Abstract
Aim
To compare 6‐month adherence, persistence and treatment patterns among patients initiating once‐weekly glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), dulaglutide versus semaglutide, and dulaglutide versus exenatide BCise, using claims from the HealthCore Integrated Research Database.
Materials and methods
Patients aged ≥18 years, with type 2 diabetes, ≥1 claim for dulaglutide, semaglutide or exenatide BCise during the index period February 2018 to December 2018 (index date = earliest GLP‐1RA fill date), no claim for GLP‐1RAs in the 6‐month pre‐index period, and continuous enrolment 6 months pre‐ and post‐index were included. Dulaglutide users were propensity‐matched 1:1 to semaglutide users (3852 pairs) or exenatide BCise users (1879 pairs). The proportions of adherent (proportion of days covered ≥80%) patients were compared using chi‐squared tests. Persistence, measured as days to discontinuation, was analysed using a Cox regression model.
Results
Matched cohorts (dulaglutide:semaglutide and dulagutide:exenatide BCise) were balanced in baseline characteristics and the mean age was 54 and 55 years, respectively, with approximately 51% and 49% women, respectively. At 6 months, significantly more dulaglutide users were adherent than semaglutide (59.7% vs. 42.7%; P <0.0001) or exenatide BCise users (58.1% vs. 40.3%; P <0.0001). Cox regression showed that dulaglutide users were less likely to discontinue therapy than semaglutide (hazard ratio [HR] 0.71, 95% confidence interval [CI] 0.66, 0.76) or exenatide BCise users (HR 0.59, 95% CI 0.53, 0.65; P <0.0001, both).
Conclusion
At 6‐month follow‐up, a higher proportion of patients initiating dulaglutide were adherent to and persistent with their treatment, compared to matched patients initiating either semaglutide or exenatide BCise.
Keywords: dulaglutide, exenatide, GLP‐1, type 2 diabetes
1. INTRODUCTION
Diabetes is a complex, multifactorial disease that affects an estimated 34 million individuals in the United States. 1 Type 2 diabetes (T2D) accounts for 90% of people with diabetes and is a major contributor to global morbidity and mortality. 2 , 3 , 4 The American Diabetes Association and European Association for the Study of Diabetes recommend diet and exercise as initial treatment for people with T2D, followed by initiation of metformin. 5 , 6 Treatment intensification to oral anti‐hyperglycaemic drug (OAD) combination therapy or an injectable agent is required over time for patients who are unable to achieve and maintain glycaemic control with metformin alone. 7 Current guidelines advise the use of sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) after metformin therapy as options in patients with cardiovascular (CV) comorbidities, since these agents may offer multiple benefits including glycaemic control, a low risk of hypoglycaemia, potential for weight loss, and proven CV benefits for some agents. 5 , 6
Medication adherence and persistence are important to T2D disease management. Higher medication adherence and persistence are associated with better glycaemic control and reduced healthcare resource utilization. 8 , 9 , 10 Most studies report an association between higher medication adherence rates and greater glycated haemoglobin (HbA1c) reduction. 8 , 11 Better medication adherence has also been shown to be associated with an overall reduction in healthcare utilization. 12 Published studies show wide variability in medication adherence to OADs in patients with T2D, ranging from 38% to 93%, 13 , 14 , 15 , 16 whereas adherence rates range from 38% to 61% for non‐insulin injectables, such as GLP‐1RAs. 17 , 18 , 19 , 20
Several once‐daily and once‐weekly injectable and oral GLP‐1RA agents for the treatment of T2D are available in the US market. These GLP‐1RAs not only differ in their clinical profiles, but also have substantial differences in dosing regimens, need/length of dose titration, and administration device features such as need for reconstitution, single‐dose versus multi‐dose devices, needle handling and ease of use. These differences in profile may play an important role in treatment adherence, persistence and eventually real‐world effectiveness of GLP‐1RAs. Previous real‐world studies have evaluated and compared adherence and persistence of once‐daily, twice‐daily and once‐weekly injectable GLP‐1RAs. Studies have shown that once‐weekly GLP‐1RAs, such as dulaglutide, have demonstrated better adherence compared to once‐daily liraglutide and once‐weekly exenatide. 17 , 19 , 21
Recently, semaglutide became available in the United States (December 2017), adding to once‐weekly options that include dulaglutide and exenatide; and exenatide once‐weekly is now available in a new formulation and device: exenatide BCise (October 2017). No published data are currently available comparing adherence outcomes of these three once‐weekly GLP‐1RAs in the United States. Previous retrospective real‐world studies were limited to comparing adherence and persistence outcomes of once‐weekly versus once‐daily GLP‐1RAs and once‐weekly dulaglutide versus exenatide once weekly in the old formulation and device. To enhance our understanding of adherence outcomes, the present study compared adherence, persistence and treatment patterns among patients initiating the once‐weekly GLP‐1RAs dulaglutide, semaglutide and exenatide BCise.
2. MATERIALS AND METHODS
2.1. Data source
This retrospective real‐world observational study used data from the HealthCore Integrated Research Database (HIRD). The HIRD is a health insurance database comprising administrative claims and clinical data integrated across data sources and types, which are acquired from multiple health plans representative of members in all 50 US states. Overall, individuals in HIRD are representative of the US population, with the ≥65 years age group being underrepresented in the HIRD but still accounting for a substantial sample size. 22 Beginning in 2006 through the most recent calendar quarter, data sources consist of professional claims, facility claims, outpatient pharmacy claims, outpatient laboratory results and health plan enrolment information. Key data elements captured by the system include demographics, prescription data (eg, national drug codes), diagnosis data (International Classification of Diseases [ICD]‐10‐Clinical Modification [CM] codes), laboratory test results, physician characteristics, and inpatient and outpatient utilisation and cost data. Data were made available for analysis in a Health Insurance Portability and Accountability Act (HIPAA)‐compliant, de‐identified research database, and did not involve the collection, use or transmittal of individually identifiable data. Institutional review board approval to conduct this study was not required.
