Abstract
Objectives
This study aims to evaluate the efficacy of extracorporeal shock wave therapy (ESWT) in carpal tunnel syndrome (CTS) compared to the wrist splint treatment.
Patients and methods
Between April 2016 and March 2017, a total of 189 patients (22 males, 167 females, mean age 48.8±9.5 years, range, 24 to 70 years) with mild-to-moderate CTS were included in this double-blind, prospective, randomized, placebo-controlled study. The patients were divided into four treatment groups using stratified randomization: splint group (Group 1, n=47), splint+ESWT (Group 2, n=47), ESWT (Group 3, n=45), and splint+placebo ESWT (Group 4, n=50). All patients were evaluated at baseline, and one and three months. Pain using the Visual Analog Scale (VAS), finger pinch strength, Boston Carpal Tunnel Questionnaire (BCTQ), Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), and electrophysiological examination were assessed.
Results
A total of 168 patients completed the study. There was no significant difference among the four groups in terms of age, sex, comorbid diseases, symptom duration, VAS-pain, BCTQ, and LANSS scores (p>0.05). Pain and functionality significantly improved in all groups (p<0.05). In the group with ESWT and using wrist splint combined, a greater improvement of the hand function and electrophysiological measures was observed.
Conclusion
Our study results show that ESWT is a valuable and reliable treatment modality for mild-to-moderate CTS.
Keywords: Carpal tunnel syndrome, high-energy shock waves, neuropathic pain
Introduction
Carpal tunnel syndrome (CTS) develops as a result of median nerve compression and it is the most common entrapment neuropathy.[1] Paresthesia and pain in the first three fingers and the radial half of the fourth finger are often aggravated at night.[2] The incidence of CTS increases in diabetes, inflammatory arthritis, amyloidosis, hypothyroidism, wrist fractures, and pregnancy.[3]
Conservative treatments may be considered for mild and moderate CTS, while surgical options are more applicable for severe CTS.[4] Non-surgical treatment options are splinting, steroid injections, non- steroidal anti-inflammatory drugs (NSAIDs), vitamin B6 supplement, and physical therapy treatments such as therapeutic ultrasound, transcutaneous electrical nerve stimulation, contrast baths, tendon and nerve gliding exercises.[4]
High amplitude acoustic waves which focus on a region of the body are mentioned as extracorporeal shock wave therapy (ESWT).[5] The shock waves are characterized by high positive pressure up to 100 Megapascals (MPa), fast peak duration (10 to 30 nanosec), and short pulse duration (5 microsec). Two different effects of shock waves, direct and indirect have been reported. Positive pressure and short increase time are responsible for direct effect, causing high stress on substance interfaces, while tensile wave is responsible for indirect effect (cavitations). These waves can accelerate cell regeneration by increasing the permeability of the neuron cell membrane and neovascularization in the tissue. For analgesic mechanisms, theories such as cell membrane damage by free radicals, nociceptor blockade, and central control of sensory input have been proposed.[6] Radial extracorporeal shock wave therapy (rESWT) that are not focused with low energy have been used as an easier and more effective method for shock wave technology. They can be used in superficial tendinopathies and muscle disorders.[7] The ESWT is frequently applied for musculoskeletal disorders as lateral epicondylitis, nonunions, calcific tendinitis, Achilles tendinitis, plantar fasciitis, and patellar tendinitis, and beyond this, it has become a novel option in CTS patients.[5]
A limited number of studies with CTS patients which were about the efficacy of ESWT have been documented with favorable results.[8-13] However, these studies indicate no clear consensus about the density and frequency of shock wave energy. In the present study, we aimed to investigate the efficacy of ESWT based on functional, electrophysiological and clinical parameters including the neuropathic pain compared to wrist splint treatment.
Patients and Methods
This single-center, double-blind, prospective, randomized, placebo-controlled study was conducted at Physical Medicine and Rehabilitation outpatient clinic of Ankara Numune Training and Research Hospital between April 2016 and March 2017. Standard electrophysiological tests for on both hands were applied to the patients with CTS pre-diagnosis. A total of 189 (22 males, 167 females, mean age 48.8±9.5 years, range, 24 to 70 years) of 323 patients diagnosed with mild-to-moderate CTS were included. In addition, 134 were excluded before randomization according to study exclusion criteria. Exclusion criteria were as follows: cervical radiculopathy, brachial plexopathy, polyneuropathy and other upper extremity entrapment neuropathies, previous wrist fracture, cervical spinal and wrist surgeon history, steroid injection for CTS, wrist deformity preventing splint use, malign tumoral mass, thrombosis predisposition, <18 years old, pregnancy, and receiving dialysis treatment. All patients were evaluated three times in total: at baseline (pre-treatment) and at one and three months after treatment. A written informed consent was obtained from each patient. The study protocol was approved by the Ankara Numune Training and Research Hospital Ethics Committee (No. 829/2016). The study was conducted in accordance with the principles of the Declaration of Helsinki.
