Abstract
Objective
The effects of anticonvulsants on lipids are the subject of considerable concern and investigation, but there are almost no data on this issue from randomized trials. We evaluated serum lipid profiles in adults with newly diagnosed epilepsy, following randomization to lacosamide (LCM) or carbamazepine (CBZ) monotherapy.
Methods
We analyzed data from a Phase 3, international, randomized, double‐blind trial of LCM vs CBZ for the initial treatment of focal epilepsy. Serum lipid profiles in patients not taking lipid‐lowering agents and providing blood samples under fasting conditions before treatment, and following 3 or 12 months of treatment with LCM or CBZ at various doses were analyzed.
Results
At 12 months, 271 patients satisfied the inclusion criteria for the analysis. No change was observed in LCM‐treated patients for total cholesterol, cholesterol fractions, or triglycerides. CBZ‐treated patients showed an increase of 21.1 mg/dL in total cholesterol, 12.6 mg/dL in low‐density lipoprotein (LDL) cholesterol, 12.5 mg/dL in non–high density lipoprotein (non‐HDL) cholesterol, and 8.5 mg/dL in HDL cholesterol; triglycerides remained unchanged. The proportion of patients with elevated total cholesterol levels (above the upper limit of the reference range) did not change in the LCM treatment group (37.0% at Baseline; 34.8% at 12 months), but increased from 30.8% (at Baseline) to 49.6% (at 12 months) in the CBZ treatment group.
Significance
This study provides Class II evidence that CBZ elevates serum lipids, whereas LCM has no effect on lipids. It supports LCM as an appropriate choice for new‐onset focal epilepsy.
Keywords: antiepileptic drug, monotherapy, newly diagnosed epilepsy, serum lipid levels, vascular risk factors
Key Points.
Anticonvulsant drugs that affect cytochrome P450 (CYP) enzymes may also affect metabolic pathways related to serologic vascular risk markers
After 12 months of lacosamide monotherapy, serum lipid levels did not change from baseline
Carbamazepine monotherapy resulted in significant increases in cholesterol and its fractions after 3 and 12 months
These results provide Class II evidence that carbamazepine increases serum lipids, whereas lacosamide has no impact on lipid levels
1. INTRODUCTION
The profusion of anticonvulsant drugs during the past 25 years has yielded a large group of compounds with very distinct structures and chemical properties. Because this “class” of drugs is not a class in a pharmacologic sense, each drug must be studied individually to ascertain its effects.
Lacosamide (LCM) is a chemically unique anticonvulsant that has been increasingly adopted for the treatment of focal epilepsy. Lacosamide works by selectively enhancing the slow inactivation of voltage‐gated sodium channels, a mode of action that is different from that first‐generation drugs such as carbamazepine (CBZ) and phenytoin, which affect fast inactivation of the sodium channels. 1 , 2 Lacosamide also differs from the latter agents in having no clinically relevant impact on the cytochrome P450 (CYP) enzyme system. 3 This is relevant not merely from the standpoint of drug interactions, but also because CYP enzymes have many effects on endogenous metabolic pathways, such that perturbation of the CYP system can induce a number of metabolic effects. 4
Among the more important of these effects concerns the pathways related to the metabolism of cholesterol and other serologic markers of vascular risk. Evidence from both cross‐sectional and repeated‐measures studies suggest that CBZ is associated with significant elevations of serum cholesterol, a phenomenon that may be mediated by its effects on CYP51A1. 5 The same studies also suggest that CBZ elevates levels of lipoprotein(a) and C‐reactive protein (CRP), which are additional and independent vascular risk markers. 6 There is very little by way of randomized evidence for this, however, with only a single paper published in which the authors examine the effect of CBZ on serum lipids in a randomized fashion. 7 That study was performed with older patients only (mean age approximately 70.5 years), and yielded complex data that did not wholly fit the narrative of the literature, for reasons that remain unclear. 7 Thus, there is a need for additional randomized data on the impact of CBZ on lipid levels.
Although LCM is not known to affect serum lipids, this has not been systematically examined in a way that allows comparison with any other anticonvulsant aside from a single, small, repeated‐measures study showing that switching young male patients from CBZ to LCM reduced serum lipid concentrations. 8
Here we present an analysis of serologic data from a randomized monotherapy trial comparing the controlled‐release formulation of CBZ with LCM for the treatment of new‐onset focal epilepsy. 9 This was primarily an efficacy and safety trial, demonstrating that the efficacy of LCM was noninferior to CBZ, and that LCM was generally well tolerated. Serologic monitoring of lipid levels during the trial enabled analysis of comparative data on the effects of LCM on lipid levels in a randomized fashion for the first time. Although the analysis is secondary and post hoc, the objective nature of the outcome measure removes many potential sources of bias, yielding data that should reflect a true comparison of the effect of the two drugs on lipid serology.
