Abstract
Background
Whether low muscle mass is a risk factor for disability and mortality is unclear. Associations between approximations of muscle mass (including lean mass from dual-energy x-ray absorptiometry [DXA]), and these outcomes are inconsistent.
Methods
Muscle mass measured by deuterated creatine (D3Cr) dilution and appendicular lean mass (ALM, by DXA) were assessed at the Year 14 Visit (2014–2016) of the prospective Osteoporotic Fractures in Men study (N = 1,425, age 77–101 years). Disability in activities of daily living (ADLs), instrumental ADLs, and mobility tasks was self-reported at the Year 14 visit and 2.2 years later; deaths were centrally adjudicated over 3.3 years. Relative risks and 95% confidence intervals (CI) were estimated per standard deviation decrement with negative binomial, logistic regression, or proportional hazards models.
Results
In age- and clinical center-adjusted models, the relative risks per decrement in D3Cr muscle mass/wgt was 1.9 (95% CI: 1.2, 3.1) for incident self-reported ADL disability; 1.5 (95% CI: 1.3, 1.9) for instrumental ADL disability; and 1.8 (95% CI: 1.5, 2.2) for mobility disability. In age-, clinical center-, and weight-adjusted models, the relative risks per decrement in D3Cr muscle mass was 1.8 (95% CI: 1.5, 2.2) for all-cause mortality. In contrast, lower DXA ALM was not associated with any outcome. Associations of D3Cr muscle mass with these outcomes were slightly attenuated after adjustment for confounding factors and the potentially mediating effects of strength and physical performance.
Conclusions
Low muscle mass as measured by D3Cr dilution is a novel risk factor for clinically meaningful outcomes in older men.
Keywords: Muscle mass, Disability, Death
There is strong evidence that poor muscle function (quantified as strength and power) and impaired physical performance (gait speed, repeat chair stands) are related to mortality and disability (1–4). However, although low muscle mass would then theoretically predispose individuals to disability and mortality, the evidence for the association between low muscle mass and these outcomes is less clear. For example, appendicular lean body mass (ALM, kg) derived from dual x-ray absorptiometry (DXA) standardized to body height (m2) (5,6) is a commonly used approximation of muscle mass that is not consistently and independently related to disability (7,8), although the apparent modest association with mortality is somewhat better characterized (9). This has led some to conclude that muscle mass is relatively unimportant in classifying the risk of disability or death in older adults (10,11). Indeed, more recently published consensus definitions of sarcopenia, such as the European Working Group on Sarcopenia in Older People updated 2019 guidelines (EWGSOP2) have shifted in focus definition of sarcopenia from low muscle mass (ie, low DXA lean mass) toward muscle function (ie, muscle strength) (12). Furthermore, although composite dentitions of sarcopenia (based on the combined presence of low lean mas, low grip strength, and/or low gait speed) are associated with adverse health outcomes in older adults (13), it is often unclear which component(s) of sarcopenia are driving these associations: often it is low grip strength or low walking speed, not low lean mass, that identifies those at risk (1,7).
We posit that the lack of consistent association between low DXA lean mass and disability risk is due to problems inherent in the use of DXA to approximate muscle mass. DXA does not measure muscle mass directly, rather DXA-derived total body lean mass, and includes tissue from organs such as the kidney and liver, as well as fibrotic and other lean tissue. Operationally, lean mass from DXA is usually analyzed as ALM that is the nonbone, nonfat component of the arms and legs that includes muscle, fibrotic and connective tissue, and water. Because DXA inherently measures muscle mass inaccurately, it is possible that a more direct and accurate measure of muscle mass may be related to risk of disability even when DXA lean mass is not. A newly available method to assess muscle mass can investigate this problem: the D3-creatine dilution method measures total body skeletal muscle mass with a simple, direct, and clinically feasible procedure that has been validated in humans (14). Recent studies confirm that low muscle mass by D3Cr dilution was strongly related to weakness, poor physical performance, increased likelihood of prevalent disability, incident short-term mobility limitations, and incident serious injurious falls in older men while DXA ALM was not (15). Taken together, we posit that this new, accurate assessment of muscle mass will demonstrate more robust associations with disability and mortality outcomes compared with the association of DXA measures of lean mass with these outcomes.
Therefore, we aimed to test the hypothesis that low muscle mass as measured by the D3Cr dilution method is independently associated with an increased risk of self-reported disability in activities of daily living (ADL), instrumental ADLs (IADLs), or mobility. Because much of the association of muscle mass with these outcomes may act through strength and performance, we also aimed to test whether any association observed between D3Cr muscle mass and disability or mortality was independent of these potential mediators. Finally, we hypothesized that low DXA ALM would not be a risk factor for disability or mortality. These hypotheses were tested in the prospective Osteoporotic Fractures in Men (MrOS) study, a prospective cohort of community-dwelling older men.
Methods
MrOS Cohort
In 2000–2002, 5,994 ambulatory community-dwelling men aged ≥65 and older without bilateral hip replacements were enrolled in MrOS, a multicenter cohort study of aging and osteoporosis (16,17). All men provided written informed consent separately for the general measures at Visit 4/follow-up and then also for the D3-creatine protocol specifically. The study was approved by the Institutional Review Board at each center, including the clinical sites and Coordinating Center (California Pacific Medical Center). In 2014–2016, 2,786 survivors were contacted to participate in “Visit 4” (Year 14) clinic visit. Of these, 362 refused participation, 583 completed questionnaires only, and 1,841 completed questionnaires and at least part of the clinic visit (Supplementary Figure 1).
