Abstract
Acquired hemophilia A (AHA) is a severe auto‐immune bleeding disorder. Treatment of AHA is burdensome and optimal management is still unresolved. Therefore a retrospective nationwide multi‐center cohort study (1992‐2018) was performed to evaluate clinical presentation and treatment efficacy and safety of AHA in the Netherlands. Multivariate logistic and Cox regression analysis was used to study independent associations between patient characteristics and clinical outcomes. A total of 143 patients (median age 73 years; 52.4% male) were included with a median follow‐up of 16.8 months (IQR 3.6‐41.5 months). First‐line immunosuppressive treatment was mostly steroid monotherapy (67.6%), steroids/cyclophosphamide (11.9%) and steroids/rituximab (11.9%), with success rates of 35.2%, 80.0% and 66.7% respectively, P < .05. Eventually 75% of patients achieved complete remission (CR). A high anti‐FVIII antibody titer, severe bleeding and steroid monotherapy were associated with lower CR rates. Infections, the most important adverse event, occurred significantly more often with steroid combination therapy compared to steroids alone (38.7% vs 10.6%; P = .001). Overall mortality was 38.2%, mostly due to infections (19.2%) compared to 7.7% fatal bleeds. Advanced age, underlying malignancy and ICU admission were predictors for mortality. This study showed that AHA is characterized by significant disease‐related and treatment‐related morbidity and mortality. A high anti‐FVIII titer, severe bleeding and steroid monotherapy were associated with a lower CR rate. The efficacy of steroid combination therapies however, was overshadowed by higher infection rates and infections represented the most important cause of death. The challenging and delicate balance between treatment effectivity and safety requires ongoing monitoring of AHA and further identification of prognostic markers.
1. INTRODUCTION
Acquired hemophilia A (AHA) is a clinically challenging bleeding disorder, which is caused by the presence of antibodies against factor VIII (FVIII). These antibodies to FVIII partially or completely neutralize its coagulant function or cause an accelerated clearance. 1 The resulting lack of FVIII activity causes spontaneous and sometimes life‐threatening bleeding.
AHA affects mainly elderly patients and in about 50% an underlying cause can be identified, predominantly including malignancies or auto‐immune disorders. 2 , 3 , 4 , 5 , 6
The estimated incidence of AHA is approximately 1.5 cases per million persons/y. 4 The rarity of the disease often results in a significant delay in diagnosis and start of appropriate treatment, further increasing morbidity and mortality. Treatment of AHA requires a dual approach, which includes both the control of bleeding with hemostatic agents as well as inhibitor eradication with immunosuppressive drugs. During the past decades, mortality due to bleeding dramatically decreased from about 22% in the early 1980s to 3%‐4% reported in more recent studies, which is attributed to earlier diagnosis, the introduction of bypassing agents, and prompt inhibitor eradication. 2 , 3 , 5 , 7 However, this apparent success is overshadowed by the high rate of treatment‐related side effects, including infections and sepsis, which nowadays seem to be a major contributor to the morbidity and mortality in AHA patients. The challenge is to balance the minimization of bleeding risk by rapid inhibitor eradication with the risk of treatment‐related side effects, especially in an elderly and frail population.
This delicate balance urges the need for identification of prognostic patient characteristics to help tailor the intensity of immunosuppressive therapy. In the GTH‐AH 01/2010 study of Tiede et al a FVIII concentration <1 IU/dL, a poor performance status and the presence of anti‐FVIII IgA antibodies were associated with lower complete remission (CR) rates and overall survival. 5 , 8 However, to our knowledge, no protocols use these potential predictors as criteria to guide therapy.
Due to the challenge of performing randomized controlled trials in rare diseases like AHA, observational studies provide the main source of clinical data.
A retrospective analysis of Dutch patients diagnosed with AHA from 1992 to 2018, based on the Dutch national hemophilia complication registry, was performed to evaluate clinical presentation as well as efficacy and safety of treatment in AHA.
2. METHODS
2.1. Study population
In this multicenter retrospective cohort study the Dutch national hemophilia complication registry (KWARK database) was used to identify patients diagnosed with AHA between January 1992 and December 2018.
The KWARK database was founded by the Dutch Society of Hemophilia Physicians, representing all Dutch hemophilia treatment centers (HTCs). In the Netherlands care of (acquired) hemophilia patients is concentrated in these HTCs. Currently, there are six centers. For each participating center approval of the local medical ethics committee was obtained before data collection was started.
2.2. Definitions and data collection
All patients with a clinical diagnosis of AHA were included in this study. AHA was defined as an acquired bleeding tendency with a plasma FVIII activity below 50 IU/dL and detection of anti‐FVIII antibodies. Patients with other coagulation inhibitors or patients with congenital hemophilia A were excluded. There was no age restriction.
Paper and electronic patient files were reviewed to collect detailed information regarding demographic data, comorbidities, clinical presentation, plasma FVIII and anti‐FVIII (aFVIII) titers at presentation and follow‐up, bleeding episodes and hemostatic treatment, immunosuppressive regimen, response to treatment, adverse events, relapse and outcome at final follow‐up. Data collection was performed in 2018; patients were followed until death or last follow‐up. Both FVIII activity and aFVIII titers were measured in the local laboratories and were reported in IU/dL and Bethesda Units (BU) respectively. Regarding the measurement of aFVIII titer, the dilution closest to a 50% inhibition of FVIII in normal plasma is selected to estimate the inhibitor titer. 9 Since the introduction in 1995 all laboratories use the Nijmegen modification of the Bethesda assay. In two patients, included before 1995, the original Bethesda assay was used. All laboratories take part of an external quality assessment program of the European Concerted Action on Thrombosis (ECAT) Foundation. 10 , 11
A treatment line was defined as any (immunosuppressive) treatment modality. A treatment episode represented the treatment lines required for achieving complete remission (CR). Treatment course is defined as all treatment episodes together (in case of relapsing disease).
