Abstract
Aims
The safety and efficacy of the novel selective cardiac myosin activator, omecamtiv mecarbil, in patients with heart failure with reduced ejection fraction (HFrEF) is being tested in the Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure (GALACTIC‐HF) trial. Here we describe the baseline characteristics of participants in GALACTIC‐HF and how these compare with other contemporary trials.
Methods and results
Adults with established HFrEF, New York Heart Association (NYHA) functional class ≥II, ejection fraction ≤35%, elevated natriuretic peptides and either current hospitalization for heart failure or history of hospitalization/emergency department visit for heart failure within a year were randomized to either placebo or omecamtiv mecarbil (pharmacokinetic‐guided dosing: 25, 37.5, or 50 mg bid). A total of 8256 patients [male (79%), non‐white (22%), mean age 65 years] were enrolled with a mean ejection fraction 27%, ischaemic aetiology in 54%, NYHA class II 53% and III/IV 47%, and median N‐terminal pro‐B‐type natriuretic peptide 1971 pg/mL. Heart failure therapies at baseline were among the most effectively employed in contemporary heart failure trials. GALACTIC‐HF randomized patients representative of recent heart failure registries and trials with substantial numbers of patients also having characteristics understudied in previous trials including more from North America (n = 1386), enrolled as inpatients (n = 2084), systolic blood pressure <100 mmHg (n = 1127), estimated glomerular filtration rate <30 mL/min/1.73 m2 (n = 528), and treated with sacubitril/valsartan at baseline (n = 1594).
Conclusions
GALACTIC‐HF enrolled a well‐treated, high‐risk population from both inpatient and outpatient settings, which will provide a definitive evaluation of the efficacy and safety of this novel therapy, as well as informing its potential future implementation.
Keywords: Heart failure, Omecamtiv mecarbil, Cardiac myosin activator, Inotrope, Myotrope, Cardiovascular outcomes trial
Graphical representation of the GALACTIC‐HF trial design, enrolment and baseline characteristics. ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; CRT, cardiac resynchronization therapy (biventricular pacemaker); CV, cardiovascular; EF, ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, Hew York Heart Association; SBP, systolic blood pressure.

Introduction
The pathogenesis of heart failure with reduced ejection fraction (HFrEF) is characterized primarily by a decrease in systolic function independent of the specific aetiology. Contemporary therapies do not directly address this fundamental defect, but rather act on the compensatory pathways that are stimulated by this loss of function. Omecamtiv mecarbil 1 is a cardiac myosin activator 2 and the first of a novel class of myotropes, 3 agents that directly improve myocardial function by selectively increasing cardiac sarcomere function. Clinical studies in healthy volunteers 4 and patients with stable chronic 5 and acute heart failure 6 show that omecamtiv mecarbil improves cardiac performance. In the COSMIC‐HF study, 7 patients with chronic HFrEF treated with omecamtiv mecarbil for 20 weeks had improved systolic function and structure demonstrated by increased systolic ejection time, 8 , 9 , 10 fractional shortening, ejection fraction and stroke volume as well as decreased left ventricular systolic dimensions and volumes. Omecamtiv mecarbil treatment also led to reductions in N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and heart rate, consistent with decreased ventricular stress and less neurohormonal activation. Importantly, the significant reductions in left ventricular diastolic and systolic dimensions and volumes were suggestive of beneficial reverse ventricular remodelling. These findings suggest that omecamtiv mecarbil might reduce both heart failure hospitalizations 11 and mortality. 12
The Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in Heart Failure (GALACTIC‐HF) trial is the first to examine whether selectively increasing cardiac contractility in patients with HFrEF will result in improved clinical outcomes. 13 Due to the absence of adverse effects on blood pressure, heart rate, renal function or potassium homeostasis with omecamtiv mecarbil, GALACTIC‐HF enrolled a broad range of patients from both the inpatient and outpatient settings, many of whom have been specifically excluded in other contemporary heart failure trials. GALACTIC‐HF tests the hypotheses that omecamtiv mecarbil can safely improve symptoms, prevent clinical heart failure events, and delay cardiovascular death in patients with HFrEF.
Methods
The GALACTIC‐HF (ClinicalTrials.gov NCT02929329; EU Clinical Trials Register 2016‐002299‐28) trial is a multicentre, randomized, double‐blind, placebo‐controlled, event‐driven cardiovascular outcomes trial to evaluate the efficacy, safety and tolerability of omecamtiv mecarbil in patients with chronic HFrEF. 13
Summary of GALACTIC‐HF design
People aged 18–85 years with a history of symptomatic [New York Heart Association (NYHA) functional class II–IV] HFrEF (ejection fraction ≤ 35%), optimally treated with standard of care pharmacologic and device therapy for HFrEF were eligible. Participants were currently hospitalized for heart failure (inpatients; approximately 25% of enrolment) or within 1 year had either an urgent visit to the emergency department for heart failure or a hospitalization for heart failure (outpatients). In addition, patients had NT‐proBNP concentration ≥400 pg/mL or B‐type natriuretic peptide (BNP) ≥125 pg/mL at screening (if in atrial fibrillation/flutter: NT‐proBNP ≥1200 pg/mL or BNP ≥375 pg/mL). Key exclusion criteria included: current haemodynamic or clinical instability requiring mechanical or intravenous medication, systolic blood pressure (SBP) <85 mmHg, estimated glomerular filtration rate (eGFR) <20 mL/min/1.73 m2, recent acute coronary syndrome events or cardiovascular procedures (including planned procedures), and other conditions with reduced life expectancy <2 years or that would adversely affect participation in the trial. A full description of the eligibility criteria has been published. 13
Participants were randomized 1:1 to oral administration of either placebo or omecamtiv mecarbil twice daily (pharmacokinetic‐guided dosing: 25, 37.5, or 50 mg bid). The primary objective of the GALACTIC‐HF trial is to determine in patients with HFrEF on standard of care heart failure therapy whether omecamtiv mecarbil is superior to placebo in reducing cardiovascular death or heart failure events. A heart failure event is defined as an urgent, unscheduled clinic/office/emergency department visit or hospital admission with a primary diagnosis of heart failure where the patient exhibited new or worsening symptoms of heart failure on presentation, had objective evidence of new or worsening heart failure, and received initiation or intensification of treatment specifically for heart failure. Additional secondary and exploratory outcomes have been published. 13 The study is endpoint‐driven and will end after accumulation of approximately 1590 cardiovascular deaths.
Comparator studies
Three recent registries [European Society of Cardiology Heart Failure Long‐Term Registry (ESC HF Long‐Term Registry), 14 Asian Sudden Cardiac Death in Heart Failure (ASIAN‐HF) registry, 15 , 16 Change the Management of Patients with Heart Failure (CHAMP‐HF) registry 17 , 18 ] from varied international regions were reviewed to provide ‘real‐world’ context for the GALACTIC‐HF population. Moreover, patient characteristics from four contemporary Phase III clinical trials of heart failure pharmacologic therapies [Prospective comparison of ARNi with ACEi to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM‐HF), 19 Dapagliflozin And Prevention of Adverse outcomes in Heart Failure (DAPA‐HF), 20 Vericiguat global study in subjects with heart failure with reduced ejection fraction (VICTORIA) 21 and Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction (EMPEROR‐Reduced) 22 ] were compared to those in GALACTIC‐HF. GALACTIC‐HF was designed to enrol patients with HFrEF from both the inpatient and outpatient settings. While many Phase III trials have been conducted to evaluate new intravenous therapies for patients with acute heart failure, three major trials [Efficacy of Vasopressin Antagonism In Heart Failure Outcome Study with Tolvaptan (EVEREST), 23 , 24 Aliskiren Trial on Acute Heart Failure Outcomes (ASTRONAUT), 25 and Comparison of Sacubitril‐Valsartan vs. Enalapril on Effect on NT‐proBNP in Patients Stabilized from an Acute Heart Failure Episode (PIONEER‐HF) 26 ] have enrolled patients stabilized during an admission for heart failure and treated with chronic oral therapies. These trials have been used to provide the context for GALACTIC‐HF participants enrolled as inpatients.