D3-Creatine Dilution
The D3-creatine dilution method involves a participant ingesting a 30-mg dose of stable isotope labeled creatine (D3-creatine), and providing a fasting, morning urine sample 72–144 hours (3–6 days) later in which D3-creatinine, unlabeled creatinine, and creatine are measured using high performance liquid chromatography and tandem mass spectroscopy (MS/MS); these measures are then included in an algorithm to determine total body creatine pool size and thus skeletal muscle mass as previously described (18). Importantly, because the enrichment of creatinine is measured (ie, the ratio of D3-creatinine to unlabeled creatinine), this method is not dependent on creatinine clearance or renal function. The method does not require any special dietary control other than the need for a fasting morning spot urine sample.
Self-reported Incident Disability Outcomes
Men answered questions at the Year 14 visit (Visit 4) and a follow-up mailed questionnaire 2.2 ± 0.3 years later (Year 16 questionnaire) about their ability to complete a number of tasks across three domains: activities of daily living (ADLs; eating or feeding oneself, toileting, transferring, bathing/showering, dressing); IADLs (preparing meals, doing heavy housework, shopping for groceries/clothes, managing money, managing medications, driving); and mobility (walking two to three blocks on level ground, climbing 10 steps without resting, and carrying or lifting 10 pounds). Men reporting inability to perform one or more individual tasks within each domain were considered to have a disability for that domain. More details about disability assessed are provided in Supplementary Materials.
Mortality
Men were contacted every 4 months after the Year 14 contact. Clinic staff was usually notified of a participant’s death when following up on missed contacts. More detail about adjudication of mortality is provided in Supplementary Materials.
DXA and Other Measurements
ALM and body fat were assessed by whole-body DXA scans (Hologic 4500 scanners, Waltham, MA; details are provided in Supplementary Materials) (19). Weight was measured on a balance beam or digital scale and height by wall-mounted stadiometers. Other covariate information is described in Supplementary Materials.
Study Sample
We invited 1,841 men who attended the Year 14 clinic visit to complete the D3-creatine dilution protocol, and 1,641 agreed to participate (Supplementary Figure 1). Of these, 187 were excluded for protocol violations that included incorrect timing of the dose or urine collection (either less than 72 hours or more than 144 hours between the dose and collection) or forgetting to take the dose or provide the specimen. Six samples were lost by the clinical center or laboratory, and 23 men were excluded because of outlying values for total muscle mass/wgt more than 2 SD from the mean, most of which included values that exceeded 100% of body weight. Thus, 1,425 men had valid measures of D3Cr muscle mass, of whom 1,400 had complete data on vital status and were included in the mortality analysis. Men missing data on the incident disability outcomes or those with prevalent disability for that outcome were excluded from that specific analysis (N = 182 for prevalent ADL disability; 376 for prevalent IADL disability; and 269 for prevalent IADL disability). This left 1,243 men in the analysis of incident ADL disability, 1,049 men in the analysis of incident IADL disability, and 1,156 men in the incident mobility disability analysis.
Statistical Analysis
We compared characteristics of participants by median split of D3Cr muscle mass/wgt using t-tests and Wilcoxon rank-sum tests as appropriate. Negative binominal regression was used to estimate the relative risk and 95% confidence interval for incident IADL disability, incident ADL disability, and incident mobility disability outcomes, and also separately for disability of each individual task. The negative binomial models did not converge for both D3Cr muscle mass/wgt and DXA ALM/ht2 for the outcomes of dressing, toileting, transferring, and managing money, in which case we used logistic regression to calculate the odds ratio (OR, which estimates the relative risk in this instances as the outcome is rare). For disability outcomes, D3Cr muscle mass/wgt was the primary predictor variable because exploratory analyses revealed that both D3Cr muscle mass and weight were independent predictors of the disability outcomes, and the absolute value of the ratio of the beta coefficients for the log values of these variables was approximately 1 (indicating that a ratio of D3Cr muscle mass to weight is the most appropriate predictor for disability outcomes). To account for body size in DXA ALM models, primary models used ALM/ht2 as the predictor. Proportional hazards models were used to estimate the hazard ratio and 95% confidence interval for mortality outcomes. Weight was not strongly related to mortality, so D3Cr muscle mass (unadjusted for body size) was analyzed as the primary predictor variable; the mortality models also included weight as a covariate. Analogously, we used DXA ALM (not standardized to body size) as the primary predictor for mortality models. D3Cr muscle mass/wgt, D3Cr muscle mass (not standardized to body size), DXA ALM/ht2, and DXA ALM (not standardized to body size) were normally distributed and were analyzed as continuous values with the relative risk expressed per SD increment, and also by quartiles. Secondarily, we also examined the associations DXA ALM (or DXA ALM/ht2), DXA ALM/BMI, DXA ALM/wgt with the outcomes. We adjusted all models for age and clinical center. Then we further adjusted for potential confounding variables unlikely to be on the causal pathway between low muscle mass and disability (or mortality) and adjusted all models for this parsimonious set of variables. We considered confounders selected a priori that likely to be associated with both muscle mass and mortality or disability outcomes. The factors included race, lifestyle habits (smoking, alcohol use); self-reported health and comorbid conditions that are likely to have effects on muscle mass (chronic heart failure, chronic obstructive pulmonary disease, diabetes, and myocardial infarction); activity level and exhaustion; cognitive function; and percent fat and history of weight change since MrOS enrollment. Height was also included as a covariate in multivariate models (except in those models where ALM/ht2 was a predictor.) Weight was also included as a covariate in multivariate models (except in those models were D3Cr muscle mass/wgt was a predictor). Smoking status was not assessed in 68 men; those missing these data were included in multivariate models as a separate group in order to increase the analysis sample for the multivariate model.
