 468 , 469 , 470 , 471 , 472 , 473 Numerous molecular network interactions (including feedback regulation of the neuroendocrine and immune systems) resulting in agonist‐dependent antagonism are presumably the most suitable model for understanding the mechanism of action of adaptogens. 16
Interactive pathway analysis has demonstrated that adaptogens targets mediators of extracellular communications, intracellular networks, and signaling pathways, which are involved in stress‐induced and aging‐related disorders such as chronic inflammation, atherosclerosis, neurodegenerative cognitive impairment, metabolic disorders, and cancer. 17 , 23 , 24 , 25 , 368 Importantly, the effect on every disease is multitargeted. As an example, Robiola regulates 22 genes, which are deregulated in mood disorders, including 14 genes that are deregulated in depression: ADRA2B, AQP4, CACNB2, CCKBR, CHRNA1, CHRNB4, CHRNG, ESR1, GRIA3, GRIN1, KCNK2, MYOM1, NCAM1, and PDE11A. 24 , 275
5.1.3. Mechanisms underlying the cytoprotective, antioxidant, and antitoxic activities of adaptogens
Cytoprotective, antioxidant, and antitoxic effects of various adaptogenic preparations have been shown in many isolated cells as experimental models and (in vitro and ex vivo) in animals. 1 , 2 , 3 , 4 , 9 , 26 , 27 , 69 , 71 , 474 Extensive research on E. senticosus reveals its antitoxic, neuroprotective, hepatoprotective, cardioprotective, antioxidant, immunomodulating, and antiviral activities along with stress‐protective, antifatigue, hypoglycaemic, antidepressant and antiproliferative effects. 3 , 8 , 46 , 474 , 475 , 476 , 477 , 478 , 479 , 480 , 481 As an example, E. senticosus inhibits the cadmium‐induced apoptosis and mitosis of hepatocytes in mice, and significantly decreased cadmium concentration in their liver and blood. 478 It has been shown that hepatoprotective effect of E. senticosus extract is triggered by upregulation of expression Nrf2 and activation of innate antioxidant enzymes that increase the ratio of reduced versus oxidized glutathione in liver homogenate and serum. 226 Repeated administration of E. senticosus preparation decreased isoproterenol induced cardiotoxicity and increased ventricular fibrillation threshold in rats with post‐infarction cardiosclerosis. 480 , 481 Eleutherococcus reduces the toxic effects of cytostatic drugs (cyclophosphane, etimidine, benzotef, sarcolisyne, ribomicine, 6‐mercaptopurine, dopane thiophosphamide, trichlortriethylamin) as chemotherapy including loss of body weight, increased mortality, decreased life span, reduction of tumor growth, thymic involution, haematopoiesis, and immunosupression. Adjuvant treatment with Eleutherococcus significantly increases the survival of rodents following etimidine treatment for carcinoma (100% survival vs. 70% control [etimidine]). Similarly, adjuvant treatment with Eleutherococcus significantly increases survival following thiophosphamide treatment (85% survival vs. 47% control [thiophosphamide]). 482 , 483 , 484 , 485
The antioxidant and hepatoprotective activity of Eleutherococcus and Andrographis paniculate preparations have been reviewed in EMA assessment report. 3 , 4 The chemopreventive effects of A. paniculata and Andrographolide against cyclophosphamide (CTX)‐induced urothelial toxicity were previously demonstrated. 486 Both substantially lowered the elevated levels of IL‐2 and IFN‐γ and reduce CTX‐induced toxicity during CTX treatment. 486 In another study, the aqueous extract of A. paniculata was shown to attenuate gentamicin‐induced nephrotoxicity decreasing blood levels of urea, creatinine, and urea nitrogen levels in rats. 487
A. paniculata and andrographolide exhibit an extremely wide array of pharmacological activities 4 , 425 , 488 , 489 , 490 , 491 , 492 including adaptogenic, 160 antioxidant, chemopreventive, 4 and neuroprotective activities. 361 , 493 , 494 , 495 , 496
The cytoprotective effect of several adaptogenic plant extracts on chemotherapeutics‐induced dramatic impact on transcriptome‐wide RNA microarray profiles of neuroglia cells culture have been recently studied. 497 , 498 , 499 The fixed combination 5‐fluorouracil, epirubicin, and cyclophosphamide (FEC) has been shown to deregulate 67 genes involved in the reduction of neuronal development, 37 genes involved in development of the sensory system, 12 genes involved in axon extension, and 3 genes involved in neuronal migration. Pretreatment of cells with A. paniculata prevented the FEC‐induced deregulation of genes involved in regulation of neuronal death, neurogenesis, and other vital functions in the nervous system. Similar cytoprotective effects exhibit a fixed combination of A. paniculate with E. senticosis, which prevented the FEC‐induced deregulation of gene expression involved in migration of T98G neuroglia cells, axon extension, conduction of nerves, and other neuronal functions associated with cognitive impairments. Adaptogens significantly modify FEC‐induced deregulation of genes involved in the regulation of cell morphology, synaptic, mitochondrial function, and protein‐related functions suggesting their potential neuroprotective and hepatoprotective effects, which are associated with FEC‐induced adverse events in cancer chemotherapy. The authors concluded that adjuvant treatment with adaptogens can prevent mild cognitive impairments and “chemobrain” effect associated with cancer chemotherapy. 497 It is noteworthy that adaptogens potentiate the cytotoxic effects of chemotherapeutics in human T98G glioblastoma cells. 498
There are several mechanisms underlying the cytoprotective and antitoxic effects of adaptogens.
One of them is the Nrf2/antioxidant response element (ARE) signaling pathway, which a key defense response signaling pathway regulating the expression of phase II detoxifying enzymes in response to toxic stimuli (Figure 8).
Figure 8.
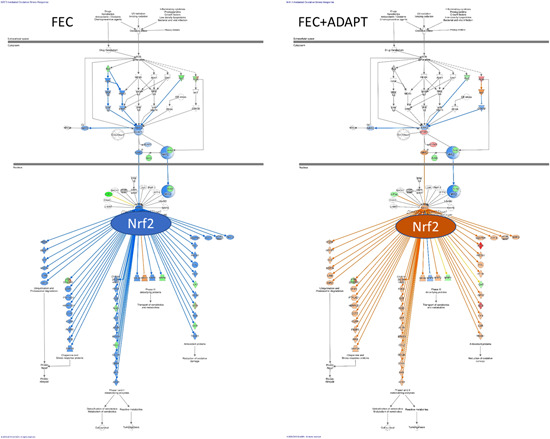
Adaptogens exhibit antioxidant and detoxifying effects presumably by activation of the Nrf2/ARE pathway. Nrf2 is a principal regulator of redox homeostasis normally retained in the cytoplasm by association Kelch‐like ECH‐associated protein‐1 (Keap1). Upon exposure of cells to oxidative stress, Nrf2 is phosphorylated in response to PKC, phosphatidylinositol 3‐kinase (PI3K), and MAPK pathways. After phosphorylation, this complex dissociates and Nrf2 translocates to the nucleus where it binds to the ARE and triggers expression of antioxidant and detoxifying genes including superoxide dismutase, glutathione S‐transferase, NAD(P)H quinone oxidoreductase 1, and heme oxygenase 1. Thus, activation of Nrf2 translocation or upregulation of gene expression resulting in activation of the Nrf2 signaling pathway is the key mechanism of the cellular defense response 500 , 501 associated with the antioxidant effects of medicinal plants, and particularly of adaptogenic plants, which are useful in stress‐ and aging‐related diseases [Color figure can be viewed at wileyonlinelibrary.com]
An imbalance between the production of reactive oxygen radicals and their degradation results in oxidative stress. Reactive intermediates interact with polyunsaturated fatty acids, proteins, and RNA and DNA fragments, initiating numerous redox reactions that damage many cellular components such as the membrane, mitochondria, and nucleus, which leads to dysfunction of cellular processes and homeostasis, and triggers apoptosis and necrosis. Oxidative stress is increased in chronic inflammation and aging‐related disorders including atherosclerosis, angiogenesis, and neurodegeneration. 502 The feedback cellular response is associated with activation of defence mechanisms including induction of antioxidant and detoxifying enzymes and molecular chaperones. Several adaptive signaling pathways such as p38, PKC, ERK, JNK, and PI3K signaling may activate Nrf2. Two other adaptive signaling pathways involving NF‐κB and FOXO transcription factors are important in neuronal stress adaptation. 503 , 504 , 505 , 506
Although adaptogens at high concentrations are potent radical scavengers, in lower amounts, they may activate some intracellular adaptive stress response signaling pathways resulting in the expression of cytoprotective proteins including neurotrophic factors, protein chaperones, antioxidant and phase II enzymes, and antiapoptotic proteins. One of them is transcription factor Nrf2. 151
The beneficial effects of adaptogens appear to be related, at least in part, to their ability to activate the Nrf2/ARE pathway (Figure 8) and regulate the number of genes playing important roles in activation of the production of antioxidant and detoxifying proteins and genes involved in the reduction of oxidation damage (Figure 9).
Figure 9.
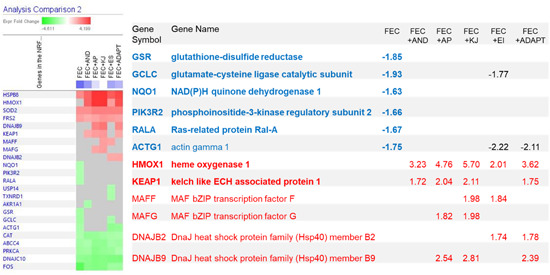
Adaptogens prevents the chemotherapy (FEC)‐induced downregulation of genes activating production of antioxidant and detoxifying proteins and upregulates genes involved in reduction of oxidation damage via Nrf2 signaling. Upregulated genes are shown in red, whereas downregulated genes are shown in blue [Color figure can be viewed at wileyonlinelibrary.com]
Other possible cytoprotecting mechanisms of adaptogens related to drug toxicity, oxidative stress, chronic inflammation, and aging‐related disorders include their effects on Hsp70 and FOXO expression (Figure 10).
Figure 10.
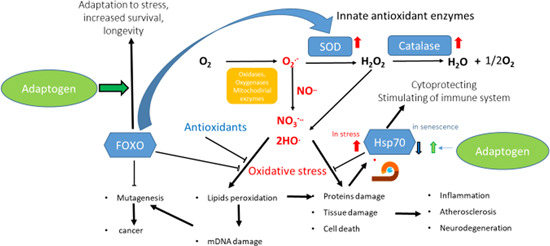
Hypothetical mechanism of action of adaptogens in the regulation of the innate antioxidant system and oxidative stress‐induced apoptosis in aging. According to the free radical theory of aging, the organisms are continuously exposed to reactive oxygen species containing molecules/species (ROS), which are produced as by‐products of normal cellular metabolism. When the innate antioxidant system (glutathione peroxidase, superoxide dismutase, and catalase) incompletely deactivates ROS, increasing cellular oxidative damage induces irreversible functional changes leading to early senescence and to aging‐associated diseases. Oxidative stress triggers many signaling pathways, including FOXO and Hsp70 mediated pathways. Adaptogens upregulate Hsp70, which directly regulates FOXO signaling and promote translocation of FOXO/DAF‐16 to nucleus triggering activation antioxidant systems and antiaging programs. Updated from authors’ drawings 16 [Color figure can be viewed at wileyonlinelibrary.com]
5.1.4. Aging‐associated disorders
Aging‐associated disorders arise from declining capabilities to cope with stress, to sustain cellular and system homeostasis, and to maintain physiological functions. These disorders are associated with neurodegeneration (common for Alzheimer's disease, Parkinson's disease, and senile dementia), atherosclerosis (cause of cardiovascular and cerebrovascular diseases), immune regulation (dysregulated in cancer, autoimmune, and chronic inflammatory diseases), and endocrine/metabolic dysfunction (imbalanced in diabetes and obesity).
Overproduction of ROS in stress‐induced condition leads to destruction of proteins, including those triggering genetic programs of cellular senescence and cell death (apoptosis). Attenuation of functions, increasing damage to proteins, and toxic protein aggregates initiate aging‐related changes leading to disease, senescence, and reduced life span. In aging cells, substantially decreased expression of heat shock protein Hsp70 and its precursor, heat shock transcription factor HSF1, correlates with a decreased ability to cope with stress. 507 , 508 When cells are exposed to stress resulting in protein damage, HSF1 initiates the production of molecular chaperone Hsp70, 508 which repairs proteins by folding denatured parts of proteins and promotes the degradation of irreversibly damaged proteins and their aggregates. In addition, Hsp70 directly protects cells against switch to apoptosis. Decreased expression of HSF1 and Hsp70 in brain cells is observed in Alzheimer's disease. 509 , 510 It is associated with the accumulation of protein aggregates of β‐amyloid peptide and cytoskeletal protein. 511 Aging‐related decline of hepatic Hsp70 expression results in decreased liver detoxification 512 and protection from toxic substances. 513 Decrease of Hsp70 is coupled with upregulation of stress‐activated protein kinase (JNK) dependent apoptosis and progression of cancer Stress‐induced decline in induction of Hsp70 observed in humans, is associated with aging and aging‐related disease. 514 Amazingly, in some individuals which are more than 100 years old, Hsp70 does not decrease with age. 515
In young age, the balance between pro‐ and antiaging JNK‐mediated programs is shifted in favor of Hsp70 (Figure 11). Apparently, oxidative stress does not affect survival and reproduction of young cells because stress‐activated Hsp70 blocks JNK‐stimulated apoptosis. Enhanced levels of Hsp70 correlate with increased life span. In contrast, with age, when induction of Hsp70 is reduced, the balance shifts in favor of the aging and apoptosis programs. Consequently, even weak oxidative stress can induce the degeneration of neuronal cells and the progression of aging‐related diseases. The ability to respond effectively to stress by generating increased Hsp70 correlates with high adaptability and increased life span. 516 Thus, the onset of neurodegenerative diseases and other aging‐related illnesses may be delayed by modulating these two pathways. 517
Figure 11.
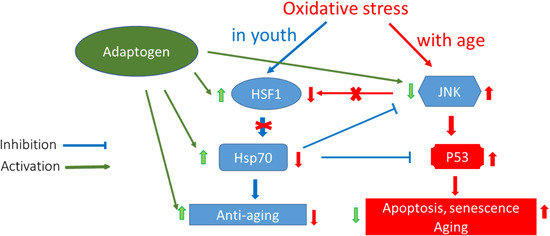
Effects of age and adaptogens on longevity regulatory pathways during oxidative stress. HSF1, heat shock factor 1; Hsp70, heat shock protein 70; JNK, JN kinase; P‐53, p‐53 transcription factor;↓ or ←, for activation; x, for blocking; |, for inhibition; bold text for the prevailing process [Color figure can be viewed at wileyonlinelibrary.com]
The adaptogens R. rosea, S. chinensis, and E. senticossus, alone and in combination, up‐regulate transcription factor HSF1, and increase generation of molecular chaperon Hsp70 in vitro and in vivo. 19 , 20 , 21 , 28 , 42 , 518 , 519 , 520 , 521 Adaptogens also inhibit stress‐activated protein kinase JNK, 18 a key mediator of apoptosis and aging (Figure 11). Furthermore, adaptogens trigger translocation of transcription factor DAF‐16 (FOXO) from the cytoplasm into the nucleus. 95 The protective effect against myocardial ischemia‐reperfusion injury via increase of Hsp25 and Hsp70 expression in rat hearts was described for schizandrin B, an active constituent of S. chinensis. 518 Induction of Hsp27 and Hsp70 genes and protein expression were observed in a dose‐dependent manner after oral administration of schizandrin B to rats. 520
The prolongation of life span and increased survival under stress after treatment with preparations from R. rosea, S. chinensis, E. senticossus, W. somnifera, and P. ginseng have been shown. in the fruit fly Drosophila melanogaster, 370 , 371 , 522 , 523 the nematode Caenorhabditis elegans, 95 , 317 , 524 and yeast Saccharomyces cerevisiae. 525 Oral supplementation with salidroside or extracts of E. senticosus, S. chinensis, and R. rosea significantly decreased stress‐induced elevation of p‐SAPK/p‐JNK in rabbits subjected to restraint stress. 18 Based on these observations, it was suggested that adaptogens acts as mild stressors inducing enhanced stress resistance and an extended life span. 368
Adaptogens regulate G‐protein signaling phosphatidylinositol and phospholipase C pathways (Figure 4). R. rosea, S. chinensis, and E. senticossus up‐regulate the expression of PLCB1 gene, which encodes phosphoinositide‐specific phospholipase C (PLC), and the PI3KC2G gene, which encodes PI3Ks. 23 G‐protein activated, phospholipase C (PLC) catalyzes the hydrolysis of phosphatidylinositol 4,5‐bisphosphate (PIP2) into diacylglycerol (DAG) and inositol‐1,4,5‐triphosphate (IP3) that is involved in numerous intracellular signaling pathways associated with various diseases including depression and cancer. DAG triggers protein kinase C (PKC), which phosphorylates numerous proteins and plays an important role in tumor progression. PI3K is a key upstream mediator of intracellular signaling related to regulation of NF‐kB‐mediated defence responses and apoptosis as well as to long‐term potentiation of neurotransmission, which improves memory and learning. 526 , 527
R. rosea, S. chinensis, and E. senticossus downregulate the CETP gene 23 expression, which regulate the biosynthesis of cholesteryl ester transfer protein that facilitates the transport of cholesterol esters and triglycerides between low‐density lipoproteins (LDL) and high‐density lipoproteins (HDL). 528 Inhibiting of expression of CETP may be helpful in the treatment of atherosclerosis, cardiovascular and metabolic diseases. 529
Adaptogens downregulate the ESR1 gene. 23 ESR1 encodes estrogen receptor α (ERα), which is overexpressed in some cancers. 530 , 531 , 532 Downregulation of expression of ESR1 by Rhodiola and other adaptogens may be effective for preventing and treating some aging‐related cancers such as breast cancer, ovarian, colon, prostate, and endometrial cancers. 23 , 24 The neuroprotective effects of adaptogens 533 , 534 , 535 , 536 , 537 , 538 , 539 , 540 , 541 , 542 may be also partially associated with the upregulation of ESR1 in glia cells, as estrogen signaling through ERα decreases the inflammatory neurodegeneration via its effect on astrocytes. Since pretreatment with adaptogens is known to adapt the cell to stress 12 , 95 , 351 it is possible that the adaptogens‐mediated downregulation of ERα gene expression signals the glia cells to initiate feedback regulation of ERα. This concept is usually associated with inflammation, a protective reaction to infection (‘turn on’ defense system). A feedback mechanism that downregulates pathogen‐induced inflammatory response is triggered (e.g., increased secretion of cortisol and release of anti‐inflammatory cytokines) to prevent an overreaction (“turn off” defence system). Because mild stress is generally a protective reaction to activate innate immunity, in this context, adaptogens initiate stimulation of the innate defence system, including ERα, as one element of the stress system.
Along with canonical mode of steroid receptor action related to regulation of transcription of target genes in the nucleus, estrogen activated membrane bound CRα triggers PI3K/PLC signaling pathways in brain cells to modulate neuronal function and apoptosis. 434 , 444 Estrogen treatment attenuates transcription at estrogen response elements sites in glioma cells. 444 Since Rhodiola upregulates PLCB1, PI3KC2G, and c‐AMP related genes, and modulates NO and JNK it was suggested that Rhodiola and estrogens interfere with each other in some way. 340 While both are neuroprotective, it is remaining unclear whether they are mimetic and in competition or are naturally antagonistic.
Since adaptogens downregulate adenylate cyclase (AC) and upregulate phosphodiesterase (PDE) genes’ expression Panossian et al. 2013, concluded that adaptogens decrease the level of the cyclic adenosine monophosphate (cAMP) in brain cells. 23 The authors suggested that cAMP‐mediated prefrontal cortex signaling model 542 , 543 , 544 provides one possible explanation of CNS stimulating activity of adaptogens. 23 Working memory is preserved by recurrent excitation as well as by functional interaction of G‐proteins coupled α2‐adrenoreceptors with colocalized in dendritic spines hyperpolarization‐activated channels (HCN) of prefrontal cortical neurons, Figure 12. This interaction is mediated via endogenous cAMP, which at high level promotes opening, while at low level, induces blockage of HCN channels. HCN channels opening shunts synaptic transmission onto dendritic spines, decreases cognitive performance in animals performing working memory tasks and thus improving quality of arousal and working memory, which is essential for abstract thinking, planning, and executive functions. 542 , 543 , 544
Figure 12.
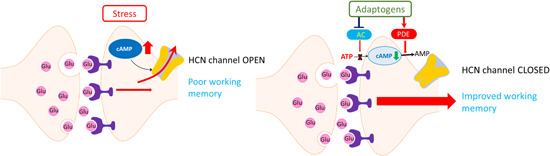
Effect on cAMP‐mediated signaling in prefrontal cortex neurons. HCN channel opening shunts synaptic inputs onto dendritic spines and reduces strength of prefrontal cortex network activity. cAMP opens HCN channel that decreases the efficacy of cortical inputs. Adaptogens downregulate AC and upregulate PDE that decreases cAMP level in brain cells followed by closing of HCN channel. That increases efficacy of synaptic imputes, neuronal activity and working memory. 23 cAMP, cyclic adenosine monophosphate; HCN, hyperpolarization‐activated channels [Color figure can be viewed at wileyonlinelibrary.com]
Stimulation of α 2A‐adrenoreceptor decreases cAMP level and blockade of HCN channels that enhances the working memory in behavioral studies. Overall, cAMP inhibition strengthens connectivity of prefrontal cortex neuronal networks, while excessive cAMP has negative effect on the network strength. 542 , 543 , 544 Since adaptogens downregulate AC and upregulate PDE that decreases cAMP level in brain cells (Figure 3), it was hypothesized that beneficial effects of adaptogens on stress‐induced and aging‐related weakening of cognitive functions in some extend is associated with their effect on cAMP–HCN mediated signaling pathway, 23 which is increasing in stress. 542 , 543 , 544 This hypothesis is consistent with studies in which adaptogens improved cognitive function in humans. 27
Overproduction of ROS and their inadequate elimination by the innate antioxidant system in aging relate directly to transcription factors that control the expression of genes associated with cell proliferation, inflammation, and ROS production. As an example, age‐related changes in the expression of AP‐1, NF‐kB, FoxO, and Nrf2 transcription factor‐mediated signaling pathways in vascular smooth muscle cells lead to the progression of inflammaging (i.e., aging‐related low‐grade chronic inflammation) and atherosclerosis. 502
Aging is associated with the activation of AP‐1, NF‐kB, and Nrf2 and inhibition of FoxO transcription factor‐mediated intracellular signaling pathways. 502 Translocation of NF‐kB into the nucleus triggers the expression of multiple genes involved in inflammation. 502
Adaptogens downregulate NF‐kB translocation and expression, NF‐kB signaling, and NF‐kB mediated inflammation 326 , 336 , 434 , 436 , 437 , 441 , 545 , 546 , 547 , 548 , 549 , 550 , 551 , 552 , 553 , 554 , 555 , 556 , 557 , 558 , 559 , 560 ;
adaptogens downregulate Fos and Jnk, the components of AP‐1 transcription factor (Table 9), which regulates many genes involved in cell proliferation, migration, ROS production, and extracellular matrix degradation 17 , 502 , 561 ;
adaptogens switch FoxO‐dependent responses from apoptosis promotion to stress‐resistance in response to oxidative stress 95 ; and
adaptogens activate Nrf2 signaling. Normally, Nrf2 is located in bound form in the cytosol with reduced kelch‐like ECH‐associated protein 1 (Keap1). Dissociated Nrf2 translocates to the nucleus, where it triggers the transcription of phase II or other adaptive response genes, including enzymes involved in GSH metabolism, NQO1,2, and HO‐1. 226 , 550 , 553 , 554 , 558 , 561 , 562 , 563 , 564 , 565 , 566 , 567 , 568 , 569 , 570 , 571 , 572 , 573 , 574 , 575 , 576 , 577
The efficacy of adaptogens in the treatment of acute respiratory tract diseases is possibly also partially associated with the downregulation of proinflammatory NF‐kB signaling in various cells and tissues involved in the acute inflammatory response.
In conclusion, the observed beneficial effects of adaptogens in aging‐related disorders (Table 10) include neurodegeneration, atherosclerosis, and impaired apoptosis. 23 , 368 , 369
Table 10.
Aging‐associated diseases and genes involved in pathogenesis and progression, up‐ or downregulated by adaptogens tested in isolated neuroglia cells
| Cancer and gastrointestinal | Adenocarcinoma, prostate carcinoma, colon and colorectal cancer, cancer, gastrointestinal tumor, gastric mucosa atrophy | 132 genes |
|---|---|---|
| Organismal injury and abnormalities | Physical disability | PDE11A, PDE3A, PDE4Dupregulated |
| Degeneration of retinal cone cells—inhibition | AIPL1downregulated, CNGB3upregulated | |
| Atrophy of gastric mucosa | CCKBRdownregulated | |
| Hypoestrogenism | ESR1downregulated | |
| Postmenopausal vulvar atrophy | ESR1б MTNR1Adownregulated | |
| Pain—inhibition | KCNK10, PDE11A, PDE3A, PDE4D, SCN2Bupregulated | |
| Cone dystrophy | CDHR1, ESR1downregulated | |
| Pelvic organ prolapses | CNGB3, SERPINA1upregulated | |
| Dermatological | Rosacea | AKR1D1upregulated, MMP8downregulated |
| Inflammatory and pulmonary | Pulmonary emphysema‐ inhibition | PDE11A, PDE3A, PDE4D, SERPINA1upregulated |
| Bronchiectasis | PDE11A, PDE3A, PDE4Dupregulated | |
| Chronic bronchitis | MMP8, MTNR1Adownregulated | |
| Neurological and psychological | Non‐24‐h sleep‐wake disorder | MTNR1Adownregulated |
| Sleep‐wake schedule disorder | PDE3Aupregulated | |
| Urological | Recurrent urinary tract infection | ESR1downregulated |
| Cardiovascular | Ischemic cardiomyopathy | PDE11A, PDE3A, PDE4D, PPP1R1Aupregulated |
| Cholesteryl ester transfer protein deficiency | CETPdownregulated | |
| Angina pectoris | PDE11A, PDE3A, PDE4Dupregulated | |
| Cerebral small vessel disease | PDE3Aunregulated | |
| Skeletal and connective tissue | Osteochondrodysplasia | COL9A1downregulated, PDE4Dupregulated |
| Metabolic | Estrogen resistance | ESR1downregulated |
5.1.5. Adaptogens in regulation of energy homeostasis
Adaptogens prevent stress‐induced increases in NO, and as such, ATP production remains efficient and performance and endurance are increased. 18 Putative mechanisms of ATP generation are shown in Figure 13.
