Abstract
The aim of this study was to investigate the appearance of a disturbed oropharyngeal microbiota during hospitalization and explore the patient characteristics that maybe associated with such a disturbance. Oropharyngeal swabs were collected from 134 patients at hospital admission and every 3–4 days thereafter. The samples were cultivated to determine the presence of a disturbed microbiota, which, in turn, was subcategorized into respiratory tract pathogens, gut microbiota and yeast species. Demographics, medical history data and hospitalization events were compared. The percentage of disturbed oropharyngeal microbiota increased significantly with length of stay (LOS). Receiving antibiotic treatment during the hospitalization tended to be associated with a disturbed microbiota (OR 2.75 [0.99–7.60]). Proton pump inhibitor (PPI) medication and receiving antibiotics before hospitalization were associated with the development of a disturbed oropharyngeal microbiota with colonization of gut pathogens (OR 3.49 [1.19–10.2] and OR 4.52 [1.13–18.1], respectively), while acute hospital admission was associated with a lower risk of colonization of gut pathogens (OR: 0.23 [0.074–0.72]). The risk of developing a disturbed oropharyngeal microbiota increased with LOS in hospitalized patients. PPI medication and receiving antibiotics before hospitalization were independent risk factors for developing oropharyngeal colonization of gut pathogens.
Keywords: Oropharyngeal microbiota, PPI, hospitalization, antibiotics, nosocomial infection
The microbiota of the oropharyngeal tract normally comprises a large variety of bacteria that helps maintain a balanced local environment with regard to aspects such as saliva pH and orodental health (1). Illness and medication can disturb this balance and thereby allow pathogens to colonize the oropharyngeal tract or facilitate the overgrowth of certain other species (2, 3). Examples of such opportunistic pathogens are gut bacteria, bacteria from the upper respiratory tract, and yeast species, as well as any combination of these three groups. Presence of oropharyngeal pathogens, even at low numbers, can be identified by conventional culture techniques. Microaspiration of these pathogens can lead to colonization of the lower respiratory tract and increase the risk of nosocomial pneumonia (NP) (4, 5). Colonization of the oropharyngeal tract by gut bacteria is associated with general severity of illness (3) and with proton pump inhibitor (PPI) medication; the latter relation has been shown in intensive care cohorts (6) as well as in non‐ICU cohorts at hospital admission (7). PPI use has been associated with a distorted gut microbiota, which in turn can lead to development of enteric infections in general and Clostridium difficile infection specifically (8, 9).
PPI use also increases the risk of developing NP (10, 11), hospital‐acquired pneumonia (HAP) and the more serious ventilator‐associated pneumonia (VAP) (12, 13, 14). The incidence of NP is as high as 5–10 cases per 1000 hospitalizations, and NP is the leading cause of mortality due to hospital‐acquired infections (15). An NP diagnosis increases hospital length of stay (LOS) by 7–10 days, and VAP prolongs mechanical ventilation times as well as ICU LOS (16). Also, hospital costs have been shown to rise by a factor of 5 in non‐ICU patients with a diagnosis of HAP (17). Therefore, early detection of a disturbed oropharyngeal microbiota, along with preservation of a normal oropharyngeal microbiota, may be a way to reduce the occurrence of NP to the benefit of the patient, the health care system and society.
The primary aim of the present study was to investigate the appearance of a disturbed oropharyngeal microbiota during the period of hospitalization. Our second objective was to identify the hospitalization events and/or patient characteristics associated with the development of a disturbed oropharyngeal microbiota during hospitalization. Given its primary and secondary objectives, the study also has the potential of describing a baseline from which potential interventions to maintain a normal oropharyngeal microbiota can be hypothesized.
MATERIALS AND METHODS
This clinical study was conducted at Skane University Hospital, Lund, Sweden, using an observational, longitudinal and comparative approach.
Ethical approval
The study protocol was reviewed and approved by the Regional Ethical Review Board, Lund, Sweden (no. 2013/764). Informed consent, including permission to collect and publish anonymous data, was obtained from all patients at enrolment.
Study population
During the period 6 February 2014 to 1 February 2017, 145 patients were enrolled in the study using the following inclusion criteria: age ≥ 18 years; possible to obtain the first oropharyngeal swab (OPS) within 24 h of hospital admission; expected LOS of> 72 h. The exclusion criterion was hospitalization in the preceding 14 days. Patient enrolment occurred during a 3‐year period with the aim of including as many patients as possible during this time with the resources at hand. Being an observational and non‐interventional study, no a priori power calculations were performed.
The patients were identified and enrolled at nine different wards: a medical high‐dependency unit, a surgical high‐dependency unit, two orthopaedic wards, two surgical wards and three internal medicine wards. Patients who changed wards during their stay were still eligible to remain in the study, and swabs were collected according to protocol.