2.2. Patient selection
An overview of patient selection is provided in Figure S1. This analysis included adults with T2D who initiated dulaglutide, semaglutide or exenatide BCise between February 2018 and December 2018 (index period; index date = earliest GLP‐1RA fill during this period). The study period was from August 2017 to June 2019.
Patients with ≥1 pharmacy claim for dulaglutide, semaglutide or exenatide BCise during the index period, continuous enrolment in the 6 months pre‐index (baseline) and 6 months post‐index (follow‐up), and with ≥1 medical claim for T2D over the baseline period were included. Patients with ≥1 medical claim for type 1 diabetes or ≥1 pharmacy claim for any GLP‐1RA (or GLP‐1RA/insulin fixed‐ratio combination) prescription during the 6‐month baseline period or ≥1 pharmacy claim for GLP‐1RAs other than the index drugs or ≥1 pharmacy claim for GLP‐1RA/insulin fixed‐ratio combination at the index date were excluded from the study. Additionally, patients with a diagnosis of gestational diabetes or bariatric surgery during the study period were excluded.
2.3. GLP‐1RA index dose
Two once‐weekly index doses were available for dulaglutide: 0.75 mg (low dose) or 1.5 mg (high dose). Index doses for once‐weekly semaglutide included 0.25‐mg and 0.5‐mg doses (low dose) or a 1.0‐mg dose (high dose). Once‐weekly exenatide BCise was available in only one dose, 2.0 mg.
2.4. Patient baseline characteristics
Key patient baseline characteristics included gender, age and comorbidity indices. Baseline comorbidities captured using ICD‐10‐CM codes from medical claims included CV disease, dyslipidaemia, hypertension and obesity. The Quan–Charlson Comorbidity Index 23 and adapted Diabetes Complications Severity Index 24 were calculated to describe the comorbidity burden.
2.5. Adherence outcome measures
Adherence was measured by proportion of days covered (PDC) and calculated as the number of days with drug on hand in the 6‐month follow‐up period divided by the number of days in the follow‐up period, regardless of discontinuation. In cases where patients had overlapping days' supply, the start of the new fill was adjusted to begin after the previous fill was considered to have run out. A patient with a PDC ≥80% was considered adherent, as this threshold is considered clinically relevant and is widely used in the literature. 15 , 18 , 19 , 21 , 25
2.6. Persistence outcome measures
Persistence was defined as the number of days of continuous therapy from the initiation of index treatment to discontinuation or end of the 6‐month follow‐up period. Discontinuation of index medication was defined as failure to refill the index medication within the allowable gap period of 45 days (primary analysis) or 60 days (sensitivity analysis) after the depletion of the previous fill's supply. Patients who did not discontinue during the entire follow‐up period were considered persistent.
2.7. Other treatment‐pattern‐related outcome measures
The mean number of index medication fills, and the proportion of patients with second and fourth fills were assessed. Dose patterns for dulaglutide and semaglutide cohorts were also examined. Dose patterns for the exenatide BCise cohort were not assessed as this product is only available as a single dose strength. Proportions of patients belonging to each of the following four mutually exclusive groups were evaluated: (a) start on low dose, never use high dose (low dose only); (b) start on high dose, never use low dose (high dose only); (c) start on low dose, change to high and stay on high (low dose to high dose); and (d) all others.
Use of other anti‐hyperglycaemic medications was evaluated over the baseline and 6‐month follow‐up periods and included insulin (any type) and OADs.
2.8. Subgroup analysis
Adherence and persistence outcomes were evaluated for the following subgroups: (a) age (<65 vs. ≥65 years); (b) index dose (low vs. high); (c) dose patterns (as described above), and (d) baseline use of insulin (with vs. without insulin). Within each cohort, chi‐squared tests were conducted to compare adherence and persistence outcomes between subgroups based on the four categories.
2.9. Statistical analysis
Descriptive statistics including means, SD values, and absolute/relative frequencies for continuous and categorical data, respectively, were generated. Since patients were not randomized to treatment, propensity‐score matching was used to adjust for confounders for each comparison: dulaglutide versus semaglutide and dulaglutide versus exenatide BCise. The propensity score was defined as the probability of being classified as a dulaglutide initiator versus a semaglutide initiator or dulaglutide versus an exenatide BCise initiator, conditional on a patient's observed baseline characteristics. The propensity score was estimated by logistic regression, with baseline characteristics as covariates. Propensity scores for the dulaglutide versus semaglutide cohorts were calculated using the baseline covariates age, gender, region, prescribing healthcare provider specialty, diagnosis of obesity, presence of insulin fill, sulphonylurea fill, SGLT2 inhibitor fill, index dose, endocrinologist visit, and number of prescription classes filled. For the dulaglutide versus exenatide BCise cohort, baseline variables for propensity scoring were age, gender, region, prescribing healthcare provider specialty, and plan type including Medicare Advantage. Patients were matched on the propensity scores in a 1:1 ratio using optimal matching. In addition, patients were matched exactly based on age category (age <65 and ≥65 years) and index dose for the dulaglutide and semaglutide comparison. Absolute standardized differences of <0.10 were considered to denote balance in baseline characteristics between the cohorts. 26 , 27 For both comparisons, after propensity‐score matching, cohorts were finalized before the outcome analyses were conducted.
