Abstract
Presentation of autoimmune hepatitis (AIH) can differ from nonacute to acute autoimmune hepatitis (A‐AIH) with jaundice and acute severe autoimmune hepatitis (AS‐AIH) with jaundice and coagulopathy. The aim of the study was to evaluate the short‐term prognosis of different presentations of AIH and the influence of liver function improvement on short‐term prognosis. In this single‐center retrospective cohort study, AIH patients with repeatedly tested liver function at diagnosis and during at least 1 year of follow‐up were included. A‐AIH was defined as bilirubin >45 µmol and international normalized ratio (INR) <1.5. AS‐AIH was defined as bilirubin level >45 µmol/L and INR ≥1.5. Of the 81 included patients, 17 (21%) presented with A‐AIH, and 14 (17%) presented with AS‐AIH. After the start of immunosuppressive therapy, bilirubin, albumin, and INR normalized in 70%, 77%, and 69%, respectively, in a median of 2.6 months, 3 months, and 4 weeks, respectively, in patients with A‐AIH and AS‐AIH. Liver transplantation (LT)–free survival rate was 100% in nonacute AIH, 94% in A‐AIH, and 57% in AS‐AIH at 12 months after diagnosis. An increase of INR or bilirubin at 2 weeks was the best predictive factor for the need of LT within 12 months with a Youden’s index of 0.85. A‐AIH was present in 21%, and AS‐AIH was present in 17% of AIH patients. In the majority of patients, bilirubin, albumin, and INR normalized in the first months of treatment. Deterioration of liver function after 2 weeks of treatment should lead to rapid evaluation for LT and consideration of second‐line medication.
Short abstract
https://www.wileyhealthlearning.com/Activity/7201351/disclaimerspopup.aspx
Abbreviations
- A‐AIH
acute autoimmune hepatitis
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- AS‐AIH
acute severe autoimmune hepatitis
- AST
aspartate aminotransferase
- GGT
gamma‐glutamyltransferase
- HAI
histological activity index
- IgG
immunoglobulin G
- INR
international normalized ratio
- LT
liver transplantation
- MELD
Model for End‐Stage Liver Disease
- PPV
positive predictive value
- ROC
receiver operating characteristic
- SBP
spontaneous bacterial peritonitis
Autoimmune hepatitis (AIH) is a chronic inflammatory liver disease characterized by hypergammaglobulinemia, circulating autoantibodies, and interface hepatitis with plasma cells on histology, which is usually responsive to immunosuppressive therapy. Presentation can vary from asymptomatically raised liver enzymes to jaundice and liver failure.( 1 , 2 )
In a subset of AIH patients, hepatitis causes a decrease in excretory and synthetic function of the liver reflected by increased serum bilirubin, decreased albumin levels, and prolonged international normalized ratio (INR). Presentation with decreased liver function is termed acute presentation of AIH. Acute presentation can be divided into 2 different categories: acute autoimmune hepatitis (A‐AIH) presenting with jaundice but without coagulopathy and acute severe autoimmune hepatitis (AS‐AIH) presenting with jaundice and coagulopathy.( 3 ) AS‐AIH can present with or without hepatic encephalopathy (AS‐AIH with or without acute liver failure).( 3 )
For most AIH patients, the prognosis is good with treatment with corticosteroids and thiopurines. Patients without cirrhosis have the same life expectancy as the general population.( 4 ) However, for AS‐AIH the prognosis seems worse and reported liver transplantation (LT)‐free survival rates differ between 20% and 90%.( 5 , 6 , 7 , 8 , 9 ) In 1988, Czaja et al. reported that no improvement of serum bilirubin after 2 weeks was a bad prognostic sign.( 10 ) This finding was only confirmed in a cohort of 11 patients.( 11 ) More recently, the worsening of INR was reported as a bad prognostic sign in a cohort of 17 patients.( 7 )
The aim of this study was to evaluate the relation between liver function, improvement of liver function, and short‐term prognosis in a larger cohort of patients presenting with A‐AIH and AS‐AIH.
Patients and Methods
In this single‐center retrospective cohort study, all consecutive patients with a diagnosis of AIH between 1995 and 2018 in Leiden University Medical Center, a tertiary referral center for liver disease and LT, were eligible for inclusion. All patients with available sequential liver function tests and at least 1 year of follow‐up or death or LT within a year were included. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the local ethical committee. All surviving patients gave informed consent. No organs from executed prisoners were used.
For diagnosis of AIH, the simplified International Autoimmune Hepatitis Group criteria were used.( 12 ) A‐AIH was defined as serum bilirubin level >45 µmol/L and INR <1.5. AS‐AIH was defined as serum bilirubin level >45 µmol/L and INR ≥1.5.( 3 ) If hepatic encephalopathy was present, AS‐AIH was accompanied by acute or acute‐on‐chronic liver failure.( 3 , 13 ) Because of a limited number of patients, patients with AS‐AIH with acute liver failure were not analyzed separately.
