Abstract
Animal studies show that high‐salt diet affects T‐cell subpopulations, but evidence in humans is scarce and contradictory. This pilot study investigated the effect of a 2‐week high‐salt diet on T‐cell subpopulations (ie, γδ T cells, Th17 cells, and regulatory T cells) in five healthy males. The mean (SD) age of the participants was 33 (2) years, with normal body mass index, kidney function, and baseline blood pressure. In terms of phenotype, there was an isolated increase of CD69 expression in Vδ1 T cells (P = .04), which is an early activation marker. There were no statistically significant changes or trends in any of the other tested markers or in the Th17 or regulatory T‐cell subsets. The increase in CD69 was strongly correlated to increases in 24‐hour urinary sodium excretion (r = .93, P = .02). These results of this pilot may motivate the use of longer dietary salt interventions in future studies on salt and adaptive immune cells.
Keywords: blood pressure, gamma delta T cells, regulatory T cells, salt, sodium, T cells, Th17 cells
1. INTRODUCTION
Recent evidence demonstrates that excessive salt (NaCl) intake induces pro‐inflammatory priming of innate immune cells, which may play a role in the deleterious outcomes of salt consumption. In both animal and human studies, monocytes and macrophages showed pro‐inflammatory signs after 1‐2 weeks salt loading. 1 , 2 , 3 , 4 , 5 For the adaptive immune system, evidence in humans is limited and is in contrast with animal‐derived findings. In mouse models, salt induces Th17 cells and inhibits regulatory T cells, while the exact opposite effect was observed in humans. 6 , 7 , 8 , 9 Also, a special subset of T lymphocytes that has not been studied in the context of salt involves TCRγδ T cells, which express T‐cell receptors (TCR) composed of γ and δ chains and play a role in a variety of inflammatory diseases. Recently, these cells were shown to mediate angiotensin‐II‐induced hypertension in mice and to correlate with systolic blood pressure in humans. 10 Given the increasingly recognized notion that immune cells contribute to salt‐sensitive hypertension, 2 , 5 the effect of salt on TCRγδ T cells, in conjunction with the other T‐cell subsets, merits exploration.
We performed a pilot study on 5 healthy males that were subjected to a 2‐week low‐salt diet and high‐salt diet, in randomized order and without a washout period in between. All participants provided written informed consent, and the study was approved by the local ethics committee and in accordance with the Declaration of Helsinki. The trial was registered in The Netherlands Trial Register (NTR4785). Participants could prepare their own meals, with the help of dietary lists. For the low‐salt diet, an average of 3 grams salt/day was achieved, and for the high‐salt diet 17 grams salt/day, based on 24‐hour urine sodium. Other nutrients like potassium and urea were not changed (data not shown). Blood pressure was measured with an automatic 24‐hour ambulatory blood pressure device (Mobil‐O‐Graph®). PBMCs were isolated from heparinized blood samples and stored in liquid nitrogen. Live TCRγδ+TCRαβ‐CD3+ T cells and TCR Vδ1+ and TCRV δ2+ therein were analyzed for activation assessing CD25, CD69, Ki67, and granzyme B (Figure 1A+B). Also, we analyzed numbers of Th17 (RORγT + CD161+CCR4 + CCR6+RORCD4 + CD3+) and regulatory T cells (FOX‐P3 + CD127‐CD25 + CD4+CD3 + and helios + CD127‐CD25 + CD4+CD3+) and their expression of Ki67. To detect differences between diets, data were tested with a paired t test. Prior to significance testing, data were log‐transformed. Associations were tested using Spearman's coefficients with Z‐scores in case of paired data. A P‐value of < .05 was considered significant.
Figure 1.