All outcome measures were compared between matched cohorts. The proportions of adherent and persistent patients were evaluated using chi‐squared tests. Persistence, measured as days to discontinuation, was analysed as a time‐to‐event outcome. Patients were required to have a minimum follow‐up of 6 months so censoring only occurred at the end of this period. Kaplan–Meier estimates were calculated and the log‐rank test was used to compare survival curves between cohorts. In addition, hazard ratios (HRs) along with 95% confidence intervals (CIs) were calculated using Cox proportional hazard models, with days to discontinuation as the outcome and cohort as the independent variable.
An α level of 0.05 was used to identify statistical significance and no adjustments were made for multiple comparisons. Variables were created and analysed using the Instant Health Data platform (BHE, Boston, Massachusetts) and SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Study population
Prior to propensity‐score matching, 12 919 patients met the criteria for the dulaglutide cohort, 3852 for semaglutide and 1879 for exenatide BCise, which comprised the pre‐matching treatment analysis population (Figure S1 and Table S1). Following propensity‐score matching, the dulaglutide versus semaglutide cohort consisted of 3852 pairs and the dulaglutide versus exenatide BCise cohort consisted of 1879 pairs, which comprised the post‐matching treatment analysis population (Figure S1 and Table 1). Unless otherwise indicated, the results presented are for the post‐propensity‐matching treatment analysis population (matched cohorts).
TABLE 1.
Baseline characteristics of propensity matched cohorts
| Matched DU vs. SEMA cohorts | Matched DU vs. EBCise cohorts | |||||
|---|---|---|---|---|---|---|
| Characteristics | DU (N = 3852) | SEMA (N = 3852) | Std diff | DU (N = 1879) | EBCise c (N = 1879) | Std diff |
| Women a , % | 51.6 | 50.9 | 0.01 | 48.4 | 48.4 | 0.00 |
| Age a , years, mean (SD) | 53.5 (9.8) | 53.6 (9.6) | 0.01 | 54.8 (10.1) | 54.8 (10.2) | 0.00 |
| QCI score b , mean (SD) | 0.9 (1.4) | 0.8 (1.3) | 0.07 | 0.9 (1.4) | 0.8 (1.3) | 0.05 |
| aDCSI score b , mean (SD) | 0.9 (1.3) | 0.8 (1.3) | 0.05 | 0.9 (1.3) | 0.8 (1.3) | 0.05 |
| Selected comorbidities b , % | ||||||
| Cardiovascular diseases | 14.1 | 14.1 | 0.00 | 14.7 | 14.7 | 0.00 |
| Dyslipidaemia | 71.8 | 73.5 | 0.04 | 70.7 | 73.6 | 0.06 |
| Hypertension | 73.1 | 73.9 | 0.02 | 74.0 | 73.0 | 0.02 |
| Obesity | 36.9 | 38.2 | 0.03 | 30.8 | 31.1 | 0.01 |
| Index dose, % | ||||||
| Low dose (DU 0.75 mg; SEMA 0.25/0.5 mg) | 77.2 | 77.2 | 0.00 | 64.6 | Only 1 dose available | |
| High dose (DU 1.5 mg, SEMA 1.0 mg) | 22.8 | 22.8 | 0.00 | 35.4 | ||
| Antihyperglycaemic medication use b , % | ||||||
| Insulin | 31.9 | 32.1 | 0.00 | 29.9 | 28.6 | 0.03 |
| OADs | 85.3 | 85.3 | 0.00 | 85.8 | 85.8 | 0.00 |
| Metformin | 72.7 | 71.8 | 0.02 | 72.3 | 70.1 | 0.05 |
| SGLT2 inhibitors | 27.1 | 29.2 | 0.05 | 24.3 | 26.3 | 0.05 |
| DPP‐4 inhibitors | 26.5 | 25.4 | 0.03 | 25.6 | 25.8 | 0.00 |
| Sulphonylureas | 23.6 | 25.5 | 0.04 | 33.9 | 30.1 | 0.08 |
| TZDs | 5.5 | 6.6 | 0.05 | 6.9 | 8.1 | 0.05 |
|
Number of OAD classes Mean (SD) |
1.6 (1.0) | 1.6 (1.1) | 0.00 | 1.6 (1.1) | 1.6 (1.0) | 0.00 |
Abbreviations: aDCSI, adapted Diabetes Complications Severity Index; DPP‐4, dipeptidyl peptidase‐4; DU, dulaglutide; EBCise, exenatide BCise; OAD, oral anti‐hyperglycaemic drug; QCI, Quan–Charlson Comorbidity Index; SEMA, semaglutide; SGLT2, sodium‐glucose co‐transporter 2; Std diff, standardized difference; TZD, thiazolidinediones.
Demographic characteristics were evaluated on index date.
Clinical characteristics were assessed over the 6‐month pre‐index period.
Only exenatide BCise users were included for this study. Standardized differences of ≤0.10 were used to indicate cohort balance. Propensity scores for the dulaglutide vs. semaglutide cohorts were calculated using baseline covariates age, gender, region, prescribing healthcare provider specialty, diagnosis of obesity, presence of insulin fill, sulphonylurea fill, SGLT2 inhibitor fill, index dose, endocrinologist visit, and number of prescription classes filled. For the dulaglutide vs. exenatide BCise cohort, baseline variables for propensity scoring were age, gender, region, prescribing healthcare provider specialty, and plan type including Medicare Advantage.