Data, including baseline characteristics, laboratory results before and during treatment, and treatment and survival data, were retrieved from a local database and electronic patient files. Definitions for treatment response were according to the European Association for the Study of the Liver guidelines: biochemical remission was defined as normalization of aminotransferases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]) and immunoglobulin G (IgG), and partial response was defined as an improvement of aminotransferases and IgG without normalization.( 14 ) Liver histology was used to determine the presence of liver cirrhosis. The available liver biopsies of A‐AIH and AS‐AIH patients were scored by a liver pathologist for inflammation using the histological activity index (HAI) as modified by Ishak et al.( 15 )
For total and conjugated serum bilirubin, values of 20 and 5 µmol/L, respectively, were used for the upper limit of normal. For time until normalization of bilirubin, the conjugated bilirubin was used to correct for patients with Gilbert’s syndrome. The lower limit of normal for albumin was 34 g/L, and for INR, the upper limit of normal was <1.5. Patients taking oral anticoagulants were excluded from INR analysis, and for calculation of the Model for End‐Stage Liver Disease (MELD) score in these patients, an INR of 1.0 was used.
All available serum conjugated and total bilirubin, albumin, creatinine, and INR values before start of treatment until LT or during 3 years of treatment were collected from patient files. Values before the start of treatment and at 2 weeks after the start of treatment were used as prognostic factors. All available values were used to determine the time to normalization of bilirubin, albumin, and INR.
Statistical Analysis
Statistical analysis was done with SPSS, version 25 (IBM, Armonk, NY). The Kolmogorov‐Smirnov test and the Shapiro‐Wilk test were used to test normal distribution. Data are presented as median (range), unless indicated otherwise. Mann‐Whitney U test or Wilcoxon signed rank test was used to test significance of continuous variables, and chi‐square was used for categorical variables. Kaplan‐Meier survival analysis was used to assess the time until normalization, with log‐rank test for differences. Predictors for 12‐month transplant‐free survival were assessed using Youden’s index and receiver operating characteristic (ROC) analysis. P < 0.05 was considered significant.
Results
Of the 81 included AIH patients, 50 (62%) patients had a nonacute presentation; 17 (21%) patients presented with A‐AIH; and 14 (17%) patients presented with AS‐AIH. In 5 of the 14 (36%) patients with AS‐AIH, hepatic encephalopathy was present (thus fulfilling the criteria for acute or acute‐on‐chronic liver failure).
Baseline characteristics are shown in Table 1. In 2 patients the presentation of AIH was associated with the use of nitrofurantoin and valproic acid, and a drug‐induced AIH could not be excluded. In the other patients, no drugs were involved. Underlying cirrhosis was present in 18 (36%) patients with nonacute presentation, 5 (29%) of the patients with A‐AIH, and in 8 (57%) of the patients with AS‐AIH (P = 0.25). Patients with A‐AIH and AS‐AIH versus patients with nonacute presentation had significantly higher levels of AST (1286 and 1029 versus 205 U/L, respectively; P < 0.001) and ALT (1275 versus 1130 versus 226 U/L, respectively; P < 0.001). The difference between AST and ALT for the A‐AIH and AS‐AIH groups was nonsignificant (P = 0.46 for AST; P = 0.84 for ALT). IgG g/l levels were also significantly higher in patients with A‐AIH and AS‐AIH compared with patients with a nonacute presentation (25.1 versus 27.5 versus 21.7; P = 0.02). The difference in IgG between A‐AIH and AS‐AIH was nonsignificant (P = 0.60).
Table 1.
Baseline Characteristics of Patients With Nonacute AIH, A‐AIH, and AS‐AIH
| Nonacute AIH Group (n = 50) | A‐AIH Group (n = 17) | AS‐AIH Group (n = 14) | P Value* | |
|---|---|---|---|---|
| Sex, female | 37 (74) | 12 (71) | 11 (79) | 0.88 |
| Age, years | 56 (14‐77) † | 49 (13‐71) | 48 (11‐64) | 0.07 |
| Cirrhosis | 18 (36) | 5 (29) | 8 (57) | 0.25 |
| HAI | — | 11.5 (1‐18) ‡ | 12 (1‐18) § | 0.91 |
| Biochemical variables | ||||
| IgG, g/l | 21.7 (8.2‐72) | 25.1 (11.4‐49.4) | 27.5 (19‐43) | 0.019 |
| Total bilirubin, µmol/L | 17 (5‐47) | 112 (51‐595) | 179 (49‐563) | <0.001 |
| AST, U/L | 205 (30‐1630) | 1286 (100‐2527) | 1029 (103‐2582) | <0.001 |
| ALT, U/L | 226 (35‐1602) | 1275 (38‐3400) | 1130 (66‐2703) | <0.001 |
| Alkaline phosphatase, U/L | 154 (48‐603) | 200 (85‐410) | 246 (125‐588) | 0.12 |
| GGT, U/L | 191 (27‐1122) | 145 (36‐1317) | 109 (33‐1098) | 0.26 |
| Albumin, g/L | 41 (25‐50) | 35 (15‐44) | 29 (22‐45) | <0.001 |
| INR | 1.1 (0.9‐1.3) | 1.2 (1.0‐1.5) | 1.6 (1.5‐2.8) | <0.001 |
| Platelets, ×109/L | 190 (53‐487) | 179 (55‐297) | 174 (38‐420) | 0.53 |
| MELD score | 7 (6‐17) | 16 (11‐22) | 21 (16‐27) | <0.001 |
Data are given as n (%) or median (range).