Results
GALACTIC‐HF baseline characteristics
Nearly 11 000 people were screened for enrolment in GALACTIC‐HF and approximately 25% did not meet eligibility criteria (online supplementary Table S1 ). From 6 January 2017 to 9 July 2019, 8256 participants were randomized at 945 sites in 35 countries. The baseline characteristics of these participants are presented in Table 1 and in Figure 1 . The participants were on average 65 years of age, 21% female, and 78% self‐identified white race recruited from a wide range of regions (33% Eastern Europe/Russia; 23% Western Europe/South Africa/Australasia; 19% Latin and South America; 17% North America; 8% Asia). Comorbidities were common in the participants, including 62% with coronary artery disease, 42% history of atrial fibrillation/flutter, 70% hypertension, and 40% diabetes mellitus. The median (Q1–Q3) eGFR was 59 (44–74) mL/min/1.73 m2 and 52% had chronic kidney disease stage III–V. The mean left ventricular ejection fraction was 27%, predominantly due to an ischaemic aetiology (54%). Participants had mild–moderate symptom limitation, with 53% patients in NYHA functional class II and 47% in NYHA functional class III/IV. The mean Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ‐TSS) was 66 (where 100 is the least symptom burden). Mean (standard deviation) SBP was 117 (15) mmHg and the mean heart rate was 72 (12) bpm. NT‐proBNP was substantially elevated [median (Q1–Q3): 1971 (961–4033) pg/mL], with modestly elevated high‐sensitivity troponin I [median (Q3): 0.030 (0.049) ng/mL; upper limit of 95% confidence interval: 0.014 ng/mL]. Patients were well treated with guideline‐recommended heart failure therapies at baseline with 87% receiving an angiotensin‐converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB)/angiotensin receptor–neprilysin inhibitor (ARNi) (19.3% ARNi), 94% beta‐blocker, and 77% mineralocorticoid receptor antagonist (MRA). Approximately two‐thirds of the participants were receiving triple therapy (ACEi/ARB/ARNi + beta‐blocker + MRA). Almost 32% of the patients had an implantable cardioverter defibrillator and 14% had cardiac resynchronization therapy at baseline.
Table 1.
Baseline characteristics of GALACTIC‐HF patients
| Overall (n = 8256) | Current HF hospitalization (‘Inpatient’) (n = 2084) | Recent HF hospitalization or ED visit within 1 year (‘Outpatient’) (n = 6172) | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 64.5 (11.3) | 65.0 (11.3) | 64.4 (11.4) |
| Female sex, n (%) | 1756 (21.3) | 411 (19.7) | 1345 (21.8) |
| Race, n (%) | |||
| White | 6421 (77.8) | 1706 (81.9) | 4715 (76.4) |
| Asian | 710 (8.6) | 184 (8.8) | 526 (8.5) |
| Black or African American | 562 (6.8) | 105 (5.0) | 457 (7.4) |
| Other a | 563 (6.8) | 89 (4.3) | 474 (7.7) |
| Ethnicity, Hispanic/Latino, n (%) | 1771 (21.5) | 355 (17.0) | 1416 (22.9) |
| Geographic region, n (%) | |||
| Eastern Europe/Russia | 2705 (32.8) | 915 (43.9) | 1790 (29.0) |
| Western Europe/South Africa/Australasia | 1921 (23.3) | 486 (23.3) | 1435 (23.3) |
| Latin and South America | 1574 (19.1) | 326 (15.6) | 1248 (20.2) |
| US and Canada | 1386 (16.8) | 180 (8.6) | 1206 (19.5) |
| Asia | 670 (8.1) | 177 (8.5) | 493 (8.0) |
| Clinical characteristics | |||
| Medical conditions, n (%) | |||
| Coronary artery disease | 5144 (62.3) | 1317 (63.2) | 3827 (62.0) |
| Myocardial infarction | 3457 (41.9) | 893 (42.9) | 2564 (41.5) |
| Percutaneous coronary intervention | 2452 (29.7) | 599 (28.7) | 1853 (30.0) |
| Coronary artery bypass grafting | 1319 (16.0) | 320 (15.4) | 999 (16.2) |
| Peripheral artery disease | 847 (10.3) | 215 (10.3) | 632 (10.2) |
| Stroke | 753 (9.1) | 197 (9.5) | 556 (9.0) |
| Atrial fibrillation or flutter history | 3472 (42.1) | 995 (47.7) | 2477 (40.1) |
| Hypertension | 5800 (70.3) | 1495 (71.7) | 4305 (69.8) |
| Hypercholesterolaemia | 4553 (55.1) | 1094 (52.5) | 3459 (56.0) |
| Type 2 diabetes mellitus | 3313 (40.1) | 870 (41.7) | 2443 (39.6) |
| Chronic kidney disease | 2977 (36.1) | 809 (38.8) | 2168 (35.1) |
| Chronic obstructive pulmonary disease | 1344 (16.3) | 354 (17.0) | 990 (16.0) |
| Asthma | 440 (5.3) | 92 (4.4) | 348 (5.6) |
| HF history | |||
| LVEF (%), mean (SD) | 26.6 (6.3) | 26.5 (6.4) | 26.6 (6.2) |
| MAGGIC score, mean (SD) | 23.3 (6.3) | 25.0 (6.3) | 22.8 (6.3) |
| NYHA class, n (%) | |||
| II | 4391 (53.2) | 767 (36.8) | 3624 (58.7) |
| III | 3616 (43.8) | 1190 (57.1) | 2426 (39.3) |
| IV | 248 (3.0) | 126 (6.0) | 122 (2.0) |
| Ischaemic HF aetiology, n (%) | 4458 (54.0) | 1148 (55.1) | 3310 (53.6) |
| KCCQ total symptom score, mean (SD) | 66.4 (25.1) | 52.6 (25.4) | 71.0 (23.2) |
| Vitals and laboratory parameters | |||
| Body mass index (kg/m2), mean (SD) | 28.5 (6.2) | 28.0 (6.1) | 28.6 (6.2) |
| SBP (mmHg), mean (SD) | 117 (15) | 114 (14) | 117 (16) |
| Heart rate (bpm), mean (SD) | 72 (12) | 73 (12) | 72 (12) |
| NT‐proBNP (pg/mL), median (Q1–Q3) | 1971 (961–4033) | 2457 (1185–5073) | 1858 (900–3749) |
| hsTnI (ng/mL), median (Q3) | 0.030 (0.049) | 0.036 (0.066) | 0.029 (0.044) |
| eGFR (mL/min/1.73 m2), median (Q1–Q3) | 59 (44–74) | 54 (41–70) | 60 (45–75) |
| Stage ≤2: >60 | 3922 (47.7) | 838 (40.2) | 3084 (50.0) |
| Stage 3: 30–59 | 3806 (46.1) | 1077 (51.7) | 2729 (44.2) |
| Stage 4: 15–29 | 523 (6.3) | 169 (8.1) | 354 (5.7) |
| Stage 5: <15 | 5 (<0.1) | 0 (0.0) | 5 (<0.1) |
| Medications and cardiac devices, n (%) | |||
| ACEi, ARB, or ARNi | 7161 (86.7) | 1729 (83.0) | 5432 (88.0) |
| ARNi | 1594 (19.3) | 328 (15.7) | 1266 (20.5) |
| BB | 7763 (94.0) | 1931 (92.7) | 5832 (94.5) |
| MRA | 6358 (77.0) | 1686 (80.9) | 4672 (75.7) |
| (ACEi, ARB, or ARNi) + MRA + BB | 5367 (65.0) | 1360 (65.3) | 4007 (64.9) |
| Digitalis glycosides | 1380 (16.7) | 356 (17.1) | 1024 (16.6) |
| SGLT2 inhibitors | 219 (2.7) | 56 (2.7) | 163 (2.6) |
| Ivabradine | 533 (6.5) | 156 (7.5) | 375 (6.1) |
| CRT | 1156 (14.0) | 267 (12.8) | 889 (14.4) |
| ICD | 2614 (31.7) | 598 (28.7) | 2016 (32.7) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; CRT, cardiac resynchronization therapy; ED, emergency department; eGFR, estimated glomerular filtration rate; HF, heart failure; hsTnI, high‐sensitivity troponin I; ICD, implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation; SGLT2, sodium–glucose co‐transporter 2.
