Abstract
Asymmetrically substituted tertiary phosphines and quaternary phosphonium salts are used extensively in applications throughout industry and academia. Despite their significance, classical methods to synthesize such compounds often demand either harsh reaction conditions, prefunctionalization of starting materials, highly sensitive organometallic reagents, or expensive transition‐metal catalysts. Mild, practical methods thus remain elusive, despite being of great current interest. Herein, we describe a visible‐light‐driven method to form these products from secondary and primary phosphines. Using an inexpensive organic photocatalyst and blue‐light irradiation, arylphosphines can be both alkylated and arylated using commercially available organohalides. In addition, the same organocatalyst can be used to transform white phosphorus (P4) directly into symmetrical aryl phosphines and phosphonium salts in a single reaction step, which has previously only been possible using precious metal catalysis.
Keywords: alkylation, arylation, phosphane, phosphorus, photocatalysis
Driven by light: A mild and facile photoredox approach towards asymmetrically substituted phosphines and phosphonium salts is reported. Blue‐light irradiation of mono‐ and diphenylphosphine with various aryl and alkyl iodides, diisopropylethylamine (DIPEA), and the organic photocatalyst 3DPAFIPN affords the desired products in good‐to‐excellent yields. In addition, the same method transforms white phosphorus (P4) directly into symmetrical aryl phosphines and phosphonium salts.

Introduction
Tertiary phosphines (PR3) and the related quaternary phosphonium salts (PR4 +) are of great significance in both industrial and academic chemistry. As such, the development of new methods for the synthesis of these compounds remains an important, ongoing research challenge. In particular, there is a strong desire to develop new routes for the preparation of asymmetrically substituted PR2R′ and PR3R′+ products, which find extensive uses throughout chemistry. For example, the former are ubiquitous in the fields of coordination chemistry (including for biomedical applications) and catalysis, where they are used as ‘designer’ and chelating ligands with well‐defined and optimized steric and electronic properties. [1] The latter, meanwhile, are used as phase transfer catalysts, [2] and components of ionic liquids, [3] among a number of other applications. [4] Unfortunately, the number of practical methods for the preparation of these compounds remains limited, which creates a barrier to research and can curtail developments in these fields.
Classical methods for the preparation of asymmetrical tertiary phosphines (I, III, see Figure 2) involve the nucleophilic substitution of halophosphines with organometallic reagents, reaction of metal phosphides with organic halides, and reduction of mixed phosphine oxides (Figure 1 a). [5] However, these methods can suffer from the use of hazardous or sensitive reagents, harsh reaction conditions, difficult procedures with problematic reproducibility and/or poor product yields. Transition metal (Pd, Ni, Cu) catalyzed condensations of secondary phosphines with organic halides or pseudo halides may also be employed, but often require an expensive transition metal catalyst and forcing reaction conditions (Figure 1 b, top). [6] Alternatively, hydrophosphination of alkenes or alkynes [7] can be catalyzed by transition metal complexes (Fe, Ni, Pd, Cu), [8] or by rare‐earth‐metal complexes (La, Yb), [9] or may proceed without a catalyst in certain cases (Figure 1 b, bottom). [10] While sometimes very effective, these reactions can only be used to introduce alkyl substituents with a β‐H atom, and often suffer from issues of regioselectivity. Very recently, radical cross‐coupling of N‐hydroxyphthalimide esters with chlorophosphines was reported, mediated either by a metal reductant (Zn) or an iridium photocatalyst. [11] While this protocol provided a broad range of tertiary phosphines, pre‐functionalization steps were required to obtain the activated esters, which limits the attractiveness of these reactions.
Figure 2.

Overview of the broad scope of symmetrically and asymmetrically substituted phosphines and phosphonium salts accessed in this work, and the corresponding product numbering scheme. R=alkyl, Ar=aryl.
Figure 1.

General methods to synthesize asymmetrical tertiary phosphines and quaternary phosphonium salts; R, R′=aryl, alkyl; M=metal; X=leaving group.
Asymmetrical quaternary phosphonium salts (II, IV, see Figure 2) are mostly synthesized from tertiary phosphines through nucleophilic attack on alkyl or aryl (pseudo) halides and transition metal catalyzed (Pd, Ni) arylation using haloarenes at (very) high temperatures (>140 °C; Figure 1 c), [12] although a small number of metal‐free reactions have also been reported. [13] One of these involves the trapping of in situ generated arynes by tertiary phosphines but provides only poor regioselectivity. [13a] Another recently developed reaction of PPh3 with bromoarenes in phenol requires very high temperature (180 °C). [13b] Alternatively, visible light‐driven reactions with iodonium salts have been reported, either mediated by a Ru‐based photosensitizer, [14] or using a metal free protocol facilitated by the formation of an electron donor‐acceptor complex formed from iodonium salts and tertiary phosphines (Figure 1 d). [15] In both cases, the need for prior synthesis of the iodonium salts places a limit on the overall usefulness of the reaction. On the other hand, there are only a very few examples which enable the synthesis of asymmetrical quaternary phosphonium salts from secondary phosphines or chlorophosphines using the conventional strategy of exploiting transition metal (Pd, Ni) catalysts at elevated temperatures (>150 °C). [16] Thus, despite a variety of methods having been reported for the synthesis of asymmetrical tertiary phosphines and quaternary phosphonium salts, a mild, practical and general method for their formation from readily‐available precursors remains elusive.