Figure 13.
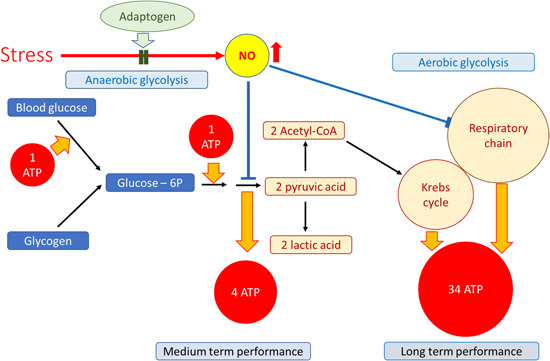
Schematic representation of the potential molecular mechanism by which generation of nitric oxide (NO) strongly inhibits the production of cellular energy through two mechanisms: inhibition of mitochondrial respiration by reversible (from constitutive isoforms of nitric oxide synthase [NOS]) and irreversible (from inducible NOS [iNOS]) inhibition of cytochrome P450 578 ; and glycolysis inhibition through modification of the SH‐groups of glyceraldehyde 3‐phosphate dehydrogenase. 579 In anaerobic glycolysis, muscle glycogen is converted to lactic acid via glucose‐6‐phosphate, yielding three ATP molecules for each glucose residue. Aerobic oxidation of glucose is required for the sustained exercise and provides 34 ATP molecules per glucose residue via the Krebs cycle and the respiratory chain, a process that occurs 1 min after anaerobic ATP generation. If a sufficient supply of ATP is not generated, anaerobic glycolysis is continued. NO levels increase during stress, consequently decreasing performance by inhibiting ATP production. Adaptogens prevent stress‐induced increases in NO, 18 and as such, ATP production remains efficient and performance and endurance are increased. 26 Updated from authors’ drawings 26 [Color figure can be viewed at wileyonlinelibrary.com]
In addition to this possible source of ATP generation stimulated by adaptogens, apparently there are some other mechanisms of regulation of energy homeostasis by adaptogens. Thus, adaptogens presumably decrease c‐AMP level in brain cells by deregulation the expression of genes involved in regulation of cAMP. 23 Consequently, low levels of c‐AMP decreases protein kinase A (PKA) overall activity. PKA activation effects vary with the cell type; for example, PKA stimulates lipases in adipocytes, while in myocytes and hepatocytes PKA increases glucose formation and its catabolic transformation to pyruvate (glycolysis). This provides free energy in the form of adenosyl triphosphate (ATP) and nicotine adenine dinucleotide, reduced (NADH), which are important in stress response. The regulation of cAMP levels and PKA activity is one of mechanisms of regulation of energy homeostasis, somewhat a metabolic switch between catabolism and anabolism. Stress‐induced catabolic transformations are induced by downregulation of cAMP and PKA by adaptogens. Apparently, PKA is involved in “energy‐saving” effect of adaptogens that favors ATP‐consuming anabolic pathways. Increased intracellular ATP levels and prevention of ATP‐conversion (to cAMP) is due to an inhibition of adenylate cyclase by adaptogens. An increased storage of ATP seems to represent an energy source for other ATP‐dependent metabolic conversions. This is consistent with the concept of ATP generation induced by adaptogens and their potential benefits in aging‐related diseases and fatigue. 23
5.2. Synergy and antagonism of several plants as background for the discovery of new drugs with better efficacy and safety
Kampo tradition uses fixed combinations of medicinal plants in standardized proportions. The idea to combine two or more plants or substances, which will be stronger than any ingredient alone, is very attractive for several reasons: the ingredients may have different targets and mechanisms of action in human organisms, and therefore better effect as a combination; and the combination can be used at lower doses and may be less toxic if any ingredient contains a toxic impurity. The ingredients can also act synergistically, thereby providing new unique effects that are not possible to obtain by any ingredient independently. Synergistically means that the combination is active, while the ingredients separately are inactive. 17 , 23 , 25 , 451 Synergy can be also interpreted as the generation of new pharmacological activity, which is only specific for the combination of two or more ingredients. 17 , 23 , 451 , 580 This is a fantastic phenomenon that has yet to be fully understood; however, it is has been observed in various interactions at different intracellular, extracellular, organism, social, and other levels of communications.
This comparison is in line with our observations made during our analysis of the gene expression transcriptome‐wide microarray profiles of isolated neuroglia cells after incubation with several adaptogenic plant extracts, their combinations, and purified compounds. 17 , 23 , 25 , 451 It was concluded this experimental model is very useful for assessing synergistic and antagonistic interactions of various plant extracts, with the aim to discover an unexpected pharmacological activity of new combinations or to rid of adverse effects of ingredients due to their interactions within intracellular molecular networks. 17 , 25 Further downstream effect analysis of mRNA microarray data enables prediction of pharmacological effects of fixed combinations. 17 , 25 , 451
For example, it was found that the fixed combination of Eleutherococcus and Andrographis might be useful for the treatment of encephalitis because of the synergistic inhibition of the expression of a number of genes of the molecular network involved in the development of encephalitis, whereas neither Eleutherococcus nor Andrographis individually has an effect on these genes. 25 Although microarray analysis did not provide the final proof of the efficacy of this fixed combination in humans with encephalitis after its oral administration, it provided information regarding its predictable (z‐score > 2) effects on diseases and biological functions as well as insights into putative genes and directions for future research and possible implementation into practice.
This approach was implemented for the assessment of the synergistic and antagonistic interactions of R. rosea (RR), W. somnifera (WS), E. senticosus (ES), Rhaponticum carthamoides (RC), Bryonia alba (BA), and melatonin (M), with the purpose of predicting the potential pharmacological and toxicological profiles of their combinations (RR–BA, RR–WS, WS–M, RC–ES–WS). 17 , 25 , 451
It was found that WS in combination with melatonin synergistically deregulates several genes involved in the regulation of glucagon, the main catabolic hormone that increases the concentration of glucose and fat in the bloodstream, suggesting that WS–M might be useful for the prevention of type 2 diabetes. 17 Another synergistic interaction of WS with RR induced the deregulation of 20 genes, 10 of which contribute to the predicted activation of neuronal development, suggesting a beneficial effect of this combination on age‐related decline in memory and cognitive functions. 17
These models take into account interactions within the biological network, which are important if medications act at various targets in the network or if homeostatic feedback mechanisms are effective. System pharmacology models are useful to describe synergistic mechanisms of action of complex combinations of medicinal plants. The term synergy is appropriate for interactions of two or more ingredients leading to qualitatively new pharmacological effects, e.g., to the expression of genes that cannot be obtained by any single ingredient independently. On the contrary, antagonism occurs as a result of the interaction of several ingredients in a combination, which leads to the absence, reduction or prevention of the effects of any individual ingredient in this combination. 17 , 23 , 25 , 452
6. CHALLENGES AND REGULATORY ISSUES
6.1. Terminology
The term adaptogens, like the terms antioxidants and vitamins, is not yet commonly used to refer to a distinct pharmacological group despite the fact that the terms adaptogenic activity and adaptogen have been adopted by drug regulatory authorities and general practitioners in Europe, the United States, and Asia.
In 2008, the European Medicines Agency published “Reflection Paper on the Adaptogenic Concept,” which was based on and which refers to the 18 review articles published in 1947–2005 mainly including studies on Eleutherococcus and a few other adaptogens. 581 It this review, HMPC (anonymous authors) concluded:
The principle of an adaptogenic action needs further clarification and studies in the preclinical and clinical area. As such, the term is not accepted in pharmacological and clinical terminology that is commonly used in the EU.
The HMPC is aware of the fact that numerous preclinical and clinical studies have been performed with the view to proving the concept of an adaptogen. However, the clinical data have a number of shortcomings such as deficiencies in the description of inclusion and exclusion criteria, description of the medication, diagnosis, study design, analysis, etc. There is a wide range of clinical conditions that have been investigated and in some studies the number of patients was very small. None of the studies would be sufficient to substantiate efficacy of Eleutherococcus preparations in a clearly defined clinical condition, although, in total, the data available are sufficient to justify further research into the concept of adaptogens.
As the term “adaptogen” is considered not appropriate for a marketing authorization, more clinical studies and data on the efficacy in a well‐defined clinical condition would be necessary.
The concept of adaptogens is sufficient to be considered in the assessment of traditional herbal medicinal products (e.g., monograph on Eleutherococcus root). 3
Since the publication of “Reflection Paper on the Adaptogenic Concept,” an enormous number of studies that significantly enrich the current knowledge of the pharmacology, clinical efficacy, and mechanisms of action of adaptogens have been published. The term adaptogens has been used in numerous publications indexed in PubMed that clearly show that the statement “as such, the term is not accepted in pharmacological and clinical terminology that is commonly used in the EU” is now far from reality.
Various adaptogenic herbal medicinal products, which are formally divided into two categories (EC–traditional, used for at least 30 years, including at least 15 years within the EU; and well‐established use, used within the EU for at least 10 years, with recognized efficacy and an acceptable level of safety), have a commonly acceptable level of safety and efficacy in various diseases. Nevertheless, more evidence from large‐scale, well‐controlled clinical trials of high‐quality uniform botanicals and their phase III pharmacovigilance data are essential for further implementation in common practice, at least for decreasing the risk of disease progression and as adjuvant therapy in infections and chronic diseases.
In other assessment reports, 1 , 2 the HMPC concluded that the preparations of three adaptogenic plants can be officially used as traditional herbal medicinal products.
Rhodiola: for temporary relief of symptoms of stress, such as fatigue and sensation of weakness, in Austria, Italy, the Netherlands, Spain, Sweden, and the United Kingdom. 1
Ginseng 2 and Eleutherococcus 3 : for the relief of symptoms of asthenia (abnormal loss of strength and energy) such as tiredness and weakness in France, Germany, Lithuania, Poland, Spain, and Sweden. 2 , 3
Furthermore, several Eleutherococcus products have marketing authorization in Germany and Denmark as herbal medicinal products with well‐established use as tonics for invigoration in individuals with fatigue and impairment, in decreasing capability and power of concentration as well as against tiredness, and in periods of convalescence. 3
Similarly, a large number of ginseng products have marketing authorization in Austria, Belgium, Denmark, France, Germany, Ireland, Latvia, Poland, Portugal, and Spain as herbal medicinal products indicated for use “as a tonic in case of tiredness and weakness and decreased mental and physical capacity as well as in concentration,” in “asthenia, such as lack of concentration, fatigue, weakness, tiredness, lack of vitality or in convalescence,” and in “exhaustion fatigue and at convalescence; can be tried in lack of concentration in middle‐aged and elderly when other causes to the condition have been excluded.” 2
6.2. Consistency of the results of clinical studies
The most important challenge is related to the evidence of the clinical efficacy of adaptogens for the treatment of many stress‐induced and aging‐related diseases, which should be demonstrated in large‐scale randomized, double‐blind, comparator‐controlled unbiased clinical studies.
Studies on well‐defined preparations often show contradictory results, which is a common trait of herbal preparations per se. Although numerous studies of adaptogens have suggested an advantageous safety and tolerability profile as compared with conventional drugs, we also acknowledge several disadvantages of herbal preparations per se. Herbal preparations, although standardized to active constituents, are still very complex mixtures of many compounds and may have variable positive and negative effects depending on factors that have a crucial impact on the reproducibility of pharmacological activity (e.g., growing conditions, regional territory, and genus differences). Difficulties in manufacturing place herbal preparations at a disadvantage against conventional drugs, which are single compounds that remain identical and reproducible from batch to batch during production. In addition, various differently standardized herbal preparations of the same medicinal plant may have different pharmacokinetic and pharmacodynamic dose–effect responses. For example, the maximal active antifatigue, antidepressant, and antistressor dose of the SHR‐5 brand of R. rosea extract 355 , 356 , 357 , 360 might be inactive for a different extract of R. rosea despite the fact that both products are extracted from Rhodiola roots that have different chemical compositions. 350 , 582 Finally, we acknowledge the difficulties in producing herbal medicinal products that provide reproducible effectiveness over time, and this represents a serious challenge and limitation of herbal medicinal products and dietary supplements in general. However, despite these limitations, the development of herbal preparation for the prevention and treatment of many diseases is of great interest and has promising potential for safe, effective, and affordable therapies with superior tolerability and a low incidence of adverse events. More evidence from properly controlled clinical studies is required to support health claims and indications of pharmaceutical‐grade herbal preparations for use in disease treatment.
6.3. Network pharmacology and systems biology models for assessment of pleotropic activity of adaptogens
Adaptogens are the multitaskers in terms of their pharmacological effects.
Adaptogens have pharmacologically pleiotropic effects, including antistress/antifatigue, stimulating, tonic, antidepressant, neuroprotective, cardioprotective, hepatoprotective, gastroprotective, antioxidant, autoinflammatory, immunomodulatory, antitumor, antiviral, antibacterial, and hypoglycemic activity. 1 , 2 , 3 , 4 This polyvalent activity is due to their action on genes encoding hormones, transcription factors, and other regulatory proteins, which play a key role in the regulation of many canonical intracellular signaling pathways and molecular networks as well as extracellular communication in the neuroendocrine–immune system.
The specificity of the pharmacological action of various adaptogenic plants depends on both the chemical compositions of the extracts and the dose. Product‐specific (or compound‐specific) activity is theoretically possible to achieve in the smallest dose/concentration, when a compound selectively interacts with only one receptor type, which can trigger minimal signaling pathways in a molecular network. Although the effector (ligand) molecule at higher doses can nonspecifically interact with numerous molecules of several networks, this may cause both feedback downregulations and antagonistic interactions of various molecular networks, resulting in quite different pharmacological responses and toxic effects.
At low and normal doses, adaptogens act as mild stress mimetics, increasing the homeostatic range (Figure 1) and resulting in increased resistance to stress. At higher doses, they may suppress inflammation and therefore prevent premature aging and maintain health and vitality. This is the “specific” difference of adaptogens, which activate adaptive signaling pathways and increase survival and resiliency from stress, from some other natural compounds, the so‐called PAINS (PAn‐assay Interference compouNdS), such as toxoflavin, epigallocatechin gallate, genistein, and resveratrol. Quercetin, β‐sitosterol, rutin, and curcumin do not comply with these criteria, despite nonspecific pleiotropic effects in numerous in vitro experiments. 583
Adaptogens stimulate neurogenesis and exhibit neuroprotective activity, suggesting their potential benefits in neurodegenerative disorders. Surprisingly, they trigger apoptotic signaling pathways associated with antitumor activity. Regulation of both stress‐resistance and proapoptotic genes is not necessarily a paradox. Adaptogens stimulate mediators of the stress response and transcription factors, 17 which may orchestrate different patterns of gene expression based on the dose of adaptogens, perhaps activating stress‐resistance genes normal or small doses, but proapoptotic genes at high doses beyond a certain threshold. Possibly, adaptogens regulate different genes in different cell types, causing apoptosis in some cells (e.g., cancer cells) while promoting survival in others (e.g., in neurons and glia cells). Importantly, the induction of apoptosis by adaptogens may cause the death of damaged or abnormal cells, which may extend the lifespan of the entire organism.
The adaptogenic process is can be studied very well using “systems biology” and “network pharmacology” approaches, which has the potential to provide plant‐based treatments for complex diseases, chronic conditions, and syndromes. This is a remarkably complex system of synergistic interactions of molecular networks and cellular communication systems that quite literally add up to more than the sum of the parts. It also requires a detailed understanding of disease concepts, as we have outlined in this MS and second, the use of suitable pharmacological models to understand such effects. There can be no one to one correlation between use as an adaptogens and a specific model, and the suitability of a model needs to be assessed carefully before starting experimental approaches. Such approaches can help understanding these complex systems better and this is a key challenge in the future.
6.4. Safety and pharmacokinetic of adaptogens
The published literature on Rhodiola, Eleutherococcus, Withania, ginseng, and Schisandra does not provide reasons for safety concerns, and herbal preparations containing adaptogens are not harmful when prepared and used in specified conditions. No serious adverse events have been reported from clinical trials, epidemiological studies, or pharmacovigilance reporting that can be clearly correlated with the ingestion of adaptogens (EMA assessment reports 2012–2014). 1 , 2 , 3 , 4 It might be suggested that such a high tolerance in humans and a low rate of adverse events might be due to their poor absorption and low bioavailability. However, the data available on absorption, distribution, metabolism, and excretion of the active constituents of adaptogenic plants show that some of these have high bioavailability and are quickly absorbed and widely distributed in all organs and tissues involved in the regulation of the neuroendocrine–immune system. Others are metabolized into more active metabolites or significantly affect gut microbiota, which plays an important role in the maintenance of homeostasis and the development of several chronic diseases, including colitis‐associated colorectal cancer, among others.
Thus, the earliest pharmacokinetic studies of adaptogens 584 , 585 , 586 that intraperitoneally administered 3H‐labeled eleutheroside B (with radioactivity localized in the aglycone, 52 µCi/mmol) in rats demonstrated that eleutheroside B is quickly absorbed into the blood and distributed in the liver, kidneys, adrenals, pancreas, thymus, spleen, heart, testes, brain, and hypophysis. The extent to which a compound is distributed throughout the body has a large impact on its therapeutic utility.
The highest concentration of the label in the blood was observed 15 min after administration of the 3H‐labeled eleutheroside B, and this concentration dropped sharply within 4 h and was eliminated mainly through the renal system with urine. Approximately 35% of the labeled drug was eliminated via the urine 2 h after administration, 55% after 4 h, and 90% after 48 h. A small amount of radioactivity (2.5%–3%) was eliminated with the feces. Most of the labeled drug accumulated rapidly in the organs and tissues. In fact, after only 15 min, 88% was absorbed and retained at a high level for a rather long duration. After 8 h, up to 30% of the administered drug was still retained in the organs and tissues. This represents an exceptionally high level of incorporation of labeled eleutheroside B into the liver and kidneys with subsequent rapid removal from these organs. The high levels of labeled eleutheroside B in the pancreas can probably be attributed to its active participation in the digestion process and to its synthesis of two important hormones: insulin and glucagon. The accumulation of eleutheroside B in the adrenals for up to 4 h suggests its influence on the hypophysis–adrenal cortical system. In the brain, a minimal level of incorporation of the radioactive label with an insignificant reduction over time was observed. Eleutheroside B does not pass the blood–brain barrier. 584 , 585 , 586 Interestingly, the bioavailability of individual eleutherosides B and E after oral administration of an aqueous extract of E. senticosus was significantly increased as compared with the oral administration of single compounds. Both eleutherosides are metabolized and excreted primarily from the liver and kidney. 587 The absorption of orally administered eleutherosides 588 and isofraxidin 589 was also rapid, with the maximum concentration noted at 0.4 and 0.2 h, respectively.
Lipophilic compounds such as lignans are well distributed in the tissues and organs, where their content is higher than that in blood plasma. 590
The absorption of any potential therapeutic is a critical consideration, especially for oral dosing. Many pharmacokinetic studies of other adaptogens, including clinical trials, have been performed. 339 , 584 , 585 , 586 , 587 , 588 , 589 , 590 , 591 , 592 , 593 , 594 , 595 , 596 , 597 , 598 , 599 , 600 , 601 , 602 , 603 , 604 , 605 , 606 , 607 , 608 , 609 , 610 , 611 Some of these studies provided evidence of the level and steady‐state concentration of active compounds in the blood of human subjects who received oral administration of the herbal drugs in therapeutic doses. These concentrations were in line with those used in in vitro studies. 591 , 598 As an example, the concentration of andrographolide (the active compound of Andrographis and its combination with Eleutherococcus) in blood plasma of human subjects was approximately 3.5 µM at 2 h after drug uptake, 591 which is adequate for exhibiting an anti‐PAF effect in vitro (EC50, 5 µM). 440 A comparison of the results obtained in humans and rats showed that the pharmacokinetics of andrographolide are similar in both species. It was found that andrographolide is rapidly and almost totally absorbed (T1/2abs of about 25 min) into the blood (bioavailability = 91%, F = 0.91) after oral administration of Andrographis extract at a therapeutic dose (20 mg/kg). Thus, in the absorption phase, the concentration of andrographolide in the blood is not significantly changed during the first 1.5 h and increases to a maximal level 2 h after oral administration. It binds intensively with blood proteins and is redistributed between blood and tissues within 1 to 2 h. The elimination half‐time is in the range of 2–7 h. 591 The tissue distribution study of andrographolide revealed the highest tissue concentration in kidney, followed by the liver, spleen, and brain, whereas an almost identical concentration was observed in the heart and lungs. 592
The pharmacokinetics of three active compounds (tyrosol, rhodioloside, and rosavin) of R. rosea extracts were studied in rodents 597 , 598 , 599 , 600 , 601 , 602 , 603 and healthy volunteers. 597 Salidroside was quickly absorbed into the blood of rats (t max = 1 h; bioavailability: 75%–90%) and metabolized to tyrosol within 2 h after oral administration of the R. rosea extract. The concentration of tyrosol attained its maximum value within 1.5–2.0 h and then decreased exponentially to basal level within 3 h after oral administration of the extract. Many of the measured pharmacokinetic parameters of purified salidroside were significantly different when the pure compound was administered (C max, V dis, AUC, t‐1/2, and higher t max and CI) rather than the plant extract. Rosavin had a lower bioavailability (20%–26%) and was eliminated from the blood within 2 h. The pharmacokinetics of rosavin in humans are different from that in rats. For example, both t max (2 h) and elimination rate were longer in humans after oral administration of Rhodiola tablets in a therapeutic daily dose. The maximal concentration and elimination half‐life of salidroside were two‐ to threefold higher than those of rosavin. The elimination of salidroside from the blood was 1.8‐fold longer than the elimination time of rosavin. The beneficial effect of R. rosea on mental performance in humans, which was observed 1 h after oral administration and lasted for more than 3 h, is worth noting. During this time period, the concentration of salidroside in human blood was about 587 ng/ml after 1 h and 483 ng/ml after 4 h. 597 It was found that after intravenous administration, salidroside was extensively metabolized to tyrosol and then distributed to various organs and cleared rapidly. The highest levels of p‐tyrosol were detected in the heart, followed by the spleen, kidney, liver, and lungs. 603
It should be noted that many of the measured pharmacokinetic parameters of purified salidroside were significantly different when the pure compound was administered (C max, Vdis, AUC, t‐1/2, and higher t max and CI) rather than the total plant extract, 597 indicating an interaction with the other constituents of the plant extract.
It should also be emphasized that the biological activity of the preparations of R. rosea is not entirely due to salidroside and tyrosol but rather to the entire complex of substances that are extracted from R. rosea. That is also true for ginseng, Schisandra, and presumably for all other adaptogens.
The pharmacokinetics of different active ginseng compounds have been studied in both animals and humans. 2 The bioavailability of ginsenosides is low after oral administration, but the pharmacokinetic behavior differs among various ginsenosides. 35 , 611 The highly glycosylated ginsenosides Rb1, Rb2, Rc, Rd., Re, Rg1, and Rg2 have poor stability in the gastrointestinal tract, and they are easily converted into monoglycosides and aglycone ginsenosides (e.g., CK, Rh2, Rh1, and F1) by gastric acid and/or the intestinal flora. 35 , 611 , 612 , 613 , 614 , 615 , 616
After oral administration, blood concentrations of ginsenosides are high, but their absorption rate is low. Both the absorption profile of ginsenosides in the intestinal mucosa and the availability of intact ginsenosides and their metabolites from the intestines are exceptionally low. 35 , 611 The maximal concentration of ginsenosides in plasma is reached within 2 h, suggesting that they are rapidly absorbed and distributed in tissues. Rg1, Re, Rb1, and Rc reach the brain, but their concentrations decline rapidly over time. 35 , 617 Rg1 and Re are more readily distributed in the brain, and they are considered the main components directly affecting the neurons of the CNS. 617 The plasma level of ginsenosides indicates that protopanaxadiol ginsenosides have higher concentrations and longer half‐lives than protopanaxatriol ginsenosides. 35 , 617 After the biotransformation of ginsenosides, the microbiota in the gut produces deglycosylated products. 358 , 618 , 619 , 620 The intestinal bacteria isolated from human feces and some food‐derived microorganisms as well as fungi from soil around ginseng roots convert glycosylated ginsenosides to compound K, 612 , 620 , 621 which has great potential for cancer chemoprevention. 342 Compound K was the only ginsenoside detected in plasma and urine after the oral administration of Rb1. 622 Deglycosylated products are better absorbed than ginsenosides based on their greater ability to permeate biological membranes. 623
EMA assessment of the available literature suggests that ginsenoside metabolites contribute substantially to the pharmacological effects of ginseng. 2 The metabolites are well distributed to most of the tissues. 593 It was concluded that metabolites of ginsenosides produced by gut microbiota might be more biologically active than their precursors. 2 The results of recent studies 624 , 625 are in line with this conclusion: the ginseng preparation with a higher content of rare ginsenosides was more active in its ability to prevent symptoms of stress such as fatigue, impaired memory, reduced concentration, and attention deficit related to daily work in healthy subjects 624 as well as enhanced long‐term potentiation in rat hippocampal slices. 625 An active ginseng metabolite may differ in distribution and clearance from its parent compound, and the parent compound and its metabolite may be bioactive by similar or different mechanisms. 342
The results of herb–drug interaction studies of various adaptogens are contradictory. 1 , 2 , 3 , 4 , 598 , 626 , 627 , 628 Interactions with some CYP isoenzymes have been observed in vitro studies only in high concentrations of herbal extracts that are far beyond their blood levels when taken in the standard therapeutic doses and not associated with active markers. 1 , 598 , 626 , 627 , 628 Few poorly conducted clinical studies in limited number of healthy subjects (lack of placebo, proper randomization, procedure for treatment compliance, pharmacokinetic data, sufficiently controlled consumption of CYP‐active food ingredients, etc.) do not provide strong, evidence supporting the clinical relevance of the interaction effects observed in vitro.
