Abstract
Background
Despite recent therapeutic advances, life expectancy in persons with congenital hemophilia A (PwcHA) remains below that of the non‐HA population. As new therapies are introduced, a uniform approach to the assessment of mortality is required for comprehensive evaluation of risk–benefit profiles, timely identification of emerging safety signals, and comparisons between treatments.
Objectives
Develop and test a framework for consistent reporting and analysis of mortality across past, current, and future therapies.
Patients/Methods
We identified known causes of mortality in PwcHA through literature review, analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS) database, and expert insights. Leading causes of death in general populations are those recognized by the Centers for Disease Control and Prevention and the World Health Organization. We developed an algorithm for assessing fatalities in PwcHA and used this to categorize FAERS data as a proof of concept.
Results
PwcHA share mortality causes with the non‐HA population including cardiovascular disease, malignancy, infections, pulmonary disease, dementias, and trauma/suicide. Causes associated with HA include hemorrhage, thrombosis, human immunodeficiency virus, hepatitis C virus, and liver dysfunction. We propose an algorithm employing these classes to categorize fatalities and use it to classify FAERS fatality data between 01/01/2000 and 03/31/2020; the most common causes were hemorrhage (22.2%) and thrombosis (10.4%).
Conclusions
A conceptual framework for examining mortality in PwcHA receiving any hemophilia therapy is proposed to analyze and interpret fatalities, enabling consistent and objective assessment. Application of the framework using FAERS data suggests a generally consistent pattern of reported mortality across HA treatments, supporting the utility of this unified approach.
Keywords: algorithms, cause of death, data analysis, hemophilia A, mortality
Essentials.
Uniform reporting of mortality in persons with congenital hemophilia A (PwcHA) is vital.
The authors have developed and tested a framework for mortality reporting in PwcHA.
Application of the framework to fatality data provides first proof of concept.
We propose application of this framework to enable consistent assessment of mortality in PwcHA.
1. BACKGROUND
People with congenital hemophilia A (PwcHA) are reported to have a shorter life expectancy than that of the general population. 1 , 2 , 3 This is partially due to the residual effects of contamination of blood products with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) that were used for the treatment of hemophilia during the 1980s, 4 and partially due to a higher rate of hemorrhagic death associated with hemophilia A (HA) compared with the general population. 5 Furthermore, up to one third of people with severe congenital HA develop factor VIII (FVIII) inhibitors, rendering them at a higher risk of severe bleed‐related complications known to be associated with shorter life expectancy. 6 Management of PwcHA with FVIII inhibitors is associated with poorer outcomes due to the reduced efficacy of bypassing agents (BPAs) in the treatment and prevention of bleeds, their pharmacokinetic properties, burdensome administration, 7 and their pro‐thrombotic risk. 8 , 9
A recent systematic literature review noted that mortality rates and ratios in PwcHA have generally decreased over the past several decades. 5 The review also found that the most common causes of death changed over time, reflecting the diminishing effect of HIV/HCV infections and generally increasing life expectancy. 10 , 11 A study of causes of death in PwcHA in the Netherlands found that approximately 80% of deaths from acquired immunodeficiency syndrome (AIDS) occurred before 1995, when effective antiviral treatment for HIV first became available. 1 Similarly, antiviral therapies for people with HCV are now available, and offer sustained virologic responses or cure in >95% of patients. 12 However, prior to their availability, some PwcHA may have experienced irreversible liver disease with progression to cirrhosis and hepatocellular carcinoma (HCC). 13 The increasing life expectancy in PwcHA is associated with a shift in causes of death toward those associated with aging in the general population, such as cardiovascular disease (CVD) and malignancy. The literature review also highlighted that causes of death reported among PwcHA varied across populations, countries, and time. Similarly, reporting of cause of death within post‐marketing reports is also highly heterogeneous. Furthermore, spontaneous post‐marketing reports of adverse events (AEs) often lack key clinical contextual details surrounding the cause of death, which makes the interpretation of such safety data difficult at best.
The HA treatment landscape is rapidly evolving with several different therapies approved or under investigation. Novel treatments include emicizumab, a bispecific, humanized, monoclonal antibody that bridges activated factor IX (FIX) and factor X (FX), thereby restoring the function of missing activated FVIII in PwcHA; 14 it is approved for PwcHA of all ages regardless of FVIII inhibitor status. Other non‐factor replacement therapies still in development include: a ribonucleic acid (RNA) interference molecule targeting antithrombin, 15 a monoclonal antibody targeting tissue factor pathway inhibitor, 16 and a bioengineered serine protease inhibitor that targets activated protein C. 17 Adeno‐associated virus (AAV) gene therapies for HA, which promise continuous endogenous expression of FVIII through transfer of a functional B‐domain‐deleted FVIII gene, are also in development. 18 , 19 While newer agents offer the prospect of improved efficacy, 15 , 16 , 20 improved quality of life, 21 and decreased treatment burden, 21 safety experience in the real world is less developed. Moreover, given their different mechanisms of action, their safety profiles may differ from those of traditional coagulation therapies. 22 As new therapies and iterations of current factor replacement therapeutic approaches enter the market, it is reasonable to expect a further evolution in mortality, including shifts in the causes of mortality, together with improvements in patient quality of life and life expectancy.
These points demonstrate the need for a unified approach to reporting fatal events and causes of death to better understand mortality in PwcHA. This approach could ultimately enable improved risk–benefit assessments and allow monitoring of the beneficial impact of new therapies on the HA community as disease management advances. As such, a globally applicable conceptual framework for cross‐examining mortality in PwcHA that can be used to fully and consistently report, analyze, and interpret fatalities for all hemophilia therapies is needed. Here we aim to develop a mortality framework, integrating current data and medical practice pertaining to fatal events in PwcHA, which can be used to aid reporting and classification retrospectively, currently, and into the future. We then assess the utility of this framework by applying it to causes of death in PwcHA contemporaneous to coagulation product use, as reported to the United States Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) database.
2. METHODS
2.1. Development of the framework
Causes of mortality in PwcHA were compiled from several sources. First, a prospective systematic literature review was undertaken. 5 A comprehensive search of Medline, Embase, the Cochrane Central Register of Controlled Studies, clinicaltrials.gov, and published conference abstracts was conducted on February 5, 2020 using the search terms h(a)emophilia, therapy, mortality, or cause of death to identify studies published between January 2010 and February 2020. The search was updated on March 17, 2020 to include observational studies that did not mention specific therapies. Second, the FAERS database was analyzed for causes of fatality in people with HA treated with FDA‐approved coagulation products reported to FAERS in the last 20 years. Last, clinical insights from practicing doctors in the United States, Italy, UK, and South Africa, and insights from hemophilia patient support organizations were collected. The leading causes of death in the United States and worldwide general (non‐hemophilia) populations were sourced from the Centers for Disease Control and Prevention (CDC) 23 and the World Health Organization (WHO), respectively. 24
This information was collated and used to develop an algorithm for assessing fatalities in PwcHA. Causes of mortality were categorized into HA‐associated and non‐HA‐associated causes, and further grouped into subcategories that reflected the leading causes of death for the general population and those most relevant to PwcHA.