In recent years, visible‐light photoredox catalysis has become a powerful synthetic tool that has enabled the development of many novel organic transformations. [17] We recently demonstrated the visible light‐driven, iridium‐catalyzed direct functionalization of white phosphorus, which gives triarylphosphines and tetraarylphosphonium salts under mild reaction conditions. [18] It was found that this method arylates P4 in a stepwise manner, giving rise to H2PAr, HPAr2, PAr3, and PAr4 + products in a well‐defined sequence. Building upon these observations, herein we report that the same reaction protocol can be used to provide convenient access to asymmetrical tertiary phosphines and quaternary phosphonium salts, by starting from the commercially available primary and secondary phosphines H2PPh and HPPh2. Furthermore, we show that the previously employed noble metal photocatalyst can be replaced by the inexpensive organic photocatalyst 3DPAFIPN, not only in these reactions but also in the direct arylation of P4. [19] Our protocols hence provide simple and practical synthetic access to a broad range of symmetric and asymmetric target products (Figure 1, bottom box, Figure 2).
Results and Discussion
Arylation of diphenylphosphine catalyzed by [Ir(dtbbpy)(ppy)2]PF6
Based upon our previous observations, [18] we reasoned that the commercially‐available aryl phosphines HPPh2 and H2PPh could be transformed into asymmetrical tertiary phosphines (RPPh2 or R2PPh) and quaternary phosphonium salts (R2PPh2 + or R3PPh+) through reaction with aryl iodides, mediated by the same photocatalytic system previously used for the arylation of P4. Thus, a solution containing the photocatalyst [Ir(dtbbpy)(ppy)2]PF6 ([1]PF6; dtbbpy=4,4′‐bis‐tert‐butyl‐2,2′‐bipyridine, ppy=2‐(2‐pyridyl)phenyl; structure shown in Table 1), HPPh2, the electron donor Et3N, and a model substrate 2‐iodotoluene in a CH3CN/PhH mixture (3:1) was irradiated with blue LED light (λ max=455 nm) for 18 h. Gratifyingly, this reaction was indeed found to yield 63 % of the desired tertiary phosphine product. Further investigations revealed that the yield could be further optimized to 71 % by adjusting the ratios of Et3N, aryl iodide and catalyst [1]PF6 (Table 1, I‐3). Control experiments confirmed that the reaction proceeds only in the presence of all reaction components (Et3N, aryl iodide, blue light irradiation, HPPh2 and [1]PF6; Table S1 in the Supporting Information). Meanwhile, a preliminary substrate screening showed that several other iodobenzene derivatives could also be employed in the reaction successfully (Table 1).
Table 1.
Photocatalytic synthesis of asymmetrically substituted aryldiphenylphosphines (I) and bisaryldiphenylphosphonium salts (II) from HPPh2 using [Ir(dtbbpy)(ppy)2]PF6 (1) as a photoredox catalyst.
|
|
All reactions were carried out using HPPh2 (0.1 mmol, 1 equiv), Ar−I (0.3 mmol, 3 equiv), [1]PF6 (1 mol %), and Et3N (8.6 mmol, 8.6 equiv) in CH3CN/PhH (3:1 v/v, 2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 18 h. Yields were determined by quantitative 31P{1H} NMR analysis of the reaction mixture with PPh3O as an internal standard. [a] Values in parentheses are the yields of the corresponding tetraarylphosphonium salt II. [b] Values in parentheses are the yields of the corresponding triarylphosphine I.
While these initial results clearly confirmed the validity of our proposed reaction, it was found that in most cases undesirable mixtures of phosphine (I) and phosphonium (II) products were obtained, in ratios typically between ca. 1:1 and 2:1. Interestingly, slightly higher selectivity was generally observed using electron‐poor iodoarenes, regardless of whether the major product was a phosphine or phosphonium salt. This more controlled behavior is presumably not due to difficulties in radical generation (Ar−I reduction should be more facile for electron‐poor arenes) and may instead be due to reduced reactivity of less nucleophilic aryl radicals (e.g. towards electrophilic P2Ph4; vide infra). Only ortho‐substituted aryl iodides (with the exception of 2‐iodothioanisole) selectively furnished phosphine products (I), presumably for steric reasons.
Arylation of diphenylphosphine catalyzed by 3DPAFIPN
Given the generally poor selectivity observed using [1]PF6, it was decided to further optimize the HPPh2 arylation reaction through investigation of alternative photocatalysts. In particular, it was decided to pursue the use of organic photocatalysts, in order to avoid the need to use expensive and scarce precious metals. To achieve similar reactivity, it was anticipated that a photocatalyst with comparable redox properties to [1]+ would be required. Fortunately, the recently‐developed organic photocatalyst 3DPAFIPN (2, structure shown in Table 2) has been reported to possess a reduction potential very similar to that of [1]+, while also being a competent catalyst for other photoredox reactions. [19] We were delighted to find that replacing [1]PF6 by 2 gave not only comparable, but in fact notably superior results in the arylation of HPPh2, including markedly improved selectivity for the phosphonium products II in most cases (Table 2 and vide infra). After further optimization, it was found that only 0.5 mol % of 2, and a much more modest excess of Et3N (2.4 equiv) and aryl iodide (3 equiv) in pure CH3CN under blue‐light irradiation transformed HPPh2 into a variety of desired products with generally excellent selectivity. Hence, not only could [1]PF6 be replaced with the readily available and inexpensive organophotocatalyst 2, but this substitution additionally yielded a considerable improvement in reaction performance.