So, CR was defined as a FVIII activity of ≥70 IU/dL and a negative inhibitor titer (according to the cut‐off of the local laboratories). 2 A relapse was defined as recurrence of AHA after CR. Hereby relapses during withdrawal of immunosuppressive therapy (IST) and relapses after cessation of IST were recorded separately.
Severe bleeding was classified according to the ISTH criteria as (a) fatal bleeding, and/or (b) bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular, pericardial, or intramuscular with compartment syndrome, and/or (c) bleeding causing a fall in hemoglobin level of 2.0 g/dL (1.24 mmol/L) or more or leading to transfusion of two or more units of whole blood or red cells. 12 Other bleeds were classified as non‐severe. The following treatments were classified as hemostatic therapy: bypassing agents (recombinant activated factor VII (rFVIIa) or activated prothrombin complex concentrate (aPCC)), FVIII concentrates (human (hFVIII) or recombinant porcine (pFVIII)), prothrombin complex concentrate (PCC), fresh frozen plasma (FFP) and 1‐desamino‐8‐D‐arginine‐vasopressin (DDAVP). Administration of antifibrinolytic drugs, red blood cell (RBC) and/or platelet transfusion, surgery, compression, topical hemostatic therapy, immunoabsorption and plasmapheresis were all classified as ancillary therapy.
Resolution of bleeding was based on the clinicianʼs judgment. A bleeding relapse was defined as any bleeding event at the same site within a month after resolution of the first episode and/or cessation of hemostatic therapy.
An adverse event was defined as any untoward medical occurrence in a patient using immunosuppressive (IST) and/or hemostatic therapy. The following adverse events were recorded: (ischemic or hemorrhagic) stroke, cardiac events, thromboembolic complications (venous thromboembolism), infections, steroid related adverse events (including diabetes mellitus) and steroid induced psychiatric events) and other adverse events, which could not be classified in the previous categories.
Data collection was performed by two authors (S.S. and W.D.). In case of uncertainties consensus was reached through mutual consultation.
2.3. Statistical analysis
Continuous data were reported as median and interquartile range (IQR). Differences between continuous variables and comparisons of frequencies were analyzed using the Mann‐Whitney U test (or Kruskal‐Wallis test if >2 groups) and Fisherʼs exact test respectively.
The effectivity of hemostatic agents was compared by using multivariate logistic regression analysis with age, sex, FVIII activity, anti FVIII titer and type of hemostatic agent included in the model.
Logistic regression analysis was also performed to assess predictors for CR after first‐line therapy, relapse, adverse events and overall mortality. Mortality in the subgroup of patients with stable CR that stopped IST was followed until 1 year after cessation of IST to avoid confounding by indication.
The following variables were included in the analysis for CR and relapse: age, sex, underlying condition, malignancy, comorbidity, FVIII activity and aFVIII titer at presentation, bleeding severity (severe or non‐severe) and therapy regimen (four categories: steroid monotherapy, steroids/cyclophosphamide (S/C), steroids/rituximab (S/R) and other regimens). For analysis of mortality admission to the intensive care unit (ICU) at diagnosis was additionally added.
Variables included in the model of adverse events were age, gender, underlying condition, malignancy, presence of comorbid disorder and therapy regimen. The heterogeneity in treatment course limited the assessment of its effect on adverse events. Therefore a subanalysis was performed for predictors of infections during first‐line IST.
Age, FVIII activity and the inhibitor titer were evaluated as both continuous and categorical parameter in order to obtain the best predictive model.
Univariate and multivariate Cox regression analysis were performed to assess which characteristics were associated with the time to achieve CR and the time to death. The same variables as stated above were included in these models. Patients who died before reaching CR were set to indefinite, as they could no longer achieve CR. 5
Patients with unknown remission or survival state at final follow‐up were censored from the analysis. A P value of less than .05 was considered statistically significant. Analyses were performed using IBM SPSS statistics (version 25.0).
3. RESULTS
3.1. Clinical presentation
In the period from January 1992 to December 2018 a total of 143 AHA patients (75 male, 52.4%) were reported. Median follow‐up time was 16.8 months (IQR 3.6‐41.5 months) (Table 1 ). The median age was 73 years (IQR 60‐79 years), for women this was significantly lower due to occurrence of post‐partum AHA (68 vs 76 years, P = .01); Supplemental figure 1.
TABLE 1.
Demographics and clinical presentation
| Cases/total (%) or median (IQR) | |
|---|---|
| Male | 75/143 (52.4%) |
| Age (y) | 73 (60‐79) |
| Follow‐up time (mo) | 16.8 (3.6‐41.5) |
| Clinical presentation | |
| FVIII activity, IU/dL | 2.0 (0.0‐6.0) |
| aFVIII titer, BU | 20.5 (7.7‐58.8) |
| Hb, g/dL | 9.2 (7.7‐11.3) |
| Bleeding symptoms a | 49/136 (36.0%) |
| Mild bleeding | 87/136 (64.0%) |
| Severe bleeding | 16/136 (11.8%) |
| ICU admission at diagnosis b | |
| Time until diagnosis (d) | |
| From first symptoms until diagnosis | 22 (8–77) |
| From first medical evaluation until diagnosis | 2 (0–10) |
| Identifiable cause | |
| None | 82/130 (63.1%) |
| Malignancy | 24/130 (18.5%) |
| Solid | 15/130 (11.5%) |
| Hematologic | 9/130 (6.9%) |
| Immune‐related | 13/130 (10.0%) |
| Post‐partum | 6/130 (4.6%) |
| Infection‐related | 3/130 (2.3%) |
| Drug‐induced | 2/130 (1.5%) |
Abbreviations: aFVIII, anti FVIII antibody; BU, Bethesda units; FVIII, factor VIII; ICU, intensive care unit.