To determine whether the association between D3Cr muscle mass/wgt or DXA ALM/ht2 and disability was potentially mediated by strength and physical performance, we subsequently adjusted all models for gait speed, grip strength, and chair stand rate. No clear cut-point has been established for identifying mediation. Therefore, we considered a reduction in the beta coefficient for the association of D3Cr muscle mass/wgt or DXA ALM/ht2 and the mortality or disability outcomes of at least 10% to support a hypothesis of mediation, similar to other reports (20,21). The statistical approach to quantify the discriminative ability of D3Cr muscle mass/wgt or DXA ALM/ht2 in these analyses is described in Supplementary Materials. We then repeated these models for mortality outcomes, using D3Cr muscle mass or DXA ALM (not standardized to body size) as the primary predictor variables.
All significance levels were two sided. Analyses were conducted using SAS version 9.4 (SAS Institute, Inc., Cary, NC). MrOS data from February 2019 were used (https://mrosdata.sfcc-cpmc.net/).
Role of the Funding Source
The funding source had no role in the design, collection, analysis, or interpretation of data; writing of the manuscript; or the decision to submit this paper for publication.
Results
Many of the participant characteristics varied by median split in D3Cr muscle mass/wgt status (Table 1). Of note, compared with men with higher D3Cr muscle mass/wgt, those with lower D3Cr muscle mass/wgt were older, report a race other than White, a greater number of comorbidities, lower activity, and worse self-reported health. Men with lower D3Cr muscle mass/wgt weighed more, with higher BMI (but no difference in history of weight loss) than men with higher D3Cr muscle mass/wgt. Mean D3Cr muscle mass/wgt was 0.31 (SD = 0.05) and mean D3Cr muscle mass (unadjusted for body size) was 24.1 kg (SD = 4.11). D3Cr muscle mass and height were correlated at r = .39 (p < .001).
Table 1.
Characteristics (Mean ± SD or N [%]) of the MrOS Men by Median Split in D3Cr Muscle Mass/wgt
| Characteristics | Lower D3Cr Muscle Mass < 0.30(N = 712) | Higher D3Cr Muscle Mass/wgt ≥ 0.30(N = 713) | p-Value |
|---|---|---|---|
| Age, y | 85.1 ± 4.2 | 83.3 ± 3.7 | <.001 |
| Race, nonwhite | 50 (7.0) | 91 (12.8) | <.001 |
| Alcohol intake, drinks/wk | .003 | ||
| <1 drink/wk | 375 (52.9) | 314 (44.2) | |
| 1–13 drinks/wk | 297 (41.9) | 362 (51.0) | |
| 14+ drinks/wk | 37 (5.2) | 34 (4.8) | |
| Smoking status | <.001 | ||
| Never smoker | 232 (32.6) | 306 (42.9) | |
| Past or current smoker | 441 (61.9) | 378 (53.0) | |
| Missing smoking data | 39 (5.5) | 29 (4.1) | |
| Number of comorbidities | <.001 | ||
| 0 | 412 (57.9) | 501 (70.3) | |
| 1 | 202 (28.4) | 164 (23.0) | |
| 2 or more | 98 (13.8) | 48 (6.7) | |
| History of congestive heart failure | 76 (10.7) | 40 (5.6) | <.001 |
| History of chronic obstructive pulmonary disease | 103 (14.5) | 64 (9.0) | .001 |
| History of diabetes mellitus | 134 (18.8) | 87 (12.2) | <.001 |
| History of myocardial infarction | 114 (16.0) | 75 (10.5) | .002 |
| Physical activity score (PASE) | 101.6 ± 61.7 | 134.2 ± 62.9 | <.001 |
| Exhaustion | 138 (19.4) | 57 (8.0) | <.001 |
| Modified Mini-Mental state (3MS) exam score | 91.7 ± 7.1 | 93.0 ± 6.5 | <.001 |
| Gait speed, m/s | 0.99 ± 0.25 | 1.15 ± 0.22 | <.001 |
| Number of chair stands in 10 s | 3.1 ± 1.9 | 4.3 ± 1.5 | <.001 |
| Maximum grip strength, kg | 34.1 ± 7.7 | 37.1 ± 7.7 | <.001 |
| Percent body fat | 30.6 ± 5.4 | 25.0 ± 5.1 | <.001 |
| Weight, kg | 83.7 ± 13.0 | 75.6 ± 10.5 | <.001 |
| Body mass index, kg/m2 | 28.1 ± 3.8 | 25.6 ± 3.0 | <.001 |
| Weight change since baseline, kg | −4.0 ± 7.5 | −4.3 ± 6.1 | .517 |
| Excellent/good self-rated health | 606 (85.4) | 664 (93.3) | <.001 |
| Died during follow-up | 132 (18.9) | 65 (9.3) | <.001 |
| Incident difficulty with ADL tasks | 115 (25.0) | 67 (11.4) | <.001 |
| Incident ADL disability | 17 (2.9) | 4 (0.6) | .002 |
| Incident difficulty with IADL tasks | 140 (37.0) | 118 (22.4) | <.001 |
| Incident IADL disability | 94 (20.8) | 48 (8.0) | <.001 |
| Incident difficulty with mobility tasks | 131 (33.9) | 98 (17.0) | <.001 |
| Incident mobility disability | 89 (17.1) | 27 (4.2) | <.001 |
Notes: ADL = activity of daily living; IADL = instrumental ADLs. aIncidence for difficulty or disability all each ADL, IADL, or mobility tasks was significantly lower for those with higher D3Cr muscle mass/wgt except inability to feed oneself (this was not reported by any participant); inability to dress oneself; and inability to transfer; these events were rare (N < 6 in the entire cohort for each).