Table 2.
Photocatalytic synthesis of asymmetrically substituted aryldiphenylphosphines (I) and bisaryldiphenylphosphonium salts (II) from HPPh2 using 3DPAFIPN (2) as a photoredox catalyst.
|
|
All reactions were carried out using HPPh2 (0.1 mmol, 1 equiv), Ar−I (0.3 mmol, 3 equiv), 3DPAFIPN (2, 0.5 mol %) and Et3N (0.24 mmol, 2.4 equiv) in CH3CN (2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 18 h. Yields were determined by quantitative 31P{1H} NMR analysis of the reaction mixture with PPh3O as an internal standard. [a] Values in parentheses are the yield of the corresponding triarylphosphine I. For simplicity, yields smaller than 10 % are not given (see the Supporting Information for further details). [b] Values in parentheses are the yield of the corresponding tetraarylphosphonium salt II. [c] Value in parentheses is the isolated yield for I‐1 at 1 mmol scale.
Gratifyingly, this optimized protocol was found to be compatible with iodobenzene derivatives bearing both electron‐donating and electron‐withdrawing groups, with the nature of these substituents generally having relatively little impact on the reaction outcome (Table 2). Notably, and in contrast to results obtained using [1]PF6, meta‐, and para‐substituted substrates were found to furnish exclusively the corresponding phosphonium salts Ar2PPh2 + in almost all cases (Table 2, II‐6–11, 13). Only for methyl 4‐iodobenzoate (Table 2, I‐12) was the tertiary phosphine (ArPPh2) formed preferentially, which is in line with our previous observation that strongly electron‐withdrawing groups can disfavor formation of a phosphonium cation. Conversely, for ortho‐substituted substrates significant Ar2PPh formation was observed in all cases (II‐1–5), in line with previous observations using [1]PF6 (vide supra). The reason(s) behind the increased preference for phosphonium salts II when using 2 rather than [1]PF6 are currently unclear but are presumably related to slight differences in redox characteristics and/or electron transfer kinetics. [20]
Arylation of phenylphosphine catalyzed by 3DPAFIPN
Having established the ability to arylate the secondary phosphine HPPh2, we proceeded to investigate the further extension of the scope of the arylation to the primary phosphine H2PPh. Thus, after a modest increase in the amount of iodoarene, reductant and catalyst (to reflect the larger number of arylation steps required), various additional phosphines (Ar2PPh, III) and phosphonium salts (Ar3PPh+, IV) were conveniently accessible from H2PPh in reasonable yields (Table 3). Similar patterns of functional group tolerance and selectivity were observed to the analogous reactions of HPPh2, although for ortho‐substituted substrates a noticeably increased preference for the triarylphosphine product could be detected (III‐1–6). This results in generally higher selectivity for these reactions (albeit with a slight cost in overall yield) and is consistent with the greater steric impact caused by the presence of two ortho‐substituted aryl residues within the product structures.
Table 3.
Photocatalytic synthesis of asymmetrically substituted bisarylphenylphosphines (III) and trisarylphenylphosphonium salts (IV) from H2PPh using 3DPAFIPN (2) as a photoredox catalyst.
|
|
All reactions were carried out using H2PPh (0.1 mmol, 1 equiv), Ar−I (0.5 mmol, 5 equiv), 3DPAFIPN (2 mol %) and Et3N (0.4 mmol, 4 equiv) in CH3CN/PhH (3:1 v/v, 2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 18 h. Yields were determined by quantitative 31P{1H} NMR analysis of the product mixture with PPh3O as an internal standard. [a] Values in parentheses are the yield of the corresponding tetraarylphosphonium salt IV. [b] Values in parentheses are the yield of the corresponding triarylphosphine III. For simplicity, yields smaller than 10 % of tertiary phosphine are not given in the table (see the Supporting Information for further details).
Alkylation of diphenylphosphine catalyzed by 3DPAFIPN
As well as the formation of purely aryl‐substituted products, we expected that our photocatalytic methodology should also be suitable for the formation of asymmetrical mixed aryl/alkyl phosphines and/or the corresponding phosphonium salts. Unfortunately, initial attempts to functionalize dicyclohexylphosphine (Cy2PH) using either iodobenzene or cyclohexyl iodide proved unsuccessful, typically resulting in the decomposition of Cy2PH (Schemes S1 and S2). Conversely, excellent reactivity was observed when alkyl iodides were employed in combination with HPPh2. [21] After further optimization our approach provided access to numerous alkyl‐substituted diarylphosphines RPPh2 in good to excellent yields (Table 4).
Table 4.
Photocatalytic synthesis of asymmetrically substituted mixed alkyldiphenylphosphines (V) from HPPh2 using 3DPAFIPN 2 as a photoredox catalyst.
|
|
All reactions were carried out using HPPh2 (0.2 mmol, 1 equiv), R−I (0.4 mmol, 2 equiv), 3DPAFIPN 2 (0.2 mol %) and DIPEA (0.4 mmol, 2 equiv) in CH3CN (2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 24 h. Yields were determined by quantitative 31P{1H} NMR analysis of the reaction mixture with PPh3O as an internal standard. [a] Values in parentheses are isolated yields for reactions at 1 mmol scale (for HPPh2) using 0.5 mol % 3DPAFIPN. [b] The reaction was carried out with 0.5 mol % 3DPAFIPN for 38 h, [c] R−Br was used instead of R−I.