2. MATERIAL AND METHODS
2.1. Study design
SP0993 (NCT01243177) was a Phase 3, multicenter, randomized, double‐blind, non‐inferiority trial comparing the efficacy and tolerability of LCM vs the controlled‐release formulation of CBZ monotherapy in adult patients with newly diagnosed epilepsy. 9 The trial was conducted at 185 sites in 29 countries across Europe, North America, and the Asia Pacific, in accordance with Good Clinical Practice, the Declaration of Helsinki, and local laws. The protocol was reviewed by the appropriate national regulatory agency for each site. Additional reviews were conducted by regional or local independent ethics committees or institutional review boards if deemed necessary by local requirements. All patients (or their parents or legal guardians) provided written informed consent for participation.
Detailed methodology of this trial has been published previously 9 and is summarized briefly here. Patients 16 years of age or older who had newly or recently diagnosed epilepsy with unprovoked focal (partial‐onset) seizures or generalized tonic‐clonic seizures (with neither signs of focal onset nor history/clinical/ electroencephalography (EEG) findings suggestive of idiopathic genetic generalized epilepsy) were eligible. In addition, patients were required to have experienced at least two unprovoked seizures in the previous 12 months, separated by at least 48 hours and with at least one seizure occurring in the previous 3 months. Eligible patients were randomized 1:1 to LCM or CBZ monotherapy.
The trial had a stepwise design, with three predefined target dose levels for each treatment (200, 400, or 600 mg/d LCM; 400, 800, or 1200 mg/d CBZ). Lacosamide and CBZ were initiated at 100 mg/d and 200 mg/d, respectively, and were titrated to the initial target dose level (200 mg/d LCM; 400 mg/d CBZ). If a seizure occurred during the 6‐month Evaluation period, the dose was increased to the next target dose level (LCM 400 or 600 mg/d; CBZ 800 or 1200 mg/d); if a seizure occurred during the 6‐month Evaluation period of the third dose level or during the 6‐month Maintenance period of any dose level, the patient was withdrawn from the trial. For patients who escalated to the second or third dose level but were unable to tolerate the increased dose, one dose reduction (of LCM 100 mg/d; CBZ 200 mg/d) was allowed.
2.2. Patients included in the analysis and serum lipid levels
Blood samples were collected under fasting conditions (at least 8 hours) at Baseline and at each trial visit. Lipid levels were assessed at Baseline and at 3 and 12 months after trial drug initiation. Baseline was defined as the last sample obtained before the first dose of trial medication. The 3‐month assessment included lipid level values recorded at 3 months (90 days plus a 30‐day window) of treatment; the 12‐month assessment included lipid level values recorded at 364 days (plus a 30‐day window) of treatment during the Treatment period. The analyses were performed by a central laboratory. Lipid measurements included total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C, calculated), high‐density lipoprotein cholesterol (HDL‐C), and triglycerides (TG); non‐HDL‐C was calculated from TC and HDL‐C. Results of the laboratory lipid tests were masked from the Investigators during the trial unless they exceeded alert levels, in which case the values would be revealed to the investigator.
In this post hoc analysis, we included patients treated with LCM or CBZ for at least 12 months who provided fasting blood samples, both at Baseline and at 12 months, and who did not receive lipid‐lowering agents during that time. 10 , 11 An additional analysis was performed in patients treated with LCM or CBZ for at least 3 months who provided blood samples under fasted conditions at Baseline and at 3 months, and who were not receiving lipid‐lowering agents.
2.3. Statistical analyses
Patients randomized to CBZ were combined into a single group, regardless of dose, and the same was done for LCM patients. The results of serum levels for TC, LDL‐C, HDL‐C, non‐HDL‐C, and TG at Baseline, 3 months, and 12 months were summarized. A paired t test was used to compare the means of observed lipid levels between Baseline and 3 or 12 months within each treatment group. An analysis of covariance (ANCOVA) was performed to analyze the change from Baseline at 3 and 12 months between treatment groups. The ANCOVA model included treatment as a main effect, and age, sex, body mass index, and Baseline lipid level as covariates. The least squares (LS) means of change from Baseline at 3 and 12 months were estimated. Subgroup analyses on within‐treatment group comparison and between‐treatment group comparison of change from Baseline at 3 and 12 months were performed on patients with Baseline TC or LDL‐C levels below the upper limit of the reference range and on patients with Baseline TC or LDL‐C levels above the upper limit of the reference range. The upper limit for TC was 200 mg/dL; for LDL‐C, it was 130 mg/dL for patients 18 and older and 110 mg/dL for patients under 18 years of age.
The proportions of patients who had increases in TC, LDL‐C, or non‐HDL‐C of at least 20 mg/dL or at least 40 mg/dL from Baseline at the 3‐ and 12‐month time points were compared between treatment groups using Fisher exact tests. Shifts from Baseline to 3 and 12 months in serum levels of TC and LDL‐C were calculated.
