Abstract
Background
Self-reported time spent standing has been associated with lower risk of mortality. No previous studies have examined this association using device-measured standing.
Method
This was a prospective cohort study of 5878 older (median age = 80 years), racial/ethnically diverse, community-dwelling women in the WHI Objective Physical Activity and Cardiovascular Health Study (OPACH). Women wore accelerometers for 1 week and were followed for mortality. The study applied previously validated machine learning algorithms to ActiGraph GT3X+ accelerometer data to separately measure time spent standing with and without ambulation. Cox proportional hazards models were used to estimate mortality risk adjusting for potential confounders. Effect modification by age, body mass index, moderate-to-vigorous physical activity, sedentary time, physical functioning, and race/ethnicity was evaluated.
Results
There were 691 deaths during 26 649 person-years of follow-up through March 31, 2018 (mean follow-up = 4.8 years). In fully adjusted models, all-cause mortality risk was lower among those with more standing without ambulation (quartile [Q] 4 vs Q1 HR = 0.63; 95% CI = 0.49–0.81, p-trend = .003) and more standing with ambulation (Q4 vs Q1 HR = 0.50; 95% CI = 0.35–0.71, p-trend < .001). Associations of standing with ambulation and mortality were stronger among women with above-median sedentary time (HR = 0.51; 95% CI = 0.38–0.68) compared to women with below-median sedentary time (HR = 0.80; 95% CI = 0.59–1.07; p-interaction = .02).
Conclusions
In this prospective study among older women, higher levels of accelerometer-measured standing were associated with lower risks of all-cause mortality. Standing is an achievable approach to interrupting prolonged sedentary time, and if not contraindicated, is a safe and feasible behavior that appears to benefit health in older ages.
Keywords: Accelerometer, Longevity, Physical activity
The number of women aged 65 and older in the United States is expected to reach 48.6 million by 2050 (1,2). Avoiding prolonged sedentary time and engaging in regular physical activity are key strategies for older Americans to improve prospects for healthy aging (3). Moderate-to-vigorous physical activity (MVPA) is associated with reduced risks of many age-related chronic diseases including heart disease, type 2 diabetes, certain cancers, Alzheimer’s disease, and dementia (4,5).
Low-intensity physical activities, such as standing, are important to study due to their feasibility and safety for nearly all older adults (6,7). The 2017 consensus definition of standing is “a position in which one has or is maintaining an upright position while supported by one’s feet” (8). This definition includes standing in one place and standing while walking around, 2 behaviors that have not been distinguished in previous research, referred to in this report as standing with and without ambulation. For example, 2 recent studies reported a dose–response relationship between higher self-reported standing time and reduced mortality risk (9,10). In both studies, participants were asked to report their usual standing time in hours (10) or percentage of time during the day (9) without reference to whether walking occurred during standing. Hazard ratios (HRs) indicated a 21% reduced risk of mortality in adults standing more than 8 h/d compared to those standing less than 2 h/d (10). Additionally, Katzmarzyk (9) reported a 33% reduced risk associated with standing “almost all of the time” versus “almost none of the time.” When compared to standing without ambulation, standing with ambulation could be more beneficial due to greater muscle and metabolic activation affecting glycemic control and other physiological pathways (11–14). For example, stepping significantly improve fasting insulin and HOMA-S when compared to standing still in a workplace intervention study (15). Thus, it would be useful to examine standing with and without ambulation as separate low-intensity physical activities that may reduce mortality risk.
To our knowledge, no studies of standing and mortality have used measures derived from accelerometers. Through the use of validated machine-learned algorithms specifically trained to estimate standing behaviors using raw accelerometer data from hip-worn devices, we estimated time spent standing with ambulation and without ambulation (16–18). The objectives of this study of older women were to: (i) determine associations of time spent standing with and without ambulation in relation to all-cause mortality and (ii) examine whether associations were modified by age, body mass index (BMI), physical functioning, race/ethnicity, MVPA, and/or sedentary time.
Method
Study Participants
In the Women’s Health Initiative (WHI), postmenopausal women aged 50–79 were enrolled in the WHI Clinical Trial(s) or the Observational study from 1993 to 1998 across 40 sites (19,20). The WHI Objective Physical Activity and Cardiovascular Health in Older Women (OPACH) is an ancillary study that enrolled 7058 ambulatory community-dwelling women aged 63 and older from 2012 to 2014. Details of OPACH have been previously published (21). The Institutional Review Board protocol for OPACH was approved by the Fred Hutchinson Cancer Research Center and all women provided informed consent in writing or by phone.
Classification of Standing and Standing With Ambulation
Participants were instructed to wear the hip-worn accelerometer 24 h/d except while bathing or swimming. ActiLife version 6 software was used to aggregate GT3X+ accelerometer data into 15-s epochs. Periods of accelerometer non-wear were identified using the Choi algorithm as previously described (21,22). Participant-recorded in-bed and out-of-bed times from daily sleep diaries were used to exclude sleep time. Data on time spent napping were not collected. For missing sleep times, the participant-specific average was used. If all sleep times were missing, the OPACH population average was used (in-bed time = 10:45 pm; out-of-bed time = 7:22 am).
All days with ≥10 hours of awake wear time were considered adherent. Standing without ambulation (hereafter referred to as “standing”) and standing with ambulation were computed for each participant as the number of waking minutes classified in each behavior averaged over all adherent days. This study excluded 1181 women who did not return their accelerometer (n = 338), returned an accelerometer with no usable data (n = 232), or returned an accelerometer with insufficient data to apply the machine-learned algorithms (n = 611), which resulted in a final analytic sample of 5878 women.