Incident Self-reported Disability Outcomes
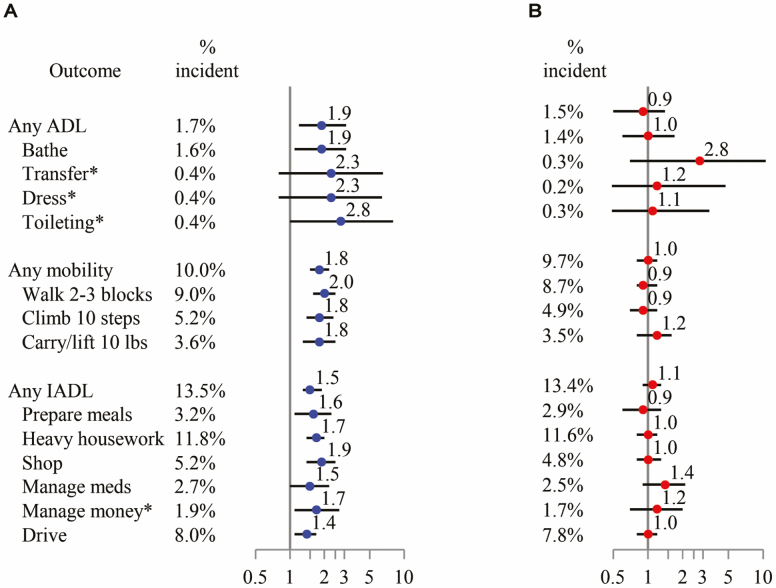
Over 2.2 years, 21 men initially free of ADL disability reported new disability in at least one ADL task (Figure 1); 142 men (13.5%) initially free of IADL disability reported new disability in at least one IADL task; and 116 men (10.0%) initially free of mobility disability reported new mobility disability. There were marked differences in incidence of self-reported ADL, IADL, and mobility difficulties and disability, both overall and by task. For example, 20.8% of men (N = 94) with lower D3Cr muscle mass/wgt had new difficulty with an IADL task, whereas only 8.0% (N = 48) of men with higher D3Cr muscle mass/wgt reported difficulty with at least one IADL task (p < .001).
Figure 1.
Age- and clinic center-adjusted relative risk for incident IADL disability and mobility disability, per SD decrement of D3Cr muscle mass/wgt (A) or appendicular lean mass (kg)/height (m2) (B). *Relative risk (95% CI) estimated using logistic regression due to small event counts.