Mann‐Whitney U test was used to determine significance for continuous variables, and chi‐square test was used for categorical variables.
All values are median (range) unless otherwise specified.
n = 10.
n = 9.
Treatment
Treatment with prednisolone was started in 46 (92%) of patients with nonacute AIH, in 17 (100%) patients with A‐AIH, and in 14 (100%) patients with AS‐AIH. Median dose of prednisolone was 0.49 mg/kg/day (range, 0.14‐1.01 mg/kg/day) in nonacute AIH, 0.50 mg/kg/day (range, 0.32‐0.97 mg/kg/day) in A‐AIH, and 0.58 mg/kg/day (range, 0.41‐1.02 mg/kg/day) in AS‐AIH (P = 0.06). Patients requiring LT received a median prednisolone dose of 0.58 mg/kg/day (range, 0.42‐1.00 mg/kg/day), which was not significantly different from A‐AIH and AS‐AIH patients not requiring LT (0.54 mg/kg/day; range, 0.32‐0.97; P = 0.499). In 2 nonacute patients, budesonide was started, and in 2 nonacute patients, no treatment was started because of only mildly raised aminotransferases. In addition to steroids, first‐line treatment in nonacute patients consisted of azathioprine in 44 patients (88%), 6‐thioguanine in 2 patients (4%), 6‐mercaptopurine in 1 (2%) patient, and mycophenolate mofetil in 1 (2%) patient. In all A‐AIH patients, azathioprine was started as first‐line therapy in addition to prednisolone. Of the AS‐AIH patients, first‐line therapy consisted of azathioprine in 9 patients, cyclosporine in 1 patient, and 6‐thioguanine in 1 patient. Three AS‐AIH patients were treated with prednisolone monotherapy. There were 4 patients with nonacute AIH and 1 patient with AS‐AIH who were using oral anticoagulants at diagnosis. The patient with AS‐AIH had jaundice and hepatic encephalopathy at diagnosis. All patients using oral coagulants were excluded from INR analysis.
Change in Liver Function
Median MELD score was 7 (range, 6‐17) in nonacute AIH patients, 16 (range, 11‐22) in A‐AIH patients, and 21 (range, 16‐27) in AS‐AIH patients (Table 1). The difference between A‐AIH and AS‐AIH was nonsignificant (P = 0.14). At 12 months of therapy, median MELD score had decreased in nonacute AIH to a median of 7 (range, 6‐16; P = 0.02). In A‐AIH patients, the median MELD score decreased from 16 (range, 11‐22) at diagnosis to 6 (range, 6‐12) at 12 months (P < 0.001). he A‐AIH and AS‐AIH patients are combined in Figure 1B, the median MELD score decreased from 21 (range, 16‐27) at diagnosis to 8 (range, 6‐12) at 12 months (P = 0.02; Fig. 1).
Fig. 1.
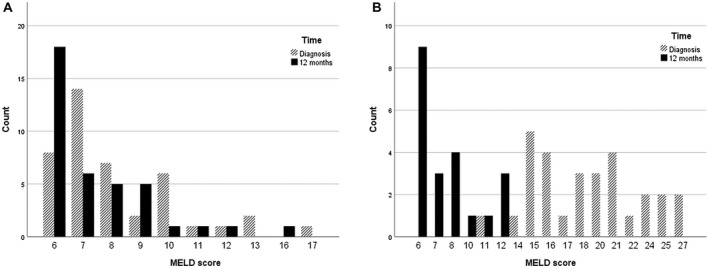
MELD score at diagnosis versus at 12 months in (A) nonacute patients (P = 0.02) and (B) A‐AIH (P < 0.001) and AS‐AIH (P = 0.02) patients.
At diagnosis, median total bilirubin was 17 µmol/L (range, 5‐47 µmol/L) in patients with nonacute AIH, and significantly higher in patients with A‐AIH (112 µmol/L; range, 51‐595 µmol/L; P < 0.001) and in patients with AS‐AIH (179 µmol/L; range, 49‐563 µmol/L; P < 0.001). The difference in bilirubin between patients with A‐AIH and AS‐AIH was nonsignificant (P = 0.65). After the start of therapy, conjugated bilirubin normalized in 21 of the 30 (70%) patients with A‐AIH and AS‐AIH within a median of 2.6 months (range, 1‐11 months; Fig. 2A). All 9 patients without normalization of conjugated bilirubin eventually progressed to LT (range, 1 week to 70 months).
Fig. 2.