2.2. Application of the framework using the FAERS database
The FAERS Public Dashboard is a web‐based tool allowing searches of AE reports, medication error reports, and product quality complaints resulting in AEs from the United States and worldwide. While useful as a data source for spontaneous voluntary reports of AEs to the FDA from the pharmaceutical industry, health‐care providers, and consumers, FAERS is limited by the presence of duplicate and incomplete reports, and lack of treatment causation or medical verification of reports. 25 A search was conducted to identify fatal events contemporaneous to all FDA‐approved coagulation FVIII (including plasma‐derived and recombinant; standard and extended half‐life), BPAs (including activated prothrombin complex concentrate [aPCC] and activated recombinant factor VII [rFVIIa]), and emicizumab (see Table S1 for search and application methodology and Table S2 in supporting information for the FVIII products included in the analysis). Where patients were exposed to multiple therapies, the first therapy reported was used for classification. Duplications and events in rare bleeding disorders other than congenital HA were excluded. The fatalities were categorized using the treating physician's assessment of death and applying the algorithm that underpins the framework.
3. RESULTS
As treatments have advanced over the past decades, particularly in well‐resourced countries, causes of mortality in PwcHA have become increasingly similar to, but are still divergent from, those of the general population. 5 PwcHA share causes of mortality with the general population including CVD, malignancy, and infections; but also retain unique disease‐ and treatment‐associated causes of death (Figure 1). With this in mind, we developed a framework consisting of two overarching categories: “HA‐associated mortality” and “non‐HA‐associated mortality.” Additionally, a category of “unspecified” is reserved for cases in which not enough information is available to adequately categorize the death.
Figure 1.
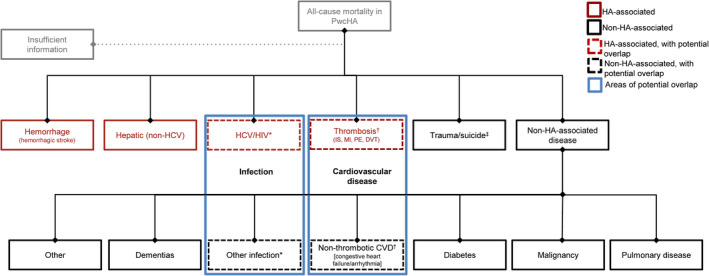
Framework for HA‐associated and non‐HA‐associated causes of death. Some HA‐associated diseases overlap with non‐HA‐associated diseases of the general population (indicated by blue boxes). *Infection is a common cause of death in the general population, but can be considered both HA associated or non‐HA associated due to the prevalence of viral infections (HCV/HIV) in PwcHA. †CVD is a common cause of death in the general population, but can be considered both HA associated or non‐HA associated due to the increased risk of thrombosis that is associated with many HA therapies. ‡Trauma/suicide is considered a distinct step in the algorithm, allowing the user to quickly and easily distinguish between cases which could or could not contribute to meaningful epidemiological analyses or yield clinical insight. CVD, cardiovascular disease; DVT, deep vein thrombosis; HA, hemophilia A; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IS, ischemic stroke; MI, myocardial infarction; PE, pulmonary embolism; PwcHA, persons with congenital hemophilia A
3.1. Categorization of HA‐associated mortality
Evidence from the systematic literature review demonstrates that HA‐associated mortality risks can be grouped into four primary categories: hemorrhage, thrombosis, HIV/HCV related, and hepatic (non‐HCV) related. 5 The rationale for these categories is discussed below. Then, secondary considerations were identified enabling a more in‐depth categorization of complex cases with multiple reported or contributory causes of death, and to avoid speculation on the specific cause of death when details are limited or difficult to interpret. This assessment should be made by the health‐care professional who has the most in‐depth knowledge of each specific case.
3.1.1. Hemorrhage
As a leading cause of death in PwcHA, 5 it is important that the risk of hemorrhage as a cause of fatality is documented as disease management improves. The risk of hemorrhage in PwcHA is multifactorial, depending on factors including: coagulation potential, presence of FVIII inhibitors, presence of comorbidities (eg, hypertension), hemostatic challenge (eg, level of physical activity, trauma, surgery), and tissue integrity (eg, inflamed versus healthy). 26 When the primary cause of death is hemorrhage, it is clinically important to analyze the hemorrhagic event further, given the high prevalence of confounding factors and the limitations of current medications. The specific cause of death can be further elucidated by considering other factors including: history and/or evidence of poor treatment response (ie, history of multiple bleeds at the same site) or life‐threatening bleeding events (eg, intracerebral hemorrhage, pseudotumor), other known risk factors (such as advanced liver disease) or use of medications (eg, anticoagulants) that increase the risk of hemorrhage, and objective evidence of loss of treatment efficacy.
3.1.2. Thrombosis
As a known but rare complication of HA treatment, some approved products have boxed warnings on their product labels regarding thrombosis. 8 , 9 , 27 Thrombosis‐related mortality is receiving increasing attention as PwcHA live longer and develop other comorbidities (eg, hypertension, atherosclerosis), 28 and as newer therapies have altered the kinetics of hemostatic protection from the frequent peaks and troughs of factor replacement toward more steady‐state coagulation potential through novel mechanisms of action (eg, FVIII replacement with bispecific antibody, messenger RNA antisense/rebalancing, gene therapy) or pharmaceutical modifications of replacement clotting factors (eg, extended half‐life products). It is crucial to determine whether there are any changes in the incidence of thrombosis caused by the novel mechanisms of action of newer treatments. Alternatively, changes in the incidence of thrombosis could be a reflection of increased life expectancy, and hence an aging population of PwcHA, whose risk of thrombosis approaches that of the general population; if the latter is the case, this would require a more evidence‐based prevention approach. Of note, channeling bias, whereby two drugs with similar therapeutic indications are prescribed to patients with different prognostic characteristics, may confound drug comparisons. 29 , 30 For example, a more convenient therapy may represent a particularly attractive option for clinically complex PwcHA who are either not candidates for burdensome prophylaxis regimens (eg, end‐of‐life care) or who have experienced insufficient effect from prior treatments. This may result in a cluster of fatal events occurring in association with the use of the new therapy which are not necessarily attributable to its mechanism of action. 29 As new therapies achieve a hemostatic status closer to normal, 31 thrombotic risk might be expected to mirror that of the general population more closely; 31 however, due to hemorrhagic risk, these PwcHA are less likely to be managed with standard prophylactic anticoagulation (eg, dual anti‐platelet therapy for cardiovascular risk) as they would in a non‐HA population.