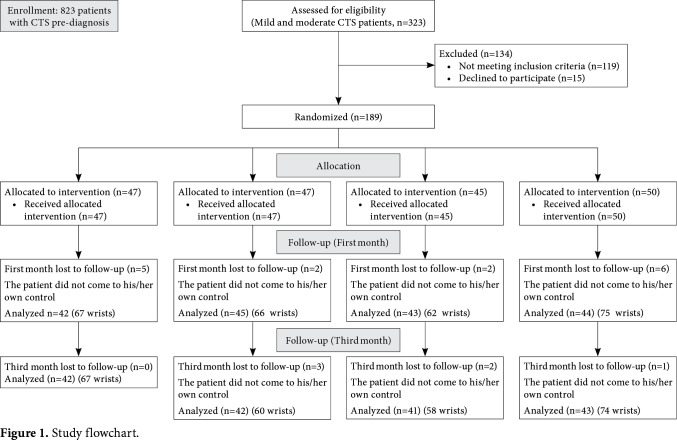
A total of 189 patients (295 wrists) were randomized to four groups by an independent researcher using stratified randomization method. In this randomization, the researcher specified stratification according to the factors (age, sex, and CTS severity) which may affect the outcomes of intervention. The patients were, then, assigned to intervention groups using a computer-generated randomization of study numbers as follows: wrist splint group (Group 1, n=47), splint+rESWT (Group 2, n=47), rESWT (Group 3, n=45), and splint+placebo rESWT (Group 4, n=50). The study flow chart is shown in Figure 1.
Figure 1. Study flowchart.
All interventions were carried out by a single physician who was blinded to the outcome measurements and randomization. Only the sound was heard without energy for placebo rESWT in Group 4 patients. All patients were informed about that they should not receive any other medical treatment such as analgesic, steroids, injections, or any alternative treatment method for pain and paresthesia due to CTS.
Wrist splint
In Groups 1, 2, and 4, a wrist splint with suitable size was advised to use every night and as much as possible during the day for three months.
Shock wave therapy
The patient was seated. The forearm and fingers were placed on the table with the palm facing up, and median nerve was found with musculoskeletal ultrasonography (USG) (LOGIQ® GE Healthcare Ultrasound, Korea). The rESWT was performed with the Vibrolith ESWT device (Elmed Medical Systems, Orlando, FL, USA) and the probe was located perpendicularly on the median nerve. The treatment area included to the proximal carpal tunnel at the level of the pisiform bone that was shown by transverse USG image. The rESWT was applied with 1,000 shots, 0.05 mJ/mm2 intensity of energy and frequency of 5 Hz. The rESWT was administered consecutively for three weeks, once a week for Group 2 and 3 patients. Pain medication and anesthesia were not needed, as there was no pain during treatment. All of the patients were evaluated for side effects and safety after each rESWT session. The patients were followed until the end of the study and no local tissue effects were reported. Complications such as pain, bleeding, or paresthesia were not seen in any of the patients.
Electrodiagnostic studies
Electrodiagnostic studies were performed with the Neuropack® 2-MEB 7102-K device (Nihon Kohden, Japan). Median and ulnar nerve motor and sensory nerve conduction studies were performed for all individuals by the same physician in a 25°C room, and skin temperature was maintained at 32.0 to 34.0°C. The recording electrodes were placed on the abductor pollicis brevis muscle and median nerve stimulated at wrist and ulnar side of brachial artery pulsation for median motor nerve conduction studies. Sensory nerve conduction values were obtained by orthodromic stimulation and were performed with stimulation from the palm and index finger and recording at the wrist. We used finger-wrist segment sensory conduction study values in our data, as this study has been shown to be the most sensitive electrophysiological examination in the CTS.[14]
Assessment measures
The patients were assessed three times in total: at the beginning and at one and three months. The Visual Analog Scale (VAS),[15] finger pinch strength, Boston Carpal Tunnel Questionnaire (BCTQ),[16,17] and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS)[18,19] were used. Electrophysiological examination was evaluated at baseline and three months.
The VAS scores are quantified digital pain and paresthesia in the last week, ranging from 0 to 10 (0: no pain/paresthesia, 10: most severe pain/ paresthesia).
For the finger pinch strength, a finger dynamometer (Baseline® hydraulic pinch gauge) was used. During the measurement, the patient was seated and the shoulder was placed in the adduction, the elbow in the 90° flexion, the forearm in the neutral position and the wrist in the 20 to 30° extension. The finger pinch was repeated three times and the average value was recorded.
The BCTQ consists of two parts: the symptom severity scale (BCTQs) with 11 questions and the functional capacity scale (BCTQf) with eight questions. Each scale score is calculated by the average values of the questions. The scores vary between one point to five point. High mean scores indicate severe symptoms and impaired functional capacity.
The LANSS pain scale is a useful test used in the differential diagnosis of neuropathic pain and nociceptive pain. The first part describes the experience with neuropathic pain (dysesthesia, autonomic dysfunction, excited pain, paroxysmal pain, thermal pain) with five questions. In the second part, allodynia with physical examination is tested by touching the painful and painless area with cotton. Pinprick sensation is also assessed in the same areas using a needle. The score ranges from 0 to 24. The test supports neuropathic pain in patients with a score of ≥12.
Statistical analysis
Study power analysis and sample size calculation were performed using the G*Power version 3.0.10 software (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany) to ensure the adequate sample size for one way analysis of variance (ANOVA): repeated measure between factors test. To obtain a power of 0.90 [α (Type I error) was 0.05 and β (Type II error) was 0.10, and four intervention groups with three repetitions] appropriate total sample size was 188 (n=47 in each group) for this study.
Statistical analysis was performed using the IBM SPSS for Windows version 20.0 software (IBM Corp., Armonk, NY, USA). Continuous variables were expressed in mean ± standard deviation or median (min-max), while categorical variables were expressed in number and frequency. The Shapiro-Wilk test was used to test normality. The chi-square test was used for comparison of nominal variables among the groups. Continuous variables were compared with ANOVA or Kruskal-Wallis analysis among four different treatment groups. The post-hoc tests (Bonferroni) were used to analyze which group was different from the other. Outcomes at three follow-up points were analyzed using two factors repeated-measures analysis of variance followed by post-hoc tests. A p value of <0.05 was considered statistically significant with 95% confidence interval (CI).