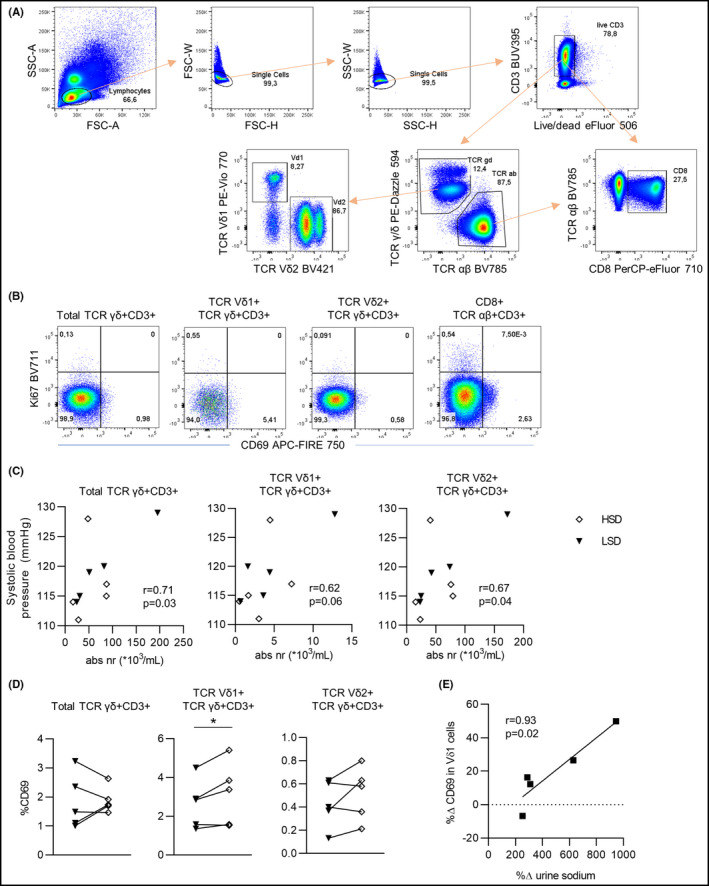
(A) Gating strategy of live single cell Vδ1 + and Vδ2 + TCRγδ+CD3 + T cells. (B) Examples of CD69 vs. Ki67 expression within the total TCRγδ T cells, Vδ1 + TCRγδ T cells, and Vδ2 + TCRγδ T cells. Expression of CD69 vs. Ki67 in total TCRαβ + CD3+CD8 + T cells is shown for comparison (right). (C) Correlation between γδ T cells, Vδ1 T cells, and Vδ2 T cells with 24‐hour ambulatory systolic blood pressure. (D) CD69 expression in Total γδ T cells, Vδ1 T cells, and Vδ2 T cells after LSD and HSD. (E) Correlation between percentage change ((HSD‐LSD)/LSD * 100) in 24‐hour urine sodium excretion (as a proxy for salt intake) and CD69 expression in Vδ1 T cells. (A‐B) Two million PBMC were first stained with the following surface monoclonal antibodies: TCR Vδ1 PE‐Vio 770 (REA173)(Miltenyi Biotec, Bergisch Gladbach, Germany), CD3 BUV395 (UCHT1) (BD Bioscience, Franklin Lakes, NJ, USA), CD8 PerCP‐eFluor 710 (SK1)(eBioscience Inc, Thermo Fisher Scientific, San Diego, CA, USA), TCR Vδ2 BV421 (B6), TCR αβ BV785 (IP26), TCR γ/δ PE‐Dazzle 594 (B1), and CD69 APC‐FIRE 750 (FN50) (BioLegend, San Diego, CA, USA). Dead cells were excluded with viability dye eFluor506 (eBioscience Inc). Secondly, the intracellular staining with anti‐Ki67 BV711 (Ki‐67)(BioLegend) was performed after the fixation and permeabilization of the cells using the FoxP3/Transcription Factor Staining Set (eBioscience Inc). Measurements were performed on an LSRFortessa flow cytometer (BD Biosciences). Data were analyzed using FlowJo version 10 (FlowJo, Ashland, OR, USA). All graphs were created using Graphpad Prism version 8.3 for Windows (GraphPad Software, La Jolla California USA). Paired t tests were used to compare data between diets, and associations were tested using Spearman's coefficients with Z‐scores in case of paired data. LSD, low‐salt diet (black triangles). HSD, high‐salt diet (white diamonds)
The mean (SD) age of the participants was 33 (2) years, and they had normal body mass index, estimated glomerular filtration rate (eGFR), and baseline blood pressure. Although high‐salt diet did not have an effect on blood pressure (117/70 vs 119/71 mmHg; P = .52/.77) or the amount of TCRγδ T cells, Vδ1 T cells, and Vδ2 T cells (the latter two representing the two major subsets of TCRγδ T cells), mean 24‐hour systolic blood pressure correlated with the total amount of TCRγδ T cells and Vδ1 and Vδ2 subsets (Figure 1C). In terms of phenotype, there was an isolated increase of CD69‐expression in Vδ1 T cells (P = .04), which is an early activation marker (Figure 1D). There were no changes in any of the other tested markers or in the Th17 or regulatory T‐cell subsets (data not shown). The increase in CD69 was strongly correlated to increases in 24‐hour urinary sodium excretion (r = .93, P = .02) (Figure 1E).