Based on ISTH criteria.
In all cases reason for ICU admission was bleeding related.
All patients presented with bleeding, which was severe in 87/136 patients (64.0%) and resulted in an admission to the ICU in 16/136 patients (11.8%). Hemostatic therapy was started in 161/218 of the recorded bleeding episodes (73.9%). The bypassing agents rFVIIa and aPCC were most frequently used, showing an overall efficacy of respectively 80.9% (56/68) and 93.4% (58/61), which was not significantly different when correcting for age, FVIII level, aFVIII titer and bleeding severity (P = .11), see Supplemental figure 2/Supplemental table 3 for details regarding hemostatic treatment and outcome.
The median patient delay, that is, the time from first symptoms until diagnosis, was 22 days (IQR 8‐77 days). Median diagnostic delay, defined as time to confirm the diagnosis of AHA after first medical evaluation, was 2 days (IQR 0‐10 days).
At diagnosis of AHA, most patients had comorbidity (125/143, 87.4%). An underlying disease for AHA was identified in 36.9% of all patients, mostly malignancies, immune‐related disorders and pregnancy‐related AHA (Table 1).
3.2. Immunosuppressive therapy (IST) and outcome
Note, IST was started in 139/143 (97.2%) patients. Details regarding outcome of the first‐line regimen and at final follow‐up were available in respectively 132/139 (95.0%) and 136/139 (97.8%) patients, Table 2 or Supplemental figure 4.
TABLE 2.
Immunosuppressive therapy (IST) and outcome
| Cases/total (%) or median (IQR) | |
|---|---|
| First line IST | |
| Steroids | 94/139 (67.6%) |
| Steroids/cyclophosphamide | 17/139 (11.9%) |
| Steroids/rituximab | 17/139 (11.9%) |
| Other steroid combination therapy a | 6/139 (4.3%) |
| IVIG | 1/139 (0.7%) |
| Rituximab | 1/139 (0.7%) |
| Treatment underlying disease | 3/139 (2.1%) |
| Complete remission (CR) after first‐line IST | 60/132 (45.5%) |
| Overall CR after first disease period | 105/132 (79.5%) |
| No. of treatment lines to reach first CR b | |
| 1 treatment line | 71/132 (53.8%) |
| 2 treatment lines | 41/132 (31.1%) |
| 3 treatment lines | 18/132 (13.6%) |
| 4 treatment lines | 1/132 (0.8%) |
| 5 treatment lines | 1/132 (0.8%) |
| Relapse during steroid withdrawal | 18/117 (15.4%) |
| ≥1 Relapse after first CR and stop IST | 23/92 (25.0%) |
| CR at data capture | 102/136 (75.0%) |
| On IST | 19/136 (14.0%) |
| Off IST | 83/136 (61.0%) |
| Time to achieve first CR, weeks | 10.7 (6.4‐22.1) |
| Time to (any) subsequent CR after relapse, weeks | 3.4 (1.5‐10.0) |
| Total duration of treatment, weeks | 24.4 (11.6‐44.0) |
| Adverse events (any) | 92/136 (67.6%) |
| Mortality at data capture | 52/136 (38.2%) |
| Mortality cause | |
| Infection c | 10/52 (19.2%) |
| Malignancy | 7/52 (13.5%) |
| Cardiac | 5/52 (9.6%) |
| Fatal bleeding | 4/52 (7.7%) |
| Respiratory insufficiency | 1/52 (1.9%) |
| Other | 7/52 (13.4%) |
| Unknown | 18/52 (34.6%) |
Note: Differences in the nominator are the result of missing data or, in case of relapse rate, result from the fact that not every patient achieved complete remission and/or IST was not withdrawn/stopped in all cases.
Steroids/mycophenolate mofetil 3/136 (2.1%), steroids/cyclophosphamide/rituximab 2/136 (1.4%), steroids/azathioprine 1/136 (0.7%).
A treatment line is defined as a certain immunosuppressive regimen (ie, steroid monotherapy or steroids/cyclophosphamide); any change in this regimen (ie, change in and/or addition of an immunosuppressive agent) is considered as a subsequent therapy line.
8/10 patients on IST, 2/10 patients off IST.
Treatment was usually started at the day of diagnosis (median 0 days [IQR 0‐3 days]). As first‐line therapy, several different treatment regimens were applied. Steroid monotherapy was most frequently used (94/139, 67.6%), followed by combination therapy of steroids with cyclophosphamide or rituximab (both in 17/139 patients, 11.9%). Treatment details regarding dose and route of administration were similar among the cohort, with prednisone administered in a dose of 1 mg/kg/d, cyclophosphamide 1‐2 mg/kg/d and rituximab 375 mg/m2 per week for 4 times, according to the national guidelines. 13
Overall 60/132 patients (45.5%) achieved complete remission (CR) after first‐line therapy, whereas 46.3% of all patients needed ≥2 therapy lines and 8.3% died before achieving CR during first‐line.
In nearly all cases (55/61, 90.2%) second‐line IST consisted of a combination of steroids and another immunosuppressive agent, most frequently cyclophosphamide or rituximab (54.1% and 21.3% respectively). Regardless of the number of therapy lines, 105/132 (79.5%) achieved CR after the first treatment episode with a median time to CR of 10.7 weeks (IQR 6.4‐22.1 weeks).