Results
Of 189 patients with mild-to-moderate CTS, 168 completed the study at the end of the third month. There were 93 (31.5%) mild and 202 (68.5%) moderate CTS patients and 113 (59.8%) of 189 patients were bilateral CTS. Baseline demographic and clinical characteristics of the patients are shown in Table 1. There was no significant difference among the groups in terms of age, sex, comorbid diseases, symptom duration, VAS pain, BCTQs, BCTQf, and LANSS scores (p>0.05).
Table 1. Baseline demographic and clinical characteristics of patients.
| Group 1 (n=47) | Group 2 (n=47) | Group 3 (n=45) | Group 4 (n=50) | P | |
| Age (year) | 48.1±10.1 | 48.4±10.1 | 50±8.6 | 48.5±9.8 | 0.325 |
| Sex | 0.298 | ||||
| Female n (%) | 40 (85.1) | 39 (83) | 41 (91.1) | 47 (94) | |
| Education duration (year) | 5.7±3.5 | 7.5±4.2 | 5.5±3.3 | 6.8±3.3 | 0.002* |
| Median (min-max) | 5 (0-5) | 6.50 (0-17) | 5 (0-13) | 5 (0-15) | |
| Education level | 0.101 | ||||
| Literate n (%) | 6 (12.8) | 5 (10.6) | 8 (17.8) | 3 (6.0) | |
| Primary school n (%) | 31 (66.0) | 20 (42.6) | 24 (53.3) | 27 (54.0) | |
| Secondary education n (%) | 8 (17.0) | 17 (36.2) | 12 (21.4) | 19 (38.0) | |
| University n (%) | 2 (4.3) | 5 (10.6) | 1 (2.2) | 1 (2.0) | |
| Comorbid disease | 0.940 | ||||
| Diabetes mellitus % | 15 | 15 | 8.8 | 22 | |
| Hypothyroidism % | 19.2 | 25.6 | 13.3 | 18 | |
| Hypertension % | 21.4 | 23.5 | 20 | 26 | |
| Symptom duration (month) | 22.2±26.9 | 33.7±38.1 | 23.5±27.3 | 24.8±31.5 | 0.152 |
| Median (min-max) | 12 (1-120) | 15 (1-120) | 18 (1-120) | 12 (1-120) | |
| VAS-night | 6.2±3 | 6.1±2.7 | 5.9±2.9 | 6.0±2.6 | 0.899 |
| Median (min-max) | 6(0-10) | 6.50 (0-10) | 6(0-10) | 6(0-10) | |
| VAS-day | 4.2±3.0 | 4.2±2.3 | 4.1±2.6 | 4.5±2.4 | 0.876 |
| Median (min-max) | 4(0-10) | 4 (0-9) | 4 (0-9) | 4(0-10) | |
| BCTQs | 2.6±0.9 | 2.5±0.7 | 2.5±0.9 | 2.5±0.7 | 0.914 |
| BCTQf | 2.2±1.0 | 2.3±0.7 | 2.2±0.8 | 2.5±0.7 | 0.480 |
| Finger pinch (kg) | 5.0±1.7 | 5.7±1.8 | 5.1±1.3 | 4.9±1.4 | 0.017# |
| LANSS | 9.9±6.8 | 9.1±5.9 | 9.6±6.2 | 9.5±6.0 | 0.900 |
| Neuropathic pain + ** n (%) | 30 (41.7) | 27 (38.6) | 26 (40.6) | 24 (29.3) | 0.361 |
| Group 1: Only wrist splint treatment; Group 2: rESWT + wrist splint treatment; Group3: Only rESWT; Group4: Placebo rESWT + wrist splint treatment; Min: Minimum; Max: Maximum; VAS: Visual Analog Scale, (0-10 cm); BCTQs: Boston Carpal Tunnel Symptom Severity Score; BCTQf: Boston Carpal Tunnel Functional Capacity Score; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs; * Difference between Group 1 and Group 2 p value=0.021; * Difference between Group 2 and Group 3 p value=0.006; # Difference between Group 2 and Group 4, p value=0.018; ** LANSS score ≥12. | |||||
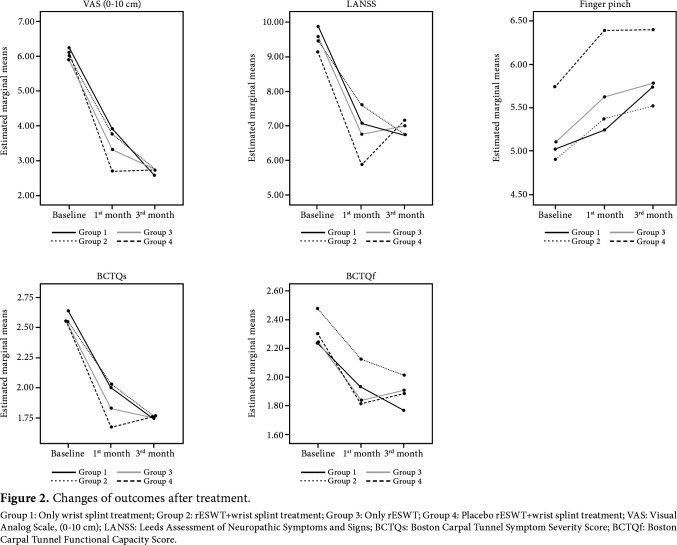
Table 2 shows the VAS, finger pinch, BCTQs, BCTQf, LANSS scores before and after treatment of the groups. All the assessments except for the finger pinch and LANSS showed a significant improvement in all four groups at one and three months, compared to baseline (p<0.05). The pinch strength showed a significant improvement in each measurement in Groups 2, 3, and 4 compared to baseline, while Group 1 did not significantly differ from the baseline at one month. These scores significantly improved at three months in Group 1.