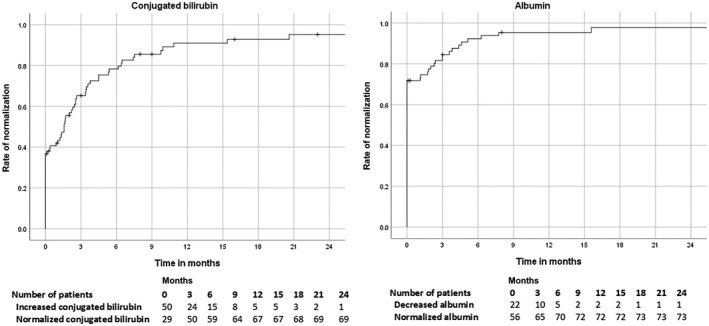
Rate of normalization of (A) conjugated bilirubin and (B) albumin in patients with A‐AIH or AS‐AIH by time in months after diagnosis.
Serum bilirubin levels decreased in 16 of the 21 A‐AIH and AS‐AIH patients in whom bilirubin was available at 2 weeks. Out of the 5 patients with an increase in bilirubin at 2 weeks after start of therapy, 4 (80%) patients received an LT in the first year. At 12 months, median bilirubin was 11 µmol/L (range, 6‐43 µmol/L) in patients with A‐AIH and 11 µmol/L (range, 6‐67 µmol/L) in patients with AS‐AIH.
At diagnosis, median albumin was 41 g/L (range, 25‐50 g/L) in nonacute AIH patients. Albumin was significantly lower in patients with A‐AIH (35 g/L; range, 15‐44 g/L; P = 0.005) and in patients with AS‐AIH (29 g/L; range, 22‐45 g/L; P = 0.001). Albumin was not significantly different in AS‐AIH patients compared with A‐AIH patients (P = 0.36). Albumin was below the reference range in 5 (11%) patients with nonacute AIH, 7 patients (41%) with A‐AIH, and 10 (71%) with AS‐AIH (P < 0.001). In 17 (77%) of these 22 patients, albumin normalized within a median of 3 months (range, 1.1‐16 months; Fig. 2B). The other 5 patients received a LT (median 3 months, range 1 week to 40 months). At 2 weeks, albumin had increased in 8 of the 11 (73%) patients with albumin below the reference range at diagnosis. Of the other 11 patients, no albumin values were available.
At diagnosis, INR was ≥1.5 in 13 of the 70 (19%) patients with available INR. In 6 nonacute patients, INR was missing, and 5 patients used oral anticoagulants. INR was ≥1.5 in 7 of the 28 (25%) patients with cirrhosis compared with 6 of the 42 (14%) patients without cirrhosis (P = 0.26). In 9 of the 13 (69%) patients with prolonged INR, the INR normalized within a median of 4 weeks (range, 1‐7 weeks). At 2 weeks, INR was available in 13 (26%) nonacute AIH patients, 8 (47%) A‐AIH patients, and 10 (71%) of the AS‐AIH patients. Vitamin K was administered to 4 (24%) A‐AIH patients and to 9 (64%) AS‐AIH patients. In 26 of 31 (84%) patients, INR decreased or remained unchanged in the first 2 weeks of treatment. INR decreased or remained unchanged in 7 of the 9 patients using vitamin K and in 19 of the 22 patients not using vitamin K (P = 0.56). In 4 of the 5 (80%) patients with an increase of INR in the first 2 weeks, an LT was necessary.
Liver biopsies of 10 A‐AIH and 9 AS‐AIH patients were available for scoring of HAI. Median HAI was 11.5 (range, 1‐18) in A‐AIH patients and 12 (range, 1‐18) in AS‐AIH patients (P = 0.91). Centrilobular necrosis (3 points of higher) was present in 6 of the 10 A‐AIH patients and in 6 of the 9 AS‐AIH patients.
Treatment Response and Short‐Term Prognosis
The 12‐month LT‐free survival rate was 100% in patients presenting with nonacute AIH. In patients with nonacute AIH, 24/45 (53%) patients were in remission at 12 months (in 5 data for remission were missing). In patients with A‐AIH, the LT‐free survival rate was 94% at 12 months, and only 1 of these patients (6%) received an LT 2 months after diagnosis (Table 2). This patient had treatment failure and developed hepatic encephalopathy after 1 month of treatment. Out of the remaining 16 patients, 10 (63%) were in remission after 12 months. In patients with AS‐AIH, the LT‐free survival rate was 57% at 12 months. Six of these patients (43%) received an LT during the first 12 months (range, 0.25 to 9 months; Table 2). Out of the remaining 8 AS‐AIH patients, 3 (38%) patients were in remission at 12 months.
Table 2.