A relapse during prednisone withdrawal occurred in 18/117 (15.4%) and another 23/92 patients (25.0%) experienced at least one relapse after cessation of IST. The median time to relapse after stop of IST was 14.7 weeks (IQR 2.9‐66.6 weeks).
At the moment of data capture 102/136 patients (75.0%) were in CR, of which 19/102 (18.6%) were still using immunosuppressive drugs.
Details regarding outcome according to underlying condition is shown in Supplemental table 5.
Six women with post‐partum AHA were included in this cohort study. Immunosuppressive therapy was started in 5/6 patients (83.3%). Almost all patients (4/5, 80%) were treated with steroid monotherapy, with a success rate of 75% (3/4). One patient was treated with rituximab monotherapy, which was changed to steroids/cyclophosphamide because of an allergic reaction. Ultimately CR was achieved in all six post‐partum AHA cases: one patient spontaneously, three after first‐line therapy with steroids alone and two after third‐line therapy.
3.3. Adverse events and mortality
A total of 212 treatment related adverse events (AEs) were recorded in 92/136 patients (67.7%), Supplemental figure 6. In 95.7% of patients (88/92) AEs were related to IST, in 3.3% (3/92) to hemostatic therapy and one patient (1.1%) experienced AEs related to both IST and hemostatic therapy.
Two patients (2/136, 1.5%) suffered from cardiac events while using aPCC: angina pectoris in one patient, myocardial infarction in the other. Another patient (1/136, 0.7%) showed clinical deterioration of an ischemic stroke after rFVIIa, which was administered because he also presented with major bleeding. A venous thromboembolism occurred in 2/136 patients (1.5%), including one patient treated with rFVIIa (0.7%).
From all IST‐related AEs, steroid induced AEs occurred most often (71/136, 52.2%), especially induction or deterioration of diabetes mellitus (44/136, 32.4%).
A total of 81 infections was seen in 49/136 patients (36.0%); most patients experienced uncomplicated infections (35/49, 71.4%) but sepsis was seen in 14/49 (28.6%). Airway/pulmonary (43.6%) and urogenital (26.9%) infections were most frequently observed. The presence of neutropenia, occurring in 15/136 (11.0%), was significantly associated with the risk of infection (P = .001).
In the multivariate model including age, sex, underlying condition, malignancy, comorbidity and first‐line IST regimen only age appeared to be independently associated with the risk of adverse events, showing a significant higher risk of AEs in patients ≥50 years with no significant difference between age categories 50‐75 years and ≥75 years (age 50‐75 years: OR 4.00 (95% confidence interval [CI] 1.32‐12.14); age ≥75 years: OR 3.13 (CI 1.09‐8.98), Supplemental table 7.
A sub analysis evaluating risk factors for infections during first‐line IST showed that any immunosuppressive regimen other than steroid monotherapy was independently associated with higher infection rates (steroids/cyclophosphamide [S/C] OR 5.33 [CI 1.53‐18.56], steroids/rituximab (S/R) OR 5.92 [CI: 1.67‐20.97], other regimens: OR 3.80 [CI 0.82‐17.70]; P = .011).
Overall mortality at end of follow‐up was 38.2% [52/136]), including 29 patients (55.8%) with active AHA, 21 patients (40.4%) in CR and two patients (3.8%) with an unknown outcome at the time of death. Infections (10/52, 19.2%) and malignancies (7/52, 13.5%) represented the most important causes of death, whereas 4/52 patients (7.7%) died because of fatal bleeding, Table 2. Note, IST contributed to death in 15.4% (8/52), which was based on both the use of immunosuppression and a cause of death related to an infection.
3.4. Comparison of immunosuppressive regimens
Effectivity and safety of the first‐line therapy regimens was evaluated. Baseline characteristics (sex, age, FVIII activity, aFVIII titer, bleeding severity, underlying disorders and comorbidity) were similar among the different regimens (Supplemental table 8). Outcome of steroid monotherapy was compared to steroids with cyclophosphamide (S/C), steroids with rituximab (S/R), any other steroid based regimen (S/O) and treatment of the underlying disorder (Figure 1 / Supplemental table 9). The CR rates were significantly lower with steroid monotherapy (35.2%) compared to every steroid combination therapy (varying from 67.7% to 83.3%), P < .05. In the univariate analysis, time to achieve CR and therapy duration (among patients with CR) were similar among the treatment groups.
FIGURE 1.
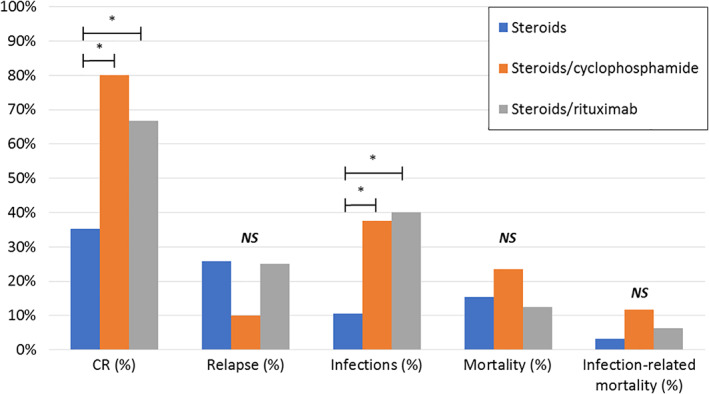
Outcome of first‐line immunosuppressive therapy. CR, complete remission. Mortality and infection‐related mortality represent mortality rates during first‐line treatment period. *P value <.05 (Fisher exact test). NS, not significant. The three most frequent used first‐line immunosuppressive regimens are compared. Data of outcome in other steroid‐based regimens and treatment of the underlying conditions is not shown [Color figure can be viewed at wileyonlinelibrary.com]
Although the overall adverse event rate was similar, steroid combination therapies (S/C and S/R) and treatment of the underlying disorder were associated with a significantly higher infection rate than steroid monotherapy (respectively 37.5%, 40.0% and 66.7% vs 10.6%, P < .05).