Overall, the pharmacokinetics of various compounds from adaptogenic plant extracts is different depending on their chemical structure, lipophilicity, water solubility, metabolic activity, concentration as well as the presence of other bioactive compounds in test samples. Adaptogens are distributed in all organs and tissues involved in the regulation neuroendocrine‐immune
system where they trigger the expression of hormones and key metabolic regulators of defense responses and cellular homeostasis. That is one of the likely explanations of the pleotropic effects of adaptogens. Finally, some adaptogens actively interact with gut microbiota that results in prevention of progression of chronic inflammatory diseases.
7. OVERALL CONCLUSIONS
The adaptogenic concept does not have a long history as analogues of TMS, even though adaptogenic plants have been used in TMS as rejuvenating herbs, qi tonics, rasayanas, and restoratives for centuries and are formally considered to be “traditional” by drug regulatory authorities in Europe and the United States. It is supported by an evidence‐based approach and statistical assessments of pharmacological and clinical studies of efficacy and safety of standardized herbal medicinal products as well as their mechanisms of action. The efficacy of plants used in TMS has been investigated using modern theories and methods of system biology and network pharmacology. In this review, we summarized our knowledge about common adaptogenic plants used as officinal medical preparations in USSR/Russia and in traditional Chinese medicine, Ayurveda medicine, and other TMS and alternative medical systems, and to provide a modern rationale for their use in the treatment of stress‐induced and aging‐related disorders. Overall, the basic principles of TMS are in line with those of the adaptogenic concept, which uses systems biology and network pharmacology models to understand the fundamentals of TMS such as “life vital energy”/qi (Chinese)/prana (Indian)/pneuma (Greece)/zorutyun (Armenian)/od (German)/ruah (Hebrew), and mana (Polynesian), which are related to adaptability. Yin‐yang balance can be interpreted as “homeostasis”, whereas “shanghuo”—as a state of threatened homeostasis and decreased resistance to stress, which is increased by adaptogens.
Adaptogens play key roles in defending organisms against environmental challenges including harmful bacteria, diseases carried by insects, excessive ultraviolet rays from the sun, and challenges from pollution, excess heat and cold, and hypoxia.
The key to understanding adaptogens is their role in establishing and maintaining adaptive homeostasis by building the body's natural resistance to stressors, which may be physical, chemical, biological, and psychological in nature. Adaptogens function like stress vaccines to activate the body's defence system and metabolic rate, reversing the negative physical effects of stress and restoring the body's balance and health.
If the immune system is not functioning properly by overreacting or underreacting to challenges, adaptogens help restore the proper immune response.
If the immune system is overly active, triggering allergies and asthma, rheumatoid arthritis or lupus, adaptogens lower the immune system's response and returns it to a normal level.
If the immune system is underactive, leading to frequent colds, bronchitis, sinus or ear infections, and even pneumonia or causing anemia or digestive problems such as ulcers or chronic diarrhea, adaptogens can help strengthen the immune response, thereby ending the cycle of illness.
If the brain chemistry is unbalanced, adaptogens can restore the balance, having profound effects on cognitive function, memory, and mood.
The power of adaptogens goes far beyond the immune system.
Adaptogens can correct imbalances in cellular division cycles that cause cells to divide in an uncontrolled manner, eventually causing cancer.
Adaptogens have a potential to prevent or postpone chronic diseases associated with aging, recognizing their uncanny ability to fix what's wrong, boost what's right, keep the body in balance, and prevent the body's functions from deteriorating.
Adaptogenic effects like those seen in Ginseng, Rhodiola, Eleutherpcoccus, Withania, and Schisandra have been scientifically validated as being effective against chronic inflammation, atherosclerosis, neurodegenerative cognitive impairment (e.g., Alzheimer's disease and other forms of dementia), metabolic disorders, diabetes, cancer and a host of other aging‐related diseases.
Overall, in this review for the first time we compare and analyze common basic principles, concepts, and uses of adaptogenic plants using a cross‐cultural, comparative approach. We demonstrate that the concept of adaptogens provides a scientific rationale for adaptogenic plants traditionally used in stress‐induced and aging‐related diseases. In conclusion, the basic principles of TMS are in line with those of the adaptogenic concept, which uses systems biology and network pharmacology models to understand the fundamentals of TMS.
CONFLICT OF INTERESTS
Alexander G. Panossian is self‐employed by the research and development company, Phytomed AB. He has an Independent Contractor Agreement with Europharma USA Inc. He has no shares or financial interest in any pharmaceutical company. All other authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Alexander G. Panossian initiated this project, planned and wrote the first and final draft of the manuscript. All other authors added specific parts, critically reviewed and edited the drafts, and approved the final version of the manuscript.
Biographies
Alexander Panossian, Ph.D., Dr.Sc, is the Founder of Phytomed AB in Sweden and Head of Research & Development of Europharma USA Inc. since 2016. He has multiple advanced degrees in bioorganic chemistry and chemistry of natural and physiologically active compounds. He completed his doctorate in organic chemistry at the Yerevan State University in Armenia in 1971 and obtained his scientific degrees from Moscow Institute of Bioorganic Chemistry in 1975 and Moscow Institute of Fine Chemical Technology in 1986. Dr. Panossian was made a full professor of bioorganic chemistry and chemistry of natural and physiologically active compounds in the Russian Federation, and later served as Director of the Laboratory of Quality Control of Drugs of Medical Drug Agency of the Republic of Armenia (1993–2003). In 2003, he moved to Sweden to act as head of research and development at the Swedish Herbal Institute. He has the honor of participating as a guest scientist in the Laboratory of Nobel Laureate Bengt Samuelsson at the Karolinska Institute in Stockholm (1982–1983), at Munich University (1993–1995), and at King College in London, 1996. He was the editor‐in‐chief of Phytomedicine, International Journal of Phytopharmacology and Phytotherapy (Elsevier, Germany) from 2014 to 2017. He has authored or co‐authored about 200 articles in peer‐reviewed journals. His main research interest is focused on plant adaptogens, anti‐stress compounds that are involved in the regulation of neuroendocrine and immune system.
Professor Dr. Prof. h. c. mult. Thomas Efferth is chair of the Department of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, Johannes Gutenberg University, Mainz, Germany. He is a biologist by training (Technical University of Darmstadt, Germany). His doctoral thesis was completed at the German Cancer Research Center (DKFZ), Heidelberg, Germany (1990). Dr. Efferth was awarded the Ludolf‐Krehl‐Prize of the Southwest German Association for Medicine (1991), the Willmar‐Schwabe‐Award of the German Society for Medicinal Plant Research (2006), the citizen medal of the City of Heidelberg, Germany (2008), the CESAR Award for Translational Oncology (2011), the SCENTEDdrop Award on medicinal and flagrant herbs (2015), and the Qihuang International Award of the Chinese Association of Chinese Medicine (2017). Since 2018, he is a full member of the World Academy of Sciences. He headed a research group for Pharmaceutical Biology at DKFZ (2005–2009) and was an adjunct professor (apl.) at the University of Heidelberg (2007–2009). In 2009, he took over the Chair of Pharmaceutical Biology (full professorship) at the Johannes Gutenberg University, Mainz. Furthermore, he is an honorary professor at the Northeast Forestry University, Harbin, and at the Zhejiang Chinese Medical University, Hangzhou, China. He is a visiting professor at the Zhejiang University of Science and Technology, Hangzhou, China and an honorary adjunct professor at the Chinese University Hong Kong.
Alexander Shikov, Ph.D., Dr. pharm.Sci., is the Professor at the Department of Pharmaceutical Formulations at the St. Petersburg State Chemical Pharmaceutical University since 2016. He completed his Ph.D. at the Saint‐Petersburg State Chemical Pharmaceutical Academy in 1995 and obtained his Dr. Pharm.sci. degree from Saint‐Petersburg State Chemical Pharmaceutical Academy in 2006. Dr. Shikov began his career with the St‐Petersburg State Chemical Pharmaceutical Academy in 1992 as a professor assistant at the department of drugs technology and phytopreparations. He became a Deputy of the general director in science at the Interregional Center “Adaptogen,” St‐Petersburg, Russia in 1998, deputy of the general director at the St‐Petersburg institute of Pharmacy in 2008, and was promoted to Professor at the Department of Pharmacology of the North West State Medical University named after I.I. Mechnikov, St‐Petersburg, Russia in Sept. 2009. He served as associate editor of Journal of Ethnopharmacology (Elsevier BV, The Netherlands) from 2015, associate editor of Frontiers in Pharmacology Ethnopharmacology (Frintiers Media S.A.) from 2018, and as member of editorial board Phytomedicine, International Journal of Phytopharmacology and Phytotherapy (from 2009), Chinese Herbal Medicine (from 2013), Chinese Journal of Natural Medicine (from 2013), Synergy (from 2013), and World Journal of Traditional Chinese Medicine (from 2014). He has authored and co‐authored about 200 articles in Russian and international peer‐reviewed journals. His main research interest is focused on plant adaptogens, standardization of natural products (herbal and marine), pharmacokinetic of natural compounds, and approaches to improvement of bioavailability of natural molecules.
Olga Pozharitskaya, Ph.D.(pharm)., is the senior scientist at the Murmansk Marine Biological Institute, Kola Scientific Center of the Russian Academy of Sciences since 2020. She completed her Ph.D. at the Saint‐Petersburg State Chemical Pharmaceutical Academy in 1998. Dr. Pozharitskaya began her career with the St‐Petersburg State Chemical Pharmaceutical Academy in 1989 as a scientist at the department of drugs technology and phytopreparations. She became a head of the department of new technologies at the Interregional Center “Adaptogen”, St‐Petersburg, Russia in 2002, deputy of the general director in standardization and new technologies at the St‐Petersburg institute of Pharmacy in 2008. She has authored or co‐authored about 180 articles in Russian and international peer‐reviewed journals. Her main research interest is focused on natural product research, plant adaptogens, chemistry and pharmacology of marine organisms and seaweeds, pharmacokinetic of natural compounds and improvement of bioavailability of natural molecules.
Dr. rer.nat. Kenny Kuchta, FLS, is an honorary Professor at the Zhejiang Institute of TCM and Natural Medicine, Hangzhou, China. He is currently establishing Special Research on Far Eastern Medicine at Göttingen University, Germany. He obtained his doctorate in 2012 at the Chair of Pharmaceutical Biology at the University of Leipzig with a focus on pharmacognosy. He became University lecturer (Koushi) and faculty member at Sanyo Gakuen University, Okayama, Japan in 2012, with a research focus on East Asian Drugs of Kampo Medicine as chair of the Teaching and Research Center for Complementary and Alternative Medicine, which was subsequently restructured to the chair of Natural Products Chemistry Research in 2014. For his research on this field, he was awarded the Egon Stahl Award 2014 of the Society for Medicinal Plant and Natural Product Research (GA). From 2015 to 2017, he served as a visiting Professor at the National Institute of Health Science (NIHS), Division of Pharmacognosy, Phytochemistry, and Narcotics, funded by the Japan Society for the Promotion of Science (JSPS) of the Japanese Ministry of Education, Science and Technology as Research fellow with a focus on phytochemistry, regulatory implications for the internationalization of Kampo medicine and the implementation of Western phytotherapy in Japan. Furthermore, he serves as an advisor of the “Kampo Industralization Consortium”—founded amongst others by the Japanese prefectures Kanagawa, Toyama, and Nara as well as Mitsubishi Research Institute. He has co‐authored about 40 articles in peer‐reviewed journals and several book contributions, for example, the 13th edition of the “Lehrbuch Phytotherapie.”
Professor Pulok K. Mukherjee is working as the Director, Institute of Bioresources and Sustainable Development Department of Biotechnology (IBSD‐DBT), Ministry of Science and Technology, Govt of India Imphal, Manipur, India. He was formerly the Director, School of Natural Product Studies, Jadavpur University, Kolkata, India. His research/academic works highlights on traditional medicine inspired drug discovery from Indian medicinal plants to make them available from “Farm to Pharma.” He has to his credit more than 200 publications in peer reviewed impact journals, several patents. Prof. Mukherjee has authored/edited 5 books and 18 book chapters. His research publications have a cumulative Impact Factor of 241; h‐Index‐ 60, i10‐index—252; which has been cited for over >18,755 times. Dr. Mukherjee is a Fellow of the Royal Society of Chemistry (FRSC), Fellow of National Academy of Sciences, India (FNASc) and has been awarded with so many laurels from Govt. of India and abroad; to name a few: awarded with the prestigious Commonwealth Academic Staff Fellowship from Association of Commonwealth Universities [ACU], UK; TATA innovation fellowship, by Department of Biotechnology, Govt. of India; Outstanding Service Award from Drug Information association [DIA], USA; Career Award for Young Teacher from All India Council for Technical Education (AICTE), Govt. of India; Best Pharmaceutical Scientist of the Year, from the Association of Pharmaceutical Teachers’ of India (APTI); IASTAM Award for Contributions to Development of Ayurvedic and Herbal Pharmaceutics by Indian Association for the Study of Traditional Asian Medicine (IASTAM) and many others.
Subhadip Banerjee is a senior research scholar at the School of Natural Product Studies, Jadavpur University where he is pursuing his doctoral research under Prof. Pulok K. Mukherjee Director, IBSD‐DBT since 2016 after graduating from the same university with a major in Ayurveda Pharmacy. He obtained his M.Pharm. (Ayurveda) in pharmacology in the year 2015. His work focuses on establishing integrated approaches for drug development from Indian traditional medicine to understand the synergy based on network pharmacology and metabolomics. His work on traditional medicine inspired drug discovery based on systems biology approaches focus on development of leads from natural resources as adaptogens, hypolipidemic and immunomodulatory agents for safe health care.
Michael Heinrich is a Professor of Ethnopharmacology and Medicinal Plant Research (Pharmacognosy) and was until recently the head of the research cluster “Biodiversity and Medicines” at the UCL School of Pharmacy. He currently serves as the joint chair of UCL's Research Ethics Committee (with Dr. L. Ang, Institute of Education). The group's research is based on a transdisciplinary perspective integrating approaches from the biomedical and social sciences with an overall aim of tackling the fast changing global health needs. Key areas of interest include the prevention and early stage management of diabetes/metabolic syndrome and cancer chemoprevention based on the use of traditional medicines as well as value chains of (herbal) medicinal products. The research integrates methodological approaches from ethnopharmacology, natural product research, public health research, and anthropology. He is Specialty Editor in Chief of Frontiers in Pharmacology (Ethnopharmacology) and Reviews Editor of the Journal of Ethnopharmacology as well as an associate editor of the Journal of Pharmacy and Pharmacology, among other roles.
Wanying Wu is a professor and doctoral supervisor at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, China. She received her Ph.D. degree from Beijing University of Chinese Medicine and finished her postdoctoral fellowship at the School of Pharmaceutical Sciences, Peking University. After that, Dr. Wu joined the Shanghai Institute of Materia Medica and was later promoted to associate professor and professor. She is now the committee of the 11th Chinese Pharmacopoeia Commission, a member of the United States Pharmacopeia (USP) Herbal Medicines Compendium—East Asia Expert Panel, director and Secretary General of the Specialty Committee of traditional Chinese Medicine (TCM) Pharmaceutical Analysis of World Federation of Chinese Medicine Societies, the editor of the SCI journal Phytomedicine and the English edition of Chinese herbal medicine. As the major performer, she established 32 TCM standards that were accepted by USP or European Pharmacopoeia. Another major accomplishment is that she developed an innovative TCM prescription named “Dan Qi Tong Mai Tablet” that had been approved by CFDA for a clinical trial in 2009 in China. Her research interests are mainly in the systematic analysis of complex TCM systems by exploring the mechanisms of TCM pharmacology and its bioactive ingredients, establishing modern standards of TCM quality control, and promoting the drug discovery from TCM.
Dr. De‐an Guo serves as director of the Shanghai TCM Research Center as well as The National Engineering Laboratory for TCM Standardization Technologies which focused on the new technology development for TCM chemical analysis and international standard elaboration of TCM quality at Shanghai Institute of Materia Medica, Chinese Academy of Sciences. He received his Ph.D. Degree of Pharmacognosy at Beijing Medical University in 1990 and engaged in his postdoctoral research at Texas Tech University (1993–1996). He has been serving as the chair of Natural Medicine Expert Committee of Chinese Pharmacopoeia Commission since 2000, expert member of Botanical and Herbal Medicine Expert Committee United States Pharmacopoeia (2008‐present) and TCM Working Party Expert Member of European Pharmacopoeia, EDQM (2014‐present). He is also the editor in chief, associate editors or editorial board members of 18 international journals. To date, he has published 470 papers cited in SCI journals with 10,000 plus citations. He received a number of renowned national and international awards including National Natural Science Award and Science and Technology Progress Award, American Botanical Council Norman Farnsworth Award, American Society of Pharmacognosy Varo Tyler Prize, Cheung An Tak International Award for Outstanding Contribution to Chinese Medicine, National Innovation & Contending Prize, Ho Leung Ho Lee Foundation Prize, China Standard Innovation Award, and so forth. His main research focus is on the comprehensive phytochemical analysis and quality standard elaboration of herbal medicines, mainly traditional Chinese medicines.
Professor em. Dr. Dr. h.c.mult. Hildebert Wagner is one of the most outstanding scientists in the field of phytopharmacology. He headed the Institute of Pharmaceutical Biology, Ludwig‐Maximilian University, Munich Germany since 1965 for more than 25 years. His extraordinary scholarly activity and research have garnered him worldwide recognition. Multiple awards and prizes, including the Egon Stahl Gold Medal and, honorary doctorates, have been given to Prof. Wagner over the course of his long research and scholarly career. He has also been Distinguished Visiting Professor (Columbus, OH, USA), Dean of the Faculty of Chemistry/Pharmacy (Munich), Member of the Hungarian Academy of Science, Ph.D. honoris causae of the Universities of Budapest and Debrecen (Hungary), Dijon (France), Helsinki and Iaci. He is a Member of the Editorial/Advisory Boards of several journals in the field of natural products research, including Phytochemistry, Journal of Ethnopharmacology, and Journal of Natural Products. In June 1994 Professor Wagner and Professor Norman Farnsworth, became editors of the newly established journal Phytomedicine, International Journal of Phytotherapy and Phytopharmacognosy. He was Editor in Chief of the journal for 18 years. He has published more than 950 (!) original papers, 40 review articles, and several handbooks. Coveringa very wide range of research areas in pharmacognosy, from the phytochemical analysis of numerous plants and their secondary metabolites (e.g., alkaloids, cardiac glycosides, flavonoids, and lignans) to pharmacological studies of various natural compounds in bioassays, animals and human clinical studies, mainly associated with defense responses, immune systems, and adaptation to stress. His TLC and HPLC atlases are unique encyclopedias of plant analysis used in the industry for quality control of herbal substances and herbal preparations. His research interests are mainly related to chemistry, quality proof, and pharmacology of medicinal plants of Traditional Chinese Medicine, Synergy effects of herbal drug combinations for multitarget therapy, Genomic and Proteonomic biotechnology for evaluation of synergy effects of drug combinations and adaptogens.
Panossian AG, Efferth T, Shikov AN, et al. Evolution of the adaptogenic concept from traditional use to medical systems: Pharmacology of stress‐ and aging‐related diseases. Med Res Rev. 2021;41:630–703. 10.1002/med.21743
REFERENCES
- 1.EMA/HMPC/232100/2011. Assessment report on Rhodiola rosea L., rhizoma et radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as amended traditional use). Final. 27 March 2012.
- 2.EMA/HMPC/321232/2012. Assessment report on Panax ginseng C.A. Meyer, radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use). Final. 25 March 2014.
- 3.EMA/HMPC/680615/2013. Assessment report on Eleutherococcus senticosus (Rupr. et Maxim.) Maxim., radix. Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use). Final. 25 March 2014.
- 4.EMA/HMPC/320433/2012. Assessment report on Andrographis paniculata Nees, folium. Based on Article 10a of Directive 2001/83/EC as amended (well‐established use). Based on Article 16d (1), Article 16f and Article 16h of Directive 2001/83/EC as amended (traditional use). Final. 27 August 2014.
- 5. Samuelsson G, Bohlin L. Drugs of Natural Origin: A Treatise of Pharmacognosy. 6th ed. Stockholm: Swedish Academy of Pharmaceutical Sciences; 2009:776. [Google Scholar]
- 6. Lazarev NV. General and specific in action of pharmacological agents. Farmacol Toxicol. 1958;21:81‐86. [Google Scholar]
- 7. Lazarev NV, Ljublina EI, Ljublina MA. State of nonspecific resistance. Patol Fiziol Exp Terapia. 1959;3:16‐21. [PubMed] [Google Scholar]
- 8. Brekhman II, Dardymov IV. New substances of plant origin which increase nonspecific resistance. Annu Rev Pharmacol. 1969;9:419‐430. [DOI] [PubMed] [Google Scholar]
- 9. Farnsworth NR, Waller D, Strelkova LM. Use of Eleutherococcus senticosus in United States: problems, prospects and literature update. Proceedings of the Second International Symposium on Eleutherococcus Vladivostok: Far Eastern Scientific Center, USSR Academy of Sciences; 1986:47‐51.