Thrombotic complications have been reported in PwcHA using clotting factor concentrates and BPAs. 32 , 33 For example, myocardial infarction (MI) and pulmonary embolism (PE) have been observed in PwcHA taking either recombinant FVIII, aPCC, or rFVIIa 34 , 35 , 36 , 37 and more recently these complications have been observed in those using non‐factor substitution treatments. 27 Based on three thrombotic microangiopathies (TMAs) and two thrombotic events (TEs) that occurred during the development program, the emicizumab label describes a known risk of TE/TMA due to a drug–drug interaction associated with an average cumulative dose of aPCC >100 U/kg/d for ≥24 hours. 38 , 39 As of December 31, 2019, one additional TMA event has been reported in a PwcHA who also received emicizumab concomitant to aPCC (at a cumulative dose of >100 U/kg/d for ≥24 hours). 40 None of these events were fatal; however, one individual later died due to an ongoing rectal hemorrhage (unrelated to emicizumab). This individual refused potentially life‐saving blood transfusions, and the TMA was reported as resolving at the time of death. 41 In 2017, a patient without FVIII inhibitors enrolled in the fitusiran open‐label extension trial 15 , 42 receiving concomitant FVIII experienced a serious TE (cerebral sinus vein thrombosis) resulting in death. 27 More recently, two phase III clinical trials and one phase II clinical trial for concizumab were paused due to the occurrence of non‐fatal TEs in three patients enrolled in the phase III program. 43
Due to this developing evidence, the evolving safety profiles of emerging therapies, and as coagulation levels approach that of the general population, an assessment of thrombotic risk in PwcHA is required. Thrombosis can be further categorized according to secondary factors, including whether the demonstrated clot was arterial or venous, or associated with other risk factors for thrombosis. Furthermore, reporting of pertinent negative findings or a description of key diagnostic tests performed (or not) can aid in understanding a given case to avoid speculation.
3.1.3. HIV/HCV
A high proportion of fatalities arise in PwcHA with advancing comorbidities associated with HIV and HCV infections (eg, cirrhosis), and complications associated with its treatment (eg, dyslipidemia associated with HIV therapy). Complications from HCV and HIV infection have historically been reported as primary causes of death in the HA population, responsible for 22% and 26% of HA deaths, respectively, from 1992 to 2001; 1 however, risk of HIV infection from contaminated products was almost completely eliminated with the removal of HIV from plasma‐derived coagulation products and the generation of recombinant products. 5 Although antiviral therapies for HCV and HIV are now available, there remains a population of PwcHA who experienced irreversible harm from active viral infections prior to these therapies becoming available. Moreover, while sustained antiviral responses to HCV therapy reduce the risk for HCC, it is not completely eliminated. 44 Additionally, therapies for HCV and HIV are not equally accessible around the world. 45 The long‐term effects of HIV infection remain unknown; this therefore needs to be considered as a secondary factor, as does antiretroviral therapy for HIV, which has been associated with a number of AEs, including increased risk of sudden cardiac death, hyperlipidemia, diabetes, and CVD. 46 , 47 , 48 It will be important to maintain awareness of such AEs when considering causes of death for PwcHA and HIV. As such, to further determine the specific cause of death and underlying risks relating to HIV/HCV infection, secondary factors should be considered, such as whether the cause of death was specifically related to cirrhosis/advanced liver failure/liver cancer or whether the patient had been previously treated for HCV.
3.1.4. Hepatic (non‐HCV)
Non‐HCV hepatic mortality may also be an HA‐associated mortality risk in the future. AAV‐mediated gene therapy for HA, for example, relies on the transduction of hepatocytes to produce FVIII; 49 therefore, both short‐ and long‐term safety follow‐up on potential liver toxicity is warranted. 50 Furthermore, persons enrolled in clinical trials for AAV liver‐directed gene therapy for HA have experienced alanine transaminase (ALT) elevations triggering corticosteroid administration to prevent loss of efficacy. 18 , 19 In addition, the possibility of off‐target effects of gene therapy has been discussed, 51 as well as the potential for genotoxicity due to the integration of viral vector deoxyribonucleic acid, 49 and the risk of hepatic malignancies. 52 These factors have the potential to develop into a signal which could be identified using the proposed mortality framework. This category will also capture hepatic cases in which insufficient evidence is provided on the contribution of HCV.
3.2. Categorization of non‐HA‐associated mortality
Categorization of non‐HA‐associated mortality is critical for the monitoring of emerging signals. General mortality risks can first be categorized as “traumatic/suicide” or “non‐traumatic/suicide,” allowing the user of the algorithm to quickly distinguish between cases that may or may not contribute to meaningful epidemiological analyses or yield clinical insights pertinent to HA. Based on the leading causes of death in the United States and worldwide populations from the CDC 23 and WHO, 24 non‐traumatic general mortality risks can be categorized into six further categories: dementias, infection, cardiovascular (non‐thrombotic heart disease), diabetes, malignancy, and pulmonary disease; or other.
The global incidence of cardiovascular‐related deaths in the general population is high; 23 , 24 in the United States in 2017, “diseases of the heart” accounted for 23.3% of total deaths and “cerebrovascular diseases” (ie, stroke) accounted for a further 5.0% of the total deaths. 23 , 53 Studies reporting the proportion of deaths due to CVD in PwcHA range from 4% 54 to 25.5%, 55 although a number of studies suggest that this risk may be lower than in the general population. 54 , 55 , 56 , 57 , 58 However, determining the association between cardiovascular mortality and HA is complicated by the fact that cardiovascular risks overlap between HA‐associated and non‐HA‐associated mortality causes (Figure 1); and there is conflicting evidence on the level of cardiovascular (including thrombotic) risk in PwcHA relative to the general population. 58 , 59 A recent real‐world study found no evidence of a difference in the risk of MI in PwcHA relative to non‐HA counterparts, 58 although further studies are required to determine cardiovascular risk in PwcHA. Another consideration is that PwcHA with cardiovascular risks may not receive anticoagulation prophylaxis when needed, as would the non‐HA population, which could contribute to the risk for a cardiovascular event. Due to concern over thrombosis in PwcHA, and a heightened concern with procoagulant therapeutics versus other non‐thrombotic cardiac events such as congestive heart failure, congenital heart disease, pericarditis, and arrhythmia, cardiovascular causes must be considered to be overlapping HA‐associated and non‐HA‐associated mortality, but must still be captured as distinct causes in the algorithm in order to distinguish between HA‐associated and non‐HA‐associated cardiovascular death. This further speaks to the importance of detailed reporting around cardiovascular death in order to minimize over‐ or under‐estimation of thrombotic risk. Another consideration is that improvements in treatments that increase life expectancy may be associated with a greater incidence of cardiovascular thromboses, as PwcHA begin to develop similar age‐related comorbidities to the general population.