3.2. Patient demographics and clinical characteristics
Table S1 and Table 1 show the demographic and clinical characteristics of the dulaglutide versus semaglutide and dulaglutide versus exenatide BCise cohorts before and after propensity‐score matching, respectively. Before matching, notable (standardized difference >0.10) between‐group differences for the dulaglutide versus semaglutide cohort were mean age (54.6 vs. 53.6 years), proportion of patients with obesity (30.5% vs. 38.2%), index dose (low dose, 65.3% vs. 77.2%; high dose, 34.7% vs. 22.9%), and use of certain OADs (SGLT2 inhibitors, 22.7% vs. 29.2%; sulphonylureas, 33.3% vs. 25.5%). There were no notable between‐group differences for the dulaglutide versus exenatide BCise cohort prior to matching. Besides the differences noted above, both cohorts were well balanced with regards to other clinical characteristics such as diabetes‐related comorbidities.
In the matched dulaglutide versus semaglutide cohort, the mean age was 54 years and 51% were women. A high proportion of patients had hypertension (73.1% vs. 73.9%), and dyslipidaemia (71.8% vs. 73.5%). Other prevalent complications included obesity (36.9% vs. 38.2%) and CV disease (14.1% in both). At baseline, 85% of patients were using at least one OAD, and 32% were using insulin. The matched dulaglutide versus exenatide BCise cohort was similar, with a mean age of 55 years and 48% women. A high proportion of patients had hypertension (74.0% vs. 73.0%) and dyslipidaemia (70.7% vs. 73.6%), and other prevalent comorbidities were obesity (30.8% vs. 31.1%) and CV disease (14.7% in both). At baseline, 86% of patients were using at least one OAD, and ~ 30% were using insulin. Demographics and clinical characteristics were well balanced in the two matched cohorts.
3.3. Adherence
Dulaglutide initiators had significantly higher mean (SD) PDC (75% [28] vs. 67% [28]; P <0.0001) and proportion of adherent patients (PDC ≥80%: 59.7% vs. 42.7%; P <0.0001) compared with semaglutide initiators, respectively, at 6‐month follow‐up (Figure 1). Dulaglutide initiators also had significantly higher mean (SD) PDC (75% [28] vs. 63% [30]; P <0.0001) and proportion of adherent patients (PDC ≥80%: 58.1% vs. 40.3%; P <0.0001) compared with exenatide BCise initiators, respectively (Figure 1).
FIGURE 1.
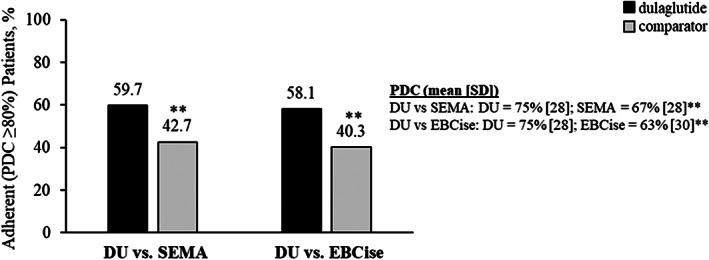
Adherence of matched cohorts during 6‐month follow‐up. **P <0.0001 vs. dulaglutide. DU, dulaglutide; EBCise, exenatide BCise; PDC, proportion of days covered; SEMA, semaglutide
3.4. Persistence
Using a 45‐day permissible gap, the mean (SD) number of days on dulaglutide and semaglutide therapy was 143.6 (58.2) days and 129.9 (64.2) days, respectively (P <0.0001 [Figure 2A]). A significantly greater proportion of dulaglutide initiators was persistent than semaglutide initiators (69.2% vs. 59.2%; P <0.0001 [Figure 2A]). Similar results were observed in the dulaglutide and exenatide BCise cohorts, where the mean (SD) number of days on therapy using a 45‐day permissible gap was 142.0 (58.4) days and 121.4 (62.3) days, respectively (P <0.0001; Figure 2B). Dulaglutide initiators were significantly more persistent than exenatide BCise initiators (67.9% vs. 50.6%; P <0.0001 [Figure 2B]). Cox proportional hazard models showed that patients initiating dulaglutide were significantly less likely to discontinue therapy than those initiating semaglutide (HR 0.71, 95% CI 0.66–0.76; P <0.0001) or exenatide BCise (HR 0.59, 95% CI 0.53–0.65; P <0.0001 [Figure 2A,B]).
FIGURE 2.