Includes American Indian or Alaska native, native Hawaiian or other Pacific Islander, or multiple self‐identified races.
Figure 1.
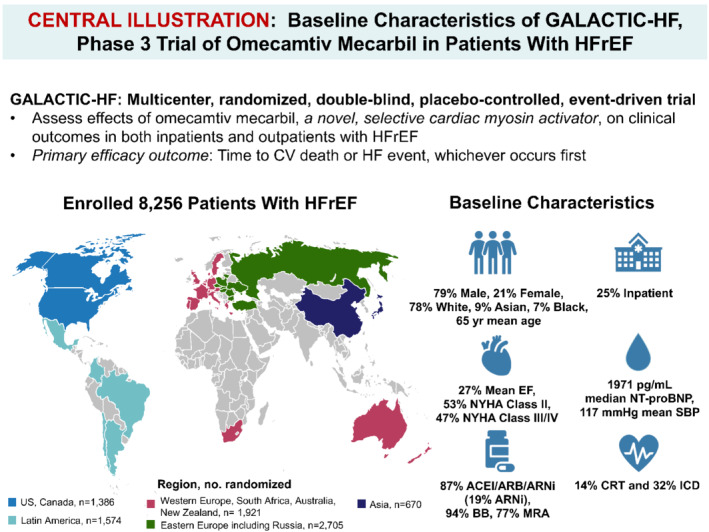
Graphical representation of the GALACTIC‐HF trial design, enrolment and baseline characteristics. ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor‐neprilysin inhibitor; BB, beta‐blocker; CRT, cardiac resynchronization therapy (biventricular pacemaker); CV, cardiovascular; EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, Hew York Heart Association; SBP, systolic blood pressure.
Participants in GALACTIC‐HF were enrolled from both the inpatient and outpatient clinical settings. Of the 8256 participants, 2084 (25.2%) were enrolled as inpatients after stabilization during a hospitalization for heart failure with a greater percentage of the inpatient cohort enrolled in Eastern Europe and Russia (Table 1 ). As might be anticipated, the participants enrolled during a heart failure hospitalization also had a higher prevalence of chronic kidney disease and history of atrial fibrillation/flutter, had more symptomatic heart failure (worse baseline NYHA functional class, MAGGIC score, and KCCQ total symptom score), lower SBP and eGFR, and higher NT‐proBNP and high sensitivity troponin I concentrations compared to those enrolled as outpatients. Although the use of heart failure therapy was lower at baseline in the participants enrolled as inpatients, 83% were treated with ACEi/ARB/ARNi (16% ARNi), 93% with beta‐blockers, 81% with MRA and 65% with triple therapy [(ACEi/ARB/ARNi) + beta‐blocker + MRA].
The absence of adverse effects on renal function and blood pressure in prior studies with omecamtiv mecarbil permitted enrolment of patients in GALACTIC‐HF with eGFR and SBP lower than levels often excluded from HFrEF clinical trials. Over 500 patients with eGFR <30 mL/min/1.73 m2 participated (Table 2 ) and there were over 1100 participants in GALACTIC‐HF with SBP <100 mmHg (Table 2 ). GALACTIC‐HF will provide important insights into these two groups of patients that have been underrepresented in other contemporary clinical trials.
Table 2.
Selected subgroups in GALACTIC‐HF
| Renal function | SBP | |||
|---|---|---|---|---|
| eGFR <30 mL/min/m2 (n = 528) | eGFR ≥30 mL/min/m2 (n = 7728) | <100 mmHg (n = 1127) | ≥100 mmHg (n = 7129) | |
| Demographics | ||||
| Age (years), mean (SD) | 70.5 (8.8) | 64.1 (11.4) | 63.3 (11.9) | 64.7 (11.3) |
| Female sex, n (%) | 175 (33.1) | 1581 (20.5) | 248 (22.0) | 1508 (21.2) |
| Race, n (%) | ||||
| White | 450 (85.2) | 5971 (77.3) | 789 (70.0) | 5632 (79.0) |
| Asian | 19 (3.6) | 691 (8.9) | 188 (16.7) | 522 (7.3) |
| Black or African American | 24 (4.5) | 538 (7.0) | 72 (6.4) | 490 (6.9) |
| Other a | 35 (6.6) | 528 (6.8) | 78 (6.9) | 485 (6.8) |
| Geographic region, n (%) | ||||
| Eastern Europe/Russia | 139 (26.3) | 2566 (33.2) | 136 (12.1) | 2569 (36.0) |
| Western Europe/South Africa/Australasia | 196 (37.1) | 1725 (22.3) | 363 (32.2) | 1558 (21.9) |
| Latin and South America | 86 (16.3) | 1488 (19.3) | 214 (19.0) | 1360 (19.1) |
| US and Canada | 90 (17.0) | 1296 (16.8) | 237 (21.0) | 1149 (16.1) |
| Asia | 17 (3.2) | 653 (8.4) | 177 (15.7) | 493 (6.9) |
| Clinical characteristics | ||||
| Medical conditions, n (%) | ||||
| Coronary artery disease | 397 (75.2) | 4747 (61.4) | 627 (55.6) | 4517 (63.4) |
| Myocardial infarction | 282 (53.4) | 3175 (41.1) | 455 (40.4) | 3002 (42.1) |
| Peripheral artery disease | 88 (16.7) | 759 (9.8) | 99 (8.8) | 748 (10.5) |
| Stroke | 63 (11.9) | 690 (8.9) | 111 (9.8) | 642 (9.0) |
| Atrial fibrillation or flutter history | 282 (53.4) | 3190 (41.3) | 509 (45.2) | 2963 (41.6) |
| Hypertension | 403 (76.3) | 5397 (69.8) | 551 (48.9) | 5249 (73.6) |
| Hyperlipidaemia | 352 (66.7) | 4201 (54.4) | 568 (50.4) | 3985 (55.9) |
| Type 2 diabetes mellitus | 287 (54.4) | 3026 (39.2) | 404 (35.8) | 2909 (40.8) |
| Chronic kidney disease | 466 (88.3) | 2511 (32.5) | 407 (36.1) | 2570 (36.0) |
| Chronic obstructive pulmonary disease | 92 (17.4) | 1252 (16.2) | 152 (13.5) | 1192 (16.7) |
| HF history | ||||
| LVEF (%), mean (SD) | 26.7 (6.2) | 26.6 (6.3) | 24.0 (6.2) | 27.0 (6.2) |
| Ischaemic HF, n (%) | 353 (66.9) | 4105 (53.1) | 542 (48.1) | 3916 (54.9) |
| NYHA class, n (%) | ||||
| II | 210 (39.8) | 4181 (54.1) | 557 (49.4) | 3834 (53.8) |
| III | 295 (55.9) | 3321 (43.0) | 523 (46.4) | 3093 (43.4) |
| IV | 23 (4.4) | 225 (2.9) | 47 (4.2) | 201 (2.8) |
| KCCQ total symptom score, mean (SD) | 60.2 (26.7) | 66.8 (25.0) | 64.3 (26.0) | 66.7 (25.0) |
| MAGGIC score, mean (SD) | 30.4 (5.5) | 22.9 (6.1) | 26.1 (6.5) | 22.9 (6.2) |
| Vitals and laboratory parameters | ||||
| Body mass index (kg/m2), mean (SD) | 28.7 (5.7) | 28.5 (6.2) | 26.28 (5.6) | 28.81 (6.2) |
| SBP (mmHg), mean (SD) | 114.4 (16.7) | 116.6 (15.2) | 92.6 (4.6) | 120.2 (12.8) |
| Heart rate (bpm), mean (SD) | 70.4 (11.5) | 72.5 (12.2) | 72.5 (12.2) | 72.3 (12.1) |
| NT‐proBNP (pg/mL), median (Q1–Q3) | 4525.0 (2082.0–8435.0) | 1885.0 (924.0–3768.0) | 2927.0 (1466.5–5835.0) | 1858.0 (903.0–3775.0) |
| hsTnI (ng/mL), median (Q3) | 0.040 (0.075) | 0.030 (0.048) | 0.030 (0.052) | 0.030 (0.049) |
| eGFR (mL/min/1.73 m2), median (Q1–Q3) | 25.7 (23.1–28.0) | 60.4 (47.3–75.4) | 55.2 (40.7–70.6) | 59.3 (44.7–74.4) |
| Stage ≤2: >60 | – | 3922 (50.8) | 461 (40.9) | 3461 (48.5) |
| Stage 3: 30–59 | – | 3806 (49.2) | 561 (49.8) | 3245 (45.5) |