3. RESULTS
3.1. Patient demographics and characteristics
Of the 886 patients who received at least one dose of trial medication (LCM n = 444; CBZ‐CR n = 442), 9 271 patients (LCM, n = 138; CBZ, n = 133; Table 1, Figure S1), and 431 patients (LCM, n = 210; CBZ, n = 221; Table S1, Figure S2) satisfied the criteria for inclusion in the 12‐ and 3‐month analyses on lipid levels, respectively. 9
Table 1.
Patient demographics and Baseline characteristics (12‐mo population)
|
Lacosamide (n = 138) |
Carbamazepine (n = 133) |
Total (n = 271) |
|
|---|---|---|---|
| Age, mean (SD), y | 41.0 (16.7) | 37.4 (15.5) | 39.2 (16.2) |
| <25 y, n (%) | 28 (20.3) | 37 (27.8) | 65 (24.0) |
| 25 to <45 y, n (%) | 57 (41.3) | 50 (37.6) | 107 (39.5) |
| 45 to <65 y, n (%) | 36 (26.1) | 39 (29.3) | 75 (27.7) |
| ≥65 y, n (%) | 17 (12.3) | 7 (5.3) | 24 (8.9) |
| Male, n (%) | 78 (56.5) | 83 (62.4) | 161 (59.4) |
| Weight, kg, mean (SD) | 71.1 (15.7) | 72.1 (15.2) | 71.6 (15.5) |
| Body mass index, mean (SD), kg/m2 | 24.5 (4.5) a | 24.5 (4.3) | 24.5 (4.4) b |
| <25, n (%) | 79 (57.2) | 76 (57.1) | 155 (57.2) |
| 25 to <30, n (%) | 42 (30.4) | 47 (35.3) | 89 (32.8) |
| ≥30, n (%) | 16 (11.6) | 10 (7.5) | 26 (9.6) |
| Missing, n (%) | 1 (0.7) | 0 | 1 (0.4) |
| Comorbid endocrine disorders, metabolism and nutrition disorders, social circumstances, and vascular disorders reported by ≥2% of total patients i | |||
| Hypertension, n (%) | 22 (15.9) | 22 (16.5) | 44 (16.2) |
| Postmenopause, n (%) | 9 (6.5) | 6 (4.5) | 15 (5.5) |
| Hypothyroidism, n (%) | 2 (1.4) | 5 (3.8) | 7 (2.6) |
| Menopause, n (%) | 4 (2.9) | 3 (2.3) | 7 (2.6) |
| Total cholesterol | |||
| Patients with levels below upper limit of the reference range, n (%) c | 87 (63.0) | 92 (69.2) | 179 (66.1) |
| Patients with levels above upper limit of the reference range, n (%) d | 51 (37.0) | 41 (30.8) | 92 (33.9) |
| LDL cholesterol | |||
| Patients with levels below upper limit of the reference range, n (%) e | 102 (73.9) | 105 (80.2) g | 207 (77.0) h |
| Patients with levels above upper limit of the reference range, n (%) f | 36 (26.1) | 26 (19.8) g | 62 (23.0) h |
Abbreviations: LDL, low‐density lipoprotein; SD, standard deviation.
n = 137.
n = 270.
Total cholesterol levels ≤200 mg/dL.
Total cholesterol levels >200 mg/dL.
LDL‐cholesterol levels ≤130 mg/dL.
LDL‐cholesterol levels >130 mg/dL.
n = 131.
n = 269.
Hepatobiliary disorders were also evaluated; however, no preferred term was reported by ≥2% Total patients.
Baseline characteristics were generally similar in patients on LCM and CBZ. However, in the 12‐month population, a smaller proportion of patients randomized to LCM were <25 years of age and a slightly larger proportion were ≥65 years of age, compared with patients randomized to CBZ (Table 1). The highest percentage of patients randomized to both LCM and CBZ were in the 25 to <45 years of age category. In addition, more patients randomized to LCM vs CBZ had Baseline TC (12‐month population) or LDL‐C (3‐ and 12‐month populations) levels above the upper limit of the reference range (Table 1; Table S1).
3.2. Dose level
At 12 months after treatment initiation, the large majority of patients were taking the lower medication doses: 200 mg/d LCM (93.5%) or 400 mg/d CBZ (91.7%). In addition, 3.6% of LCM patients were on 400 mg/d and 2.9% were taking 600 mg/d. Of the CBZ patients, 6.0% of patients were on 800 mg/d CBZ and 2.3% of patients were on 1200 mg/d CBZ (Table S2). The corresponding proportions of patients at 3 months were: 72.9% on 200 mg/d LCM, 73.8% on 400 mg/d CBZ; 17.6% on 400 mg/d LCM, 19.0% on 800 mg/d CBZ; and 9.5% on 600 mg/d LCM, 7.2% on 1200 mg/d CBZ target dose levels.