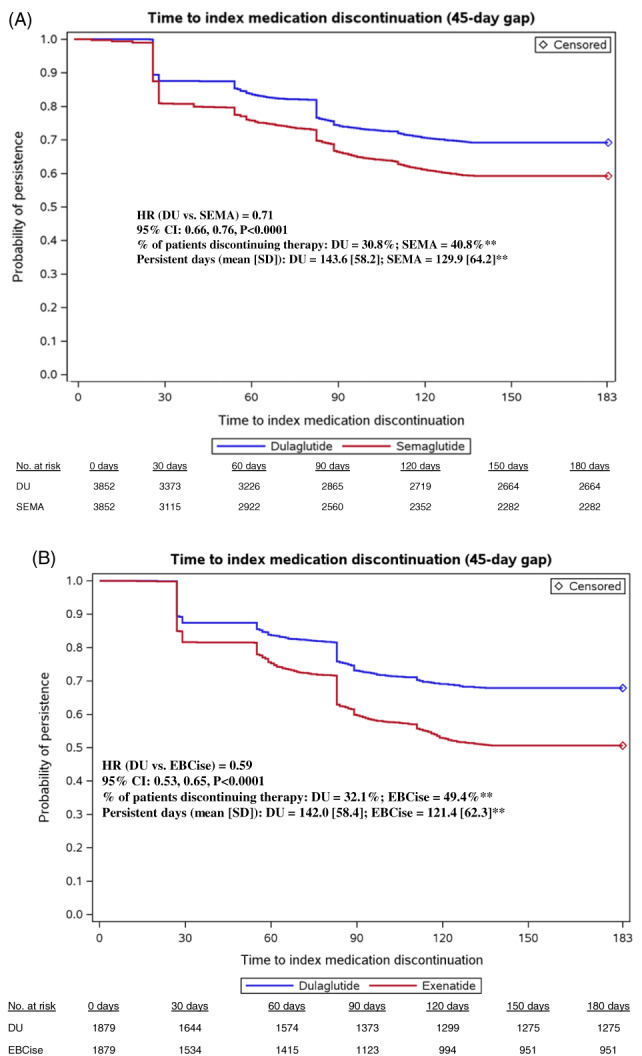
Persistence of matched cohorts during 6‐month follow‐up. **P < .0001 vs dulaglutide. CI, confidence interval; DU, dulaglutide; EBCise, exenatide BCise; HR, hazard ratio; No., number; SEMA,semaglutide
Using a 60‐day permissible gap, the mean (SD) number of days on dulaglutide and semaglutide therapy was 147.2 (56.2) days and 137.1 (61.2) days, respectively (P <0.0001; Table S2). A significantly greater proportion of dulaglutide initiators was persistent than semaglutide initiators (73.3% vs. 66.0%; P <0.0001 [Table S2]). The mean (SD) number of days on dulaglutide and exenatide BCise therapy was 146.0 (56.5) days and 124.1 (62.0) days, respectively (P <0.0001 [Table S2]). Dulaglutide initiators were significantly more persistent than exenatide BCise initiators (72.3% vs. 54.6%; P <0.0001 [Table S2]).
3.5. Other treatment pattern‐related outcome measures
In the dulaglutide and semaglutide matched cohorts, 77.5% of patients received the low dose (dulaglutide, 0.75 mg; semaglutide, 0.25 or 0.5 mg) as the index dose, and 22.9% received the high dose (dulaglutide, 1.5 mg; semaglutide, 1.0 mg) as the index dose. For the final fill, 54.2% versus 62.6% of dulaglutide versus semaglutide patients received the low dose, and 45.9% versus 37.5% received the high dose (P <0.0001, both). Through the 6‐month follow‐up, 52.1% versus 59.6% (P <0.0001) of patients received low dose only, 21.7% versus 20.2% (P = 0.1168) received high dose only, and 25.4% versus 17.9% (P <0.0001) initiated low dose and switched to high dose for dulaglutide compared with semaglutide, respectively.
The mean (SD) number of index drug fills was 4.2 (2.1) and 3.8 (2.0) for dulaglutide and semaglutide, respectively (P <0.0001). Two or more fills of the index drug were obtained by 87.6% and 85.3% (P = 0.0034) of patients in the dulaglutide and semaglutide cohorts, respectively; and 56.8% and 52.3% (P <0.0001) of patients obtained four or more fills of the index drug. Similar results were observed in the dulaglutide and exenatide BCise cohorts, where the mean (SD) number of index drug fills was 4.3 (2.2) and 3.6 (2.1), respectively (P <0.0001). Two or more fills of the index drug were obtained by 87.9% and 78.8% of patients in the dulaglutide and exenatide BCise cohorts, respectively; and 57.5% and 44.8% of patients obtained four or more fills of the index drug (P <0.0001, both). A summary of treatment patterns is provided in Table 2.
TABLE 2.
Treatment patterns of matched cohorts during 6‐month follow‐up
| DU vs. SEMA matched cohorts | DU vs. EBCise matched cohorts | |||
|---|---|---|---|---|
| DU, N = 3852 | SEMA, N = 3852 | DU, N = 1879 | EBCise, N = 1879 | |
| Initial dose fill, % | Only one dose available | |||
| Low | 77.5 | 77.5 | 65.6 | |
| High | 22.9 | 22.9 | 35.4 | |
| Final dose fill, % | ||||
| Low | 54.2 | 62.6** | 46.6 | |
| High | 45.9 | 37.5** | 53.5 | |
| Dosing pattern, % | ||||
| Low dose only | 52.1 | 59.6** | 44.8 | |
| High dose only | 21.7 | 20.2 | 33.5 | |
| Low dose to high dose | 25.4 | 17.9** | 20.8 | |
| All others | 1.2 | 2.7** | 1.9 | |
| Number of index drug fills | ||||
| Mean (SD) | 4.2 (2.1) | 3.8 (2.0)** | 4.3 (2.2) | 3.6 (2.1)** |
| Patients with ≥2 index drug fills, % | 87.6 | 85.3* | 87.9 | 78.8** |
| Patients with ≥4 index drug fills, % | 56.8 | 52.3** | 57.5 | 44.8** |
Abbreviations: DU, dulaglutide; EBCise, exenatide BCise; SEMA, semaglutide.
P <0.05 vs. dulaglutide.
P <0.0001 vs. dulaglutide.
At baseline, 85.3% (both cohorts) of patients were using at least one OAD and 31.9% versus 32.1% of patients were using insulin in the dulaglutide and semaglutide cohorts, respectively (Table 1). At 6‐month follow‐up, there were no significant differences in the proportion of patients using at least one OAD or in insulin use between the dulaglutide versus semaglutide cohorts and the dulaglutide versus exenatide BCise cohorts (Table 3).
TABLE 3.