- 10. Wagner H, Nörr H, Winterhoff H. Plant adaptogens. Phytomedicine. 1994;1:63‐76. [DOI] [PubMed] [Google Scholar]
- 11. Panossian A, Wikman G, Wagner H. Plant adaptogens III. Earlier and more recent aspects and concepts on their mode of action. Phytomedicine. 1999;6:287‐300. [DOI] [PubMed] [Google Scholar]
- 12. Panossian A, Gabrielian E, Wagner H. On the mechanism of action of plant adaptogens with particular reference to cucurbitacin R diglucoside. Phytomedicine. 1999;6:147‐155. [DOI] [PubMed] [Google Scholar]
- 13. EMEA/HMPC/102655/2007 . Reflection paper on the adaptogenic concept. London; 2008. http://www.ema.europa.eu/docs/en_GB/document&/WC500003646.pdf
- 14. Panossian A, Wikman G. Evidence‐based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress‐protective activity. Curr Clin Pharmacol. 2009;4:198‐219. [DOI] [PubMed] [Google Scholar]
- 15. Panossian A, Amsterdam J. Chapter 8: Adaptogens in psychiatric practice In: Gerbarg PL, Muskin PR, Brown RP, eds. Complementary and Integrative Treatments in Psychiatric Practice. Arlington, VA: American Psychiatric Publishing; 2017:155‐181. [Google Scholar]
- 16. Panossian A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann NY Acad Sci. 2017;1401:49‐64. [DOI] [PubMed] [Google Scholar]
- 17. Panossian A, Seo EJ, Efferth T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine. 2018;50:257‐284. [DOI] [PubMed] [Google Scholar]
- 18. Panossian A, Hambartsumyan M, Hovanissian A, Gabrielyan E, Wilkman G. The adaptogens Rhodiola and Schizandra modify the response to immobilization stress in rabbits by suppressing the increase of phosphorylated stress‐activated protein kinase, nitric oxide and cortisol. Drug Targets Insights. 2007;2:39‐54. [PMC free article] [PubMed] [Google Scholar]
- 19. Panossian A, Wikman G, Kaur P, Asea A. Adaptogens exert a stress‐protective effect by modulation of expression of molecular chaperones. Phytomedicine. 2009;16:617‐622. [DOI] [PubMed] [Google Scholar]
- 20. Panossian A, Wikman G, Kaur P, Asea A. Molecular chaperones as mediators of stress protective effect of plant adaptogens In: Asea AAA, Pedersen BK, eds. Heat Shock Proteins and Whole Body Physiology. Vol 5 Dordrecht, Heidelberg, London, New York: Springer; 2009:351‐364. [Google Scholar]
- 21. Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;7:481‐493. [DOI] [PubMed] [Google Scholar]
- 22. Panossian A, Wikman G, Kaur P, Asea A. Adaptogens stimulate neuropeptide Y and Hsp72 expression and release in neuroglia cells. Front Neurosci. 2012;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panossian A, Hamm R, Wikman G, Kadioglu O, Efferth T. Synergy and antagonism of active constituents of ADAPT‐232 on transcriptional level of metabolic regulation in isolated neuroglial cells. Front Neurosci. 2013;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panossian A, Hamm R, Wikman G, Efferth T. Mechanism of action of Rhodiola, salidroside, tyrosol and triandrin in isolated neuroglial cells: an interactive pathway analysis of the downstream effects using RNA microarray data. Phytomedicine. 2014;21:1325‐1348. [DOI] [PubMed] [Google Scholar]
- 25. Panossian A, Seo EJ, Wikman G, Efferth T. Synergy assessment of fixed combinations of Herba Andrographidis and Radix Eleutherococci extracts by transcriptome‐wide microarray profiling. Phytomedicine. 2015;22:981‐992. [DOI] [PubMed] [Google Scholar]
- 26. Panossian A. Adaptogens in mental and behavioral disorders. Psychiatr Clin North Am. 2013;36:49‐64. [DOI] [PubMed] [Google Scholar]
- 27. Panossian A, Wikman G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress‐protective activity. Pharmaceuticals. 2010;3:188‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asea A, Kaur P, Panossian A, Wikman G. Evaluation of molecular chaperons Hsp72 and neuropeptide Y as characteristic markers of adaptogenic activity of plant extracts. Phytomedicine. 2013;20:1323‐1329. [DOI] [PubMed] [Google Scholar]
- 29. Ahuja A, Kim JH, Kim JH, Yi YS, Cho JY. Functional role of ginseng‐derived compounds in cancer. J Ginseng Res. 2018;42:248‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amico AP, Terlizzi A, Damiani S, Ranieri M, Megna M, Fiore P. Immunopharmacology of the main herbal supplements: a review. Endocr Metab Immune Disord Drug Targets. 2013;13:283‐288. [DOI] [PubMed] [Google Scholar]
- 31. Cheng Y, Shen LH, Zhang JT. Anti‐amnestic and anti‐aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143‐149. [DOI] [PubMed] [Google Scholar]
- 32. Choi SH, Jung SW, Lee BH, et al. Ginseng pharmacology: a new paradigm based on gintonin‐lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geng J, Dong J, Ni H, et al. Ginseng for cognition. Cochrane Database Syst Rev. 2010;12:CD007769. [DOI] [PubMed] [Google Scholar]
- 34. Im DS, Nah SY. Yin and Yang of ginseng pharmacology: ginsenosides vs gintonin. Acta Pharmacol Sin. 2013;34:1367‐1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jakaria M, Haque ME, Kim J, Cho DY, Kim IS, Choi DK. Active ginseng components in cognitive impairment: Therapeutic potential and prospects for delivery and clinical study. Oncotarget. 2018;9:33601‐33620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joo SS, Yoo YM, Ahn BW, et al. Prevention of inflammation‐mediated neurotoxicity by Rg3 and its role in microglial activation. Biol Pharm Bull. 2008;31:1392‐1396. [DOI] [PubMed] [Google Scholar]
- 37. Kim JH, Yi YS, Kim MY, Cho JY. Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J Ginseng Res. 2017;41:435‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee S, Rhee DK. Effects of ginseng on stress‐related depression, anxiety, and the hypothalamic‐pituitary‐adrenal axis. J Ginseng Res. 2017;41:589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee TK, Johnke RM, Allison RR, O'Brien KF, Dobbs LJ, Jr. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237‐243. [DOI] [PubMed] [Google Scholar]
- 41. Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222‐226. [DOI] [PubMed] [Google Scholar]
- 42. Lee MS, Yang EJ, Kim JI, Ernst E. Ginseng for cognitive function in Alzheimer's disease: a systematic review. J Alzheimers Dis. 2009;18:339‐344. [DOI] [PubMed] [Google Scholar]
- 43. Lee SM, Bae BS, Park HW, et al. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J Ginseng Res. 2015;39:384‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leung KW, Wong AST. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leung KW, Yung KK, Mak NK, et al. Angiomodulatory and neurological effects of ginsenosides. Curr Med Chem. 2007;14:1371‐1380. [DOI] [PubMed] [Google Scholar]
- 46. Liang W, Ge S, Yang L, et al. Ginsenosides Rb1 and Rg1 promote proliferation and expression of neurotrophic factors in primary Schwann cell cultures. Brain Res. 2010;1357:19‐25. [DOI] [PubMed] [Google Scholar]
- 47. Liao LY, He YF, Li L, et al. A preliminary review of studies on adaptogens: comparison of their bioactivity in TCM with that of ginseng‐like herbs used worldwide. Chin Med. 2018;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Megna M, Amico AP, Cristella G, Saggini R, Jirillo E, Ranieri M. Effects of herbal supplements on the immune system in relation to exercise. Int J Immunopathol Pharmacol. 2012;25:43S‐49S. [DOI] [PubMed] [Google Scholar]
- 49. Mohanan P, Subramaniyam S, Mathiyalagan R, Yang DC. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42:123‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murthy HN, Dandin VS, Park SY, Paek KY. Quality, safety and efficacy profiling of ginseng adventitious roots produced in vitro. Appl Microbiol Biotechnol. 2018;102:7309‐7317. [DOI] [PubMed] [Google Scholar]
- 51. Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71(suppl 1):S1‐S5. [DOI] [PubMed] [Google Scholar]
- 52. Ong WY, Farooqui T, Koh HL, Farooqui AA, Ling EA. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pannacci M, Lucini V, Colleoni F, et al. Panax ginseng C.A. Mayer G115 modulates pro‐inflammatory cytokine production in mice throughout the increase of macrophage toll‐like receptor 4 expression during physical stress. Brain Behav Immun. 2006;20:546‐551. [DOI] [PubMed] [Google Scholar]
- 54. Park SE, Park C, Kim SH, et al. Korean red ginseng extract induces apoptosis and decreases telomerase activity in human leukemia cells. J Ethnopharmacol. 2009;121:304‐312. [DOI] [PubMed] [Google Scholar]
- 55. Patel S, Rauf A. Adaptogenic herb ginseng (Panax) as medical food: Status quo and future prospects. Biomed Pharmacother. 2017;85:120‐127. [DOI] [PubMed] [Google Scholar]
- 56. Qi HY, Li L, Ma H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Med Res Rev. 2018;38:625‐654. [DOI] [PubMed] [Google Scholar]
- 57. Radad K, Gille G, Liu L, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175‐186. [DOI] [PubMed] [Google Scholar]
- 58. Shen CY, Jiang JG, Yang L, Wang DW, Zhu W. Anti‐ageing active ingredients from herbs and nutraceuticals used in traditional Chinese medicine: pharmacological mechanisms and implications for drug discovery. Br J Pharmacol. 2017;174:1395‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13:209‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wiegant FAC, Limandjaja G, de Poot SAH. Plant adaptogens activate cellular adaptive mechanisms by causing mild damage In: Lukyanova L, Takeda N, Singal PK, eds. Adaptation Biology and Medicine, vol. 5: Health Potentials. New Delhi: Narosa Publishing House; 2008:319‐332. [Google Scholar]
- 61. Xie CL, Wang WW, Xue XD, Zhang SF, Gan J, Liu ZG. A systematic review and meta‐analysis of Ginsenoside‐Rg1 (G‐Rg1) in experimental ischemic stroke. Sci Rep. 2015;5:7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xie W, Zhou P, Sun Y, et al. Protective effects and target network analysis of Ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells. 2018;7:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yae S, Takahashi F, Yae T, et al. Hochuekkito (TJ‐41), a Kampo formula, ameliorates cachexia induced by colon 26 adenocarcinoma in mice. Evid Based Complement Alternat Med. 2012;2012:976926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rossijskij DM. Schisandra chinensis as a stimulant and tonic agent. Naval doctor. 1944;4:18‐22. [Google Scholar]
- 65. Karo VI. The geographic study of Schizandra stimulating effect. Scientific Papers of 3rd Year Students of the Naval Medical School. 1945;3:30‐33. [Google Scholar]
- 66. Zhestyanikov VD. Some data on the effect of the Far East Schizandra on the CNS. Scientific Papers of 3rd Year Students of the Naval Medical School. 1945;3:24‐29. [Google Scholar]
- 67. Stix G. Turbocharging the brain. Sci Am. 2009;301(46‐49):52‐55. [DOI] [PubMed] [Google Scholar]
- 68. Lebedev AA. Schizandrin—a new stimulant from Schizandra chinensis fruits. [dissertation]. Tashkent: Tashkent University; 1967.
- 69. Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail.: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118:183‐212. [DOI] [PubMed] [Google Scholar]
- 70. Brekhman II. Ginseng. Leningrad: State Publishing House of Medical Literature MEDGIZ; 1957:182. [Google Scholar]
- 71. Brekhman II. Eleutherococcus. Leningrad: Nauka; 1968:188. [Google Scholar]
- 72. Saratikov AS, Krasnov EA. Rhodiola rosea (Golden root). Tomsk: Tomsk State University Publishing House; 2004:292. [Google Scholar]
- 73. Panossian A, Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19:819‐838. [DOI] [PubMed] [Google Scholar]
- 74. Bogatova RI, Shlykova LV, Salnitsky VP, Wikman G. Evaluation of the effect of a single dose of a phytoadaptogen on the working capacity of human subjects during prolonged isolation. Aerosp Environ Med. 1997;31:51‐54. [PubMed] [Google Scholar]
- 75. Yue PY, Mak NK, Cheng YK, et al. Pharmacogenomics and the Yin/Yang actions of ginseng: antitumor, angiomodulating and steroid‐like activities of ginsenosides. Chin Med. 2007;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hovhannisyan AS, Panossian AG. Wikman G. ADAPT‐232 and ADAPT‐S for stress‐induced fatigue and recovery of trained and elite athletes: a randomized, controlled trial. J Athl Enhancement. 2015;4:4. [Google Scholar]
- 77. Fry RW, Morton AR, Keast D. Overtraining in athletes: an update. Sports Med. 1991;12:32‐65. [DOI] [PubMed] [Google Scholar]
- 78. Wang LM, Zhao X, Jx Chen, et al. Biological indicators of sub‐optimal health status. J Trad Chin Med. 2013;33:647‐650. [DOI] [PubMed] [Google Scholar]
- 79. He RR, Hiroshi K. Shanghuo syndrome in traditional Chinese medicine. World Sci Technol. 2008;10:37‐41. [Google Scholar]
- 80. Minihane AM, Vinoy S, Russell WR, et al. Low‐grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee J, Kim S‐H, Lee Y, Song S, Kim Y, Lee S. The concept of Mibyeong (sub‐health) in Korea: a Delphi study. Eur J Integr Med. 2013;5:514‐418. [Google Scholar]
- 82. Yamamoto S, Tsumura N, Nakaguchi T, et al. Principal component vector rotation of the tongue color spectrum to predict “Mibyou” (disease‐oriented state). Int J Comput Assist Radiol Surg. 2011;6:209‐215. [DOI] [PubMed] [Google Scholar]
- 83. Brekhman II. Valeology—The science of health. Moscow: Physical education and sport:; 1990:208. [Google Scholar]
- 84. Ogawa‐Ochiai K, Kawasaki K. Panax ginseng for frailty‐related disorders: a review. Front Nutr. 2018;5:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Arring NM, Millstine D, Marks LA, Nail LM. Ginseng as a treatment for fatigue: a systematic review. Altern Complement Med. 2018;24:624‐633. [DOI] [PubMed] [Google Scholar]
- 86. Kim HG, Cho JH, Yoo SR, et al. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double‐blind, placebo‐controlled trial. PLOS One. 2013;8:e61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bach HV, Kim J, Myung SK, Cho YA. Efficacy of Ginseng supplements on fatigue and physical performance: a meta‐analysis. J Korean Med Sci. 2016;31:1879‐1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Inglis JE, Lin PJ, Kerns SL, et al. Nutritional interventions for treating cancer‐related fatigue: a qualitative review. Nutr Cancer. 2019;71:21‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pharmacopoeia of the People's Republic of China 2015. 1st ed., HM He, L Cui, (Eds). Beijing: China Medical Science Press. 2017.
- 90.Ginseng Radix. WHO monographs on selected medicinal plants. World Health Organization; 1999; 1:168‐182.
- 91. Kuchta K. Traditional Japanese Kampo medicine—History of ideas and practice; Part 1: From ancient shamanic practice to the medical academies of Edo. Trad Kampo Med. 2019;6:49‐56. [Google Scholar]
- 92. Kuchta K. 3 Fachleute—3 Behandlungsstrategien: Lebensverlängerndes Phytotherapeutikum weiterhin gesucht! Zkm. 2015;6:40‐41. [Google Scholar]
- 93. Iijima K, Sun S, Cyong JC, Jyonouchi H. Juzen‐taiho‐to, a Japanese herbal medicine, modulates type 1 and type 2 T cell responses in old BALB/c mice. Am J Chin Med. 1999;27:191‐203. [DOI] [PubMed] [Google Scholar]
- 94. Kuchta K. Traditionelle Japanische Medizin—Kampo (Teil 2) Schulrichtungen und philosophische Konzepte. Z Phytother. 2014;35:128‐135. [Google Scholar]
- 95. Wiegant FA, Surinova S, Ytsma E, Langelaar‐Makkinje M, Wikman G, Post JA. Plant adaptogens increase lifespan and stress resistance in C. elegans . Biogerontology. 2009;10:27‐42. [DOI] [PubMed] [Google Scholar]
- 96. Kuchta K. Traditionelle Japanische Medizin—Kampo (Teil 3) Arzneipflanzen und Rezepturen. Z Phytother. 2014;35:172‐175. [Google Scholar]
- 97.JP XVI; 2011. http://kconsort.umin.jp/JP/JP16E035.pdf. Accessed 15 March 2020.
- 98.JP XVI; 2011. http://kconsort.umin.jp/JP/JP16E046.pdf. Accessed 15 March 2020.
- 99. Tatsumi K, Shinozuka N, Nakayama K, Sekiya N, Kuriyama T, Fukuchi Y. Hochuekkito improves systemic inflammation and nutritional status in elderly patients with chronic obstructive pulmonary disease. J Am Geriatr Soc. 2009;57:169‐170. [DOI] [PubMed] [Google Scholar]
- 100. Kiyohara H, Nagai T, Munakata K, et al. Stimulating effect of Japanese herbal (kampo) medicine, hochuekkito on upper respiratory mucosal immune system. Evid Based Complement Alternat Med. 2006;3:459‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Matsuda T, Maekawa K, Asano K, Hisamitsu T. Suppressive effect of juzen‐taiho‐to on lung metastasis of B16 melanoma cells in vivo. Evid Based Complement Alternat Med. 2011;2011:743153‐743155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kuroda M, Kotake T, Sonoda T, et al. The clinical evaluation of hochuekkito for symptoms of malignant neoplasm patients. Hinyokika Kiyo. 1985;31:173‐177. [PubMed] [Google Scholar]
- 103. Mukherjee, PK . Traditional systems of medicine and harmonization In: Mukherjee PK, ed. Quality Control and Evaluation of Herbal Drugs. Amsterdam: Elsevier; 2019:1‐28. [Google Scholar]
- 104. Mukherjee PK, Harwansh RK, Bahadur S, et al. Development of Ayurveda—Tradition to trend. J Ethnopharmacol. 2017;197:10‐24. [DOI] [PubMed] [Google Scholar]
- 105. Vyas VK, Bhandari P, Patidar R. A Comprehensive review on Withania somnifera Dunal. J Nat Remedies. 2011;11:1‐13. [Google Scholar]
- 106. Singh P, Rao RN, Reddy JR, et al. PE11, a PE/PPE family protein of Mycobacterium tuberculosis is involved in cell wall remodelling and virulence. Sci Rep. 2016;6:21624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kaur P, Makanjuola VO, Arora R, Singh B, Arora S. Immunopotentiating significance of conventionally used plant adaptogens as modulators in biochemical and molecular signalling pathways in cell mediated processes. Biomed Pharmacother. 2017;95:1815‐1829. [DOI] [PubMed] [Google Scholar]
- 108. Mukherjee PK, Banerjee S, Kar A. Exploring synergy in ayurveda and traditional Indian systems of medicine. Synergy. 2018;7:30‐33. [Google Scholar]
- 109. Mukherjee PK. Phyto‐pharmaceuticals, nutraceuticals and their evaluation In: Mukherjee PK, ed. Quality Control and Evaluation of Herbal Drugs. Amsterdam: Elsevier; 2019:707‐722. [Google Scholar]
- 110.Avicenna. Canon of Medical Science. Tashkent: Tashkent. Publ, Usb. Acad. Sci.; book 1, 1954; book 2, 1956; book 3, vol. 1, 1958, vol. 2, 1959, vol. 4, 1960 (russ.)
- 111. Amirdovlat Amasiatsi . Unnecessary for the Ignorant or a Dictionary of Medicinal Substances. Vienna: Mkhitaristov; 1926:766. [Google Scholar]
- 112. Amirdovlat Amasiatsi . Unnecessary for the ignorant. Moscow: Science; 1990:880. [Google Scholar]
- 113. Ganalanian AT. Armenian Legends. Yerevan: Acad. Sci. Arm. SSR; 1969:530. [Google Scholar]
- 114. Panossian A, Gabrielian E, Wagner H. Plant adaptogens. II. Bryonia as an adaptogen. Phytomedicine. 1997;4:83‐97. [DOI] [PubMed] [Google Scholar]
- 115. Jensen D. Uber zwei einheimische Giftpflanzen. Eine kritisch‐literarische und experimentelle Studie. Inaugural Dissertation zur Erlangung der Doktorwurde der hohen medi zinischen Fakulrat der Universitat zu Rostock, Rostock; 1914:57.
- 116. Wilson CO, Jones TE. American Drug Index. Philadelphia, PA and Montreal: J. B. Lippincott Company; 1959:671. [Google Scholar]
- 117. The Extra Pharmacopoeia. (Martindale). v. 1. 24th ed. London: Pharmaceutical Press; 1958:1362. [Google Scholar]
- 118. Osol A, Farrat GE, Beyer KH, eds. The Dispensatory of the United States of America. 25th edition Philadelphia, PA and Montreal: J. B. Lippincott Company; 1960:1608. [Google Scholar]
- 119. Hager H. Hagers Handbuch der pharmazeutischen Praxis für Apotheker, Arzneimittelhersteller, Ärzte und Medizinalbeamte. Chemikalien und Drogen (AM‐CH). Berlin: Springer; 1972:911. [Google Scholar]
- 120. Glocker M, ed. Anthroposophische Medizin. Stuttgart: Verlag Freies Geistesleben; 1993:121‐136. [Google Scholar]
- 121. Daems WE. Ita Wegman und das erste Mistelpraparat Iscar zur Krebsbehandlung In: Leroi R.Ed., Misteltherapie. Eine Antwort auf die Herausforderung Krebs . Die Pioniertat Rudolf Steiners und Ita Wegmans. Stuttgart: Verlag Freies Geistesleben; 1987:35‐44. [Google Scholar]
- 122. Patel S, Panda S. Emerging roles of mistletoes in malignancy management. Biotech. 2014;4:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Panossian A, Kocharian A, Matinian K, et al. Pharmacological activity of phenylpropanoids of the mistletoe, Viscum album L., host: Pyrus caucasica Fed. Phytomedicine. 1998;5:11‐17. [DOI] [PubMed] [Google Scholar]
- 124. Ernst E, Schmidt K, Steuer‐Vogt MK. Mistletoe for cancer? A systematic review of randomised clinical trials. Int J Cancer. 2003;107:262‐267. [DOI] [PubMed] [Google Scholar]
- 125. Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;2:CD003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ostermann T, Raak C, Büssing A. Survival of cancer patients treated with mistletoe extract (Iscador): a systematic literature review. BMC Cancer. 2009;9:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Melzer J, Iten F, Hostanska K, Saller R. Efficacy and safety of mistletoe preparations (Viscum album) for patients with cancer diseases. A systematic review. Forsch Komplementmed. 2009;16:217‐226. [DOI] [PubMed] [Google Scholar]
- 128. Kienle GS, Kiene H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142‐157. [DOI] [PubMed] [Google Scholar]
- 129. Burkhart J, Walchli C, Heusser P, Weissenstein U, Baumgartner S, Andres AC. In vitro investigation into the potential of a mistletoe extract to alleviate adverse effects of cyclophosphamide. Altern Ther Health Med. 2010;16:40‐48. [PubMed] [Google Scholar]
- 130. Calabrese EJ, Dhawan G, Kapoor R, Mattson MP, Rattan SI. Curcumin and hormesis with particular emphasis on neural cells. Food Chem Toxicol. 2019;129:399‐404. [DOI] [PubMed] [Google Scholar]
- 131. Davies KJ. Adaptive homeostasis. Mol Aspects Med. 2016;49:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cannon WB. Stresses and strains of homeostasis. Am J Med Sci. 1935;189:1‐14. [Google Scholar]
- 133. Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci. 1995;771:1‐18. [DOI] [PubMed] [Google Scholar]
- 134. Chrousos GP, Gold PW. The concept of stress system disorders: overview of behavioral and physical homeostasis. JAMA. 1992;267:1244‐1252. [PubMed] [Google Scholar]
- 135. Peck JR, Waxman D. What is adaptation and how it should be measured? J Theor Biol. 2018;447:190‐198. [DOI] [PubMed] [Google Scholar]
- 136. Melnikov VN. A quantitative method for estimating the adaptedness in a physiological study. Theor Biol Med Model. 2019;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Canguilhem G. Essai sur quelques problèmes concernant le normal et le pathologique. 1943. In: Fawcett CR, Cohen RS, trans. The Normal and the Pathological. New York: Zone Books; 1991.