A number of studies have commented on an increase in malignancy‐related mortality in PwcHA 55 , 56 , 60 which, again, is likely related to increased life expectancy. However, cancer‐related mortality rates in PwcHA are equal to or lower than those observed in the general population. 54 , 56 , 57 One exception is HCC, 56 a known complication of chronic HCV and hepatitis B virus infection; the incidence of HCC is higher in PwcHA than the general population and is an important cause of mortality in aging persons with hemophilia. 61 , 62 Careful consideration is needed to determine whether such cases should be classified under “malignancy” or “virus‐related” deaths.
3.3. Algorithm for the categorization of causes of death in PwcHA
Based on the above rationale, we propose an algorithm to easily, comprehensively, and consistently categorize fatalities in PwcHA (Figure 2). Primary questions determine the main cause of death and once the user identifies if a given case contains events related to HA or its treatment, an initial categorization is determined. Secondary questions help determine contributing factors to fatalities: for example, with medically complex cases in which the fatality is attributable to multiple causes, and cases in which loss of efficacy of the hemophilia treatment contributed to the death.
Figure 2.
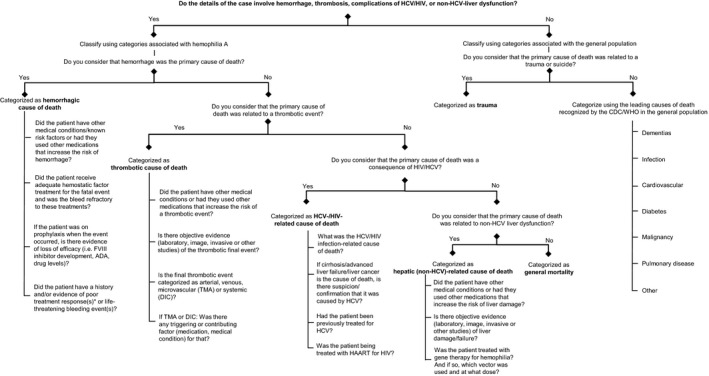
Algorithm for categorization of death. *Poor treatment response: history of multiple bleeds at the same site. ADA, anti‐drug antibodies; CDC, Centers for Disease Control and Prevention; DIC, disseminated intravascular coagulation; FVIII, factor VIII; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; TMA, thrombotic microangiopathy; WHO, World Health Organization
3.4. Checklist: key items for reporting a fatality case
A checklist has been developed to ensure that sufficient detail and context is provided in case reports to allow a comprehensive assessment of relationship of mortality to treatment and to ensure clarity on the cause of mortality when cases are complex with multiple reported or contributing causes of death (Table S3 in supporting information).
3.5. Application of the framework using FAERS data
Overall, 749 fatalities contemporaneous to FDA‐approved coagulation product use were reported to FAERS between January 1, 2000 and March 31, 2020 (Figure S1 in supporting information). When acquired HA (n = 230) was excluded, 519 fatalities were reported worldwide in PwcHA and those with unknown conditions. Using the algorithm for categorization of death (Figure 2), the 519 fatalities were categorized into one of the subgroups identified in the framework. In cases in which the information provided in the report was insufficient to identify the primary cause, the case was classified as “unspecified.”
When the data were filtered through the algorithm, the cause of death was considered to be HA associated in 195 (37.57%) cases. Of the remaining cases, 143 (27.55%) were causes of death commonly seen in non‐HA populations, and 181 (34.87%) were classified as other or unspecified causes (Figure S1).
3.5.1. Demographics and clinical characteristics
Overall, the median (range) age at time of death was 54 (0–96) years and the majority of individuals were male (Table 1). The relatedness of the fatal event to the coagulation product was not reported in the FAERS database, as it is not possible to report relatedness in that system.
Table 1.
Demographic and clinical characteristics of the 519 fatal events contemporaneous to coagulation factor use reported to FAERS
| Characteristic |
Total N = 519 |
|---|---|
| Age | |
| Median (range), years | 54 (0‐96) |
| Age classifications, n (%) | |
| <18 | 44 (8.5) |
| 18‐39 | 46 (8.9) |
| 40‐65 | 126 (24.3) |
| >65 | 93 (17.9) |
| Not reported | 210 (40.5) a |
| Sex, n (%) | |
| Male | 392 (75.5) |
| Female | 33 (6.4) |
| Not reported | 94 (18.1) |
| Indication, b n (%) | |
| HA without FVIII inhibitors | 219 (42.2) |
| HA with FVIII inhibitors | 202 (38.9) |
| Not reported | 98 (18.9) |
| Treatment | |
| FVIII | 284 (54.7) |
| rFVIIa | 94 (18.1) |
| aPCC | 125 (24.1) |
| Emicizumab | 16 (3.1) |
| Past medical history, n (%) | |
| HIV/HCV | 30 (5.8) |
| ≥1 cardiovascular risk factor c | 228 (43.9) |
| Cirrhosis | 8 (1.5) |
| Malignancy (historic or present) | 39 (7.5) |
Reporting to FAERS is voluntary, and reports can be incomplete and/or not medically verified, and do not establish causation.
Abbreviations: aPCC, activated prothrombin complex concentrate; FAERS, United States Food and Drug Administration Adverse Event Reporting System; FVIII, factor VIII; HA, hemophilia A; HCV, hepatitis C virus; HIV, human immunodeficiency virus; rFVIIa, recombinant activated factor VII.
Total sums >100% due to rounding.
Assumes that those individuals (with a diagnosis of HA) receiving bypassing agents, as indicated in their FAERS case report, were FVIII‐inhibitor positive and that those receiving FVIII were FVIII‐inhibitor negative.
Due to incomplete reporting, not all cases have sufficient information to extract cardiovascular risk factors, therefore it cannot be guaranteed that such risk factors did not contribute to the event. Factors considered to be associated with cardiovascular risk are outlined in Table S4 in supporting information. Presence of cardiovascular risk factors was also established from assessment of reported concomitant medications.
