Abstract
Objective
This article expands on research that links education and frailty among older adults by considering the role of genes associated with education.
Method
Data come from a sample of 7,064 non-Hispanic, white adults participating in the 2004–2012 waves of the Health and Retirement Study. Frailty was measured with two indices: (a) The Frailty Index which corresponds to a deficit accumulation model; and (b) The Paulson–Lichtenberg Frailty Index which corresponds to the biological syndrome/phenotype model. Genes associated with education were quantified using an additive polygenic score. Associations between the polygenic score and frailty indices were tested using a series of multilevel models, controlling for multiple observations for participants across waves.
Results
Results showed a strong and negative association between genes for education and frailty symptoms in later life. This association exists above and beyond years of completed education and we demonstrate that this association becomes weaker as older adults approach their 80s.
Discussion
The results contribute to the education–health literature by highlighting new and important pathways through which education might be linked to successful aging.
Keywords: Education, Functional health status, Genetics, Successful aging
One of the most important areas of research for social, psychological, and biological researchers involves the characterization of health and the determinants of health of aging populations (Christensen et al., 2009). One particular area that has grown over the past 25 years is research that consistently demonstrates a robust relationship between education and health (Link & Phelan, 1995). The protective effect of increasing years of education is evident across a number of morbidities and is fairly consistent across different sociodemographic groups in the United States (Zajacova & Lawrence, 2018). In this article, we focus on a critical aging-related phenotype, frailty, and evaluate the extent to which this indicator of overall health among the elderly population is linked to years of education. Previous work has shown educational gradients for frailty (Santos-Eggimann, Cuénoud, Spagnoli, & Junod, 2009; Seeman et al., 2008), but no existing work has evaluated the hypothesis that some of this association is due to genetic influences that affect education and subsequently frailty.
The purpose of this study was to explore the possibility that genes associated with educational attainment are also implicated in the reduced likelihood of frailty in late life. Importantly, we evaluate the possibility that genes related to education also predict frailty above and beyond the effect of educational attainment. Although there is some evidence that the genes associated with education have lasting benefits through the life course (Plassman, Williams, Burke, Holsinger, & Benjamin, 2010), we seek to explore how these effects manifest over chronological age. That is, are the potential effects of these genes most predictive of frailty more or less important as an individual ages? Such answers will help to elucidate the relationship effects of education on frailty by exploring the genetic mechanisms underpinning both measures.
Genetics and Years of Completed Education
A recent meta-analysis of twin and family studies show that up to 40% of variance in educational attainment can be explained by genetic factors (Branigan, McCallum, & Freese, 2013). Using genetic markers, it is now possible to quantify a portion of an individual’s propensity to educational achievement. One increasingly popular method is the polygenic score (PGS) approach (Dudbridge 2013), which has led to a number of advances over the past two decades in predicting disease (Visscher et al., 2017). Such successes are reflective of a growing consensus that complex phenotypes, such as educational attainment, are influenced by many genetic loci with very small effect sizes (i.e., highly polygenic; Visscher et al., 2017). The PGS for educational attainment (PGSeduc) is a single score representing genome-wide influence on (e.g., genetic propensity for) academic success, as measured by formal years of schooling. Initially, social scientists developed this score (Lee et al., 2018; Okbay et al., 2016; Rietveld et al.; 2013) to explain variation in schooling due to genetic factors. In line with increased sample sizes and power, the most recent score now explains roughly 11% of the variance in education (Lee et al., 2018). In early and midlife, the PGSeduc is predictive of other measures of academic success and cognitive performance (e.g., general cognitive ability, standardized exam scores, highest math course completed; Belsky et al., 2018; Lee et al., 2018). Into adulthood, the PGSeduc predicts social mobility and indicators of economic success (e.g., occupational status, asset accumulation, financial stability, and wealth at retirement; Belsky et al., 2016, 2018). Similar to other predictors, an individual’s genetic propensity provides a probabilistic estimate of an outcome rather than a deterministic one. Similar to other social predictors, the effect an individual’s PGSeduc can be modified by other protective or environmental risk factors.
Although there is robust evidence that genetic variants associated with educational attainment predict indicators of success, the relationship with indicators of health is less established but is growing. For example, Wedow and colleagues (2018) demonstrate a genetic correlation between years of completed education and regular smoking among adults in the United States. They show that this association has increased across birth cohorts and highlight the importance of social sorting mechanisms (e.g., evocative gene–environment correlations) that may influence the extent to which genetic covariance is related to overall phenotypic covariance in the population. These results add to increasing information from large consortia suggesting significant genetic overlap between genes for educational attainment and a range of other health statuses (e.g., Alzheimer’s disease, ischemic stroke, psychiatric conditions, vascular–metabolic diseases, and other physiological measures such as body mass index; Bulik-Sullivan et al., 2015; Hagenaars et al., 2016). Indirect evidence for this association comes from two additional studies. First, Marioni and colleagues (2016) found that a child’s PGSeduc was predictive of mortality, as measured by the paternal and maternal life span. Second, Boardman, Domingue, and Daw (2015) provide evidence for genetic correlation (rG) for genes linked to years of education and genes linked to self-rated health. Thus, there are clear reasons to expect that genes linked to educational attainment may influence frailty due to of a host of different cognitive, physiological, social, or psychosocial mechanisms.
Aging, Genes, and Frailty
From a medical perspective, frailty is a state of reduced physiological resilience and increased vulnerability to adverse events. Patients who are frail are more likely to be hospitalized, lose daily independence, have negative outcomes with medical procedures (e.g., poor response to surgeries), and have increased risk of death compared to non-frail individuals of the same age (Hanlon et al., 2018). Frailty can be considered a debilitating and financially costly age-related syndrome (Goldman et al., 2013), for which a growing population is at risk as demographic shifts in population age structure occur worldwide. As such, it is imperative to understand the predictors and mechanisms governing who is most at risk for frailty.