- 138. An CE, Nolty AAT, Amano SS, Rizzo AA, Buckwalter JG, Rensberger J. Heart rate variability as an index of resilience. Mil Med. 2019;185:363‐369. [DOI] [PubMed] [Google Scholar]
- 139. Selye H. Experimental evidence supporting the conception of "adaptation energy". Am J Physiol. 1938;123:758‐765. [Google Scholar]
- 140. Selye H. Forty years of stress research: principal remaining problems and misconceptions. Can Med Assoc J. 1976;115:53‐56. [PMC free article] [PubMed] [Google Scholar]
- 141. Panossian AG, Oganessian AS, Ambartsumian M, Gabrelian ES, Wagner H, Wikman G. Effects of heavy physical exercise and adaptations on nitric oxide content in human saliva. Phytomedicine. 1999;6:17‐26. [DOI] [PubMed] [Google Scholar]
- 142. Mattson MP. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008;27:155‐162. [DOI] [PubMed] [Google Scholar]
- 143. Mattson MP, Son TG, Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5:174‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Murakami A. Non‐specific protein modifications may be novel mechanism underlying bioactive phytochemicals. J Clin Biochem Nutr. 2018;62:115‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dhabhar FS. The short‐term stress response—mother nature's mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front Neuroendocrinol. 2018;49:175‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose‐response framework. Toxicol Appl Pharmacol. 2007;222:122‐128. [DOI] [PubMed] [Google Scholar]
- 147. Calabrese V, Cornelius C, Dinkova‐Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13:1763‐1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mattson MP, Cheng A. Neurohormetic phytochemicals: Low‐dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632‐639. [DOI] [PubMed] [Google Scholar]
- 150. Thorin‐Trescases N, Thorin E. Vascular aging and oxidative stress: Hormesis and adaptive cellular pathways In: Bondy S, Maiese K, eds. Aging and Age‐Related Disorders. Berlin Heidelberg: Springer Science + Business Media, LLC; 2010:309‐321. [Google Scholar]
- 151. Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. NeuroMolecular Med. 2008;10:236‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro‐resolving superfamily of mediators. J Clin Invest. 2018;128:2657‐2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Duraisami R, Mohite VA, Kasbe AJ. Anti stress, adaptogenic activity of standardized dried fruit extract of Aegle marmelos against diverse stressors. Asian J Pharm Clin Res. 2010;3:1‐3. [Google Scholar]
- 154. Lalremruta V, Prasanna GS. Evaluation of protective effect of Aegle marmelos Corr. in an animal model of chronic fatigue syndrome. Indian J Pharmacol. 2012;44:351‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Arthur ST, Zwetsloot KA, Lawrence MM, et al. Ajuga turkestanica increases Notch and Wnt signaling in aged skeletal muscle. Eur Rev Med Pharmacol Sci. 2014;18:2584‐2592. [PubMed] [Google Scholar]
- 156. Kim JH, Kim SY, Lee SY, Jang CG. Antidepressant‐like effects of Albizzia julibrissin in mice: involvement of the 5‐HT1A receptor system. Pharmacol Biochem Behav. 2007;87:41‐47. [DOI] [PubMed] [Google Scholar]
- 157. Kulkarni MP, Juvekar AR. Effect of Alstonia scholaris (Linn.) R. Br. on stress and cognition in mice. Indian J Exp Biol. 2009;47:47‐52. [PubMed] [Google Scholar]
- 158. Baliga MS. Review of the phytochemical, pharmacological and toxicological properties of Alstonia Scholaris Linn. R. Br (Saptaparna). Chin J Integr Med. 2012:1‐14. [DOI] [PubMed] [Google Scholar]
- 159. Roshan S, Khan A, Ali S. To study the effect of Allium sativum on swimming endurance, anoxia tolerance and cold stress. J Global Pharma Technol. 2010;2:27‐32. [Google Scholar]
- 160. Thakur AK, Chatterjee SS, Kumar V. Adaptogenic potential of andrographolide: an active principle of the king of bitters (Andrographis paniculata). J Tradit Complement Med. 2015;5:42‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Thakur AK, Soni UK, Rai G, Chatterjee SS, Kumar V. Protective effects of Andrographis paniculata extract and pure andrographolide against chronic stress‐triggered pathologies in rats. Cell Mol Neurobiol. 2014;34:1111‐1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Raina AP, Gupta V, Sivaraj N, Dutta M. Andrographis paniculata (Burm. f.) Wall.ex Nees (kalmegh), a traditional hepatoprotective drug from India. Genet Resour Crop Evol. 2013;60:1181‐1189. [Google Scholar]
- 163. Padma P, Chansauria JPN, Khosa RL, Ray AK. Effect of Annona muricata and Polyalthia cerasoides on brain neurotransmitters and enzyme monoamine oxidase following cold immobilization stress. J Nat Remedies. 2001;1:144‐146. [Google Scholar]
- 164. Hernandez DE, Hancke JL, Wikman G. Evaluation of the anti‐ulcer and antisecretory activity of extracts of Aralia elata root and Schizandra chinensis fruit in the rat. J Ethnopharmacol. 1988;23:109‐114. [DOI] [PubMed] [Google Scholar]
- 165. Baranov AI. Medicinal uses of ginseng and related plants in the Soviet Union: recent trends in the Soviet literature. J Ethnopharmacol. 1982;6:339‐353. [DOI] [PubMed] [Google Scholar]
- 166. Mamedov N. Adaptogenic, geriatric, stimulant and antidepressant plants of Russian Far East. J Cell Mol Biology. 2005;4:71‐75. [Google Scholar]
- 167. Shikov AN, Pozharitskaya ON, Makarov VG. Aralia elata var. mandshurica (Rupr. & Maxim.) J.Wen: an overview of pharmacological studies. Phytomedicine. 2016;23:1409‐1421. [DOI] [PubMed] [Google Scholar]
- 168. Habbu PV, Mahadevan KM, Kulkarni PV, Daulatsingh C, Veerapur VP, Shastry RA. Adaptogenic and in vitro antioxidant activity of flavanoids and other fractions of Argyreia speciosa (Burm. f) Boj. in acute and chronic stress paradigms in rodents. Indian J Exp Biol. 2010;48:53‐60. [PubMed] [Google Scholar]
- 169. Garg R, Gupta VB. Adaptogenic activity of milk and aqueous decoction of Asparagus racemosus Willd. in acute and chronic stress paradigms in mice. J Cell Tissue Res. 2010;10:2281‐2286. [Google Scholar]
- 170. Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275‐291. [DOI] [PubMed] [Google Scholar]
- 171. Bhat HP, Jakribettu RP, Boloor R, Fayad R, Baliga MS. Use of Ayurvedic medicinal plants as immunomodulators in geriatrics: preclinical studies In: Watson RR, ed. Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults. San Diego: Academic Press; 2015:143‐149. [Google Scholar]
- 172. Koner BC, Banerjee BD, Ray A. Effects of stress on gamma glutamyl transpeptidase (GGT) activity in lymphoid system of rats: modulation by drugs. Indian J Exp Biol. 1997;35:222‐224. [PubMed] [Google Scholar]
- 173. Yanpallewar SU, Sen S, Tapas S, Kumar M, Raju SS, Acharya SB. Effect of Azadirachta indica on paracetamol‐induced hepatic damage in albino rats. Phytomedicine. 2003;10:391‐396. [DOI] [PubMed] [Google Scholar]
- 174. Shahid M, Subhan F, Ahmad N, Ullah I. A bacosides containing Bacopa monnieri extract alleviates allodynia and hyperalgesia in the chronic constriction injury model of neuropathic pain in rats. BMC Complement Altern Med. 2017;17:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Singh HK, Dhawan BN. Effect of Bacopa monniera Linn. (brahmi) extract on avoidance responses in rat. J Ethnopharmacol. 1982;5:205‐214. [DOI] [PubMed] [Google Scholar]
- 176. Singh RH, Narsimhamurthy K, Singh G. Neuronutrient impact of Ayurvedic Rasayana therapy in brain aging. Biogerontology. 2008;9:369‐374. [DOI] [PubMed] [Google Scholar]
- 177. Suslov NI, Churin AA, Skurikhin EG, et al. Effect of the natural nootrope and adaptogen preparations on the bioelectric cortex activity in rats. Eksp Klin Farmakol. 2002;65:7‐10. [PubMed] [Google Scholar]
- 178. Shikov AN, Pozharitskaya ON, Makarova MN, et al. Adaptogenic effect of black and fermented leaves of Bergenia crassifolia L. in mice. J Functional Foods. 2010;2:71‐76. [Google Scholar]
- 179. Shikov AN, Pozharitskaya ON, Makarova MN, Makarov VG, Wagner H. Bergenia crassifolia (L.) Fritsch—pharmacology and phytochemistry. Phytomedicine. 2014a;21:1534‐1542. [DOI] [PubMed] [Google Scholar]
- 180. Desai SK, Desai SM, Navdeep S, Arya P, Pooja T. Antistress activity of Boerhaavia diffusa root extract and a polyherbal formulation containing Boerhaavia diffusa using cold restraint stress model. Int J Pharm Pharm Sci. 2011;3:130‐132. [Google Scholar]
- 181. Mungantiwar AA, Nair AM, Shinde UA, et al. Studies on the immunomodulatory effects of Boerhaavia diffusa alkaloidal fraction. J Ethnopharmacol. 1999;65:125‐131. [DOI] [PubMed] [Google Scholar]
- 182. Pal P, Bose S. Phytopharmacological and phytochemical review of Butea monosperma . Int J Res Pharm Biomed Sci. 2011;2:1374‐1388. [Google Scholar]
- 183. Pawar VS, Shivakumar H. A current status of adaptogens: natural remedy to stress. Asian Pac J Trop Disease. 2012;2:S480‐S490. [Google Scholar]
- 184. Kannur DM, Hukkeri VI, Akki KS. Adaptogenic activity of Caesalpinia bonduc seed extracts in rats. J Ethnopharmacol. 2006;108:327‐331. [DOI] [PubMed] [Google Scholar]
- 185. Aggarwal BB, Prasad B, Reuter S, et al. Identification of novel anti‐inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 2011;12:1595‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Pellati F, Brighenti V, Sperlea J, Marchetti L, Bertelli D, Benvenuti S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules. 2018;23:2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Somarathna KIWK, Chandola HM, Ravishankar B, Pandya KN, Attanayake AMP, Ashok BK. Evaluation of adaptogenic and anti‐stress effects of Ranahamsa Rasayanaya—a Sri Lankan classical Rasayana drug on experimental animals. Ayu. 2010;31:88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Koppula S, Kopalli SR, Sreemantula S. Adaptogenic and nootropic activities of aqueous extracts of Carum Carvi L. (Caraway) fruit: an experimental study in Wistar rats. Aust J Medical Herbal. 2009;21:72‐78. [Google Scholar]
- 189. Barbosa NR, Pittella F, Gattaz WF. Centella asiatica water extract inhibits iPLA2 and cPLA2 activities in rat cerebellum. Phytomedicine. 2008;15:896‐900. [DOI] [PubMed] [Google Scholar]
- 190. Agarwal P, Sharma B, Fatima A, Jain SK. An update on Ayurvedic herb Convolvulus pluricaulis Choisy. Asian Pac J Trop Biomed. 2014;4:245‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Gopalkrishna B, Akki KS, Patil SH, Hukkeri VI. Preliminary phytochemical investigation and in vivo antistress activity of Safed musli (Chlorophytum Borivillianum L.). Indian Drugs. 2006;43:878‐880. [Google Scholar]
- 192. Deore SL, Khadabadi SS. Screening of antistress properties of Chlorophytum borivilianum tuber. Pharmacologyonline. 2009;1:320‐328. [Google Scholar]
- 193. Graver SK, Divekar HM, Kumar R, et al. Experimental evaluation of a composite Indian herbal preparation II (CIHPII) as an adaptogen and its mechanism of action. Int J Pharmacognosy. 1995;33:148‐154. [Google Scholar]
- 194. Kenjale RD, Shah RK, Sathaye SS. Anti‐stress and anti‐oxidant effects of roots of Chlorophytum borivilianum (Santa Pau & Fernandes). Indian J Exp Biol. 2007;45:974‐979. [PubMed] [Google Scholar]
- 195. Cassani J, Ferreyra‐Cruz OA, Dorantes‐Barrón AM, Villaseñor RMV, Arrieta‐Baez D, Estrada‐Reyes R. Antidepressant‐like and toxicological effects of a standardized aqueous extract of Chrysactinia mexicana A. Gray (Asteraceae) in mice. J Ethnopharmacol. 2015;171:295‐306. [DOI] [PubMed] [Google Scholar]
- 196. Singh J, Handa G, Rao PR, Atal CK. Pangamic acid, a stamina building, antistress and antihyperlipidemic principle from Cicer arietinum L. J Ethnopharmacol. 1983;7:239‐242. [DOI] [PubMed] [Google Scholar]
- 197. Shilova IV, Suslov NI, Krasnov EA. Adaptogenic and nootropic properties of the thick extract from the aerial part of Atragene sibirica L. Plant Resources. 2001;37:78‐88. [Google Scholar]
- 198. Ishola IO, Ashorobi RB. Anti‐stress potential of aqueous root extract of Cnestis ferruginea . Int J Pharmacol. 2007;3:295‐298. [Google Scholar]
- 199. Ko KM, Leon TYY, Mak DHF, Chiu PY, Du Y, Poon MKT. A characteristic pharmacological action of ‘Yang‐invigorating’ Chinese tonifying herbs: enhancement of myocardial ATP‐generation capacity. Phytomedicine. 2006;13:636‐642. [DOI] [PubMed] [Google Scholar]
- 200. Gao SM, Liu JS, Wang M, et al. Traditional uses, phytochemistry, pharmacology and toxicology of Codonopsis: a review. J Ethnopharmacol. 2018;219:50‐70. [DOI] [PubMed] [Google Scholar]
- 201. Gupta GL, Fernandes J. Protective effect of Convolvulus pluricaulis against neuroinflammation associated depressive behavior induced by chronic unpredictable mild stress in rat. Biomed Pharmacother. 2019;109:1698‐1708. [DOI] [PubMed] [Google Scholar]
- 202. Ramchandani D, Ganeshpurkar A, Bansal D, Karchuli MS, Dubey N. Protective effect of Curculigo orchioides extract on cyclophosphamide‐induced neurotoxicity in murine model. Toxicol Int. 2014;21:232‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Wong RWK, Rabie B, Bendeus M, Hägg U. The effects of rhizoma curculiginis and rhizoma drynariae extracts on bones. Chin Med. 2007;2:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Nie Y, Dong X, He Y, et al. Medicinal plants of genus Curculigo: traditional uses and a phytochemical and ethnopharmacological review. J Ethnopharmacol. 2013;147:547‐563. [DOI] [PubMed] [Google Scholar]
- 205. Mohanty I, Dharamvir SA, Dinda A, Joshi S, Talwar K, Gupta SK. Protective effects of Curcuma longa on ischemia‐reperfusion induced myocardial injuries and their mechanisms. Life Sci. 2004;75:1701‐1711. [DOI] [PubMed] [Google Scholar]
- 206. Volkova LA, Urmantseva VV, Burgutin AB, Nosov AM. Adaptogenic action of the complex of phenylpropanoids on Dioscorea deltoidea cell culture under abiotic stress. Rus J Plant Physiol. 2013;60:235‐243. [Google Scholar]
- 207. Singh N, Nath R, Gupta ML. A pharmacological evaluation of anti‐stress activity of Diospyros peregrina Gurke. Indian J Pharmacol. 1988;20:102‐108. [Google Scholar]
- 208. Saggu S, Kumar R. Effect of seabuckthorn leaf extracts on circulating energy fuels, lipid peroxidation and antioxidant parameters in rats during exposure to cold, hypoxia and restraint (C–H–R) stress and post stress recovery. Phytomedicine. 2008;15:437‐446. [DOI] [PubMed] [Google Scholar]
- 209. Saggu S, Divekar HM, Gupta V, Sawhney RC, Banerjee PK, Kumar R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: a dose dependent study. Food Chem Toxicol. 2007;45:609‐617. [DOI] [PubMed] [Google Scholar]
- 210. Mingyu X, Xiaoxuan S, Jinhua C. The medicinal research and development of seabuckthorn. J Water Soil Conser. 1991:1‐11. [Google Scholar]
- 211. Li F, Wang D, Jiang Z, Gao X, Zhao H. Activity stimulating osteoblast‐like cells proliferation of some traditional Chinese medicinal herbs and other plants. Pharm Biol. 2008;39:351‐356. [Google Scholar]
- 212. Sun H, Zhang Y, Zhang A, Zhang Y, Wang X. Rapid analysis of constituents and metabolites from extracts of Acanthopanax senticosus Harms leaf. In: Wang X, Zhang A, Sun H (Eds.). Serum Pharmacochemistry of Traditional Chinese Medicine Academic Press; 2017:303‐311.
- 213. Variya BC, Bakrania AK, Patel SS. Emblica officinalis (Amla): a review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol Res. 2016;111:180‐200. [DOI] [PubMed] [Google Scholar]
- 214. Pareek S, Shikov AN, Pozharitskaya ON, et al. Indian Gooseberry (Emblica officinalis Gaertn) In: Yahia EM, ed. Fruit and Vegetable Phytochemicals: Chemistry and Human Health. Wiley‐Blackwell; 2017:1077‐1105. [Google Scholar]
- 215. Oshima Y, Takata S, Hikino H, Deyama T, Kinoshita G. Anticomplementary activity of the constituents of Eucommia ulmoides bark. J Ethnopharmacol. 1988;23:159‐164. [DOI] [PubMed] [Google Scholar]
- 216. Deyama T, Nishibe S, Nakazawa Y. Constituents and pharmacological effects of Eucommia and Siberian ginseng. Acta Pharmacol Sin. 2001;22:1057‐1070. [PubMed] [Google Scholar]
- 217. Xiao YP, Zeng J, Jiao LN, Xu XY. Review for treatment effect and signaling pathway regulation of kidney‐tonifying traditional Chinese medicine on osteoporosis. Zhongguo Zhong Yao Za Zhi. 2018;43:21‐30. [DOI] [PubMed] [Google Scholar]
- 218. Siripurapu KB, Gupta P, Bhatia G, Maurya R, Nath C, Palit G. Adaptogenic and anti‐amnesic properties of Evolvulus alsinoides in rodents. Pharmacol Biochem Behav. 2005;81:424‐432. [DOI] [PubMed] [Google Scholar]
- 219. Gupta P, Siripurapu KB, Ahmad A, Palit G, Arora A, Maurya R. Anti‐stress constituents of Evolvulus alsinoides: an ayurvedic crude drug. Chem Pharm Bull. 2007;55:771‐775. [DOI] [PubMed] [Google Scholar]
- 220. Singh A. Review of ethnomedicinal uses and pharmacology of Evolvulus alsinoides L. Ethnobot Leaflets. 2010;12:734‐740. [Google Scholar]
- 221. Kothiyal P, Ratan P. Antistress effect of Fagopyrum esculentum in rats subjected to forced swimming endurance test. Pharmacologyonline. 2011;3:290‐296. [Google Scholar]
- 222. Son YK, Lee MH, Han YN. A new antipsychotic effective neolignan from Firmiana simplex . Arch Pharm Res. 2005;28:34‐38. [DOI] [PubMed] [Google Scholar]
- 223. Ghosal S, Singh AAK, Biswas K. New 6‐aryl‐2‐pyrones from Gentiana pedicellata . Planta Med. 1983;49:240‐243. [DOI] [PubMed] [Google Scholar]
- 224. Rai D, Bhatia G, Sen T, Palit G. Antistress effects of Ginkgo biloba and Panax ginseng: a comparative study. J Pharmacol Sci. 2003;93:458‐464. [DOI] [PubMed] [Google Scholar]
- 225. Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Wang X, Hai CX, Liang X, Yu SX, Zhang W, Li YL. The protective effects of Acanthopanax senticosus Harms aqueous extracts against oxidative stress: role of Nrf2 and antioxidant enzymes. J Ethnopharmacol. 2010;127:424‐432. [DOI] [PubMed] [Google Scholar]
- 227. Patwardhan B, Mutalik G, Tillu G. Longevity, Rejuvenation, and Rasayana In: di Sarsina PR, Tassinari M, eds. Integrative Approaches for Health: Biomedical Research, Ayurveda and Yoga. Boston. MA: Academic Press; 2015:259‐291. [Google Scholar]
- 228. Mendes FR, Carlini EA. Brazilian plants as possible adaptogens: an ethnopharmacological survey of books edited in Brazil. J Ethnopharmacol. 2007;109:493‐500. [DOI] [PubMed] [Google Scholar]
- 229. Galvão SMP, Marques LC, Oliveira MGM, Carlini EA. Heteropterys aphrodisiaca (extract BST0298): a Brazilian plant that improves memory in aged rats. J Ethnopharmacol. 2002;79:305‐311. [DOI] [PubMed] [Google Scholar]
- 230. Fraga GA, Balogun SO, Pascqua ED, et al. Heteropterys tomentosa A. Juss: toxicological and adaptogenic effects in experimental models. Nutr Health. 2017;23:289‐298. [DOI] [PubMed] [Google Scholar]
- 231. Natraj GR, Nanjappaiah HM, Hugar S. Evaluation of adaptogenic potential of Hibiscus cannabinus in acute stress induced mice. Pharmacologyonline. 2011;2:508‐513. [Google Scholar]
- 232. Puri S, Kumar B, Debnath J, et al. Comparative pharmacological evaluation of adaptogenic activity of Holoptelea integrifolia and Withania somnifera . Int J Drug Dev Res. 2011;3:84‐98. [Google Scholar]
- 233. Srivastava J, Prasad SK, Dwivedi KN, Pandey HP. Holoptelea integrifolia Planch: a potential Ayurvedic medicinal plant. Int J Pharm Sci Rev Res. 2013;21:281‐286. [Google Scholar]
- 234. Ghosal S, Jaiswal DK, Singh SK, Srivastava RS. Dichotosin and dichotosinin, two adaptogenic glucosyloxy flavans from Hoppea dichotoma . Phytochemistry. 1985;24:831‐833. [Google Scholar]
- 235. Kumar V, Singh PN, Bhattacharya SK. Anti‐stress activity of Indian Hypericum perforatum L. Indian J Exp Biol. 2001;39:344‐349. [PubMed] [Google Scholar]
- 236. Nagasirisha M, Saleem TM. Effect of whole plant of Rostellularia diffusa Willd. on experimental stress in mice. Pharmacogn Mag. 2014;10(Suppl 3):S614‐S621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lakshmi BVS, Sudhakar M. Adaptogenic activity of Lagenaria siceraria: an experimental study using acute stress models on rats. J Pharmacol Toxicol. 2009;4:300‐306. [Google Scholar]
- 238. López‐Fando A, Gómez‐Serranillos MP, Iglesias I, Lock O, Upamayta UP, Carretero ME. Lepidium peruvianum chacon restores homeostasis impaired by restraint stress. Phytother Res. 2004;18:471‐474. [DOI] [PubMed] [Google Scholar]
- 239. Kour K, Sharma N, Chandan BK, Koul S, Sangwan PL, Bani S. Protective effect of Labisia pumila on stress‐induced behavioral, biochemical, and immunological alterations. Planta Med. 2010;76:1497‐1505. [DOI] [PubMed] [Google Scholar]
- 240. Podkolzin AA, Dontsov VI, Sychev IA, Kobeleva GY, Kharchenko ON. Immunomodulating, antianemic, and adaptogenic effects of polysaccharides from plaster clover (Melilotus officinalis). Bull Exp Biol Med. 1996;121:597‐599. [PubMed] [Google Scholar]
- 241. Aji BM, Effraim KD, Onyeyili IP. Anti‐stress activity of Mitragyna africanus (Willd), stembark extract. Sciences (New York). 2001;1:105‐107. [Google Scholar]
- 242. Meera S, Nagarjuna CG. Antistress and immunomodulatory activity of aqueous extract of Momordica charantia . Phcog Mag. 2009;5:69‐73. [Google Scholar]
- 243. Nade VS, Kawale LA, Naik RA, Yadav AV. Adaptogenic effect of Morus alba on chronic footshock‐induced stress in rats. Indian J Pharmacol. 2009;41:246‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Shukla KK, Mahdi AA, Ahmad MK, Jaiswar SP, Shankwar SN, Tiwari SC. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complement Alternat Med. 2010;7:137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Saraf MN, Sanaye MM, Mengi SA. Antifatigue effect of Murraya koenigii . Pharmacologyonline. 2011;2:1025‐1037. [Google Scholar]
- 246. Koul S, Chaudhary A. Radical scavenging and antistress activity of Mussaenda frondosa roots (Rubiaceae). Pharmacologyonline. 2011;1:1091‐1097. [Google Scholar]
- 247. Mukherjee D, Khatua TN, Venkatesh P, Saha BP, Mukherjee PK. Immunomodulatory potential of rhizome and seed extracts of Nelumbo nucifera Gaertn. J Ethnopharmacol. 2010;128:490‐494. [DOI] [PubMed] [Google Scholar]
- 248. Mukherjee PK, Balasubramanian R, Saha K, Saha BP, Pal M. A review on Nelumbo nucifera Gaertn. Anc Sci Life. 1996;15:268‐276. [PMC free article] [PubMed] [Google Scholar]
- 249. Roshan S, Khan A, Taznee B, Ali S. To study the effect of Nigella sativa on various biochemical parameters on stress induced in albino rats. Int J Pharm Pharm Sci. 2010;2:185‐189. [Google Scholar]
- 250. Singh N. Antistress activity of Ocimum sanctum. L. Ind J Med Res. 1981;73:443‐451. [PubMed] [Google Scholar]
- 251. Ahumada F, Trincado MA, Arellano JA, Hancke J, Wikman G. Effect of certain adaptogenic plant extracts on drug‐induced narcosis in female and male mice. Phytother Res. 1991;5:29‐31. [Google Scholar]
- 252. Anju L. Adaptogenic and anti‐stress activity of Ocimum sanctum in mice. Res J Pharm Biol Chem Sci. 2011;2:670‐678. [Google Scholar]
- 253. Shikov AN, Pozharitskaya ON, Makarov VG, Yang WZ, Guo DA. Oplopanax elatus (Nakai) Nakai: chemistry, traditional use and pharmacology. Chin J Nat Med. 2014;12:721‐729. [DOI] [PubMed] [Google Scholar]
- 254. Park HJ, Kim DH, Park SJ, Kim JM, Ryu JH. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Dua PR, Shanker G, Srimal RC, et al. Adaptogenic activity of Indian Panax pseudoginseng . Indian J Exp Biol. 1989;27:631‐634. [PubMed] [Google Scholar]
- 256. Hussain S, Hore DK. Collection and conservation of major medicinal pants of Darjeeling and Sikkim Himalayas. Indian J Trad Knowledge. 2007;6:352‐357. [Google Scholar]
- 257. Adkar PP, Jadhav PP, Ambavade SD, Bhaskar VH, Shelke T. Adaptogenic activity of lyophilized hydroethanol extract of Pandanus odoratissimus in Swiss albino mice. Int Sch Res Notices. 2014;2014:429828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Espinola EB, Dias RF, Mattei R, Carlini EA. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J Ethnopharmacol. 1997;55:223‐229. [DOI] [PubMed] [Google Scholar]
- 259. Sipahimalani A, Nörr H, Wagner H. Phenylpropanoid glycosides and tetrahydrofurofuranlignan glycosides from the adaptogenic plant drugs Tinospora cordifola and Drypetes roxburghii . Planta Med. 1994;60:596‐597. [DOI] [PubMed] [Google Scholar]
- 260. Yadav V, Chatterjee SS, Majeed M, Kumar V. Long lasting preventive effects of piperlongumine and a Piper longum extract against stress triggered pathologies in mice. J Intercult Ethnopharmacol. 2015;4:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Yadav V, Chatterjee SS, Majeed M, Kumar V. Preventive potentials of piperlongumine and a Piper longum extract against stress responses and pain. J Tradit Complement Med. 2016;6:413‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Padma P, Chansouria JPN, Khosa RL. Polyalthia cerasoides—a possible antistress drug. Indian J Nat Prod. 2000;16:20‐23. [Google Scholar]
- 263. Filaretov AA, Bogdanova TS, Mityushov MI, Podvigina TT, Srailova GT. Effect of adaptogens on activity of the pituitary‐adrenocortical system in rats. Biul Eks Biol Med. 1986;101:573‐574. [PubMed] [Google Scholar]
- 264. Shikov AN, Lazukina MA, Pozharitskaya ON, et al. Pharmacological evaluation of Potentilla alba L. in mice: adaptogenic and central nervous system effects. Pharm Biol. 2011;49:1023‐1028. [DOI] [PubMed] [Google Scholar]
- 265. Giri M, Rao PM, Jayaveera KN. Evaluation of adaptogenic activity of Prunella vulgaris . Int J Pharm Sci Res. 2011;8:62‐65. [Google Scholar]
- 266. Lakshmi BVS, Sudhakar M. Screening of Psidium guajava leaf extracts for antistress activity in different experimental animal models. Phcog Res. 2009b;1:359‐366. [Google Scholar]
- 267. Piato AL, Detanico BC, Linck VM, Herrmann AP, Nunes DS, Elisabetsky E. Anti‐stress effects of the “tonic” Ptychopetalum olacoides (Marapuama) in mice. Phytomedicine. 2010;17:248‐253. [DOI] [PubMed] [Google Scholar]
- 268. Pramanik SS, Sur TK, Debnath PK, Bhattacharya D. Effect of Pueraria tuberose tuber extract on chronic foot shock stress in wistar rats. Nepal Med Coll J. 2010;12:234‐238. [PubMed] [Google Scholar]
- 269. Kokoska L, Janovska D. Chemistry and pharmacology of Rhaponticum carthamoides: a review. Phytochemistry. 2009;70:842‐855. [DOI] [PubMed] [Google Scholar]
- 270. Wang S, Wang EP. Studies on the chemical components of Rhodiola crenulata . Acta Pharmaceutica Sinica. 1992;27:117‐120. [PubMed] [Google Scholar]
- 271. Tao H, Wu X, Cao J, et al. Rhodiola species: a comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med Res Rev. 2019;39:1779‐1850. [DOI] [PubMed] [Google Scholar]
- 272. Grace MH, Yousef GG, Kurmukov AG, Raskin I, Lila MA. Phytochemical characterization of an adaptogenic preparation from Rhodiola heterodonta . Nat Prod Commun. 2009;4:1053‐1058. [PMC free article] [PubMed] [Google Scholar]
- 273. Yunuskhodjaev AN, Iskandarova SF, Kurmukov A, Saidov SA. Study of adaptogenic properties and chronic toxicity of extract of Rhodiola heterodonta . Eur J Natural History. 2014;2:35‐38. [Google Scholar]
- 274. Gupta V, Saggu S, Tulsawani RK, Sawhney RC, Kumar R. A dose dependent adaptogenic and safety evaluation of Rhodiola imbricata Edgew, a high altitude rhizome. Food Chem Toxicol. 2008;46:1645‐1652. [DOI] [PubMed] [Google Scholar]
- 275. Amsterdam JD, Panossian AG. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine. 2016;23:770‐783. [DOI] [PubMed] [Google Scholar]
- 276. Perfumi M, Mattioli L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res. 2007;21:37‐43. [DOI] [PubMed] [Google Scholar]
- 277. Ip SP, Che CT, Leung PS. Association of free radicals and the tissue renin‐angiotensin system: prospective effects of Rhodiola, a genus of Chinese herb, on hypoxia‐induced pancreatic injury. JOP. 2001;2:16‐25. [PubMed] [Google Scholar]
- 278. Khanum F, Bawa AS, Singh B. Rhodiola rosea: a versatile adaptogen. Compr Rev Food Sci Food Saf. 2005;4:55‐62. [DOI] [PubMed] [Google Scholar]