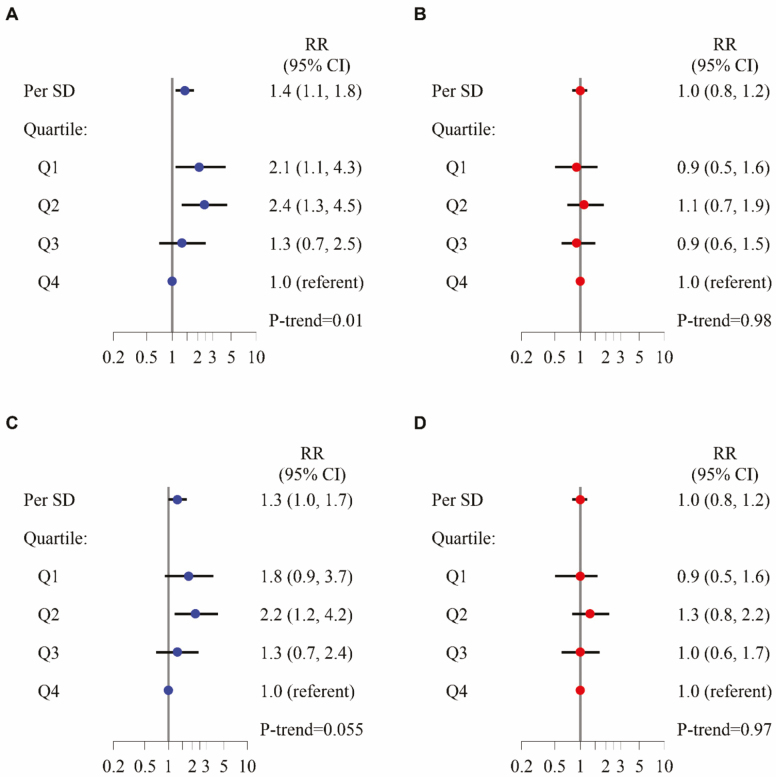
After adjustment for age and clinical center, each SD decrement in D3Cr muscle mass/wgt was associated with a 1.9-fold increased risk of self-reported incident ADL disability, 1.8-fold increased risk of self-reported incident mobility disability, and 1.5-fold increased risk of self-reported incident IADL disability (Figure 1). Furthermore, the point estimate for the relative risk between D3Cr muscle mass/wgt and incident disability in each functional task (eg, bathing, walking two to three blocks, preparing meals) was consistently between 1.4 and 2.8, but these did not always reach statistical significance as some outcomes were rare. The associations remained mostly unchanged after adjustment for potentially confounding factors (Figures 2A and B and 3A and B). In contrast to the association between low D3Cr muscle mass/wgt, with the incident disability outcomes, there was no significant association between DXA ALM/ht2 and risk of incident ADL disability, incident mobility disability, or incident IADL disability overall; for any individual functional task; or after adjustment for covariates and mediators. Results of secondary analyses for disability (mediation, discrimination, and use of alternative DXA lean mass metrics including DXA ALM, DXA ALM/BMI, DXA ALM/wgt) are described in Supplementary Materials.
Figure 2.
Multivariable-adjusted relative risk for incident IADL disability, by quartiles and per SD increment of D3Cr muscle mass/wgt (A, C) or DXA appendicular lean mass (kg)/height (m2) (B, D). Multivariable models adjusted for age, race, clinical center, alcohol use, smoking status, comorbidities, physical activity, percent fat, exhaustion, cognitive function, self-reported health status, and weight change. D3Cr muscle mass/wgt models also adjusted for height (A, B). Models further adjusted for strength and physical performance covariates also include chair stands, gait speed, and grip strength (C, D).
Figure 3.
Multivariable-adjusted relative risk for incident mobility disability, by quartiles and per SD increment of D3Cr muscle mass/wgt (A, C) or DXA appendicular lean mass(kg)/height (m2) (B, D). Multivariable models adjusted for age, race, clinical center, alcohol use, smoking status, comorbidities, physical activity, percent fat, exhaustion, cognitive function, self-reported health status and weight change. D3Cr muscle mass/wgt models also adjusted for height (A, B). Models further adjusted for strength and physical performance covariates also include chair stands, gait speed, and grip strength (C, D).
Mortality
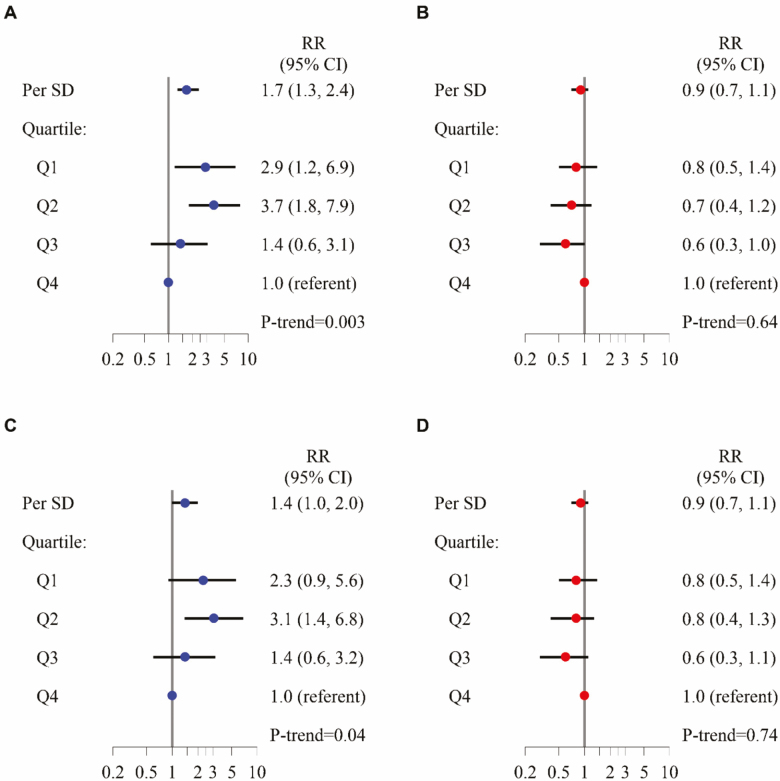
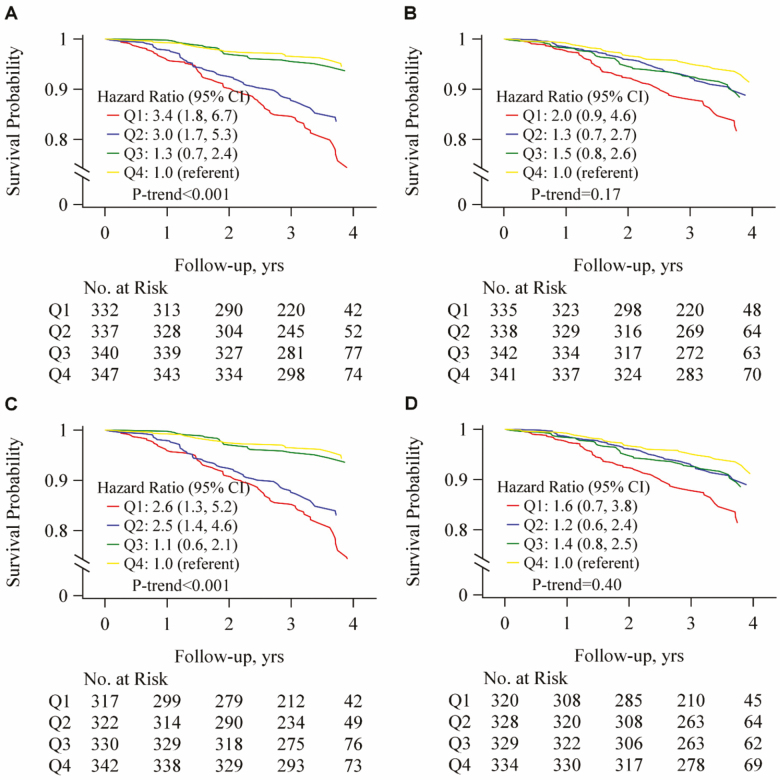
Over 3.3 years of follow-up, 197 (14.1%) of participants died, including 91 (26.0%) of men in the lowest D3Cr muscle mass quartile; 61 (17.6%) of the men in the second D3Cr muscle mass quartile; 25 (7.2%) of the men in the third D3Cr muscle mass quartile; and only 20 (5.6%) of the men in the highest D3Cr muscle mass quartile (p < .001). After adjustment for potentially confounding variables, both the risk across quartiles of D3Cr muscle mass and mortality (relative risk, Q1 vs Q4: 3.4, 95% confidence interval: 1.8, 6.7) and the association across quartiles of DXA ALM and mortality (relative risk, Q1 vs Q4, 2.0, 95% confidence interval: 0.9, 4.6) remained elevated. Only D3Cr muscle mass remained independently associated with mortality after adjustment for strength and physical performance (Figure 4). Results of secondary analyses for mortality (mediation, discrimination, and use of alternative DXA lean mass metrics including DXA ALM/ht2, DXA ALM/BMI, DXA ALM/wgt) are described in Supplementary Materials.
