Abstract
Aim
Recently, microRNA‐149 (miR‐149) has been indicated to act as an oncogene or a tumor suppressor in various malignant tumors, while its inner mechanisms in recurrent miscarriage (RM) are still in infancy. Therein, this study intends to decode the mechanism of miR‐149 in RM.
Methods
miR‐149 and RUNX2 expression in the chorionic tissues of normal pregnant women and RM patients were first examined, and the correlation between miR‐149 and RUNX2 was analyzed. Subsequently, miR‐149 was upregulated in HTR‐8 cells or downregulated in BEWO cells, and then the changes in biological functions of trophoblasts in RM were detected. Furthermore, the expression of PTEN/Akt signaling pathway‐related factors in trophoblasts was detected by western blot analysis.
Results
miR‐149 expression was increased while RUNX2 expression was suppressed in RM patients, and miR‐149 was negatively correlated with RUNX2. Overexpressed miR‐149 induced cell apoptosis and inhibited cell activity, while reduced miR‐149 in trophoblasts contributed to opposite experimental results. Moreover, miR‐149 promoted the expression of PTEN and inhibited Akt phosphorylation by targeting RUNX2, thereby inhibiting trophoblast activity and promoting their apoptosis.
Conclusion
Our study demonstrates that miR‐149 knockdown halted the RM development through upregulating RUNX2 and activation of the PTEN/Akt signaling pathway.
Keywords: cell activity, microRNA‐149, PTEN/Akt signaling pathway, recurrent miscarriage, RUNX2
Introduction
Recurrent miscarriage (RM) is defined as the spontaneous loss of no less than three consecutive pregnancies in the first trimester. RM usually influences 1–2% of women, but half of the cases have no identifiable reason. 1 RM can be regarded as a primary or secondary process based upon if a live birth has happened at some time. 2 Established risk factors for RM are multifactorial, such as successive previous pregnancy losses, structural uterine anomalies, parental chromosomal anomalies, as well as maternal thrombophilia disorders. 3 Treatment strategies like Aspirin and low molecular weight heparin are considered the standard medications in RM, but their benefit on live birth rate has only been proved in a few placebo‐controlled trials. New treatment options, such as drugs like tumor necrosis factor inhibitors and granulocyte colony‐stimulating factor, have been evidenced to be beneficial for RM therapy. 4 Given the severity and complex mechanism of RM, the need to unearth novel therapy is in urgency.
MicroRNAs (miRNAs) are post‐transcriptional modulators that are able to bind specifically to the 3′‐untranslated region (3′‐UTR) of target mRNAs, resulting in translational inhibition. 5 Lately, miRNAs have been established to function in numerous aspects of tumor development, tumorigenesis and metastasis. 6 In addition, many candidate miRNAs have been detected to be promising biomarkers for some kinds of specific pregnancy disorders. 7 For instance, five miRNAs (hsa‐miR‐517c, hsa‐miR‐519a‐1, hsa‐miR‐522, hsa‐miR‐520h and hsa‐miR‐184) were found to be overexpressed in the decidua of patients suffered from recurrent spontaneous abortion. 8 miR‐133a has also been implicated to be related to recurrent spontaneous abortion. 9 Has‐miR‐149 is positioned on chromosome 2, and it is a vital miRNA implicated in human malignancies. 10 Intriguingly, miR‐149 could act as an oncogene or a tumor suppressor, which is dysregulated in various malignant tumors. 11 A prior sequencing study has revealed that many differentially expressed miRNAs are related to preeclampsia development, and miR‐149 is elevated during preeclampsia. 12 Runx transcription factors are demonstrated to be evolutionarily‐conserved modulators of cell fate, among which there are three family members in mammals (RUNX1‐3). 13 RUNX2 has been identified to be expressed in the human placenta, especially in villous cytotrophoblast and extravillous trophoblast cell populations. 14 In combination, the integrated functions of miR‐149 and RUNX2 in RM is still in ambiguity. Moreover, trophoblast invasion has been found to be closely related to pathologies during pregnancy such as preeclampsia, RM, stillbirth and spontaneous abortion. 15 Thereafter, this study is initiated to uncover their underlying mechanism in RM using a cell model with trophoblasts.
Methods
Ethical approval
This study was carried out with the approval and supervision of the ethics committee of Tianjin Central Hospital of Obstetrics and Gynecology, and the informed consents were obtained from all patients.
Antibodies, reagents and plasmids
The antibodies used for western blot analysis: RUNX2 (#12556, CST, Danvers, MA), Akt1 (ab235958, Abcam, Cambridge, UK), Akt1 phospho S473 (ab194201, Abcam), PTEN (#9188, CST), GAPDH (ab181602, Abcam), horseradish peroxidase (HRP)‐labeled IgG (ab6721, Abcam). The antibodies used for immunofluorescence assay: E‐cadherin (#14472, CST), Vimentin (#5741, CST), Alexa Fluor 488 labeled goat anti‐mouse IgG (ab150077, Abcam). A PTEN signaling pathway‐specific inhibitor SF1670 was purchased from Abcam (#ab141303). miR‐149 mimic/inhibitor and negative controls were purchased from GenePharma (Shanghai, China).
Clinical sample collection
This study enrolled 21 RM patients with normal karyotype, 9 RM patients with abnormal karyotype as well as 13 patients received induced abortion at the reproductive medicine center of Tianjin Central Hospital of Obstetrics and Gynecology from June 2016 to December 2017. The following cases were excluded: (a) Symptoms of endocrine or metabolic diseases, such as hypothyroidism and diabetes; (b) Abnormal karyotype analysis; (c) Uterine abnormalities confirmed by pelvic examination and ultrasound and (d) antiphospholipid syndrome. RM was defined as two or more consecutive miscarriages during the first 20 weeks of pregnancy. Clinical baseline information for patients is listed in Table 1.
Table 1.