3.5.2. Framework categorization
In total, 26.4% (137/519) of cases reported to FAERS did not indicate a cause of death. Of those that did report a cause of death (382/519; 73.6%), hemorrhage was the most frequently reported across all products (115/382; 30.1%; Figure 3). Of the 115 hemorrhage‐related mortalities, 56 (48.7%) were classified as intracranial, 23 (20.0%) as gastrointestinal, and 36 (31.3%) as other or unspecified. All 10 deaths caused by HIV or HCV occurred in PwcHA receiving FVIII products and accounted for just 2% of deaths reported to FAERS across this 20‐year period. Thrombosis‐related deaths occurred in PwcHA receiving three out of four types of coagulation treatment, the exception being emicizumab. Deaths attributable to malignancy, infection, and non‐thrombotic CVD were reported with all four treatment types.
Figure 3.
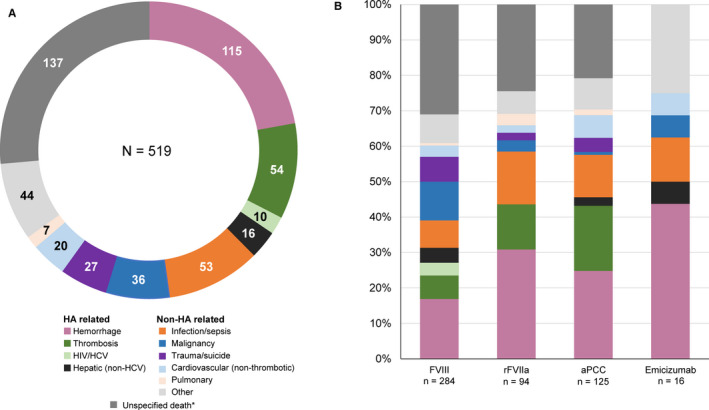
Causality of 519 fatal events reported to FAERS categorized through the HA mortality algorithm. A, Total. B, By coagulation product. *Unspecified deaths can be HA related or non‐HA related. aPCC, activated prothrombin complex concentrate; FAERS, United States Food and Drug Administration Adverse Event Reporting System; FVIII, factor VIII; HA, hemophilia A; HCV, hepatitis C virus; HIV, human immunodeficiency virus; rFVIIa, activated recombinant factor VII
4. DISCUSSION
Here, we provide a new model of evidence‐based approach for the classification of fatal events in PwcHA. As the treatment armamentarium broadens for PwcHA, it is important that physicians, patients, and the hemophilia community have a uniform means of describing mortality that can provide context for interpreting potential safety signals that arise from existing and newer therapies with novel mechanisms of action. Recent publications indicate that disease‐related and treatment‐associated mortality in HA is an active area of interest for the hemophilia community, 63 , 64 , 65 and recent publications on newer HA therapies have been highly cited. 15 , 18 , 20 , 66
The objective of creating this framework was to provide a uniform approach for the analysis and documentation of HA fatalities that is accessible to the wider community. This provides guidance to objectively and efficiently analyze and attribute causes for fatal events, particularly in complex cases. This framework is intended to be adaptable to a changing landscape and it is hoped that this will permit identification and monitoring of emerging safety signals. This framework can be applied by health authorities, clinicians, industry, and patients to understand and share fatal events with a common vernacular to achieve greater consistency in AE reporting and understanding, and to capture the public health impact of innovation in drug development on clinical outcomes in congenital HA. In developing countries where large‐scale reporting databases may not be available, the framework could instead be adopted at a center level. It may also be adapted to standardize the examination of fatal events in persons with other rare disorders. The algorithm and checklist presented here aim to identify key features that should be included in mortality reporting to encourage consistent reporting of fatal events in PwcHA.
Disparities between publications that report deaths in PwcHA remain a hindrance to case classification, 5 in part due to the complexity of cases with multiple reported or contributing causes of death. Furthermore, there are issues surrounding the non‐uniform reporting of HA‐associated deaths to regulatory authorities; and lack of classification of fatalities within regulatory databases such as FAERS complicates their interpretation. In addition, information from databases such as FAERS can have limitations, such as incomplete information for adequate categorization, lack of independent verification, and no certainty that a specific product caused the reaction. Furthermore, without a systematic or structured methodology, the depth of reporting and relevance of details such as concomitant medications and concurrent conditions is often lacking.
However, the categories identified herein, which tailor for the HA population, enable fatality categorization within datasets, as shown in the application of the framework to FAERS data. Detailed and timely case information is crucial to ensure that the community is able to accurately evaluate the evidence of mortality risk in PwcHA. Emerging therapies may influence mortality in a number of ways. Treatments with improved efficacy may result in lower rates of hemorrhage with a consequent increase in life expectancy, causing a shift in causes of mortality in PwcHA toward that of the general population (eg, CVD) and an increase in deaths due to age‐related diseases in PwcHA (eg, dementia, malignancy). Furthermore, as treatment efficacy improves and PwcHA live longer, the incidence of thrombotic events (eg, stroke, MI) is likely to increase due to age and comorbidities. These events will need to be carefully considered to determine whether or not they are associated with HA or its treatment. The use of antiplatelet/anticoagulation therapy for CVD prevention and treatment has the potential to improve our understanding of CVD comorbidities in PwcHA and could ultimately lead to the development of tailored guidelines for best practice in this population. On the other hand, emerging treatments may also come with their own unique attributes and associated risk factors because different therapies use distinct strategies to approach normalization of hemostasis, and hence may have different safety profiles. Emerging safety concerns in the general population may also influence HA‐associated concerns. For example, non‐alcoholic steatohepatitis and/or non‐alcoholic fatty liver disease may pose an emerging hepatic risk to PwcHA given the increasing global rates of obesity. 67 A study in children with hemophilia found that obesity was more common in children with severe hemophilia versus the general population, and persistent elevation of ALT was most likely to occur in association with obesity rather than chronic viral hepatitis. 68
The framework can be applied to monitor AEs for existing therapies, which may otherwise be under‐reported. The Weber effect is an established bias in AE reporting, which describes that AE reporting peaks 2 years after a drug has been on the market and subsequently decreases with longer time on the market. 69 As such, it is important to consider that the safety record of established agents may also be incomplete due to under‐reporting. 70 A 2006 systematic literature review found that under‐reporting is problematic for all therapeutics and one reason for under‐reporting was uncertainty about the drug that caused the AE. 70 This illustrates the need for a clearly defined framework for categorizing cause of death and elucidating the contributing factors, and how such a framework may improve AE reporting. However, to establish the current baseline mortality rate for PwcHA, further studies and registries are required.