Table 2. Clinical outcomes before and after treatment.
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
| Mean±SD | P | Mean±SD | P | Mean±SD | P | Mean±SD | P | |
| Pre-treatment | 6.0±3.0 | 5.9±2.8 | 5.8±2.8 | 5.7±2.9 | ||||
| 1st month | 3.9±3.0 | <0.001 | 2.6±2.2 | <0.001 | 3.3±2.8 | <0.001 | 3.8±2.5 | <0.001 |
| 3rd month | 2.6±3.1 | <0.001 | 2.7±3.5 | <0.001 | 2.8±3.0 | <0.001 | 2.7±2.9 | <0.001 |
| VAS-day | 4.0±3.0 | 4.1±2.4 | 4.0±2.4 | 4.3±2.5 | ||||
| 1st month | 2.9±2.5 | <0.001 | 2.2±1.7 | <0.001 | 2.3±1.9 | <0.001 | 3.4±2.6 | <0.001 |
| 3rd month | 2.4±3.0 | <0.001 | 2.4±2.6 | <0.001 | 2.0±2.1 | <0.001 | 2.1±2.2 | <0.001 |
| Finger pinch (kg) | 4.8±1.7 | 5.5±1.9 | 5.0±1.3 | 4.7±1.6 | ||||
| 1st month | 5.3±1.6 | 0.083 | 6.2±2.3 | <0.001 | 5.6±1.5 | <0.001 | 5.4±1.6 | <0.001 |
| 3rd month | 5.7±1.7 | <0.001 | 6.4±2.0 | <0.001 | 5.8±1.4 | <0.001 | 5.5±1.6 | <0.001 |
| BCTQs | 2.6±0.9 | 2.6±0.7 | 2.6±0.9 | 2.5±0.8 | ||||
| 1st month | 2.0±0.8 | <0.001 | 1.7±0.5 | <0.001 | 1.8±0.7 | <0.001 | 2.0±0.8 | <0.001 |
| 3rd month | 1.7±0.7 | <0.001 | 1.8±0.7 | <0.001 | 1.8±0.7 | <0.001 | 1.8±0.8 | <0.001 |
| BCTQf | 2.3±0.9 | 2.3±0.7 | 2.3±0.7 | 2.5±0.7 | ||||
| 1st month | 1.9±0.8 | <0.001 | 1.9±0.6 | <0.001 | 1.9±0.7 | <0.001 | 2.1±0.7 | <0.001 |
| 3rd month | 1.8±0.8 | <0.001 | 1.9±0.7 | <0.001 | 1.9±0.8 | <0.001 | 2.0±0.7 | <0.001 |
| LANSS | 10.1±6.7 | 9.3±6.5 | 9.9±6.4 | 9.7±6.0 | ||||
| 1st month | 7.1±6.6 | 0.003 | 6.1±5.8 | <0.001 | 6.8±6.2 | 0.003 | 7.5±6.8 | 0.007 |
| 3rd month | 6.8±7.1 | 0.006 | 7.6±6.1 | 0.126 | 7.5±6.8 | 0.026 | 7.0±6.5 | 0.001 |
| Group 1: Only wrist splint treatment; Group 2: rESWT + wrist splint treatment; Group 3: Only rESWT; Group 4: Placebo rESWT + wrist splint treatment; SD: Standard deviation; VAS: Visual Analog Scale, (0-10 cm); BCTQs: Boston Carpal Tunnel Symptom Severity Score; BCTQf: Boston Carpal Tunnel Functional Capacity Score; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs. N values for groups were for 1st month: Group 1 n=42 (67 wrists), Group 2 n=45 (66 wrists), Group 3 n=43 (62 wrists), Group 4 n=44 (75 wrists) N values for groups were for 3rd month: Group 1 n=42 (67 wrists), Group 2 n=42 (60 wrists), Group 3 n=41 (58 wrists), Group 4 n=43 (74 wrists) | ||||||||
The improvements in clinical variables were compared among the four groups. There was no significant difference in all clinical variables except for finger pinch. In Group 2, baseline - first-month finger pinch increase was higher than Groups 1 and 4, and the baseline - third-month finger pinch increase was higher than Group 4 (Table 3). Figure 2 shows the improvements of outcomes.
Table 3. Changes in outcome measurements from baseline to first and third months.