| Stage 4: 15–29 | 523 (99.1) | – | 104 (9.2) | 419 (5.9) |
| Stage 5: <15 | 5 (0.9) | – | 1 (<0.1) | 4 (<0.1) |
| Medications and cardiac devices, n (%) | ||||
| ACEi, ARB, or ARNi | 379 (71.8) | 6782 (87.8) | 957 (84.9) | 6204 (87.0) |
| ARNi | 110 (20.8) | 1484 (19.2) | 344 (30.5) | 1250 (17.5) |
| BB | 480 (90.9) | 7283 (94.2) | 1030 (91.4) | 6733 (94.4) |
| MRA | 294 (55.7) | 6064 (78.5) | 893 (79.2) | 5465 (76.7) |
| (ACEi, ARB, or ARNi) + MRA + BB | 217 (41.1) | 5150 (66.6) | 711 (63.1) | 4656 (65.3) |
| Digitalis glycosides | 54 (10.2) | 1326 (17.2) | 223 (19.8) | 1157 (16.2) |
| SGLT2 inhibitors | 7 (1.3) | 212 (2.7) | 44 (3.9) | 175 (2.5) |
| Ivabradine | 40 (7.6) | 493 (6.4) | 79 (7.0) | 454 (6.4) |
| CRT | 130 (24.6) | 1026 (13.3) | 248 (22.0) | 908 (12.7) |
| ICD | 235 (44.5) | 2379 (30.8) | 502 (44.5) | 2112 (29.6) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; hsTnI, high‐sensitivity troponin I; ICD, implantable cardioverter defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation; SGLT2, sodium–glucose co‐transporter 2.
Includes American Indian or Alaska native, native Hawaiian or other Pacific Islander, or multiple self‐identified races.
Comparison of GALACTIC‐HF baseline characteristics to other heart failure populations
The baseline characteristics of participants in GALACTIC‐HF are compared to the population of patients in a number of registries (online supplementary Table S2 ) as well as four major contemporary trials (PARADIGM‐HF, DAPA‐HF, VICTORIA and EMPEROR‐Reduced) of pharmacologic therapies in participants enrolled as outpatients with HFrEF (Table 3 ). To provide context for GALACTIC‐HF participants enrolled in‐hospital, their baseline characteristics were compared to patients from the EVEREST, ASTRONAUT, and PIONEER‐HF trials (Table 4 ). The key selection criteria for these trials are presented in online supplementary Table S3 .
Table 3.
Baseline characteristics and treatments in GALACTIC‐HF, EMPEROR‐Reduced, VICTORIA, DAPA‐HF and PARADIGM‐HF trials
| GALACTIC‐HF (n = 8256) | EMPEROR‐Reduced (n = 3730) | VICTORIA (n = 5050) | DAPA‐HF (n = 4744) | PARADIGM‐HF (n = 8442) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years), mean (SD) | 64.5 (11.3) | 66.9 (11.0) | 67.3 (12.2) | 66.4 (11) | 63.8 (11.4) |
| Female sex, n (%) | 1756 (21.3) | 893 (23.9) | 1208 (23.9) | 1109 (23.4) | 1832 (22.0) |
| Race, n (%) | |||||
| White | 6421 (77.8) | 2629 (70.5) | 3239 (64.2) | 3333 (70.3) | 5544 (65.7) |
| Asian | 710 (8.6) | 672 (18.0) | 1132 (22.4) | 1116 (23.5) | 1509 (17.9) |
| Black or African American | 562 (6.8) | 257 (6.9) | 249 (4.9) | 226 (4.8) | 428 (5.1) |
| Geographic region, n (%) | |||||
| Eastern Europe/Russia | 2705 (32.8) | All Europe 1353 (36.3) | 1694 (33.5) | 1604 (33.8) | 2826 (33.5) |
| Western Europe/South Africa/Australasia | 1921 (23.3) | 889 (17.6) | 550 (11.6) | 2051 (24.3) | |
| Latin and South America | 1574 (19.1) | 1286 (34.5) | 724 (14.3) | 817 (17.2) | 1433 (17.0) |
| US and Canada | 1386 (16.8) | 425 (11.4) | 560 (11.1) | 677 (14.3) | 602 (7.1) |
| Asia Pacific | 670 (8.1) | 493 (13.2) | 1183 (23.4) | 1069 (23.1) | 1487 (17.6) |
| Index event, n (%) | |||||
| Inpatient for HF | 2084 (25.2) | N/A | a | N/A | N/A |
| HF hospitalization within 3 months | 2992 (36.2) | 3366 (66.7) | 368 (7.8) | 1611 (19.1) | |
| IV diuretic for HF within 3 months (no hospitalization) | N/A | 813 (16.1) | N/A | N/A | |
| HF hospitalization 3–6 months | 1523 (18.4) | 871 (17.2) | 410 (8.6) | 1009 (12.0) | |
| HF hospitalization >6 months | 1636 (19.8) | N/A | 1473 (31.0) | 2632 (31.2) | |
| Clinical characteristics | |||||
| Medical conditions, n (%) | |||||
| Coronary artery disease | 5144 (62.3) | 1710 (45.8) | 2944 (58.3) | – | 4796 (57.1) |
| Myocardial infarction | 3457 (41.9) | 1623 (43.5) | 1977 (44.5) | 3634 (43.0) | |
| Peripheral artery disease | 847 (10.3) | 261 (7.0) | 630 (12.5) | 324 (6.8) | |
| Stroke | 753 (9.1) | 421 (11.3) | 578 (11.5) | 472 (9.9) | 725 (8.7) |
| Atrial fibrillation or flutter history | 3472 (42.1) | 1369 (36.7) | 2268 (44.9) | 1818 (38.3) | 3091 (37.0) |
| Hypertension | 5800 (70.3) | 2698 (72.3) | 3995 (79.1) | (74) | 5940 (71.2) |
| Type 2 diabetes mellitus | 3313 (40.1) | 1856 (49.8) | 2369 (46.9) | 1983 (41.8) | 2907 (34.9) |
| Chronic obstructive pulmonary disease | 1344 (16.3) | 443 (11.9) | 867 (17.2) | 585 (12.3) | 1080 (12.9) |
| HF history | |||||
| LVEF (%), mean (SD) | 26.6 (6.3) | 27.5 (6.1) | 28.9 (8.3) | 31.1 (6.8) | 29.5 (6.2) |
| Ischaemic HF aetiology, n (%) | 4458 (54.0) | 1929 (51.7) | 2674 (56.4) | 5036 (59.7) | |
| NYHA class, n (%) | |||||
| I | 0 | 0 | 2 (0.0) | 0 | 389 (4.7) |
| II | 4391 (53.2) | 2800 (75.1) | 2975 (59.0) | 3203 (67.5) | 5919 (70.9) |
| III | 3616 (43.8) | 910 (24.4) | 2003 (39.7) | 1498 (31.6) | 2018 (24.1) |
| IV | 248 (3.0) | 20 (0.5) | 66 (1.3) | 43 (0.9) | 60 (0.7) |
| KCCQ total symptom score, median (Q1–Q3) | 68.8 (49.0–87.5) | 77.1 (58.3–91.7) | 83.3 (67.7–95.8) | ||
| MAGGIC score, median (Q1– Q3) | 23 (19–28) | 23 (18–27) | 22 (18, 25) | 20 (16–24) | |
| Vitals and laboratory parameters | |||||
| Body mass index (kg/m2), mean (SD) | 28.5 (6.2) | 27.9 (5.4) | 27.8 (5.9) | 28.2 (6.0) | 28.2 (5.5) |
| SBP (mmHg), mean (SD) | 117 (15) | 122 (15) | 121 (16) | 122 (16) | 121 (15) |
| Heart rate (bpm), mean (SD) | 72.4 (12.1) | 71.3 (11.8) | 73.1 (13.0) | 71.5 (11.7) | 72 (12) |
| NT‐proBNP (pg/mL), median (Q1–Q3) | 1971 (962–4033) |