Anti‐hyperglycaemic medication use of matched cohorts during 6‐month follow‐up
| DU vs. SEMA matched cohorts | DU vs. EBCise matched cohorts | |||
|---|---|---|---|---|
| Anti‐hyperglycaemic medication use, % | DU, N = 3852 | SEMA, N = 3852 | DU, N = 1879 | EBCise, N = 1879 |
| Insulin | 34.1 | 32.1 | 31.6 | 31.1 |
| OADs | 81.8 | 80.7 | 82.4 | 84.5 |
| Metformin | 69.9 | 67.5* | 68.6 | 67.9 |
| SGLT2 inhibitors | 26.7 | 28.8* | 24.4 | 29.7* |
| DPP‐4 inhibitors | 15.0 | 11.8** | 14.6 | 16.7 |
| Sulphonylureas | 21.9 | 20.4 | 29.5 | 26.8 |
| TZDs | 5.6 | 5.7 | 6.7 | 9.2* |
Abbreviations: DPP‐4, dipeptidyl peptidase‐4; DU, dulaglutide; EBCise, exenatide BCise; OAD, oral anti‐hyperglycaemic drug; SEMA, semaglutide; SGLT2, sodium‐glucose co‐transporter‐2; TZD, thiazolidinediones.
P <0.05 vs. dulaglutide.
P <0.0001 vs. dulaglutide.
3.6. Subgroup analysis
For the dulaglutide cohort, a greater proportion of patients who started on the low dose and those who started on the low dose then switched to and remained on the high dose were adherent compared to those who started on the high dose. Patients initiating dulaglutide in the absence of basal insulin use at baseline had a greater proportion who were adherent compared to those with basal insulin use at baseline. No other subgroup differences were observed for the dulaglutide cohort. A similar adherence pattern by dose was also observed for the semaglutide cohort; however, no other subgroup differences were observed within the semaglutide cohort. No subgroup differences were observed within the exenatide BCise cohort. A summary of adherence by subgroup is provided in Table S3.
Similar patterns for the subgroups were observed for the proportion of persistent patients within the dulaglutide and semaglutide cohort. Dulaglutide and semaglutide initiators starting on the low dose were more persistent than those starting on the high dose; those starting on the low dose then switching to and remaining on the high dose were more persistent than those only prescribed the high dose. No other subgroup differences were observed within the dulaglutide or semaglutide cohorts. No subgroup differences were observed within the exenatide BCise cohort. A summary of persistence by subgroup is provided in Table S4.
4. DISCUSSION
To our knowledge, this is the first study to report comparative results on medication adherence and persistence for dulaglutide versus semaglutide and versus exenatide BCise using real‐world data. Patients with T2D initiating dulaglutide showed better adherence and persistence over the 6‐month follow‐up period and had lower rates of treatment discontinuation compared with propensity‐score‐matched patients initiating semaglutide or exenatide BCise. The observed adherence rates for the dulaglutide cohorts were consistent with previous reports. Prior US studies reported 6‐month dulaglutide adherence rates of 54% to 61%, 17 , 18 which are similar to the 6‐month adherence rates reported in the present study (58% to 61%). Similarly, our observed 6‐month exenatide BCise adherence rate of 40.3% was within previously reported adherence rates (38% to 51% 17 , 18 , 20 ); however, it should be noted that the present study only included patients that used exenatide BCise, whereas other reports may have included more than one exenatide formulation. Given its recent approval, prior US studies regarding semaglutide adherence rates were not available at the time of the present study.
Through the 6‐month follow‐up period, greater proportions of patients initiating semaglutide or exenatide BCise discontinued therapy than those who initiated dulaglutide. The observed proportions of patients who discontinued therapy for the dulaglutide cohorts were consistent with prior reports. Prior 6‐month US studies reported 26% to 37% of dulaglutide‐treated patients discontinued therapy, 17 , 18 which is similar to the proportion of patients who discontinued dulaglutide in the present study (31% to 32%).
Analysis of treatment patterns among dulaglutide and semaglutide cohorts showed that patients who initiated the low dose and then switched to the high dose were more adherent across both cohorts compared to those with other dose patterns. Randomized controlled trials of dulaglutide and semaglutide have demonstrated dose‐dependent efficacy and gastrointestinal adverse events reporting, which in turn can potentially impact adherence and persistence. 28 , 29 Given this, it is important to understand adherence and persistence outcomes by dosing pattern in real‐world settings. Across all dose patterns, the dulaglutide cohort had better adherence compared to the semaglutide cohort. Although the present study did not assess reasons for higher adherence rates observed in the dulaglutide cohorts, differences in patients' preference for the injection device, administration, and/or tolerability profiles for the GLP‐1RAs may have been factors. In a study evaluating patient preference for dulaglutide and semaglutide injection devices among injection‐naïve patients with T2D receiving OADs, a greater proportion of patients preferred the dulaglutide device than the semaglutide device (84.2% vs. 12.3%; P <0.0001). 30 Patients perceived the dulaglutide device as having greater ease of use compared with the semaglutide device (86.8% vs. 6.8%; P <0.0001). 30 Patients' preference for a device may contribute to better willingness to use a medication, which could result in improved medication adherence and patient outcomes. Better adherence with dulaglutide versus semaglutide may also have been attibutable to differences in adverse event profiles. In a head‐to‐head clinical trial of dulaglutide versus semaglutide, numerically higher proportions of patients discontinued semaglutide due to adverse events compared with dulaglutide‐treated patients for both low‐ (8% vs. 5%) and high‐ (10% vs. 7%) dose patient cohorts. 31 Additionally, numerically higher proportions of semaglutide‐treated patients reported gastrointestinal adverse events compared with dulaglutide‐treated patients in the low‐dose cohort; gastrointestinal adverse events also led to a greater proportion of treatment discontinuations for semaglutide‐treated patients. 31
Treatment adherence is essential to achieving glycaemic control in patients with T2D. Prior studies have shown that adherent patients have greater reduction in HbA1c compared with non‐adherent patients. 19 , 32 , 33 Furthermore, 75% of the efficacy gap in HbA1c reduction between clinical trial and real‐world data is attributable to poor patient adherence. 33 Achieving glycaemic control may also offer long‐term cost savings. 34 Although achieving glycaemic control through improved medication adherence can contribute to higher diabetes‐related pharmacy costs, such costs may be offset by lower diabetes‐related medical costs. 19 , 35 , 36 In the present study, dulaglutide cohorts achieved better adherence compared with semaglutide and exenatide BCise cohorts, which could potentially yield improved patient outcomes as has been observed in prior reports evaluating the association between glycaemic control and adherence. 19 , 33
Literature reviews regarding interventions focused on improving T2D‐specific medication adherence and persistence have reported use of patient education programmes for disease management and medication use, intensive behavioural support, medication reminders, T2D care journals and daily logs, community pharmacist support, and other collaborative efforts between providers and patients. 16 , 37 , 38 , 39 , 40 Despite the availability of multifaceted interventions, long‐term, sustained improvements in medication adherence remain problematic for T2D treatment. 16 Long‐term poor adherence and persistence can increase the financial burden of T2D, 8 , 9 thus it is essential to identify treatment options that offer sustained glycaemic control and medication adherence.