Time spent standing and standing with ambulation were classified based on validated machine-learned algorithms specifically for older women (17). The algorithm, which included a random forest classifier and subsequent hidden Markov model, was developed using first-person images that were collected from 39 older women approximately every 20 seconds for up to 7 days using a SenseCam worn around the neck along with a hip-worn accelerometer. Internal validation was completed using leave-one-out cross-validation. The averaged balanced accuracy for behaviors learned using the machine learning algorithm was 67% for standing, 79% for standing with ambulation, and 84% for walking/running compared to staff annotated SenseCam images (17,23). To apply this algorithm to the OPACH study, every 1-minute window of raw (30 Hz) triaxial acceleration data was converted into 41 descriptive statistical features, such as: the mean and SD of each axis, the roll, pitch, and yaw angles, the direction of acceleration, low-pass filters with several frequency cutoffs, and several features of the frequency domain after Fourier transformation was applied to the vector magnitude signal.
Covariates
Based on previous literature, covariates selected included age, race/ethnicity (white, black, Hispanic), education (high school/general education development or less, some college, college graduate or more), current smoking, alcohol consumption (non-drinker, <1 drink/wk, 1–2 drinks/wk, 3–4 drinks/wk, 5–6 drinks/wk, every day, and unknown), BMI (underweight, normal, overweight, obese), self-rated health (fair or poor, good, excellent, or very good), multimorbidity as the number of chronic conditions (0, 1–2, ≥3 including cardiovascular disease, cancer, cognitive impairment, depression, diabetes, osteoarthritis, history of frequent falls [2+/y], chronic obstructive pulmonary disease, and cerebrovascular disease), physical function (RAND-36 10-item physical function subscale, range 0–100, higher score reflects higher function), and residualized minutes spent in accelerometer-measured sedentary time and MVPA (23). For covariates that could change over time, the most recent measurement on or before the OPACH baseline was used. Accelerometer-measured sedentary time and MVPA were classified using cutpoints specifically calibrated to OPACH women from a lab-based study conducted among 200 women aged 60–91 (24). The cutpoints were applied to 15-second epoch accelerometer counts as follows: sedentary (≤18), light physical activity (19–518), and MVPA (≥519).
Follow-up and Mortality Ascertainment
As part of ongoing mortality surveillance for the broader Women’s Health Initiative cohort, deaths were ascertained through March 31st, 2018 using annual mailed outcome questionnaires, systematic searches of the National Death Index, hospital records, obituaries, and proxy queries (25). Vital status was known for 96.6% of WHI participants as of March 1st, 2018. Follow-up time ranged from 2.2 to 6.0 years with an average of 4.8 years.
Statistical Analysis
Participant characteristics were reported across quartiles of combined standing time. To assess the differences across quartiles, Pearson’s chi-squared tests were used for categorical variables and F-tests for continuous variables. Pearson correlation coefficients were calculated to assess the degree of linear correlation between the machine-learned standing measures and accelerometer-derived intensity of physical activity measures (sedentary, light physical activity, MVPA).
To account for variations in accelerometer wear, standing and standing with ambulation were adjusted for awake accelerometer wear time using the residuals method (26). Cox proportional models were used to estimate HRs and 95% confidence intervals comparing higher quartiles of standing time to the lowest quartile for each standing variable. Time to event was computed as days from OPACH baseline to date of death or last medical history update. Potential confounding by covariates was assessed using progressively adjusted models as follows: Model 1 included age and race/ethnicity; Model 2 included Model 1 covariates and education, self-rated health, number of chronic conditions, current smoking status, alcohol consumption, physical functioning, and BMI; Model 3 included Model 2 covariates and minutes of MVPA. Additionally, a sensitivity analysis was conducted that excluded deaths within the first year of follow-up to evaluate potential reverse causation. P values for trend were calculated using Cox models testing the continuous functional form of the standing variables for each model. Multiple imputation was used to impute data for missing covariates in Models 2 and 3. Multivariate imputation with chained equations was used to produce 100 datasets with imputed missing data. Effect estimates were calculated for all imputed datasets and pooled to produce a final effect estimate (27).
The consistency of associations across key subgroups of participants was examined for categories of race/ethnicity (white, black, Hispanic), age (<80 and ≥80 years), BMI (<30 and ≥30 kg/m2), MVPA (<44 and ≥44 min/d; median split), sedentary time (<558 and ≥558 min/d; median split), and physical function (<75 and ≥75; median split). Due to lack of meaningful thresholds for dichotomizing accelerometer-measured sedentary time and MVPA, median splits were used to display the results and test for statistical interaction. A sensitivity analysis additionally tested the potential effect modifiers as continuous variables. Hazard ratios for mortality were computed using Model 3 comparing the 75th and 25th percentiles of standing (interquartile range [IQR] = 46.2 min/d) and standing with ambulation (IQR = 115.7 min/d) in each category and statistical interaction was tested by adding the multiplicative term for the continuous exposure variable and each stratification variable. Based on the results of the interaction testing, the dose–response associations of Model 3 for both standing behaviors were plotted by high and low sedentary time using a restricted cubic spline function tested using 3 or 4 knots. Because no meaningful differences were observed between 3 and 4 knots, the results were plotted showing 3 knots to minimize the degrees of freedom. Visual inspection in addition to a Wald test was used to test for nonlinearity. All analyses were conducted using R packages (R Foundation for Statistical Computing, Vienna, Austria).