Notably, and unlike to the results obtained using aryl iodides as radical precursors, tertiary phosphines were formed as the sole products in all cases. This transformation was found to be effective for primary long chain alkyl iodides such as n‐octyl iodide and n‐butyl iodide as well as for the more sterically encumbered neopentyl iodide. Excellent conversions were also achieved using various substituted benzyl bromide substrates, [22] leading to formation of the corresponding benzylic phosphines. Such benzylic phosphines have recently found application as ligands within Ir‐ or Pt‐ complexes with potential uses in electroluminescent devices and OLEDs. [23] A range of electron deficient groups (such as F (V‐6), Cl (V‐7), CF3 (V‐8)) at the 4‐position were tolerated, with the exception of NO2 (V‐9), possibly due to side‐reactions at the NO2 group itself. In addition to primary alkyl iodides, cyclic and acyclic secondary iodides could also be transformed into the corresponding tertiary phosphine products V in reasonable yields, as could tertiary alkyl iodides. It should be noted that for the isopropyl and tert‐butyl iodide substrates the corresponding products (V‐12, V‐13) could also be prepared in moderate yield (35 %, 50 %) in the absence of the photocatalyst 2, although no product was observed upon exclusion of light (Table S3). For other substrates, however, control experiments confirmed that a productive reaction is achieved only if all reaction components are present, including the photocatalyst.
Alkylation of phenylphosphine catalyzed by 3DPAFIPN
Similar success was achieved for the alkylation of the primary phosphine H2PPh, providing access to a broad range of dialkyl‐substituted phenyl phosphines R2PPh VI in good yields (Table 5). Again, a range of primary and secondary alkyl iodides could be employed as coupling partners, as could several benzyl bromides, although attempts to use tert‐butyl iodide were unsuccessful, presumably for steric reasons. In contrast to the formerly described alkylation of HPPh2, which gave exclusively tertiary phosphine products, here, in many cases triple alkylation of H2PPh was found to furnish the phosphonium salts R3PPh+ with high selectivity. This is probably at least in part due to steric factors, given the small profile of most of the primary alkyl moieties. Notably, for the benzyl bromide substrates either the R3PPh+ or R2PPh products can be accessed with high selectivity, simply by altering the molar ratio with H2PPh.
Table 5.
Photocatalytic synthesis of asymmetrically substituted mixed bisalkylphenylphosphines (VI) and trisalkylphenylphosphonium salts (VII) from H2PPh using 3DPAFIPN (2) as a photoredox catalyst.
|
|
All reactions were carried out using HPPh2 (0.2 mmol, 1 equiv), R−I (0.8 mmol, 4 equiv), 3DPAFIPN (2) (0.2 mol %) and DIPEA (0.3 mmol, 3 equiv) in CH3CN (2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 24 h. Yields were determined by quantitative 31P{1H} NMR analysis of the reaction mixture with PPh3O as an internal standard. [a] Reaction was carried out with 0.5 mol % 3DPAFIPN. [b] R−Br was used instead of R−I. [c] 3 equiv of R−Br were used. [d] Broad signals were observed in 31P{1H} NMR experiments and hence yield was not calculated. [e] Values in parentheses are isolated yields for reactions at 1 mmol scale (for H2PPh) using 0.5 mol % 3DPAFIPN.
Mechanistic investigations
To acquire mechanistic insights, the optimized reaction between HPPh2 and cyclohexyl iodide (Cy‐I) was chosen as a model system for further study. Radical inhibition experiments showed that addition of 2,2,6,6‐tetramethyl‐1‐piperidinyloxy (TEMPO) as a radical scavenger to this reaction completely suppressed the formation of the expected product PPh2Cy (V‐10), supporting the involvement of a radical pathway (Scheme S3). [24] In addition, 31P{1H} NMR monitoring of the model reaction showed complete consumption of HPPh2 and rapid formation of the diphosphine P2Ph4 in less than 2 h. The same reaction gave only traces of product in the absence of photocatalyst 2. Notably, no formation of P2Ph4 was observed in the absence of Cy‐I, even with extended reaction times (Table S4). These observations suggest that product formation may not proceed through direct alkylation of HPPh2 per se but rather through the intermediate formation of P2Ph4. This intermediate is presumably formed via dimerization of Ph2P. radicals that are in turn formed by abstraction of an H atom from HPPh2 (Scheme 1, step iv) by photochemically‐generated Cy. radicals. [11] The formation of organyl radicals was confirmed by EPR measurements: when a CH3CN solution of 2, diisopropylethylamin (DIPEA), Cy‐I and the spin trap N‐tert‐butyl‐α‐phenylnitrone (PBN) was irradiated with blue LED light, the formation of a spin adduct with hyperfine couplings of aN=15.2 G, aH=2.5 G was observed (Figure S6), which might correspond to the known spin adduct Cy‐PBN or a related adduct.[ 25 , 26 ] Finally, fluorescence quenching experiments were performed to confirm that of the reaction components present in the model reaction mixture (DIPEA, Cy‐I, H2PPh, P2Ph4) only DIPEA effectively quenches the photoexcited state of photocatalyst 2 in CH3CN (Figure S7).
Scheme 1.

Proposed mechanism for the formation of an asymmetrical tertiary phosphine from Cy‐I (cyclohexyl iodide) and HPPh2.