Though aging is the greatest known risk factor for frailty (Fried et al., 2001), not all aging individuals become frail. Importantly, those with lower levels of education are far more likely than those with higher levels of education to evidence symptoms of frailty at an early age (Santos-Eggimann et al., 2009). There are two competing hypotheses regarding the influence of education across the life course which may explain the related gradients for frailty. The cumulative advantage hypothesis posits that educational gradients should increase with age (O’Rand, 2006). Alternatively, the age as a leveler hypothesis suggests these differences are diminished later in life (Elo & Preston, 1996). To date, there is evidence for both perspectives depending on the specific measure of frailty, the age composition of the study, and whether or not frailty is measured as a static indicator of current health or a within person measure of change in indicators of frailty over time (Yang & Lee, 2010). Importantly, research describing the roles of genetics and epigenetics across the life course has shown clear evidence that genetic influences on phenotypic plasticity will reduce significantly as individuals age (Li & Tollefsbal, 2016) which is very much in line with the age as leveler hypothesis.
Bringing PGSeduc to bear on this question may shed light on the relevance of these two age-related models. Previous research has shown that roughly 45% of frailty is genetically oriented (Young, Glaser, Spector, & Steves, 2016). Although individuals will vary in their relative genetic risk for frailty, they will also vary in exposure to environmental risk exposures across their lifetime. To examine the relevance of the two age-related models discussed earlier we consider the following changes in the association of the PGSeduc with frailty increasing ages:
Cumulative advantage: the association PGSeduc with frailty is the same across ages among adults in mid to late life; cumulative advantage may have origins in genes linked to educational success.
Age as a leveler: the effect of the PGSeduc on frailty will decrease with increasing age; age levels the playing field with respect to genetic influence on the educational determinants of frailty.
Method
Sample
The data come from the Health and Retirement Study (HRS), a biennial panel study tracking physical, emotional, and economic well-being during the transition into older age (Juster & Suzman, 1995). These respondents were born between 1900 and 1970 with the interquartile range of birth years spanning from 1930 to 1950. The HRS is sponsored by the National Institute on Aging (NIA U01AG009740, RC2AG036495, and RC4AG039029) and was conducted by the University of Michigan. This study focuses on a sample of 7,064 HRS respondents with genotype data and had one or more frailty measure (please see Figure S1 for inclusion criteria). Descriptive statistics for all variables are provided in Table 1. Because of potential complications due to population stratification and the use of the PGSeduc values across socially identified racial and ethnic groups, our analyses are limited to those who identify as non-Hispanic and white. Age range spans from mid-to-late life.
Table 1.
Descriptive Statistics Stratified by Polygenic Scores for Educational Attainment
| Total | Low PGS | Avg. PGS | High PGS | p< | |
|---|---|---|---|---|---|
| Frailty measures | |||||
| Paulson–Lichtenberg Frailty Index (PLFI) | 1.23 (1.18) | 1.30 (1.22) | 1.25 (1.19) | 1.15 (1.15) | 0.0001 |
| PLFI ≥ 3 (%) | 15.47 | 17.05 | 15.77 | 13.69 | 0.0191 |
| Frailty Index (FI) | 22.77 (12.62) | 24.10 (13.22) | 22.99 (12.68) | 21.23 (11.75) | 0.0001 |
| FI ≥ 25 (%) | 37.05 | 41.37 | 37.92 | 31.85 | 0.0001 |
| Sociodemographic | |||||
| Female | 59.68 | 61.21 | 58.68 | 59.15 | 0.1801 |
| Age (years) | 68.91 (10.23) | 68.10 (9.89) | 69.16 (10.29) | 69.48 (10.44) | 0.0001 |
| Education (years) | 13.28 (2.49) | 12.38 (2.37) | 13.28 (2.38) | 14.17 (2.38) | 0.0001 |
| Cohort (%) | |||||
| No cohort | 1.33 | 1.58 | 1.25 | 1.17 | 0.0001 |
| AHEAD | 5.89 | 4.44 | 6.19 | 7.03 | |
| CODA | 14.32 | 12.11 | 14.99 | 15.84 | |
| HRS | 39.81 | 41.46 | 39.24 | 39.03 | |
| War Babies | 16.87 | 17.91 | 17.45 | 15.24 | |
| Early Baby Boomers | 17.74 | 18.76 | 16.83 | 17.62 | |
| Mid Baby Boomer | 4.06 | 4.04 | 4.05 | 4.07 | |
| Genetic information | |||||
| Education PGS | 0.00 (1.00) | −1.08 (0.52) | 0.00 (0.25) | 1.10 (0.53) | 0.0001 |
| PC1 | 0.00 (1.00) | 0.02 (1.03) | 0.01 (1.01) | −0.03 (0.96) | 0.0023 |
| PC2 | 0.00 (1.00) | 0.02 (0.96) | 0.00 (0.98) | −0.02 (1.06) | 0.0305 |
| PC3 | 0.00 (1.00) | −0.01 (0.86) | 0.03 (0.99) | −0.02 (1.13) | 0.0007 |
| PC4 | 0.00 (1.00) | −0.03 (0.98) | 0.02 (0.97) | 0.02 (1.04) | 0.0003 |
| PC5 | 0.00 (1.00) | 0.01 (1.00) | 0.04 (0.99) | −0.05 (1.01) | 0.0001 |
| No. of full sample | 7,064 | 2,360 | 2,356 | 2,348 |
Note: Data come from the Health and Retirement Study (HRS). Statistics reflect mean and standard deviations, unless otherwise noted as percentage of sample (%). Sample size for all sociodemographic, cohort, and genetic information reflects the full sample. Sample size for the FI includes n = 7,064 individuals, with n = 34,969 observations whereas ample size for PLFI was slightly reduced, with n = 5,301 individuals, with n = 12,952 observations. Cohort names include: AHEAD = the study of Assets and Health Dynamics Among the Oldest Old; CODA = Children of Depression; HRS= original Health and Retirement cohort; PC = principal component; PGS = polygenic score.
Measures
PGS for Educational Attainment
The PGSeduc is a measure of the genes associated with education. We used PGSs based on results from the most recent Social Science Genetic Association Consortium Genome Wide Association Study of educational attainment (Lee et al., 2018). We use a publically available score available through the HRS, which was constructed using LDpred software (Vilhjálmsson et al., 2015), all available single nucleotide polymorphisms, and standard quality control procedures. Full description of the score construction is detailed elsewhere (Okbay, Benjamin, & Visscher, 2019).