Results
After excluding women who were missing data or had data that could not be processed by the machine-learned algorithm (n = 1811), there were 5878 women in the analytic sample. The 1811 excluded women were slightly older and reported lower self-rated health, a higher number of chronic conditions on average, and lower physical function scores compared to women included in the study (Supplementary Table 1). During 26 649 person-years of follow-up time in this study, 691 deaths occurred. On average, women spent 0.9 h/d standing (SD = 0.7 h/d) and 3.2 h/d standing with ambulation (SD = 1.5 h/d). Table 1 presents the baseline characteristics of women stratified by quartile of combined standing and standing with ambulation. Women in the lowest quartile of combined standing and standing with ambulation (<3.0 h/d) when compared to the remaining quartiles were more likely to be older, obese, have worse self-rated health, have a greater number of chronic conditions, and have lower physical function scores (all p-trend < .001). The distributions of baseline characteristics were similar when comparing the lowest quartile of standing (<2.5 h/d) and standing with ambulation (<0.5 h/d) to higher quartiles (Supplementary Tables 2 and 3).
Table 1.
Baseline Characteristics by Average Daily Quartiles of Combined Standing Time (n = 5878): OPACH (2012–2014)
| Characteristics | Combined Standing Time Quartiles | ||||
|---|---|---|---|---|---|
| <3.0 h | 3.0–3.9 h | 4.0–5.1 h | >5.1 h | p | |
| n | 1470 | 1469 | 1469 | 1470 | |
| Age category (y), % (n) | <.001* | ||||
| 60–<70 | 6.5 (96) | 9.0 (132) | 12.9 (189) | 13.6 (200) | |
| 70–<80 | 33.9 (498) | 37.8 (556) | 42.1 (619) | 46.3 (681) | |
| 80–<90 | 52.9 (777) | 48.7 (715) | 41.4 (608) | 38.4 (565) | |
| 90+ | 6.7 (99) | 4.5 (66) | 3.6 (53) | 1.6 (24) | |
| Race/ethnicity, % (n) | <.001* | ||||
| White | 58.1 (854) | 51.4 (755) | 47.4 (697) | 42.0 (618) | |
| Black | 32.6 (479) | 33.8 (497) | 33.4 (490) | 33.3 (489) | |
| Hispanic | 9.3 (137) | 14.8 (217) | 19.2 (282) | 24.7 (363) | |
| Education, % (n) | <.001* | ||||
| HS/GED or less | 17.3 (255) | 19.1 (280) | 19.9 (292) | 23.7 (349) | |
| Some college | 40.5 (596) | 37.0 (544) | 38.9 (572) | 36.9 (542) | |
| College graduate or more | 41.8 (614) | 42.8 (629) | 40.6 (596) | 39.0 (574) | |
| NA | 0.3 (5) | 1.1 (16) | 0.6 (9) | 0.3 (5) | |
| Current smoker, % (n) | 4.3 (63) | 2.5 (36) | 1.7 (25) | 2.0 (30) | <.001* |
| Alcohol consumption, % (n) | <.001* | ||||
| Never | 38.0 (559) | 33.8 (496) | 32.3 (475) | 32.2 (473) | |
| <1/wk | 32.0 (471) | 31.7 (466) | 32.7 (481) | 30.0 (441) | |
| 1–2/wk | 8.0 (118) | 9.1 (134) | 9.9 (146) | 11.2 (165) | |
| 3–4/wk | 4.4 (64) | 6.4 (94) | 7.1 (105) | 7.1 (105) | |
| 5–6/wk | 3.4 (50) | 5.4 (80) | 5.2 (77) | 6.1 (90) | |
| Everyday | 4.1 (61) | 5.4 (79) | 6.0 (88) | 5.2 (77) | |
| NA | 10.0 (147) | 8.2 (120) | 6.6 (97) | 8.1 (119) | |
| BMI (kg/m2) category, % (n) | <.001* | ||||
| Underweight (<18.5) | 0.9 (13) | 0.8 (12) | 1.7 (25) | 2.0 (29) | |
| Normal (18.5–24.9) | 18.9 (278) | 24.7 (363) | 31.3 (460) | 41.8 (615) | |
| Overweight (25.0–29.9) | 28.5 (419) | 38.0 (558) | 36.6 (538) | 32.8 (482) | |
| Obese (≥30) | 43.9 (645) | 31.1 (457) | 24.4 (358) | 18.1 (266) | |
| NA | 7.8 (115) | 5.4 (79) | 6.0 (88) | 5.3 (78) | |
| Self-rated health, % (n) | <.001* | ||||
| Excellent or very good | 41.1 (604) | 50.0 (735) | 54.7 (804) | 57.1 (840) | |
| Good | 43.5 (639) | 40.6 (596) | 37.8 (555) | 35.6 (523) | |
| Fair or poor | 15.2 (223) | 9.1 (133) | 7.4 (108) | 6.7 (99) | |
| NA | 0.3 (4) | 0.3 (5) | 0.1 (2) | 0.5 (8) | |
| No. chronic conditions, % (n)a | <.001* | ||||
| 0 | 13.5 (198) | 15.9 (233) | 18.9 (278) | 22.3 (328) | |
| 1 | 29.0 (427) | 35.2 (517) | 36.0 (529) | 37.1 (546) | |
| 2 | 27.8 (409) | 27.4 (403) | 26.8 (394) | 24.4 (358) | |
| 3+ | 29.3 (430) | 21.0 (309) | 18.0 (264) | 15.6 (230) | |
| NA | 0.4 (6) | 0.5 (7) | 0.3 (4) | 0.5 (8) | |
| RAND physical function, mean (SD)b | 56.4 (28.2) | 68.1 (25.1) | 73.5 (23.5) | 77.9 (21.2) | <.001* |
| MVPA time, mean (SD)c | 28.8 (19.6) | 44.1 (25.5) | 56.7 (30.3) | 73.5 (38.6) | <.001* |
Notes: BMI = body mass index; GED = general educational development; HS = high school; MVPA = moderate-to-vigorous physical activity; NA = missing; OPACH = Objective Physical Activity and Cardiovascular Health.
aConditions include cardiovascular disease, cancer, cognitive impairment, depression, osteoarthritis, history of falls, chronic obstructive pulmonary disease, diabetes, and cerebrovascular disease. bRAND-36 10-item physical function subscale, range 0–100, higher score reflects higher physical function. cAdjusted for awake wear time using the residuals method.