Based on our observations, a catalytic cycle can be proposed, which is summarized in Scheme 1. Irradiation of photocatalyst 2 with blue light generates the excited species 2* [E 1/2(PC*/PC.−)=+1.09 V vs. SCE)], which undergoes reductive quenching in the presence of DIPEA (E 1/2 ox=+0.65 V vs. SCE), resulting in the simultaneous formation of the DIPEA radical cation (DIPEA.+) and the strong reductant 2 .− [E 1/2 (PC/PC.−)=−1.59 V vs. SCE] (step i, ii). [19] This is capable of generating a cyclohexyl radical (Cy., step iii) through one‐electron reduction of Cy‐I, which also closes the photoredox catalytic cycle. [27] The radicals (Cy.) thus generated can then abstract a hydrogen radical from HPPh2, producing Ph2P. radicals that rapidly self‐couple to form the intermediate diphosphine P2Ph4 (step iv, v). This step also accounts for the need for at least a 1 equiv excess of the organohalide in all reactions. Subsequent radicals Cy. can then attack the P‐P bond of P2Ph4, releasing the product tertiary phosphine PPh2Cy alongside Ph2P., which can self‐couple as before (step v).
Given the proposed involvement of P2Ph4 as a reaction intermediate, the direct reaction of P2Ph4 with selected alkyl iodides was also investigated. Thus, the reaction of P2Ph4 with Cy‐I or 1‐iodoadamantane and DIPEA in a 1:1.5:1.5 molar ratio (per phosphorus atom) was confirmed to provide the expected products V‐10 and V‐14, in 85 % and 43 % yields, respectively (Scheme 2, path A). Unfortunately, the formation of tertiary phosphine was not observed with primary alkyl halides such as benzyl bromide and n‐octyl iodide suggesting that different mechanisms may be operative in these cases (Scheme S4; for a discussion of the mechanism of phosphonium salt formation see section 4.5 of the Supporting Information).
Scheme 2.

Synthesis of tertiary phosphines and PCP pincer ligand from P2Ph4. Reaction conditions: A) P2Ph4 (0.2 mmol,1 equiv), alkyl iodide (0.6 mmol, 1.5 equiv based on phosphorus atom), 3DPAFIPN 2 (0.5 mol %), DIPEA (0.6 mmol, 1.5 equiv based on phosphorus atom), CH3CN (2 mL), N2 atmosphere, blue LED (λ max=455 nm), 24 h. B) P2Ph4 (0.15 mmol,1.5 equiv), 1,3‐bis(bromomethyl)benzene (0.1 mmol, 1 equiv), 3DPAFIPN (0.5 mol %), DIPEA (0.4 mmol, 4 equiv), CH3CN (1.5 mL), PhH (0.5 mL), N2 atmosphere, blue LED (λ max=455 nm), 24 h. a) Isolated yield for reactions at 0.5 mmol scale (for 1,3‐bis(bromomethyl)benzene).
Application to the synthesis of a PCP pincer ligand
As a further demonstration of the utility of this method, we sought to synthesize a PCP pincer ligand starting from P2Ph4. These ligands are well known and widely exploited in organometallic chemistry. [28] We had previously been disappointed to find that attempts to prepare these products starting from HPPh2 were unsuccessful. [29] However, to our satisfaction, blue LED irradiation of a 1:1.5:4 molar ratio of P2Ph4,1,3‐bis(bromomethyl)benzene and DIPEA in CH3CN/PhH in the presence of 0.5 mol % of 3DPAFIPN led to selective formation of 1,3‐bis(diphenylphosphinomethyl)benzene V‐15 in 61 % isolated yield (Scheme 2, path B).
Application of 3DPAFIPN in P4 functionalization
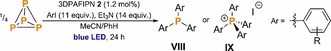
Finally, having demonstrated the ability of organic photocatalyst 2 to mediate the arylation and alkylation of primary and secondary phenyl phosphines, we were interested to establish whether the same catalyst could also mediate the formation of these phosphines from P4, and thus act as a competent catalyst for the direct transformation of P4 into triarylphosphines and tetraarylphosphonium salts, in an analogous manner to the precious metal catalyst [1]+. Thus, our previously reported procedure for the catalytic phenylation of P4 with iodobenzene was repeated using organic photocatalyst 2 (1.2 mol %, Table 6). Direct catalyst replacement in this manner yielded the tetra‐arylated phosphonium salt PPh4I in 60 % yield and with complete selectivity.
Table 6.
Direct P4 functionalization using 3DPAFIPN (2) as a photoredox catalyst.
|
| ||||
|---|---|---|---|---|
|
Entry |
R |
Product No. |
[PAr4]+I− |
PAr3 |
|
1 |
H |
IX‐1 |
60 (24 %) |
– |
|
2 |
3‐Me |
IX‐2 |
37 |
– |
|
3[a] |
4‐Me |
IX‐3 |
29 |
– |
|
4 |
3‐OMe |
IX‐4 |
45 |
– |
|
5 |
4‐OMe |
IX‐5 |
19 |
– |
|
6 |
3‐COOMe |
IX‐6 |
20 |
– |
|
7 |
4‐COOMe |
VIII‐1 |
– |
31 |
|
8 |
2‐Me |
VIII‐2 |
– |
54 (43 %)[b] |
|
9[a] |
2‐OMe |
VIII‐3 |
– |
24 (12 %)[b] |
|
10 |
2‐SMe |
VIII‐4 |
– |
24 (16 %)[b] |
|
11[c] |
Ph3SnCl |
VIII‐5 |
– |
56 |
All reactions were carried out using P4 (0.025 mmol, 1 equiv), Ar−I (1.1 mmol, 11 equiv based on phosphorus atom), 3DPAFIPN 2 (1.2 mol % based on phosphorus atom) and Et3N (1.4 mmol, 14.4 equiv based on phosphorus atom) in CH3CN/PhH (3:1 v/v, 2 mL) under an N2 atmosphere and blue LED irradiation (λ max=455 nm) for 24 h. Yields were determined by quantitative 31P{1H} NMR analysis of the reaction mixture with PPh3O as an internal standard. [a] 30 h reaction time. [b] Values in parentheses are isolated yields for reactions at 1 mmol scale. [c] Ar−I was replaced by Ph3SnCl.