Patient data
A standardized case report form was used to record the following patient data: age, gender, smoking status, alcohol consumption, physical fitness, body mass index (BMI), diabetes, ongoing systemic cortisone treatment, PPI medication, ongoing antibiotic treatment initiated >24 h before admission (referred to as ‘antibiotics before hospitalization’), lung disease at admission and whether the patient admission was acute or planned. The following hospitalization events were recorded: antibiotic treatment lasting >24 h during hospitalization (referred to as ‘antibiotics during hospitalization’), abdominal surgery during hospitalization, and occurrence of hospital‐acquired pneumonia. Patients admitted for elective orthopaedic surgery who only received a perioperative three‐dose regime of either cloxacillin or clindamycin were not classified as ‘antibiotics during hospitalization’, since that prophylactic perioperative treatment lasted for <24 h, the rationale being the vast scope of evidence, which suggests that short perioperative antibiotic prophylaxis does not markedly influence the oropharyngeal milieu (18). Definitions of variables and abbreviations are presented in Table 1.
Table 1.
Definitions of variables (short designations within parentheses)
| Variable | Definition |
|---|---|
| Age | dichotomous yes/no, >70 years |
| Gender | dichotomous yes/no, male |
| Smoking status | dichotomous yes/no, current or ex‐smoker |
| Alcohol consumption | dichotomous yes/no, alcohol intake> 2 times/week |
| Physical fitness | dichotomous yes/no, ability to climb two flights of stairs |
| Body mass index (BMI) | dichotomous yes/no, BMI> 35 |
| Occurrence of diabetes (diabetes) | dichotomous yes/no |
| Ongoing systemic cortisone treatment (cortisone treatment) | dichotomous yes/no |
| PPI medication | dichotomous yes/no |
| Ongoing antibiotic treatment initiated> 24 h before admission (antibiotics before hospitalisation) | dichotomous yes/no |
| Lung disease at admission | dichotomous yes/no |
| Acute admission to hospital (acute admission) | dichotomous yes/no |
| Antibiotic treatment for> 24 h during hospitalization (antibiotics during hospitalization) | dichotomous yes/no |
| Hospital‐acquired pneumonia | dichotomous yes/no |
| Gastrointestinal surgery during hospitalization (abdominal surgery) | dichotomous yes/no |
Oropharyngeal sample collection
Sterile Nylon® flocked swabs with 1 mL liquid Amies medium (ESwab™ 480C, COPAN Diagnostics Inc., Murrieta, CA, USA), were used to collect the samples. A tongue depressor and a flashlight were used to gain access to and visualize the pharynx. The swab was inserted into the posterior pharynx and rubbed over both the tonsillar pillars and the posterior oropharynx, carefully avoiding touching the tongue, teeth and gums. The sample was then transported to the Department of Microbiology for cultivation analyses.
The first OPS was collected within 24 h of the hospital admission (day 1) and the procedure was repeated on day 3 and approximately every fourth day thereafter throughout the patient’s entire LOS. In all other respects, the patients received standard care according to their diagnoses and the clinical decisions of the responsible physicians.
Microbiological procedures
The OPSs were processed by extended microbiological procedures at the Department of Clinical Microbiology, Skane University Hospital in Lund. The laboratory is accredited by the accreditation body (SWEDAC) designated by the Swedish government and is formally recognized as competent according to European and international standards.
For bacteria cultivation, sampling media were inoculated on five types of agar plates (three selective, one differentiating and one non‐selective). All plates were produced in‐house, sometimes using commercially available media components (5% horse blood, haematin agar and Uriselect 4 agar), as listed below:
Agar with 5% horse blood (LabM, Heywood, Lancashire, UK) supplemented with 10 mg/L colistin and 15 mg/L nalidixic acid with an optochin disc (selective)
Agar with 5% horse blood supplemented with 2 mg/L gentamicin and 25 mg/L nalidixic acid for Gram‐positive cocci including S. pneumonia (selective)
Haematin agar (Oxoid™, Thermo Science, Basingstoke, UK) supplemented with 300 mg/L bacitracin for fastidious Gram‐negative rods including H. influenzae (selective)
Uriselect 4 agar (Bio‐Rad Laboratories, Copenhagen, Denmark) supplemented with 10 mg/L vancomycin for non‐fastidious Gram‐negative rods (differentiating)
Haematin agar with a colistin disc (non‐selective)
The plates were inspected for growth after 16 and 40 h of aerobic, anaerobic or CO2 incubation at 35–37 °C. If an inspection result was ambiguous at 40 h, the plate was incubated for an additional 24 h to obtain a more definite result. Species identification of bacteria was performed using matrix‐assisted laser desorption/ionization time‐of‐flight (MALDI‐TOF) mass spectrometry (MALDI Biotyper Microbial Identification System, Bruker, Boston, MA, USA).