3.3. Changes in lipid levels from Baseline
In the 12‐month population, mean Baseline lipid levels were similar between patients randomized to LCM and CBZ (Figure 1). After 12 months of LCM monotherapy, mean TC, LDL‐C, non‐HDL‐C, HDL‐C, and TG levels did not change from Baseline (P > .05 for all comparisons; Figure 1). In the CBZ group, significant increases in levels of TC, LDL‐C, non‐HDL‐C, and HDL‐C, were observed after 12 months vs Baseline (all P‐values <.0001; Figure 1). The difference in the mean change from Baseline between LCM and CBZ was also significant (P < .001 for all cholesterol measures). Results were very similar in the 3‐month population (Figure S3).
Figure 1.
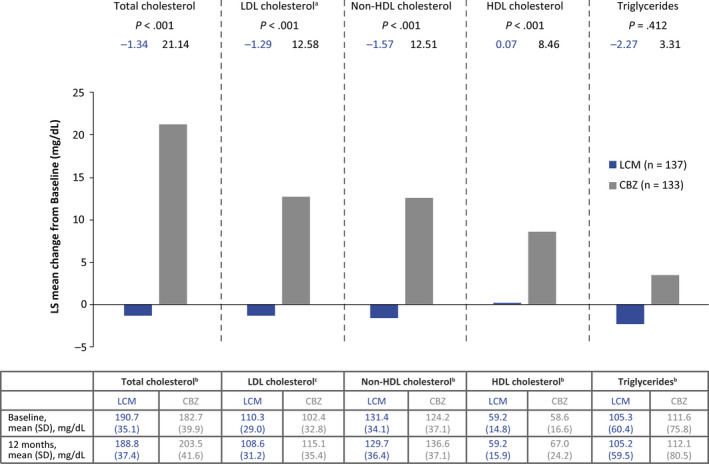
Least‐squares mean change in lipid levels from Baseline at 12 mo (ANCOVA) (12‐mo population). The ANCOVA model included treatment as a main effect and age, sex, body mass index, and Baseline lipid level as covariates; an = 131 for CBZ; bn = 138 for LCM, n = 133 for CBZ; cn = 138 for LCM, n = 131 for CBZ. Table includes within treatment group comparison 12‐month vs Baseline, pairedttest; P‐values were based on means of observed values (unadjusted): LCM: Total cholesterol P> .05; LDL cholesterol P > .05; non‐HDL cholesterol P> .05; HDL cholesterol P> .05; triglyceridesP >.05. CBZ: Total cholesterol P<.0001; LDL cholesterol P< .0001; non‐HDL‐cholesterol P< .0001; HDL cholesterol P< .0001; triglycerides P> .05. ANCOVA, analysis of covariance; CBZ, carbamazepine; HDL, high‐density lipoprotein; LCM, lacosamide; LDL, low‐density lipoprotein
3.4. Proportion of patients with lipid levels above the upper limit of the reference range
In the 12‐month population, 37.0% of patients taking LCM and 30.8% of patients taking CBZ had TC levels above the upper limit of the reference range (200 mg/dL) at Baseline. LDL‐C above the limit of the reference range (130 mg/dL for adults, and 110 mg/dL for those under 18 years of age) was observed in 26.1% of patients taking LCM and 19.8% of patients taking CBZ (Table 1).
In the LCM group, the proportion of patients with TC or LDL‐C levels above the upper limit of the reference range remained almost unchanged from Baseline after 12 months of treatment (TC 34.8%; LDL‐C 23.9%) (Figure 2). In the CBZ group, the proportion of patients with TC or LDL‐C levels above the upper limit of the reference range was markedly higher after 12 months compared with Baseline (TC 49.6%; LDL‐C 29.0%) (Figure 2). Results at the 3‐month time point were similar (Figure S4).
Figure 2.