To adjust for confounders of treatment selection, propensity‐score matching was used to ensure balance in measured baseline characteristics; however, as is typical of observational studies, the present study was limited by the potential for bias attributable to unmeasured confounders. Medical and pharmacy claims were used for data collection, which may have included undetected imputation errors. Some patient information (such as duration of diabetes or education) and provider characteristics that may be associated with the outcomes of interest were not available for analysis. Pharmacy claims are indicative of a prescription fill; however, it is unknown whether patients used the medication as prescribed. Additionally, pharmacy claims do not capture prescription fills purchased with cash or over the counter. Lastly, all patients included in the study were enrolled in US commercial health insurance plans and met all inclusion/exclusion criteria. Study results may not be generalizable to patients who were not selected for the treatment cohorts, or to those with other types of health insurance or those who are uninsured or reside outside of the United States.
In conclusion, this analysis is the first to examine real‐world adherence and persistence for three once‐weekly injectable GLP‐1RAs in the United States. The results of this study showed that more patients receiving dulaglutide for treatment of T2D were adherent and persistent at 6 months compared with those receiving semaglutide or exenatide BCise. Given the importance of adherence and its role in glycaemic control, these results should be considered when selecting a treatment option for improving outcomes in patients with T2D.
CONFLICTS OF INTEREST
R.M., M.Y. and M.K. are employees and stockholders of Eli Lilly and Company. B.N. and M.G. are employees of HealthCore Inc., a wholly owned subsidiary of Anthem Inc. HealthCore, Inc. was under contract with Eli Lilly and Company for the conduct of the study.
AUTHOR CONTRIBUTIONS
R.M., M.Y. and M.K. contributed to the study concept and design, data interpretation, and manuscript preparation and critical review. B.N. and M.G. contributed to the study concept and design, data collection, data interpretation and manuscript preparation and critical review. All authors have provided final approval of the manuscript. Some of the data were presented at the 80th annual meeting for the American Diabetes Association held virtually June 12–16, 2020.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14195.
Supporting information
Figure S1. Patient selection.
Table S1. Baseline characteristics of pre‐matched cohorts.
Table S2. Persistence outcomes for matched cohorts during 6‐month follow‐up using 60‐day gap.
Table S3. Adherence of matched cohorts by subgroups during 6‐month follow‐up.
Table S4. Persistence of matched cohorts by subgroups during 6‐month follow‐up.
ACKNOWLEDGMENTS
The authors thank Oralee Varnado, PhD for writing contributions.
Mody R, Yu M, Nepal B, Konig M, Grabner M. Adherence and persistence among patients with type 2 diabetes initiating dulaglutide compared with semaglutide and exenatide BCise: 6‐month follow‐up from US real‐world data. Diabetes Obes Metab. 2021;23:106–115. 10.1111/dom.14195
Funding information This work was supported by Eli Lilly and Company.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020. 2020. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 10, 2020.
- 2. Alwan A. Global Status Report on Noncommunicable Diseases 2010. https://www.who.int/nmh/publications/ncd_report2010/en/. Accessed August 28, 2020.