E: 1887 (1077–3429) P: 1926 (1153–3525) |
2816 (1556–5314) | 1437 (857–2649) | 1608 (886–3221) |
| eGFR (mL/min/1.73 m2), mean (SD) | 60.3 (21.8) | 62.0 (21.6) | 61.5 (27.2) | 65.8 (19.4) | 70 (20) |
| Stage ≤2: ≥60 | 3922 (47.5) | 1929 (51.7) | 2335 (47.1) | 2782 (58.6) | 5654 (67.0) |
| Stage ≥3: <60 | 4334 (52.5) | 1799 (48.3) | 2624 (52.0) | 1962 (40.2) | 2745 (33.0) |
| Medications and cardiac devices, n (%) | |||||
| ACEi, ARB, or ARNi | 7161 (86.7) | 3327 (89.2) | 3700 (73.4) | 4476 (94.4) | 8339 (100) |
| ARNi | 1594 (19.3) | 727 (19.5) | 731 (14.5) | 508 (10.7) | N/A |
| BB | 7763 (94.0) | 3533 (94.7) | 4691 (93.1) | 4558 (96.1) | 7811 (93.6) |
| MRA | 6358 (77.0) | 2661 (71.3) | 3545 (70.3) | 3370 (71.0) | 4671 (55.3) |
| (ACEi, ARB, or ARNi) + MRA + BB | 5367 (65.0) | 3009 (59.7) | 3097 (65.3) | (≤55.3) | |
| Digitalis glycosides | 1380 (16.7) | 594 (15.9) | 887 (18.7) | 2539 (30.2) | |
| ICD | 2614 (31.7) | 1171 (31.4) | 1399 (27.8) | 1242 (26.2) | 1243 (14.9) |
| CRT | 1156 (14.0) | 442 (11.8) | 739 (14.7) | 354 (7.5) | 574 (6.8) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; CRT, cardiac resynchronization therapy; E, empagliflozin group; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IV, intravenous; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; N/A, not available; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; P, placebo group; SBP, systolic blood pressure; SD, standard deviation.
VICTORIA also enrolled inpatients, although these data were not available at this time.
Table 4.
Baseline characteristics and treatment in stabilized inpatients in contemporary trials
| GALACTIC‐HF (‘inpatient’) (n = 2084) | EVEREST (n = 4133) | ASTRONAUT (n = 1615) | PIONEER‐HF (n = 881) | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years), mean (SD) or median (Q1–Q3) | 65.0 (11.3) | 65.8 (11.8) | 64.6 (12.2) | 62 (53–71) |
| Female sex, n (%) | 411 (19.7) | 25.6 | 368 (22.7) | 246 (27.9) |
| Race, n (%) | ||||
| White | 1706 (81.9) | 3533 (85.5) | 1140 (70.6) | 515 (58.5) |
| Asian | 184 (8.8) | N/A | 336 (20.8) | |
| Black or African American | 105 (5.0) | 310 (7.5) | 78 (4.8) | 316 (35.9) |
| Other b | 89 (4.3) | 290 (7.0) | 61 (3.8) | 50 (5.7) |
| Hispanic ethnicity, n (%) | 355 (17.0) | 201 (4.9) | 75 (8.5) | |
| Geographic region, n (%) | ||||
| Eastern Europe/Russia | 915 (43.9%) | 1619 (39.2) | 498 (30.4) | – |
| Western Europe/South Africa/Australasia | 486 (23.3%) | 564 (13.6) | 407 (24.8) | – |
| Latin and South America | 326 (15.6%) | 699 (16.9) | 165 (10.1) | – |
| US and Canada | 180 (8.6%) | 1251 (30.2) | 124 (7.6) | 881 (100) |
| Asia | 177 (8.5%) | – | 445 (27.2) | – |
| Clinical characteristics | ||||
| Medical conditions, n (%) | ||||
| Coronary artery disease | 1317 (63.2) | 2911 (70.4) | 881 (54.6) | 244 (27.7) |
| Myocardial infarction | 893 (42.9) | 2084 (50.4) | 689 (42.7) | 62 (7.0) |
| Percutaneous coronary intervention | 599 (28.7) | 738 (17.9) | 323 (20.0) | 8 (0.9) |
| Coronary artery bypass grafting | 320 (15.4) | 862 (20.9) | 279 (17.3) | 35 (4.0) |
| Peripheral artery disease | 215 (10.3) | 866 (21.0) | 95 (10.8) | |
| Stroke | 197 (9.5) | 471 (11.4) | 87 (9.9) | |
| Atrial fibrillation or flutter history | 995 (47.7) | 1790 (43.3) | 676 (41.9) | 407 (46.2) |
| Hypertension | 1495 (71.7) | 2932 (70.9) | 1225 (75.9) | 753 (85.6) |
| Type 2 diabetes mellitus | 870 (41.7) | 1598 (38.7) | 662 (41.0) | 168 (19.1) |
| Chronic kidney disease | 809 (38.8) | 1107 (26.8) | 332 (20.6) | 250 (29.1) |
| Chronic obstructive pulmonary disease | 354 (17.0) | 416 (10.1) | 322 (19.9) | |
| HF history | ||||
| LVEF (%), mean (SD) or median (Q1–Q3) | 26.5 (6.4) | 27.5 (8.1) | 27.9 (7.3) | 25 (20–30) |
| Ischaemic HF aetiology, n (%) | 1148 (55.1) | 2672 (64.6) | 1027 (63.6) | |
| NYHA class at randomization, n (%) | ||||
| II | 767 (36.8) | 513 (31.8) | 112 (19.4) | |
| III | 1190 (57.1) | 2404 (59.4) | 903 (55.9) | 297 (51.6) |
| IV | 126 (6.0) | 1622 (40.1) | 139 (8.6) | 54 (9.4) |
| Vitals and laboratory parameters | ||||
| Body mass index (kg/m2), mean (SD) or median (Q1, Q3) | 28.0 (6.1) | 27.2 (6.2) | 30 (26–37) | |
| SBP (mmHg), mean (SD) or median (Q1, Q3) | 114 (14) | 121 (19) | 123 (13) | 118 (110–132) |
| Heart rate (bpm), mean (SD) or median (Q1, Q3) | 73 (12) | 80 | 78 (16) | 80 (72–91) |
| NT‐proBNP (pg/mL), median (Q1–Q3) at randomization | 2457 (1185–5073) | 2718 (1531–5235) | 2701 (1490–5218) | |
| hsTnI (ng/mL), median (Q3) | 0.036 (0.066) | 0.035 (0.08) | 0.032 (0.050) | |
| eGFR (mL/min/1.73 m2), mean (SD) or median (Q1–Q3) | 54.4 (41.3–70.2) | 66.7 (19.9) | 59 (48–71) | |
| Medications and cardiac devices, n (%) | ||||
| ACEi, ARB, or ARNi | 1729 (83.0) | 3479 (84.2) | 1360 (84.2) | 422 (47.9) a |
| BB | 1931 (92.7) | 2903 (70.2) | 1333 (82.5) | 525 (59.6) |
| MRA | 1686 (80.9) | 2242 (54.2) | 921 (57.0) | 88 (10.0) |
| (ACEi, ARB, or ARNi) + MRA + BB | 1360 (65.3) | (≤54.2) | (≤57.0) | 502 (57.0) a |
| Digitalis glycosides | 356 (17.1) | 1815 (43.9) | 628 (38.9) | 76 (8.6) |
| CRT | 267 (12.8) | 109 (6.7) | 76 (8.6) | |
| ICD | 598 (28.7) | 600 (14.5) | 253 (15.7) | 250 (28.3) |
ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BB, beta‐blocker; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HF, heart failure; hsTnI, high‐sensitivity troponin I; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist; N/A, not available; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