Clinical characteristics of the study population
| Parameters | Control (n = 13) | RM (n = 21) | p Value |
|---|---|---|---|
| Maternal age (y) | 26.98 ± 3.14 | 27.51 ± 3.27 | 0.63 |
| Body mass index (kg/m2) | 21.65 ± 5.94 | 23.82 ± 6.63 | 0.34 |
| Gestation age (weeks) | 7,62 ± 1,81 | 7.96 ± 2.15 | 0.57 |
| Number of miscarriage | 0.71 ± 0.24 | 2.94 ± 0.83 | <0.01 |
| Number of live births | 1.59 ± 0.37 | 0.00 ± 0.00 | <0.01 |
| Number of pregnancies | 2.61 ± 0.52 | 2.79 ± 0.76 | 0.46 |
| Exposure to GC | 1 (7.69%) | 9 (42.86%) | <0.01 |
Abbreviation: GC, glucocorticoid.
RT‐qPCR
Total RNA in trophoblasts and placental chorionic tissues was extracted using a Trizol kit (Invitrogen; Thermo Fisher Scientific, Waltham, MA), and RNA concentration was tested by Nanodrop 2000 (Thermo Fisher Scientific). Takara Taq polymerase (Takara Bio, Inc., Otsu, Japan) was used for DNA amplification. Commentary DNA was synthesized using a PrimeScript RT kit (Takara Biotechnology, Co., Ltd., Dalian, China). qPCR was performed with the application of the SYBR Premix EX Taq Kit (Takara). The expression of miR‐149 was normalized to the endogenous expression of U6, and the relative expression of mRNAs was normalized to GAPDH. The relative expression of target genes was evaluated by the 2−ΔΔCt method. The PCR primers are listed in Table 2.
Table 2.
Primers for RT‐qPCR
| Targets | Prime sequences (5′–3′) |
|---|---|
| miR‐149 | Forward: CUUCGGAUCAGUGAAUGCUTT |
| Reverse: AGCAUUCACUGAUCCGAAGTT | |
| U6 | Forward: CTCGCTTCGGCAGCACA |
| Reverse: AACGCTTCACGAATTTGCGT | |
| RUNX2 | Forward: GCGUCUGUGUGAUUUGUAUTT |
| Reverse: AUACAAAUCACACAGACGCTT | |
| GAPDH | Forward: GCACCAGGCAGTGTAGTTAGC |
| Reverse: GTGCAGGGTCCGAGGT |
Abbreviations: RT‐qPCR, reverse transcription quantitative polymerase chain reaction; miR‐149, microRNA‐149; RUNX2, runt‐related transcription factor 2; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase.
Cell culture
The HTR‐8/SVneo trophoblast cell line was obtained from the China Center for Type Culture Collection (Beijing, China), and JAR and BEWO cells were available from the Chinese Academy of Sciences (Shanghai, China). Next, cells were cultured under standard conditions in high‐glucose DMEM/RPMI‐1640 medium/F‐12 K medium (Gibco, Life Technologies, Gaithersburg, MD) comprising 10% fetal bovine serum. HTR‐8 cells with good growth conditions were introduced with miR‐149 mimic or mimic control. After 48 h, RT‐qPCR was implemented to detect miR‐149 expression in the cells to verify the successful transfection. Subsequently, HTR‐8 cells overexpressing miR‐149 were further treated with SF1670 (2 μM) for 2 h. 16 , 17 BEWO cells with good growth conditions were introduced with miR‐149 inhibitor or inhibitor control. After 48 h, RT‐qPCR was implemented to detect miR‐149 expression in the cells to verify the successful transfection. Next, siRNA against RUNX2 was further delivered into miR‐149‐transfected cells, and RT‐qPCR was utilized to detect RUNX2 expression in the cells after 48 h to confirm the successful intervention.
CCK‐8
Cells were seeded on 96‐well plates (2000 cells in each well). Afterward, the cells were incubated for the time points of 0, 24, 48 and 72 h, separately, and each well was appended with 10 μl CCK‐8 solution (C0038, Beyotime, Shanghai, China). After 1‐h incubation, the measurement of the optical density (OD) value was carried out at 450 nm with the application of a microtiter plate reader (Molecular Devices, CA). Finally, the results were counted as the average of three measurements with the same conditions.
Colony formation assay
Twenty‐four hours after transfection, the single‐cell suspension was obtained by trypsinization. Five hundred cells were seeded in six‐well plates and subsequently, cultured with the complete medium in a 5% CO2 incubator for 2 w. Cells were fixed with 4% paraformaldehyde (PFA; Biosharp, Hefei, China) for 20 min, and stained with 1% crystal violet solution (KeyGen BioTECH, Jiangsu, China) for 15 min. The number of colony‐forming units was calculated in three random wells.
EdU staining
Cells were seeded at 5 × 104 cells/ml into a 24‐well culture plate. EdU staining was implemented using a Cell‐Light EdU DNA Cell Proliferation Kit (RiboBio, Guangzhou, Guangdong, China) in the light of the manufacturer's protocol. After that, the cells were cultured with EdU (1: 1000) for 8 h, then fixed with PFA and infiltrated with 0.3% Triton X‐100 for 10 min. Next, 4′,6‐diamidino‐2‐phenylindole was used for 5‐min nuclei staining, and images were captured using an Olympus laser scanning microscope system.
Carboxyfluorescein succinimidyl ester (CFSE)
CFSE is a fluorescent dye that penetrates the cell membrane and has succinimidyl ester specifically bound to the cell and fluorescein diacetate with non‐enzymatic hydrolysis, which makes CFSE a good cell marker. When CFSE exists in the form of two acetic acid groups and a succinimidyl ester functional group, it has no fluorescence property, but has cell membrane permeability and is able to enter cells freely. And when it spreads into the intracellular environment, the endogenous esterase can hydrolyze its acetic acid group. This form of CFSE molecule has high fluorescence activities, which can produce green fluorescence, but no longer has membrane permeability. At the same time, the succinimidyl ester group can react with the free amine group in the cytoskeleton protein, and finally, form the protein adduct with fluorescence. When cells proliferate, the fluorescent proteins are distributed equally to cells at passage two, which reduces the fluorescence intensity to half compared with cells at passage one. The fluorescence intensity of cells at passage three was weaker than that of cells at passage two. By detecting the decrease of cell fluorescence intensity, cell proliferation can be further analyzed by using a flow cytometer at 488 nm. Therefore, CellTrace CFSE Cell Proliferation Kits (Invitrogen, Carlsbad, CA) were used for analysis. Seeded at 1.0 × 105 cells/plate in the six‐well plates, cells in each group were harvested after 3‐day incubation. Analysis of cell proliferation was performed with the application of a flow cytometer (FACSCalibur, BD Biosciences). The calculation of the proliferation index was realized by Modfit LT (Verity Software House, Topsham, ME).