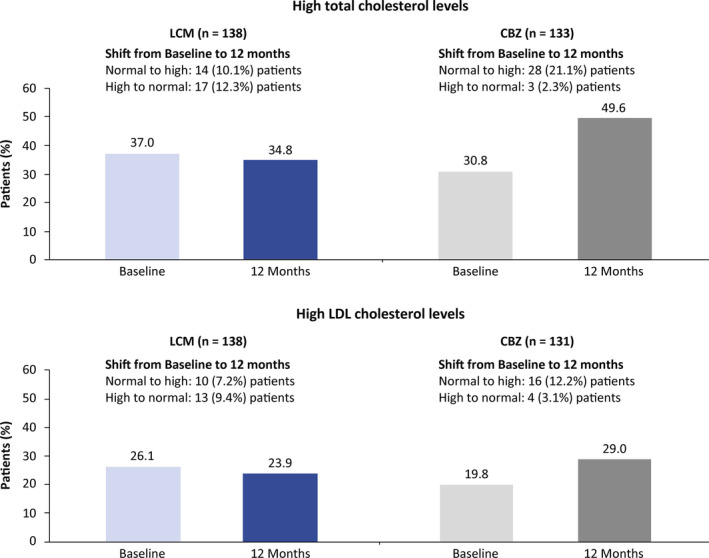
Proportion of patients with total cholesterol and/or low‐density lipoprotein (LDL) cholesterol levels above the upper limit of the reference range at Baseline and 12 mo (12‐mo population). Reference ranges for total cholesterol: normal was 130‐200 mg/dL, high was >200 mg/dL; reference ranges for LDL cholesterol: under 18 y of age, normal was 0‐110 mg/dL, high was >110 mg/dL; above 18 y of age, normal was 0‐130 mg/dL, high was >130 mg/dL. CBZ, carbamazepine; LCM, lacosamide; LDL, low‐density lipoprotein
3.5. Proportion of patients with large increases in lipid levels
For TC, LDL‐C, and non‐HDL‐C, the proportion of patients with ≥20 and ≥40 mg/dL increases between Baseline and 12 months was higher in patients treated with CBZ than in patients taking LCM (Figure 3). In the LCM group, increases of ≥20 mg/dL in TC, LDL‐C, and non‐HDL‐C were seen in 15.2%, 12.3%, and 12.3% patients, respectively; increases of ≥40 mg/dL in these levels were seen in 5.8%, 1.4%, and 3.6% patients, respectively. In patients on CBZ, the proportion of patients with an increase of ≥20 mg/dL was 48.9% for TC, 35.1% for LDL‐C, and 41.4% for HDL‐C. An increase of ≥40 mg/dL was observed in 21.8% for TC, 10.7% for LDL‐C, and 16.5% for non‐HDL‐C. Again, results at the 3‐month time point were similar (Figure S5).
Figure 3.
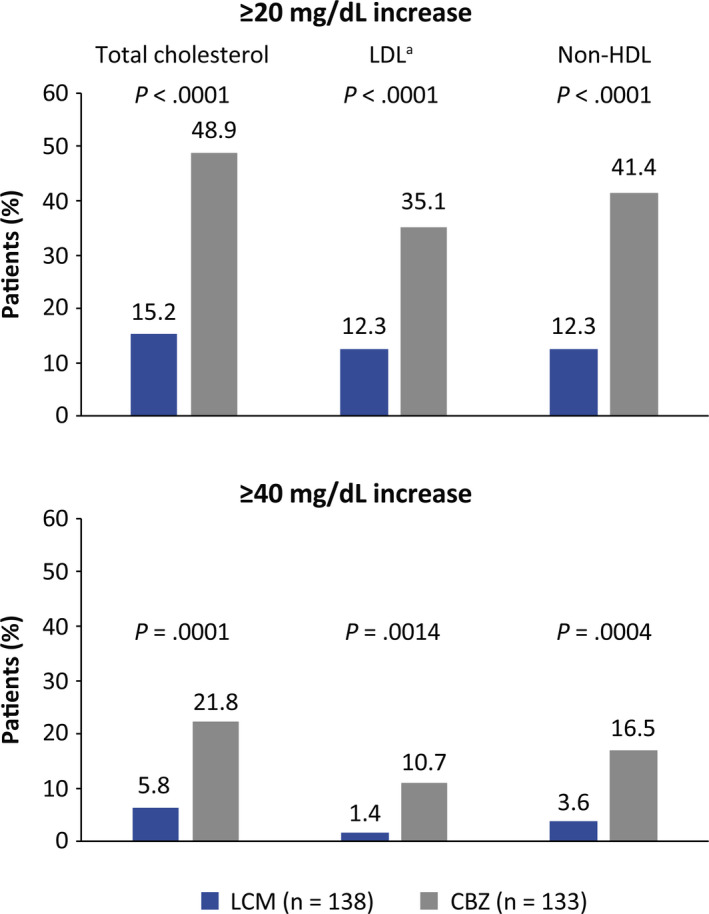
Proportion of patients with a ≥20 or ≥40 mg/dL increase in total cholesterol, LDL cholesterol, and non‐HDL cholesterol levels from Baseline to 12 mo (12‐mo population). P‐values are from Fisher exact test;an = 131 for CBZ. CBZ, carbamazepine controlled‐release; HDL, high‐density lipoprotein; LCM, lacosamide; LDL, low‐density lipoprotein; TC, total cholesterol
3.6. Relevance of baseline lipid levels on CBZ‐induced changes
A subsequent analysis was performed to determine if the impact of the drugs on lipids depended on whether or not lipids had been elevated at Baseline. Irrespective of whether TC was above or below 200 mg/dL at Baseline, or whether LDL‐C was above or below 130 mg/dL at Baseline, the effects of the anticonvulsants were similar: LCM did not increase lipid levels, whereas CBZ caused a similar rise in lipid levels in both subgroups (Table 2). Similar results were observed after 3 months of treatment (3‐month subpopulation; Table S3).