Note that PIONEER‐HF enrolled de novo HF patients and patients on ARNi were excluded, so this value represents patients on ACEi or ARB at baseline.
Includes American Indian or Alaska native, native Hawaiian or other Pacific Islander, or multiple self‐identified races.
Discussion
The GALACTIC‐HF trial was designed to provide a comprehensive assessment of the effect of chronic therapy with the cardiac myosin activator omecamtiv mecarbil on cardiovascular mortality, heart failure hospitalizations and quality of life in people with HFrEF. Participants in GALACTIC‐HF were randomized from a diverse international population representing a wide spectrum of the HFrEF clinical course. Their clinical characteristics are similar to those of patients in contemporary registries (online supplementary Table S2 ), although GALACTIC‐HF patients tended to be more symptomatic and received more guideline‐recommended medical therapy.
GALACTIC‐HF randomized patients with many similarities to those in other chronic heart failure trials (Table 3 ), though there are some interesting differences, some of which were generated by differences in the eligibility criteria (online supplementary Table S3 ). The mean ages of patients in these contemporary HFrEF trials ranged from 64–67 years and 21–24% of the participants were females. Racial characteristics differed amongst the trials, with GALACTIC‐HF enrolling a high percentage of self‐identified Black participants (6.8%) compared to the other trials (4.8–6.9%), but a substantially lower proportion of Asian patients (8.6% vs. 17.9–23.5%). These proportions reflect the geographic distribution of recruitment, where GALACTIC‐HF enrolled more patients from the United States and Canada (16.8% vs. 7.1–14.3%), but fewer participants from Asia (8.1% vs. 13.2–23.4%). Given the size of GALACTIC‐HF, these proportions translated into substantially greater absolute numbers of patients (nearly twice as many as the comparators) in North America where recruitment has been historically challenging. Comorbidities were common and similar in all of the trials with a history of atrial fibrillation present in about 37–45%, type 2 diabetes mellitus in 35–50% and hypertension in 70–79% of subjects. The participants in GALACTIC‐HF had a slightly lower ejection fraction than the other trials (26.6% vs. 27.5–31.1%) and higher NT‐proBNP concentrations compared to PARADIGM‐HF, DAPA‐HF and EMPEROR‐Reduced, the latter being potentially related to enrolling inpatients. As opposed to the other trials which enrolled patients with predominantly mild symptoms (59–75% NYHA class II), patients with a broader range of symptoms are represented in GALACTIC‐HF from mild (NYHA class II, 53%) to moderate–severe (NYHA class III/IV, 47%) symptoms.
The GALACTIC‐HF cohort is a unique population, comprising patients enrolled from both the inpatient and outpatient settings. For comparison, the VICTORIA trial 21 specifically randomized patients (n = 3366; 66.7%) in the high‐risk period within 3 months of hospitalization for heart failure. GALACTIC‐HF also has a robust representation of these high‐risk patients (n = 4976; 60.3%). In the Phase 2 trial of omecamtiv mecarbil in patients with acute heart failure, ATOMIC‐AHF, 6 606 patients were randomized to a 48 h infusion of placebo or omecamtiv mecarbil in ascending dose cohorts. In these acutely ill inpatients, omecamtiv mecarbil had a side‐effect profile similar to placebo and improved dyspnoea in the high‐dose group. In the context of these safety data and with the recognition that early initiation of disease‐modifying therapies would optimize medication adherence and its potential benefit, GALACTIC‐HF randomized 2084 patients (25.2%) in the inpatient setting. A greater proportion of subjects enrolled as inpatients had a history of atrial fibrillation/flutter and chronic renal disease compared to the outpatient cohort. While this inpatient group had the same left ventricular ejection fraction as those randomized as outpatients, the inpatients were more symptomatic as represented by worse NYHA functional class and KCCQ total symptom scores and also had lower blood pressure, worse renal function, and higher NT‐proBNP and troponin I concentrations. Three other large multicentre trials have enrolled stabilized inpatients with HFrEF who were hospitalized for acute heart failure (Table 4 ; online supplementary Table S3 ). The international trials EVEREST 23 , 24 and ASTRONAUT 25 enrolled patients with a history of HFrEF who had baseline characteristics similar to the inpatient group from GALACTIC‐HF, except for the worse renal function and lower SBP in GALACTIC‐HF. The lower heart rate in GALACTIC‐HF compared to these other trials may be reflective of the higher use of beta‐blockers at baseline. PIONEER‐HF 26 also enrolled patients who were haemodynamically stable while hospitalized for acute decompensated heart failure but only in the United States. PIONEER‐HF had a higher representation of women and Blacks compared to the other three trials, as well as higher prevalence of obesity and hypertension. Patients in PIONEER‐HF had a much lower use of renin–angiotensin system inhibitors (48% ACEi/ARB vs. 83% ACEi/ARB/ARNi), beta‐blockers (60% vs. 94%) and MRA (10% vs. 81%) compared to the inpatient group from GALACTIC‐HF, although PIONEER‐HF randomized patients with de novo heart failure such that only 65.4% of patients had a prior history of heart failure.