Flow cytometry
The transfected cells of each group were trypsinized at 37°C for 2 min. Apoptosis rates of trophoblast cells were determined using an Annexin V‐FITC Apoptosis Detection Kit (Abcam) referring to the manufacturer's protocol. Prior to counting, cells were stained with 5 μl Annexin V and propidium iodide (PI, BD Bioscience, Franklin Lakes, NJ) for 15 min at 37°C. Flow cytometer (EPICS XL; Beckman Coulter, Inc., Fullerton, CA) and FCS Express 3.0 software (DeNovo Software, Glendale, CA) was then applied to determine the apoptosis rate of transfected cells.
As for cell cycle distribution, cells were then suspended in staining solution (P4170, Sigma Aldrich) to analyze the cell cycle arrest with a flow cytometer (BD Biosciences).
Hoechst 33258 staining
For Hoechst staining, the treated ARPE‐19 cells were cultured in a six‐well plate with 2 ml culture medium containing Hoechst 33258 blue fluorescent nuclear dye (Sigma‐Aldrich) for 30 min. The nuclear morphology was measured by fluorescence microscopy.
Immunofluorescence assay
Cell slides were fixed in 4% PFA overnight and infiltrated with 0.025% Triton X‐100 in PBS. After incubation with the primary antibody and anti‐mouse IgG (Alexa Fluor 488, Abcam) secondary antibody, the slides were imaged using a laser scanning confocal microscope Zeiss LSM 510 META. Five fields of view were randomly selected for photographing.
Transwell assay
To assess cell migration and invasiveness, Transwell analysis was performed using uncoated Matrigel and Matrigel‐coated Boyden chamber (BD Biosciences), respectively. Stable cell serum was starved overnight, harvested and resuspended in a serum‐free medium. Cells (1 × 105) were then plated into the apical chamber, and a 10% FBS‐containing medium was appended to the basolateral chamber. After a 24‐h incubation, the migrated and invaded cells attached to the subsurface of the membrane were fixed with formalin, stained with 0.5% crystal violet solution and counted with a microscope.
Dual‐luciferase reporter gene assay
The synthetized 3′UTR of RUNX2 cDNA containing miR‐149 binding site and mutation site were cloned into pGL3 vector (Promega, Madison, WI) to generate pGL3‐RUNX2‐wild type (Wt) and pGL3‐RUNX2‐mutant type (Mut) plasmids, respectively. HEK293T cells were seeded into a 24‐well plate at 2 × 105 cells/well and cultured for 24 h. Next, 200 ng pGL3‐RUNX2‐Wt or pGL3‐RUNX2‐Mut plasmid was co‐transfected into 293 T cells with miR‐149 mimic or mimic control using Lipofectamine 2000 (Invitrogen). The dual‐luciferase reporter gene analysis system (Promega) was then used to assess luciferase activity. Firefly luciferase activity was standardized to renilla luciferase activity.
Western blot assay
To determine protein expression, cells were lysed in a pre‐chilled RIPA lysis buffer (Beyotime) with a freshly‐added proteinase inhibitor mixture (Hoffman‐La Roche Ltd., Basel, Switzerland). Protein concentration was quantified by a dicaprylic acid assay kit (Thermo Fisher Scientific). The same amount of protein was separated by SDS‐PAGE and subjected to western blot analysis 18 as previously described. The polyvinylidene difluoride membrane was incubated with the primary antibody and then washed three times with pre‐chilled TBST. After incubation with an HRP‐labeled secondary antibody for 2 h, the protein bands were analyzed using an ECL hypersensitivity chemiluminescence kit (Beyotime). At least three experiments were performed independently.
Statistical analysis
Statistical analysis was carried out by SPSS 22.0 software (SPSS Inc, Chicago, IL). The experiment data were depicted as a mean ± standard deviation. Comparisons between two groups were performed using unpaired t test. One‐way analysis of variance (ANOVA) or two‐way ANOVA was adopted for comparisons among multiple groups, after which Tukey's multiple comparisons test was applied for pairwise comparisons. Pearson's correlation test was used to analyze the correlation between miR‐149 and RUNX2 expression in RM patients. P < 0.05 was regarded as statistical significance.
Results
miR‐149 is highly expressed in RM patients
We first detected miR‐149 expression in placental chorionic tissues of 21 RM patients and 13 controls by RT‐qPCR. We found that miR‐149 expression in RM patients was significantly increased (Fig. 1a). We further tested the expression of miR‐149 in the placental chorionic tissues of nine patients with abnormal karyotypes and found no significant difference between the expression of miR‐149 in the chorionic tissues of patients with abnormal karyotypes and patients with RM caused by normal karyotypes (Fig. S1). Subsequently, we tested miR‐149 expression in HTR‐8, BEWO and JEG3 cells and found that miR‐149 expression in HTR‐8 cells was relatively low, thus we transfected miR‐149 mimic and corresponding mimic control into HTR‐8 cells. On the contrary, miR‐149 expression in BEWO cells was relatively high, thus we transfected miR‐149 inhibitor and corresponding inhibitor control into BEWO cells. We confirmed by RT‐qPCR that the HTR‐8 cell line overexpressing miR‐149 and the BEWO cell line with miR‐149 knockdown were successfully developed (Fig. 1b,c).
Figure 1.