Similarly, death due to infection occurred more frequently in S/C and S/R compared to steroids alone, whereas bleeding related mortality was higher in the steroid monotherapy group (Supplemental table 9). These differences were however not statistically significant. Also overall mortality during first‐line treatment was also not significantly different among the treatment groups.
3.5. Predictors of outcome
Logistic regression analysis was performed to identify predictors for CR, relapse and mortality (Supplemental table 7). Cox regression analysis was used to analyze time to achieve CR and survival time.
3.5.1. Complete remission (CR) after first‐line therapy
Multivariate logistic regression analysis showed that a high aFVIII titer (aFVIII >20 BU OR 0.33; 95% CI 0.14‐0.78; P = .012), severe bleeding (OR 0.31; 95% CI 0.13‐0.75; P = .010) and steroid monotherapy (any other regimen OR 2.10‐8.06 [range]; P = .007) were all independently associated with failure to achieve CR. In the univariate analysis a FVIII level <2 IU/dL and female sex were also associated with a lower CR rate, but this difference disappeared after correction for other factors in the multivariate analysis.
With regard to the time to achieve CR the same parameters were independently correlated to a worse outcome (aFVIII >20 BU: HR 0.38; CI 0.21‐0.70; P = .003, severe bleeding: HR 0.38; CI 0.21‐0.70; P = .002, and steroid monotherapy: HR (any other regimen) 1.99‐2.59 (range); P = .028, Figure 2.
FIGURE 2.
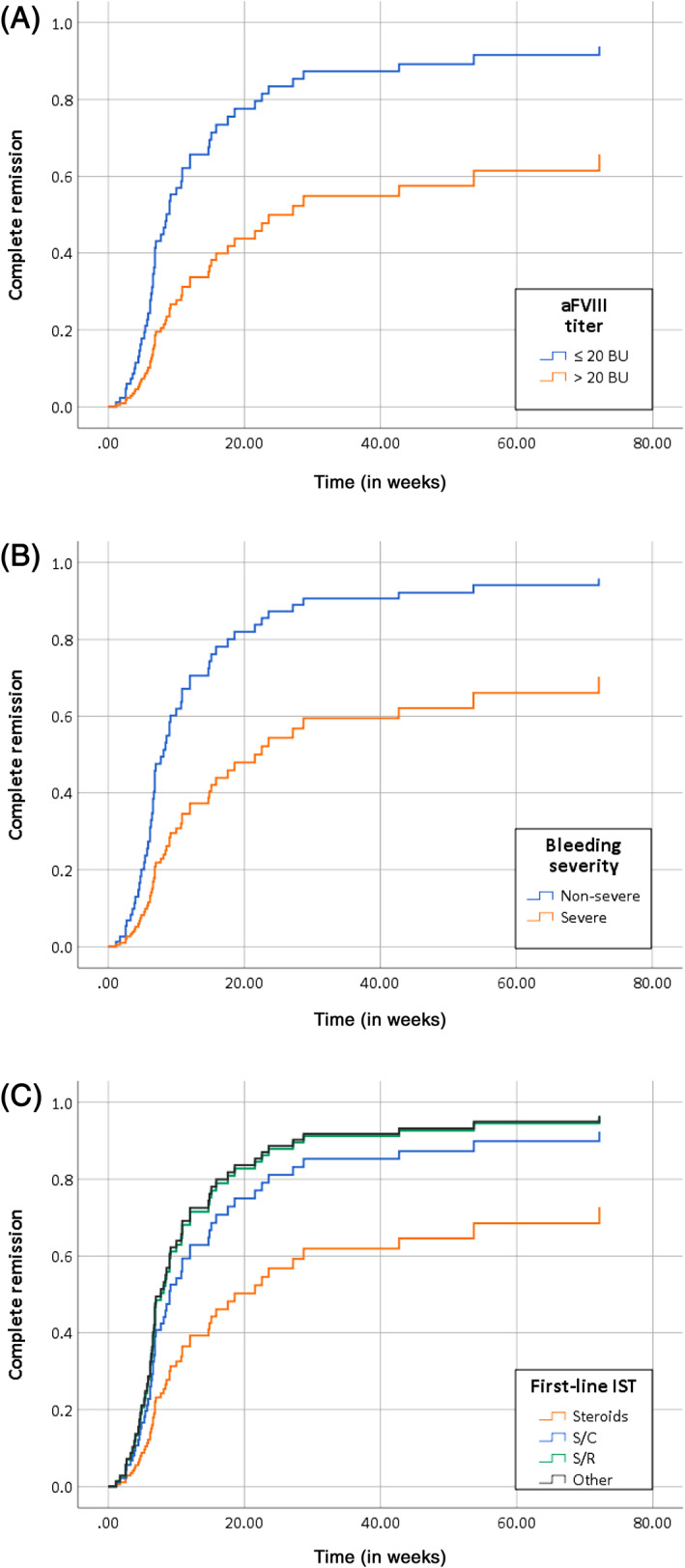
Time to complete remission by (A) inhibitor titer, (B) bleeding severity and (C) first‐line immunosuppressive therapy. Time to complete remission (CR) in weeks. BU, Bethesda units. Cox regression analysis; variables included in the model: age, sex, underlying condition, malignancy, comorbidity, FVIII activity and aFVIII titer at presentation, bleeding severity (severe or non‐severe) and first‐line therapy regimen (four categories: steroid monotherapy, steroids/cyclophosphamide (S/C), steroids/rituximab (S/R) and other regimens). An aFVIII titer >20 BU (P = .003), severe bleeding (P = .002) and steroid monotherapy (P = .028) were all inversely correlated with the time to achieve CR [Color figure can be viewed at wileyonlinelibrary.com]
When using inhibitor titer at presentation (≤20 BU) and mild bleeding as clinical markers to tailor therapy, 61.5% of this cohort would achieve CR with steroids alone compared to 7.4% in patients with aFVIII >20 BU and severe bleeding (P = .002).