Table 2.
Total cholesterol and LDL cholesterol at Baseline and 12 mo and change from Baseline to 12 mo (12‐mo population) in patients with baseline levels below or above the upper limit of the reference range
| Treatment | n | Baseline, mean (SD) | 12‐mo treatment a , mean (SD) |
Analysis of covariance model b Change in lipid levels from Baseline at 12 mo |
|||
|---|---|---|---|---|---|---|---|
| n | LS mean (SE) | P‐value (treatment) | |||||
| Total cholesterol below upper limit of the reference range at Baseline c | |||||||
| LCM, mg/dL | 87 | 170.2 (23.3) | 173.6 (32.2) | 86 | 3.0 (2.6) | <.001 | |
| CBZ, mg/dL | 92 | 162.6 (28.2) | 185.60 (32.8)*** | 92 | 23.5 (2.6) | ||
| Total cholesterol above upper limit of the reference range at Baseline d | |||||||
| LCM, mg/dL | 51 | 225.6 (21.5) | 214.9 (30.6)** | 51 | −9.3 (3.5) | <.001 | |
| CBZ, mg/dL | 41 | 228.0 (20.2) | 243.7 (29.3)*** | 41 | 15.9 (3.9) | ||
| LDL cholesterol below upper limit of the reference range at Baseline e | |||||||
| LCM, mg/dL | 102 | 97.6 (20.8) | 98.4 (26.7) | 101 | 0.8 (2.0) | <.001 | |
| CBZ, mg/dL | 105 | 90.2 (23.1) | 103.7 (27.8)*** | 105 | 13.5 (2.0) | ||
| LDL cholesterol above upper limit of the reference range at Baseline f | |||||||
| LCM, mg/dL | 36 | 146.4 (15.0) | 137.5 (24.3)* | 36 | −8.3 (3.9) | .003 | |
| CBZ, mg/dL | 26 | 151.9 (13.9) | 161.1 (23.1) | 26 | 10.5 (4.6) | ||
Abbreviations: ANCOVA, analysis of covariance; CBZ, carbamazepine; LCM, lacosamide; LDL, low‐density lipoprotein; LS, least squares; SD, standard deviation; SE, standard error.
The 12‐mo lipid levels included lipid values collected at 364 d (plus a 30‐d window) of treatment during the Treatment period.
The ANCOVA model included treatment as a main effect and age, sex, body mass index, and Baseline lipid level as covariates.
Total cholesterol levels ≤200 mg/dL.
Total cholesterol levels >200 mg/dL.
LDL‐cholesterol levels ≤130 mg/dL.
LDL‐cholesterol levels >130 mg/dL. Within treatment group comparison 12‐mo vs Baseline; paired t test.
P < .05.
P < .01.
P < .001.
4. DISCUSSION
Randomized data on the effects of anticonvulsants on lipids have been scarce, with nearly all studies having been conducted using a cross‐sectional or repeated‐measures design. The only randomized study in this area published to date yielded data that were somewhat inconsistent, for reasons that remain unclear. 7 Here, we demonstrate in an unambiguous fashion, using a randomized design, that CBZ administration leads to elevated serum lipid concentrations. The magnitude of this increase is quite consistent with that seen in other studies, all of which show that CBZ increases TC by an average of 20‐25 mg/dL. 6 , 10 , 11 , 12 Showing this in a randomized study is important, because such a design is the best way to minimize possible bias. Even findings of the repeated‐measures studies, in which patients were switched from CBZ to other anticonvulsants, cannot exclude the possibility that certain patients were more likely to be switched than others; this could, theoretically, affect the generalizability of the findings. Randomization eliminates this potential bias, providing both definitive and generalizable demonstration of the hyperlipidemic properties of CBZ.
The randomized design of this study also permitted verification of other findings. Analyses carried out at 3 and 12 months indicate that the hyperlipidemic effect of CBZ is present early and remains unchanged over time, consistent with earlier work. 13 Our subgroup analysis indicates that CBZ raises the level of lipids comparably, regardless of baseline lipid levels. This has not been examined previously, to our knowledge.
Furthermore, we demonstrate here that LCM has no appreciable effect on lipids. This is consistent with the lack of effect of the drug on the CYP system. It is noteworthy that LCM, which acts on the sodium channel, does not alter lipid levels; the same appears to be true of lamotrigine (LTG). 13 This suggests that the lipid‐elevating properties of CBZ and phenytoin are very likely unrelated to their anticonvulsant mechanism at the sodium channel. The accumulated evidence from this and other studies is consistent with the hypothesis that it is the CYP induction effects of CBZ (and other anticonvulsants such as phenytoin) that are responsible for lipid elevation.