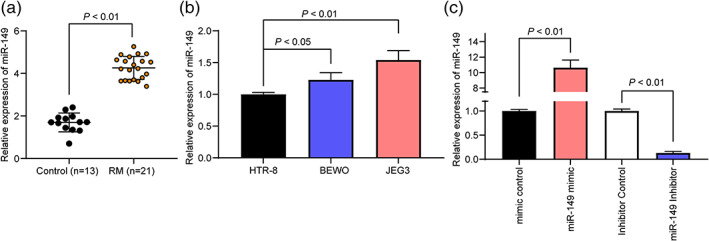
miR‐149 is highly expressed in RM patients. (a) miR‐149 expression in placental chorionic tissues of 21 RM patients and 13 controls detected by RT‐qPCR. (b) miR‐149 expression in HTR‐8, BEWO and JEG3 cells detected by RT‐qPCR. (c) RT‐qPCR was utilized to detect miR‐149 expression in HTR‐8 and BEWO cells. Comparisons between the two groups were performed using an unpaired t test (panel a). One‐way ANOVA was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test (panel b and c).
miR‐149 inhibitor promotes the activity of trophoblasts
According to CCK‐8 assay, we found that in HTR‐8 cells overexpressing miR‐149, the OD value at 450 nm was reduced from 24 h, while in BEWO cells with low miR‐149 expression, cell activity was enhanced from 24 h (Fig. 2a). Next, we detected cell proliferation through colony formation assay and EdU staining and found that high miR‐149 expression inhibited the proliferation of HTR‐8 cells, while downregulation of miR‐149 promoted the proliferation of BEWO cells (Fig. 2b,c). Furthermore, results from CFSE and flow cytometry suggested that miR‐149 mimic reduced the number of CFSE‐positive cells in HTR‐8 cells, while miR‐149 inhibitor had the opposite effect in BEWO cells (Fig. 2d).
Figure 2.
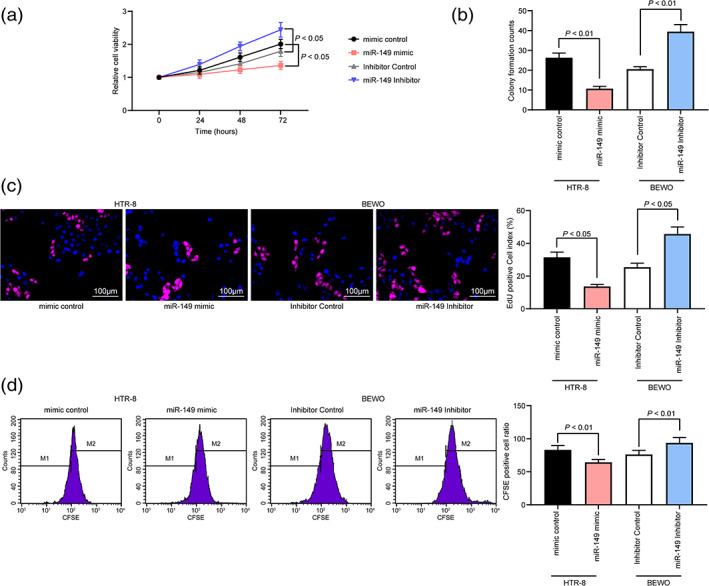
miR‐149 inhibitor promotes the activity of trophoblasts. (a) CCK‐8 assay for cellular activity of HTR‐8 and BEWO cells. (b and c) Detection of cell proliferation levels by colony formation assay and EdU staining. (d) Cells were stained with CFSE and the cells were tested for cell viability by flow cytometry. Two‐way (panel a) or one‐way ANOVA (panel b–d) was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test.
miR‐149 inhibitor suppresses trophoblast apoptosis
Then, we further detected the number of apoptotic cells by Hoechst 33258 staining. The results showed that miR‐149 overexpression resulted in an elevated number of apoptotic HTR‐8 cells, while inhibition of miR‐149 in BEWO cells reduced cell apoptosis (Fig. 3a). Subsequently, we further assessed the cell cycle distribution by flow cytometry. The corresponding results implied that HTR‐8 cells with miR‐149 overexpression arrested more cells in the G0/G1 phase and fewer cells in the S phase, with no significant changes in the G2/M phase. On the contrary, in BEWO cells with inhibited miR‐149, more cells arrested in the S phase, while fewer cells in the G0/G1 phase (Fig. 3b). This shows that miR‐149 promotes G0/G1 cell cycle arrest in cells, while inhibition of miR‐14 promotes cellular progression. Therefore, we further detected cell apoptosis by flow cytometry. We found that overexpression of miR‐149 promoted apoptosis in HTR‐8 cells, but inhibition of miR‐149 reduced apoptosis in BEWO cells (Fig. 3c).
Figure 3.
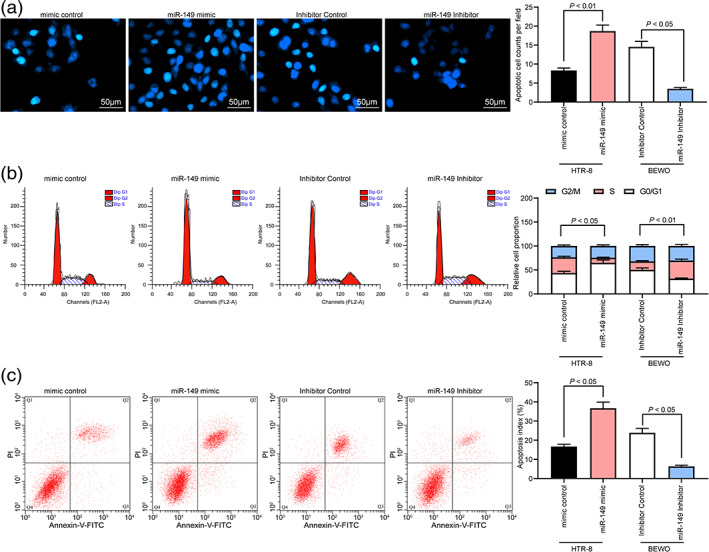
miR‐149 inhibitor inhibits trophoblast apoptosis. (a) The number of apoptotic cells detected by Hoechst 33258 staining. (b) Cell cycle distribution of cells in each group detected by flow cytometry. (c) Cell apoptosis rate in each group detected by flow cytometry. One‐way ANOVA (panel a–c) was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test.