3.5.2. Relapse
Using the same model as used for prediction of CR, none of the included parameters appeared to be an independent predictor for the risk of relapse (data not shown).
3.5.3. Mortality
Age (age ≥75 years: OR 4.40; CI 1.77‐10.94; P = .001), the presence of a malignancy (OR 9.74; CI 3.12‐30.43; P < .001) and ICU admission at diagnosis (OR 8.44; CI 1.89‐37.75; P = .005) were independent predictors for mortality. The reason for ICU admission was severe bleeding in all cases. Evaluation of survival time showed that the same parameters were significantly associated with a worse outcome (age ≥75 years: HR 2.71, CI 1.39‐5.26, P = .003; malignancy: HR 3.62, CI 1.88‐6.97, P < .001; ICU admission: HR 3.61, CI 1.64‐7.97, P = .001).
4. DISCUSSION
In this study we describe the clinical presentation and outcome of a large cohort of patients with AHA treated in the Netherlands during the last 27 years. The results of this study emphasize that AHA is a serious disorder, which affects mostly elderly and frail patients and is characterized by severe bleeding symptoms and a protracted, burdensome treatment. Inhibitor eradication by immunosuppressive therapy, although successful in most of the cases, is associated with a high rate of adverse events and secondary infections represent the most important cause of death.
Compared to previous publications, the present study confirms that AHA mostly affects elderly patients given a median age over 70 years. 2 , 3 , 4 , 5 An underlying disorder was identified in almost 37% of all patients, most frequently malignancies and auto‐immune disorders, which is in line with other large registries. 2 , 3 , 4 , 5
In this study the majority of patients presented with severe bleeding and more than two‐thirds required hemostatic therapy. Similar to the European Acquired Haemophilia Registry (EACH2), rVIIa and aPCC were associated with a success rate over 80% and a low risk of adverse events. 14
Compared to the favorable risk–benefit ratio of bypassing agents, the immunosuppressive treatment in AHA is more troublesome. The CR rate after first‐line therapy in this cohort was around 50%. An important finding in our study was the striking difference of CR rates between first‐line steroid monotherapy and steroid combination therapy: 35.2% vs 73.3% (CR rate of S/C and S/R combined). This difference in success rate was also shown in the EACH2. 15
Regarding predictors for CR after first‐line therapy a high aFVIII titer, severe bleeding and steroid monotherapy were all independently associated with lower CR rates and a longer time to achieve CR.
The relationship between aFVIII titer and inhibitor eradication was described by the EACH2 and GTH‐AH 01/2020 study as well. 5 , 15 In line with these studies, the median aFVIII titer (≤or >20 BU in our cohort) was used as cut‐off.
Of note, this study showed that aFVIII titer and bleeding severity were independently correlated with outcome. Up to now, this is the first time bleeding severity is identified as prognostic marker for achieving complete remission, but many studies did not include this parameter in the multivariate analysis.
As stated above, this study demonstrated the superior efficacy of steroid/cyclophosphamide combination therapy compared to steroid monotherapy, which is in line with the EACH2 and CARE registries. 15 , 16 We additionally showed that steroids combined with rituximab results in significantly higher remission rates as well.
In line with many AHA registries, this study emphasizes the high rate of adverse events, especially infections. 5 , 15 , 17 This is however the first study evaluating risk‐factors for treatment‐related adverse events, which provides valuable information to guide therapy. In our analysis only age appeared to be significantly correlated with the overall adverse events rate. Since many patients received more than one type of IST, it was difficult to assess the influence of a certain immunosuppressive regimen. Therefore a sub analysis was performed to detect predictors for infections during first‐line treatment. This multivariate analysis showed that steroid combination therapy (both S/C and S/R) is the main risk factor. Further research is warranted to clarify both patient‐ and treatment‐related predictors for adverse events in AHA.
The overall mortality rate of AHA patients in this cohort is high. Strikingly not bleeding, but infections represent the most important cause of death, and in about one in seven patients death was associated with the use of immunosuppressive drugs. The high rate of infection‐related mortality is emphasized by several other reports as well. 3 , 4 , 5 , 18 , 19 , 20 It should however be addressed that bleeding‐related mortality could be underestimated as a result of selection bias, as patients presenting with major bleeding may die before being diagnosed with AHA.
Predictors for a poor survival in this cohort were increasing age, the presence of a malignancy and ICU admission at diagnosis. No correlation between FVIII, aFVIII or the immunosuppressive regimen and survival was found. These results are comparable with most of the AHA cohort studies. 3 , 15 , 16 , 20 In the GTH‐AH 01/2020 study some additional potential negative predictors for survival were described, which include a FVIII activity <1 IU/dL, a poor WHO‐performance status and the presence of aFVIII IgA. 5 , 8 In this cohort many patients died of an AHA unrelated cause or were already in CR, which could explain the lack of identified” AHA‐specific” markers and the identification of general markers of a poor survival instead.