Frailty Indices
Two metrics of frailty were used to assess five waves of the HRS (2004–2012). These waves were selected due to consistency in item measurement and have also been used in previous research on frailty (Mezuk, Lohman, Rock, & Payne, 2016). When possible, items were pulled from a harmonized longitudinal file prepared by the RAND Center on the Study of Aging (RAND, 2014). When unavailable in the RAND file, matching items were identified in the biennial files. For both metrics of frailty, scores are treated as both continuous and categorical. Continuous measures reflect an unweighted count of the number of health problems (i.e., symptoms, signs, or functional impairments), which are collectively referred to as “deficits.” Counting deficits stratifies respondents based on their level of functional decline, and thus, their degrees of vulnerability (Rockwood et al., 2005). Categorical measures were treated as binary and are based on clinical cutoffs that have emerged to classify an individual’s frailty status (i.e., frail or non-frail).
Paulson–Lichtenberg Frailty Index
On the basis of the biological syndrome/phenotype model described by Fried and colleagues (2001), the Paulson–Lichtenberg Frailty Index (PLFI) is a self-report measure created for use in the HRS (Paulson & Lichtenberg, 2015). The PLFI included five symptoms: wasting (i.e., individual reported loss of at least 10% of body weight over a 2-year period), weakness (i.e., “Because of health problems, do you have any difficulty with lifting or carrying weights over 10 pounds, like a heavy bag of groceries”), slowness (i.e., “Because of a health problem, do you have any difficulty with getting up from a chair after sitting for long periods), fatigue (i.e., “Have you had any of the following persistent or troublesome problems: sever fatigue or exhaustion?”), and falls (“Have you fallen down in the past 2 years”). The continuous PLFI measure had a range of 0–5, whereas the binary measure of frailty status used the established cutoff of PLFI more than 3 as indicative of “frail” (Paulson & Lichtenberg, 2015).
Deficit Accumulation Model Frailty Index
Frailty in the HRS was also operationalized using the deficit accumulation model put forth by Rockwood and colleagues (2005). Although the original Frailty Index (FI) measured 70 deficits, an FI has been generalized as the calculated ratio of health deficits out of the total number of possible deficits measured (e.g., 28 deficits present/70 measured deficits indicates an FI of .40). A validated 30-item FI of self-reported health measures was developed for use in the HRS and was recreated using the item list published in Mezuk and colleagues, (2016). Items included a variety of deficits such as difficulties with activities of daily living (e.g., dressing, bathing, toileting, cooking, shopping, changes activities of daily living), problems with pain (e.g., general pain, back pain, headache), problems with worsening memory or dementia, disturbances in sleep (e.g., persistent fatigue, problems falling or staying asleep), motor impairment (e.g., falling, impaired mobility, gross motor impairment, fine motor impairment), and presence of health conditions (e.g., incontinence, cancer, arthritis, psychiatric conditions, depression, lung disease, respiratory problems, diabetes, stroke, angina, heart failure, heart attack, or high blood pressure). For ease of interpretation, the continuous FI was coded as a percentage ranging from 0% to 100% of deficits, whereas frailty status was defined as having 25% or more deficits present (Rockwood et al., 2005).
Analysis
Statistical analyses were conducted using Stata (StataCorp, 2011). Simple association of frailty scores with PGS was done by chi-squared test of independence. Because frailty was repeatedly assessed, we use all data across waves. Multilevel regression with the xtmixed procedure in Stata was used to model the association of PGSeduc and frailty score in which observations are nested within people. We report the intraclass correlation coefficient for each model. Similarly, multilevel logistic regression was used with the xtmelogit command in Stata to model PGSeduc and frailty status (frail/non-frail). Level 1 error variance was assumed to be (Guo & Zhao, 2000). All models control for the top five principal components (PCs) calculated among the European Ancestry population in the study to control for effects of population stratification or spurious association due noncausal allele frequency differences across ancestry groups (Patterson, Price, & Reich, 2006).
Results
Table 1 presents one-way analysis of variance test of the mean differences of our four frailty metrics across low, average, and high PGSeduc categories. These results provide the first evidence for our hypothesis linking genes related to educational outcomes and the indicators of frailty among adults transitioning to older ages. The PLFI is highest among those with the lowest PGSeduc ( = 1.30) and lowest among those with the highest PGSeduc ( = 1.15). Similar decreases in mean frailty are seen for the FI as the PGSeduc increases, where the lowest PGSeduc ( = 41.37) and lowest among those with the highest PGSeduc ( = 31.85).
Table 1 also presents the associations high PGSeduc categories and a binary indicators of frailty status. Although only 13.69% of those with the highest education PGSeduc were in a frail state according to the PLFI, 17.05% of those with the lowest education PGSeduc scores were in a frail PLFI state. This same association is seen with the threshold for the FI. Specifically, although only 31.85% of those with high education PGSeduc scores were frail at the time of the interview, 41.37% of those with low education PGSeduc were frail according to the FI. As with the continuous indicators, both binary assessments of frailty status were statistically significant (p < .019 and < .0001, respectively).
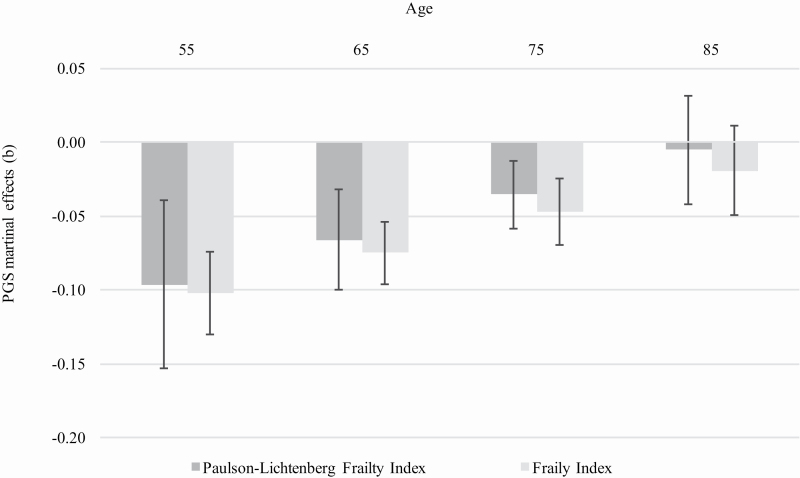
Table 2 presents the results from multilevel analyses of our frailty metrics with observations nested within individuals. We present a series of three models for each continuous indicator of frailty. The first regresses the frailty score on the education PGSeduc with controls for gender, age, cohort, and the top five PCs. The second introduces a control for years of education and the third introduces the interaction between the PGSeduc and age to evaluate the possibility of age-related changes in the influence of the PGSeduc on frailty. Model 1 provides a baseline indicator for the association of educational attainment on PLFI. The effect (b = −.15) suggests increases in educational attainment confers a protective effect on frailty. Model 2 introduces the independent effect of the PGSeduc controlling for the effect to actual educational attainment. The effect (b = −.13) suggests that a 1-SD increase in the PGSeduc reduces frailty by .13 points. This provides evidence for our primary hypothesis. Namely, that genes linked to educational attainment provides health protections that are above and beyond each additional year of formal education. Model 3 evaluates our interest in this association as individual’s age. Accordingly, we introduce an interaction between age and the education PGSeduc. As shown in Model 3, the interaction is positive and significant which suggests that the protective effects of the education PGSeduc on frailty diminish with age. To better gauge the meaning of this interaction, we used the postestimation margins command in Stata to retrieve parameter estimates and confidence intervals for age-specific slopes for the education PGSeduc—Frailty association. These estimates are shown graphically in Figure 1.