The application of this algorithm using FAERS data suggests a generally consistent pattern of reported mortality across treatments, with all FDA‐approved hemostatic products having reported fatalities in PwcHA due to infection, CVD, and malignancies. However, more than one quarter of fatalities reported did not specify a cause of death and were classed as “unspecified,” indicating a need for improved reporting in PwcHA. The application of this framework to data such as FAERS highlights the utility of such a unified approach across all hemostatic agents. In addition, the framework has been applied to categorize causes of death in PwcHA contemporaneous to emicizumab use as assessed by reporters and documented in the Roche Emicizumab Global Safety Database. This demonstrated the absence of trends that point to unique risks associated with the administration of emicizumab prophylaxis in PwcHA. 71
5. CONCLUSIONS
In summary, novel therapies have the potential to significantly influence short‐ and long‐term outcomes in PwcHA, both in terms of overall survival and recognized causes of death. A conceptual framework for cross‐examining causes of mortality for established and non‐factor replacement therapies alike is needed to understand and gain actionable insights into mortality in PwcHA. Here we have demonstrated how the conceptual framework can be used to put into context the public reports of fatalities in PwcHA using any kind of anti‐hemophilia therapy and, importantly, this presents a rare opportunity to document the impact of innovation in drug development on mortality. We propose that this new and robust tool be integrated into the evaluation of mortality in PwcHA.
CONFLICTS OF INTEREST
None of the authors received honoraria or fees for their contribution to the development of this article or supplement. P.K., R.H.K., and T.C. are current employees of and hold shares in Genentech, Inc. C.D.F. and F.S. are current employees of and hold shares in F. Hoffmann‐La Roche Ltd. Outside of this article, most authors received fees for participation in activities funded by F. Hoffmann‐La Roche Ltd and/or Genentech, Inc. as described in the following disclosures, with the exception of G.F.P. S.W.P. has received consultancy for Apcintex, Bayer, BioMarin, Catalyst Biosciences, CSL Behring, HEMA Biologics, Freeline Therapeutics, Novo Nordisk, Pfizer, F. Hoffmann‐La Roche Ltd/Genentech, Inc. Sangamo Therapeutics, Sanofi, Takeda, Spark Therapeutics, and uniQure, has received research funding from Siemens, and is a member of the board of directors/advisory committee for the Medical and Scientific Advisory Council to the National Hemophilia Foundation and the Medical Advisory Board to World Federation of Hemophilia. R.K.‐J. has received consultancy from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, and Genentech, Inc.; has received research funding from CSL Behring, Genentech, Inc., and Spark; has received honoraria from Chugai Pharmaceutical Co., BioMarin, CSL Behring, CRISPR Therapeutics, and Genentech, Inc.; and has participated in speakers’ bureaus for F. Hoffmann‐La Roche Ltd. J.N.M. has received consultancy from CSL Behring, Catalyst Biosciences, Freeline Therapeutics, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda; has received research funding from BioMarin, CSL Behring, Freeline Therapeutics, Novo Nordisk, Novartis, Pfizer, Sanofi, F. Hoffmann‐La Roche Ltd, and uniQure; has participated in a speakers’ bureau for CSL Behring, Catalyst Biosciences, Novo Nordisk, F. Hoffmann‐La Roche Ltd, Sanofi, Spark, and Takeda; and sits on the board of directors/advisory committee for the South Africa Medical Research Council, the Wits Health Consortium, and Colleges of Medicine of South Africa. G.F.P. is a leader of WFH and NHF; has received honoraria from Griffols; has received fees for consultancy/advice for BioMarin, CRISPR Therapeutics, Generation Bio, Geneception, Pfizer, Takeda, and Third Rock Ventures; and has patent fees from Sanofi. F.P. has received honoraria for participating as a speaker at satellite symposia organized by F. Hoffmann‐La Roche Ltd, Sanofi, Sobi, Spark, and Takeda and has participated in advisory boards for F. Hoffmann‐La Roche Ltd, Sanofi, and Sobi. C.R.M.H. has received consultancy and honoraria from Shire, Alnylam, Novo Nordisk, Sobi, and F. Hoffmann‐La Roche Ltd, has participated in speakers’ bureaus for Shire, Sobi, F. Hoffmann‐La Roche Ltd, and Pfizer; has received research funding from Bayer, Shire, Sobi, Novo Nordisk, and Pfizer; and has received travel and accommodation expenses from F. Hoffmann‐La Roche Ltd, Sobi, CSL Behring, and Shire.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design of the work, as well as the analysis and interpretation of data for the work. All authors revised the manuscript critically and provided final approval of the version to be published. They all agree to be accountable for all aspects of the work.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Michelle Rice of the National Hemophilia Foundation, USA; Sonji Wilkes of the Hemophilia Federation of America, USA; Mark Skinner of the Institute for Policy Advancement Ltd, USA; Brian O'Mahony of Irish Haemophilia Society, Ireland; and Declan Noone of the European Haemophilia Consortium, Belgium, for initial guidance and advice; as well as Lucy Lee, PharmD, MPH, of Genentech, Inc. and Guillermo Tobaruela, MPharm, MSc, of F. Hoffmann‐La Roche Ltd for insightful contributions to the drafts. Editorial assistance for the development of this article, under the direction of the authors, was provided by Rebecca A. Bachmann, PhD; Alex Coulthard, BSc; and Bonnie Nicholson, PhD; of Gardiner‐Caldwell Communications, Macclesfield, UK and funded by F. Hoffmann‐La Roche Ltd.
Pipe SW, Kruse‐Jarres R, Mahlangu JN, et al. Establishment of a framework for assessing mortality in persons with congenital hemophilia A and its application to an adverse event reporting database. J Thromb Haemost. 2021;19(Suppl. 1):21–31. 10.1111/jth.15186
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 28 October 2020
REFERENCES
- 1. Plug I, Van Der Bom JG, Peters M, et al. Mortality and causes of death in patients with hemophilia, 1992–2001: a prospective cohort study. J Thromb Haemost. 2006;4:510‐516. [DOI] [PubMed] [Google Scholar]
- 2. Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815‐825. [DOI] [PubMed] [Google Scholar]
- 3. Mannucci PM. Benefits and limitations of extended plasma half‐life factor VIII products in hemophilia A. Expert Opin Investig Drugs. 2020;29:303‐309. [DOI] [PubMed] [Google Scholar]
- 4. Franchini M, Mannucci PM. Past, present and future of hemophilia: a narrative review. Orphanet J Rare Dis. 2012;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hay C, Nissen F, Pipe SW. Mortality in congenital hemophilia A – a systematic literature review. J Thromb Haemost. 2021;19:(Suppl. 1):6‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giangrande PLF, Hermans C, O'Mahony B, et al. European principles of inhibitor management in patients with haemophilia. Orphanet J Rare Dis. 2018;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365‐3372. [DOI] [PubMed] [Google Scholar]
- 8. FEIBA product monograph . FDA 1986 [Last updated: November 2013]. https://www.fda.gov/media/78852/download. Accessed May 2, 2020.