We confirm the relationship between TCRγδ T cells and blood pressure, 10 and show that a 2‐week high‐salt diet increases CD69 in Vδ1 T cells. The fact that none of the other markers are altered may imply that salt has no profound effect on TCRγδ T cells. On the other hand, the isolated increase of this early activation marker—which correlated strongly with the amount of salt intake—may also be the first subtle sign of salt‐induced activation of this subset. Although a 2‐week high‐salt diet did induce pro‐inflammatory priming of innate immune cells, 1 it cannot be excluded that for the slower‐acting adaptive immune system longer salt interventions are needed to observe actual effects. Especially relative to life span, the duration of salt exposure in animal studies studying T‐cell populations largely exceed those in humans. With regard to the earlier reported inconsistent data on salt‐induced changes of Th17 and Tregs, 3 , 4 , 5 our pilot was not able to throw more light on the direction of the effects in humans. We want to emphasize that by definition our pilot is underpowered. Although we did not observe effect sizes indicating that a larger sample size would lead to different results (ie, there were no statistical trends that are thought to become significant with a larger sample size), future larger‐sized studies are warranted, specifically in patient groups at risk for hypertension development. Such studies may, however, be considered to explore the effect of longer intervention durations based on our preliminary pilot data and preferentially characterize immune cells at several points in time to elucidate the undoubtedly complex interplay of different immune cells in response to salt overload and blood pressure.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
LV contributed to conceptualization. LV contributed to funding acquisition. EFEW, EBMR, NDB, and EMS contributed to investigation. EBMR and NDB contributed to methodology. LV and FJB contributed to supervision. EFEW and EBMR contributed to visualization. EFEW and EBMR contributed to roles/writing—original draft. All authors contributed to writing—review and editing.
ACKNOWLEDGEMENTS
This research was funded by a Dutch Kidney Foundation Junior Kolff grant (KJPB 11.22), Senior Kolff grant (18OKG12), and ZonMW Clinical Fellowship grant (90700310) to LV. The authors thankfully acknowledge the contribution of Frank van Diemen (Amsterdam UMC, dept. of Internal Medicine) to the statistical analyses.
Wenstedt EFE, Remmerswaal EBM, van der Bom‐Baylon ND, Schrooten EM, Bemelman FJ, Vogt L. The effect of high‐salt diet on t‐lymphocyte subpopulations in healthy males—A pilot study. J Clin Hypertens. 2020;22:2152–2155. 10.1111/jch.14049
Funding information
LV was supported by a Dutch Kidney Foundation Junior Kolff grant (KJPB 11.22), Senior Kolff grant (18OKG12), and ZonMW Clinical Fellowship grant (90700310).
REFERENCES
- 1. Wenstedt EF, Verberk SG, Kroon J, et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight. 2019;4(21):e130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mattson DL. Immune mechanisms of salt‐sensitive hypertension and renal end‐organ damage. Nat Rev Nephrol. 2019;15(5):290‐300. [DOI] [PubMed] [Google Scholar]
- 3. Binger KJ, Gebhardt M, Heinig M, et al. High salt reduces the activation of IL‐4‐ and IL‐13‐stimulated macrophages. J Clin Invest. 2015;125(11):4223‐4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jantsch J, Schatz V, Friedrich D, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage‐driven host defense. Cell Metab. 2015;21(3):493‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rucker AJ, Rudemiller NP, Crowley SD. Salt, Hypertension, and Immunity. Annu Rev Physiol. 2018;80:283‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496(7446):518‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125(11):4212‐4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo T, Ji WJ, Yuan F, et al. Th17/Treg Imbalance Induced by Dietary Salt Variation Indicates Inflammation of Target Organs in Humans. Sci Rep. 2016;6:26767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilck N, Matus MG, Kearney SM, et al. Salt‐responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551(7682):585‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caillon A, Mian MOR, Fraulob‐Aquino JC, et al. gammadelta T Cells Mediate Angiotensin II‐Induced Hypertension and Vascular Injury. Circulation. 2017;135(22):2155‐2162. [DOI] [PubMed] [Google Scholar]