Table 2.
Frailty and Genes Associated With Educational Attainment: Multilevel Model Parameter Estimates
| Paulson–Lichtenberg Frailty Index | Frailty Index | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 3B | 4 | 5 | 6 | 6B | |||||||||
| Sex (male = 0) | ||||||||||||||||
| Female | 0.314 | *** | 0.314 | *** | 0.315 | *** | 0.958 | *** | 2.522 | *** | 2.539 | *** | 2.549 | *** | 0.849 | *** |
| Age (Z) | 0.596 | *** | 0.597 | *** | 0.595 | *** | 1.880 | *** | 4.909 | *** | 4.916 | *** | 4.910 | *** | 1.387 | *** |
| Cohort (no cohort) | ||||||||||||||||
| AHEAD | −7.922 | *** | −7.533 | *** | −7.489 | *** | −2.273 | *** | ||||||||
| CODA | 0.172 | ** | 0.167 | ** | 0.174 | ** | 0.583 | ** | −7.364 | *** | −7.081 | *** | −6.935 | *** | −1.903 | *** |
| HRS | 0.384 | *** | 0.374 | *** | 0.381 | *** | 1.349 | *** | −6.104 | *** | −5.930 | *** | −5.744 | *** | −1.501 | *** |
| War Babies | 0.686 | *** | 0.673 | *** | 0.678 | *** | 2.446 | *** | −3.662 | ** | −3.549 | ** | −3.371 | ** | −0.662 | ** |
| Early Baby Boomer | −2.751 | * | −2.692 | * | −2.525 | * | −0.713 | * | ||||||||
| Mid Baby Boomer | −1.158 | −0.995 | −0.813 | −0.179 | ||||||||||||
| Genetic principal components (z) | ||||||||||||||||
| PC1 | −0.034 | ** | −0.035 | ** | −0.034 | ** | −0.174 | *** | −0.545 | *** | −0.559 | *** | −0.561 | *** | −0.161 | *** |
| PC2 | −0.013 | −0.013 | −0.013 | −0.034 | −0.202 | −0.203 | −0.200 | −0.064 | ||||||||
| PC3 | 0.015 | 0.014 | 0.014 | 0.049 | 0.207 | 0.195 | 0.196 | 0.014 | ||||||||
| PC4 | −0.012 | −0.011 | −0.011 | −0.024 | −0.272 | * | −0.258 | * | −0.256 | * | −0.097 | * | ||||
| PC5 | 0.004 | 0.002 | 0.002 | 0.076 | 0.037 | 0.010 | 0.010 | 0.017 | ||||||||
| Education (Z) | −0.147 | *** | −0.132 | *** | −0.132 | *** | −0.412 | *** | −2.105 | *** | −1.827 | *** | −1.828 | *** | −0.579 | *** |
| PGS education (Z) | −0.044 | ** | −0.059 | ** | −0.205 | ** | −0.811 | *** | −0.762 | *** | −0.240 | *** | ||||
| Age × PGS | 0.039 | * | 0.178 | ** | 0.378 | *** | 0.269 | *** | ||||||||
| Intercept | 0.432 | *** | 0.442 | *** | 0.434 | *** | −5.661 | *** | 26.954 | *** | 26.783 | 26.588 | *** | −0.325 | ||
| Level 1 | 0.686 | 0.685 | 0.611 | 3.287 | 43.825 | 43.824 | 43.834 | 3.287 | ||||||||
| Level 2 | 0.613 | 0.612 | 0.686 | 4.699 | 99.634 | 99.060 | 98.727 | 8.412 | ||||||||
| ICC (ρ) | 0.472 | 0.472 | 0.529 | 0.588 | 0.695 | 0.693 | 0.693 | 0.719 | ||||||||
| N (observations) | 12,952 | 12,952 | 12,952 | 12,952 | 34,969 | 34,969 | 34,969 | 34,969 | ||||||||
| N (individuals) | 5,301 | 5,301 | 5,301 | 5,301 | 7,064 | 7,064 | 7,064 | 7,064 | ||||||||
Note: Data come from the Health and Retirement Study (HRS). Cell entries represent parameter estimates from a multilevel model in which observations are nested within individuals. PGS = polygenic score; Z = z-scored. Cohort names include: AHEAD = the study of Assets and Health Dynamics Among the Oldest Old (born before 1924), CODA = Children of Depression (born 1924–1930), HRS = original Health and Retirement cohort (born 1931–1941), War Babies (born 1942–1947), Early Baby Boomer (born 1948–1953), and Mid Baby Boomer (born 1954–1959). ICC = intraclass correlation coefficient; PC = principal component.
*p < .05. **p < .01. ***p < .001.
Figure 1.
PGS × Age interaction on risk of frailty status. PGS = polygenic score.
A very similar story is shown in Models 4–6 in which the dependent variable is now the deficits accumulation model or FI. In Model 4, the baseline association for educational attainment (b = −2.105) is evident above and beyond controls for gender, age, cohort, and the top five PCs. The effect in Model 5 (b = −0.81) represents the reduction in FI for a 1-SD increase in PGSeduc. Again, there is an independent influence of genes associated with education that do not operate through actual educational attainment. Similarly, the association is reduced as people age as indicated by the positive interaction in Model 6 (b = .38), which is statistically significant. The functional form of this interaction and the age thresholds at which education PGSeduc is linked to frailty for the FI is nearly identical to the PLFI despite differences in the conceptual models.