- 9. NovoSeven product monograph . FDA 1999 [Last updated: July 2014]. https://www.fda.gov/media/70442/download. Accessed May 2, 2020.
- 10. Antiretroviral Therapy Cohort Collaboration . Life expectancy of individuals on combination antiretroviral therapy in high‐income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marcus JL, Hurley LB, Chamberland S, et al. Life expectancy of insured people with and without hepatitis C virus infection, 2007–2017. Open Forum Infect Dis. 2020;7:ofaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holmes JA, Rutledge SM, Chung RT. Direct‐acting antiviral treatment for hepatitis C. Lancet. 2019;393:1392‐1394. [DOI] [PubMed] [Google Scholar]
- 13. Sherman KE, Rockstroh J, Thomas D. Human immunodeficiency virus and liver disease: an update. Hepatology. 2015;62:1871‐1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitazawa T, Esaki K, Tachibana T, et al. Factor VIIIa‐mimetic cofactor activity of a bispecific antibody to factors IX/IXa and X/Xa, emicizumab, depends on its ability to bridge the antigens. Thromb Haemost. 2017;117:1348‐1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377:819‐828. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro A, Castaman G, Cepo K, et al. Efficacy and safety of subcutaneous prophylaxis with concizumab in patients with hemophilia a or B with inhibitors: results from EXPLORER 4, a Phase 2, randomized, open‐label, controlled trial. Blood. 2019;134:1139. [Google Scholar]
- 17. Polderdijk SG, Adams TE, Ivanciu L, Camire RM, Baglin TP, Huntington JA. Design and characterization of an APC‐specific serpin for the treatment of hemophilia. Blood. 2017;129:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear follow‐up of AAV5‐hFVIII‐SQ gene therapy for hemophilia A. N Engl J Med. 2020;382:29‐40. [DOI] [PubMed] [Google Scholar]
- 19. Rangarajan S, Walsh L, Lester W, et al. AAV5‐Factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519‐2530. [DOI] [PubMed] [Google Scholar]
- 20. Mahlangu J, Oldenburg J, Paz‐Priel I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors. N Engl J Med. 2018;379:811‐822. [DOI] [PubMed] [Google Scholar]
- 21. Oldenburg J, Mahlangu JN, Bujan W, et al. The effect of emicizumab prophylaxis on health‐related outcomes in persons with haemophilia A with inhibitors: HAVEN 1 Study. Haemophilia. 2019;25:33‐44. [DOI] [PubMed] [Google Scholar]
- 22. Franchini M, Mannucci PM. Non‐factor replacement therapy for haemophilia: a current update. Blood Transfus. 2018;16:457‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . Leading Causes of Death. https://www.cdc.gov/nchs/fastats/leading‐causes‐of‐death.htm. Accessed April 21, 2020.
- 24. World Health Organization . The top 10 causes of death. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐death. Accessed April 21, 2020.
- 25. FDA . FDA Adverse Event Reporting System (FAERS) Public Dashboard. https://fis.fda.gov/sense/app/d10be6bb‐494e‐4cd2‐82e4‐0135608ddc13/sheet/7a47a261‐d58b‐4203‐a8aa‐6d3021737452/state/analysis. Accessed August 2, 2020.
- 26. Srivastava A, Brewer AK, Mauser‐Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1‐e47. [DOI] [PubMed] [Google Scholar]
- 27. Tiede A. Thromboembolic risks of non‐factor replacement therapies in hemophilia. Hamostaseologie. 2017;37:307‐310. [DOI] [PubMed] [Google Scholar]
- 28. Larsen JB, Nielsen KBJ, Poulsen LH, Bor MV. Arterial and venous thrombosis in haemophilia patients: experiences from a Danish haemophilia centre. Acta Haematol. 2017;138:91‐95. [DOI] [PubMed] [Google Scholar]
- 29. Petri H, Urquhart J. Channeling bias in the interpretation of drug effects. Stat Med. 1991;10:577‐581. [DOI] [PubMed] [Google Scholar]
- 30. Hudson M, Suissa S. Avoiding common pitfalls in the analysis of observational studies of new treatments for rheumatoid arthritis. Arthritis Care Res. 2010;62:805‐810. [DOI] [PubMed] [Google Scholar]
- 31. Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia A and inhibitors‐a single‐center cohort. Ped Blood Cancer. 2019;66:e27886. [DOI] [PubMed] [Google Scholar]
- 32. van Vulpen LF, Saccullo G, Iorio A, Makris M. The current state of adverse event reporting in hemophilia. Expert Rev Hematol. 2017;10:161‐168. [DOI] [PubMed] [Google Scholar]
- 33. Morfini M, Rapisarda CAP. Safety of recombinant coagulation factors in treating hemophilia. Expert Opin Drug Saf. 2019;18:75‐85. [DOI] [PubMed] [Google Scholar]
- 34. Higgins P, Brown SA. Pulmonary embolus in a haemophilia patient. J Paediatr Child Health. 2013;49:423. [DOI] [PubMed] [Google Scholar]
- 35. Davoodabadi A, Adib MM, Keleidari B. Post splenectomy fatal pulmonary embolism in a patient with moderate hemophilia A. Iran J Med Sci. 2011;36:136‐140. [PMC free article] [PubMed] [Google Scholar]
- 36. Gunaldi M, Helvaci A, Yildirim ND, Kiskac M, Kucukkaya RD. Acute myocardial infarction in a patient with hemophilia A and factor V Leiden mutation. Cardiol J. 2009;16:458‐461. [PubMed] [Google Scholar]
- 37. Zupancic‐Salek S, Vodanovic M, Pulanic D, Skoric B, Matytsina I, Klovaite J. A case report of acute inferior myocardial infarction in a patient with severe hemophilia A after recombinant factor VIII infusion. Medicine. 2017;96:e9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Genentech, Inc . HEMLIBRA® (emicizumab‐kxwh) injection for subcutaneous use, prescribing information. Initital U.S. approval: 2017. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761083s000lbl.pdf. Accessed April 28, 2020.