As shown in Table 6, a range of substituted aryl iodides were amenable to this photocatalytic strategy, including both electron rich and electron deficient arenes. The scope and selectivity of the reaction were found to mirror those previously observed using [1]+ as a catalyst, again offering selective triarylphosphine formation for ortho‐substituted aryliodides as well as for the electron‐poor para‐methyl benzoate derivative. By replacing the aryl iodide with Ph3SnCl it was possible to prepare the potentially useful ‘P3−‘ triply stannylated synthon (Ph3Sn)3P. Although the yields of phosphines and phosphoniums obtained are typically slightly reduced compared to the analogous reactions catalyzed by [1]+, the ability to use an inexpensive organic photocatalyst in place of a precious metal complex represents a significant improvement to the practicality and attractiveness of this synthetic method.
Conclusions
We have developed a mild and versatile, visible‐light‐mediated approach for the selective formation of a broad scope of asymmetrical aryl/aryl and aryl/alkyl tertiary phosphines and quaternary phosphonium salts, using stable, commercially available organic halides in combination with phenyl‐substituted primary and secondary phosphines (Scheme 3). Optimal results are obtained using low loadings of an inexpensive organic photocatalyst, resulting in a mild and versatile synthetic method for the preparation of these valuable compounds and providing an attractive alternative to previously developed Ir‐photocatalyzed protocols. The same organic photocatalyst can also be used to replace the precious metal photoredox catalyst in our previously reported photocatalytic arylation of P4, significantly improving the synthetic feasibility of this important transformation.
Scheme 3.

Organic photocatalytic synthesis of asymmetrically substituted tertiary phosphines and quaternary phosphonium salts.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Financial support by the European Research Council (ERC CoG 772299), the Alexander von Humboldt foundation (fellowship to D.J.S.), the DFG research unit FOR2177, and the DFG graduate program „Chemical Photocatalysis“ (GRK 1626) is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
P. B. Arockiam, U. Lennert, C. Graf, R. Rothfelder, D. J. Scott, T. G. Fischer, K. Zeitler, R. Wolf, Chem. Eur. J. 2020, 26, 16374.
Contributor Information
Prof. Dr. Kirsten Zeitler, Email: kzeitler@uni-leipzig.de.
Prof. Dr. Robert Wolf, Email: robert.wolf@ur.de.
References
- 1.
- 1a. Liu N., Li X., Sun H., J. Organomet. Chem. 2011, 696, 2537–2542; [Google Scholar]
- 1b. Khan H., Badshah A., Said M., Murtaza G., Ahmad J., Jean-Claude B. J., Todorova M., Butler I. S., Appl. Organomet. Chem. 2013, 27, 387–395; [Google Scholar]
- 1c. Khan H., Badshah A., Said M., Murtaza G., Sirajuddin M., Ahmad J., Butler I. S., Inorg. Chim. Acta 2016, 447, 176–182; [Google Scholar]
- 1d. Potgieter K., Engelbrecht Z., Naganagowda G., Cronjé M. J., Meijboom R., J. Coord. Chem. 2017, 70, 2644–2658; [Google Scholar]
- 1e. Yilmaz V. T., Icsel C., Turgut O. R., Aygun M., Evren E., Ozdemir I., Inorg. Chim. Acta 2020, 500, 119220. [Google Scholar]
- 2.
- 2a. Enders D., Nguyen T. V., Org. Biomol. Chem. 2012, 10, 5327–5331; [DOI] [PubMed] [Google Scholar]
- 2b. Liu S., Kumatabara Y., Shirakawa S., Green Chem. 2016, 18, 331–341; [Google Scholar]
- 2c. Golandaj A., Ahmad A., Ramjugernath D., Adv. Synth. Catal. 2017, 359, 3676–3706. [Google Scholar]
- 3.
- 3a. McNulty J., Nair J. J., Cheekoori S., Larichev V., Capretta A., Robertson A. J., Chem. Eur. J. 2006, 12, 9314–9322; [DOI] [PubMed] [Google Scholar]
- 3b. Cao H., McNamee L., Alper H., J. Org. Chem. 2008, 73, 3530–3534; [DOI] [PubMed] [Google Scholar]
- 3c. Ferreira A. F., Simões P. N., Ferreira A. G. M., J. Chem. Thermodyn. 2012, 45, 16–27; [Google Scholar]
- 3d. Cassity C. G., Mirjafari A., Mobarrez N., Strickland K. J., O'Brien R. A., Davis J. H., Chem. Commun. 2013, 49, 7590–7592. [DOI] [PubMed] [Google Scholar]
- 4.