Cultivation and differentiation of Candida spp. were based on colony appearance on CHROM Candida agar (CHROMagar, Hägersten, Sweden) after 48 h of incubation at 35 °C.
Definitions of culture findings
For a sample to be considered representative of ‘oropharyngeal microbiota’, several bacterial species normally found in the oropharynx cavity were required to grow on the non‐selective haematin plate as determined by visual inspection by experienced senior microbiologists. In the oropharynx, the genera (the taxonomic rank above species) most commonly found are Streptococcus, Prevotella, Capnocytophaga, Rothia, Campylobacter, Veillonella, Neisseria and Haemophilus (19, 20), followed by a large group of less common genera. The plates were subsequently inspected for signs of a disturbed oropharyngeal microbiota, which required growth of species not normally found in the oropharyngeal cavity or overgrowth of normal oropharyngeal microbiota and/or overgrowth of yeast species.
Samples with a disturbed oropharyngeal microbiota were further assigned to one of three categories: gut microbiota, respiratory tract pathogens or yeast species (see Fig. 1), to aid the analysis, improve understanding of the underlying pathogenesis, and elucidate the results. This classification system with three categories has been used previously (7) and was developed in collaboration with senior microbiologists at the Department of Clinical Microbiology, and senior consultant specialists in Infectious Disease at the Skane University Hospital in Lund.
Fig 1.
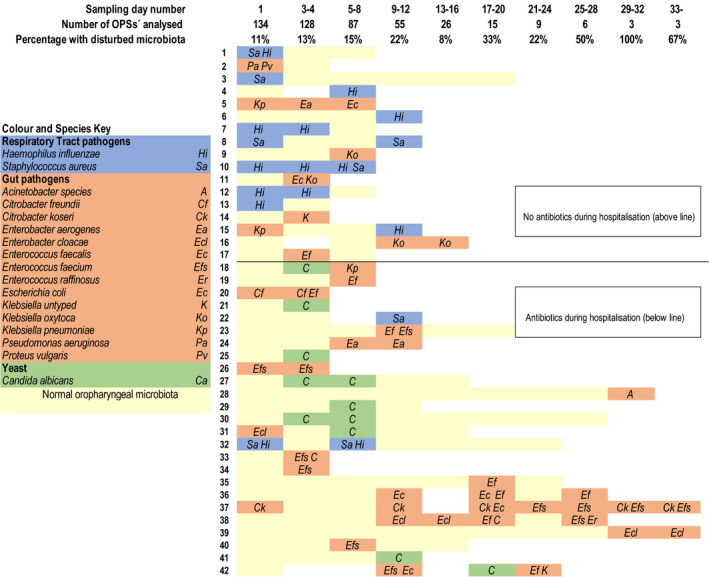
Oropharyngeal swab (OPS) culture results for the 42 patients who had at least one OPS sample with a disturbed microbiota during their hospitalization. Each horizontal bar represents the duration of a patient’s hospitalization, and the colour indicates the OPS result for each sampling occasion (day number): yellow = normal, blue = respiratory pathogens, terracotta = gut microbiota, green = yeast species for each sampling time point (sampling day number). The figure presents the number of OPSs collected/analysed and the percentage of OPSs with a disturbed microbiota for the total cohort at each sampling time point. The patients are dichotomized according to whether they did or did not receive antibiotics for >24 h during the hospitalization.
Statistical analyses
Continuous variables were presented with median, minimum and maximum values. Dichotomous variables were presented as number and as percentage of total number. For subjects with a normal oropharyngeal microbiota at inclusion, a univariable logistic regression was used to analyse the association between the patients’ characteristics (predicting variables) and the development of any type of disturbed oral microbiota (dependent variable), and also, specifically, the development of a disturbed oral microbiota with colonization of gut pathogens.. Thereafter, a multivariable logistic regression model using the three strongest predicting variables from the univariable analysis regarding colonization of gut pathogens was constructed in which one additional potential explanatory variable was added to determine whether the model improved or did not improve by including a fourth variable. Fisher’s exact test was used to assess the relationship between potential risk factors and HAP.
Statistical analyses were performed using IBM SPSS Statistics 22 for Windows (IBM Corporation, Armonk, NY, USA). Odds ratios (ORs) are presented with a 95% confidence interval (CI). P < 0.05 was regarded as significant, and all statistical tests were two tailed.
RESULTS
Initially, 145 patients were included and provided a first OPS sample. Eleven patients were subsequently excluded due to non‐adherence to protocol, 10 due to <2 OPSs during the hospital stay, and one due to inclusion >24 h after hospital admission. Thus, 134 patients met all inclusion criteria and contributed a total of 466 OPSs. The median number of OPSs per patient was 3 (2, 3, 4, 5, 6, 7, 8, 9, 10, 11), and all OPSs were representative of oropharyngeal microbiota.