We replicate the results using a binary indicator of frailty status with an a priori threshold for each measure described earlier (Models 3B and 6B). As the pattern of results was nearly identical to those using the continuous indicators, we show only the full interaction model in Table 2 for simplicity. Again, the effect of the PGSeduc exists above and beyond educational attainment and the effect is the most evident among those aged 75 years and younger and nonexistent among those aged 80 years and older using logit models.
Finally, given the potential for the PGSeduc × Age interaction to be influenced by other confounders (Keller, 2014), we also refit the models to include the interactions between the PGSeduc, age, and all other covariates, including sex, PCs, and education. Interactions with cohort were excluded due to collinearity with cohort. Although the p value for the PGSeduc main effect was attenuated slightly, the overall results consistent and the substantive conclusions of our results remain the same.
Discussion
A breadth of work has established a link between educational attainment and health (Zajakova & Lawrence, 2018). Recently, attention has been directed at extending this research to study the link between educational attainment and an age-related health outcome, frailty. In our current study, we replicated these robust associations between years of completed education and two indicators of frailty among adults transitioning to older ages in the United States. More importantly, to the best of our knowledge, our study is the first to explore whether genes related to educational attainment (e.g., as measured by PGSeduc) are also associated with frailty regardless of one’s education. Across two frailty metrics we found that genes related to educational attainment predict frailty over and above an individual’s years of education. That is, among adults with comparable years of education, those with higher PGSeduc scores had better general health (i.e., less functional decline) compared to their similarly educated peers with lower PGSs. This held true using both a deficit accumulation model (FI) and Fried and colleagues (2001) biological syndrome/phenotype model (PLFI). Similarly, participants with higher PGSeduc were less likely to be classified as “frail” based on established cutoffs. Standing alone, this finding may not be entirely surprising. Controlling for PGSeduc reduced the main effect of education by 10.2% and 13.2% for the PLFI and FI frailty measures, respectively.
Accordingly, the residual effect of PGSeduc on frailty suggests that there is something critical in the education–health process that also operates above and beyond years of completed education. In their comprehensive review, Zajakova and Lawrence (2018: 275) discuss what they call a “signaling or credentialing perspective” in which the attainment of specific degrees provides new sources of human and social capital that frames an individual as productive or skilled. Accordingly, it may be that individuals with higher PGSeduc scores present themselves in a manner that is concordant with a successful or productive individual and thus create a response from others in which these signals lead to a positive framing of an individual that exists above and beyond their years of education. This is what is referred to as an evocative gene–environment correlation (rGE) because an individual’s cumulative genotype may evoke environments that are associated with both increasing education and reduced risks of frailty. This perspective is in line with our findings but our analyses cannot rule out other explanations.
Although we did not explore other intermediate phenotypes between the PGSeduc and frailty in our main analysis, several cognitive, physiological, social, or psychosocial mediators have been put forth. For example, in order to link an individual’s PGSeduc inherited at birth to downstream educational success, it is tempting to conceptualize this score as a measure of general aptitude or cognitive ability. However, it is equally plausible that PGSeduc contributes to general health which in turn influences years of education. Both explanations are in line with the idea that individuals that start with higher cognitive or physiological “reserves” will take longer to reach frailty status. Other potential mechanisms may be social or psychological in nature. A critical psychological mechanism could be the role of self-efficacy or mastery in which individuals with higher educational PGS may also have higher levels of efficacy that lead to both educational success, increased resilience, and delayed frailty onset (Stretton Latham, Carter, Lee, & Anderson, 2006). This same perspective is supported by work linking education to hopelessness and other indicators of sense of control (Mitchell, Ailshire, Brown, Levine, & Szanton, 2016). It is possible that the education PGS is involved in complex processes that reduce the sense of hopelessness and subsequently increase the likelihood of completing education. This same reduced hopeless is then instrumentally linked to reduced onset of frailty. Further, a recent study using similar metrics in the HRS highlighted the role for depression symptomology as an overlapping construct for frailty, which is also linked to educational attainment (Lohman, Dumenci, & Mezuk, 2016). Finally, it is possible that there is an indirect effect of social origin (Belsky et al., 2018) wherein PGSeduc is correlated among relatives, in that those with higher scores are also more likely to benefit from the social advantages correlated with relative’s PGSeduc. We posit multiple mechanisms are at play, given the complex interaction between genes with environment to produce behavior. We encourage future researchers to consider potential mechanisms as well as evocative rGE explanation in future work to better understand the physiological, cognitive, social, or psychosocial mediators through which genes associated with education may reduce the likelihood of frailty regardless of one’s level of education.
Another key finding of our study was that the association between PGSeduc scores and frailty diminished with age. That is to say, genes related to educational attainment are more strongly predictive of frailty for participants aged 75 years and younger, and the effect was nearly absent beyond age 80. This is an important finding that is consistent with previous analyses of age trends in allostatic load (Crimmins et al., 2003), where age-related increases in allostatic load flattened at the oldest ages (Seeman et al., 2008). For education-related differences in physical performance tasks (e.g., grip strength, balance, walking speed, and chair stands), the effect also diminishes at advanced ages (80+ years; Vaupel et al., 1998). Given that the direct effect of education on health diminishes with age, we would expect that genes associated with education would also have less of an effect on health at advanced ages. Overall, the result that PGSeduc is less predictive of frailty beyond age 80 when birth cohorts are combined is supportive of the age as leveler hypothesis; although, attention should be paid to intracohort heterogeneity (Yang & Lee, 2010).
In ancillary analyses, we tested whether several intermediate phenotypes could account for the independent effect of the PGSeduc on frailty, which does not operate through actual educational attainment. These potential mediators included the following variables measured at each wave: self-reported health (on a five-point scale from excellent to poor), self-reported self-efficacy (mean score across five items, rated on a five-point scale from low to high), and depression (as measured by the Center for Epidemiologic Studies Depression scale, from zero to seven symptoms present). Finally, we tested a measure of wealth as a mediator. As described in Belsky and colleagues, (2018), this measure combines multiple household measures of wealth, corrected for age, skew, and inflation, to reflect life course social attainment. Despite some attenuation of the effect of the PGSeduc on both the PLFI and FI, no potential mediator was able render the effect of genes insignificant.