Clinical Characteristics of Patients Requiring LT During the First Year of Treatment
| Patient Number | Clinical Presentation | Treatment | Treatment Response | Months to LT | MELD at Diagnosis | MELD at LT |
|---|---|---|---|---|---|---|
| Acute AIH group | ||||||
| 1 | Cirrhosis | Prednisolone | Treatment failure | 2 | 20 | 30 |
| Ascites | Azathioprine/mycophenolate mofetil* | |||||
| Encephalopathy after 4 weeks | ||||||
| AS‐AIH group | ||||||
| 1 | Acute liver failure | Prednisolone | Treatment failure | 0.25 | 24 | 37 |
| 2 | Cirrhosis | Prednisolone | Treatment failure | 0.5 | 25 | 29 |
| Encephalopathy | ||||||
| Ascites | ||||||
| 3 | Acute liver injury | Prednisolone | Treatment failure | 1 | 24 | 44 |
| 4 | Cirrhosis | Prednisolone | Treatment failure | 3 | 27 | 24 |
| SBP | Azathioprine/mycophenolate mofetil † | |||||
| Ascites | ||||||
| 5 | Cirrhosis | Prednisolone | Treatment failure | 8 | 16 | 15 |
| Encephalopathy | Cyclosporine | |||||
| 6 | Cirrhosis | Prednisolone | Incomplete response | 9 | 18 | 9 |
| Hepatopulmonary syndrome | Thioguanine |
Azathioprine was replaced by mycophenolate mofetil due to insufficient treatment response.
Azathioprine was replaced by mycophenolate mofetil due to adverse effects related to the azathioprine.
Several factors at baseline and at 2 weeks of treatment were analyzed for sensitivity and specificity to predict need for LT within 1 year in all included patients (Table 3). At diagnosis INR ≥1.5 was the best predictive factor with a sensitivity of 86%, a specificity of 89%, and a Youden’s index of 0.75. At 2 weeks, an increase of INR had a sensitivity of 80%, a specificity of 96%, and a Youden’s index of 0.76. An increase of bilirubin at 2 weeks had a sensitivity of 80%, a specificity of 92%, and a Youden’s index of 0.72. An increase of INR or bilirubin at 2 weeks was the best predictive factor with a sensitivity of 100%, a specificity of 85%, and a Youden’s index of 0.85. ROC analysis for INR, MELD score, and HAI yielded similar cutoff points to the values used: 1.5 for INR, 15 for MELD, and 12 for HAI (Supporting Table 1).
Table 3.
Presentation of Patients Requiring LT Compared With Patients Not Needing an LT Within 12 Months
| LT (n = 7) | No LT (n = 74) | Sensitivity, % | Specificity, % | PPV, % | Youden’s Index | |
|---|---|---|---|---|---|---|
| Diagnosis | ||||||
| Cirrhosis | 5/7 (71) | 26/74 (35) | 71 | 66 | 16 | 0.37 |
| Decompensated cirrhosis | 5/7 (71) | 6/74 (8) | 71 | 92 | 45 | 0.63 |
| Encephalopathy | 4/7 (57) | 1/74 (1) | 57 | 99 | 80 | 0.56 |
| Ascites | 3/7 (43) | 6/74 (8) | 43 | 92 | 33 | 0.35 |
| SBP | 2/7 (29) | 2/74 (3) | 29 | 97 | 50 | 0.26 |
| INR ≥1.5 | 6/7 (86) | 7/63 (11)* | 86 | 89 | 46 | 0.75 |
| MELD score >15 at diagnosis | 7/7 (100) | 21/64 (33) | 100 | 67 | 25 | 0.67 |
| Histology | ||||||
| HAI >12 | 2/3 (66) † | 8/16 (50) † | 66 | 50 | 20 | 0.16 |
| Centrilobular necrosis | 2/3 (66) † | 10/16 (63) † | 66 | 37 | 17 | 0.03 |
| 2 weeks | ||||||
| Increase of bilirubin | 4/5 (80) ‡ | 2/26 (8)§ | 80 | 92 | 67 | 0.72 |
| Decrease of albumin | 1/4 (25) ‡ | 8/27 (30) ‡ | 25 | 70 | 3 | −0.05 |
| Increase of INR | 4/5 (80) * , ‡ | 1/26 (4) * , ‡ | 80 | 96 | 80 | 0.76 |
| Increase of bilirubin or INR | 5/5 (100) * , ‡ | 3/20 (15) * , ‡ , § | 100 | 85 | 63 | 0.85 |
| Decrease MELD >2 points | 3/4 (75) ‡ | 13/26 (50) ‡ | 75 | 50 | 19 | 0.25 |
Data are given as n (%) unless otherwise noted.
Patients using oral anticoagulants were excluded from this analysis.
Only liver biopsies of acute and AS‐AIH patients were scored.
Laboratory values were not measured at 2 weeks in the remaining patients.
11 patients with normal bilirubin at diagnosis were excluded.
Discussion
In this retrospective cohort of AIH patients from a transplantation center, 21% presented with A‐AIH and 17% presented with AS‐AIH. Patients presenting with A‐AIH and AS‐AIH had higher aminotransferases and IgG compared with those with a nonacute presentation. LT‐free survival was 94% at 12 months after diagnosis in patients with A‐AIH because 1 patient needed an LT due to treatment failure and development of hepatic encephalopathy. In patients with AS‐AIH with or without liver failure, the LT‐free survival was only 57% at 12 months after diagnosis.