- 4a. Deng Z., Lin J.-H., Xiao J.-C., Nat. Commun. 2016, 7, 13651; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4b. Toda Y., Gomyou S., Tanaka S., Komiyama Y., Kikuchi A., Suga H., Org. Lett. 2017, 19, 5786–5789; [DOI] [PubMed] [Google Scholar]
- 4c. Guo S., Mi X., Tetrahedron Lett. 2017, 58, 2881–2884; [Google Scholar]
- 4d. Gupta M., Yan D., Xu J., Yao J., Zhan C., ACS Appl. Mater. Interfaces 2018, 10, 5569–5576; [DOI] [PubMed] [Google Scholar]
- 4e. Gupta M., Yan D., Yao J., Zhan C., ACS Appl. Mater. Interfaces 2018, 10, 35896–35903. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Bhattacharya A. K., Thyagarajan G., Chem. Rev. 1981, 81, 415–430; [Google Scholar]
- 5b. Wauters I., Debrouwer W., Stevens C. V., Beilstein J. Org. Chem. 2014, 10, 1064–1096; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Hérault D., Nguyen D. H., Nuel D., Buono G., Chem. Soc. Rev. 2015, 44, 2508–2528. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Oshiki T., Imamoto T., J. Am. Chem. Soc. 1992, 114, 3975–3977; [Google Scholar]
- 6b. Gelman D., Jiang L., Buchwald S. L., Org. Lett. 2003, 5, 2315–2318; [DOI] [PubMed] [Google Scholar]
- 6c. Allen D. V., Venkataraman D., J. Org. Chem. 2003, 68, 4590–4593; [DOI] [PubMed] [Google Scholar]
- 6d. Sun M., Zang Y.-S., Hou L.-K., Chen X.-X., Sun W., Yang S.-D., Eur. J. Org. Chem. 2014, 6796–6801; [Google Scholar]
- 6e. Yang J., Xiao J., Chen T., Han L.-B., J. Organomet. Chem. 2016, 820, 120–124; [Google Scholar]
- 6f. Yu R., Chen X., Wang Z., Tetrahedron Lett. 2016, 57, 3404–3406; [Google Scholar]
- 6g. Hirai Y., Uozumi Y., Synlett 2017, 28, 2966–2970. [Google Scholar]
- 7.
- 7a. Koshti V., Gaikwad S., Chikkali S. H., Coord. Chem. Rev. 2014, 265, 52–73; [Google Scholar]
- 7b. Bange C. A., Waterman R., Chem. Eur. J. 2016, 22, 12598–12605. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Kazankova M. A., Efimova I. V., Kochetkov A. N., Afanas'ev V. V., Beletskaya I. P., Dixneuf P. H., Synlett 2001, 0497–0500; [Google Scholar]
- 8b. Shulyupin M. O., Kazankova M. A., Beletskaya I. P., Org. Lett. 2002, 4, 761–763; [DOI] [PubMed] [Google Scholar]
- 8c. Kazankova M. A., Shulyupin M. O., Borisenko A. A., Beletskaya I. P., Russ. J. Org. Chem. 2002, 38, 1479–1484; [Google Scholar]
- 8d. Leyva-Pérez A., Vidal-Moya J. A., Cabrero-Antonino J. R., Al-Deyab S. S., Al-Resayes S. I., Corma A., J. Organomet. Chem. 2011, 696, 362–367; [Google Scholar]
- 8e. Gallagher K. J., Webster R. L., Chem. Commun. 2014, 50, 12109–12111; [DOI] [PubMed] [Google Scholar]
- 8f. Gallagher K. J., Espinal-Viguri M., Mahon M. F., Webster R. L., Adv. Synth. Catal. 2016, 358, 2460–2468. [Google Scholar]
- 9.
- 9a. Hu H., Cui C., Organometallics 2012, 31, 1208–1211; [Google Scholar]
- 9b. Liu B., Roisnel T., Carpentier J.-F., Sarazin Y., Chem. Eur. J. 2013, 19, 13445–13462; [DOI] [PubMed] [Google Scholar]
- 9c. Trifonov A. A., Basalov I. V., Kissel A. A., Dalton Trans. 2016, 45, 19172–19193; [DOI] [PubMed] [Google Scholar]
- 9d. Selikhov A. N., Plankin G. S., Cherkasov A. V., Shavyrin A. S., Louyriac E., Maron L., Trifonov A. A., Inorg. Chem. 2019, 58, 5325–5334. [DOI] [PubMed] [Google Scholar]
- 10.
- 10a. Mimeau D., Delacroix O., Gaumont A.-C., Chem. Commun. 2003, 2928–2929; [DOI] [PubMed] [Google Scholar]
- 10b. Mimeau D., Delacroix O., Join B., Gaumont A.-C., C. R. Chim. 2004, 7, 845–854; [Google Scholar]
- 10c. Moglie Y., González-Soria M. J., Martín-García I., Radivoy G., Alonso F., Green Chem. 2016, 18, 4896–4907; [Google Scholar]
- 10d. Bissessar D., Egly J., Achard T., Steffanut P., Bellemin-Laponnaz S., RSC Adv. 2019, 9, 27250–27256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin S., Haug G. C., Nguyen V. T., Flores-Hansen C., Arman H. D., Larionov O. V., ACS Catal. 2019, 9, 9764–9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Migita T., Nagai T., Kiuchi K., Kosugi M., Bull. Chem. Soc. Jpn. 1983, 56, 2869–2870; [Google Scholar]
- 12b. Kolodiazhnyi O. I., in Phosphorus Ylides: Chemistry and Applications in Organic Synthesis Wiley-VCH: New York, 1999, pp. 11–29; [Google Scholar]
- 12c. Marcoux D., Charette A. B., Adv. Synth. Catal. 2008, 350, 2967–2974; [Google Scholar]
- 12d. Marcoux D., Charette A. B., J. Org. Chem. 2008, 73, 590–593. [DOI] [PubMed] [Google Scholar]
- 13.