The baseline patient characteristics and hospitalization characteristics of the cohort are presented in Table 2. Forty‐five patients (34%) were treated with PPI before and during their hospitalization. Twelve patients (9%) had antibiotic treatment before hospitalization, most of them with flucloxacillin or clindamycin. Sixty‐six (49%) received antibiotics during hospitalization. Sixteen patients (12%) had ongoing systemic cortisone treatment at admission. Ninety‐two (69%) of the 134 patients were acutely admitted to the hospital, and the most common reason for hospitalization in those cases was acute or elective abdominal or orthopaedic surgery.
Table 2.
Patient characteristics
| Variable | Patient cohort, n = 134 |
|---|---|
| Age, years | 72 (23–97) |
| Gender, male | 69 (51%) |
| Smoking status | 38 (28%) |
| Alcohol consumption | 77 (57%) |
| Physical fitness | 85 (63%) |
| Body mass index | 26 (15–43) |
| Diabetes | 22 (16%) |
| Cortisone treatment | 16 (12%) |
| Proton pump inhibitor medication | 45 (34%) |
| Antibiotics before hospitalization | 12 (9%) |
| Lung disease at admission | 29 (22%) |
| Acute admission | 92 (69%) |
| Antibiotics during hospitalization | 66 (49%) |
| Hospital‐acquired pneumonia | 6 (4.5%) |
| Abdominal surgery | 29 (22%) |
See Table 1 for definitions of the variables. Age and BMI are presented as median (range). The other variables are presented as number (valid percentages).
Figure 1 presents the microbiological results for the 42 patients who presented with any type of disturbed oropharyngeal microbiota on any of the sampling occasions. Approximately 22% of these OPSs had polymicrobial results (i.e. they met the definitions of more than one of the three subclasses of disturbed microbiota). We found that the longer the hospital stay, the greater the proportion of collected OPSs with a disturbed microbiota. The majority of OPSs that were collected after day 12 and showed a disturbed oropharyngeal microbiota were subclassed as gut microbiota (Fig. 1).
In 119 patients (89%), the first OPS (at admission) was normal. In this group, the univariable analyses showed that antibiotics given before and during hospitalization predicted development of a disturbed oropharyngeal microbiota (Table 3). In the multivariable analyses, antibiotics during hospitalization were the only variable close to being statistically significant for the occurrence of a disturbed oropharyngeal microbiota in this group (P = 0.052). Restricting the univariable analyses to colonization of gut pathogens showed that PPI medication and antibiotics before hospitalization were associated with an increased risk of colonization of gut pathogens, whereas acute hospital admission was associated with a decreased risk (Table 4). In the best‐fitting multivariable regression model, ongoing PPI medication was associated with an increased risk of colonization of gut pathogens, and acute hospital admission was associated with a decreased risk of developing such a disturbed microbiota (Table 5).
Table 3.
Univariable logistic regression analysis of occurrence of a disturbed oropharyngeal microbiota during hospitalization in 119 subjects with a normal microbiota at admission
| Variable | n | OR (95% CI) | P value |
|---|---|---|---|
| Age, years | 0.99 (0.97–1.02) | 0.654 | |
| ≤70 | 57 | 1.20 (0.51–2.83) | 0.683 |
| >70 | 62 | ||
| Gender | |||
| Female | 58 | 1.52 (0.64–3.63) | 0.346 |
| Male | 61 | ||
| Smoking status | |||
| No | 86 | 0.69 (0.25–1.89) | 0.469 |
| Yes | 33 | ||
| Alcohol consumption | |||
| No | 46 | 2.10 (0.81–5.46) | 0.127 |
| Yes | 73 | ||
| Physical fitness | |||
| No | 39 | 0.78 (0.32–1.92) | 0.592 |
| Yes | 80 | ||
| Body mass index | 119 | ||
| ≤35 | 108 | 2.11 (0.57–7.84) | 0.264 |
| >35 | 11 | ||
| Diabetes | |||
| No | 97 | 2.35 (0.86–6.40) | 0.096 |
| Yes | 22 | ||
| Cortisone treatment | |||
| No | 104 | 2.63 (0.84–8.23) | 0.095 |
| Yes | 15 | ||
| Proton pump inhibitor medication | |||
| No | 80 | 2.36 (0.98–5.69) | 0.056 |
| Yes | 39 | ||
| Antibiotics before hospitalization | |||
| No | 109 | 3.95 (1.05–14.9) | 0.042 |
| Yes | 10 | ||
| Lung disease at admission | |||
| No | 95 | 0.87 (0.29–2.61) | 0.808 |
| Yes | 24 | ||
| Acute admission | |||
| No | 38 | 0.49 (0.20–1.19) | 0.116 |
| Yes | 81 | ||
| Antibiotics during hospitalization | |||
| No | 59 | 2.95 (1.17–7.43) | 0.021 |
| Yes | 60 | ||
| Abdominal surgery | |||
| No | 92 | 1.62 (0.61–4.26) | 0.330 |
| Yes | 27 | ||
Table 4.