| Group 1 | Group 2 | Group 3 | Group 4 | P | |
| Mean difference±SD | Mean difference±SD | Mean difference±SD | Mean difference±SD | ||
| VAS-night Baseline-1stmonth |
-2.3±0.4 | -3.4±0.4 | -2.6±0.4 | -2.2±0.3 | >0.05 |
| Baseline-3rdmonth | -3.7±0.4 | -3.4±0.6 | -3.1±0.4 | -3.3±0.4 | >0.05 |
| VAS-day Baseline-1stmonth |
-1.3±0.3 | -1.9±0.2 | -1.8±0.3 | -1.1±0.2 | >0.05 |
| Baseline-3rdmonth | -1.8±0.4 | -1.8±0.4 | -2.1±0.4 | -2.4±0.3 | >0.05 |
| Finger pinch (kg) Baseline-1stmonth |
0.2±0.1 | 0.6±0.1 | 0.5±0.1 | 0.5±0.1 | <0.05* |
| Baseline-3rdmonth | 0.7±0.2 | 0.7±0.1 | 0.7±0.1 | 0.6±0.1 | <0.05** |
| BCTQs Baseline-1stmonth |
-0.06±0.1 | -0.9±0.1 | -0.7±0.1 | -0.5±0.1 | >0.05 |
| Baseline-3rdmonth | -0.9±0.1 | -0.8±0.1 | -0.8±0.1 | -0.8±0.1 | >0.05 |
| BCTQf Baseline-1stmonth |
-0.3±0.1 | -0.5±0.1 | -0.4±0.1 | -0.4±0.1 | >0.05 |
| Baseline-3rdmonth | -0.5±0.1 | -0.4±0.1 | -0.3±0.1 | -0.5±0.1 | >0.05 |
| LANSS | |||||
| Baseline-1stmonth | -2.8±0.8 | -3.3±0.8 | -2.8±0.8 | -1.9±0.6 | >0.05 |
| Baseline-3rdmonth | -3.1±1.0 | -2.0±1.0 | -2.6±1.0 | -2.7±0.7 | >0.05 |
|
Group 1: Only wrist splint treatment; Group 2: rESWT + wrist splint treatment; Group 3: Only rESWT, Group 4: Placebo rESWT + wrist splint treatment, n: number of patients; SD: Standard deviation; VAS: Visual Analog Scale, (0-10 cm); BCTQs: Boston Carpal Tunnel Symptom Severity Score; BCTQf: Boston Carpal Tunnel Functional Capacity Score; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs; *Baseline-first month change in finger pinch: difference between group 2 and 1 p=0.031; difference between group 2 and 4 p value=0.019; ** Baseline-third month change in finger pinch: difference between group 2 and 4; p value=0.006. N values for groups were for 1st month: Group 1 n=42 (67 wrists), Group 2 n=45 (66 wrists), Group 3 n=43 (62 wrists), Group 4 n=44 (75 wrists) N values for groups were for 3rd month: Group 1 n=42 (67 wrists), Group 2 n=42 (60 wrists), Group 3 n=41 (58 wrists), Group 4 n=43 (74 wrists) | |||||
Figure 2. Changes of outcomes after treatment. Group 1: Only wrist splint treatment; Group 2: rESWT+wrist splint treatment; Group 3: Only rESWT; Group 4: Placebo rESWT+wrist splint treatment; VAS: Visual Analog Scale, (0-10 cm); LANSS: Leeds Assessment of Neuropathic Symptoms and Signs; BCTQs: Boston Carpal Tunnel Symptom Severity Score; BCTQf: Boston Carpal Tunnel Functional Capacity Score.
Nerve conduction studies were performed at baseline and three months (Table 4). In Group 1, the median nerve motor amplitude (mMNA) value increased after the treatment. In Group 2, median nerve motor conduction velocity (mMNCV) increased, while median nerve sensory distal latency (mSNDL) shortened after treatment. In Group 4, mSNDL was found to be shortened.
Table 4. Nerve conduction studies at baseline and three months.
| Group 1 | Group 2 | Group 3 | Group 4 | |||||
| Mean±SD | p | Mean±SD | p | Mean±SD | p | Mean±SD | p | |
| mMNDL (msec) | 0.57 | 0.58 | 0.93 | 0.38 | ||||
| Baseline | 4.1±0.7 | 4.3±0.7 | 4.1±0.7 | 4.1±0.7 | ||||
| 3rd month | 4.1±1.1 | 4.3±0.7 | 4.1±0.7 | 4.0±0.8 | ||||
| mMNA(mV) | 0.03* | 0.16 | 0.14 | 0.73 | ||||
| Baseline | 10.5 ±3.5 | 10.4±3.7 | 10.2±3.7 | 11.3±4.4 | ||||
| 3rd month | 11.5±3.8 | 11.3±4.5 | 11.1±3.5 | 11.5±3.9 | ||||
| mMNCV (m/sec) | 0.29 | <0.01* | 0.78 | 0.64 | ||||
| Baseline | 56.9±4.6 | 54.6±3.8 | 56.0±4.7 | 55.1±3.7 | ||||
| 3rd month | 56.2±3.0 | 55.8±3.9 | 55.8±3.9 | 54.8±3.7 | ||||
| mSNDL (msec) | 0.08 | 0.01* | 0.16 | 0.02* | ||||
| Baseline | 3.5±0.5 | 3.7±0.6 | 3.5±0.5 | 3.4±0.6 | ||||
| 3rd month | 3.3±1.0 | 3.4±1.0 | 3.4±0.8 | 3.2±0.8 | ||||
| mSNA (gV) | 0.19 | 0.02* | 0.51 | 0.22 | ||||
| Baseline | 13.8±7.1 | 12.8±7.2 | 12.2±5.6 | 12.3±5.9 | ||||
| 3rd month | 12.6±7.1 | 11.1±5.7 | 11.8±6.2 | 13.12±5.6 | ||||
| mSNCV (m/sec) | 0.13 | 0.40 | 0.92 | 0.5 | ||||
| Baseline | 34.6±4.5 | 32.8±5.4 | 33.5±4.5 | 35.1±5.2 | ||||
| 3rd month | 33.0±10.1 | 32.1±9.2 | 33.4±7.8 | 34.5± 9.0 | ||||
| Group 1: Only wrist splint treatment; Group 2: rESWT+wrist splint treatment; Group 3: Only rESWT; Group 4: Placebo rESWT+wrist splint treatment; SD: Standard deviation; mMNDL: Median verve motor distal latency; mMNA: Median motor nerve amplitude; mMNCV: Median motor nerve conduction velocity; mSNDL: Median sensory nerve (2. Finger-wrist) distal latency; mSNA: Median sensory nerve (2. Finger-wrist) amplitude; mSNCV: Median sensory nerve (2. Finger-wrist) conduction velocity; msec: Milisecond; mV: Milivolt; m/sec: Meter/second; pV: Microvolt; * P value<0.05. N values for groups were for 1s month: Group 1 n=42 (67 wrists), Group 2 n=45 (66 wrists), Group 3 n=43 (62 wrists), Group 4n=44 (75 wrists) N values for groups were for 3rd month: Group 1 n=42 (67 wrists), Group 2 n=42 (60 wrists), Group 3 n=41 (58 wrists), Group 4n=43 (74 wrists) | ||||||||
Changes in the nerve conduction studies were compared among the four groups. In Group 2, the increase in mMNCV was higher than Group 1. The results are summarized in Table 5.