In the few publications on AS‐AIH, the reported survival rates are between 20% and 90%.( 5 , 6 , 7 , 8 , 9 ) Studies are difficult to compare because the definitions for AS‐AIH differ. The definitions used in the current study were recently proposed by Rahim et al.( 3 )
Three studies included patients with coagulopathy. Two studies excluded patients with cirrhosis. Survival rates differed greatly between the studies. Yeoman et al. reported 32 patients with a median INR of 2.3 with a transplant‐free survival rate of 20%.( 5 ) In the study of Zachou et al., 33 of the 34 (97%) patients with a median INR of 1.52 survived without LT with a median follow‐up of 5 years. All patients in this report were treated with high‐dose corticosteroids.( 6 ) The study of Moenne‐Loccoz et al. did not exclude patients with cirrhosis and had a transplant‐free survival rate of 60% in 17 patients with a median INR of 2.3 with a mean follow‐up of approximately 4 years.( 7 ) The difference in survival in the different studies may partly be explained by higher severity of disease reflected by the severity of coagulopathy in the studies of Yeoman et al.( 5 ) and Moenne‐Loccoz et al.( 7 ) In the current study, the severity of coagulopathy was comparable to the study of Zachou et al.( 6 ) with a median INR of 1.6, but patients with cirrhosis were included.
Two other studies included patients with coagulopathy and hepatic encephalopathy. The first study excluded patients with cirrhosis and reported a transplant‐free survival rate of 90% after 6 months in 32 patients.( 8 ) The second study included patients with cirrhosis and reported a lower survival rate of 41% in 40 steroid‐treated patients.( 9 )
In the current retrospective cohort, in 38% of the patients, excretory dysfunction of the liver, as reflected by jaundice at diagnosis, was present. This is comparable to previous studies in which 40%‐65% of AIH patients presented with jaundice.( 16 , 17 , 18 ) Coagulopathy, a sign of decreased synthetic function of the liver, was present in 17% of AIH patients at diagnosis. Excretory and synthetic function of the liver improved in the first months after the start of immunosuppressive treatment in most patients. Serum bilirubin, albumin, and INR normalized within a median of 2.6 months, 3 months, and 4 weeks, respectively, after the start of therapy. This is consistent with a previous study reporting an increase of albumin, antipyrine clearance, and decrease of bilirubin after 6 months of immunosuppressive treatment in AIH patients.( 19 )
A lack of improvement in excretory liver function, as reflected by an increase in bilirubin, or worsened synthetic liver function, as reflected by INR, after the start of therapy were the best predictors for the need for LT. An increase of bilirubin during treatment as a worrisome sign was already reported by Czaja et al. in 1988.( 10 ) Another study in 11 patients found an increase of bilirubin at 2 weeks to be predictive for liver‐related death or transplantation.( 11 ) INR of >2.5 at diagnosis and a lack of improvement in INR were predictive for LT or death in a study with 17 patients.( 7 ) Another study reported that a decrease of <2 points in MELD score—consisting of creatinine, bilirubin, and INR—after 7 days of treatment was predictive for treatment failure.( 20 ) Consistent with the current study, a Japanese group also reported that the lack of improvement of bilirubin and INR is predictive for the need of LT and that patients in whom bilirubin and INR do not improve after 2 weeks of corticosteroid therapy should be considered for LT.( 21 ) In these patients, nonstandard treatment therapies like tacrolimus may be considered during screening and waiting for LT.( 22 )
Interestingly, MELD at diagnosis of AIH does not predict LT requirement. In chronic liver disease in general, the 1‐year survival rates with and without LT are similar in patients with a MELD score of approximately 15‐17.( 23 ) A MELD score above 15 is therefore often considered a valid argument for listing a patient for LT. However, median MELD score decreased with immunosuppressive treatment during the first year from a median of 16 to 6 in the A‐AIH group and from a median of 21 to 8 in the AS‐AIH group. With treatment, the vast majority of patients with MELD >15 at diagnosis of AIH did not require LT. On the basis of the MELD score, approximately 40% of patients would have required LT if left untreated, which is consistent with the poor survival in AIH before prednisolone treatment became common in the 1970s.( 24 , 25 , 26 )
The HAI, as a measure for liver inflammation, did not differ between A‐AIH and AS‐AIH. It also did not predict the risk of LT in the first year. The HAI was developed for chronic hepatitis, but no specific scoring systems exist for A‐AIH or AS‐AIH.( 15 ) Centrilobular necrosis is described more often in A‐AIH and AS‐AIH compared with nonacute AIH.( 27 , 28 ) In this study, centrilobular necrosis was indeed frequently present in A‐AIH and AS‐AIH, but it did not predict for the need for LT in the first year.
In approximately 30% of the patients, cirrhosis was present at diagnosis regardless of age.( 29 ) In patients with AS‐AIH, the prevalence of previously undiagnosed underlying cirrhosis was almost twice as high at 57%. Strictly speaking, these patients have an acute‐on‐chronic presentation of AIH. In a previous study, AIH was diagnosed in 2.9% of patients presenting with acute‐on‐chronic liver failure.( 30 ) In studies that did not exclude patients with underlying cirrhosis, 20%‐40% of the patients with AS‐AIH had an acute‐on‐chronic presentation.( 9 , 31 ) In these patients, AIH was diagnosed at a very late stage, which demonstrates that it can sometimes be difficult to diagnose AIH in earlier stages. Physicians should be aware that patients presenting with presumed acute liver failure due to AIH can, in fact, have acute‐on‐chronic liver failure.