Univariable logistic regression analysis of colonization of gut pathogens in the oropharyngeal swabs during hospitalization in 119 subjects with a normal microbiota at admission
| Variable | n | OR (95% CI) | P value |
|---|---|---|---|
| Age, years | 0.99 (0.96–1.02) | 0.426 | |
| ≤70 | 57 | 0.91 (0.33–2.47) | 0.846 |
| >70 | 62 | ||
| Gender | |||
| Female | 58 | 1.23 (0.45–3.36) | 0.693 |
| Male | 61 | ||
| Smoker | |||
| No | 86 | 0.71 (0.22–2.34) | 0.572 |
| Yes | 33 | ||
| Alcohol consumption | |||
| No | 46 | 2.49 (0.77–8.10) | 0.129 |
| Yes | 73 | ||
| Physical fitness | |||
| No | 39 | 1.32 (0.43–4.01) | 0.625 |
| Yes | 80 | ||
| Body mass index | |||
| ≤35 | 108 | 2.32 (0.55–9.76) | 0.249 |
| >35 | 11 | ||
| Diabetes | |||
| No | 97 | 2.66 (0.87–8.11) | 0.086 |
| Yes | 22 | ||
| Cortisone treatment | |||
| No | 104 | 2.34 (0.65–8.37) | 0.192 |
| Yes | 15 | ||
| Proton pump inhibitor medication | |||
| No | 80 | 4.10 (1.44–11.6) | 0.008 |
| Yes | 39 | ||
| Antibiotics before hospitalization | |||
| No | 109 | 4.52 (1.13–18.1) | 0.033 |
| Yes | 10 | ||
| Lung disease at admission | |||
| No | 95 | 1.16 (0.34–3.90) | 0.814 |
| Yes | 24 | ||
| Acute admission | |||
| No | 38 | 0.23 (0.082–0.66) | 0.006 |
| Yes | 81 | ||
| Antibiotics during hospitalization | |||
| No | 59 | 2.21 (0.77–6.34) | 0.141 |
| Yes | 60 | ||
| Abdominal surgery | |||
| No | 92 | 1.90 (0.64–5.67) | 0.247 |
| Yes | 27 | ||
Table 5.
Multivariable logistic regression analysis of colonization of gut pathogens in the oropharyngeal swabs during hospitalization in 119 subjects with a normal microbiota at admission
| Variable | n | OR (95% CI) | P value |
|---|---|---|---|
| Proton pump inhibitor | |||
| No | 59 | 2.10 (0.60–7.28) | 0.243 |
| Yes | 60 | ||
| Antibiotics before hospitalization | |||
| No | 109 | 2.08 (0.41–10.4) | 0.375 |
| Yes | 10 | ||
| Acute admission | |||
| No | 38 | 0.23 (0.074–0.72) | 0.012 |
| Yes | 81 | ||
| Antibiotics during hospitalization | |||
| No | 59 | 2.10 (0.60–7.28) | 0.243 |
| Yes | 60 | ||
Both PPI medication and antibiotics before hospitalization were associated with an increased risk of acquiring HAP (Table 6).
Table 6.
Hospital‐acquired pneumonia (n = 5)
| Variable | Hospital‐acquired pneumonia | P value 1 | |
|---|---|---|---|
| No | Yes | ||
| (n = 114) | (n = 5) | ||
| no. (%) | no. (%) | ||
| Proton pump inhibitor medication | |||
| No | 79 (69.3) | 1 (20.0) | 0.039 |
| Yes | 35 (30.7) | 4 (80.0) | |
| Antibiotics before hospitalization | |||
| No | 108 (94.7) | 1 (20.0) | <0.001 |
| Yes | 6 (5.3) | 4 (80.0) | |
| Acute admission | |||
| No | 36 (31.6) | 2 (40.0) | 0.654 |
| Yes | 78 (68.4) | 3 (60.0) | |
| Antibiotics during hospitalization | |||
| No | 59 (51.8) | 0 (0.0) | 0.057 |
| Yes | 55 (48.2) | 5 (100.0) | |
Fisher’s exact test.
Cox regression analyses of the data gave no additional information regarding the development of a disturbed oropharyngeal microbiota and its correlation to LOS.