Adverse effects on renal function are a major impediment to the initiation, up‐titration and maintenance of many current heart failure therapies. Omecamtiv mecarbil has demonstrated no significant difference from placebo with respect to renal function or related adverse events in Phase I or II clinical studies. Consequently, the entry criterion for renal function in GALACTIC‐HF was among the lowest of any contemporary clinical trial, enrolling patients with an eGFR ≥20 mL/min/1.73 m2 who were not on dialysis. GALACTIC‐HF enrolled 528 patients with eGFR <30 mL/min/1.73 m2, representing 6.4% of total enrolment. These patients are a distinct group with a greater proportion being women and older age, enrolled as inpatients with lower SBP, more comorbidities and ischaemic aetiology of heart failure, more symptomatic heart failure (worse NYHA class, MAGGIC and KCCQ total symptom scores) and markedly higher NT‐proBNP, increased troponin, on less guideline‐recommended medical therapy, yet a greater proportion of device therapy, compared to those with better renal function. Patients with an eGFR ≥20 mL/min/1.73 m2 were also enrolled in EMPEROR‐Reduced. These patients are typically a poorly studied group in HFrEF therapeutic trials and were excluded from ASTRONAUT, 25 PARADIGM‐HF, 19 PIONEER‐HF 26 and DAPA‐HF. 27 In the VICTORIA trial 21 of the soluble guanylate cyclase activator vericiguat, patients with an eGFR ≥15 mL/min/1.73 m2 were enrolled and there were 506 patients enrolled with an eGFR ≤30 mL/min/1.73 m2 (10.2% of total enrolment).
Many patients with HFrEF have lower blood pressure, especially those on maximal neurohormonal antagonist therapy, yet due to the hypotensive effects of many investigational therapies, these patients have been excluded from contemporary clinical trials. Trials with aliskiren (ASTRONAUT, 23 , 24 SBP <110 mmHg), sacubitril/valsartan (PARADIGM‐HF, 19 PIONEER‐HF 26 ), vericiguat (VICTORIA 21 ) and empagliflozin (EMPEROR‐Reduced 22 ) excluded patients with SBP <100 mmHg, while DAPA‐HF 27 (dapagliflozin) excluded those with SBP <95 mmHg. Omecamtiv mecarbil has no vasodilating properties and does not adversely affect blood pressure, so the GALACTIC‐HF trial was able to study patients with SBP ≥85 mmHg, randomizing 1127 patients (13.7%) with a baseline SBP <100 mmHg. These patients were slightly younger and more frequently enrolled as inpatients, with lower left ventricular ejection fraction, mildly increased NT‐proBNP and troponin, decreased eGFR, fewer with ischaemic aetiology of heart failure, and more symptomatic heart failure (worse NYHA class, MAGGIC and KCCQ total symptom scores), compared to participants with higher SBP. GALACTIC‐HF provides a unique opportunity to prospectively evaluate a therapy in this large, but underserved group of patients with HFrEF.
Comprehensive background heart failure therapy is not only important for the appropriate care of the participants but is also essential to the evaluation of the additional therapeutic benefit of a study drug. Patients in GALACTIC‐HF received amongst the most comprehensive baseline heart failure therapy in contemporary clinical trials. Implantable cardioverter defibrillators were in 32% (15–31% in PARADIGM‐HF, DAPA‐HF, VICTORIA, and EMPEROR‐Reduced) and cardiac resynchronization therapy was present in 14% of patients in GALACTIC‐HF at baseline (7–15% in the other four trials). In terms of baseline medical therapies employed, 87% of participants in GALACTIC‐HF received an ACEi, ARB or ARNi, compared to 73–94% in PARADIGM‐HF, DAPA‐HF, VICTORIA, and EMPEROR‐Reduced. Beta‐blockers were administered to 94% of patients in GALACTIC‐HF, similar to the 93–96% of patients in the other trials. MRA administration was more common in GALACTIC‐HF (77% compared to 55–71% in the other trials). The absence of adverse effects of omecamtiv mecarbil on potassium homeostasis may have accounted for the investigators' comfort in enrolling patients with the highest percentage of MRA use in any contemporary clinical trial. Unlike the PARADIGM‐HF, PIONEER‐HF, ASTRONAUT, and EVEREST trials, GALACTIC‐HF had no exclusion criterion for potassium plasma concentrations. Triple therapy (ACEi/ARB/ARNi + beta‐blocker + MRA) was present in 65% of the GALACTIC‐HF patients compared to less than 55% to 65% in the other three contemporary trials. Relative to DAPA‐HF (n = 508, 11%), VICTORIA (n = 731, 14%) and EMPEROR‐Reduced (n = 727; 19.5%), almost 1600 patients (over 19%) in GALACTIC‐HF were treated with sacubitril/valsartan at baseline. As might be expected given the different timing of regulatory approvals and accessibility of sacubitril/valsartan worldwide, there was considerable regional variation in baseline ARNi use, ranging from approximately 5% of patients in Eastern Europe to almost one‐third of patients in North America and Western Europe.
A recent study demonstrated that failure to initiate or up‐titrate guideline‐recommended medical therapies to target doses is most frequently due to physiologic factors, such as blood pressure, heart rate, renal function, or potassium concentrations rather than physician inertia. 28 In Phase I and II clinical studies of omecamtiv mecarbil, there was no adverse effect on blood pressure, heart rate, renal function, or potassium homeostasis. These findings suggest that omecamtiv mecarbil should not interfere with baseline guideline‐recommended medical therapy and has the potential to facilitate initiation or up‐titration of these therapies through improved cardiac function.
Limitations
GALACTIC‐HF is one of the most inclusive contemporary clinical trials, but there are some important limitations. Underrepresentation of racial groups and women in clinical trials is a continuing concern. 29 Only 7% of the GALACTIC‐HF participants were Black; however, a total of 562 Black participants were enrolled, representing over 100 more Black patients than were randomized in PARADIGM‐HF and over twice that enrolled in VICTORIA (n = 249), DAPA‐HF (n = 226) or EMPEROR‐Reduced (n = 257). Moreover, in the United States, over 29% of the GALACTIC‐HF participants self‐reported race as Black, more than twice the corresponding proportion of U.S. population (13.4%). Asian, Pacific Islander and other non‐white racial groups have limited representation in this trial. In addition, women continue to constitute a minority of patients with HFrEF in both registries and clinical trials. 30 Approximately 21% of the participants in GALACTIC‐HF were female, constituting a database of over 1700 women with HFrEF in whom the effects of omecamtiv mecarbil can be evaluated. The recent compelling trial results from DAPA‐HF 27 and EMPEROR‐Reduced 22 have established a role for the sodium–glucose co‐transporter 2 (SGLT2) inhibitors in the treatment of patients with HFrEF, but these data did not enter clinical practice or guidelines until after GALACTIC‐HF had completed enrolment. Only 219 (2.7%) patients in GALACTIC‐HF were on SGLT2 inhibitors at baseline, but given that the mechanism of action of the cardiac myosin activator omecamtiv mecarbil has no apparent significant overlap with that of the SGLT2 inhibitors, there is minimal biological plausibility that they would interfere with the other's effects on clinical outcomes, and there is the suggestion that they could be complementary or even synergistic.