Figure 4.
Multivariable-adjusted hazard ratio for all-cause mortality, by quartiles of D3Cr muscle mass (A, C) or DXA appendicular lean mass(kg) (B, D). Multivariable models adjusted for age, race, clinical center, alcohol use, smoking status, comorbidities, physical activity, percent fat, exhaustion, cognitive function, self-reported health status, weight change, weight, and height (A, B). Models further adjusted for strength and physical performance covariates also include chair stands, gait speed, and grip strength (C, D).
Discussion
Low D3Cr muscle mass, a direct assessment of muscle mass, was strongly associated with a higher risk of mortality and self-reported disability in older men. These associations were largely independent of potentially confounding factors such as age, comorbidity, health habits, and activity level and were only partially mediated by muscle strength and physical performance. The absolute difference the incidence of these important clinical outcomes was large: for example, 26% of men in the lowest quartile of D3Cr muscle mass died while only 6% of men in the highest D3Cr muscle mass quartile died. In contrast, low DXA lean mass (a traditional approximation of muscle mass) was not strongly related to risk of mortality or disability (Figure 4).
Our results clarify a conundrum presented by previous work: that is, how was it possible that deficits in strength and performance were associated with poor outcomes (1–3) while the quantity of muscle (when approximated by DXA lean mass) was not (7,8)? This issue has led to significant controversy about the inclusion of lean mass in operational definitions of sarcopenia, a geriatric condition with an ICD-10 code (M62.84) that lacks consensus for clinical diagnostic standards (22). Data herein suggest that it may be possible to define sarcopenia by the presence of low D3Cr muscle mass or D3Cr muscle mass/wgt, but further research is needed confirm these findings in other populations including younger adults, women, and other racial and ethnic groups. In addition, other estimates of muscle mass or size include magnetic resonance imaging, bioelectrical impedance analysis, and computerized tomography—including peripheral quantitative computerized tomography. These techniques are either expensive, inaccurate, do not assess total body muscle mass, or carry radiation exposure, which limit clinical uptake and application. Furthermore, imaging-based methods (magnetic resonance, computerized tomography) require arduous postacquisition processing. Nonetheless, low amounts of muscle volume or cross-sectional are from these measurements have generally been associated with poor outcomes in older adults, although perhaps to a lesser extent than strength or physical performance (23–26).