DISCUSSION
In this study, we found that a significant proportion of a mixed patient cohort admitted to hospital developed a disturbed oropharyngeal microbiota during their hospitalization, and that the proportion of included patients who had a disturbed microbiota increased with the length of hospital stay. In patients with a long LOS and a disturbed oropharyngeal microbiota, gut species most commonly caused the disturbance. The risk of developing HAP was increased among patients treated with PPI and/or antibiotics before their hospital admission, but the numbers were small (N = 5). PPI medication was an independent risk factor for colonization of gut pathogens. The subgroup acutely admitted to hospital had a lower risk of acquiring gut microbiota disturbance.
The oropharyngeal bacterial microbiota has a strong impact on the microenvironment in the lower respiratory tract, presumably due to continuous microaspiration of bacteria into the lungs (5). Consequently, a disturbed oropharyngeal microbiota may be a precursor to pneumonia.
In this study, we identified PPI medication as a risk factor for colonization of gut pathogens in the oropharynx and also as a risk factor for developing HAP. These observations corroborate previous studies showing that PPI medication was associated with the development of VAP and HAP (10, 13), and also changes in oropharyngeal microbiota (21). Ongoing antibiotic treatment initiated> 24 h before admission was a risk factor for occurrence of any type of disturbed microbiota during hospitalization. It is well known that antibiotics change the normal microbiota, primarily in the large intestine but also in the oropharyngeal and respiratory tract (22, 23, 24, 25). There are indications that antibiotic‐induced microbiota changes arise more slowly in the oropharynx as compared with other parts of the gastrointestinal tract (23, 26). If a patient undergoes antibiotic therapy before being hospitalized, the effect of that treatment on the oropharyngeal microbiota may therefore occur during the subsequent hospital stay. Ongoing antibiotic treatment> 24 h prior to admission may also be a marker of overall fragility and ‘degree of illness’. As stated by Johanson et al. (3), ‘the appearance of a Gram‐negative bacillary microbiota in our patients correlated best with a clinical impression of the degree of illness’.
Those admitted acutely for hospital care in our cohort had a reduced risk of oropharyngeal colonization of gut pathogens. This suggests that there may be differences between patients admitted for elective procedures and those acutely admitted with respect to characteristics not accounted for in this study. A potential explanation is that electively admitted patients’ general health as a whole was undermined by a chronic disorder, whereas a greater proportion of those receiving emergency care were quite healthy prior to their hospitalization.
Our results are important. To our knowledge, we are the first to have performed consecutive sampling in a mixed cohort of hospitalized ward patients. We are the only group to have used the subcategorization of respiratory tract pathogens, yeast and gut pathogens when studying and describing microbiota changes over time. In an earlier study by our group, which involved intensive care patients as well as ward patients, PPI treatment was associated with the gut‐pathogen colonization subclass of disturbed oropharyngeal flora (7). Those results strengthened our belief that it is rational to differentiate between different types of oropharyngeal microbiota disturbances when trying to understand potential causes and cures. Our present results support this notion, since it appears that the pathogenesis of overgrowth of already existing microbiota and yeasts differs from the pathogenesis of gut flora colonization.
The vast majority of previous research into pathophysiology, prevention and treatment of HAP comes from ICU environments and may therefore not be fully applicable or relevant to a general ward cohort setting (27). Only a few previous studies have investigated the incidence of HAP and HAP‐associated risk factors outside an ICU setting (28, 29, 30). Whereas these studies analysed risk factors associated with HAP, our study aimed at identifying potential pre‐stages to HAP in a relatively large, non‐selected, consecutive cohort of ward patients, which has not been done before.
Our findings reinforce the need for vigilance in the care of patients who have risk factors associated with the development of a disturbed oropharyngeal microbiota. Patients admitted with ongoing antibiotic treatment and/or PPI medication may well benefit from more aggressive physiotherapy aimed at maximizing lung aeration and minimizing aspiration; cough training, basic lung expansion therapy and an upright position are all relatively easily accomplished prophylactic measures. Careful consideration of whether there is a real or a relative indication for PPI treatment is also called for, as is stringent and optimized antibiotic stewardship to avoid unnecessary and potentially harmful antibiotics. Administration of probiotics may also promote and/or restore a normal microbiota (31, 32, 33, 34), but its potential needs further exploration.