Several limitations of this study need to be addressed. Most important is the retrospective design, with consequently the risk of information bias and confounding by indication. Secondly, a small portion (<25%) of patients was also included in the EACH2. Finally, we cannot exclude selection bias, since the number of included patients is lower than expected based on the incidence of AHA. Except for patients who died because of bleeding before being diagnosed with AHA, this selection bias could result in an overestimation of disease severity, as patients with only mild symptoms may be treated at non‐academic hospitals and are not registered in the KWARK database. It is noteworthy that the number of AHA registrations was especially low during the first years of the founding of the KWARK database. Patient identification was more complete during the last decade of the cohort, as 100/143 (69.9%) of all included patients were identified between 2010 and 2018, corresponding to an average of more than 10 patients per year.
Besides these limitations, detailed data was collected from a relatively large cohort of patients with a long follow‐up duration. The reported demographic and disease characteristics are consistent with previous registries, confirming that this study describes a representative cohort of AHA patients and therefore provides valuable information to increase the knowledge of this severe bleeding disorder.
The most important challenge nowadays seems to be finding the optimal balance between treatment efficacy and toxicity. Therefore an initial attempt was made to detect prognostic markers for both successful outcome as well as adverse events.
This study demonstrated that both inhibitor titer and bleeding severity are independent prognostic markers for CR, that the superiority of steroid combination regimens comes with the drawback of higher infection rates and that increasing age is the main risk factor for adverse events. A combination of a low aFVIII titer (≤ 20 BU) with mild bleeding characterizes a population with a good prognosis, which could benefit from the less toxic steroid monotherapy. In other patients steroids combined with cyclophosphamide or rituximab remains the preferred treatment strategy because of the significant higher CR rates. Crucial in this group is the prevention of infections, either by antibiotic prophylaxis or anti‐neutropenic measures (if applicable). Unfortunately the application of antibiotic prophylaxis was not systematically recorded in this cohort.
Abovementioned strategy to use prognostic markers as guidance to tailor IST is in line with recently published recommendations of the diagnosis and treatment of AHA. 21 Mostly based on the results of the GTH‐AH 01/2010 study, the authors of this article suggest that patients with FVIII ≥1 IU/dL and an inhibitor titer ≤20 BU should receive first‐line treatment with steroids alone, whereas patients with FVIII <1 IU/dL and an inhibitor titer >20 BU should receive combination therapy. 5 , 21
Ongoing registry data collection and/or a meta‐analysis of the current evidence are warranted for further clarification of predictive markers, ideally resulting in the stratification of patients regarding both prognosis of AHA and the risk of adverse events, which is essential to optimally tailor immunosuppressive therapy. Furthermore the role of prophylactic antibiotics and other measures to prevent infections is an interesting, but largely unexplored area in AHA.
5. CONCLUSION
In conclusion, this study describes the experience with AHA in the Netherlands during the last 25 years. It shows that AHA is a severe bleeding disorder, which is associated with significant morbidity and mortality, not only bleeding‐, but especially also treatment‐related. A high aFVIII titer, severe bleeding and steroid monotherapy were independently associated with a lower remission rates. On the other side the superior efficacy of steroid combination regimens, that is, steroids/cyclophosphamide and steroids/rituximab, is partly overshadowed by increased infection rates. Moreover infections represent the most important cause of death. Prompt inhibitor eradication by immunosuppressive therapy to reduce the bleeding risk, while at the same time minimalizing the risk of adverse events is a balancing act in the typically old and frail AHA patient. Ongoing research and further clarification of prognostic markers is essential to find the optimal balance.
CONFLICT OF INTEREST
F.L. has received unrestricted research grants from CSL Behring, Takeda and UniQure. He is consultant for Takeda, UniQure of which fees go to the institute. J.E. has received research support from CSL Behring (funds to the institute) and an honorarium for educational activity from Roche (funds to the institute). K. Meijer reports grants, travels support and speaker fees from Bayer; grants and speaker fees from Sanquin; grants from Pfizer; speaker fees from Boehringer Ingelheim, BMS and Aspen, and consulting fees from UniQure, outside the submitted work. L.V. has received a speakerʼs fee from Pfizer and has performed consultancy for Tremeau (all fees were paid to the institute). R.S. has received research support from Bayer, Baxter, CSL Behring, NovoNordisk, Pfizer and Sovi (funds to the institute). All other authors declare no conflict of interests.
AUTHOR CONTRIUBTIONS
Sarah J. Schep and Wobke E.M. van Dijk collected the data. Sarah J. Schep analyzed the data and wrote the manuscript, with support from Lize F.D. van Vulpen, Kathelijn F. Fischer and Roger E.G. Schutgens All other authors contributed with patient identification and access to medical charts and provided feedback for the final version of the manuscript.
Supporting information
Supplement S1. Age distribution in AHA (N = 143).
Supplement S2. Bleeding site in AHA.
Supplement S3. Bleeding treatment and outcome.
Supplement S4. Disease course and outcome of IST in AHA.
Supplement S5. Outcome of AHA per underlying condition.
Supplement S6. Adverse events.
Supplement S7. Predictors of outcome in AHA by multivariate analysis.
Supplement S8. Baseline characteristics among first‐line immunosuppressive therapies.
Supplement S9. Comparison first‐line treatment immunosuppressive therapies.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of all participation centers of this study.