- 3. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81‐S90. [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Prevention and Control . National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 5. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes‐2020. Diabetes Care. 2020;43(Suppl 1):S98‐S110. [DOI] [PubMed] [Google Scholar]
- 6. Buse JB, Wexler DJ, Tsapas A, et al. Update to: Management of Hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2019;43(2):487‐493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blonde L, Raccah D, Lew E, et al. Treatment intensification in type 2 diabetes: a real‐world study of 2‐OAD regimens, GLP‐1 RAs, or basal insulin. Diabetes Ther. 2018;9(3):1169‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74‐109. [DOI] [PubMed] [Google Scholar]
- 9. Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care. 2012;35(12):2533‐2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller BR, Nguyen H, Hu CJ, Lin C, Nguyen QT. New and emerging drugs and targets for type 2 diabetes: reviewing the evidence. Am Health Drug Benefits. 2014;7(8):452‐463. [PMC free article] [PubMed] [Google Scholar]
- 11. Nichols GA, Rosales AG, Kimes TM, Tunceli K, Kurtyka K, Mavros P. The change in HbA1c associated with initial adherence and subsequent change in adherence among diabetes patients newly initiating metformin therapy. J Diabetes Res. 2016;2016:9687815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521‐530. [DOI] [PubMed] [Google Scholar]
- 13. Cramer JA, Benedict A, Muszbek N, Keskinaslan A, Khan ZM. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farr AM, Sheehan JJ, Curkendall SM, Smith DM, Johnston SS, Kalsekar I. Retrospective analysis of long‐term adherence to and persistence with DPP‐4 inhibitors in US adults with type 2 diabetes mellitus. Adv Ther. 2014;31(12):1287‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krass I, Schieback P, Dhippayom T. Adherence to diabetes medication: a systematic review. Diabet Med. 2015;32(6):725‐737. [DOI] [PubMed] [Google Scholar]
- 16. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016;10:1299‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alatorre C, Fernandez Lando L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon‐like peptide‐1 receptor agonists: higher adherence and persistence with dulaglutide compared with once‐weekly exenatide and liraglutide. Diabetes Obes Metab. 2017;19(7):953‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mody R, Grabner M, Yu M, et al. Real‐world effectiveness, adherence and persistence among patients with type 2 diabetes mellitus initiating dulaglutide treatment. Curr Med Res Opin. 2018;34(6):995‐1003. [DOI] [PubMed] [Google Scholar]
- 19. Mody R, Huang Q, Yu M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21(4):920‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu M, Xie J, Fernandez Lando L, Kabul S, Swindle RW. Liraglutide versus Exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther. 2016;38(1):149‐160. [DOI] [PubMed] [Google Scholar]
- 21. Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon‐like peptide‐1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31(11):1119‐1133. [DOI] [PubMed] [Google Scholar]
- 22. Wasser T, Wu B, Ycas J, Tunceli O. Applying weighting methodologies to a commercial database to project US census demographic data. Am J Accountable Care. 2015;9(15):33‐38. [Google Scholar]
- 23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 24. Chang HY, Weiner JP, Richards TM, Bleich SN, Segal JB. Validating the adapted diabetes complications severity index in claims data. Am J Manag Care. 2012;18(11):721‐726. [PubMed] [Google Scholar]
- 25. Pharmacy Quality Alliance . Adherence: PQA Adherence Measures. https://www.pqaalliance.org/adherence-measures. Accessed August 11, 2020.
- 26. Fairies DE, Leon AC, Haro JM, Obenchain RL. Analysis of Observational Health Care Data Using SAS. Cary, NC: SAS Institute, Inc; 2010. [Google Scholar]
- 27. Stuart EA. Matching methods for casual inference: a review and a look forward. Stat Sci. 2010;25(1):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goldenberg RM, Steen O. Semaglutide: review and place in therapy for adults with type 2 diabetes. Can J Diabetes. 2019;43(2):136‐145. [DOI] [PubMed] [Google Scholar]
- 29. Jendle J, Grunberger G, Blevins T, Giorgino F, Hietpas RT, Botros FT. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev. 2016;32(8):776‐790. [DOI] [PubMed] [Google Scholar]
- 30. Matza LS, Boye KS, Stewart KD, et al. Assessing patient PREFERence between the dulaglutide pen and the semaglutide pen: a crossover study (PREFER). Diabetes Obes Metab. 2020;22(3):355‐364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pratley RE, Aroda VR, Lingvay I, et al. Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open‐label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6(4):275‐286. [DOI] [PubMed] [Google Scholar]
- 32. Buysman EK, Liu F, Hammer M, Langer J. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther. 2015;32(4):341‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carls GS, Tuttle E, Tan RD, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real‐world use of GLP‐1 RA and DPP‐4 therapies in patients with type 2 diabetes. Diabetes Care. 2017;40(11):1469‐1478. [DOI] [PubMed] [Google Scholar]
- 34. Mody R, Huang Q, Yu M, et al. Clinical and economic outcomes among injection‐naive patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real‐world setting: the DISPEL study. BMJ Open Diabetes Res Care. 2019;7(1):e000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kennedy‐Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee WC, Balu S, Cobden D, Joshi AV, Pashos CL. Prevalence and economic consequences of medication adherence in diabetes: a systematic literature review. Manag Care Interface. 2006;19(7):31‐41. [PubMed] [Google Scholar]
- 37. Sapkota S, Brien JA, Greenfield J, Aslani P. A systematic review of interventions addressing adherence to anti‐diabetic medications in patients with type 2 diabetes: impact on adherence. PLoS One. 2015;10(2):e0118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vignon Zomahoun HT, de Bruin M, Guillaumie L, et al. Effectiveness and content analysis of interventions to enhance oral antidiabetic drug adherence in adults with type 2 diabetes: systematic review and meta‐analysis. Value Health. 2015;18(4):530‐540. [DOI] [PubMed] [Google Scholar]
- 39. Williams JL, Walker RJ, Smalls BL, Campbell JA, Egede LE. Effective interventions to improve medication adherence in type 2 diabetes: a systematic review. Diabetes Manag. 2014;4(1):29‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zullig LL, Gellad WF, Moaddeb J, et al. Improving diabetes medication adherence: successful, scalable interventions. Patient Prefer Adherence. 2015;9:139‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient selection.
Table S1. Baseline characteristics of pre‐matched cohorts.
Table S2. Persistence outcomes for matched cohorts during 6‐month follow‐up using 60‐day gap.
Table S3. Adherence of matched cohorts by subgroups during 6‐month follow‐up.
Table S4. Persistence of matched cohorts by subgroups during 6‐month follow‐up.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