All patients with A‐AIH and AS‐AIH were treated with prednisolone. A daily prednisolone dose of 0.5‐1 mg/kg/day is advised in chronic AIH.( 14 ) On the basis of limited evidence, 1 mg/kg/day is suggested in AS‐AIH.( 14 ) The ideal dose of prednisolone in AS‐AIH is still unknown. In AIH in general, there was no difference in remission rate between patients treated with <0.5 mg/kg/day compared with those treated with ≥0.5 mg/kg/day.( 32 ) In the current study, median dose of prednisolone in AS‐AIH was lower than suggested, but no difference was found between patients with and without transplantation. High‐dose prednisolone can increase the risk of infections. In AIH patients, more serious infections after transplant were reported compared with transplanted patients with primary biliary cirrhosis, primary sclerosing cholangitis, or alcoholic liver disease.( 33 ) More research is necessary to determine the ideal prednisolone dose for AS‐AIH.
There are some limitations for the current data. Patients with acute liver failure due to AIH may have been missed because the simplified AIH criteria were used. In patients with A‐AIH or AS‐AIH autoantibodies and hypergammaglobulinemia are less often present and the simplified criteria were not developed for acute liver failure due to AIH.( 12 , 28 ) Furthermore, this retrospective cohort study was performed in an LT center, which could have led to an overrepresentation of patients with decreased liver function at diagnosis and a relatively higher number of LTs. Because of the retrospective design of the study, not all data were complete in all patients. The number of patients is limited, but the definitions proposed by Rahim et al.( 3 ) do seem to be a useful risk stratification. Also, this is one of the few studies with numbers comparable to the literature on this clinically relevant subject.
In conclusion, AS‐AIH is a severe liver disease with a 12‐month survival rate of only 57%. In most patients, synthetic and excretory liver function normalized with immunosuppressive therapy within the first months. An increase of bilirubin or INR during the first 2 weeks should lead to rapid evaluation for LT and consideration of second‐line medication.
Supporting information
Table S1
Maaike Biewenga was supported by an unrestricted grant from Zambon Pharma.
Earn MOC for this article: https://www.wileyhealthlearning.com/Activity/7201351/disclaimerspopup.aspx
References
- 1. van Gerven NM, de Boer YS, Mulder CJ, van Nieuwkerk CM, Bouma G. Auto immune hepatitis. World J Gastroenterol 2016;22:4651‐4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology 2005;42:53‐62. [DOI] [PubMed] [Google Scholar]
- 3. Rahim MN, Liberal R, Miquel R, Heaton ND, Heneghan MA. Acute severe autoimmune hepatitis: corticosteroids or liver transplantation? Liver Transpl 2019;25:946‐959. [DOI] [PubMed] [Google Scholar]
- 4. van den Brand FF, van der Veen KS, de Boer YS, van Gerven NM, Lissenberg‐Witte BI, Beuers U, et al. Increased mortality among patients with vs without cirrhosis and autoimmune hepatitis. Clin Gastroenterol Hepatol 2019;17:940‐947. [DOI] [PubMed] [Google Scholar]
- 5. Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al‐Chalabi T, Wendon JA, et al. Prognosis of acute severe autoimmune hepatitis (AS‐AIH): the role of corticosteroids in modifying outcome. J Hepatol 2014;61:876‐882. [DOI] [PubMed] [Google Scholar]
- 6. Zachou K, Arvaniti P, Azariadis K, Lygoura V, Gatselis NK, Lyberopoulou A, et al. Prompt initiation of high‐dose i.v. corticosteroids seems to prevent progression to liver failure in patients with original acute severe autoimmune hepatitis. Hepatol Res 2019;49:96‐104. [DOI] [PubMed] [Google Scholar]
- 7. Moenne‐Loccoz R, Severac F, Baumert TF, Habersetzer F. Usefulness of corticosteroids as first‐line therapy in patients with acute severe autoimmune hepatitis. J Hepatol 2016;65:444‐446. [DOI] [PubMed] [Google Scholar]
- 8. Anastasiou OE, Dogan‐Cavus B, Kucukoglu O, Baba H, Kahraman A, Gerken G, et al. Corticosteroid therapy improves the outcome of autoimmune hepatitis‐induced acute liver failure. Digestion 2018;98:104‐111. [DOI] [PubMed] [Google Scholar]
- 9. Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, et al. Fulminant presentation of autoimmune hepatitis: clinical features and early predictors of corticosteroid treatment failure. Eur J Gastroenterol Hepatol 2015;27:644‐648. [DOI] [PubMed] [Google Scholar]
- 10. Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid‐treated severe autoimmune chronic active hepatitis. Gastroenterology 1988;95:448‐453. [DOI] [PubMed] [Google Scholar]