Schep SJ, van Dijk WEM, Beckers EAM, et al. Treatment of acquired hemophilia A, a balancing act: results from a 27‐year Dutch cohort study. Am J Hematol. 2021;96:51–59. 10.1002/ajh.26009
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cugno M, Gualtierotti R, Tedeschi A, Meroni PL. Autoimmunity Reviews Autoantibodies to coagulation factors: from pathophysiology to diagnosis and therapy. Autoimmun Rev. 2014;13(1):40‐48. 10.1016/j.autrev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 2. Knoebl P, Marco P, Baudo F, et al. Demographic and clinical data in acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). J Thromb Haemost. 2012;10(4):622‐631. 10.1111/j.1538-7836.2012.04654.x. [DOI] [PubMed] [Google Scholar]
- 3. Borg JY, Guillet B, Le Cam‐Duchez V, Goudemand J, Lévesque H. Outcome of acquired haemophilia in France: the prospective SACHA (Surveillance des Auto antiCorps au cours de lʼHémophilie Acquise) registry. Haemophilia. 2013;19(4):564‐570. 10.1111/hae.12138. [DOI] [PubMed] [Google Scholar]
- 4. Collins PW, Hirsch S, Baglin TP, et al. Acquired hemophilia A in the United Kingdom: a 2‐year national surveillance study by the United Kingdom Haemophilia Centre Doctors' Organisation. Blood. 2007;109(5):1870‐1877. www.iosrjournals.org. [DOI] [PubMed] [Google Scholar]
- 5. Tiede A, Klamroth R, Scharf RE, et al. Prognostic factors for remission of and survival in acquired hemophilia A (AHA): results from the GTH‐AH 01/2010 study. Blood. 2015;125(7):1091‐1098. 10.1182/blood-2014-07-587089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Napolitano M, Siragusa S, Mancuso S, Kessler CM. Acquired haemophilia in cancer: A systematic and critical literature review. Haemophilia. 2018;24(1):43‐56. 10.1111/hae.13355. [DOI] [PubMed] [Google Scholar]
- 7. Green D, Lechner K. A survey of 215 non‐hemophilic patients with inhibitors to factor VIII. Thromb Haemost. 1981;45(3):200‐203. [PubMed] [Google Scholar]
- 8. Tiede A, Hofbauer CJ, Werwitzke S, et al. Anti‐factor VIII IgA as a potential marker of poor prognosis in acquired hemophilia A: results from the GTH‐AH 01/2010 study. Blood. 2016;127(19):2289‐2297. 10.1182/blood-2015-09-672774. [DOI] [PubMed] [Google Scholar]
- 9. Duncan E, Collecutt M, Street A. Nijmegen‐Bethesda assay to measure factor VIII inhibitors Haemostasis. Methods in Molecular Biology (Methods and Protocols). Totowa, NJ: Humana Press; 2013:331‐333. [DOI] [PubMed] [Google Scholar]
- 10. Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser‐Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73(2):247‐251. [PubMed] [Google Scholar]
- 11. Meijer P, Verbruggen B. The between‐laboratory variation of factor VIII inhibitor testing: the experience of the external quality assessment program of the ECAT foundation. Semin Thromb Hemost. 2009;35(8):786‐793. 10.1055/s-0029-1245111. [DOI] [PubMed] [Google Scholar]
- 12. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119‐2126. 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 13. van Hemofiliebehandelaars NV. Richtlijn: diagnostiek en behandeling van hemofilie en aanverwante hemostasestoornissen. Alphen aan den Rijn: Van Zuiden; 2009. [Google Scholar]
- 14. Baudo F, Collins P, Huth‐Kühne A, et al. Management of bleeding in acquired hemophilia A: results from the European Acquired Haemophilia (EACH2) Registry. Blood. 2012;120(1):39‐46. 10.1182/blood-2012-02-408930. [DOI] [PubMed] [Google Scholar]
- 15. Collins P, Baudo F, Knoebl P, et al. Immunosuppression for acquired hemophilia A: results from the European Acquired Haemophilia Registry (EACH2). Blood. 2012;120(1):47‐55. 10.1182/blood-2012-02-409185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun B. Outcome of CARE: a 6‐year national registry of acquired haemophilia A in China. Br J Haematol. 2019;187:653‐665. 10.1111/bjh.16128. [DOI] [PubMed] [Google Scholar]
- 17. Jayakar J, OʼNeill N, Yan M, et al. Retrospective review of acquired haemophilia A from the largest Canadian haemophilia treatment centre. Haemophilia. 2018;24(5):383‐387. 10.1111/hae.13598. [DOI] [PubMed] [Google Scholar]
- 18. Mizrahi T, Doyon K, Dubé E, et al. Relapse pattern and long ‐ term outcomes in subjects with acquired haemophilia A. Haemophilia. 2019;25(2):252‐257. 10.1111/hae.13685. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa Y, Yanagisawa K, Uchiumi H, Ishizaki T, Mitsui T, Gouda F. Clinical characteristics and outcomes of acquired hemophilia A: experience at a single center in Japan. Int J Hematol. 2017;106(1):82‐89. 10.1007/s12185-017-2210-8. [DOI] [PubMed] [Google Scholar]
- 20. Delgado J, Jimenez‐Yuste V, Hernandez‐Navarro F, Villar A. Acquired haemophilia: review and meta‐analysis focused on therapy and prognostic factors. Br J Haematol. 2003;121:21‐35. [DOI] [PubMed] [Google Scholar]
- 21. Tiede A, Collins P, Knoebl P, et al. International recommendations on the diagnosis and treatment of acquired hemophilia A. Haematologica. 2020;105(7):1791‐1801. 10.3324/haematol.2019.230771. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement S1. Age distribution in AHA (N = 143).
Supplement S2. Bleeding site in AHA.
Supplement S3. Bleeding treatment and outcome.
Supplement S4. Disease course and outcome of IST in AHA.
Supplement S5. Outcome of AHA per underlying condition.
Supplement S6. Adverse events.
Supplement S7. Predictors of outcome in AHA by multivariate analysis.
Supplement S8. Baseline characteristics among first‐line immunosuppressive therapies.
Supplement S9. Comparison first‐line treatment immunosuppressive therapies.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