There are several strengths of our study. Although there is general agreement that frailty is a measure of vulnerability and decline, there is no consensus on a uniform definition or optimal theoretical model. Although different operationalizations may reflect different etiological mechanisms (Rockwood, Andrew, & Mitnkitski, 2007), most metrics are most are valid predictors of subsequent morbidity and mortality (Theou Brothers, Mitnitski, & Rockwood, 2013) and may be reflecting a unified construct. Therefore, we examined two metrics designed to measure complementary but divergent models of frailty in the HRS (Cigolle et al., 2009): the PLFI (Paulson & Lichtenburg, 2015) which is a best fit measure biological syndrome/phenotype model and a 30-item FI (Mezuk et al., 2016) measuring deficit accumulations across multiple waves of data. A major strength of this study is that we found converging evidence for the across these indices. For both models, we show an independent effect of the PGSeduc above and beyond years of education, on frailty. Our results are largely consistent whether we use a continuous metric or binary indicator of a frailty status with an a priori threshold. Similarly, we find that both models of frailty support the age as a leveler hypothesis for the effects of genes associated with education across the life course. This convergence suggests our results may extend to a unified construct of frailty, which may be a marker of biological age. Finally, few studies have attempted to parse the effects of education from underlying genetic propensity for educational attainment. Unlike a majority of work, our study begins to disentangle this effect to illuminate possible underlying mechanisms.
Several limitations should be considered when interpreting these findings. The predictive power of a PGS in a replication sample may be attenuated with differences across studies in genotyping platforms, environmental contexts, or demographic characteristics (e.g., age, cohort, sex, or ethnicity; Wray et al., 2013). That is, the PGSeduc score may be most predictive in replication samples that closely match the discovery sample. For instance, it has been shown that the magnitude of genetic influences varies across birth cohort (Domingue, Conley, Fletcher, & Boardman, 2016) and historical periods (Boardman, Blalock, & Pampel, 2010). Although we control for mean differences in cohort frailty within the HRS, the PGS is limited by the cohort composition of the original discovery sample on which it was calculated from. Similarly, previous analyses have shown significant reductions in the effect sizes for in African ancestry samples when using European-derived scores (Duncan et al., 2018). As a baseline, the effect of PGSeduc on educational attainment was attenuated by 85% in African American samples compared to European American samples in the HRS (Lee et al., 2018). Owing to limited sample size and power necessary to detect effects in the African American sample, we too limited the main analysis participants of non-Hispanic European ancestry. Importantly, others have made it clear that the reduced genetic associations among African American samples are due in large part to significant differences in the typical economic, social, and physical environments of across racial and ethnic groups (Boardman, Barnes, Wilson, Evans, & Mendes de Leon, 2012). That is, the social environment may be responsible for the onset and magnitude of frailty among residents of economically disorganized communities and small genetic influences are potentially rendered relatively less important. Although not the goal of this specific article, we encourage future work to evaluate comparable hypotheses with the full sample of respondents from the HRS when more diverse discovery samples and PGS become available.
It is also important to consider that we may be tapping into a survivor effect wherein those with worse physical function and lower education die at younger ages and are not included in the sample of aged participants (Welmer et al., 2013). Indeed, it is well documented among the oldest cohorts of the HRS that population surviving into later waves have relatively high education, wealth, and better health compared to the full sample (Zajacova & Burgard, 2013). There are similar documented selection effects into surviving until the first wave of genotypic data was collected in the HRS (i.e., 2006), wherein those participants who were still living reported higher education and better health (Domingue et al., 2017). Further, this selection is demonstrated by higher mean PGSeduc for older cohorts which results from the loss participants with low scores. (Domnigue et al., 2017). That is, participants with the lowest PGSeduc, education, and health were less to survive to be genotyped in the first place, much less to the later waves of data collection. To evaluate the extent to which selection may have influenced our estimates, we compared the mean PGSeduc and mean years of education for those who died over our sample window to those who did not die. As expected, the groups differed in terms of mean standardized PGSeduc (alive = .01, deceased = −.08) and years of education (alive = 13.27 and deceased = 12.35). Importantly, we evaluated the extent to which the effect of PGSeduc on years of education was dependent upon mortality status with a simple interaction model. The main effect of the PGSeduc on years of education (e.g., among those who remained alive across all five waves) was b = .84, p < .001, the main effect of death was b = −.47, p < .001, but the interaction was not statistically significant (b = −.025, p < .728). The negative coefficient suggests that the association between PGSeduc and education is weaker among those who died (e.g., upwardly biased among those who lived) but the magnitude of this interaction compared to the main PGS effect and the p value (<.728) provide evidence that this form of selection is not likely to bias our estimates.
Finally, we evaluated our hypotheses with men and women together. Compared to men, women tend to evidence higher levels of frailty, become frail earlier in the life course, but the effect of frailty on mortality is significantly less among women compared to men suggesting the composition of frail individuals may be very different for older men and women (Gordon et al. 2017). Although we controlled for gender in our models, we did not specifically evaluate the possibility that these associations are systematically different as a function of gender identity. We encourage future work to consider the role of gender as a key mechanism linking genes associated with education, years of completed education, frailty, and survival.
In conclusion, our study is the first to show that part of the protective educational effect on frailty is related to genes associated with education itself. Further contributing to the larger body of work for studying the educational effects on aging, we find this effect becomes less predictive of frailty in older ages. Future work exploring underlying mechanisms that link genes and frailty may help explain disparities evident in who ages successfully.
Funding
This work was supported by a National Institute on Aging training grant (NIA T32AG052371). Data collection for the Health and Retirement Study (HRS) was also supported by the National Institute on Aging (NIA U01AG009740), and is conducted by the University of Michigan. Genotyping funds for this project were provided by National Institutes of Health’s (NIH) Director’s Opportunity for Research awards using American Reinvestment and Recovery Act funds (RC2 AG036495-01, RC4 AG039029-01).
Author Contributions
B. M. Huibregtse and B. L. Newell-Stamper were responsible for data cleaning and creating frailty indices. J. D. Boardman performed data analysis. All authors were involved in conceptualizing the study and contributed to draft writing and revision.
Conflict of Interest
None reported.