Our study has limitations. Investigation of a larger cohort for a longer period would have been preferable, enabling us to also evaluate whether a subset of our cohort developed clinical symptoms associated with, for example, pneumonia. Modern Swedish healthcare, however, is characterized by very early hospital discharges, and only a small proportion of our cohort was hospitalized more than a week, which reduced the possibility of identifying long‐term alterations in the oropharyngeal microbiota and any late clinical deterioration. Furthermore, classic cultivation techniques have the disadvantage of not disclosing slow‐growing anaerobes or non‐cultivable bacterial species. Hence, we cannot know if such species were present in our cohort. The rationale for using accredited classic cultivation techniques is that we have striven to align with current clinical practice, where OPS sampling routinely provides the treating clinician with guidance in choosing the right treatment for the patient when an emerging airway infection is suspected. While molecular genomic techniques might have enhanced our ability to detect and identify specific pathogens in our samples (35, 36), these techniques introduce other sources of errors and are generally more suitable when searching for specific culprit pathogenic bacteria or when analysing samples that ideally should not host a microbiota, for example, cerebral spinal fluid. There are, however, some well‐performed recent studies where deep sequencing of the amplified 16S rRNA gene has been used to show oropharyngeal microbiota changes associated with PPI medication and antibiotic therapy (21, 24, 25). Their results are in line with our results, but it needs to be emphasized that the study populations differ, since those studies were performed on out‐of‐hospital patients with chronic disease undergoing long‐term PPI/antibiotic treatment.
Based on the results of this clinical observational study, we conclude that the oropharyngeal microbiota seems to undergo changes during hospitalization. We note that risk factors for disturbance of the oropharyngeal microbiota and for the development of HAP include antibiotic exposure, length of hospital stay and the use of PPI medication. Considering the insights gained in this investigation, it would be interesting to pursue this research area by determining whether different types of interventions can attenuate the aforementioned processes. Interventional ‘care bundles’ could potentially include intentional discontinuation of PPI treatment, vigorous physiotherapy, vigilant antibiotic stewardship and probiotic treatment.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
FUNDING
This work was funded by research grants from Swedish Region Skane, with no specific grant from any other funding agency.
ACKNOWLEDGEMENTS
Thank you Maria Celander, Department of Clinical Microbiology in Skane University Hospital, for valuable help regarding interpretation and understanding of the results and methodology.
Tranberg A, Samuelsson C, Klarin B. Disturbance in the oropharyngeal microbiota in relation to antibiotic and proton pump inhibitor medication and length of hospital stay. APMIS. 2020; 129: 14–22.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W, et al. The human oral microbiome. J Bacteriol 2010;192:5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jia G, Zhi A, Lai PFH, Wang G, Xia Y, Xiong Z, et al. The oral microbiota – a mechanistic role for systemic diseases. B Dent J 2018;224:447–55. [DOI] [PubMed] [Google Scholar]
- 3. Johanson WG, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients: emergence of gram‐negative bacilli. N Engl J Med 1969;281:1137–40. [DOI] [PubMed] [Google Scholar]
- 4. Robinson J. Colonization and infection of the respiratory tract: what do we know? Paediatr Child Health 2004;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two‐way street. Mucosal Immunol 2017;10:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frandah W, Colmer‐Hamood J, Mojazi Amiri H, Raj R, Nugent K. Oropharyngeal flora in patients admitted to the medical intensive care unit: clinical factors and acid suppressive therapy. J Med Microbiol 2013;62(Pt 5):778–84. [DOI] [PubMed] [Google Scholar]
- 7. Tranberg A, Thorarinsdottir HR, Holmberg A, Schott U, Klarin B. Proton pump inhibitor medication is associated with colonisation of gut flora in the oropharynx. Acta Anaesthesiol Scand 2018;62:791–800. [DOI] [PubMed] [Google Scholar]
- 8. Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology 2015;149:883–85.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA 2009;301:2120–28. [DOI] [PubMed] [Google Scholar]
- 11. Bateman BT, Bykov K, Choudhry NK, Schneeweiss S, Gagne JJ, Polinski JM, et al. Type of stress ulcer prophylaxis and risk of nosocomial pneumonia in cardiac surgical patients: cohort study. BMJ 2013;347:f5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid‐suppressive drugs and risk of pneumonia: a systematic review and meta‐analysis. CMAJ 2011;183:310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MacLaren R, Reynolds PM, Allen RR. Histamine‐2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med 2014;174:564–74. [DOI] [PubMed] [Google Scholar]