Our findings differ from those of the only other comparative randomized study to look at vascular risk markers in patients treated with anticonvulsants. 7 The latter investigation found that lipid fractions in patients treated with CBZ were elevated over those of non‐inducing agents—in this case, LTG and levetiracetam (LEV)—to varying degrees, which were significant in some cases and borderline or not significant in other cases. There were even some differences observed between the LTG‐ and LEV‐treated groups. This was in contrast to other research, 6 for reasons that were unclear. The present study yielded data very much aligned with those in the existing literature, suggesting that the former study, insofar as its results were inconsistent with existing literature, may simply have been a statistical fluke.
One major limitation of our study is the number of variables that may influence lipid levels for which we have no data and therefore cannot account for; these include physical exercise, smoking, and alcohol consumption. The ANCOVA model in our analysis included only treatment as a main effect, with age, sex, Baseline body mass index, and Baseline lipid level as covariates. Given the randomized nature of treatment assignment, and the fact that each patient served as their own control, we believe it is doubtful that the variables for which data were not collected or included in the ANCOVA model, would account for our findings. Another limitation is that this study was a post hoc analysis of a clinical trial powered for efficacy against seizures and not for serologic outcomes; as such, our findings should be interpreted with that in mind. This is very unlikely to have affected the findings, as the outcome measures in this analysis are objective and not subject to biased reporting or interpretation. Furthermore, the population analyzed only included patients not taking lipid‐lowering agents and who provided blood samples at fasted conditions at two timepoints, which limited the number of patients analyzed. In addition, the Baseline characteristics of the patients included in this lipid analysis were generally similar to the overall trial population. 9 Nevertheless, the number of individuals included in this analysis was more than sufficient to detect any change in lipids that would have been clinically relevant.
Several anticonvulsants have now been shown to be noninferior to CBZ in randomized initial monotherapy trials. 14 , 15 , 16 The use of a noninducing agent avoids both the chronic effects on lipids and the numerous drug interactions seen with CBZ, both of which are well documented and clinically relevant. 17 , 18 Consideration should be given to checking serum lipids in patients treated with CBZ (and possibly other serologic risk markers such as CRP and lipoprotein(a) in patients who may be at risk). When levels of these markers are found to be elevated, it may be difficult to treat the patients given the interaction between CBZ and most β‐hydroxy β‐methylglutaryl‐CoA reductase inhibitors. 7 It also is poor practice to use a medicine to treat the side effects of another medicine. In such cases, switching from CBZ to a noninducing agent will result in rapid and complete reversal of hyperlipidemic effects. This has been demonstrated for several agents, including LCM. 8 , 12
In conclusion, we have demonstrated two important outcomes from our analysis using data from a randomized clinical trial: first, that CBZ unequivocally produces increases in serum lipids; and second, that LCM does not increase lipid levels. These data add to the body of literature suggesting that noninducing anticonvulsants should be strongly preferred for treatment of focal seizures.
CONFLICT OF INTERESTS
Dr Mintzer has served as a speaker and has received research funding from UCB Pharma. Svetlana Dimova, Björn Steiniger‐Brach, Marc De Backer, Daya Chellun, and Robert Roebling are employees of UCB Pharma. Ying Zhang was an employee of UCB Pharma at the time this analysis was conducted. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Appendix S1
ACKNOWLDGMENTS
This trial and post hoc analysis were funded by UCB Pharma. The authors thank the patients and their caregivers, the clinical project team, and the investigators and their teams who contributed to this trial. The authors also acknowledge Fabien Debailleul, PhD (UCB Pharma, Brussels, Belgium) for managing the development of the manuscript, and Emily Chu, PhD (Evidence Scientific Solutions, London, UK) and Richard Fay, PhD, CMPP (Evidence Scientific Solutions, Philadelphia, PA) for editorial assistance, which was funded by UCB Pharma. The sponsor was responsible for the design of the trial and post hoc analysis, and for the analysis of data collected by the investigators. Data were interpreted by the authors and the sponsor. The sponsor was involved in the review of the manuscript and the decision to submit for publication. All authors approved the final version of the manuscript for publication.
Mintzer S, Dimova S, Zhang Y, et al. Effects of lacosamide and carbamazepine on lipids in a randomized trial. Epilepsia. 2020;61:2696–2704. 10.1111/epi.16745
DATA AVAILABILITY STATEMENT
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents, which may include analysis‐ready data sets, study protocol, annotated case report form, statistical analysis plan, data set specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and an assigned data‐sharing agreement will need to be executed. All documents are available in English only, for a pre‐specified time, typically 12 months, on a password‐protected portal.