miR‐149 inhibitor enhances migration and invasion of trophoblasts
The next step was to determine E‐cadherin and Vimentin expression in HTR‐8 and BEWO cells by immunofluorescence assay. The findings outlined that overexpressed miR‐149 promoted E‐cadherin expression and suppressed Vimentin expression in HTR‐8 cells, while downregulation of miR‐149 in BEWO cells diminished E‐cadherin expression and enhanced Vimentin expression (Fig. 4a). Next, the migratory and invasive abilities of trophoblasts determined by Transwell assay suggested that upregulated miR‐149 in HTR‐8 cells reduced the number of HTR‐8 cells migrated/invaded into the basolateral compartment, but in BEWO cells with inhibited miR‐149, the number of cell migration and invasion was elevated (Fig. 4b,c).
Figure 4.
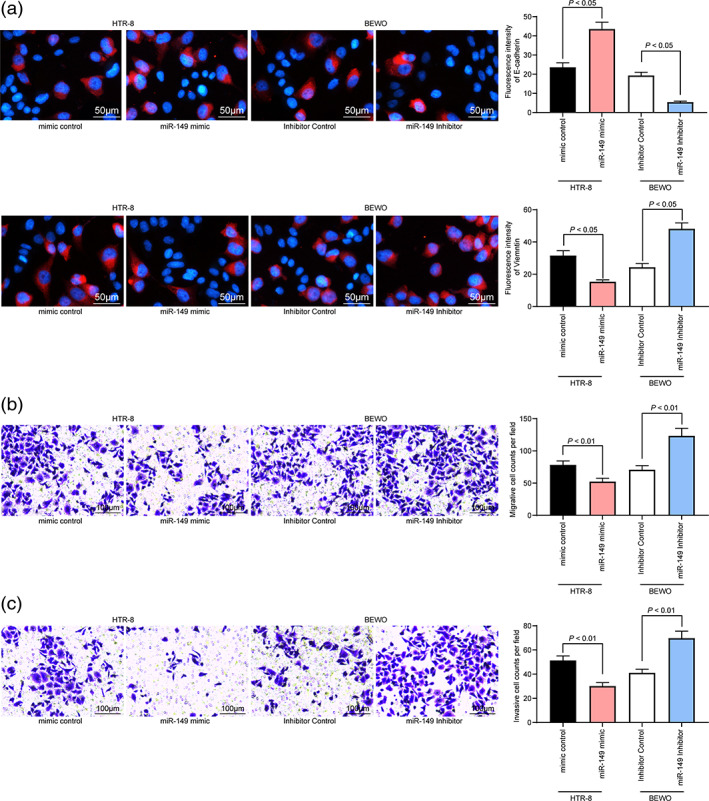
miR‐149 inhibitor promotes migration and invasion of trophoblasts. (a) Expression of E‐cadherin and Vimentin in HTR‐8 and BEWO cells determined by immunofluorescence assay. (b and c) The migration and invasion of HTR‐8 and BEWO cells determined by Transwell assay. One‐way ANOVA (panel a–c) was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test. [Correction added on 1 October, after first online publication: Figure 4 has been amended.]
miR‐149 blunts the PTEN/Akt signaling pathway by targeting RUNX2
In order to further verify the downstream molecular mechanism of miR‐149, we predicted the possible targeting mRNAs of miR‐149 through TargetScan, StarBase, miRDB and miRTarBase, and we screened a total of five genes that were related to miR‐149 (Fig. 5a). Subsequently, we predicted the binding sequence of miR‐149 to RUNX2 through StarBase (Fig. 5b). Based on this binding sequence, we designed a luciferase activity experiment to verify the binding relationship between miR‐149 and RUNX2 and found that luciferase activity was reduced in 293T cells transfected with miR‐149 mimic and RUNX2‐Wt (Fig. 5c). Meanwhile, RT‐qPCR was performed to detect the mRNA expression of RUNX2 in placental chorionic tissues of 21 RM patients and 13 controls. We found that the mRNA expression of RUNX2 in RM patients was reduced, and miR‐149 was negatively correlated with RUNX2 (Fig. 5d,e). Therefore, RUNX2 expression in HTR‐8 and BEWO cells was determined, the results of which mirrored that highly expressed miR‐149 inhibited RUNX2 expression, while the intervention of miR‐149 elevated RUNX2 expression (Fig. 5f,g). Moreover, PTEN/Akt signaling pathway activation was determined. It was found that overexpressed miR‐149 promoted PTEN expression and restricted phosphorylation of Akt in HTR‐8 cells, thereby promoting cell apoptosis. However, miR‐149 inhibitor in BEWO cells exhibited completely opposite experimental results (Fig. 5h).
Figure 5.
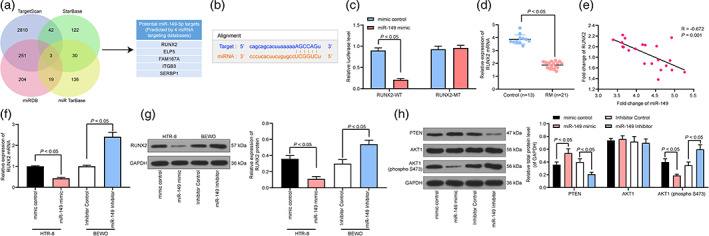
miR‐149 disrupts the PTEN/Akt signaling pathway by targeting RUNX2. (a) TargetScan, StarBase, miRDB and miRTarBase predicted possible targeting mRNAs of miR‐149. (b) StarBase predicted the targeted binding sequence of miR‐149 and RUNX2. (c) Luciferase activity experiment verified the binding relationship between miR‐149 and RUNX2. (d) The mRNA expression of RUNX2 in placental chorionic tissues of 21 RM patients and 13 controls was detected by RT‐qPCR. (e) Pearson's correlation test analyzed the relationship between miR‐149 and RUNX2 in RM patients. (f and g). The mRNA and protein expression of RUNX2 in HTR‐8 and BEWO cells determined by RT‐qPCR and western blot analysis. (h) The protein expression of PTEN and Akt1 as well as the extent of Akt1 phosphorylation determined by western blot analysis. Comparisons between the two groups were performed using an unpaired t test (panel d). Two‐way ANOVA (panel c and h) or one‐way ANOVA (panel f and g) was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test.