Table 5. Changes in outcome measurements from baseline to third month.
| Group 1 | Group 2 | Group 3 | Group 4 | p | |
| Mean difference±SD | Mean difference±SD | Mean difference±SD | Mean difference±SD | ||
| mMNDL (msec) Baseline-3rdmonth |
0.1±0.1 | 0.0±0.1 | 0.0±0.1 | -0.1±0.1 | 0.180 |
| mMNA(mV) Baseline-3rdmonth |
1.0±0.5 | 0.9±0.7 | -0.8±0.6 | 0.2±0.6 | 0.563 |
| mMNCV (m/sec) Baseline-3rdmonth |
0.2±0.1 | 1.2±0.4 | -0.2±0.6 | -0.3±0.6 | 0.026* |
| mSNDL (msec) Baseline-3rdmonth |
-0.7±0.1 | -0.3±0.1 | -0.2±0.1 | -0.3±0.1 | 0.077 |
| mSNA (gV) Baseline-3rdmonth |
-1.2±0.9 | -1.7±0.7 | -0.5±0.7 | 0.8±0.7 | 0.555 |
| mSNCV (m/sec) Baseline-3rdmonth |
-1.6±1.1 | -0.7±1.0 | -0.1±0.7 | -0.6±0.8 | 0.222 |
| Group 1: Only wrist splint treatment; Group 2: rESWT+wrist splint treatment; Group 3: Only rESWT; Group 4: Placebo rESWT+wrist splint treatment; SD: Standard deviation; mMNDL: Median verve motor distal latency; mMNA: Median motor nerve amplitude; mMNCV: Median motor nerve conduction velocity; mSNDL: Median sensory nerve (2. Finger-wrist) distal latency; mSNA: Median sensory nerve (2. Finger-wrist) amplitude; mSNCV: Median sensory nerve (2. Finger-wrist) conduction velocity; msec: Milisecond; mV: Milivolt; m/sec: Meter/second; pV: Microvolt; * Difference between Group 1 and 2 p value=0.026; Difference between Group 1 and 4 p value=0.005. N values for groups were for 1st month: Group 1 n=42 (67 wrists), Group 2 n=45 (66 wrists), Group 3 n=43 (62 wrists), Group 4 n=44 (75 wrists) N values for groups were for 3rd month:Group 1 n=42 (67 wrists), Group 2 n=42 (60 wrists), Group 3 n=41 (58 wrists), Group 4 n=43 (74 wrists) | |||||
Discussion
In the current study, we attempted to explore the effect of rESWT for CTS treatment. To the best of our knowledge, this prospective, randomized, placebo- controlled study was conducted with the largest number of patients on this subject. Our results indicated that rESWT reduced pain and disability. On the other hand, when wrist splint was used together with rESWT, the improvement of the hand function and mMNCV increase was higher than the splint or rESWT alone.
The low-energy ESWT was applied to the nerve tissue in animal experiments and contributed functional improvement without any side effects.[20,21] Current studies have proven that high-density ESWT is harmless to peripheral nerves, and reduced peripheral nerve motor conduction velocity has been shown to resolve within two weeks without any strength loss and functional failure.[22,23] Neurological complications related to ESWT have not been described in the literature. In recent years, the ESWT has emerged as an alternative treatment for peripheral neuropathies such as interdigital neuroma, distal symmetric polyneuropathy and CTS.[8-13,24,25]
Currently, standard guidelines for the application frequency and total dose for the use of ESWT in CTS patients have not been published. There are studies in the literature with single sessions, three sessions, and four sessions. It has been shown that single-session ESWT has a short-term effect.[12] There is no study comparing three and four sessions of ESWT. Since most of the studies were conducted with three-session ESWT, we preferred three-session rESWT in our study.[8,12]
The energy intensity, total shots, and frequency of ESWT application have shown diversity depending on the number of sessions.[8-13] Reported pulse repetition frequency varies between 3 Hz and 5 Hz[8-13] and practice with 5 Hz is more common.[8,9,12] Studies have demonstrated the intensity of energy and total shots in a range of 0.03 mJ/mm2 to 0.15 mJ/mm2 and 800 to 2,500 shots, respectively. In the aforementioned studies, the ESWT group benefited clinically from all applications.[8-13] In the present study, the more frequently used applications in literature was preferred and rESWT was applied with 1,000 shots, 0.05 mJ/mm2 intensity of energy, and frequency of 5 Hz.
Most of the studies show the application area for ESWT by visualizing the median nerve with USG.[8-10,12] To the best of our knowledge, there are two studies utilizing no USG.[11,13] The location of the median nerve was confirmed by USG and, then, ESWT was performed in our study.