*p < .05.
Standing had low correlations with standing with ambulation (r = .17) and MVPA (−.08), and moderate correlations with sedentary time (−.30) and light physical activity (r = .42). In contrast, standing with ambulation had moderate-to-strong correlations with MVPA (r = .66), light PA (r = .77), and sedentary time (r = −.86) (Supplementary Table 4). Examination of the distribution of accelerometer-derived physical activity intensity within the standing component showed slightly greater light physical activity (53%) than sedentary time (45%) and a very small proportion of MVPA (2%). In contrast, the distribution within the standing with ambulation component was primarily light physical activity (68%), followed by sedentary time (16%) and a small amount of MVPA (16%) (Supplementary Figure 1).
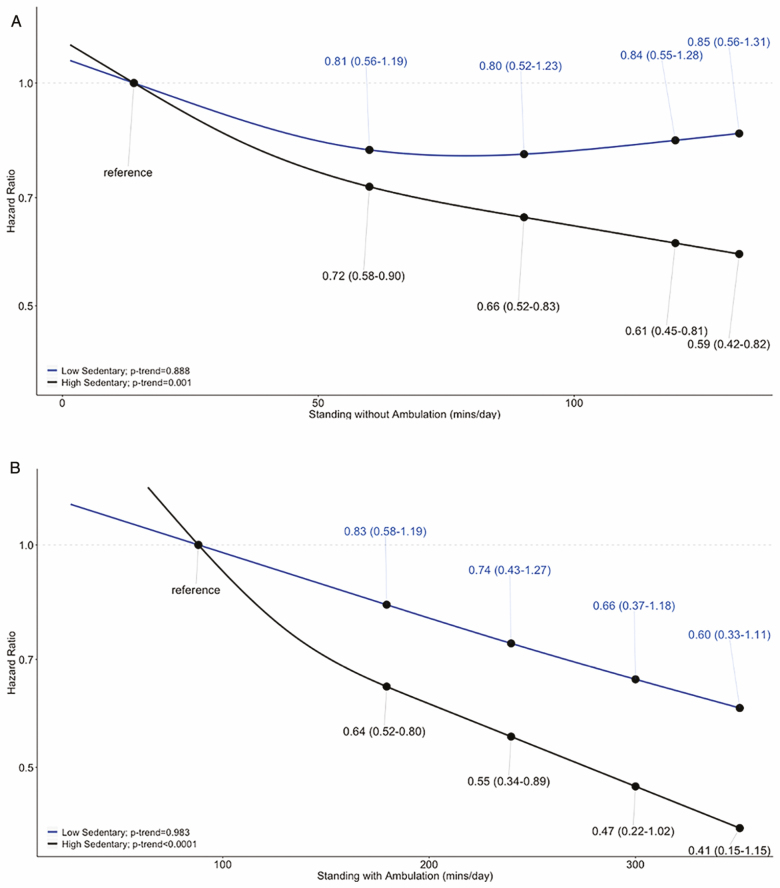
The crude mortality rates per 1000 person-years across decreasing quartiles of standing ranged from 21.3 in Q4 to 34.8 in Q1 and across decreasing quartiles of standing with ambulation from 11.5 in Q4 to 50.0 in Q1 (Table 2). In fully adjusted models (Model 3), reduced risks of mortality were observed in a dose–response pattern with higher quartiles of standing time when compared to the lowest quartile (Q1 reference; Q2 HR = 0.86; 95% CI = 0.69–1.09; Q3 HR = 0.76; 95% CI = 0.60–0.97; Q4 HR = 0.63; 95% CI = 0.49–0.81; p-trend = .003) (Table 2). Similar but somewhat stronger associations were observed across increasing quartiles of standing with ambulation (Q1 reference; Q2 HR = 0.66; 95% CI = 0.52–0.82; Q3 HR = 0.67; 95% CI = 0.51–0.87; Q4 HR = 0.50; 95% CI = 0.35–0.71; p-trend < .001; Table 2) when compared to the lowest quartile. The dose–response associations with mortality risk, tested using the fully adjusted Model 3 and a restricted cubic spline analyses, were statistically significant and found to be non-linear for both standing (p-overall < .001; p-non-linear = .03) and standing with ambulation (p-overall < .001; p-non-linear = .007). After excluding deaths within the first year of baseline, the reduced risk of mortality and dose–response pattern remained similar for higher quartiles of both standing variables when compared to the lowest quartile within the fully adjusted models (Supplementary Table 5).
Table 2.