- 39. Langer AL, Etra A, Aledort L. Evaluating the safety of emicizumab in patients with hemophilia A. Expert Opin Drug Saf. 2018;17:1233‐1237. [DOI] [PubMed] [Google Scholar]
- 40. Lee L, Moreno K, Kuebler P, et al. Summary of thrombotic or thrombotic microangiopathy events in persons with hemophilia A taking emicizumab In: Poster presented at the NHF Bleeding Disorders Virtual Conference, August 1–8, 2020 (poster no #35). [Google Scholar]
- 41. Khoo L, Matthews S, Kershaw G, et al. Case report of a fatal rectal haemorrhage in a person with severe haemophilia A receiving emicizumab and high‐dose bypassing agents in the HAVEN 1 study. Haemophilia. 2020;00:1‐4. [DOI] [PubMed] [Google Scholar]
- 42. Pasi KJGP, Mant T, Creagh MD, et al. Fitusiran, an investigational RNAi therapeutic targeting antithrombin for the treatment of hemophilia: interim results from a phase 2 extension study in patients with hemophilia A or B with and without inhibitors. Res Pract Thromb Haemost. 2017;1:25. [Google Scholar]
- 43. Novo Nordisk . Press release: Novo Nordisk pauses the clinical trials investigating concizumab (anti‐TFPI mAB) in haemophilia A and B with or without inhibitors. 2020. https://www.globenewswire.com/news‐release/2020/03/16/2001361/0/en/Novo‐Nordisk‐pauses‐the‐clinical‐trials‐investigating‐concizumab‐anti‐TFPI‐mAB‐in‐haemophilia‐A‐and‐B‐with‐or‐without‐inhibitors.html. Accessed March 2, 2020.
- 44. Su F, Ioannou GN. The impact of direct‐acting antiviral therapy for hepatitis C on hepatocellular carcinoma risk. Curr Hepatol Rep. 2018;17:377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. World Health Organization . Global report on access to hepatitis C treatment: Focus on overcoming barriers. 2016. https://www.who.int/hepatitis/publications/hep‐c‐access‐report/en/. Accessed June 2, 2020.
- 46. Brouillette J, Cyr S, Fiset C. Mechanisms of arrhythmia and sudden cardiac death in patients with HIV infection. Can J Cardiol. 2019;35:310‐319. [DOI] [PubMed] [Google Scholar]
- 47. Montessori V, Press N, Harris M, Akagi L, Montaner JSG. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. 2004;170:229‐238. [PMC free article] [PubMed] [Google Scholar]
- 48. Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59:1891‐1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Doshi BS, Arruda VR. Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol. 2018;9:273‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) . Human Gene Therapy for Hemophilia: Guidance for Industry. 2020. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/human‐gene‐therapy‐hemophilia. Accessed June 2, 2020.
- 51. Rodriguez‐Merchan EC. What´s new in gene therapy of hemophilia. Curr Gene Ther. 2018;18:107‐114. [DOI] [PubMed] [Google Scholar]
- 52. Pipe SW. Gene therapy for hemophilia. Ped Blood Cancer. 2018;65:e26865. [DOI] [PubMed] [Google Scholar]
- 53. Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep. 2019;68:1‐77. [PubMed] [Google Scholar]
- 54. Lovdahl S, Henriksson KM, Baghaei F, et al. Incidence, mortality rates and causes of deaths in haemophilia patients in Sweden. Haemophilia. 2013;19:362‐369. [DOI] [PubMed] [Google Scholar]
- 55. Jardim LL, van der Bom JG, Caram‐Deelder C, Gouw SC, Leal Cherchiglia M, Meireles RS. Mortality of patients with haemophilia in Brazil: first report. Haemophilia. 2019;25:e146‐e152. [DOI] [PubMed] [Google Scholar]
- 56. Tagliaferri A, Rivolta GF, Iorio A, et al. Mortality and causes of death in Italian persons with haemophilia, 1990–2007. Haemophilia. 2010;16:437‐446. [DOI] [PubMed] [Google Scholar]
- 57. van de Putte DEF, Fischer K, Pulles AE, et al. Non‐fatal cardiovascular disease, malignancies, and other co‐morbidity in adult haemophilia patients. Thromb Res. 2012;130:157‐162. [DOI] [PubMed] [Google Scholar]
- 58. Faghmous I, Flores C, Sarouei K, et al. Estimating the risk of myocardial infarction in persons with hemophilia A using a machine‐learning approach with US claims data In: Proceedings of the 61st ASH (American Society of Hematology) Annual Meeting & Exposition. Orlando, Florida, USA, December, 7‐10, 2019. [Google Scholar]
- 59. Pocoski J, Ma A, Kessler CM, Boklage S, Humphries TJ. Cardiovascular comorbidities are increased in U.S. patients with haemophilia A: a retrospective database analysis. Haemophilia. 2014;20:472‐478. [DOI] [PubMed] [Google Scholar]
- 60. Eckhardt CL, Loomans JI, van Velzen AS, et al. Inhibitor development and mortality in non‐severe hemophilia A. J Thromb Haemost. 2015;13:1217‐1225. [DOI] [PubMed] [Google Scholar]
- 61. Colombo M, Mannucci PM, Brettler DB, et al. Hepatocellular carcinoma in hemophilia. Am J Hematol. 1991;37:243‐246. [DOI] [PubMed] [Google Scholar]
- 62. Shetty S, Sharma N, Ghosh K. Epidemiology of hepatocellular carcinoma (HCC) in hemophilia. Crit Rev Oncol Hematol. 2016;99:129‐133. [DOI] [PubMed] [Google Scholar]
- 63. Aledort LM. Deaths associated with emicizumab in patients with hemophilia A. N Engl J Med. 2019;381:1878‐1879. [DOI] [PubMed] [Google Scholar]
- 64. Buckner TW, Watson C, Recht M. Emicizumab in hemophilia A. N Engl J Med. 2020;382:785‐786. [DOI] [PubMed] [Google Scholar]
- 65. Kruse‐Jarres R, Hay CRM. Emicizumab in hemophilia A. N Engl J Med. 2020;382:785. [DOI] [PubMed] [Google Scholar]
- 66. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 67. Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Revel‐Vilk S, Komvilaisak P, Blanchette V, et al. The changing face of hepatitis in boys with haemophilia associated with increased prevalence of obesity. Haemophilia. 2011;17:689‐694. [DOI] [PubMed] [Google Scholar]
- 69. Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti‐inflammatory drugs In: Rainsford KD, Velo GD, editors. Side‐effects of Anti‐inflammatory Drugs, Advances in Inflammation Research. New York, NY: Raven Press; 1984. [Google Scholar]
- 70. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29:385‐396. [DOI] [PubMed] [Google Scholar]
- 71. Peyvandi F, Mahlangu J, Pipe SW, et al. Application of a hemophilia mortality framework to the Emicizumab Global Safety Database. J Thromb Haemost. 2021;19:(Suppl. 1):32‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