Silencing of RUNX2 or inhibition of the PTEN/Akt signaling pathway neutralizes the protective effect of the miR‐149 inhibitor
In the next step, we added the PTEN signaling pathway inhibitor SF1670 to miR‐149‐overexpressing cells, and further inhibited RUNX2 expression in BEWO cells with miR‐149 inhibitor. Western blot analysis and RT‐qPCR exhibited that further knockdown of RUNX2 expression in the presence of miR‐149 inhibitor significantly activated the PTEN/AKT signaling pathway (Fig. 6a). Meanwhile, cell proliferation ability was detected by EdU staining and CFSE staining, and the results revealed that SF1670 treatment inhibited the blocking effect of miR‐149 mimic on HTR‐8 cell activities, and inhibited apoptosis, as well as promoted cell migration and invasion. However, after RUNX2 was further silenced in BEWO cells, the cell activity was reduced, apoptotic cells were increased and the cell migration and invasion capabilities were decreased (Fig. 6b–f).
Figure 6.
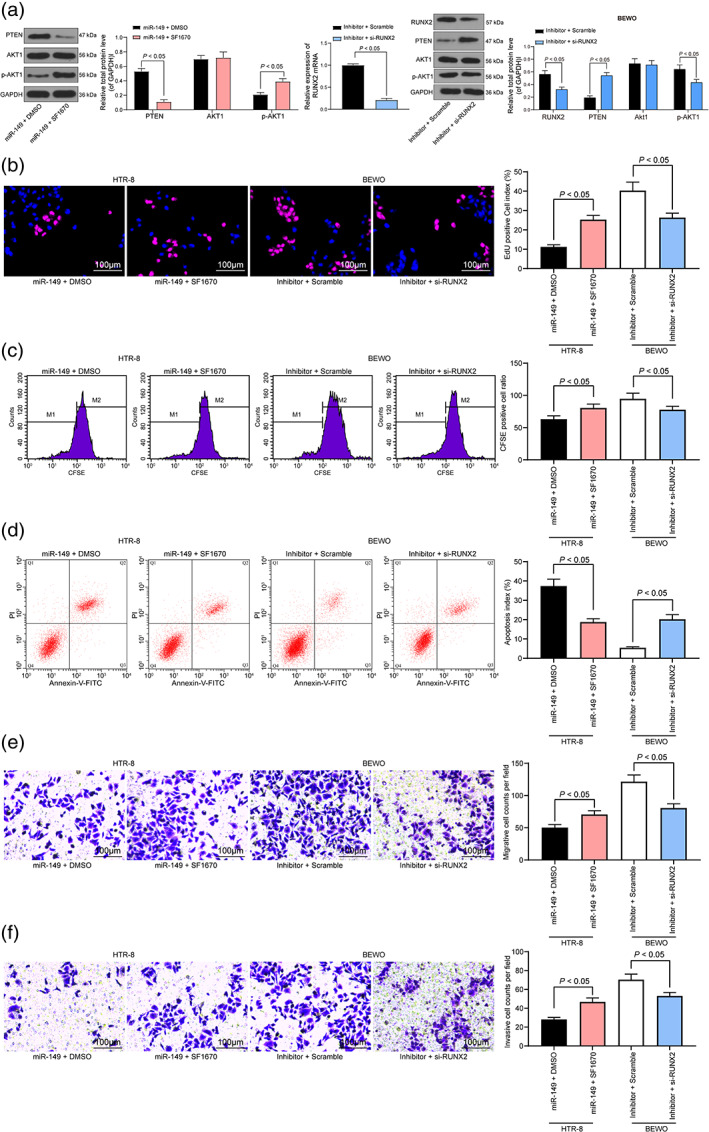
Silencing of RUNX2 or inhibition of the PTEN/Akt signaling pathway neutralizes the protective effect of miR‐149 inhibitor. The PTEN signaling pathway inhibitor SF1670 was added to miR‐149‐overexpressing cells, and RUNX2 was further inhibited in BEWO cells that interfered with miR‐149. (a) The expression of RUNX2, PTEN and Akt1 as well as the extent of Akt1 phosphorylation verified by western blot analysis and RT‐qPCR. (b) Detection of cell proliferation by EdU staining. (c) Cells were stained with CFSE, and cells were tested for cell viability by flow cytometry. (d) Detection of cell apoptosis by flow cytometry. (e and f) Transwell assay detected the migration and invasion of HTR‐8 cells and BEWO cells. Two‐way ANOVA (panel a) or one‐way ANOVA (panel b–f) was adopted for comparisons among multiple groups, followed by Tukey's multiple comparisons test.
Discussion
Recurrent miscarriage is a relatively rare disease with an estimated morbidity of 1–2%, while its etiology is not recognized in more than 50% of cases despite of years of investigation. 19 Evidence has recently indicated that miRNAs play a role in the pregnancy establishment, embryo‐maternal communication, embryo implantation, as well as embryo development. 20 , 21 Owing to this, a multidisciplinary regimen in RM evaluation is essential to unlock the risk of RM. Therefore, this study is launched to find out the relevance of miR‐149 in trophoblasts in RM.