Supplementary Material
References
- Belsky D. W., Domingue B. W., Wedow R., Arseneault L., Boardman J. D., Caspi A., . . . Harris K. M (2018). Genetic analysis of social-class mobility in five longitudinal studies. Proceedings of the National Academy of Sciences, 115, E7275–E7284. doi:10.1073/pnas.1801238115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky D. W., Moffitt T. E., Corcoran D. L., Domingue B., Harrington H., Hogan S., . . . Caspi A (2016). The genetics of success: How single-nucleotide polymorphisms associated with educational attainment relate to life-course development. Psychological Science, 27, 957–972. doi:10.1177/0956797616643070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J. D., Barnes L. L., Wilson R. S., Evans D. A., & Mendes de Leon C. F (2012). Social disorder, APOE-E4 genotype, and change in cognitive function among older adults living in Chicago. Social Science and Medicine (1982), 74, 1584–1590. doi:10.1016/j.socscimed.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J. D., Blalock C. L., & Pampel F. C (2010). Trends in the genetic influences on smoking. Journal of Health and Social Behavior, 51, 108–123. doi:10.1177/0022146509361195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J. D., Domingue B. W., & Daw J (2015). What can genes tell us about the relationship between education and health? Social Science and Medicine (1982), 127, 171–180. doi:10.1016/j.socscimed.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branigan A. R., McCallum K. J., & Freese J (2013). Variation in the heritability of educational attainment: An international meta-analysis. Social Forces, 92, 109–140. doi:10.1093/sf/sot076 [Google Scholar]
- Bulik-Sullivan B., Finucane H. K., Anttila V., Gusev A., Day F. R., Loh P. R., . . . Neale B. M.; ReproGen Consortium ; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3. (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47, 1236–1241. doi:10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K., Doblhammer G., Rau R., & Vaupel J. W (2009). Ageing populations: The challenges ahead. Lancet (London, England), 374, 1196–1208. doi:10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigolle C. T., Ofstedal M. B., Tian Z., & Blaum C. S (2009). Comparing models of frailty: The health and retirement study. Journal of the American Geriatrics Society, 57, 830–839. doi:10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- Crimmins E. M., Johnston M., Hayward M., & Seeman T (2003). Age differences in allostatic load: An index of physiological dysregulation. Experimental Gerontology, 38, 731–734. doi:10.1016/S0531-5565(03)00099-8 [DOI] [PubMed] [Google Scholar]
- Domingue B. W., Belsky D. W., Harrati A., Conley D., Weir D. R., & Boardman J. D (2017). Mortality selection in a genetic sample and implications for association studies. International Journal of Epidemiology, 46, 1285–1294. doi:10.1093/ije/dyx041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue B. W., Conley D., Fletcher J., & Boardman J. D (2016). Cohort effects in the genetic influence on smoking. Behavior Genetics, 46, 31–42. doi:10.1007/s10519-015-9731-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudbridge F. (2013). Power and predictive accuracy of polygenic risk scores. PLoS Genetics, 9, e1003348. doi:10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, L., Shen, H., Gelaye, B., Meijsen, J., Ressler, K., Feldman, M., ... & Domingue, B. (2019). Analysis of polygenic risk score usage and performance in diverse human populations. Nature Communications, 10, 3328. doi:10.1038/s41467-019-11112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo I. T., & Preston S. H (1996). Educational differentials in mortality: United States, 1979–1985. Social Science and Medicine (1982), 42, 47–57.doi:10.1016/0277-9536(95)00062-3 [DOI] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., . . . McBurnie M. A.; Cardiovascular Health Study Collaborative Research Group (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 56, M146–M156. doi:10.1093/gerona/56.3.m146 [DOI] [PubMed] [Google Scholar]
- Goldman D. P., Cutler D., Rowe J. W., Michaud P. C., Sullivan J., Peneva D., & Olshansky S. J (2013). Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Affairs (Project Hope), 32, 1698–1705. doi:10.1377/hlthaff.2013.0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. H., Peel N. M., Samanta M., Theou O., Howlett S. E., & Hubbard R. E (2017). Sex differences in frailty: A systematic review and meta-analysis. Experimental Gerontology, 89, 30–40. doi:10.1016/j.exger.2016.12.021 [DOI] [PubMed] [Google Scholar]
- Guo G., & Zhao H (2000). Multilevel modeling for binary data. Annual Review of Sociology, 26, 441–462. doi:10.1145/annurev.soc.26.1.441 [Google Scholar]
- Hagenaars S. P., Harris S. E., Davies G., Hill W. D., Liewald D. C., Ritchie S. J., . . . Deary I. J.; METASTROKE Consortium, International Consortium for Blood Pressure GWAS ; SpiroMeta Consortium; CHARGE Consortium Pulmonary Group, CHARGE Consortium Aging and Longevity Group. (2016). Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Molecular Psychiatry, 21, 1624–1632. doi:10.1038/mp.2015.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon P., Nicholl B. I., Jani B. D., Lee D., McQueenie R., & Mair F. S (2018). Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health, 3, e323–e332. doi:10.1016/S2468-2667(18)30091-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster F. T., & Suzman R (1995). An overview of the Health and Retirement Study. Journal of Human Resources, 30: S7–S56. doi:10.2307/146277 [Google Scholar]
- Keller M. C. (2014). Gene × environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biological Psychiatry, 75, 18–24. doi:10.1016/j.biopsych.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. J., Wedow R., Okbay A., Kong E., Maghzian O., Zacher M., . . . Fontana M. A (2018). Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nature Genetics, 50, 1112–1121. doi:10.1038/s41588-018-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., & Tollefsbol T. O (2016). Age-related epigenetic drift and phenotypic plasticity loss: Implications in prevention of age-related human diseases. Epigenomics, 8, 1637–1651. doi:10.2217/epi-2016-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link B. G., & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, Spec No, 80–94. doi:10.2307/2626958 [PubMed] [Google Scholar]