REFERENCES
- 1. Errington AC, Stohr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage‐gated sodium channels. Mol Pharmacol. 2008;73:157–69. [DOI] [PubMed] [Google Scholar]
- 2. Doty P, Hebert D, Mathy FX, Byrnes W, Zackheim J, Simontacchi K. Development of lacosamide for the treatment of partial‐onset seizures. Ann N Y Acad Sci. 2013;1291:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawello W, Stockis A, Andreas JO, Dimova S. Advances in epilepsy treatment: lacosamide pharmacokinetic profile. Ann N Y Acad Sci. 2014;1329:18–32. [DOI] [PubMed] [Google Scholar]
- 4. Mintzer S. Metabolic consequences of antiepileptic drugs. Curr Opin Neurol. 2010;23:164–9. [DOI] [PubMed] [Google Scholar]
- 5. Mintzer S, Mattson RT. Should enzyme‐inducing antiepileptic drugs be considered first‐line agents? Epilepsia. 2009;50(Suppl 8):42–50. [DOI] [PubMed] [Google Scholar]
- 6. Mintzer S, Skidmore CT, Abidin CJ, Morales MC, Chervoneva I, Capuzzi DM, et al. Effects of antiepileptic drugs on lipids, homocysteine, and C‐reactive protein. Ann Neurol. 2009;65:448–56. [DOI] [PubMed] [Google Scholar]
- 7. Mintzer S, Trinka E, Kraemer G, Chervoneva I, Werhahn KJ. Impact of carbamazepine, lamotrigine, and levetiracetam on vascular risk markers and lipid‐lowering agents in the elderly. Epilepsia. 2018;59:1899–907. [DOI] [PubMed] [Google Scholar]
- 8. Elger CE, Rademacher M, Brandt C, Elmoufti S, Dedeken P, Eckhardt K, et al. Changes in hormone and lipid levels in male patients with focal seizures when switched from carbamazepine to lacosamide as adjunctive treatment to levetiracetam: A small phase IIIb, prospective, multicenter, open‐label trial. Epilepsy Behav. 2016;62:1–5. [DOI] [PubMed] [Google Scholar]
- 9. Baulac M, Rosenow F, Toledo M, Terada K, Li T, De Backer M, et al. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled‐release carbamazepine in patients with newly diagnosed epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2017;16:43–54. [DOI] [PubMed] [Google Scholar]
- 10. Isojarvi JI, Pakarinen AJ, Myllyla VV. Serum lipid levels during carbamazepine medication. A prospective study. Arch Neurol. 1993;50:590–3. [DOI] [PubMed] [Google Scholar]
- 11. Bramswig S, Sudhop T, Luers C, von Bergmann K, Berthold HK. Lipoprotein(a) concentration increases during treatment with carbamazepine. Epilepsia. 2003;44:457–60. [DOI] [PubMed] [Google Scholar]
- 12. Mintzer S, Skidmore CT, Rankin SJ, Chervoneva I, Pequinot E, Capuzzi DM, et al. Conversion from enzyme‐inducing antiepileptic drugs to topiramate: effects on lipids and C‐reactive protein. Epilepsy Res. 2012;98:88–93. [DOI] [PubMed] [Google Scholar]
- 13. Mintzer S, Miller R, Shah K, Chervoneva I, Nei M, Skidmore C, et al Long‐term effect of antiepileptic drug switch on serum lipids and C‐reactive protein. Epilepsy Behav. 2016;58:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brodie MJ, Perucca E, Ryvlin P, Ben‐Menachem E, Meencke HJ, Levetiracetam Monotherapy Study Group . Comparison of levetiracetam and controlled‐release carbamazepine in newly diagnosed epilepsy. Neurology. 2007;6(68):402–8. [DOI] [PubMed] [Google Scholar]
- 15. Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled‐release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurol. 2012;11:579–88. [DOI] [PubMed] [Google Scholar]
- 16. Trinka E, Ben‐Menachem E, Kowacs PA, Elger C, Keller B, Loffler K, et al. Efficacy and safety of eslicarbazepine acetate versus controlled‐release carbamazepine monotherapy in newly diagnosed epilepsy: A phase III double‐blind, randomized, parallel‐group, multicenter study. Epilepsia. 2018;59:479–91. [DOI] [PubMed] [Google Scholar]
- 17. Wurden C, Levy R. Carbamazepine: interactions with other drugs In: Levy R, Mattson R, Meldrum B, Perucca E, editors. Antiepileptic Drugs. Philadelphia, PA: Lippincott Williams & Wilkins, 2002; p. 247–261. [Google Scholar]
- 18. Brodie MJ, Mintzer S, Pack AM, Gidal BE, Vecht CJ, Schmidt D. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia. 2013;54:11–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents, which may include analysis‐ready data sets, study protocol, annotated case report form, statistical analysis plan, data set specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and an assigned data‐sharing agreement will need to be executed. All documents are available in English only, for a pre‐specified time, typically 12 months, on a password‐protected portal.