Romeo et al.[26] used three sessions of ESWT in 40 patients who had CTS surgery and whose pain continued three months later. They found a significant decrease in pain, edema, and redness scores of the patients. The authors concluded that ESWT was a valuable, reliable, and non-invasive modality for reducing postoperative symptoms. The efficacy of ESWT in patients with CTS as the primary treatment was first reported by Seok et al.[9] In this study comparing ESWT with steroid injection, ESWT was shown to be as effective as corticosteroid injection in terms of pain and functional improvement, but improvement of nerve conduction studies was only observed in the injection group.[9] In a study evaluating conservative treatment options for CTS, oral nutraceutical capsule treatments and ESWT were compared in two groups and patients were followed for six months.[10] In both groups pain, function, mMNDL, and mSNCV were improved at six months. Paoloni et al.[13] compared ESWT, ultrasound, and cryo-ultrasound in patients with CTS, and there was an improvement in pain and function in all three groups; however, the symptomatic improvement was higher in the ESWT group than in the other two groups. In the study of Wu et al.,[8] rESWT and placebo rESWT were compared and all of the patients were also used wrist splint. Both intervention and control group had an improvement in pain, function, and mSNCV. The pain and Boston scores had a higher improvement in the intervention group. Vahdatpour et al.[11] divided patients into two groups and one group received four sessions ESWT. All patients included in the study were treated with wrist splint for three months, B1 vitamin supplement for one month, and NSAID treatment for two weeks. Both groups showed an improvement in symptoms, function, and nerve conduction studies in the first three months. However, the decrease of symptoms in the ESWT group was significant at six months.[11] In a study investigating the number of rESWT sessions in CTS, the patients were divided into three groups: the first group was a single-session rESWT group, the second group was a three-session rESWT group, and the third group was placebo rESWT group.[12] In addition, a night wrist splint was given to all participants. Three-session rESWT group showed a greater functional improvement compared to the other two groups.
In the literature, studies investigating the efficacy of ESWT in the treatment of CTS showed improved pain and function after ESWT.[8-12] In our study, unlike previous studies, only rESWT was performed without wrist splint in one of the four treatment groups, and this group had reduced pain and improved functional scores as least as the patients with wrist splint treatment. In the group with splint and rESWT together, the increase in finger pinch was better than the other groups at one and three months. Previous reports showed improvements in terms of mSNCV and mMNDL at three months.[8-12] In the present study, when the four groups were compared with each other, the group receiving splint combined with rESWT had the highest mMNCV increase. Wrist splints are known as the most popular treatment modality with moderate evidence for CTS treatment. Our results indicated that alone rESWT was as effective as splint therapy, and rESWT provided more functional enhancement and improvement in nerve conduction studies, when used with wrist splint. This finding suggests that rESWT should not be ignored in the conservative treatment of CTS.
Peripheral and central sensitization mechanisms have been described in patients with CTS. Repeated sensory stimuli after peripheral injury have been shown to cause changes in the dorsal horn and neuroplastic reorganization. The presence of neuropathic pain varies between 47.6 and 65% in patients with CTS.[27-29] In our study, neuropathic pain was detected in 37.2% of the patients. Decreased LANSS scores after hand wrist splint and/or rESWT treatment were observed. Based on these findings, we can speculate that conservative treatments are useful in neuropathic complaints, such as other symptoms for CTS patients.
The main limitation of the present study is the lack of a treatment group receiving only placebo rESWT, as in previous studies in the literature. In our study, wrist splint was used in the placebo rESWT group not to leave CTS patients untreated. Of note, four treatment groups were created to obviate this limitation.
In conclusion, rESWT is a valuable and practical treatment modality without serious side effects. It also reduces pain, neuropathic symptoms, disability, and improves electrophysiological findings in patients with mild-to-moderate CTS.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Papanicolaou GD, McCabe SJ, Firrell J. The prevalence and characteristics of nerve compression symptoms in the general population. J Hand Surg Am. 2001;26:460–466. doi: 10.1053/jhsu.2001.24972. [DOI] [PubMed] [Google Scholar]
- 2.Bouhassira D, Attal N. Diagnosis and assessment of neuropathic pain: the saga of clinical tools. S74-83Pain. 2011;152(3 Suppl) doi: 10.1016/j.pain.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Alfonso C, Jann S, Massa R, Torreggiani A. Diagnosis, treatment and follow-up of the carpal tunnel syndrome: a review. Neurol Sci. 2010;31:243–252. doi: 10.1007/s10072-009-0213-9. [DOI] [PubMed] [Google Scholar]
- 4.Uchiyama S, Itsubo T, Nakamura K, Kato H, Yasutomi T, Momose T. Current concepts of carpal tunnel syndrome: pathophysiology, treatment, and evaluation. J Orthop Sci. 2010;15:1–13. doi: 10.1007/s00776-009-1416-x. [DOI] [PubMed] [Google Scholar]
- 5.Baloğlu İ, Özsoy MH, Aydınok H, Lök V. Ortopedi ve Travmatolojide Şok Dalga Tedavisi. TOTBİD Dergisi. 2005;4:33–49. [Google Scholar]
- 6.Dıraçoğlu D. Kas-iskelet sistemi hastalıklarında ekstrakorporal şok dalga tedavisi. Turkiye Klinikleri J PM&R. 2004;4:106–104. [Google Scholar]