To begin with, the role of miR‐149 in RM has been explored and the results outlined that miR‐149 is highly expressed in RM patients. Besides, we also identified that overexpressed miR‐149 promoted cell apoptosis and inhibited the activity of trophoblasts in RM while reducing miR‐149 expression in trophoblasts exhibited opposite experimental results. Some of the miRNAs have been implied to be involved in platelet aggregation, 22 placental immune activation, 23 along with trophoblast invasion, 24 among which there is miR‐149‐5p. 25 An article has revealed that higher miR‐149 T > C polymorphism was detected in idiopathic recurrent pregnancy loss cases in contrast to healthy controls. 26 Meanwhile, another study has revealed that inhibition of miR‐149 strengthened cell invasive and migratory potentials of trophoblasts in preeclampsia. 27 Moreover, the functions of miR‐149 in human diseases have been explored. Yang et al. have identified elevated miR‐149‐5p in non‐small cell lung cancer tissues with the application of either small RNA‐sequencing or RT‐qPCR. 28 Besides, upregulated miR‐149‐3p was found in melanoma patients, and miR‐149‐3p was of significance in the diagnosis of early‐stage melanoma. 29 A recent study highlighted that the possibility of having the identical karyotype pattern (recurrent normal or recurrent abnormal) in previous and subsequent abortion is elevated compared with chance. 30 Our further investigation revealed that miR‐149 expression exhibited no significant difference between RM patients with normal or abnormal karyotype, indicating that miR‐149 may not be responsible for RM due to chromosomal abnormalities. Nevertheless, the potentials of miR‐149 in RM should be further confirmed.
Knowingly, the change of miRNA expression and function might be resulted from different mechanisms, such as improper histone modification, DNA copy number aberrations, alteration in miRNAs processing pathway as well as genetic mutations in miRNA genes. 31 In this current study, we recognized that miR‐149 was able to target RUNX2, promote PTEN expression and inhibit Akt phosphorylation. RUNX2 was initially identified as a controller of osteoblast differentiation and chondrocyte maturation. 32 A report has suggested that RUNX2 is expressed in mouse placenta. 33 Moreover, RUNX2 has been discussed in cancer biology due to its functions in controlling cancer cell development. 34 However, the target relationship of miR‐149 and RUNX2 was scarcely explored before. Emerging evidences have concentrated on the binding of miR‐149 with other target genes. For instance, a research was launched to elucidate whether ENG was involved in trophoblast invasion modulated by miR‐149‐5p, and the results of which outlined that there was a binding site between ENG and miR‐149‐5p in human trophoblasts. 35 Another study has demonstrated binding sites between PGF 3′‐UTR and miR‐149, and PGF was decreased in HTR‐8/SVneo cells upon treatment of miR‐149 mimic, while its expression was restored in trophoblasts with miR‐149 inhibitor transfection. 27 Furthermore, as for the correlation of miR‐149 with the PTEN/Akt signaling pathway, an article has demonstrated that miR‐149 exerted its biological functions in ovarian cancer cells through the modulation of the PI3K/Akt axis. 36 However, how miR‐149 regulated the PTEN/Akt signaling pathway in RM needs deep verification.
Finally, the combination of miR‐149 inhibitor and si‐RUNX2 was observed to activate the PTEN/Akt signaling pathway, as manifested by promoted PTEN expression and lowered Akt phosphorylation. It was thus concluded that miR‐149 modulated the PTEN/Akt signaling pathway by targeting RUNX2. To further validate the involvement of the PTEN/Akt signaling pathway in RM, the impact of SF1670 on HTR‐8 cells was monitored by EdU and CFSE staining, flow cytometry as well as Transwell assays. Our data demonstrated that SF1670‐induced modifications at molecular levels can be translated to increased cell viability, migration and invasion in miR‐149 mimic‐transfected cells. Moreover, the results of flow cytometry showed SF1670 attenuated the number of apoptotic cells, indicating that SF1670 inhibited miR‐149 mimic‐induced apoptotic cell death. These results and the aforementioned points suggest the protective role for miR‐149 inhibitor against RM in vitro was partially mediated by the PTEN/Akt signaling pathway.
In summary, this study highlights that miR‐149 impairs the PTEN/Akt signaling pathway by targeting RUNX2, thereby inhibiting trophoblast activity and promoting its apoptosis in RM. This article may facilitate for the discovery of novel therapeutic agents for this disease. More researches are still required for further confirmation and extension in a larger cohort.
Disclosure
None declared.
Supporting information
Figure S1 miR‐149 expression between RM patients with normal (n = 21) or abnormal (n = 9) karyotype pattern.
References
- 1. Duckitt K, Qureshi A. Recurrent miscarriage. BMJ Clin Evid 2011; 2011: 1409. [PMC free article] [PubMed] [Google Scholar]
- 2. Garrido‐Gimenez C, Alijotas‐Reig J. Recurrent miscarriage: Causes, evaluation and management. Postgrad Med J 2015; 91: 151–162. [DOI] [PubMed] [Google Scholar]
- 3. Lund M, Kamper‐Jorgensen M, Nielsen HS, Lidegaard O, Andersen AM, Christiansen OB. Prognosis for live birth in women with recurrent miscarriage: What is the best measure of success? Obstet Gynecol 2012; 119: 37–43. [DOI] [PubMed] [Google Scholar]
- 4. Toth B, Jeschke U, Rogenhofer N et al Recurrent miscarriage: Current concepts in diagnosis and treatment. J Reprod Immunol 2010; 85: 25–32. [DOI] [PubMed] [Google Scholar]
- 5. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 2009; 11: 228–234. [DOI] [PubMed] [Google Scholar]
- 6. Melo SA, Esteller M. Dysregulation of microRNAs in cancer: Playing with fire. FEBS Lett 2011; 585: 2087–2099. [DOI] [PubMed] [Google Scholar]
- 7. Zhao Z, Moley KH, Gronowski AM. Diagnostic potential for miRNAs as biomarkers for pregnancy‐specific diseases. Clin Biochem 2013; 46: 953–960. [DOI] [PubMed] [Google Scholar]