- 13a. Rémond E., Tessier A., Leroux F. R., Bayardon J., Jugé S., Org. Lett. 2010, 12, 1568–1571; [DOI] [PubMed] [Google Scholar]
- 13b. Huang W., Zhong C.-H., ACS Omega 2019, 4, 6690–6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fearnley A. F., An J., Jackson M., Lindovska P., Denton R. M., Chem. Commun. 2016, 52, 4987–4990. [DOI] [PubMed] [Google Scholar]
- 15. Bugaenko D. I., Volkov A. A., Livantsov M. V., Yurovskaya M. A., Karchava A. V., Chem. Eur. J. 2019, 25, 12502–12506. [DOI] [PubMed] [Google Scholar]
- 16.
- 16a. Cristau H.-J., Chêne A., Christol H., J. Organomet. Chem. 1980, 185, 283–295; [Google Scholar]
- 16b. Nowrouzi N., Keshtgar S., Bahman Jahromi E., Tetrahedron Lett. 2016, 57, 348–350; [Google Scholar]
- 16c. Wan W., Yang X., Smith R. C., Chem. Commun. 2017, 53, 252–254. [DOI] [PubMed] [Google Scholar]
- 17.
- 17a. Ravelli D., Fagnoni M., Albini A., Chem. Soc. Rev. 2013, 42, 97–113; [DOI] [PubMed] [Google Scholar]
- 17b. Romero N. A., Nicewicz D. A., Chem. Rev. 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]
- 17c. Luo K., Yang W.-C., Wu L., Asian J. Org. Chem. 2017, 6, 350–367; [Google Scholar]
- 17d. Twilton J., Le C., Zhang P., Shaw M. H., Evans R. W., MacMillan D. W. C., Nat. Rev. Chem. 2017, 1, 0052; [Google Scholar]
- 17e. Wang C.-S., Dixneuf P. H., Soulé J.-F., Chem. Rev. 2018, 118, 7532–7585; [DOI] [PubMed] [Google Scholar]
- 17f. Marzo L., Pagire S. K., Reiser O., König B., Angew. Chem. Int. Ed. 2018, 57, 10034–10072; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 10188–10228. [Google Scholar]
- 18. Lennert U., Arockiam P. B., Streitferdt V., Scott D. J., Rödl C., Gschwind R. M., Wolf R., Nat. Catal. 2019, 2, 1101–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Speckmeier E., Fischer T. G., Zeitler K., J. Am. Chem. Soc. 2018, 140, 15353–15365. [DOI] [PubMed] [Google Scholar]
- 20.For example, the reduction potential of 2 is reported to be slightly lower than that of [1]+. Thus, Ar−I reduction may be more facile using this photocatalyst, which may promote complete arylation.
- 21.For base-mediated addition of alkyl halides to HPPh2, see Honaker M. T., Sandefur B. J., Hargett J. L., McDaniel A. L., Salvatore R. N., Tetrahedron Lett. 2003, 44, 8373–8377. [Google Scholar]
- 22.Attempts to use other alkyl bromides were unsuccessful, presumably due to their overly negative reduction potentials.
- 23.
- 23a. Akihiro O., Yoshiaki T., (Showa Denko K. K., Japan), JP 2007169475A, 2007;
- 23b. Akihiro O., Yoshiaki T., (Showa Denko K. K., Japan), JP 2008010647A, 2008;
- 23c. Ch Y., Huang L.-M., (National Tsing Hua University), US 8722885 B1, 2014.
- 24.Formation of the radical adduct TEMPO-Cy could be detected by GC-MS (see the Supporting Information for further details).
- 25. Iwahashi H., Ishikawa Y.-i., Sato S., Koyano K., Bull. Chem. Soc. Jpn. 1977, 50, 1278–1281. [Google Scholar]
- 26.Due to the line broadening of the spectrum recorded at room temperature the formation of other alkyl or aryl radicals cannot be definitively excluded (see the Supporting Information for further details).
- 27.An alternative mechanism could involve iodide ion abstraction (XAT) mediated by DIPEA-derived radicals, as described in Constantin T., Zanini M., Regni A., Sheikh N. S., Juliá F., Leonori D., Science 2020, 367, 1021–1026. [DOI] [PubMed] [Google Scholar]
- 28.
- 28a. van der Boom M. E., Milstein D., Chem. Rev. 2003, 103, 1759–1792; [DOI] [PubMed] [Google Scholar]
- 28b. Shih W.-C., Ozerov O. V., Organometallics 2015, 34, 4591–4597. [Google Scholar]
- 29.For base-mediated coupling of HPPh2 and 1,3-bis(bromomethyl)benzene, see Kozhanov K. A., Bubnov M. P., Cherkasov V. K., Vavilina N. N., Efremova L. Y., Artyushin O. I., Odinets I. L., Abakumov G. A., Dalton Trans. 2008, 2849–2853, and references therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary