Abstract
Objective
Fenfluramine has been shown to provide clinically meaningful and statistically significant reductions in convulsive seizure frequency in children and adolescents (aged 2‐18 years) with Dravet syndrome in two randomized, placebo‐controlled clinical trials. The objective of this analysis was to assess longer‐term safety and efficacy of fenfluramine in patients who completed one of the double‐blind studies and entered an open‐label extension (OLE) study.
Methods
Patients enrolling in the OLE study initiated fenfluramine at 0.2 mg/kg/d regardless of their treatment assignment in the double‐blind study. After 4 weeks, the fenfluramine dose could be titrated based on efficacy and tolerability to maximum of 0.7 mg/kg/d (absolute maximum 27 mg/d) or maximum of 0.4 mg/kg/d (absolute maximum 17 mg/d) in patients receiving concomitant stiripentol. The number and type of seizures were recorded daily in an electronic diary, and safety, including echocardiography, was assessed at Months 1, 2, and 3, and at 3‐month intervals thereafter.
Results
A total of 232 patients were enrolled as of March 13, 2018. During this analysis period, patients were treated for a median 256 days (range = 46‐634 days). Over the entire OLE analysis period, the median decrease in convulsive seizure frequency compared to baseline in the double‐blind studies was −66.8% (range = −100% to 234.9%; P < .001). The median reduction in seizure frequency was similar in patients <6 (−75.7%) and ≥6 years old (−64.7%). The most commonly reported adverse events included pyrexia (21.6%), nasopharyngitis (19.4%), and decreased appetite (−15.9%). No valvular heart disease (VHD) or pulmonary arterial hypertension (PAH) was observed.
Significance
Study results demonstrate that fenfluramine provides clinically meaningful (≥50%) seizure frequency reduction over an extended period in patients with Dravet syndrome. No patient developed VHD or PAH, and fenfluramine was generally well tolerated.
Keywords: Dravet syndrome, epilepsy, fenfluramine
Key Points.
Patients with Dravet syndrome were treated with fenfluramine for a median 256 days in this open‐label extension study
Fenfluramine provided sustained reduction in convulsive seizure frequency, with a median reduction of 66.8%
More than 40% of patients experienced profound (>75%) reduction in convulsive seizure frequency
Anticonvulsive effectiveness was similar in patients <6 years old (−75.7%) and ≥6 years old (−64.7%)
Fenfluramine was well tolerated, with no observations of cardiac valvulopathy or pulmonary hypertension
1. INTRODUCTION
Dravet syndrome is a rare, severe, treatment‐resistant developmental and epileptic encephalopathy 1 that typically begins during the first year of life. It is characterized by focal and generalized seizures, developmental slowing and often regression, cognitive impairment, and elevated mortality risk due to status epilepticus and sudden unexpected death in epilepsy (SUDEP). 2 , 3 Treatment of Dravet syndrome remains suboptimal despite regimens that include multiple antiepileptic drugs (AEDs) as well as ketogenic diets. Pharmacoresistance is illustrated by a survey of 274 European patients with Dravet syndrome conducted by Aras et al, 4 who found that about two‐thirds of patients were taking three or four AEDs. Despite these multiple drug regimens, 45% of patients continued to experience four or more tonic‐clonic seizures per month. Researchers have found that even drugs to which patients initially respond may become less effective over time. 5
The antiseizure activity of fenfluramine was first reported in the 1980s in small case series and other observational studies of children with photosensitive or self‐induced epilepsy. 6 Some of the patients in these original studies were later diagnosed with Dravet syndrome, and these patients and other patients with Dravet syndrome provided the basis for two long‐term, open‐label, cohort studies, which found that fenfluramine provided sustained, long‐term, clinically meaningful seizure reduction. 7 , 8 , 9 The antiseizure activity of fenfluramine in Dravet syndrome is thought to be mediated by both its serotonergic activity and its interactions with sigma‐1 receptors. 10 , 11
Two phase 3, randomized, placebo‐controlled, double‐blind clinical trials of fenfluramine for treatment of patients with Dravet syndrome have recently been reported. 12 , 13 In both studies, low‐dose fenfluramine (≤0.7 mg/kg/d) was added to each patient's current antiepileptic treatment regimen, which resulted in substantial and statistically significant reductions in convulsive seizure frequency compared to placebo treatment. A total of 35% to 50% of patients treated with fenfluramine experienced a profound (≥75%) reduction in convulsive seizure frequency compared with only 2% in the placebo group. In these short‐term phase 3 trials, no patient developed valvular heart disease or pulmonary arterial hypertension, and the drug was generally well tolerated. Here we present an analysis of the safety and efficacy of fenfluramine in patients from these two core clinical trials who entered a long‐term, open‐label extension (OLE) study (NCT02823145).
2. MATERIALS AND METHODS
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the institutional review board or ethics committee at each study site before the study began. All patients or their legal representatives provided written informed consent before enrolling in the study.
Patients with Dravet syndrome between the ages of 2 and 18 years who satisfactorily completed any of the core phase 3 clinical trials (NCT02926898, NCT02682927, NCT02826863) were eligible to enroll in the OLE study. Key exclusion criteria included known hypersensitivity to fenfluramine or any excipients in the study medication; current or past history of cardiovascular or cerebrovascular disease, myocardial infarction, or stroke; current cardiac valvulopathy or pulmonary hypertension that the investigator, the parent, the International Pediatric Cardiology Advisory Board, the Independent Data Safety Monitoring Committee, or the Sponsor deemed clinically significant enough to warrant discontinuation of study medication; and current or recent history of anorexia nervosa, bulimia, or depression.
At the end of the core phase 3 clinical trial, patients treated with >0.2 mg/kg/d fenfluramine underwent a downtitration to 0.2 mg/kg/d, whereas all other patients, including those in the placebo group, underwent a dummy downtitration. The primary purpose of the downtitration was to maintain blinding of the core phase 3 clinical trials. Fenfluramine HCl oral solution (Fintepla; Zogenix, Inc) was administered at equal doses with food, once in the morning and once in the evening, approximately 12 hours apart. All patients in the OLE study started treatment with a dose of 0.2 mg/kg/d for the first 4 weeks, regardless of the treatment or dose received in the core study. After 4 weeks, the dose could be titrated based on effectiveness and tolerability, as is typically done in clinical practice with other AEDs. Dose changes were made in 0.2 mg/kg/d increments with a minimum of 2 weeks between steps. The daily fenfluramine dose was limited to 0.7 mg/kg/d (maximum 26 mg/d) or, in patients concomitantly treated with stiripentol, 0.4 mg/kg/d (maximum 17 mg/d). The protocol allowed earlier titration if clinically meaningful worsening in seizure type or frequency was seen during the first 4 weeks of the study. Patients who remained on a stable dose of fenfluramine for 6 months could have doses of concomitant AEDs reduced; however, patients had to remain on at least one other background AED as well.
The type and number of convulsive seizures were recorded by the caregiver in an electronic diary. Convulsive seizures were defined as hemi‐clonic, tonic, clonic, tonic‐clonic, generalized tonic‐clonic, and focal with clearly observable motor signs. Baseline seizure frequency for each patient was established during the 6‐week baseline period in the core double‐blind clinical trial. Monthly seizure frequency was defined as the number of seizures per 28 days.
Seizure‐related outcomes of interest included change from baseline in convulsive seizure frequency for the entire OLE analysis period, and from the start of Month 2 of the OLE study (when dose titration could begin) through each patient's final OLE study visit. In addition, the proportions of patients who achieved ≥25%, ≥50%, ≥75%, and 100% reduction in convulsive seizure frequency were determined. Overall improvement in patient status was assessed on the Clinical Global Impression of Improvement (CGI‐I) Scale by the site primary investigator and by the parent/caregiver. The CGI‐I asks each assessor to rate the change observed in a patient on a 7‐point Likert scale, with 1 indicating “very much improved,” 4 indicating “no change,” and 7 indicating “very much worse.”
Treatment‐emergent adverse events (TEAEs) were recorded primarily at study visits, either in person or by telephone. Echocardiography was performed at OLE study entry, after 4 to 6 weeks, and every 3 months thereafter to assess cardiac valve function and valve morphology and to estimate pulmonary arterial pressure.
Patients who had enrolled in the OLE study by March 13, 2018, and who received at least one dose of fenfluramine in the OLE study were included in this analysis. Safety data were collected through April 27, 2018, and patients with ≥1 month of electronic diary seizure documentation by June 8, 2018, were included in the analysis. Change in seizure frequency from baseline was assessed by a Wilcoxon signed‐rank test, and other outcomes are presented with descriptive statistics.
3. RESULTS
A total of 232 patients had enrolled in the OLE study and had received at least one dose of fenfluramine as of the analysis cutoff date of March 13, 2018. Sixteen patients had enrolled in the OLE study after participating in the drug‐drug interaction pharmacokinetic portion of one of the core trials (NCT02926898); because baseline seizure frequency was not established in this cohort of 16 patients, their results are not included in the assessment of efficacy but are included in the safety summary. Patient demographics and baseline characteristics are presented in Table 1. A total of 22 patients (9.5%) discontinued participation in the OLE study. Reasons for discontinuation included lack of efficacy (n = 16; 6.9%; median duration of treatment = 146 days; range = 57‐465 days); withdrawal by patient/caregiver (n = 3; 1.3%; duration of treatment = 133, 178, 227 days); adverse events (n = 1; 0.4%; 71 days); death due to SUDEP (n = 1; 0.4%; 112 days); and physician decision (n = 1; 0.4%; 82 days).
Table 1.
Patient demographics and baseline characteristics
| N | 232 |
| Age, y | |
| Mean ± SD | 9.1 ± 4.7 |
| Median (min, max) | 9.0 (2, 19) |
| Age group, n (%) | |
| <6 y | 65 (25.0) |
| 6‐18 y | 166 (71.6) |
| >18 y | 1 (0.4) |
| Sex, n (%) | |
| Male | 128 (55.2) |
| Female | 104 (44.8) |
| Race, n (%) | |
| White | 172 (74.1) |
| Black or African American | 1 (0.4) |
| Asian | 9 (3.9) |
| American Indian or Alaska Native | 2 (0.9) |
| Other | 13 (5.6) |
| Not reported a | 35 (15.1) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 23 (9.9) |
| Not Hispanic or Latino | 159 (68.5) |
| Not reported a | 47 (20.3) |
| Unknown | 3 (1.3) |
| Region, n (%) | |
| North America | 111 (47.8) |
| Europe and Australia | 121 (52.2) |
| BMI, kg/m2 | |
| Mean ± SD | 17.9 ± 4.2 |
| Median (min, max) | 17.0 (11.8, 39.7) |
| Concomitant antiepilepsy drugs, n (%) | |
| Valproate (all forms) | 173 (74.6) |
| Clobazam | 159 (68.5) |
| Stiripentol | 63 (27.2) |
| Topiramate | 63 (27.2) |
| Levetiracetam | 58 (25.0) |
| Clonazepam | 33 (14.2) |
| Bromides (all forms) | 24 (10.3) |
| Zonisamide | 21 (9.1) |
| Treatment in core phase 3 trial, n (%) | |
| Placebo | 64 (27.6) |
| Fenfluramine 0.7 mg/kg/d | 35 (15.1) |
| Fenfluramine 0.4 mg/kg/d | 21 (9.1) |
| Fenfluramine 0.2 mg/kg/d | 54 (23.3) |
| Not reported b | 58 (25.0) |
| Baseline convulsive seizure frequency (seizures/28 d) | |
| N | 216 c |
| Mean ± SD | 44.0 ± 113 |
| Median | 19.7 |
| Minimum, maximum | 0, d 1464 |
Privacy laws in some regions/countries precluded disclosure of certain personal information.
These patients enrolled from a phase 3 trial that is not yet unblended.
Sixteen patients who enrolled in the pharmacokinetic cohort of Study 2 13 did not have baseline seizure frequency determined during the study and were not included in the efficacy analysis of the OLE study.
All patients had >0 seizures during baseline. The minimum value of 0 is due to an error in the seizure diary of a patient in a study that is still blinded.
The median duration of treatment with fenfluramine in this analysis period was 256 days (range = 58‐634 days). The distribution of mean daily doses for each patient over the course of treatment in the OLE study was as follows: >0 to 0.2 mg/kg/d (n = 29; 13%), >0.2 to <0.3 mg/kg/d (n = 66; 28%), 0.3 to 0.5 mg/kg/day (n = 76; 33%), and >0.5 to 0.7 mg/kg/d (n = 61; 26%). The distribution of doses in patients receiving or not receiving concomitant stiripentol is presented in Table S1. The mean daily dose calculated over the entire OLE study treatment period for all patients was 0.40 ± 0.15 (±SD) mg/kg/d. In patients treated with concomitant stiripentol, the mean daily dose was 0.32 ± 0.12 mg/kg/d, and in patients not concomitantly receiving stiripentol, the mean daily dose was 0.44 ± 0.12 mg/kg/d. During the first 18 months of treatment in the OLE study, <5% of patients had a downward dose adjustment at any study visit.
The baseline median convulsive seizure frequency before the start of treatment in the double‐blind studies for this group of patients was 19.7 seizures per month (see Table 1). Consistent with findings in the completed phase 3 clinical trials, 12 , 13 fenfluramine provided a median 70.6% reduction in convulsive seizure frequency at the end of Month 2 of the OLE study (after the mandatory 0.2 mg/kg/d dosing during Month 1), and this clinically meaningful reduction was maintained throughout the entire assessment period, which comprised nearly 24 months of treatment (median = 256 days; range = 57‐634 days; Figure 1). The median percent reduction in convulsive seizure frequency at the analysis was similar in patients <6 years old (−75.7%) and ≥6 years old (−64.7%) (Figure S1). A total of 77.8%, 64.4%, and 41.2% of patients demonstrated ≥25%, ≥50%, and ≥75% reduction in convulsive seizure frequency, respectively (Figure 2). Durability of this response was assessed in two ways. On a population level, Figure 2 illustrates the proportion of patients who demonstrated ≥25%, ≥50%, ≥75%, or 100% reduction in convulsive seizures at each assessment point during the OLE study. At the individual patient level, we assessed the percentage of days a responder spent at that given level of response during participation in the OLE study. Those patients who experienced a ≥50% reduction in convulsive seizure frequency spent an average of 80% of the days they participated in the OLE study at this level of response, and those patients who experienced a ≥75% reduction in convulsive seizure frequency spent an average of 68% of the days they participated in the OLE study at this level of response (Figure 3). Six patients (2.8%) were seizure‐free during their entire observation period (62‐545 days). During the period immediately following initiation of dose titration (ie, from Month 2 until the end of the observation period), 14 patients (6.5%) were seizure‐free. Rescue medication was used for a median 0.3 days per month during the OLE study compared with a median 0.7 days during baseline periods of the double‐blind studies. A total of 34.9% of patients did not use any rescue medication during the entire analysis period.
Figure 1.
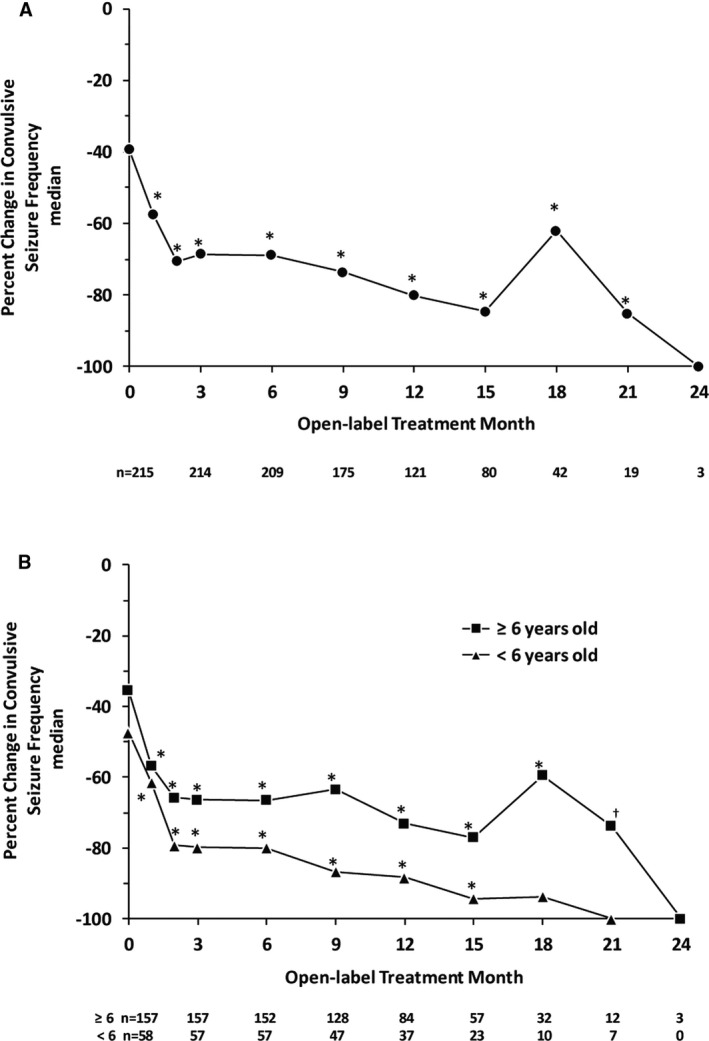
Median change from baseline in convulsive seizure frequency during treatment with fenfluramine in the open‐label extension study in the entire study population (Panel A) and in patients <6 and ≥6 years old (Panel B). OLE, open‐label extension. The number of patients assessed at each time point is shown below the x‐axes. Each point represents the cumulative change from baseline up to that time point. The decrease in patient number is due primarily to staggered entry into the OLE study—not to patient withdrawal. *P < .001, †P = .002 compared with no change (Wilcoxon signed‐rank test)
Figure 2.
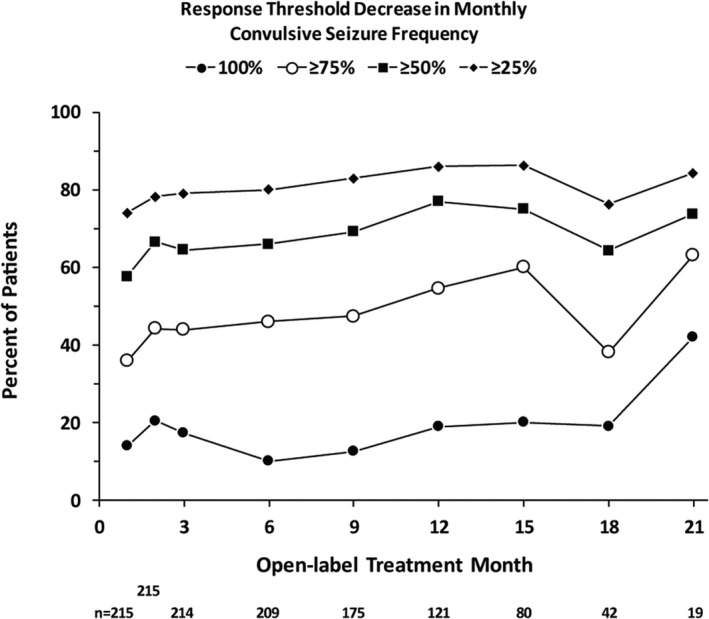
Convulsive seizure responder rates over time in the open‐label extension study (OLE). The total number of patients assessed at each time point is shown below the x‐axis. The decrease in patient number is due primarily to staggered entry into the OLE study—not to patient withdrawal. The 24‐mo time point has been omitted for clarity. All three patients with a 24‐mo assessment demonstrated 100% reduction in convulsive seizure frequency
Figure 3.
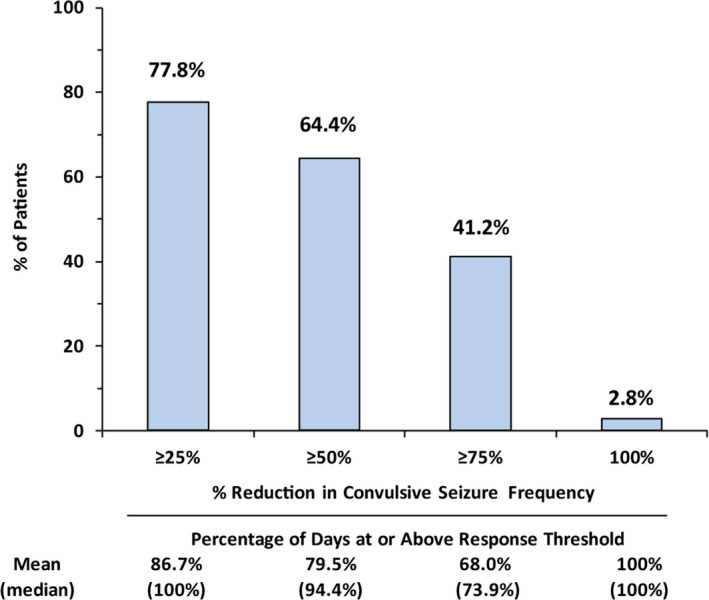
Antiseizure responder analysis for patients treated in the open‐label extension study
A similar percentage of patients was rated as “much improved” or “very much improved” on the CGI‐I by caregivers (61.1%) or investigators (65.9%) at the most recent assessment. As illustrated in Figure 4, the percentage of patients who were rated as “much improved” or “very much improved” increased over time, further demonstrating the durability of the effect of fenfluramine.
Figure 4.
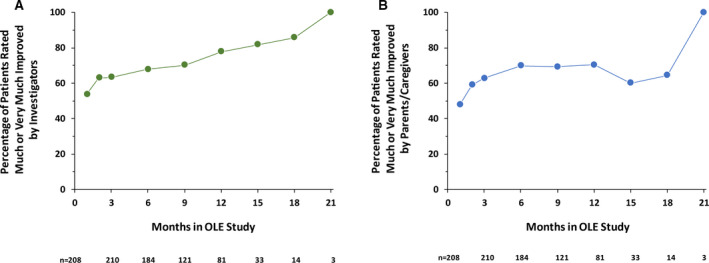
Percentage of patients rated “much improved” or “very much improved” by investigators or parents/caregivers in the OLE study. OLE, open‐label extension. The decrease in patient number is due primarily to staggered entry into the OLE study—not to patient withdrawal
TEAEs were reported for 89.7% of patients in the OLE study. TEAEs occurring in ≥10% of study participants are presented in Table 2. One patient, a 2‐year‐old boy, died in his sleep on OLE Study Day 88; his cause of death was reported as SUDEP that was unrelated to study treatment. Decreased appetite occurred in 37 patients (15.9%), and loss of body weight was reported as a TEAE in 12 patients (5.2%). During the OLE study period, loss of appetite resolved in 19 of 37, with median time to resolution of 30 days (range = 6‐61 days). A total of 31 patients (13.4%) lost ≥7% of body weight at some time during the OLE study period, and 13 of these patients (42%) recovered the weight lost over 1‐9 months. Four patients (1.7%) experienced weight gain ≥7% while treated with fenfluramine during the OLE study. Echocardiographic examination revealed that all patients continued to have normal valve function and morphology during the entire OLE analysis period (a detailed description of these results will be reported separately). No patients developed pulmonary arterial hypertension.
Table 2.
Treatment‐emergent adverse events occurring in ≥10% of patients
| TEAE | n (%) |
|---|---|
| Pyrexia | 50 (21.6) |
| Nasopharyngitis | 45 (19.4) |
| Decreased appetite | 37 (15.9) |
| Influenza | 27 (11.6) |
| Seizure | 26 (11.2) |
| Diarrhea | 25 (10.8) |
| Upper respiratory tract infection | 24 (10.3) |
4. DISCUSSION
The robust convulsive seizure reductions associated with fenfluramine for treatment of Dravet syndrome observed in this OLE study considerably extend the results observed in two phase 3 randomized, placebo‐controlled trials. 12 , 13 During Study 1, in which concomitant stiripentol use was not allowed, the fenfluramine 0.7 mg/kg/d group experienced a median 74.9% reduction in convulsive seizure frequency compared with baseline over the 14‐week treatment period. 12 During the second study (Study 2), in which all patients were also treated with stiripentol, clobazam, and/or valproate, among other AEDs, the fenfluramine 0.4 mg/kg/d group experienced a median 63.1% reduction in convulsive seizure frequency compared to baseline over the 15‐week treatment period. 13 Results of the present OLE study over a median 256 days (37 weeks) demonstrate that antiseizure responses in the core clinical trials were sustained throughout the longer OLE study analysis period. This sustained anti‐seizure response is consistent with the experience of two cohorts of patients with Dravet syndrome who have been treated with fenfluramine for up to 30 years. 7 , 8 , 9 It is important to note that no new safety issues emerged during the OLE study analysis period, and no abnormalities were noted on any echocardiogram. The overall dropout rate remained low (9.5%), supporting continued tolerability of fenfluramine during long‐term treatment, as seen during the median treatment period of 256 days. This dropout rate compares favorably to that of recent open‐label studies of stiripentol for treatment of Dravet syndrome (10 of 41 patients [24%; median treatment duration = 7 months] and 10 of 64 patients [16%; median treatment duration = 208 weeks] withdrew early due to lack of efficacy or adverse events 14 , 15 ); and of cannabidiol for Dravet syndrome (75 of 264 patients [28%] withdrew due to patient/guardian decision [n = 21; 8%], adverse events [n = 17; 6%], investigator decision [n = 16; 6%], or other reasons [8%] during a median exposure of 9 months). 16
An important component of the present study was that all patients started treatment with fenfluramine in the OLE study at a dose of 0.2 mg/kg/d. Trial design required that all patients who had been treated with 0.7 or 0.4 mg/kg/d in the double‐blind clinical trials had to undergo downtitration during transition to the OLE study. Starting all patients at the lower dose and then initiating dose titration after 4 weeks of treatment allowed a more complete assessment of the effectiveness of lower doses of fenfluramine for patients with Dravet syndrome. However, the fact that about one‐third of patients underwent down‐titration from a higher dose of fenfluramine may have accounted for the lower median percent reduction in seizure frequency observed during Month 1 of the OLE study compared with all subsequent months (see Figure 1), during which the dose was titrated to maximum effectiveness and tolerability. Of note, the balance between effectiveness and tolerability was achieved by 74% of patients at doses <0.52 mg/kg/d.
Longitudinal studies of patients with Dravet syndrome suggest that patients experiencing higher rates and greater severity of seizures are at greater risk for poor long‐term neurodevelopmental outcomes, as well as greater risk for SUDEP. 2 , 17 , 18 In the present study, patients treated with fenfluramine who attained a clinically meaningful (ie, ≥50%) reduction in convulsive seizure frequency spent an average of 80% of their days in the OLE study at this level of response. Also noteworthy was that patients experiencing a ≥75% reduction in convulsive seizure frequency spent an average of 68% of their days in the OLE study at this level of response. The ability to consistently achieve and maintain these levels of treatment response has the potential to positively influence longer‐term cognitive function. In addition, such maintenance of a profound convulsive seizure reduction may likely reduce the risk of SUDEP. 19
Fenfluramine is known to have anorectic effects 20 ; thus findings of appetite suppression and weight loss as reported in the OLE study were not unexpected. However, it appears that these TEAEs resolve over time and/or can be effectively managed, as about 50% of patients who had appetite loss during the OLE study experienced its resolution later in the study. Similarly, approximately 40% of patients who had lost ≥7% of their baseline body weight (an arbitrary threshold used in this study) experienced stability of body weight or weight gain while remaining in the OLE study.
In the past, when it was marketed as an anorectic drug for adults, fenfluramine, typically at doses of 60 to 1201 mg/d, was associated with an increased incidence of valvular heart disease and pulmonary arterial hypertension, 21 , 22 and these serious adverse events contributed to its withdrawal from global markets in 1997. 23 Because of this history, systematic intensive monitoring of cardiac valve function and morphology, as well as pulmonary arterial pressures, was performed in the OLE study to closely evaluate the long‐term safety of fenfluramine when used to treat children and young adults with Dravet syndrome. Echocardiography revealed no evidence of valvular heart disease, as evidenced by valve regurgitation, valve thickening, or restrictive valve motion, in any patient during up to 634 days of treatment with fenfluramine in the OLE study. In addition, no evidence of pulmonary arterial hypertension was observed during the OLE analysis period. Of note, in the two Belgian cohorts of patients with Dravet syndrome who have been treated with fenfluramine for up to 30 years, none of the 19 patients has developed valvular heart disease or pulmonary arterial hypertension. 24
4.1. Study limitations
Conclusions regarding efficacy and tolerability of fenfluramine for treatment of Dravet syndrome are limited by the study's open‐label design. The post hoc analysis of the relationship between the magnitude of the antiseizure response and improvement in executive function included only a subset of patients from the first double‐blind study to be completed because patients from the second double‐blind study had not yet been treated long enough in the OLE study to be included. The CGI‐I instrument requires a comparison to the baseline condition of the patient and therefore may be influenced by recall bias.
5. CONCLUSIONS
This OLE study has found that the profound reductions in convulsive seizure frequency observed in two core randomized, placebo‐controlled clinical trials of fenfluramine continued throughout the entire OLE analysis period, with a median 70.6% reduction in convulsive seizure frequency after optimization of dosing, and were sustained for a median 256 days of treatment. In addition, fenfluramine continued to be well tolerated, as evidenced by the low dropout rate in the OLE study. It is important to note that no valvular heart disease or pulmonary arterial hypertension was observed during longitudinal echocardiographic assessments performed during the OLE study. Clinically meaningful and ongoing marked seizure reduction maintained by treatment over a substantial period of time may also be clinically important, given the negative impact of frequent convulsive seizures on long‐term neurodevelopmental outcomes.
6. MEETING PRESENTATIONS
Portions of the results of this study have been presented previously at several meetings. 25 , 26 , 27 , 28 , 29
CONFLICTS OF INTEREST
JS is an advisor to Zogenix, Inc, and has received travel and grant support as an investigator from Zogenix. IES has received grant support as an investigator from Zogenix. LL is an advisor to Zogenix; has received grants and fees from Zogenix; and has a patent for the use of fenfluramine for treatment of Dravet syndrome and infantile epilepsies that is assigned to his institution and licensed to Zogenix. RN reports receiving research support from Zogenix outside the submitted work. MP is a member of the advisory board and has received fees from Zogenix. DT has received consulting fees from Zogenix. TP is a consultant to Zogenix and has received fees from Zogenix. BG, ML, AA, AG, GM, and GF are employees of Zogenix, Inc. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
AUTHOR CONTRIBUTIONS
AG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for acquisition, analysis, or interpretation of data. All authors were responsible for concept and design of the manuscript, participated in all stages of drafting of the manuscript, and critically revised the manuscript for important intellectual content. All authors approved the final version submitted for publication.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This study was funded by Zogenix, Inc (Emeryville, CA, USA). The authors received professional medical writing assistance that was provided by Edward Weselcouch, PhD, of PharmaWrite (Princeton, NJ, USA), and was funded by Zogenix, Inc.
Sullivan J, Scheffer IE, Lagae L, et al. Fenfluramine HCl (Fintepla®) provides long‐term clinically meaningful reduction in seizure frequency: Analysis of an ongoing open‐label extension study. Epilepsia. 2020;61:2396–2404. 10.1111/epi.16722
Funding information
This study was funded by Zogenix, Inc (Emeryville, CA, USA). Several of the funder's employees are authors of this paper and, accordingly, participated in the design and conduct of this study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
The cardiovascular safety results of this study are presented in detail in the accompanying article by Lai et al in this issue of Epilepsia.
DATA AVAILABILITY STATEMENT
Zogenix does not have a data‐sharing policy.
REFERENCES
- 1. Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. [DOI] [PubMed] [Google Scholar]
- 2. Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43–7. [DOI] [PubMed] [Google Scholar]
- 3. Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64(Pt A):69–74. [DOI] [PubMed] [Google Scholar]
- 4. Aras LM, Isla J, Mingorance‐Le MA. The European patient with Dravet syndrome: results from a parent‐reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104–9. [DOI] [PubMed] [Google Scholar]
- 5. Rosati A, Boncristiano A, Doccini V, Pugi A, Pisano T, Lenge M, et al. Long‐term efficacy of add‐on stiripentol treatment in children, adolescents, and young adults with refractory epilepsies: a single center prospective observational study. Epilepsia. 2019;60(11):2255–62. [DOI] [PubMed] [Google Scholar]
- 6. Schoonjans A‐N, Lagae L, Ceulemans B. Low‐dose fenfluramine in the treatment of neurologic disorders: experience in Dravet syndrome. Ther Adv Neurol Disord. 2015;8:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ceulemans B, Boel M, Leyssens K, Van Rossem C, Neels P, Jorens PG, et al. Successful use of fenfluramine as an add‐on treatment for Dravet syndrome. Epilepsia. 2012;53(7):1131–9. [DOI] [PubMed] [Google Scholar]
- 8. Ceulemans B, Schoonjans A‐S, Marchau F, Paelinck B, Lagae L. Five‐year extended follow‐up of 10 Dravet patients treated with fenfluramine. Epilepsia. 2016;57(7):e129–34. [DOI] [PubMed] [Google Scholar]
- 9. Schoonjans A, Paelinck BP, Marchau F, Gunning B, Gammaitoni A, Galer BS, et al. Low‐dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 2017;24(2):309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodriguez‐Munoz M, Sanchez‐Blazquez P, Garzon J. Fenfluramine diminishes NMDA receptor‐mediated seizures via its mixed activity at serotonin 5HT2A and type 1 sigma receptors. Oncotarget. 2018;9(34):23373–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuller RW, Snoddy HD, Robertson DW. Mechanisms of effects of d‐fenfluramine on brain serotonin metabolism in rats: uptake inhibition versus release. Pharmacol Biochem Behav. 1988;30(3):715–21. [DOI] [PubMed] [Google Scholar]
- 12. Lagae L, Sullivan J, Knupp K, Laux L, Polster T, Nikanorova M, et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2019;394:2243–54. [DOI] [PubMed] [Google Scholar]
- 13. Nabbout R, Mistry A, Zuberi S, Villeneuve N, Gil‐Nagel A, Sanchez‐Carpintero R, et al. Fenfluramine for treatment‐resistant seizures in patients with Dravet syndrome receiving stiripentol‐inclusive regimens: a randomized clinical trial. JAMA Neurol. 2019;77(3):300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myers KA, Lightfoot P, Patil SG, Cross JH, Scheffer IE. Stiripentol efficacy and safety in Dravet syndrome: a 12‐year observational study. Dev Med Child Neurol. 2018;60(6):574–8. [DOI] [PubMed] [Google Scholar]
- 15. Yamamoto Y, Takahashi Y, Ikeda H, Imai K, Kagawa Y, Inoue Y. Impact of CYP2C19 phenotypes on clinical efficacy of stiripentol in Japanese patients with Dravet syndrome. Ther Drug Monit. 2019;42(2):302–8. [DOI] [PubMed] [Google Scholar]
- 16. Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al. Long‐term cannabidiol treatment in patients with Dravet syndrome: an open‐label extension trial. Epilepsia. 2019;60(2):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long‐term follow‐up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51(6):1043–52. [DOI] [PubMed] [Google Scholar]
- 18. Wolff M, Casse‐Perrot C, Dravet C. Severe myoclonic epilepsy of infants (Dravet syndrome): natural history and neuropsychological findings. Epilepsia. 2006;47(Suppl 2):45–8. [DOI] [PubMed] [Google Scholar]
- 19. Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674–80. [DOI] [PubMed] [Google Scholar]
- 20. Pinder RM, Brogden RN, Sawyer PR, Speight TM, Avery GS. Fenfluramine: a review of its pharmacological properties and therapeutic efficacy in obesity. Drugs. 1975;10(4):241–323. [DOI] [PubMed] [Google Scholar]
- 21. Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. Appetite‐suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335(9):609–16. [DOI] [PubMed] [Google Scholar]
- 22. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, et al. Valvular heart disease associated with fenfluramine‐phentermine. N Engl J Med. 1997;337(9):581–8. [DOI] [PubMed] [Google Scholar]
- 23. Onakpoya IJ, Heneghan CJ, Aronson JK. Worldwide withdrawal of medicinal products because of adverse drug reactions: a systematic review and analysis. Crit Rev Toxicol. 2016;46(6):477–89. [DOI] [PubMed] [Google Scholar]
- 24. Schoonjans AS, Marchau F, Paelinck BP, Lagae L, Gammaitoni A, Pringsheim M, et al. Cardiovascular safety of low‐dose fenfluramine in Dravet syndrome: a review of its benefit‐risk profile in a new patient population. Curr Med Res Opin. 2017;33(10):1773–81. [DOI] [PubMed] [Google Scholar]
- 25. Lagae L, Sullivan J, Nabbout R, Pringsheim M, Farfel G, Galer BS, et al. Fintepla® (fenfluramine HCl oral solution) provides long‐term clinically meaningful reduction in seizure frequency: results of an open‐label extension study. Presented at: American Epilepsy Society Annual Meeting; New Orleans, LA. November 30‐December 4, 2018.
- 26. Lagae L, Zuberi S, Villeneuve N, Mayer T, Jacobs Le‐Van J, Sanchez‐Carpintero R, et al. Fenfluramine HCl provides long‐term clinically meaningful reduction in seizure frequency: results of an open‐label extension study. Presented at: International Epilepsy Colloquium; Lyon, France. May 26–28, 2019.
- 27. Pringsheim M, Kluger G, Brandl U, Jacobs J, Mayer T, Panzer A, et al. Fenfluramine HCl provides long‐term clinically meaningful reduction in seizure frequency: results of an open‐label extension study. Presented at: Annual Meeting of the Austrian and German Societies for Epileptology and the Swiss Epilepsy League; Basel, Switzerland. May 8–11, 2019.
- 28. Pringsheim M, Kluger G, Brandl U, Julia J, Mayer T, Panzer A, et al. Fenfluramine HCl provides long‐term clinically meaningful reduction in seizure frequency: results of an open‐label extension study. Presented at: Annual Meeting of the German Society for Pediatric and Adolescent Medicine; Munich, Germany. September 11–14, 2019.
- 29. Zuberi SM, Cross JH, Sunny P, Iyer A, Farfel G, Galer B, et al. Fenfluramine HCl provides long‐term clinically meaningful reduction in seizure frequency: results of an open‐label extension study. Presented at: London‐Innsbruck Colloquium on Status Epilepticus and Acute Seizures; London, UK, April 7–9, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Zogenix does not have a data‐sharing policy.