- 8. Dong F, Zhang Y, Xia F et al Genome‐wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction 2014; 148: 33–41. [DOI] [PubMed] [Google Scholar]
- 9. Wang X, Li B, Wang J et al Evidence that miR‐133a causes recurrent spontaneous abortion by reducing HLA‐G expression. Reprod Biomed Online 2012; 25: 415–424. [DOI] [PubMed] [Google Scholar]
- 10. Xu K, Liu X, Mao X et al MicroRNA‐149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem 2015; 35: 499–515. [DOI] [PubMed] [Google Scholar]
- 11. He Y, Yu D, Zhu L, Zhong S, Zhao J, Tang J. miR‐149 in human cancer: A systemic review. J Cancer 2018; 9: 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep 2015; 12: 527–534. [DOI] [PubMed] [Google Scholar]
- 13. Owens TW, Rogers RL, Best S et al Runx2 is a novel regulator of mammary epithelial cell fate in development and breast cancer. Cancer Res 2014; 74: 5277–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng B, Zhu H, Klausen C, Ma L, Wang YL, Leung PC. GnRH regulates trophoblast invasion via RUNX2‐mediated MMP2/9 expression. Mol Hum Reprod 2016; 22: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huppertz B. Traditional and new routes of Trophoblast invasion and their implications for pregnancy diseases. Int J Mol Sci 2019; 21: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Prasad A, Jia Y et al Pretreatment with phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibitor SF1670 augments the efficacy of granulocyte transfusion in a clinically relevant mouse model. Blood 2011; 117: 6702–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montales MT, Simmen RC, Ferreira ES, Neves VA, Simmen FA. Metformin and soybean‐derived bioactive molecules attenuate the expansion of stem cell‐like epithelial subpopulation and confer apoptotic sensitivity in human colon cancer cells. Genes Nutr 2015; 10: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang YH, Wang Y, Yusufali AH et al Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med 2014; 12: 483–494. [DOI] [PubMed] [Google Scholar]
- 19. Alijotas‐Reig J, Garrido‐Gimenez C. Current concepts and new trends in the diagnosis and management of recurrent miscarriage. Obstet Gynecol Surv 2013; 68: 445–466. [DOI] [PubMed] [Google Scholar]
- 20. Liang J, Wang S, Wang Z. Role of microRNAs in embryo implantation. Reprod Biol Endocrinol 2017; 15: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu W, Niu Z, Li Q, Pang RT, Chiu PC, Yeung WS. MicroRNA and embryo implantation. Am J Reprod Immunol 2016; 75: 263–271. [DOI] [PubMed] [Google Scholar]
- 22. Askie LM, Duley L, Henderson‐Smart DJ, Stewart LA, Group PC . Antiplatelet agents for prevention of pre‐eclampsia: A meta‐analysis of individual patient data. Lancet 2007; 369: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 23. Liao Y, Lonnerdal B. miR‐584 mediates post‐transcriptional expression of lactoferrin receptor in Caco‐2 cells and in mouse small intestine during the perinatal period. Int J Biochem Cell Biol 2010; 42: 1363–1369. [DOI] [PubMed] [Google Scholar]
- 24. Luo R, Shao X, Xu P et al MicroRNA‐210 contributes to preeclampsia by downregulating potassium channel modulatory factor 1. Hypertension 2014; 64: 839–845. [DOI] [PubMed] [Google Scholar]
- 25. Guo L, Tsai SQ, Hardison NE et al Differentially expressed microRNAs and affected biological pathways revealed by modulated modularity clustering (MMC) analysis of human preeclamptic and IUGR placentas. Placenta 2013; 34: 599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alipour M, Abtin M, Hosseinzadeh A, Maleki M. Association between miR‐146a C > G, miR‐149 T > C, miR‐196a2 T > C, and miR‐499 a > G polymorphisms and susceptibility to idiopathic recurrent pregnancy loss. J Assist Reprod Genet 2019; 36: 2237–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao Y, Guo X, Li Y, Sha W, She R. The decreased lncRNA ZEB2‐AS1 in pre‐eclampsia controls the trophoblastic cell line HTR‐8/SVneo's invasive and migratory abilities via the miR‐149/PGF axis. J Cell Biochem 2019; 120: 17677–17686. [DOI] [PubMed] [Google Scholar]
- 28. Yang C, Sun C, Liang X, Xie S, Huang J, Li D. Integrative analysis of microRNA and mRNA expression profiles in non‐small‐cell lung cancer. Cancer Gene Ther 2016; 23: 90–97. [DOI] [PubMed] [Google Scholar]
- 29. Fogli S, Polini B, Carpi S et al Identification of plasma microRNAs as new potential biomarkers with high diagnostic power in human cutaneous melanoma. Tumour Biol 2017; 39: 1010428317701646. [DOI] [PubMed] [Google Scholar]
- 30. Nikitina TV, Sazhenova EA, Zhigalina DI, Tolmacheva EN, Sukhanova NN, Lebedev IN. Karyotype evaluation of repeated abortions in primary and secondary recurrent pregnancy loss. J Assist Reprod Genet 2020; 37: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of microRNAs in cancer. Curr Biol 2014; 24: R762–R776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prince M, Banerjee C, Javed A et al Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J Cell Biochem 2001; 80: 424–440. [DOI] [PubMed] [Google Scholar]
- 33. Blyth K, Vaillant F, Jenkins A et al Runx2 in normal tissues and cancer cells: A developing story. Blood Cells Mol Dis 2010; 45: 117–123. [DOI] [PubMed] [Google Scholar]
- 34. Pratap J, Lian JB, Javed A et al Regulatory roles of Runx2 in metastatic tumor and cancer cell interactions with bone. Cancer Metastasis Rev 2006; 25: 589–600. [DOI] [PubMed] [Google Scholar]
- 35. Xiaobo Z, Qizhi H, Zhiping W, Tao D. Down‐regulated miR‐149‐5p contributes to preeclampsia via modulating endoglin expression. Pregnancy Hypertens 2019; 15: 201–208. [DOI] [PubMed] [Google Scholar]
- 36. Zhao LW, Yu AJ, Zhang YJ, Wang XC, Han B, Wang XH. MicroRNA‐149 suppresses the malignant phenotypes of ovarian cancer via downregulation of MSI2 and inhibition of PI3K/AKT pathway. Eur Rev Med Pharmacol Sci 2020; 24: 55–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 miR‐149 expression between RM patients with normal (n = 21) or abnormal (n = 9) karyotype pattern.