Some have concluded that muscle mass per se is unimportant in terms of identifying older adults at risk of poor outcomes (10,11). Our results question this paradigm, in that D3Cr muscle mass/wgt itself is associated with disability and D3Cr muscle mass is associated with mortality in older men even when strength and performance are taken into account. Our results also suggest that the role of muscle mass in the day-to-day health of older adults has been largely overlooked by medical providers. Routine measurement of muscle size, strength, or physical performance is not common in clinical settings despite evidence that deficits in these domains are strongly related to adverse outcomes (1–3,15). Gait speed is already considered by some to be the “sixth vital sign” (27); grip strength can be measured inexpensively and quickly. It is conceivable that the highly feasible D3Cr muscle mass test also has considerable clinical utility although it is not yet available for routine clinical use. As shown in Supplementary Materials, D3Cr muscle mass may improve discrimination for prediction of disability or mortality suggesting that this measure may have clinical utility. However, larger data sets with the D3Cr muscle mass measures are needed to clarify when and in whom these tests, alone or in combination, should be considered. Further research is also needed to inform treatment decisions for those with low muscle mass—treatment would currently be limited to recommendations to exercise interventions or rehabilitation, as no pharmacological treatments are approved to lower the risk of these outcomes in older adults (28).
We observed consistent associations between low D3Cr muscle mass/wgt and increased risk of each individual ADL, mobility, and IADL task, regardless of whether the task is largely dependent on intact cognitive function (such as ability to manage money or medications) or on physical function (such as ability to complete heavy housework). Given the strong relation between low D3Cr muscle mass/wgt and decreased strength and poor physical performance (15), we would expect that low D3Cr muscle mass/wgt would be related to those tasks that depend on intact physical functioning. The reason for the association between low D3Cr muscle mass/wgt and tasks dependent on intact cognitive function are less clear. One explanation is that there is a single underlying factor (such as comorbid disease burden) that causes parallel declines in both D3Cr muscle mass/wgt and cognitive function. However, results were robust to adjustment for these factors. Another intriguing speculation is that low D3Cr muscle mass/wgt is associated subsequent decline in cognitive performance resulting in an inability to perform IADLs tasks, which are more cognitively demanding. We previously found that men with lower D3Cr muscle mass/wgt had lower scores on a global cognitive function examination (15). In addition, myokines (hormones released by skeletal muscle with local and systemic effects) may coordinate exercise-induced adaptations in the brain (29). Furthermore, observational evidence suggests long-term exposure to exercise may delay cognitive decline in older adults (30), although these results do not extend to all randomized trials of exercise interventions (31). The inter-relationship of muscle mass to cognitive function as an explanation for the association between low D3Cr muscle mass/wgt and IADL disability warrants further investigation.
Both low D3Cr muscle mass and low DXA ALM were related to increased risk of mortality, even after accounting for body size, comorbidities, health status and history of weight loss. (History of weight loss remained an independent predictor of mortality after accounting for either low D3Cr muscle mass or low DXA ALM.) Although associations observed in the present study were independent of comorbid conditions, randomized studies of older adults undergoing caloric restriction to induce weight loss, a situation where weight loss is not confounded by underlying disease, have found no association between weight loss and mortality (32), suggesting that residual uncontrolled confounding may be an explanation for our findings. However, change in D3Cr muscle mass has only been measured in a small subset of 40 MrOS men (33) and studies to alter levels of D3Cr muscle mass in randomized settings (such as with resistance training or caloric restriction) have not been completed. Until such data are available, the causal relation between low muscle mass (or low lean mass) and mortality cannot be established.
There are numerous strengths to our study including the clinical importance of the study outcomes; its prospective design; and its extensive characterization of participants who allowed for us to establish temporal order of associations and to control for potentially confounding and mediating factors. However, a few limitations should be noted. First, disability was only assessed at two time points, which may have led to underreporting (34), potentially biasing associations observed toward the null. Second, very few men developed ADL disability, limiting power to detect clinically meaningful associations and the ability to control for multiple potentially confounding factors simultaneously. Third, our analyses of the meditation effect of physical performance/strength on the D3Cr muscle mass → mortality or disability association did not follow formal causal mediation procedures; future work will investigate whether the presence of mediation suggested in these analyses is, in fact, causal, and whether other factors (such nutrition or inflammation) may also mediate these associations. Finally, the MrOS cohort is comprised of very old, mostly white men, it is unknown whether similar results would be found in women, the younger old, or other race or ethnic groups.
In sum, we have demonstrated a strong and independent relationship between low D3Cr muscle mass and greater risk of incident self-reported disability and mortality in older men that was not explained by body size, comorbidity, health status, activity, or other factors. Low DXA lean mass, a commonly used approximation of muscle mass was, at best, modestly related to these outcomes. Low muscle mass, when measured accurately, is a risk factor for meaningful clinical outcomes in older men.
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Funding for the D3Cr muscle mass measure was provided by NIAMS (grant number R01 AR065268). GlaxoSmithKline provided in-kind support by providing the d3-creatine dose and analysis of urine samples.
Author Contributions
P.M.C. drafted the manuscript, and all other authors completed critical review of the paper. P.M.C. developed the initial study design. P.M.C., E.O., S.R.C., J.A.C., D.M.K., K.E.E., and W.J.E. obtained funding. T.B. completed statistical analyses. P.M.C. had full access to the data and takes final responsibility for the decision to submit this paper for publication.
Conflict of Interest
Dr. Cawthon is a consultant to BioAge Labs and has grants from Abbott and Nestle to her institution, all for work unrelated to this paper. Although Dr. Evans is listed as an inventor on the issued patents, he derives no income from this intellectual property. Dr. Stone and Ms. Blackwell receive salary support through a grant from Merck, Inc. to their institution for work unrelated to this paper. All other authors report nothing to disclose.