Associations of Standing and Standing With Ambulation Time and Prospective Mortality (n = 5878): OPACH (2012–2018)
| Standing Time Quartilesa,b | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | p-trend | |
| <0.41 h | 0.42–0.71 h | 0.72–1.18 h | 1.19–6.78 h | ||
| Rate of eventsc (# deaths) | 34.8 (231) | 25.6 (170) | 22.3 (149) | 21.3 (141) | |
| Model 1d | 1 (ref.) | 0.77 (0.63–0.94) | 0.71 (0.58–0.87) | 0.64 (0.52–0.79) | <.001* |
| Model 2e | 1 (ref.) | 0.85 (0.67–1.08) | 0.78 (0.61–1.00) | 0.68 (0.53–0.88) | .013* |
| Model 3f | 1 (ref.) | 0.86 (0.69–1.09) | 0.76 (0.60–0.97) | 0.63 (0.49–0.81) | .003* |
| Standing With Ambulation Time Quartilesc | |||||
| Q1 | Q2 | Q3 | Q4 | p-trend | |
| <2.14 h | 2.15–3.04 h | 3.04–4.07 h | 4.08–10.38 h | ||
| Rate of eventsc (# deaths) | 50.0 (313) | 25.0 (168) | 19.3 (130) | 11.5 (80) | |
| Model 1d | 1 (ref.) | 0.56 (0.47–0.68) | 0.50 (0.41–0.62) | 0.34 (0.27–0.44) | <.001* |
| Model 2e | 1 (ref.) | 0.62 (0.50–0.78) | 0.59 (0.46–0.77) | 0.40 (0.30–0.55) | <.001* |
| Model 3f | 1 (ref.) | 0.66 (0.52–0.82) | 0.67 (0.51–0.87) | 0.50 (0.35–0.71) | <.001* |
Notes: Q = quartile; ref. = reference. The associations presented are hazard ratios with 95% confidence intervals.
aStanding refers to standing without ambulation. bStanding and standing with ambulation measures were adjusted for awake wear time in accelerometer. cThe rate of events is the crude rate per 1000 person-years and the associations presented are hazard ratios with 95% confidence intervals. dModel 1 adjusted for age and race/ethnicity. eModel 2 adjusted for Model 1 + education, self-rated health, number of chronic conditions, current smoking status, frequency of alcohol intake, RAND-36 10-item physical function subscale, and body mass index. fModel 3 adjusted for Model 2 + accelerometer-measured moderate-to-vigorous physical activity minutes.
*p < .05.
Although in the same direction, HRs for the risk of mortality were stronger for standing with ambulation among women with high (HR = 0.51; 95% CI = 0.38–0.68) versus low sedentary time (HR = 0.80; 95% CI = 0.59–1.07; p-interaction = .02) (Table 3, Figure 1). A stronger association of standing and mortality was also observed among women with high (HR = 0.77; 95% CI = 0.67–0.90) versus low sedentary time (HR = 0.97; 95% CI = 0.82–1.14; p-interaction = .13 when tested using the median split, p-interaction = .01 when tested using continuous terms) (Table 3, Figure 1; Supplementary Table 6). The interaction of standing with ambulation and MVPA was statistically significant when MVPA was tested as a continuous variable (p = .04); however, standing with ambulation was associated with reduced risks of mortality in both high and low MVPA strata (Supplementary Table 6). No differences in associations stratified by age, BMI, physical functioning, or race/ethnicity were observed.
Table 3.
Risk of Mortality by Interquartile Range Increase of Standing and Standing With Ambulation Stratified by Baseline Characteristics (n = 5878): OPACH (2012–2018)
| Number of Deaths | Standinga (IQR = 116 min) | p-interaction | Standing With Ambulation (IQR = 135 min) | p-interaction | |
|---|---|---|---|---|---|
| HR (95% CI)b,c | HR (95% CI)b,c | ||||
| Age | .84 | .67 | |||
| 80 y | 130 | 0.81 (0.62–1.04) | 0.66 (0.45–0.98) | ||
| ≥80 y | 561 | 0.82 (0.73–0.92) | 0.60 (0.49–0.74) | ||
| BMI | .89 | .34 | |||
| < 30 kg/m2 | 493 | 0.85 (0.75–0.95) | 0.67 (0.55–0.82) | ||
| ≥ 30 kg/m2 | 168 | 0.81 (0.64–1.02) | 0.54 (0.35–0.83) | ||
| MVPA | .95 | .19 | |||
| < 44 min/d | 498 | 0.85 (0.75–0.95) | 0.58 (0.47–0.71) | ||
| ≥ 44 min/d | 193 | 0.84 (0.65–1.08) | 0.71 (0.54–0.94) | ||
| Sedentary time | .13 | .02* | |||
| < 558 min/d | 210 | 0.97 (0.82–1.14) | 0.80 (0.59–1.07) | ||
| ≥ 558 min/d | 481 | 0.77 (0.67–0.90) | 0.51 (0.38–0.68) | ||
| Physical functioning | .44 | .40 | |||
| <75 | 474 | 0.84 (0.74–0.95) | 0.56 (0.44–0.71) | ||
| ≥75 | 208 | 0.80 (0.65–0.98) | 0.72 (0.54–0.97) | ||
| Race/ethnicity | .52 | .71 | |||
| White | 488 | 0.85 (0.75–0.97) | 0.64 (0.51–0.80) | ||
| Black | 152 | 0.79 (0.63–0.98) | 0.61 (0.41–0.90) | ||
| Hispanic | 51 | 0.77 (0.51–1.17) | 0.54 (0.29–0.99) |
Notes: BMI = body mass index; HR = hazard ratio; IQR = interquartile range; MVPA = moderate-to-vigorous physical activity.
aStanding refers to standing without ambulation. bThe associations presented are HRs with 95% confidence intervals. cModels presented adjusted for age, race/ethnicity, education, self-rated health, number of chronic conditions, current smoking status, frequency of alcohol intake, RAND-36 10-item physical function subscale, BMI, and residualized MVPA minutes. Models assessing stratification of or interaction with MVPA did not include residualized MVPA minutes.
*p < .05.
Figure 1.
Continuous dose-response association of standing with and without ambulation with mortality stratified by high and low sedentary time. Note: The top panel shows the risk of mortality among those with low sedentary time (blue line: <558 min/d on average) and high sedentary time (black line: ≥558 min/d on average) at 0, 50, and 100 min of average standing time over 7 d compared to the reference of 14 min of average standing time over 7 d. The bottom panel shows the risk of mortality among those with low sedentary time (blue line: <558 min/d on average) and high sedentary time (black line: ≥558 min/d on average) at 100, 200, and 300 min of average standing with ambulation time over 7 d compared to the reference of 88 min of average standing time over 7 d.
Discussion
This is the first time, to our knowledge, that accelerometer-measured standing and standing with ambulation have been studied in relation to mortality risk. This study showed that standing and standing with ambulation are distinct behaviors involving different levels of physical activity intensity. Based on calibrated accelerometer cutpoints, standing is a mix of sedentary and light physical activity, while standing with ambulation is primarily light physical activity with small proportions of sedentary time and MVPA. In this racially and ethnically diverse cohort of older women, we observed strong, independent, dose–response associations between increased time spent standing and standing with ambulation and lower risk of death. The highest quartile of time spent standing and standing with ambulation had a 37% and 50% lower risk of death when compared to the lowest quartile, respectively. For both standing variables, associations with mortality were stronger among women with higher sedentary time.
Previous cohort studies have been based on self-reported indicators of standing and have not distinguished between standing and standing with ambulation. Examination of standing distinct from sedentary behavior aligns with recently agreed upon terminology developed by the Sedentary Behavior Research Network consensus project (8). Standing is also of great interest to the public health community, and to the general public, as it has been proposed as an alternative to sitting that might confer health benefits. An entire industry has capitalized on this idea by developing standing desks. These 2 common behaviors (standing and standing with ambulation) were measured by applying previously validated machine learning algorithms to raw acceleration data from hip-worn accelerometers (17). Findings from this study aligned with the self-reported standing and mortality reports published previously (9,10). Katzmarzyk (9) reported a 33% (HR = 0.67; 95% CI = 0.54–0.85) lower risk of mortality among adults, while Van der Ploeg et al. (10) reported a 21% (HR = 0.79; 95% CI = 0.67–0.93) lower mortality among women when comparing the highest level of movement category to the lowest. Katzmarzyk (9) used the 1981 Canada Fitness Survey, a representative sample of the Canadian population, which included individuals aged 18–90 years old and Van der Ploeg et al. (10) used the 45 and Up Study in Australia, which included individuals 45 to 75+ years of age. Our findings indicate similar, although stronger, associations of standing and mortality risk which could be due to more accurate classification of standing in our study using accelerometers, adjustment for health status variables, or differences in the age groups under study (28,29).
Two relatively smaller epidemiologic studies investigated standing using the thigh-worn activPal, and found that hypothetically replacing sitting time with standing time (i.e., increasing standing time), using isotemporal substitution methods, was beneficially associated with cardiometabolic biomarkers (30) and a lower odds of metabolic syndrome and type 2 diabetes (31). However, standing measured by the activPAL does not distinguish between standing and standing with ambulation (when the ambulation is at a cadence of <20 steps/min) which could lead to overestimates of standing time and its association with metabolic health. The likely overestimation of standing time can be seen in large cohorts of older adults that used activPal devices—the AusDiab cohort composed of 300 men and 398 women (mean age 57.9 ± 9.9 years) averaged 4.9 ± 1.5 h/d of standing while the Maastricht Study composed of 997 men and 1027 women (mean age 59.7 ± 8.1 years) averaged 4.3 ± 1.3 h/d of standing. Average standing time in OPACH women was 0.9 h/d. Some of this difference in standing time could be due to the older age of participants in OPACH, but much is thought to be the result of measurement error or the inclusion of ambulation with measured standing time by activPal devices (31,32).
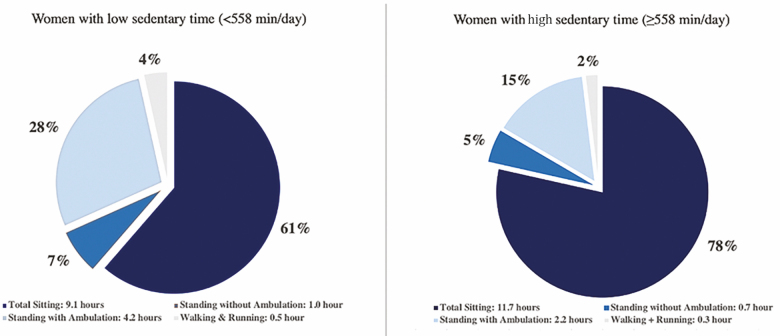
A stronger reduced risk of mortality associated with higher levels of standing with ambulation was observed among women with higher accelerometer-measured total sedentary time compared to those with lower sedentary time. On average, women with above-median sedentary time (median = 558 min/d) spent 78% of their day sitting compared to women below median sedentary time who spent 68% of their day sitting. Although the absolute time spent standing with ambulation was shorter among women with above-median sedentary time on average (2.2 h/d) compared to below median sedentary time (4.2 h/d), a stronger association between standing with ambulation and mortality was observed among women with high sedentary time (Figure 2). Median splits were used to dichotomize the physical activity intensity variables due to a lack of agreed upon meaningful thresholds that are specific to older adults; however, the results were similar when interactions were tested using continuous terms. If found to be reproducible in other studies, these results suggest that standing up, even for shorter durations of time, maybe especially valuable among women who sit the longest.
Figure 2.
Average time spent in each movement behavior stratified by high and low sedentary time. Note: Low and high sedentary time were defined using the sample median of 558 min. The average time spent in each behavior was collected over up to 7 d of accelerometry wear, excluding sleep time.
An association between time spent standing and lower risk of death is biologically plausible through mechanisms that include improved lower extremity strength and cardiometabolic health. Postural changes from sitting to standing require activation of the legs and lower abdominal/pelvic muscles to raise the body and then to maintain a standing posture. This in turn immediately increases blood pressure, heart rate, and vascular tone. The resulting increased energy expenditure, blood flow, and muscle contraction can improve endothelial function, enhance lipid metabolism, and glycemic regulation (30,33–41). To date, laboratory studies investigating these mechanisms, as well as observational studies investigating standing exposures and clinical endpoints, have not focused on the health effects of patterns of intermittent standing in older adults, an important direction for future research.
This study was a prospective cohort study of older women, half of whom were Black or Hispanic, with up to 6 years of follow-up during which over 26 000 person-years and 691 mortality events occurred. Data on important baseline characteristics and confounders were available through the rich array of prior health information collected in both OPACH and the parent WHI study. A novel aspect of our epidemiological study was the use of machine-learned algorithms to define standing exposures from accelerometer measurements up to 7 consecutive wear days under free-living conditions. The machine learning algorithm used in this study had been previously developed and validated in older women who were similar in age to the OPACH cohort. The algorithm enabled measurement of standing and standing with ambulation as mutually exclusive categories of behavior. The statistical models used to quantify associations with mortality were adjusted for accelerometer-measured MVPA and key health indicators (multimorbidity, self-rated health, and physical function), to examine standing exposures independent of these potent predictors of mortality in older women.
Potential limitations include reliance on 1 week of exposure measurement to reflect usual standing patterns, which may not fully capture standing behavior among women. However, the large number of women across a broad age range (63–99) living across the United States likely captured a profile of activity representative of that for older community-dwelling women. Replication is required to determine if the findings are generalizable to older men and younger age groups using the same machine learning algorithms that are now in the public domain (42).
In conclusion, our results suggest that greater time spent standing with or without ambulation is associated with lower risk of all-cause mortality among older women residing in the community, particularly among women with high sedentary time. It is likely that older women would find it easier to engage in standing or standing with ambulation than to engage in higher intensity behaviors such as brisk walking or running. Beyond mortality, it will be important to determine if time spent standing has other salient health benefits in terms of chronic disease prevention or maintenance of mobility. In addition, it is important to examine the length, frequency, and timing of standing and standing with ambulation bouts to examine if the variation in patterns further modifies the findings. If the present findings are replicated in other cohorts and extend to benefits on other aging phenotypes, then behavioral interventions targeting increased standing and standing with ambulation have promise to improve healthy aging (43). Standing, specifically for older adults, is generally safe and, when not contraindicated, could be promoted as an activity target even now.
Supplementary Material
Acknowledgments
We would like to acknowledge the WHI participants and staff for their participation in this important scientific endeavor. We also acknowledge the WHI investigators. The following is a short list of WHI Investigators, the full list can be found at the following site: www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller; Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg; Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Funding
This work was supported by The National Heart, Lung, and Blood Institute who provided funding for the OPACH study (grant number R01 HL105065 to A.Z.L.). Funding also came from 2 grants supported by the National Institute on Aging: A program project (P01 AG052352 to A.Z.L.) and a T32 Predoctoral Training Fellowship (T32 AG058529 to P.J.). The Women’s Health Initiative program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services (contract numbers HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C). All funding agencies had no role in the design of the study and collection, analysis, or interpretation of the data and no role in writing the manuscript.
Conflict of Interest
None declared.
Author Contributions
All authors have contributed importantly to the conception of the study design, data collection, analysis, interpretation, and/or critical revision of the manuscript and have approved the manuscript.
References
- 1. Vespa J, David MA, Medina L. Demographic turning points for the United States: population projections for 2020 to 2060. Current Population Reports, p. 25–1144, U.S. Census Bureau, Washington, DC, 2018.