- 7.Gerdesmeyer L, Frey C, Vester J, Maier M, Weil L Jr, Weil L Sr, et al. Radial extracorporeal shock wave therapy is safe and effective in the treatment of chronic recalcitrant plantar fasciitis: results of a confirmatory randomized placebo- controlled multicenter study. Am J Sports Med. 2008;36:2100–2109. doi: 10.1177/0363546508324176. [DOI] [PubMed] [Google Scholar]
- 8.Wu YT, Ke MJ, Chou YC, Chang CY, Lin CY, Li TY, et al. Effect of radial shock wave therapy for carpal tunnel syndrome: A prospective randomized, double-blind, placebo-controlled trial. J Orthop Res. 2016;34:977–984. doi: 10.1002/jor.23113. [DOI] [PubMed] [Google Scholar]
- 9.Seok H, Kim SH. The effectiveness of extracorporeal shock wave therapy vs. local steroid injection for management of carpal tunnel syndrome: a randomized controlled trial. Am J Phys Med Rehabil. 2013;92:327–334. doi: 10.1097/PHM.0b013e31826edc7b. [DOI] [PubMed] [Google Scholar]
- 10.Notarnicola A, Maccagnano G, Tafuri S, Fiore A, Pesce V, Moretti B. Comparison of shock wave therapy and nutraceutical composed of Echinacea angustifolia, alpha lipoic acid, conjugated linoleic acid and quercetin (perinerv) in patients with carpal tunnel syndrome. Int J Immunopathol Pharmacol. 2015;28:256–262. doi: 10.1177/0394632015584501. [DOI] [PubMed] [Google Scholar]
- 11.Vahdatpour B, Kiyani A, Dehghan F. Effect of extracorporeal shock wave therapy on the treatment of patients with carpal tunnel syndrome. Adv Biomed Res. 2016;5:120–120. doi: 10.4103/2277-9175.186983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke MJ, Chen LC, Chou YC, Li TY, Chu HY, Tsai CK, et al. The dose-dependent efficiency of radial shock wave therapy for patients with carpal tunnel syndrome: a prospective, randomized, single-blind, placebo-controlled trial. Sci Rep. 2016;6:38344–38344. doi: 10.1038/srep38344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoloni M, Tavernese E, Cacchio A, D’orazi V, Ioppolo F, Fini M, et al. Extracorporeal shock wave therapy and ultrasound therapy improve pain and function in patients with carpal tunnel syndrome. A randomized controlled trial. Eur J Phys Rehabil Med. 2015;51:521–528. [PubMed] [Google Scholar]
- 14.Oh SJ, editor , editors. Clinical electromyography: nerve conduction studies. 3. Baltimore: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 15.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 16.Levine DW, Simmons BP, Koris MJ, Daltroy LH, Hohl GG, Fossel AH, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg [Am] 1993;75:1585–1592. doi: 10.2106/00004623-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Sezgin M, Incel NA, Serhan S, Camdeviren H, As I, Erdoğan C. Assessment of symptom severity and functional status in patients with carpal tunnel syndrome: reliability and functionality of the Turkish version of the Boston Questionnaire. Disabil Rehabil. 2006;28:1281–1285. doi: 10.1080/09638280600621469. [DOI] [PubMed] [Google Scholar]
- 18.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 19.Yucel A, Senocak M, Kocasoy Orhan E, Cimen A, Ertas M. Results of the Leeds assessment of neuropathic symptoms and signs pain scale in Turkey: a validation study. J Pain. 2004;5:427–432. doi: 10.1016/j.jpain.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Kim SG. Effects of extracorporeal shock wave therapy on functional recovery and neurotrophin-3 expression in the spinal cord after crushed sciatic nerve injury in rats. Ultrasound Med Biol. 2015;41:790–796. doi: 10.1016/j.ultrasmedbio.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Fu M, Cheng H, Li D, Yu X, Ji N, Luo F. Radial shock wave therapy in the treatment of chronic constriction injury model in rats: a preliminary study. Chin Med J (Engl) 2014;127:830–834. [PubMed] [Google Scholar]
- 22.Wu YH, Liang HW, Chen WS, Lai JS, Luh JJ, Chong FC. Electrophysiological and functional effects of shock waves on the sciatic nerve of rats. Ultrasound Med Biol. 2008;34:1688–1696. doi: 10.1016/j.ultrasmedbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Wu YH, Lun JJ, Chen WS, Chong FC. The electrophysiological and functional effect of shock wave on peripheral nerves. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:2369–2372. doi: 10.1109/IEMBS.2007.4352803. [DOI] [PubMed] [Google Scholar]
- 24.Fridman R, Cain JD, Weil L Jr. Extracorporeal shockwave therapy for interdigital neuroma: a randomized, placebo- controlled, double-blind trial. J Am Podiatr Med Assoc. 2009;99:191–193. doi: 10.7547/0980191. [DOI] [PubMed] [Google Scholar]
- 25.Lohse-Busch H, Marlinghaus E, Reime U, Möwis U. Focused low-energy extracorporeal shock waves with distally symmetric polyneuropathy (DSPNP): a pilot study. NeuroRehabilitation. 2014;35:227–233. doi: 10.3233/NRE-141116. [DOI] [PubMed] [Google Scholar]
- 26.Romeo P, d’Agostino MC, Lazzerini A, Sansone VC. Extracorporeal shock wave therapy in pillar pain after carpal tunnel release: a preliminary study. Ultrasound Med Biol. 2011;37:1603–1608. doi: 10.1016/j.ultrasmedbio.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Gürsoy AE, Kolukısa M, Yıldız GB, Kocaman G, Celebi A, Koçer A. Relationship between electrodiagnostic severity and neuropathic pain assessed by the LANSS pain scale in carpal tunnel syndrome. Neuropsychiatr Dis Treat. 2013;9:65–71. doi: 10.2147/NDT.S38513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truini A, Padua L, Biasiotta A, Caliandro P, Pazzaglia C, Galeotti F, et al. Differential involvement of A-delta and A-beta fibres in neuropathic pain related to carpal tunnel syndrome. Pain. 2009;145:105–109. doi: 10.1016/j.pain.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 29.Myers RR. 1994 ASRA Lecture. The pathogenesis of neuropathic pain. Reg Anesth. 1995;20:173–184. [PubMed] [Google Scholar]