- 279. Patil RA, Jagdale SC, Kasture SB. Antihyperglycemic, antistress and nootropic activity of roots of Rubia cordifolia L. . Indian J Exp Biol. 2006;44:987‐992. [PubMed] [Google Scholar]
- 280. Daswani BR, Yegnanarayan R. Immunomodulatory activity of septilin, a polyherbal preparation. Phytother Res. 2002;16:162‐165. [DOI] [PubMed] [Google Scholar]
- 281. Su CY, Ming QL, Rahman K, Han T, Qin LP. Salvia miltiorrhiza: traditional medicinal uses, chemistry, and pharmacology. Chin J Nat Med. 2015;13:163‐182. [DOI] [PubMed] [Google Scholar]
- 282. Tsai CC, Huang SC, Liu JK, et al. Salvia miltiorrhiza causes tonic contraction in rat ileum through Ca2+‐calmodulin pathway. J Ethnopharmacol. 2012;142:694‐699. [DOI] [PubMed] [Google Scholar]
- 283. Brekhman II. Man and Biologically active Substances. The Effect of Drugs, Diet and Pollution on Health. Oxford: Pergamon Press Ltd; 1980:89. [Google Scholar]
- 284. Lu Y, Chen DF. Analysis of Schisandra chinensis and Schisandra sphenanthera . J Chromatogr A. 2009;1216:1980‐1990. [DOI] [PubMed] [Google Scholar]
- 285. Wei B, Li Q, Fan R, et al. UFLC‐MS/MS method for simultaneous determination of six lignans of Schisandra chinensis (Turcz.) Baill. in normal and insomniac rats brain microdialysates and homogenate samples: towards an in‐depth study for its sedative‐hypnotic activity. J Mass Spectrom. 2013;48:448‐458. [DOI] [PubMed] [Google Scholar]
- 286. Udintsev SN, Krylova SG, Konovalova ON. Correction of hormonal‐metabolic disturbances in rats by natural adaptogens during development of an adaptation syndrome and function tests with dexamethasone and ACTH. Bull Exp Biol Med. 1991;112:1739‐1741. [PubMed] [Google Scholar]
- 287. Kim EH, Shim B, Kang S, et al. Anti‐inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126:320‐331. [DOI] [PubMed] [Google Scholar]
- 288. Kholodova Y. Phytoecdysteroids: biological effects, application in agriculture and complementary medicine (as presented at the 14‐th Ecdysone Workshop, July, 2000, Rapperswil, Switzerland). Ukrains' kyi biokhimichnyi zhurnal. 1999;73:21‐29. [PubMed] [Google Scholar]
- 289. Sumanth M, Mustafa SS. Antistress, adoptogenic activity of Sida cordifolia roots in mice. Indian J Pharm Sci. 2009;71:323‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Bathori M, Kalman A, Toth G, et al. Ecdysteroids of Silene italica ssp. nemoralis, novel approaches of ecdysteroid therapy. Acta Pharm Hung. 2004;74:131‐141. [PubMed] [Google Scholar]
- 291. Zhao XX, Peng C, Zhang H, Qin LP. Sinomenium acutum: a review of chemistry, pharmacology, pharmacokinetics, and clinical use. Pharm Biol. 2012;50:1053‐1061. [DOI] [PubMed] [Google Scholar]
- 292. Mohan M, Attarde D, Momin R, Kasture S. Antidepressant, anxiolytic and adaptogenic activity of torvanol A: an isoflavonoid from seeds of Solanum torvum . Nat Prod Res. 2013;27:2140‐2143. [DOI] [PubMed] [Google Scholar]
- 293. Punegov VV, Sychov RL, Zainullin VG, Fedorov VN, Punegova NV. Extraction of ecdysteron‐80 from Serratula coronata L. and assessment of its pharmacological action. Part I. Adaptogenic, gastroprotective, thermoprotective, and antihypoxic activity. Pharmaceutical Chemistry J. 2008;42:446‐451. [Google Scholar]
- 294. Van Wyk BE, Albrecht C. A review of the taxonomy, ethnobotany, chemistry and pharmacology of Sutherlandia frutescens (Fabaceae). J Ethnopharmacol. 2008;119:620‐629. [DOI] [PubMed] [Google Scholar]
- 295. Singh AK, Dhamanigi SS, Asad M. Anti‐stress activity of hydro‐alcoholic extract of Eugenia caryophyllus buds (clove). Indian J Pharmacol. 2009;41:28‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Belapurkar P, Goyal P, Tiwari‐Barua P. Immunomodulatory effects of Triphala and its individual constituents: a review. Indian J Pharm Sci. 2014;76:467‐475. [PMC free article] [PubMed] [Google Scholar]
- 297. Ploberger F. A Tibetan herbal formula understood from a phytotherapeutical perspective of TCM. Asian Med. 2015;10:353‐364. [Google Scholar]
- 298. Upadhay AK, Kumar K, Kumar A, Mishra HS. Tinospora cordifolia (Wild) Hook. f. and Thoms. (Guduchi)—validation of the Ayurvedic pharmacology through experimental and clinical studies. Int J Ayurveda Res. 2010;1:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Choudhry N, Singh S, Siddiqui MB, Khatoon S. Impact of seasons and dioecy on therapeutic phytoconstituents of Tinospora cordifolia, a Rasayana drug. BioMed Res Int. 2014;2014:902138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Saha S, Ghosh S. Tinospora cordifolia: one plant, many roles. Anc Sci Life. 2012;31:151‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Shivakumar H, Javed T, Prakash T, Rao RN, Swamy BJ, Goud AV. Adaptogenic activity of ethanolic extract of Tribulus terrestris L. J Nat Remedies. 2006;6:87‐95. [Google Scholar]
- 302. Singh B, Gupta DK, Chandan BK. Adaptogenic activity of a glyco‐peptido‐lipid fraction from the alcoholic extract of Trichopus zeylanicus Gaertn. Phytomedicine. 2001;8:283‐291. [DOI] [PubMed] [Google Scholar]
- 303. Singh B, Chandan BK, Sharma N, Singh S, Khajuria A, Gupta DK. Adaptogenic activity of glyco‐peptido‐lipid fraction from the alcoholic extract of Trichopus zeylanicus Gaerten (part II). Phytomedicine. 2005;12:468‐481. [DOI] [PubMed] [Google Scholar]
- 304. Pawar VS, Hugar S. A current status of adaptogens: natural remedy to stress. Asian Pacific J Tropical Disease. 2012;2:S480‐S490. [Google Scholar]
- 305. Pawar VS, Hugar S. Adaptogenic activity of Trigonella foenum graecum (L.) seeds in rodents exposed to anoxia and immobilization stress. Asian Pac J Trop Biomed. 2012;2:S208‐S211. [Google Scholar]
- 306. Pawar VS, Shivakumar H. Antistress activity of Trigonella foenum graecum L. seeds using swimming endurance and cold stress in rodents. Indian Drugs. 2011a;48:56‐61. [Google Scholar]
- 307. Bin‐Hafeez B, Haque R, Parvez S, Pandey S, Sayeed I, Raisuddin S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int Immunopharmacol. 2003;3:257‐265. [DOI] [PubMed] [Google Scholar]
- 308. Kulkarni PM, Archana RJ. Effect of roots of Tylophora indica (Burm. f.) on stress and anxiety in animal models. Int J Pharm. 2009;8:1‐5. [Google Scholar]
- 309. Della Valle V. Uncaria tomentosa. G Ital Dermatol Venereol. 2017;152:651‐657. [DOI] [PubMed] [Google Scholar]
- 310. Sreemantula S, Nammi S, Kolanukonda R, Koppula S, Boini KM. Adaptogenic and nootropic activities of aqueous extract of Vitis vinifera (grape seed): an experimental study in rat model. BMC Complement Altern Med. 2005;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Singh N, Nath R, Lata A, Singh SP, Kohli RP, Bhargava KP. Withania somnifera (Ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen). Pharm Biol. 1982;20:29‐35. [Google Scholar]
- 312. Archana R, Namasivayam A. Antistressor effect of Withania somnifera . J Ethnopharmacol. 1998;64:91‐93. [DOI] [PubMed] [Google Scholar]
- 313. Shireen T, Sahni YP. Anti‐stress activity of Withania somnifera (Ashwagandha) on solar radiation induced heat stress in goats. Indian J Small Ruminants. 2012;18:64‐68. [Google Scholar]
- 314. Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8:208‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Kulkarni SK, Dhir A. Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093‐1105. [DOI] [PubMed] [Google Scholar]
- 316. Raut A, Tadvi F, Kene K, et al. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integrative Med. 2012;3:111‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Kumar R, Gupta K, Saharia K, Pradhan D, Subramaniam JR. Withania somnifera root extract extends lifespan of Caenorhabditis elegans . Ann Neurosci. 2013;20:13‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Thakur AK, Dey A, Chatterjee SS, Kumar V. Reverse Ayurvedic pharmacology of Ashwagandha as an adaptogenic anti‐diabetic plant: a pilot study. Current Tradit Med. 2015;1:51‐61. [Google Scholar]
- 319. Lakshmi BVS, Sudhakar M. Attenuation of acute and chronic restraint stress‐induced perturbations in experimental animals by Zingiber officinale Roscoe. Food Chem Toxicol. 2010;48:530‐535. [DOI] [PubMed] [Google Scholar]
- 320. Lv C, Huang Y, Liu ZX, Yu D, Bai ZM. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 2016;17:41‐47. [DOI] [PubMed] [Google Scholar]
- 321. Li H, Chen C. Inhibition of autophagy enhances synergistic effects of Salidroside and anti‐tumor agents against colorectal cancer. BMC Complement Altern Med. 2017;17:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Lin X, Liu Y, Ma L, et al. Amelioration of experimental autoimmune encephalomyelitis by Rhodiola rosea, a natural adaptogen. Biomed Pharmacother. 2020;125:109960. [DOI] [PubMed] [Google Scholar]
- 323. Kang DY, Sp N, Kim DH, et al. Salidroside inhibits migration, invasion and angiogenesis of MDA‑MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int J Oncol. 2018;53:877‐885. [DOI] [PubMed] [Google Scholar]
- 324. Qi Z, Qi S, Ling L, Lv J, Feng Z. Salidroside attenuates inflammatory response via suppressing JAK2‐STAT3 pathway activation and preventing STAT3 transfer into nucleus. Int Immunopharmacol. 2016;35:265‐271. [DOI] [PubMed] [Google Scholar]
- 325. Qi Z, Tang T, Sheng L, et al. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src‑associated signaling pathway activation and heat shock protein 70 expression. Mol Med Rep. 2018;18:147‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Xu F, Xu J, Xiong X, Deng Y. Salidroside inhibits MAPK, NF‐kB, and STAT3 pathways in psoriasis‐associated oxidative stress via SIRT1 activation. Redox Rep. 2019;24:70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Song F, Zeng K, Liao L, Yu Q, Tu P, Wang X. Schizandrin A inhibits microglia‐mediated neuroninflammation through inhibiting TRAF6‐NF‐κB and Jak2‐Stat3 signaling pathways. PLOS One. 2016;11:e0149991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Dai X, Yin C, Guo G, et al. Schisandrin B exhibits potent anticancer activity in triple negative breast cancer by inhibiting STAT3. Toxicol Appl Pharmacol. 2018;358:110‐119. [DOI] [PubMed] [Google Scholar]
- 329. Hsu JH, Chang PM, Cheng TS, et al. Identification of Withaferin A as a potential candidate for anti‐cancer therapy in non‐small cell lung cancer Cancers (Basel). 11, 2019:1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Choi BY, Kim BW. Withaferin‐A inhibits colon cancer cell growth by blocking STAT3 transcriptional activity. J Cancer Prev. 2015;20:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Sinha P, Ostrand‐Rosenberg S. Myeloid‐derived suppressor cell function is reduced by Withaferin A, a potent and abundant component of Withania somnifera root extract. Cancer Immunol Immunother. 2013;62:1663‐1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427:24‐29. [DOI] [PubMed] [Google Scholar]
- 333. Munagala R, Kausar H, Munjal C, Gupta RC. Withaferin A induces p53‐dependent apoptosis by repression of HPV oncogenes and upregulation of tumor suppressor proteins in human cervical cancer cells. Carcinogenesis. 2011;32:1697‐1705. [DOI] [PubMed] [Google Scholar]
- 334. Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Li J, Liu T, Zhao L, et al. Ginsenoside 20(S)‑Rg3 inhibits the Warburg effect through STAT3 pathways in ovarian cancer cells. Int J Oncol. 2015;46:775‐781. [DOI] [PubMed] [Google Scholar]
- 336. Han S, Jeong AJ, Yang H, et al. Ginsenoside 20(S)‐Rh2 exerts anti‐cancer activity through targeting IL‐6‐induced JAK2/STAT3 pathway in human colorectal cancer cells. J Ethnopharmacol. 2016;194:83‐90. [DOI] [PubMed] [Google Scholar]
- 337. Park S, Lee HJ, Jeong SJ, et al. Inhibition of JAK1/STAT3 signaling mediates compound K‐induced apoptosis in human multiple myeloma U266 cells. Food Chem Toxicol. 2011;49:1367‐1372. [DOI] [PubMed] [Google Scholar]
- 338. Zhang X, Zhang S, Sun Q, Jiao W, Yan Y, Zhang X. Compound K induces endoplasmic reticulum stress and apoptosis in human liver cancer cells by regulating STAT3. Molecules. 2018;23:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Kim D, Park M, Haleem I, et al. Natural product ginsenoside 20(S)‐25‐methoxyl‐dammarane‐3β, 12β, 20‐triol in cancer treatment: a review of the pharmacological mechanisms and pharmacokinetics. Front Pharmacol. 2020;11:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Ai HH, Zhou ZL, Sun LG, et al. 20 (S)‐25‐methoxyl‐dammarane‐3b, 12b, 20‐triol negatively regulates activation of STAT3 and ERK pathways and exhibits anti‐cancer effects in HepG2 cells. Apoptosis. 2017;22:1404‐1418. [DOI] [PubMed] [Google Scholar]
- 341. Qin H, Li W, Sun Y, et al. 20(S)‐25‐methoxyldammarane‐ 3b, 12b, 20‐triol attenuates endoplasmic reticulum stress via ERK/MAPK signaling pathway. Eur J Pharmacol. 2018;836:75‐82. [DOI] [PubMed] [Google Scholar]
- 342. Qi LW, Wang CZ, Du GJ, Zhang ZY, Calway T, Yuan CS. Metabolism of ginseng and its interactions with drugs. Curr Drug Metab. 2011;12:818‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Yao H, Wan JY, Zeng J, et al. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol Lett. 2018;15:8339‐8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Chen L, Chen MY, Shao L, et al. Panax notoginseng saponins prevent colitis‐associated colorectal cancer development: the role of gut microbiota. Chin J Nat Med. 2020;18:500‐507. [DOI] [PubMed] [Google Scholar]
- 345.346 Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Zhao G, Shi A, Fan Z, Du Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol Rep. 2015;33:2553‐2560. [DOI] [PubMed] [Google Scholar]
- 347. Zhang Z, Yang W, Ma F, et al. Enhancing the chemotherapy effect of Apatinib on gastric cancer by co‐treating with salidroside to reprogram the tumor hypoxia micro‐environment and induce cell apoptosis. Drug Deliv. 2020;27:691‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Wang CZ, Calway T, Yuan CS. Herbal medicines as adjuvants for cancer therapeutics. Am J Chin Med. 2012;40:657‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Lee J, Lee E, Kim D, Lee J, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009;122:143‐148. [DOI] [PubMed] [Google Scholar]
- 350. Panossian AG. Adaptogens: tonic herbs for fatigue and stress. Altern Complement Ther. 2003;9:327‐332. [Google Scholar]
- 351. Boon‐Niermeijer EK, van den Berg A, Vorontsova ON, Bayda LA, Malyshev IY, Wiegant FAC. Enhancement of adaptive resistance against a variety of chronic stress conditions by plant adaptogens: protective effects on survival and embryonic development of Lymnaea stagnalis . Adapt Med. 2012;4:233‐244. [Google Scholar]
- 352. Panossian A, Wagner H. Adaptogens. A review of their history, biological activity, and clinical benefits. HerbalGram. 2011;90:52‐63. [Google Scholar]
- 353. Aslanyan G, Amroyan E, Gabrielyan E, Nylander M, Wikman G, Panossian A. Double‐blind, placebo‐controlled, randomized study of single dose effects of ADAPT‐232 on cognitive functions. Phytomedicine. 2010;17:494‐499. [DOI] [PubMed] [Google Scholar]
- 354. Narimanian M, Badalyan M, Panosyan V, et al. Impact of ChisanR (ADAPT‐232) on the quality ‐ of ‐ life and its efficacy as an adjuvant in the treatment of acute non‐specific pneumonia. Phytomedicine. 2005;12:723‐729. [DOI] [PubMed] [Google Scholar]
- 355. Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmström C, Panossian A. Clinical trial of Rhodiola rosea L. extract SHR‐5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61:343‐348. Erratum in: Nord J Psychiatry. 61, 503. [DOI] [PubMed] [Google Scholar]
- 356. Darbinyan V, Kteyan A, Panossian A, Gabrielian E, Wikman G, Wagner H. Rhodiola rosea in stress induced fatigue—a double blind cross‐over study of a standardized extract SHR‐5 with a repeated low‐dose regimen on the mental performance of healthy physicians during night duty. Phytomedicine. 2000;7:365‐371. [DOI] [PubMed] [Google Scholar]
- 357. Olsson EM, von Schéele B, Panossian AG. A randomized double‐blind placebo controlled parallel group study of SHR‐5 extract of Rhodiola rosea roots as treatment for patients with stress related fatigue. Planta Med. 2009;75:105‐112. [DOI] [PubMed] [Google Scholar]
- 358. Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21:841‐860. [DOI] [PubMed] [Google Scholar]
- 359. Facchinetti F, Neri I, Tarbusi M. Eleutherococcus senticocus reduces cardiovascular stress response in healthy subjects: randomized, placebo‐controlled trial. Stress Health. 2002;18:11‐17. [Google Scholar]
- 360. Mao JJ, Xie SX, Zee J, et al. Rhodiola rosea versus sertraline for major depressive disorder: a randomized placebo‐controlled trial. Phytomedicine. 2015;22:394‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Bertoglio JC, Baumgartner M, Palma R, et al. Andrographis paniculata decreases fatigue in patients with relapsing‐remitting multiple sclerosis: a 12‐month double‐blind placebo‐controlled pilot study. BMC Neurol. 2016;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Lopresti AL, Smith SJ, Malvi H, Kodgule R. An investigation into the stress‐relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: a randomized, double‐blind, placebo‐controlled study. Medicine. 2019;98:e17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Gannon JM, Brar J, Rai A, Chengappa KNR. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo‐controlled clinical trial. Ann Clin Psychiatry. 2019;31:123‐129. [PubMed] [Google Scholar]
- 364. Baek JH, Heo JY, Fava M, et al. Effect of Korean Red Ginseng in individuals exposed to high stress levels: a 6‐week, double‐blind, randomized, placebo‐controlled trial. J Ginseng Res. 2019;43:402‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Cave AE, Chang DH, Münch GW, Steiner GZ. Efficacy of Cognition SupportFormula® on cognitive function in older adults with subjective cognitive impairment: a protocol for a 26‐week, randomised, double‐blind, placebo‐controlled trial. Trials. 2019;20:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Jeong HG, Ko YH, Oh SY, Han C, Kim T, Joe SH. Effect of Korean Red Ginseng as an adjuvant treatment for women with residual symptoms of major depression. Asia Pac Psychiatry. 2015;7:330‐336. [DOI] [PubMed] [Google Scholar]
- 367. Hartz AJ, Bentler S, Noyes R, et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. 2004;34:51‐61. [DOI] [PubMed] [Google Scholar]
- 368. Panossian A, Gerbarg P. Potential use of plant adaptogens in age‐related disorders In: Lavretsky H, Sajatovic M, Reynolds CF, III, eds. Complementary, Alternative, and Integrative Interventions in Mental Health and Aging. New York: Oxford University Press; 2016:197‐211. [Google Scholar]
- 369. Panossian A, Wikman G. Evidence based efficacy and effectiveness of Rhodiola SHR‐5 extract in treating stress‐ and age‐associated disorders In: Cuerrier A, Ampong‐Nyarko K, eds. Rhodiola rosea, Series: Traditional Herbal Medicines for Modern Times. Boca‐Raton London New‐York: CRC Press, Taylor & Francis Group; 2014:203‐221. [Google Scholar]
- 370. Gospodaryov DV, Yurkevych IS, Jafari M, Lushchak VI, Lushchak OV. Lifespan extension and delay of age‐related functional decline caused by Rhodiola rosea depends on dietary macronutrient balance. Longev Health Span. 2013;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Jafari M, Felgner JS, Bussel II, et al. Rhodiola: a promising anti‐aging Chinese herb. Rejuvenation Res. 2007;10:587‐602. [DOI] [PubMed] [Google Scholar]
- 372. Lee NH, Son CG. Systematic review of randomized controlled trials evaluating the efficacy and safety of ginseng. J Acupunct Meridian Stud. 2011;4:85‐97. [DOI] [PubMed] [Google Scholar]
- 373. Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55:567‐575. [DOI] [PubMed] [Google Scholar]
- 374. Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323‐344. [DOI] [PubMed] [Google Scholar]
- 375. Li W, Liu M, Feng S, et al. Acanthopanax for acute ischaemic stroke. Cochrane Database Syst Rev. 2009;3:CD007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Man C, Dai Z, Fan Y. Dazhu Hongjingtian preparation as adjuvant therapy for unstable Angina Pectoris: a meta‐analysis of randomized controlled trials. Front Pharmacol. 2020;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Ma GP, Zheng Q, Xu MB, et al. Rhodiola rosea L. improves learning and memory function: preclinical evidence and possible mechanisms. Front Pharmacol. 2018;9:1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Yu L, Qin Y, Wang Q, et al. The efficacy and safety of Chinese herbal medicine, Rhodiola formulation in treating ischemic heart disease: a systematic review and meta‐analysis of randomized controlled trials. Complement Ther Med. 2014;22:814‐825. [DOI] [PubMed] [Google Scholar]
- 379. Korczak D, Wastian M, Schneider M. Therapy of the burnout syndrome. GMS Health Technol Assess. 2012;8:Doc05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Ishaque S, Shamseer L, Bukutu C, Vohra S. Rhodiola rosea for physical and mental fatigue: a systematic review. BMC Complement Altern Med. 2012;12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Hung SK, Perry R, Ernst E. The effectiveness and efficacy of Rhodiola rosea L.: a systematic review of randomized clinical trials. Phytomedicine. 2011;18:235‐244. [DOI] [PubMed] [Google Scholar]
- 382. Sarris J. Herbal medicines in the treatment of psychiatric disorders: a systematic review. Phytother Res. 2007;21:703‐716. [DOI] [PubMed] [Google Scholar]
- 383. Pratte MA, Nanavati KB, Young V, Morley CP. An alternative treatment for anxiety: a systematic review of human trial results reported for the Ayurvedic herb ashwagandha (Withania somnifera). J Altern Complement Med. 2014;20:901‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Nasimi Doost Azgomi R, Zomorrodi A, Nazemyieh H, et al. Effects of Withania somnifera on reproductive system: a systematic review of the available evidence. BioMed Res Int. 2018;2018:4076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Ng QX, Loke W, Foo NX, et al. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother Res. 2020;34:583‐590. [DOI] [PubMed] [Google Scholar]
- 386. Durg S, Bavage S, Shivaram SB. Withania somnifera (Indian ginseng) in diabetes mellitus: a systematic review and meta‐analysis of scientific evidence from experimental research to clinical application. Phytother Res. 2020;34:1041‐1059. [DOI] [PubMed] [Google Scholar]
- 387. Ayati Z, Sarris J, Chang D, Emami SA, Rahimi R. Herbal medicines and phytochemicals for obsessive‐compulsive disorder. Phytother Res. 2020;2020(34):1889‐1901. [DOI] [PubMed] [Google Scholar]
- 388. Pérez‐Gómez J, Villafaina S, Adsuar JC, Merellano‐Navarro E, Collado‐Mateo D. Effects of Ashwagandha (Withania somnifera) on VO2max: a systematic review and meta‐analysis. Nutrients. 2020;12:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Ann Pharmacother. 2006;40:83‐95. [DOI] [PubMed] [Google Scholar]
- 390. An X, Zhang AL, Yang AW, et al. Oral ginseng formulae for stable chronic obstructive pulmonary disease: a systematic review. Respir Med. 2011;105:165‐176. [DOI] [PubMed] [Google Scholar]
- 391. Seida JK, Durec T, Kuhle S. North American (Panax quinquefolius) and Asian Ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. Evid Based Complement Alternat Med. 2011;2011:282151‐282157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Durg S, Shivaram SB, Bavage S. Withania somnifera (Indian ginseng) in male infertility: an evidence‐based systematic review and meta‐analysis. Phytomedicine. 2018;50:247‐256. [DOI] [PubMed] [Google Scholar]
- 394. Farnsworth NR, Kinghorn AD, Soejarto DD, Waller DP. Siberian Ginseng (Eleutherococcus senticosus): current status as an adaptogen In: Wagner H, Hikino H, Farnsworth NR, eds. Economic and Medicinal Plant Research. Vol 1 London: Academic Press; 1985:217‐284. [Google Scholar]
- 395. Wang Y, Jung YJ, Kim KH, et al. Antiviral activity of fermented ginseng extracts against a broad range of influenza viruses. Viruses. 2018;10:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Kim EH, Kim SW, Park SJ, et al. Greater efficacy of black ginseng (CJ EnerG) over red ginseng against lethal influenza A virus infection. Nutrients. 2019;11:1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Lee JS, Hwang HS, Ko EJ, et al. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients. 2014;6:517‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Lee JS, Ko EJ, Hwang HS, et al. Antiviral activity of ginseng extract against respiratory syncytial virus infection. Int J Mol Med. 2014;34:183‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Park EH, Yum J, Ku KB, et al. Red Ginseng‐containing diet helps to protect mice and ferrets from the lethal infection by highly pathogenic H5N1 influenza virus. J Ginseng Res. 2014;38:40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Yoo DG, Kim MC, Park MK, et al. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012;15:855‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Dong W, Farooqui A, Leon AJ, Kelvin DJ. Inhibition of influenza A virus infection by ginsenosides. PLOS One. 2017;12:e0171936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Yin SY, Kim HJ, Kim HJ. A comparative study of the effects of whole red ginseng extract and polysaccharide and saponin fractions on influenza A (H1N1) virus infection. Biol Pharm Bull. 2013;36(6):1002‐1007. [DOI] [PubMed] [Google Scholar]
- 403. Iqbal H, Rhee DK. Ginseng alleviates microbial infections of the respiratory tract: a review. J Ginseng Res. 2020;44(2):194‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Scaglione F, Cattaneo G, Alessandria M, Cogo R. Efficacy and safety of the standardised ginseng extract G115® for potentiating vaccination against the influenza syndrome and protection against the common cold. Drugs Exp Clin Res. 1996;22:65‐72. [PubMed] [Google Scholar]
- 405. Lee CS, Lee JH, Oh M, et al. Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double‐blind clinical trial. J Korean Med Sci. 2012;27:1472‐1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Hancke J, Burgos R, Caceres D, Wikman G. A double‐blind study with a new monodrug Kan Jang: decrease of symptoms and improvement in the recovery from common colds. Phytother Res. 1995;9:559‐562. [Google Scholar]
- 407. Caceres DD, Hancke JL, Burgos RA, Wikman GK. Prevention of common colds with Andrographis paniculata dried extract: a pilot double blind trial. Phytomedicine. 1997;4(4):101‐104. [DOI] [PubMed] [Google Scholar]
- 408. Caceres DD, Hancke JL, Burgos RA, Sandberg F, Wikman GK. Use of visual analogue scale measurement (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA‐10 in reducing the symptoms of common cold: a randomized double blind placebo study. Phytomedicine. 1999;6:217‐223. [DOI] [PubMed] [Google Scholar]
- 409. Melchior J, Palm S, Wikman G. Controlled clinical study of standardized Andrographis paniculata extract in common cold‐a pilot trial. Phytomedicine. 1996;34:315‐318. [DOI] [PubMed] [Google Scholar]
- 410. Saxena RC, Singh R, Kumar P, et al. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine. 2010;17:178‐185. [DOI] [PubMed] [Google Scholar]
- 411. Hu XY, Wu RH, Logue M, et al. Andrographis paniculata (Chuān Xīn Lián) for symptomatic relief of acute respiratory tract infections in adults and children: a systematic review and meta‐analysis. PLOS One. 2017;12:e0181780.407‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Kupin VI, Polevaya ES, Sorokin AM. Increased immunologic reactivity of lymphocytes in oncologic patients treated with Eleutherococcus extract. In: New Data on Eleutherococcus: Proceedings of the 2nd International Symposium on Eleutherococcus, Moscow 1984, Vladivostok; 1986:216‐220.