- 2. Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes Care. 2013;36(7):1994–2000. doi: 10.2337/dc12-1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. J Am Med Assoc. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi: 10.1186/1471-2458-13-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piercy KL, Troiano RP. Physical Activity Guidelines for Americans from the US Department of Health and Human Services. Circ Cardiovasc Qual Outcomes. 2018;11(11):e005263. doi: 10.1161/CIRCOUTCOMES.118.005263 [DOI] [PubMed] [Google Scholar]
- 6. Sparling PB, Howard BJ, Dunstan DW, Owen N. Recommendations for physical activity in older adults. Br Med J. 2015;350:h100. doi: 10.1136/bmj.h100 [DOI] [PubMed] [Google Scholar]
- 7. Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3 Suppl. 2):172–183. doi: 10.1016/s0749-3797(03)00182-x [DOI] [PubMed] [Google Scholar]
- 8. Tremblay MS, Aubert S, Barnes JD, et al. ; SBRN Terminology Consensus Project Participants Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14(1):75. doi: 10.1186/s12966-017-0525-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Katzmarzyk PT. Standing and mortality in a prospective cohort of Canadian adults. Med Sci Sports Exerc. 2014;46(5):940–946. doi: 10.1249/MSS.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 10. van der Ploeg HP, Chey T, Ding D, Chau JY, Stamatakis E, Bauman AE. Standing time and all-cause mortality in a large cohort of Australian adults. Prev Med. 2014;69:187–191. doi: 10.1016/j.ypmed.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 11. Júdice PB, Hamilton MT, Sardinha LB, Zderic TW, Silva AM. What is the metabolic and energy cost of sitting, standing and sit/stand transitions? Eur J Appl Physiol. 2016;116(2):263–273. doi: 10.1007/s00421-015-3279-5 [DOI] [PubMed] [Google Scholar]
- 12. Tikkanen O, Haakana P, Pesola AJ, et al. Muscle activity and inactivity periods during normal daily life. PLoS ONE. 2013;8(1):e52228. doi: 10.1371/journal.pone.0052228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coyle EF. Physical activity as a metabolic stressor. Am J Clin Nutr. 2000;72(Suppl. 2):512S–520S. doi: 10.1093/ajcn/72.2.512S [DOI] [PubMed] [Google Scholar]
- 14. Sale DG. Influence of exercise and training on motor unit activation. Exerc Sport Sci Rev. 1987;15:95–151. [PubMed] [Google Scholar]
- 15. Winkler EAH, Chastin S, Eakin EG, et al. Cardiometabolic impact of changing sitting, standing, and stepping in the workplace. Med Sci Sports Exerc. 2018;50(3):516–524. doi: 10.1249/MSS.0000000000001453 [DOI] [PubMed] [Google Scholar]
- 16. Kerr J, Carlson J, Godbole S, Cadmus-Bertram L, Bellettiere J, Hartman S. Improving hip-worn accelerometer estimates of sitting using machine learning methods. Med Sci Sports Exerc. 2018;50(7):1518–1524. doi: 10.1249/MSS.0000000000001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenberg D, Godbole S, Ellis K, et al. Classifiers for accelerometer-measured behaviors in older women. Med Sci Sports Exerc. 2017;49(3):610–616. doi: 10.1249/MSS.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ellis K, Godbole S, Marshall S, Lanckriet G, Staudenmayer J, Kerr J. Identifying active travel behaviors in challenging environments using GPS, accelerometers, and machine learning algorithms. Front Public Health. 2014;2:36. doi: 10.3389/fpubh.2014.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(Suppl. 9):S5–17. 10.1016/S1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 20. Design of the Women’s Health Initiative Clinical Trial and Observational Study. The Women’s Health Initiative Study Group. Control Clin Trial. 1998;19(1):61–109. 10.1016/S0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 21. LaCroix AZ, Rillamas-Sun E, Buchner D, et al. The Objective Physical Activity and Cardiovascular Disease Health in older women (OPACH) study. BMC Public Health. 2017;17(1):192. doi: 10.1186/s12889-017-4065-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi: 10.1249/MSS.0b013e3181ed61a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item Health Survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 24. Evenson KR, Wen F, Herring AH, et al. Calibrating physical activity intensity for hip-worn accelerometry in women age 60 to 91 years: The Women’s Health Initiative OPACH Calibration Study. Prev Med Rep. 2015;2:750–756. doi: 10.1016/j.pmedr.2015.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(Suppl. 9):S122–128. doi: 10.1016/S1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 26. Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366 [DOI] [PubMed] [Google Scholar]
- 27. Buuren Sv, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2010:1–68. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 28. Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS ONE. 2012;7(5):e36345. doi: 10.1371/journal.pone.0036345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ekelund U, Tarp J, Steene-Johannessen J, et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. Br Med J. 2019;366:l4570. doi: 10.1136/bmj.l4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van der Velde JHPM, Koster A, van der Berg JD, et al. Sedentary behavior, physical activity, and fitness—The Maastricht Study. Med Sci Sports Exerc. 2017;49(8):1583–1591. doi: 10.1249/MSS.0000000000001262 [DOI] [PubMed] [Google Scholar]
- 32. Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J. 2015;36(39):2643–2649. doi: 10.1093/eurheartj/ehv308 [DOI] [PubMed] [Google Scholar]
- 33. Olufsen MS, Ottesen JT, Tran HT, Ellwein LM, Lipsitz LA, Novak V. Blood pressure and blood flow variation during postural change from sitting to standing: model development and validation. J Appl Physiol (Bethesda, MD: 1985). 2005;99(4):1523–1537. doi: 10.1152/japplphysiol.00177.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castellano V, Olive JL, Stoner L, Black C, McCully KK. Blood flow response to a postural challenge in older men and women. Dyn Med. 2004;3(1):1. doi: 10.1186/1476-5918-3-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795 [DOI] [PubMed] [Google Scholar]
- 36. Healy GN, Dunstan DW, Salmon J, et al. Breaks in sedentary time: beneficial associations with metabolic risk. Diabetes Care. 2008;31(4):661–666. doi: 10.2337/dc07-2046 [DOI] [PubMed] [Google Scholar]
- 37. Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound. 2014;12:50. doi: 10.1186/1476-7120-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol. 2015;100(7):829–838. doi: 10.1113/EP085238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35(5):976–983. doi: 10.2337/dc11-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358–366. doi: 10.3945/ajcn.112.051763 [DOI] [PubMed] [Google Scholar]
- 41. Thosar SS, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit. 2012;18(12):RA173–RA180. doi: 10.12659/msm.883589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ellis K. TLBC: Two-Level Behavior Classification.https://rdrr.io/cran/TLBC/. 2019. Accessed January 9, 2019.
- 43. Kerr J, Patterson RE, Ellis K, et al. Objective assessment of physical activity: classifiers for public health. Med Sci Sports Exerc. 2016;48(5):951–957. doi: 10.1249/MSS.0000000000000841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.