- 14. Alshamsi F, Belley‐Cote E, Cook D, Almenawer SA, Alqahtani Z, Perri D, et al. Efficacy and safety of proton pump inhibitors for stress ulcer prophylaxis in critically ill patients: a systematic review and meta‐analysis of randomized trials. Crit Care 2016;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flanders SA, Collard HR, Saint S. Nosocomial pneumonia: state of the science. Am J Infect Control 2006;34:84–93. [DOI] [PubMed] [Google Scholar]
- 16. Nair GB, Niederman MS. Nosocomial pneumonia: lessons learned. Crit Care Clin 2013;29:521–46. [DOI] [PubMed] [Google Scholar]
- 17. Çakir Edis E, Hatipoğlu ON, Yılmam İ, Süt N. Economic burden of nosocomial pneumonia in non‐intensive care clinics. Tuberk Toraks 2015;63:8–12. [DOI] [PubMed] [Google Scholar]
- 18. Sullivan A, Edlund C, Svenungsson B, Emtestam L, Nord CE. Effect of perorally administered pivmecillinam on the normal oropharyngeal, intestinal and skin microflora. J Chemother 2001;13:299–308. [DOI] [PubMed] [Google Scholar]
- 19. Retchless AC, Kretz CB, Rodriguez‐Rivera LD, Chen A, Soeters HM, Whaley MJ, et al. Oropharyngeal microbiome of a college population following a meningococcal disease outbreak. Sci Rep 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Human pharyngeal microbiome may play a protective role in respiratory tract Infections. Elsevier Enhanced Reader. Available at: https://reader.elsevier.com/reader/sd/pii/S1672022914000485?token=77716CA9F33E483FF561FBFBCEE480B139B61AB962CEC4A3C9CB54E926A48A318B20A440E5BB42401E26F1A303716B42. Accessed 20 Jul 2020. [DOI] [PMC free article] [PubMed]
- 21. Rosen R, Hu L, Amirault J, Khatwa U, Ward DV, Onderdonk A. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. J Pediatr 2015;166:917–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaura E, Brandt BW, Teixeira de Mattos MJ, Buijs MJ, Caspers MPM, Rashid M‐U, et al. Same exposure but two radically different responses to antibiotics: resilience of the salivary microbiome versus long‐term microbial shifts in feces. MBio 2015;6:e01693–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rashid M, Weintraub A, Nord CE. Effect of new antimicrobial agents on the ecological balance of human microflora. Anaerobe 2012;18:249–53. [DOI] [PubMed] [Google Scholar]
- 24. Choo JM, Abell GCJ, Thomson R, Morgan L, Waterer G, Gordon DL, et al. Impact of long‐term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non‐cystic fibrosis bronchiectasis. mSphere 2018;3:e00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dos Santos Lopes, Santiago Guido, Brusselle G, Dauwe K, Deschaght P, Verhofstede C, et al. Influence of chronic azithromycin treatment on the composition of the oropharyngeal microbial community in patients with severe asthma. BMC Microbiol 2017;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edlund C, Nord CE. Manipulation of the oropharyngeal and intestinal microflora by norfloxacin: microbiological and clinical aspects. Scand J Infect Dis Suppl 1988;56:14–21. [PubMed] [Google Scholar]
- 27. Di Pasquale M, Aliberti S, Mantero M, Bianchini S, Blasi F. Non‐intensive care unit acquired pneumonia: a new clinical entity? Int J Mol Sci 2016;17(3):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sopena N, Heras E, Casas I, Bechini J, Guasch I, Pedro‐Botet ML, et al. Risk factors for hospital‐acquired pneumonia outside the intensive care unit: a case‐control study. Am J Infect Control 2014;42:38–42. [DOI] [PubMed] [Google Scholar]
- 29. Ewan V, Hellyer T, Newton J, Simpson J. New horizons in hospital acquired pneumonia in older people. Age Ageing 2017;46:352–58. [DOI] [PubMed] [Google Scholar]
- 30. Thompson DA, Makary MA, Dorman T, Pronovost PJ. Clinical and economic outcomes of hospital acquired pneumonia in intra‐abdominal surgery patients. Ann Surg 2006;243(4):547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008;111:1–66. [DOI] [PubMed] [Google Scholar]
- 32. Fooks LJ, Gibson GR. Probiotics as modulators of the gut flora. Br J Nutr 2002;88(Suppl 1):39. [DOI] [PubMed] [Google Scholar]
- 33. Klarin B, Wullt M, Palmquist I, Molin G, Larsson A, Jeppsson B. Lactobacillus plantarum 299v reduces colonisation of Clostridium difficile in critically ill patients treated with antibiotics. Acta Anaesthesiol Scand 2008;52:1096–102. [DOI] [PubMed] [Google Scholar]
- 34. Kujawa‐Szewieczek A, Adamczak M, Kwiecień K, Dudzicz S, Gazda M, Więcek A. The effect of Lactobacillus plantarum 299v on the incidence of Clostridium difficile infection in high risk patients treated with antibiotics. Nutrients 2015;7(12):10179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sontakke S, Cadenas MB, Maggi RG, Diniz PP, Breitschwerdt EB. Use of broad range16S rDNA PCR in clinical microbiology. J Microbiol Methods 2009;76:217–25. [DOI] [PubMed] [Google Scholar]
- 36. Harris KA, Hartley JC. Development of broad‐range 16S rDNA PCR for use in the routine diagnostic clinical microbiology service. J Med Microbiol 2003;52:685–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