- 413. Zykov MP, Protasova SF. Prospects of immunostimulating vaccination against influenza including the use of Eleutherococcus and other preparations of plant origin. In: Proceedings of New Data on Eleutherococcus, Moscow: 118‐122.
- 414. Wacker, A , Eichler, A , Lodemann, E The molecular mechanism of virus inhibition by Eleutherococcus. In: New Data on Eleutherococcus: Proceedings of the 2nd International Symposium on Eleutherococcusow 1984, Vladivostok, 1986:13‐15.
- 415. Wacker A, Eilmes HG. Virushemmung mit Eleutherokokk Fluid‐Extrakt. Erfahrungsheilkunde. 1978;27:346‐351. [Google Scholar]
- 416. Wacker A. Über die Interferon induzierende und immunstimulierende Wirkung von Eleutherococcus. Erfahrungsheilkunde. 1983;32:339‐343. [Google Scholar]
- 417. Schmolz NW, Sacher F, Aicher B. The synthesis of Rantes G‐CSF, IL‐4, IL‐5, IL‐6, IL‐12 and IL‐13 in human whole‐blood cultures is modulated by an extract from Eleutherococcus senticosus roots. Phytother Res. 2001;15:268‐270. [DOI] [PubMed] [Google Scholar]
- 418. Bohn B, Nebe CT, Birr C. Immunopharmacological effects of Eleutherococcus senticosus extract as determined by quantitative flow cytometry. Int J Immunopharmacol. 1988;10:67.3366511 [Google Scholar]
- 419. Bohn B, Nebe CT, Birr С. Flow‐cytometric studies with Eleutherococcus senticosus extract as an immunomodulatory agent. Arzneimittel‐Forsch/Drug Res. 1987;37:1193‐1196. [PubMed] [Google Scholar]
- 420. ESCOP Monographs, 2nd ed. Supplement 2009. Eleutherococci radix—Eleutheroccus. European Scientific Cooperative on Phytotherapy, editor. Thieme, Stuttgart; 2009; 110‐120.
- 421. Gabrielyan ES, Shukuryan AK, Goukasova GI, et al. A double blind, placebo‐controlled study of Andrographis paniculata fixed combination Kan Jang in the treatment of acute upper respiratory tract infections including sinusitis. Phytomedicine. 2002;9:589‐597. [DOI] [PubMed] [Google Scholar]
- 422. Kulichenko LL, Kireyeva LV, Malyshkina EN, Wikman G. A randomized, controlled study of Kan Jang versus amantadine in the treatment of influenza in Volgograd. J Herb Pharmacother. 2003;3(77):93. [PubMed] [Google Scholar]
- 423. Melchior J, Spasov AA, Ostrovskij OV, Bulanov AE, Wikman G. Double‐blind, placebo‐controlled pilot and phase III study of activity of standardized Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper‐ respiratory tract infection. Phytomedicine. 2000;7:341‐350. [DOI] [PubMed] [Google Scholar]
- 424. Spasov AA, Ostrovskiy OV, Chernikov MV, Wikman G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang(r) and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytother Res. 2004;18:47‐53. [DOI] [PubMed] [Google Scholar]
- 425. Panossian A, Wikman G. Efficacy of Andrographis paniculata in upper respiratory tract (URT) infectious diseases and the mechanism of action In: Wagner H, Ulrich Merzenich G, eds. Evidence and Rational Based Research on Chinese Drugs. Wien Heidelberg New‐York Dordrecht London: Springer Publ. Comp; 2012:137‐180. [Google Scholar]
- 426. Ding Y, Chen L, Wu W, Yang J, Yang Z, Liu S. Andrographolide inhibits influenza A virus‐induced inflammation in a murine model through NF‐κB and JAK‐STAT signaling pathway. Microbes Infect. 2017;19(605):615. [DOI] [PubMed] [Google Scholar]
- 427. Yu B, Dai CQ, Jiang ZY, et al. Andrographolide as an anti‐H1N1 drug and the mechanism related to retinoic acid‐inducible gene‐I‐like receptors signaling pathway. Chin J Integr Med. 2014;20:540‐545. [DOI] [PubMed] [Google Scholar]
- 428. Ko HC, Wei BL, Chiou WF. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus‐infected human epithelial cells. J Ethnopharmacol. 2006;2006(107):205‐210. [DOI] [PubMed] [Google Scholar]
- 429. Enmozhi SK, Raja K, Sebastine I, Joseph J. Andrographolide as a potential inhibitor of SARS‐CoV‐2 main protease: an in silico approach. J Biomol Struct Dyn. 2020;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Yan W, Chen J, Wei Z, et al. Effect of eleutheroside B1 on non‑coding RNAs and protein profiles of influenza A virus‑infected A549 cells. Int J Mol Med. 2020;45:753‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Yan W, Zheng C, He J, et al. Eleutheroside B1 mediates its anti‐influenza activity through POLR2A and N‐glycosylation. Int J Mol Med. 2018;42:2776‐2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Glatthaar‐Saalmüller B, Sacher F, Esperester A. Antiviral activity of an extract derived from roots of Eleutherococcus senticosus . Antiviral Res. 2001;50:223‐228. [DOI] [PubMed] [Google Scholar]
- 433. Jin L, Schmiech M, El Gaafary M, Zhang X, Syrovets T, Simmet T. A comparative study on root and bark extracts of Eleutherococcus senticosus and their effects on human macrophages. Phytomedicine. 2020;68:153181. [DOI] [PubMed] [Google Scholar]
- 434. Kim AY, Shim HJ, Shin HM, et al. Andrographolide suppresses TRIF‐dependent signaling of toll‐like receptors by targeting TBK1. Int Immunopharmacol. 2018;57:172‐180. [DOI] [PubMed] [Google Scholar]
- 435. Xiong WB, Shao ZJ, Xiong Y, et al. Dehydroandrographolide enhances innate immunity of intestinal tract through up‐regulation the expression of hBD‐2. DARU. 2015;23:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436. Gao H, Wang J. Andrographolide inhibits multiple myeloma cells by inhibiting the TLR4/NF‐κB signaling pathway. Mol Med Rep. 2016;13:1827‐1832. [DOI] [PubMed] [Google Scholar]
- 437. Chao WW, Kuo YH, Hsieh SL, Lin BF. Inhibitory effects of ethyl acetate extract of Andrographis paniculata on NF‐κB trans‐activation activity and LPS‐induced acute inflammation in mice. Evid Based Complement Alternat Med. 2011;2011:254531‐254539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Shao ZJ, Zheng XW, Feng T, et al. Andrographolide exerted its antimicrobial effects by upregulation of human β‐defensin‐2 induced through p38 MAPK and NF‐κB pathway in human lung epithelial cells. Can J Physiol Pharmacol. 2012;90(647):653. [DOI] [PubMed] [Google Scholar]
- 439. Burgos RA, Hidalgo MA, Monsalve J, LaBranche TP, Eyre P, Hancke JL. 14‐deoxyandrographolide as a platelet activating factor antagonist in bovine neutrophils. Planta Med. 2005;71:604‐608. [DOI] [PubMed] [Google Scholar]
- 440. Amroyan E, Gabrielian E, Panossian A, Wikman G, Wagner H. Inhibitory effect of andrographolide from Andrographis paniculata on PAF‐induced platelet aggregation. Phytomedicine. 1999;6:27‐31. [DOI] [PubMed] [Google Scholar]
- 441. Dai Y, Chen S, Chai L, Zhao J, Wang Y, Wang Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit Rev Food Sci Nutr. 2019;59(sup1):S17eS29. [DOI] [PubMed] [Google Scholar]
- 442.EFSA Consolidated List of Article 13 Health Claims of the European Food Safety Authority (EFSA). Legal and regulatory framework for herbal medicines. Association of the European Self‐Medication Industry (AESMI). Brussels, April 2010. P.151‐158.
- 443. Wankhede S, Langade D, Joshi K, Sinha SR, Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Baliga MS, Meera S, Shivashankara AR, Palatty PL, Haniadka R. The health benefits of indian traditional Ayurvedic Rasayana (anti‐aging) drugs: a review In: Watson RR. Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults. San Diego: Academic Press; 2015:151‐161. [Google Scholar]
- 445. Ruhsam M, Hollingsworth PM. Authentication of Eleutherococcus and Rhodiola herbal supplement products in the United Kingdom. J Pharm Biomed Anal. 2018;149:403‐409. [DOI] [PubMed] [Google Scholar]
- 446. Gulati K, Anand R, Ray A. Nutraceuticals as adaptogens: their role in health and disease In: Gupta RC. Nutraceuticals. Boston, MA: Academic Press; 2016:193‐205. [Google Scholar]
- 447. Shikov AN, Pozharitskaya ON, Makarov VG, Wagner H, Verpoorte R, Heinrich M. Medicinal plants of the Russian Pharmacopoeia; their history and applications. J Ethnopharmacol. 2014;154:481‐536. [DOI] [PubMed] [Google Scholar]
- 448.Estonian Ministry of Health Affairs, 1998. Regulation No. 7, Annex 1, 21st January, Government of Estonia, Tallinn.
- 449.National Pharmacopoeia Committee. Pharmacopoeia of the People's Republic of China, English ed.; National Pharmacopoeia Committee, Beijing; 2010.
- 450. Mehendale S, Aung H, Wang A, et al. American ginseng berry extract and ginsenoside Re attenuate cisplatin‐induced kaolin intake in rats. Cancer Chemother Pharmacol. 2005;56:63‐69. [DOI] [PubMed] [Google Scholar]
- 451. Panossian A, Seo EJ, Efferth T. Synergy assessments of plant extracts used in the treatment of stress and aging‐related disorders. Synergy Research. 2018;7:39‐49. [Google Scholar]
- 452. Panossian A, Seo EJ, Efferth T. Effects of anti‐inflammatory and adaptogenic herbal extracts on gene expression of eicosanoids signalling pathways in isolated brain cells. Phytomedicine. 2019;60:152881. [DOI] [PubMed] [Google Scholar]
- 453. Pearce PT, Zois I, Wynne KN, Funder JW. Panax ginseng and Eleuthrococcus senticosus extracts – in vitro studies on binding to steroid receptors. Endocrinol Jpn. 1982;29:567‐573. [DOI] [PubMed] [Google Scholar]
- 454. Huo YS, Chen YZ, Yu ZY, Zhang PY. The effect of Panax ginseng extract (GS) on insulin and corticosteroid receptors. J Tradit Chin Med. 1988;8:293‐295. [PubMed] [Google Scholar]
- 455. Lee YJ, Chung E, Lee KY, Lee YH, Huh B, Lee SK. Ginsenoside‐Rg1, one of the major active molecules from Panax ginseng, is a functional ligand of glucocorticoid receptor. Mol Cell Endocrinol. 1997;133:135‐140. [DOI] [PubMed] [Google Scholar]
- 456. Lee YJ, Cho JY, Kim JH, Park WK, Kim DK, Rhyu MR. Extracts from Schizandra chinensis fruit activate estrogen receptors: a possible clue to its effects on nitric oxide‐mediated vasorelaxation. Biol Pharm Bull. 2004;27:1066‐1069. [DOI] [PubMed] [Google Scholar]
- 457. Lee YJ, Jin YR, Lim WC, et al. Ginsenoside‐Rb1 acts as a weak phytoestrogen in MCF‐7 human breast cancer cells. Arch Pharm Res. 2003;26:58‐63. [DOI] [PubMed] [Google Scholar]
- 458. Chung E, Lee KY, Lee YJ, Lee YH, Lee SK. Ginsenoside Rg1 down‐regulates glucocorticoid receptor and displays synergistic effects with cAMP. Steroids. 1998;63:421‐424. [DOI] [PubMed] [Google Scholar]
- 459. Yan J, Liu Q, Dou Y, et al. Activating glucocorticoid receptor‐ERK signaling pathway contributes to ginsenoside Rg1 protection against β‐amyloid peptide‐induced human endothelial cells apoptosis. J Ethnopharmacol. 2013;147:456‐466. [DOI] [PubMed] [Google Scholar]
- 460. Song Y, Zhao F, Zhang L, Du Y, Wang T, Fu F. Ginsenoside Rg1 exerts synergistic anti‐inflammatory effects with low doses of glucocorticoids in vitro. Fitoterapia. 2013;91:173‐179. [DOI] [PubMed] [Google Scholar]
- 461. Gao Y, Chu S, Li J, et al. Anti‐inflammatory function of ginsenoside Rg1 on alcoholic hepatitis through glucocorticoid receptor related nuclear factor‐kappa B pathway. J Ethnopharmacol. 2015;173:231‐240. [DOI] [PubMed] [Google Scholar]
- 462. Sun XC, Ren XF, Chen L, Gao XQ, Xie JX, Chen WF. Glucocorticoid receptor is involved in the neuroprotective effect of ginsenoside Rg1 against inflammation‐induced dopaminergic neuronal degeneration in substantia nigra. J Steroid Biochem Mol Biol. 2016;155(Pt A):94‐103. [DOI] [PubMed] [Google Scholar]
- 463. Nah SY. Gintonin: a novel ginseng‐derived ligand that targets G protein‐coupled lysophosphatidic acid receptors. Curr Drug Targets. 2012;13:1659‐1664. [DOI] [PubMed] [Google Scholar]
- 464. Cao X, Jiang J, Zhang S, et al. Discovery of natural estrogen receptor modulators with structure‐based virtual screening. Bioorg Med Chem Lett. 2013;23:3329‐3333. [DOI] [PubMed] [Google Scholar]
- 465. Kim MH, Choi YY, Han JM, et al. Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)‐α/‐β. Food Funct. 2014;5:1594‐1601. [DOI] [PubMed] [Google Scholar]
- 466. Gerbarg PL, Brown RP. Pause menopause with Rhodiola rosea, a natural selective estrogen receptor modulator. Phytomedicine. 2016;23:763‐769. [DOI] [PubMed] [Google Scholar]
- 467. Bassa LM, Jacobs C, Gregory K, Henchey E, Ser‐Dolansky J, Schneider SS. Rhodiola crenulata induces an early estrogenic response and reduces proliferation and tumorsphere formation over time in MCF7 breast cancer cells. Phytomedicine. 2016;23:87‐94. [DOI] [PubMed] [Google Scholar]
- 468. Hahm ER, Lee J, Huang Y, Singh SV. Withaferin a suppresses estrogen receptor‐α expression in human breast cancer cells. Mol Carcinog. 2011;50:614‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469. Ravindran R, Sharma N, Roy S, et al. Interaction studies of Withania somnifera's key metabolite withaferin a with different receptors associated with cardiovascular disease. Curr Comput Aided Drug Des. 2015;11:212‐221. [DOI] [PubMed] [Google Scholar]
- 470. Khazal KF, Hill DL, Grubbs CJ. Effect of Withania somnifera root extract on spontaneous estrogen receptor‐negative mammary cancer in MMTV/Neu mice. Anticancer Res. 2014;34:6327‐6332. [PMC free article] [PubMed] [Google Scholar]
- 471. Lau WS, Chan RY, Guo DA, Wong MS. Ginsenoside Rg1 exerts estrogen‐like activities via ligand‐independent activation of ERalpha pathway. J Steroid Biochem Mol Biol. 2008;108:64‐71. [DOI] [PubMed] [Google Scholar]
- 472. Chan RY, Chen WF, Dong A, Guo D, Wong MS. Estrogen‐like activity of ginsenoside Rg1 derived from Panax notoginseng . J Clin Endocrinol Metab. 2002;87:3691‐3695. [DOI] [PubMed] [Google Scholar]
- 473. Cho J, Park W, Lee S, Ahn W, Lee Y. Ginsenoside‐Rb1 from Panax ginseng C.A. Meyer activates estrogen receptor‐alpha and ‐beta, independent of ligand binding. J Clin Endocrinol Metab. 2004;89:3510‐3515. [DOI] [PubMed] [Google Scholar]
- 474. Brekhman II. On antitoxic action of Eleutherococcus. Moscow: Meditsina; 1982:37. [Google Scholar]
- 475. Lin CC, Huang PC. Antioxidant and hepatoprotective effects of Acanthopanax senticosus . Phytother Res. 2000;14:489‐494. [DOI] [PubMed] [Google Scholar]
- 476. Monakhov BV. Influence of the liquid extract from the roots of Eleutherococcus senticosus on the toxicity and antitumor activity of cyclophosphan [in Russian]. Vopr Onkol. 1965;11:60‐63. [PubMed] [Google Scholar]
- 477. Sakharova TA, Revazova YA, Barenboim GM. The effect of Eleutherococcus extract on the induction of recessive lethal mutations by cyclophosphane and N‐nitrosomorpholine in Drosophila. Khim Farm Zh. 1985;19:539‐540. [Google Scholar]
- 478. Smalinskiene A, Lesauskaite V, Zitkevicius V, et al. Estimation of the combined effect of Eleutherococcus senticosus extract and cadmium on liver cells. Ann N Y Acad Sci. 2009;1171:314‐320. [DOI] [PubMed] [Google Scholar]
- 479. Park SH, Lee SG, Kang SK, Chung SH. Acanthopanax senticosus reverses fatty liver disease and hyperglycemia in ob/ob mice. Arch Pharm Res. 2006;29:768‐776. [DOI] [PubMed] [Google Scholar]
- 480. Maslov LN, Guzarova NV. Cardioprotective and antiarrhythmic properties of preparations from Leuzea carthamoides, Aralia mandshurica, and Eleutherococcus senticosus . Eksp Klin Farmakol. 2007;70:48‐54. [PubMed] [Google Scholar]
- 481. Maslov LN, Lishmanov YB, Arbuzov AG, et al. Antiarrhythmic activity of phytoadaptogens in short‐term ischemia‐reperfusion of the heart and postinfarction cardiosclerosis. Bull Exp Biol Med. 2009;147:331‐334. [DOI] [PubMed] [Google Scholar]
- 482. Monakhov BV. Reduction of the toxic effect of various antiblastic preparations by means of Eleutherococcus extract. Vopr Onkol. 1967;13:71‐76. [PubMed] [Google Scholar]
- 483. Monakhov BV. Extract of Eleutherococcus senticosus maxim and the therapeutic activity of cyclophosphane, ethymidine, and benzo‐TEPA. Vopr Onkol. 1967;13:94‐97. [PubMed] [Google Scholar]
- 484. Stukov AN. Combined action of Eleutherococcus senticosus and sarcolysin on lymphosarcoma LIO‐I in mice. Vopr Oncol. 1966;12:57‐60. [Google Scholar]
- 485. Goldberg ED, Shubina TS, Shternberg IB. Protective role of Eleutherococcus during the administration of rubomycia under experimental conditions. Antibiotiks. 1971;16:113‐114. [PubMed] [Google Scholar]
- 486. Sheeja K, Kuttan G. Ameliorating effects of Andrographis paniculata extract against cyclophosphamide‐induced toxicity in mice. Asian Pac J Cancer Prev. 2006;7:609‐614. [PubMed] [Google Scholar]
- 487. Singh P, Srivastava MM, Khemani LD. Renoprotective effects of Andrographis paniculata (Burm. f.) Nees in rats. Ups J Med Sci. 2009;114:136‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Gupta S, Mishra KP, Singh SB, Ganju L. Inhibitory effects of andrographolide on activated macrophages and adjuvant‐induced arthritis. Inflammopharmacology. 2087;26:447‐456. [DOI] [PubMed] [Google Scholar]
- 489. Islam MT. Andrographolide, a new hope in the prevention and treatment of metabolic syndrome. Front Pharmacol. 2017;8:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490. Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. Experimental and clinical pharmacology of andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med. 2013;2013:846740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Tan WSD, Liao W, Zhou S, Wong WSF. Is there a future for andrographolide to be an anti‐inflammatory drug? Deciphering its major mechanisms of action. Biochem Pharmacol. 2017;139:71‐81. [DOI] [PubMed] [Google Scholar]
- 492. Yang SL, Kuo FH, Chen PN, et al. Andrographolide suppresses the migratory ability of human glioblastoma multiforme cells by targeting ERK1/2‐mediated matrix metalloproteinase‐2 expression. Oncotarget. 2017;8:105860‐105872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Chan SJ, Wong WS, Wong PT, Bian JS. Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol. 2010;16:668‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494. Das S, Gautam N, Dey SK, Maiti T, Roy S. Oxidative stress in the brain of nicotine‐induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl Physiol Nutr Metab. 2009;34:124‐135. [DOI] [PubMed] [Google Scholar]
- 495. Rivera DS, Lindsay C, Codocedo JF, et al. Andrographolide recovers cognitive impairment in a natural model of Alzheimer's disease (Octodon degus). Neurobiol Aging. 2016;46:204‐220. [DOI] [PubMed] [Google Scholar]
- 496. Xu JD, Xing WM, Yuan TJ, Chen J, Lu H. Metabolic changes in the urine of andrographolide sodium bisulfite‐treated rats. Hum Exp Toxicol. 2016;35:162‐169. [DOI] [PubMed] [Google Scholar]
- 497. Seo EJ, Klauck SM, Efferth T, Panossian A. Adaptogens in chemobrain (Part I): Plant extracts attenuate cancer chemotherapy‐induced cognitive impairment—transcriptome‐wide microarray profiles of neuroglia cells. Phytomedicine. 2019a;55:80‐91. [DOI] [PubMed] [Google Scholar]
- 498. Seo EJ, Klauck SM, Efferth T, Panossian A. Adaptogens in chemobrain (Part II): Effect of plant extracts on chemotherapy‐induced cytotoxicity in neuroglia cells. Phytomedicine. 2019b;58:152743. [DOI] [PubMed] [Google Scholar]
- 499. Seo EJ, Klauck SM, Efferth T, Panossian A. Adaptogens in chemobrain (Part III): Antitoxic effects of plant extracts towards cancer chemotherapy‐induced toxicity ‐ transcriptome‐wide microarray analysis of neuroglia cells. Phytomedicine. 2019c;56:246‐260. [DOI] [PubMed] [Google Scholar]
- 500. Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234‐246. [DOI] [PubMed] [Google Scholar]
- 501. Reuland DJ, McCord JM, Hamilton KL. The role of Nrf2 in the attenuation of cardiovascular disease. Exerc Sport Sci Rev. 2013;41:162‐168. [DOI] [PubMed] [Google Scholar]
- 502. Li M, Fukagawa NK. Age‐related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Brunet A, Sweeney LB, Sturgill JF, et al. Stress‐dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011‐2015. [DOI] [PubMed] [Google Scholar]
- 504. Van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440‐450. [DOI] [PubMed] [Google Scholar]
- 505. Mattson MP, Meffert MK. Roles for NF‐kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852‐860. [DOI] [PubMed] [Google Scholar]
- 506. Camandola S, Mattson MP. NF‐kappa B as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11:123‐132. [DOI] [PubMed] [Google Scholar]
- 507. Heydari AR, Takahashi R, Gutsmann A, You S, Richardson A. Hsp70 and aging. Experientia. 1994;50:1092‐1098. [DOI] [PubMed] [Google Scholar]
- 508. Heydari AR, You S, Takahashi R, Gutsmann‐Conrad A, Sarge KD, Richardson A. Age‐related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res. 2000;256:83‐93. [DOI] [PubMed] [Google Scholar]
- 509. Bhat GJ, Samikkannu T, Thomas JJ, Thekkumkara TJ. Alpha‐thrombin rapidly induces tyrosine phosphorylation of a novel, 74‐78‐kDa stress response protein(s) in lung fibroblast cells. J Biol Chem. 2004;279:48915‐48922. [DOI] [PubMed] [Google Scholar]
- 510. Hands S, Sinadinos C, Wyttenbach A. Polyglutamine gene function and dysfunction in the ageing brain. Biochim Biophys Acta. 2008;1779:507‐521. [DOI] [PubMed] [Google Scholar]
- 511. Winklhofer KF, Tatzelt J, Haass C. The two faces of protein misfolding: gain‐ and loss‐of‐function in neurodegenerative diseases. EMBO J. 2008;27:336‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Gagliano N, Grizzi F, Annoni G. Mechanisms of aging and liver functions. Dig Dis. 2007;25:118‐123. [DOI] [PubMed] [Google Scholar]
- 513. Lindquist S, Craig EA. The heat‐shock proteins. Annu Rev Genet. 1988;22:631‐677. [DOI] [PubMed] [Google Scholar]
- 514. Singh R, Kølvraa S, Bross P, et al. Heat‐shock protein 70 genes and human longevity: a view from Denmark. An. N Y Acad Sci. 2006;1067:301‐308. [DOI] [PubMed] [Google Scholar]
- 515. Ambra R, Mocchegiani E, Giacconi R, et al. Characterization of the hsp70 response in lymphoblasts from aged and centenarian subjects and differential effects of in vitro zinc supplementation. Exp Gerontol. 2004;39:1475‐1484. [DOI] [PubMed] [Google Scholar]
- 516. Minois N, Khazaeli AA, Curtsinger JW. Locomotor activity as a function of age and life span in Drosophila melanogaster overexpressing hsp70. Exp Gerontol. 2001;36:1137‐1153. [DOI] [PubMed] [Google Scholar]
- 517. Perez FP, Moinuddin SS, ul ain Shamim Q, Joseph DJ, Morisaki J, Zhou X. Longevity pathways: HSF1 and FoxO pathways, a new therapeutic target to prevent age‐related diseases. Curr Aging Sci. 2012;5:87‐95. [DOI] [PubMed] [Google Scholar]
- 518. Chiu PY, Ko KM. Schisandrin B protects myocardial ischemia‐reperfusion injury partly by inducing Hsp25 and Hsp70 expression in rats. Mol Cell Biochem. 2004;266:139‐144. [DOI] [PubMed] [Google Scholar]
- 519. Hernández‐Santana A, Pérez‐López V, Zubeldia JM, Jiménez‐del‐Rio MA. Rhodiola rosea root extract protects skeletal muscle cells against chemically induced oxidative stress by modulating heat shock protein 70 (HSP70) expression. Phytother Res. 2014;28:623‐628. [DOI] [PubMed] [Google Scholar]
- 520. Li L, Zhang T, Zhou L, et al. Schisandrin B attenuates acetaminophen‐induced hepatic injury through heat‐shock protein 27 and 70 in mice. J Gastroenterol Hepatol. 2014;29:640‐647. [DOI] [PubMed] [Google Scholar]
- 521. Prodius PA, Manukhina EB, Bulanov AE, Vikman G, Malyshev II. Adaptogen ADAPT modulates synthesis of inducible stress protein HSP 70 and increases organism resistance to heat shock. Bull Eksp Biol Med. 1997;123:629‐631. [PubMed] [Google Scholar]
- 522. Schriner SE, Abrahamyan A, Avanessian A, et al. Decreased mitochondrial superoxide levels and enhanced protection against paraquat in Drosophila melanogaster supplemented with Rhodiola rosea . Free Radic Res. 2009;43:836‐843. [DOI] [PubMed] [Google Scholar]