- Lohman M., Dumenci L., & Mezuk B (2016). Depression and frailty in late life: Evidence for a common vulnerability. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 71, 630–640. doi:10.1093/geronb/gbu180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni R. E., Ritchie S. J., Joshi P. K., Hagenaars S. P., Okbay A., Fischer K., . . . Amador C (2016). Genetic variants linked to education predict longevity. Proceedings of the National Academy of Sciences, 113, 13366–13371. doi:10.1073/pnas.1605334113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B., Lohman M. C., Rock A. K., & Payne M. E (2016). Trajectories of body mass indices and development of frailty: Evidence from the health and retirement study. Obesity (Silver Spring, Md.), 24, 1643–1647. doi:10.1002/oby.21572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell U. A., Ailshire J. A., Brown L. L., Levine M. E., & Szanton E. M (2016). Education and psychosocial functioning among older adults: 4-year change in sense of control and hopelessness. The Journals of Gerontology: Series B, 73, 849–859. doi:10.1093/geronb/gbw031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A., Beauchamp J. P., Fontana M. A., Lee J. J., Pers T. H., Rietveld C. A., . . . Oskarsson S (2016). Genome-wide association study identifies 74 loci associated with educational attainment. Nature, 533, 539–542. doi:10.1038/nature17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okbay A., Benjamin D., & Visscher P (2019). SSGAC Educational Attainment: GWAS and MTAG Polygenic Scores (Ver 1.0). [PDF file] Retrieved from: https://hrs.isr.umich.edu/data-products. Accessed May 10, 2019
- O’Rand A. M. (2006). Stratification and the life course: Life course capital, life course risks, and social inequality. In R. H. Binstock, L. K. George, S. J. Cutler, J. Hendricks, & J. H. Schulz (Eds.), Handbook of aging and the social sciences (6th ed.) (pp. 145–162). Cambridge, MA: Academic Press. doi:10.1016/B978-012088388-2/50012-2 [Google Scholar]
- Patterson N., Price A. L., & Reich D (2006). Population structure and eigenanalysis. PLoS Genetics, 2, e190. doi:10.1371/journal.pgen.0020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson D., & Lichtenberg P. A (2015). The Paulson-Lichtenberg Frailty Index: Evidence for a self-report measure of frailty. Aging and Mental Health, 19, 892–901. doi:10.1080/13607863.2014.986645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B. L., Williams J. W. Jr, Burke J. R., Holsinger T., & Benjamin S (2010). Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine, 153, 182–193. doi:10.7326/0003-4819-153-3-201008030-00258 [DOI] [PubMed] [Google Scholar]
- RAND 2014. RAND HRS data, Version N; RAND HRS Family Data Files, Version C. Produced by the RAND Center for the Study of Aging, with Funding from the National Institute on Aging and the Social Security Administration. Santa Monica, CA: RAND. [Google Scholar]
- Rietveld C. A., Medland S. E., Derringer J., Yang J., Esko T., Martin N. W., . . . Koellinger P. D.; LifeLines Cohort Study (2013). GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science (New York, N.Y.), 340, 1467–1471. doi:10.1126/science.1235488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K., Andrew M., & Mitnitski A (2007). A comparison of two approaches to measuring frailty in elderly people. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 62, 738–743. doi:10.1093/gerona/62.7.738 [DOI] [PubMed] [Google Scholar]
- Rockwood K., Song X., MacKnight C., Bergman H., Hogan D. B., McDowell I., & Mitnitski A (2005). A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal, 173, 489–495. doi:10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Eggimann B., Cuénoud P., Spagnoli J., & Junod J (2009). Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64, 675–681. doi:10.1093/gerona/glp012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T., Merkin S. S., Crimmins E., Koretz B., Charette S., & Karlamangla A (2008). Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988-1994). Social Science and Medicine (1982), 66, 72–87. doi:10.1016/j.socscimed.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP. [Google Scholar]
- Stretton C. M., Latham N. K., Carter K. N., Lee A. C., & Anderson C. S (2006). Determinants of physical health in frail older people: The importance of self-efficacy. Clinical Rehabilitation, 20, 357–366. doi:10.1191/0269215506cr946oa [DOI] [PubMed] [Google Scholar]
- Theou O., Brothers T. D., Mitnitski A., & Rockwood K (2013). Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. Journal of the American Geriatrics Society, 61, 1537–1551. doi:10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- Vaupel J. W., Carey J. R., Christensen K., Johnson T. E., Yashin A. I., Holm N. V., . . . Longo V. D (1998). Biodemographic trajectories of longevity. Science, 280, 855–860. doi:10.1126/science.280.5365.855 [DOI] [PubMed] [Google Scholar]
- Vilhjálmsson B. J., Yang J., Finucane H. K., Gusev A., Lindström S., Ripke S., . . . Hayeck T (2015). Modeling linkage disequilibrium increases accuracy of polygenic risk scores. American Journal of Human Genetics, 97, 576–592. doi:10.1016/j.ajhg.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher P. M., Wray N. R., Zhang Q., Sklar P., McCarthy M. I., Brown M. A., & Yang J (2017). 10 Years of GWAS discovery: Biology, function, and translation. American Journal of Human Genetics, 101, 5–22. doi:10.1016/j.ajhg.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedow R., Zacher M., Huibregtse B. M., Mullan Harris K., Domingue B. W., & Boardman J. D (2018). Education, smoking, and cohort change: Forwarding a multidimensional theory of the environmental moderation of genetic effects. American Sociological Review, 83, 802–832. doi:10.1177/0003122418785368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welmer A. K., Kåreholt I., Rydwik E., Angleman S., & Wang H. X (2013). Education-related differences in physical performance after age 60: A cross-sectional study assessing variation by age, gender and occupation. BMC Public Health, 13, 641. doi:10.1186/1471-2458-13-641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray N. R., Yang J., Hayes B. J., Price A. L., Goddard M. E., & Visscher P. M (2013). Pitfalls of predicting complex traits from SNPs. Nature Reviews Genetics, 14, 507–515. doi:10.1038/nrg3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., & Lee L. C (2010). Dynamics and heterogeneity in the process of human frailty and aging: Evidence from the U.S. older adult population. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65B, 246–255. doi:10.1093/geronb/gbp102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. C., Glaser K., Spector T. D., & Steves C. J (2016). The identification of hereditary and environmental determinants of frailty in a cohort of UK twins. Twin Research and Human Genetics, 19, 600–609. doi:10.1017/thg.2016.72 [DOI] [PubMed] [Google Scholar]
- Zajacova A., & Burgard S. A (2013). Healthier, wealthier, and wiser: A demonstration of compositional changes in aging cohorts due to selective mortality. Population Research and Policy Review, 32, 311–324. doi:10.1007/s11113-013-9273-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajacova A., & Lawrence E. M (2018). The relationship between education and health: Reducing disparities through a contextual approach. Annual Review of Public Health, 39, 273–289. doi:10.1146/annurev-publhealth-031816-044628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.