Supplementary Material
References
- 1. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558 [DOI] [PubMed] [Google Scholar]
- 4. Wu Y, Wang W, Liu T, Zhang D. Association of grip strength with risk of all-cause mortality, cardiovascular diseases, and cancer in community-dwelling populations: a meta-analysis of prospective cohort studies. J Am Med Dir Assoc. 2017;18(6):551.e17–e35. doi: 10.1016/j.jamda.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 5. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 6. Newman AB, Kupelian V, Visser M, et al. ; Health ABC Study Investigators Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 7. Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67:28–40. doi: 10.1093/gerona/glr010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 9. Batsis JA, Mackenzie TA, Emeny RT, Lopez-Jimenez F, Bartels SJ. Low lean mass with and without obesity, and mortality: results from the 1999–2004 National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci. 2017;72(10):1445–1451. doi: 10.1093/gerona/glx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heymsfield SB, Gonzalez MC, Lu J, Jia G, Zheng J. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015;74:355–366. doi: 10.1017/S0029665115000129 [DOI] [PubMed] [Google Scholar]
- 11. Fragala MS, Kenny AM, Kuchel GA. Muscle quality in aging: a multi-dimensional approach to muscle functioning with applications for treatment. Sports Med. 2015;45:641–658. doi: 10.1007/s40279-015-0305-z [DOI] [PubMed] [Google Scholar]
- 12. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–2646. doi: 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 14. Clark RV, Walker AC, O’Connor-Semmes RL, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol. 2014;116(12):1605–1613. doi: 10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cawthon PM, Orwoll ES, Peters KE, et al. Strong relation between muscle mass determined by D3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;4(6):844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in Men Study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 17. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the Osteoporotic Fractures in Men (MrOS) study – a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 18. Shankaran M, Czerwieniec G, Fessler C. Dilution of D3-creatine to measure creatine pool size and estimate skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2018;9(3):540–546. doi: 10.1002/jcsm.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee CG, Boyko EJ, Nielson CM, et al. ; Osteoporotic Fractures in Men Study Group Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koo BB, Blackwell T, Lee HB, Stone KL, Louis ED, Redline S; Osteoporotic Fractures in Men (MrOS) Study Group Restless legs syndrome and depression: effect mediation by disturbed sleep and periodic limb movements. Am J Geriatr Psychiatry. 2016;24:1105–1116. doi: 10.1016/j.jagp.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psychol. 1997;65:599–610. doi: 10.1037//0022-006x.65.4.599 [DOI] [PubMed] [Google Scholar]
- 22. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) Code. J Am Med Dir Assoc. 2016;17:675–677. doi: 10.1016/j.jamda.2016.06.001 [DOI] [PubMed] [Google Scholar]
- 23. Tucker BM, Hsu FC, Register TC, et al. Psoas and paraspinous muscle measurements on computed tomography predict mortality in European Americans with type 2 diabetes mellitus. J Frailty Aging. 2019;8:72–78. doi: 10.14283/jfa.2019.5 [DOI] [PubMed] [Google Scholar]
- 24. Murea M, Lenchik L, Register TC, et al. Psoas and paraspinous muscle index as a predictor of mortality in African American men with type 2 diabetes mellitus. J Diabetes Complications. 2018;32:558–564. doi: 10.1016/j.jdiacomp.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lenchik L, Lenoir KM, Tan J, et al. Opportunistic measurement of skeletal muscle size and muscle attenuation on computed tomography predicts 1-year mortality in Medicare patients. J Gerontol A Biol Sci Med Sci. 2019;74:1063–1069. doi: 10.1093/gerona/gly183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74:1671–1678. doi: 10.1093/gerona/glz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23:314–322. doi: 10.1123/japa.2013-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–1161. doi: 10.1007/s12603-018-1139-9 [DOI] [PubMed] [Google Scholar]
- 29. Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol. 2018;9:698. doi: 10.3389/fneur.2018.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De D, Mukherjee S, Anand PSS, Kumar P, Suresh VR, Vijayan KK. Nutritional profiling of hilsa (Tenualosa ilisha) of different size groups and sensory evaluation of their adults from different riverine systems. Sci Rep. 2019;9:19306. doi: 10.1038/s41598-019-55845-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sink KM, Espeland MA, Castro CM, et al. ; LIFE Study Investigators Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314:781–790. doi: 10.1001/jama.2015.9617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kritchevsky SB, Beavers KM, Miller ME, et al. Intentional weight loss and all-cause mortality: a meta-analysis of randomized clinical trials. PLoS One. 2015;10:e0121993. doi: 10.1371/journal.pone.0121993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duchowny KA, Peters KE, Cummings SR, et al. Association of change in muscle mass assessed by D3-creatine dilution with changes in grip strength and walking speed. J Cachexia Sarcopenia Muscle. 11:55–61. doi: 10.1002/jcsm.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gill TM. Assessment of function and disability in longitudinal studies. J Am Geriatr Soc. 2010;58 (Suppl 2):S308–S312. doi: 10.1111/j.1532-5415.2010.02914.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.