- 523. Schriner SE, Lee K, Truong S, et al. Extension of Drosophila lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLOS One. 2013;8:e63886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Lee JH, Choi SH, Kwon OS, et al. Effects of ginsenosides, active ingredients of Panax ginseng, on development, growth, and life span of Caenorhabditis elegans . Biol Pharm Bull. 2007;30:2126‐2134. [DOI] [PubMed] [Google Scholar]
- 525. Bayliak MM, Lushchak VI. The golden root, Rhodiola rosea, prolongs lifespan but decreases oxidative stress resistance in yeast Saccharomyces cerevisiae . Phytomedicine. 2011;18:1262‐1268. [DOI] [PubMed] [Google Scholar]
- 526. Karpova A, Sanna PP, Behnisch T. Involvement of multiple phosphatidylinositol 3‐kinase‐dependent pathways in the persistence of late‐phase long term potentiation expression. Neuroscience. 2006;137:833‐841. [DOI] [PubMed] [Google Scholar]
- 527. Yang PC, Yang CH, Huang CC, Hsu KS. Phosphatidylinositol 3‐kinase activation is required for stress protocol‐induced modification of hippocampal synaptic plasticity. J Biol Chem. 2008;283:2631‐2643. [DOI] [PubMed] [Google Scholar]
- 528. Bruce C, Chouinard RA, Jr , Tall AR. Plasma lipid transfer proteins, high‐density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr. 1998;18:297‐330. [DOI] [PubMed] [Google Scholar]
- 529. Barter PJ, Brewer HB, Jr , Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160‐167. [DOI] [PubMed] [Google Scholar]
- 530. Ascenzi P, Bocedi A, Marino M. Structure–function relationship of estrogen receptor α and β: impact on human health. Mol Aspects Med. 2006;27:299‐402. [DOI] [PubMed] [Google Scholar]
- 531. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532. Fabian CJ, Kimler BF. Selective estrogen‐receptor modulators for primary prevention of breast cancer. J Clin Oncol. 2005;23:1644‐1655. [DOI] [PubMed] [Google Scholar]
- 533. Bocharov EV, Ivanova‐Smolenskaya IA, Poleshchuk VV, Kucheryanu VG, Il'enko VA, Bocharova OA. Therapeutic efficacy of the neuroprotective plant adaptogen in neurodegenerative disease (Parkinson's disease as an example). Bull Exp Biol Med. 2010;149:682‐684. [DOI] [PubMed] [Google Scholar]
- 534. Palumbo DR, Occhiuto F, Spadaro F, Circosta C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide‐induced cell death through reduction in the accumulation of intracellular calcium. Phytother Res. 2012;26:878‐883. [DOI] [PubMed] [Google Scholar]
- 535. Li X, Ye X, Li X, et al. Salidroside protects against MPP(+)‐induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res. 2011;1382:9‐18. [DOI] [PubMed] [Google Scholar]
- 536. Shi TY, Feng SF, Xing JH, et al. Neuroprotective effects of Salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2‐induced neurotoxicity in vitro. Neurotox Res. 2012;21:358‐367. [DOI] [PubMed] [Google Scholar]
- 537. Zeng KW, Zhang T, Fu H, Liu GX, Wang XM. Schisandrin B exerts anti‐neuroinflammatory activity by inhibiting the Toll‐like receptor 4‐dependent MyD88/IKK/NF‐κB signaling pathway in lipopolysaccharide‐induced microglia. Eur J Pharmacol. 2012;692:29‐37. [DOI] [PubMed] [Google Scholar]
- 538. Zhang L, Yu H, Sun Y, et al. Protective effects of salidroside on hydrogen peroxide‐induced apoptosis in SH‐SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564:18‐25. [DOI] [PubMed] [Google Scholar]
- 539. Zhang L, Yu H, Zhao X, et al. Neuroprotective effects of salidroside against beta‐amyloid‐induced oxidative stress in SH‐SY5Y human neuroblastoma cells. Neurochem Int. 2010;57:547‐555. [DOI] [PubMed] [Google Scholar]
- 540. Chen QG, Zeng YS, Qu ZQ, et al. The effects of Rhodiola rosea extract on 5‐HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine. 2009;16:830‐838. [DOI] [PubMed] [Google Scholar]
- 541. Mhyre AJ, Shapiro RA, Dorsa DM. Estradiol reduces nonclassical transcription at cyclic adenosine 3,5‐monophosphate response elements in glioma cells expressing estrogen receptor alpha. Endocrinology. 2006;147:1796‐1804. [DOI] [PubMed] [Google Scholar]
- 542. Arnsten AF. Prefrontal cortical network connections: key site of vulnerability in stress and schizophrenia. Int J Dev Neurosci. 2011;29:215‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543. Wang M, Ramos BP, Paspalas CD, et al. Alpha2A‐adrenoceptors strengthen working memory networks by inhibiting cAMP‐HCN channel signaling in prefrontal cortex. Cell. 2007;129:397‐410. [DOI] [PubMed] [Google Scholar]
- 544. Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545. Zhang A, Liu Z, Sheng L, Wu H. Protective effects of syringin against lipopolysaccharide‐induced acute lung injury in mice. J Surg Res. 2017;209:252‐257. [DOI] [PubMed] [Google Scholar]
- 546. Han SB, Yoon YD, Ahn HJ, et al. Toll‐like receptor‐mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus . Int Immunopharmacol. 2003;3:1301‐1312. [DOI] [PubMed] [Google Scholar]
- 547. Fei XJ, Zhu LL, Xia LM, Peng WB, Wang Q. Acanthopanax senticosus attenuates inflammation in lipopolysaccharide‐induced acute lung injury by inhibiting the NF‐kB pathway. Genet Mol Res. 2014;13:10537‐10544. [DOI] [PubMed] [Google Scholar]
- 548. Soo Kim H, Young Park S, Kyoung Kim E, et al. Acanthopanax senticosus has a heme oxygenase‐1 signaling‐dependent effect on Porphyromonas gingivalis lipopolysaccharide‐stimulated macrophages. J Ethnopharmacol. 2012;142:819‐828. [DOI] [PubMed] [Google Scholar]
- 549. Yamazaki T, Shimosaka S, Sasaki H, Matsumura T, Tukiyama T, Tokiwa T. (+)‐Syringaresinol‐di‐O‐beta‐D‐glucoside, a phenolic compound from Acanthopanax senticosus Harms, suppresses proinflammatory mediators in SW982 human synovial sarcoma cells by inhibiting activating protein‐1 and/or nuclear factor‐kappaB activities. Toxicol In Vitro. 2007;21:1530‐1537. [DOI] [PubMed] [Google Scholar]
- 550. Kang B, Kim CY, Hwang J, et al. Red ginseng extract regulates differentiation of monocytes to macrophage and inflammatory signalings in human monocytes. Food Sci Biotechnol. 2019;28:1819‐1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551. Borgonetti V, Governa P, Biagi M, Dalia P, Corsi L. Rhodiola rosea L. modulates inflammatory processes in a CRH‐activated BV2 cell model. Phytomedicine. 2020;68:153143. [DOI] [PubMed] [Google Scholar]
- 552. Song FJ, Zeng KW, Chen JF, et al. Extract of fructus Schisandrae chinensis inhibits neuroinflammation mediator production from microglia via NF‐κ B and MAPK pathways. Chin J Integr Med. 2019;25:131‐138. [DOI] [PubMed] [Google Scholar]
- 553. Zhang X, Lai W, Ying X, et al. Salidroside reduces inflammation and brain injury after permanent middle cerebral artery occlusion in rats by regulating PI3K/PKB/Nrf2/NFκB signaling rather than complement C3 activity. Inflammation. 2019;42:1830‐1842. [DOI] [PubMed] [Google Scholar]
- 554. Xin X, Yao D, Zhang K, et al. Protective effects of Rosavin on bleomycin‐induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed Pharmacother. 2019;115:108870. [DOI] [PubMed] [Google Scholar]
- 555. Hu R, Wang MQ, Ni SH, et al. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF‐κB/NLRP3 signaling pathway in AGEs‐induced HUVECs. Eur J Pharmacol. 2020;867:172797. [DOI] [PubMed] [Google Scholar]
- 556. Maitra R, Porter MA, Huang S, Gilmour BP. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of cystic fibrosis inflammation. J Inflamm (Lond). 2009;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557. Heyninck K, Lahtela‐Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W. Withaferin A inhibits NF‐kappaB activation by targeting cysteine 179 in IKKβ. Biochem Pharmacol. 2014;91:501‐509. [DOI] [PubMed] [Google Scholar]
- 558. Heyninck K, Sabbe L, Chirumamilla CS, et al. Withaferin A induces heme oxygenase (HO‐1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem Pharmacol. 2016;109:48‐61. [DOI] [PubMed] [Google Scholar]
- 559. Luo G, Cheng BC, Zhao H, et al. Schisandra Chinensis lignans suppresses the production of inflammatory mediators regulated by NF‐κB, AP‐1, and IRF3 in lipopolysaccharide‐stimulated RAW264.7 cells. Molecules. 2018;23:3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560. Han J, Liu L, Yu N, et al. Polysaccharides from Acanthopanax senticosus enhances intestinal integrity through inhibiting TLR4/NF‐kB signaling pathways in lipopolysaccharide‐challenged mice. Anim Sci J. 2016;87:1011‐1018. [DOI] [PubMed] [Google Scholar]
- 561. Sun K, Huang R, Yan L, et al. Schisandrin attenuates lipopolysaccharide‐induced lung injury by regulating TLR‐4 and Akt/FoxO1 signaling pathways. Front Physiol. 2018;9:1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562. Zhou F, Wang M, Ju J, et al. Schizandrin A protects against cerebral ischemia‐reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am J Transl Res. 2019;11:199‐209. [PMC free article] [PubMed] [Google Scholar]
- 563. Shaukat A, Yang C, Yang Y, et al. Ginsenoside Rb 1: a novel therapeutic agent in Staphylococcus aureus‐induced acute lung injury with special reference to oxidative stress and apoptosis. Microb Pathog. 2020;143:104109. [DOI] [PubMed] [Google Scholar]
- 564. Chen S, Li X, Wang Y, et al. Ginsenoside Rb1 attenuates intestinal ischemia/reperfusion‑induced inflammation and oxidative stress via activation of the PI3K/Akt/Nrf2 signaling pathway. Mol Med Rep. 2019;19:3633‐3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565. Saw CL, Yang AY, Cheng DC, et al. Pharmacodynamics of ginsenosides: antioxidant activities, activation of Nrf2, and potential synergistic effects of combinations. Chem Res Toxicol. 2012;25:1574‐1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566. Chu SF, Zhang Z, Zhou X, et al. Ginsenoside Rg1 protects against ischemic/reperfusion‐induced neuronal injury through miR‐144/Nrf2/ARE pathway. Acta Pharmacol Sin. 2019;40:13‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 567. Tang H, Gao L, Mao J, et al. Salidroside protects against bleomycin‐induced pulmonary fibrosis: activation of Nrf2‐antioxidant signaling, and inhibition of NF‐κB and TGF‐β1/Smad‐2/‐3 pathways. Cell Stress Chaperones. 2016;21:239‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568. Li R, Wang S, Li T, et al. Salidroside protects dopaminergic neurons by preserving complex I activity via DJ‐1/Nrf2‐mediated antioxidant pathway. Parkinsons Dis. 2019;2019:6073496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569. Gao J, Yu Z, Jing S, et al. Protective effect of Anwulignan against D‐galactose‐induced hepatic injury through activating p38 MAPK‐Nrf2‐HO‐1 pathway in mice. Clin Interv Aging. 2018;13:1859‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570. Han J, Xiao Q, Lin YH, et al. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2‐related factor 2 pathway. Neural Regen Res. 2015;10:1989‐1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Shan Y, Jiang B, Yu J, et al. Protective effect of Schisandra chinensis polysaccharides against the immunological liver injury in mice based on Nrf2/ARE and TLR4/NF‐κB signaling pathway. J Med Food. 2019;22:885‐895. [DOI] [PubMed] [Google Scholar]
- 572. Zhu Y, Zhang YJ, Liu WW, Shi AW, Gu N. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. 2016;21:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Kwon DH, Cha HJ, Choi EO, et al. Schisandrin A suppresses lipopolysaccharide‐induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF‐κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO‐1 signaling. Int J Mol Med. 2018;41:264‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Palliyaguru DL, Chartoumpekis DV, Wakabayashi N, et al. Withaferin A induces Nrf2‐dependent protection against liver injury: role of Keap1‐independent mechanisms. Free Radic Biol Med. 2016;101:116‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Mussard E, Cesaro A, Lespessailles E, et al. Andrographolide, a natural antioxidant: an update. Antioxidants (Basel). 2019;8:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576. Adeoye BO, Asenuga ER, Oyagbemi AA, Omobowale TO, Adedapo AA. The protective effect of the ethanol leaf extract of Andrographis Paniculata on cisplatin‐induced acute kidney injury in rats through nrf2/KIM‐1 signalling pathway. Drug Res (Stuttg). 2018;68:23‐32. [DOI] [PubMed] [Google Scholar]
- 577. Seo JY, Pyo E, An JP, Kim J, Sung SH, Oh WK. Andrographolide activates Keap1/Nrf2/ARE/HO‐1 pathway in HT22 cells and suppresses microglial activation by Aβ(42) through Nrf2‐related inflammatory response. Mediators Inflamm. 2017;2017:5906189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578. Brown GC. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2001;1504:46‐57. [DOI] [PubMed] [Google Scholar]
- 579. Hara MR, Cascio MB, Sawa A. GAPDH as a sensor of NO stress. Biochim Biophys Acta. 2006;1762:502‐509. [DOI] [PubMed] [Google Scholar]
- 580. Skirven TM, Osterman AL, Fedorczyk J, Amadio PC, eds. Rehabilitation of the Hand and Upper Extremity. Vol 1, 6th ed. Philadelphia, PA: Elsevier Mosby; 2011:152‐162. [Google Scholar]
- 581.EMEA/HMPC/102655/2007. Reflection paper on the adaptogenic concept. Committee on Herbal Medicinal Products; 2008:1‐6.
- 582. Punja S, Shamseer L, Olson K, Vohra S. Rhodiola rosea for mental and physical fatigue in nursing students: a randomized controlled trial. PLOS One. 2014;9:e108416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583. Bisson J, McAlpine JB, Friesen JB, Chen SN, Graham J, Pauli GF. Can invalid bioactives undermine natural product‐based drug discovery? J Med Chem. 2016;59:1671‐1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584. Barenboim GM, Sterlina AG, Bebyakova NV, Ribokas AA, Fuks BB. Investigation of the pharmacokinetics and mechanism of action of Eleutherococcus glycosides. VIII. Investigation of natural killer activation by Eleutherococcus extract. Khim Farm Zh. 1986;20:914‐917. [Google Scholar]
- 585. Bezdetko GN, German AV, Shevchenko VP, et al. Study of the pharmacokinetics and action mechanism of Eleutherococcus glycosides. I. Incorporation of tritium into eleutherosides B, Kinetics of its accumulation and excretion from the body of animal. Khim Farm Z. 1981;15:28‐33. [Google Scholar]
- 586. German AV, Bezdetko GN, Mitrokhin YI, Chivkov GN, Shevchenko VP, Barenboim GM. Dardymov. Study of the pharmacokinetics and mechanism of Eleutherococcus glycosides. II. Distribution of eleutheroside in organs and subcellular fraction. Khim Farm Zh. 1982;16:26‐30. [Google Scholar]
- 587. Feng SI, Hu FD, Zhao JX, Liu X, Li Y. Determination of eleutheroside E and eleutheroside B in rat plasma and tissue by high‐performance liquid chromatography using solid‐phase extraction and photodiode array detection. Eur J Pharm Biopharm. 2006;62:315‐320. [DOI] [PubMed] [Google Scholar]
- 588. Ma B, Zhang Q, Liu Y, et al. Simultaneous determination of Eleutheroside B and Eleutheroside E in rat plasma by high performance liquid chromatography‐electrospray ionization mass spectrometry and its application in a pharmacokinetic study. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;917‐918:84‐92. [DOI] [PubMed] [Google Scholar]
- 589. Li Q, Jia Y, Sun LX, et al. High‐performance liquid chromatographic determination of isofraxidin in rat plasma. Chromatographia. 2006;63:249‐253. [Google Scholar]
- 590. Wang Z, You L, Cheng Y, et al. Investigation of pharmacokinetics, tissue distribution and excretion of schisandrin B in rats by HPLC‐MS/MS. Biomed Chromatogr. 2018;32:2. [DOI] [PubMed] [Google Scholar]
- 591. Panossian A, Hovhannisyan A, Mamikonyan G, et al. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7:351‐364. [DOI] [PubMed] [Google Scholar]
- 592. Bera R, Ahmed SM, Sarkar L, Sen T, Karmakar S. Pharmacokinetic analysis and tissue distribution of andrographolide in rat by a validated LC‐MS/MS method. Pharm Biol. 2014;52:321‐329. [DOI] [PubMed] [Google Scholar]
- 593. Feng L, Wang L, Hu C, Jiang X. Pharmacokinetics, tissue distribution metabolism, and excretion of ginsenoside Rg 1 in rats. Arch Pharmacol Res. 2010;33:1975‐1984. [DOI] [PubMed] [Google Scholar]
- 594. Hao M, Wang W, Zhao Y, Zhang R, Wang H. Pharmacokinetics and tissue distribution of 25‐hydroxyprotopanaxadiol, an anti‐cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. Eur J Drug Metab Pharmacokinet. 2011;35:109‐113. [DOI] [PubMed] [Google Scholar]
- 595. Kim DH. Gut microbiota‐mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596. Won HJ, Kim HI, Park T, et al. Non‐clinical pharmacokinetic behavior of ginsenosides. J Ginseng Res. 2019;43:354‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 597. Panossian A, Hovhannisyan A, Abrahamyan H, Wikman G. Pharmacokinetics of active constituents of Rhodiola rosea SHR‐5 extract In: Gupta VK, ed. Comprehensive Bioactive Natural Products. Vol. 2: Efficacy, Safety & Clinical Evaluation I. Houston: Studium Press; 2010:307‐329. [Google Scholar]
- 598. Panossian A, Hovhannisyan A, Abrahamyan H, Gabrielyan E, Wikman G. Pharmacokinetic and pharmacodynamic study of interaction of Rhodiola rosea SHR‐5 extract with warfarin and theophylline in rats. Phytother Res. 2008;23:351‐357. [DOI] [PubMed] [Google Scholar]
- 599. Zhang Y, Li L, Lin L, et al. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013;79:1429‐1433. [DOI] [PubMed] [Google Scholar]
- 600. Zhang M, Hu Z, Fang B, Bao X, Xiang Z, Wang H. Pharmacokinetic study of rosavin in rat plasma with ultra performance LC‐MS/MS after intravenous and gavage administration. Bioanalysis. 2019;11:837‐845. [DOI] [PubMed] [Google Scholar]
- 601. Yu S, Liu L, Wen T, et al. Development and validation of a liquid chromatographic/electrospray ionization mass spectrometric method for the determination of salidroside in rat plasma: application to the pharmacokinetics study. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;861:10‐15. [DOI] [PubMed] [Google Scholar]
- 602. Guo N, Zhu M, Han X, Sui D, Wang Y, Yang Q. The metabolism of salidroside to its aglycone p‐tyrosol in rats following the administration of salidroside. PLOS One. 2014;9:e103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603. Sun H, Lv H, Zhang Y, Wang X, Bi K, Cao H. Pharmacokinetics of isofraxidin in rat plasma after oral administration of the extract of Acanthopanax senticosus using HPLC with solid phase extraction method. Chem Pharm Bull (Tokyo). 2007;55:1291‐1295. [DOI] [PubMed] [Google Scholar]
- 604. Sun Y, Xue J, Li B, et al. Simultaneous quantification of triterpenoid saponins in rat plasma by UHPLC‐MS/MS and its application to a pharmacokinetic study after oral total saponin of Aralia elata leaves. J Sep Sci. 2016;39:4360‐4368. [DOI] [PubMed] [Google Scholar]
- 605. Wang W, Wang GJ, Xie HT, et al. Determination of ginsenoside Rd in dog plasma by liquid chromatography‐mass spectrometry after solid‐phase extraction and its application in dog pharmacokinetics studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:8‐14. [DOI] [PubMed] [Google Scholar]
- 606. Kosman VM, Karlina MV, Pozharitskaya ON, et al. Pharmacokinetics of lignans from Schisandra chinensis . Rev Clin Pharmacol Drug Ther. 2015;13:3‐21. [Google Scholar]
- 607. Wang Z, Wu Q, Meng Y, et al. Determination and pharmacokinetic study of two triterpenoid saponins in rat plasma after oral administration of the extract of Aralia elata leaves by UHPLC‐ESI‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:164‐171. [DOI] [PubMed] [Google Scholar]
- 608. Wang Z, Wu Q, Meng Y, et al. Determination and pharmacokinetic study of two triterpenoid saponins in rat plasma after oral administration of the extract of Aralia elata leaves by UHPLC‐ESI‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;985:164‐171. [DOI] [PubMed] [Google Scholar]
- 609. Lin W, Ma J, Yu S. Pharmacokinetics of araloside X in rats and tissue distribution in mice by UPLC‐MS/MS. Latin American J Pharmacy. 2019;38:1505‐1509. [Google Scholar]
- 610. Guo DY, Zhai BT, Lv Y, et al. Tissue distribution of araloside A in rats. Zhongguo Zhong Yao Za Zhi. 2017;42:4002‐4006. [DOI] [PubMed] [Google Scholar]
- 611. Pan W, Xue B, Yang C, et al. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia. 2018;129:272‐282. [DOI] [PubMed] [Google Scholar]
- 612. Zhou SS, Xu J, Zhu H, et al. Gut microbiota‐involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci Rep. 2016;6:22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Xue P, Yao Y, Yang XS, Feng J, Ren GX. Improved antimicrobial effect of ginseng extract by heat transformation. J Ginseng Res. 2017;41:180‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614. Shen H, Leung WI, Ruan JQ, et al. Biotransformation of ginsenoside Rb1 via the gypenoside pathway by human gut bacteria. Chin Med. 2013;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615. Quan K, Liu Q, Wan JY, et al. Rapid preparation of rare ginsenosides by acid transformation and their structure‐activity relationships against cancer cells. Sci Rep. 2015;5:8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616. Yu S, Zhou X, Li F, et al. Microbial transformation of ginsenoside Rb1, Re and Rg1 and its contribution to the improved anti‐inflammatory activity of ginseng. Sci Rep. 2017;7:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617. Zhang Y, Lin L, Liu GY, Liu JX, Li T. Pharmacokinetics and brain distribution of ginsenosides after administration of sailuotong. Zhongguo Zhong Yao Za Zhi. 2014;39:316‐321. [PubMed] [Google Scholar]
- 618. Liu H, Yang J, Du F, et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290‐2298. [DOI] [PubMed] [Google Scholar]
- 619. Chi H, Ji GE. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett. 2005;27:765‐771. [DOI] [PubMed] [Google Scholar]
- 620. Chi H, Kim DH, Ji GE. Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull. 2005;28:2102‐2105. [DOI] [PubMed] [Google Scholar]
- 621. Kim DH. Gut microbiota‐mediated pharmacokinetics of ginseng saponins. J Ginseng Res. 2018;42:255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 622. Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng . J Pharm Pharmacol. 1998;50:1155‐1160. [DOI] [PubMed] [Google Scholar]
- 623. Hasegawa H, Uchiyama M. Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med. 1998;64:696‐700. [DOI] [PubMed] [Google Scholar]
- 624. Mariage PA, Hovhannisyan A, Panossian AG. Efficacy of Panax ginseng meyer herbal preparation HRG80 in preventing and mitigating stress‐induced failure of cognitive functions in healthy subjects: a pilot, randomized, double‐blind, placebo‐controlled crossover trial. Pharmaceuticals (Basel). 2020;13:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625. Dimpfel W, Schombert L, Panossian AG. Panax ginseng preparations enhance long term potentiation in rat hippocampal slices by glutamatergic NMDA and kainate receptor mediated transmission. J Altern Complement Integr Med. 2020;6:106. [Google Scholar]
- 626. Thu OKF, Spigset O, Hellum B. Noncompetitive inhibition of human CYP2C9 in vitro by a commercial Rhodiola rosea product. Pharmacol Res Perspect. 2017;5:e00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627. Thu OK, Nilsen OG, Hellum B. In vitro inhibition of cytochrome P‐450 activities and quantification of constituents in a selection of commercial Rhodiola rosea products. Pharm Biol. 2016;54:3249‐3256. [DOI] [PubMed] [Google Scholar]
- 628. Thu OK, Spigset O, Nilsen OG, Hellum B. Effect of commercial Rhodiola rosea on CYP enzyme activity in humans. Eur J Clin Pharmacol. 2016;72:295‐300. [DOI] [PubMed] [Google Scholar]