- 11. Miyake Y, Iwasaki Y, Terada R, Onishi T, Okamoto R, Sakai N, et al. Clinical characteristics of fulminant‐type autoimmune hepatitis: an analysis of eleven cases. Aliment Pharmacol Ther 2006;23:1347‐1353. [DOI] [PubMed] [Google Scholar]
- 12. Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawitt EL, et al.; for International Autoimmune Hepatitis Group . Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169‐176. [DOI] [PubMed] [Google Scholar]
- 13. Wendon J, Cordoba J, Dhawan A, et al.; for European Association for the Study of the Liver . EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol 2017;66:1047‐1081. [DOI] [PubMed] [Google Scholar]
- 14. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol 2015;63:971‐1004. [DOI] [PubMed] [Google Scholar]
- 15. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995;22:696‐699. [DOI] [PubMed] [Google Scholar]
- 16. Jiménez‐Rivera C, Ling SC, Ahmed N, Yap J, Aglipay M, Barrowman N, et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics 2015;136:e1237‐e1248. [DOI] [PubMed] [Google Scholar]
- 17. van Gerven NM, Verwer BJ, Witte BI, van Erpecum KJ, van Buuren HR, Maijers I, et al.; for Dutch Autoimmune hepatitis STUDY group . Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol 2014;49:1245‐1254. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Chalabi T, Boccato S, Portmann BC, McFarlane IG, Heneghan MA. Autoimmune hepatitis (AIH) in the elderly: a systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. J Hepatol 2006;45:575‐583. [DOI] [PubMed] [Google Scholar]
- 19. Coverdale SA, Field J, Farrell GC. How reversible is hepatic functional impairment in autoimmune hepatitis? J Gastroenterol Hepatol 2003;18:371‐375. [DOI] [PubMed] [Google Scholar]
- 20. Yeoman AD, Westbrook RH, Zen Y, Maninchedda P, Portmann BC, Devlin J, et al. Early predictors of corticosteroid treatment failure in icteric presentations of autoimmune hepatitis. Hepatology 2011;53:926‐934. [DOI] [PubMed] [Google Scholar]
- 21. Fujiwara K, Yasui S, Yokosuka O, Kato N. Acute severe autoimmune hepatitis: corticosteroids or liver transplantation? Liver Transpl 2019;25:1455‐1456. [DOI] [PubMed] [Google Scholar]
- 22. Hanouneh M, Ritchie MM, Ascha M, Ascha MS, Chedid A, Sanguankeo A, et al. A review of the utility of tacrolimus in the management of adults with autoimmune hepatitis. Scand J Gastroenterol 2019;54:76‐80. [DOI] [PubMed] [Google Scholar]
- 23. Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al.; for United Network for Organ Sharing Liver Disease Severity Score Committee . Model for end‐stage liver disease (MELD) and allocation of donor livers. Gastroenterology 2003;124:91‐96. [DOI] [PubMed] [Google Scholar]
- 24. Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med 1971;40:159‐185. [DOI] [PubMed] [Google Scholar]
- 25. Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology 1972;63:820‐833. [PubMed] [Google Scholar]
- 26. Murray‐Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet 1973;1:735‐737. [DOI] [PubMed] [Google Scholar]
- 27. Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 2006;59:246‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyake Y, Iwasaki Y, Kobashi H, Yasunaka T, Ikeda F, Takaki A, Yamamoto K. Autoimmune hepatitis with acute presentation in Japan. Dig Liver Dis 2010;42:51‐54. [DOI] [PubMed] [Google Scholar]
- 29. Baven‐Pronk M, Biewenga M, van Silfhout JJ, van den Berg AP, van Buuren HR, Verwer BJ, et al. Role of age in presentation, response to therapy and outcome of autoimmune hepatitis. Clin Transl Gastroenterol 2018;9:e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anand L, Choudhury A, Bihari C, Sharma BC, Kumar M, Maiwall R, et al.; for APASL ACLF (APASL ACLF Research Consortium) Working Party . Flare of autoimmune hepatitis causing acute on chronic liver failure: diagnosis and response to corticosteroid therapy. Hepatology 2019;70:587‐596. [DOI] [PubMed] [Google Scholar]
- 31. Sonthalia N, Rathi PM, Jain SS, Surude RG, Mohite AR, Pawar SV, et al. Natural history and treatment outcomes of severe autoimmune hepatitis. J Clin Gastroenterol 2017;51:548‐556. [DOI] [PubMed] [Google Scholar]
- 32. Pape S, Gevers TJG, Belias M, Mustafajev IF, Vrolijk JM, van Hoek B, et al. Predniso(lo)ne dosage and chance of remission in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol 2019;17:2068‐2075. [DOI] [PubMed] [Google Scholar]
- 33. Heinemann M, Adam R, Berenguer M, Mirza D, Malek‐Hosseini SA, O'Grady JG, et al.; for contributing centers and the European Liver and Intestine Transplant Association (ELITA) . Longterm survival after liver transplantation for autoimmune hepatitis: results from the European liver transplant registry. Liver Transpl 2020;26:866‐877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