Conclusion
GALACTIC‐HF is the first trial to test the hypothesis that directly improving cardiac function with the novel, selective cardiac myosin activator omecamtiv mecarbil can safely improve symptoms, prevent clinical heart failure events, and reduce the risk of cardiovascular death in patients with HFrEF. To this end, the trial enrolled patients with a wide range of symptomatic HFrEF receiving excellent medical and device heart failure therapy who were similar to those enrolled in other contemporary heart failure trials and registries. GALACTIC‐HF also randomized patients typically excluded from chronic heart failure trials including inpatients and those with severely reduced renal function or low blood pressure. GALACTIC‐HF will provide a definitive evaluation of the efficacy and safety of this novel therapy, and, if effective, inform its future implementation.
Supporting information
Appendix S1. Supporting Information
Acknowledgements
The authors thank Maya Shehayeb (Amgen, Inc.) for the provision of editorial support in collaboration with the authors.
Funding
The GALACTIC‐HF trial is funded by Amgen, Inc. and conducted in collaboration with Cytokinetics with the financial and strategic support of Servier.
Conflict of interest: Relationships with Industry and Other Entities: J.R.T. reports grants, personal fees and non‐financial support from Amgen; personal fees and non‐financial support from Cytokinetics and Servier, during the conduct of the study; grants and non‐financial support from Abbott, Bayer, Boerhinger Ingelheim, Bristol‐Myers Squibb, EBR Systems, St. Jude; personal fees and non‐financial support from AstraZeneca, Windtree Therapeutics; grants from Medtronic, personal fees from Merck; grants, personal fees and non‐financial support from Novartis, outside the submitted work. R.D. reports grants from Amgen, during the conduct of the study; grants from Dalcor, Amarin, outside the submitted work. G.M.F. reports grants and personal fees from Amgen, personal fees from Cytokinetics, during the conduct of the study; personal fees from Novartis, Medtronic, Cardionomic, BMS, Innolife, Novartis, V Wave, EBR Systems, Arena, Abbott, LivaNova, Eidos, Rocket, Reprieve, and grants from Merck, Myokardia, Bayer, outside the submitted work. J.J.V.M. reports other from Amgen, Cytokinetics, Servier, during the conduct of the study; other from Alnylam, Amgen, AstraZeneca, Bayer, BMS, Cardurion, Cytokinetics, Dal‐Cor, GSK, Novartis, Pfizer, Theracos, personal fees from Abbott, Hickma, Sun Pharmaceuticals, outside the submitted work. M.M. reports grants and other from Amgen, personal fees from Amgen, during the conduct of the study; personal fees from Consulting honoraria, outside the submitted work. S.D.S. reports grants from Amgen, during the conduct of the study; grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, Neurotronik, NIH/NHLBI, Novartis, Respicardia, Sanofi Pasteur, Theracos, personal fees from Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Sanofi‐Pasteur, Tenaya, Dinaqor, Tremeau, CellProThera, Moderna, outside the submitted work. K.F.A. reports grants and personal fees from Amgen, during the conduct of the study; grants and personal fees from Novartis, Roche Diagnostics, grants from Boehringer Ingelheim Pharmaceuticals Inc, Merck & Co, Inc, Otsuka, BMS, LivaNova, personal fees from Relypsa, Cytokinetics, Windtree Therapeutics, Inc; outside the submitted work. I.A. reports personal fees from Amgen, during the conduct of the study; personal fees from AstraZeneca, ARCA Biopharma, Boehringer Ingelheim, Boston Scientific Corporation, LivaNova, Novartis, Zensun, outside the submitted work. A.A.M. has nothing to disclose. T.B.S. reports personal fees from Amgen, during the conduct of the study; personal fees from Amgen, Sanofi Pasteur, Novartis, grants from Sanofi Pasteur, other from GE Health Care, outside the submitted work. M.B. is supported by the Deutsche Forschungsgemeinschaft (DFG, TTR 219, S‐01) and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Servier, Medtronic, Vifor, Novartis, outside the submitted work. D.B. has nothing to disclose. J.G.F.C. reports grants and personal fees from Amgen, during the conduct of the study; personal fees from Abbott, grants and personal fees from Bayer, Novartis, Pharmacosmos, Vifor, BMS, Servier, personal fees and non‐financial support from Medtronic, outside the submitted work. R.C. reports personal fess from Amgen. M.G.C.L. reports personal fees from Amgen, Vifor, personal fees and non‐financial support from Novartis, MSD, AstraZeneca, grants from CIBERCV, outside the submitted work. L.E.E. has nothing to disclose. J.C.F. reports personal fees from Amgen, during the conduct of the study; personal fees from AstraZeneca, Novartis, Johnson & Johnson, Boehringer Ingelheim/Lilly, Abbott, grants from NIH, grants from AHA, outside the submitted work. G.F. reports Trial Committee Member from Servier and from Amgen, during the conduct of the study; Committee Member in trials sponsored by Medtronic, Vifor, Novartis and Boehringer Ingelheim, outside the submitted work. C.F. reports personal fees from Servier, grants and personal fees from Vifor Pharma, other from Bayer, personal fees from AstraZeneca, Boehringer Ingelheim, Novartis, outside the submitted work. E.G. reports personal fees from Amgen, during the conduct of the study; personal fees from Boehringer Ingelheim, Merck, personal fees and non‐financial support from Servier, grants and personal fees from Novartis, outside the submitted work. J.G.H. reports grants and personal fees from AstraZeneca, Amgen, Novartis, Servier, Boehringer Ingelheim, Janssen, grants from Medtronic, Pfizer, outside the submitted work. D.E.L. reports grants and personal fees from Amgen, during the conduct of the study; grants and personal fees from Janssen, personal fees from Ortho Diagnostics, DCRI (Novartis), grants from Bayer, AstraZeneca, Critical Diagnostics, non‐financial support from Somalogic, outside the submitted work. In addition, D.E.L. has a patent Genomic predictors of BB response pending. P.M. reports grants and personal fees from Novartis, Servier, personal fees from AstraZeneca, Actelion, outside the submitted work. S.M. has nothing to disclose. E.O'M. reports other from Amgen, Cytokinetics, during the conduct of the study; other from Novartis, AstraZeneca, Merck, Bayer, Pfizer, outside the submitted work. A.P. reports grants from Vifor, Amgen, grants and personal fees from AstraZeneca, personal fees from Servier, Bayer, outside the submitted work. P.P. reports personal fees and other from Amgen, personal fees from Servier, during the conduct of the study; personal fees and other from Boehringer Ingelheim, grants, Vifor Pharma, Novartis, Bayer, Cibiem, AstraZeneca, BMS, Renal Guard Solutions, Impulse Dynamics, personal fees from Berlin Chemie, other from Abbott Vascular, outside the submitted work. F.J.A.R. reports personal fees from Novartis, Pfizer, AstraZeneca, Amgen, outside the submitted work. P.S. has nothing to disclose. K.S. has nothing to disclose. J.S. reports personal fees from Amgen during the conduct of the study; personal fees from AstraZeneca, Bayer, Boehringer Ingelheim outside the submitted work. T.M.S. has nothing to disclose. D.V. reports grants from Amgen, during the conduct of the study; grants and personal fees from Novartis, Boehringer Ingelheim, Bayer, outside the submitted work. M.B.Y. reports grants from Amgen, during the conduct of the study; grants from Bayer, Novartis, Dalcor Pharmaceuticals, outside the submitted work. F.Z. reports personal fees from Amgen, during the conduct of the study; personal fees from Janssen, Novartis, Boston Scientific, CVRx, Boehringer Ingelheim, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck, Bayer, Cellprothera, other from Cardiorenal, CVCT, outside the submitted work. L.S., J.C.L. and C.E.K. report being employees and stockholders of Amgen, Inc. C.V. is an employee of Servier. F.I.M. reports being an employee and stockholder of Cytokinetics, Inc.
References
- 1. Psotka MA, Teerlink JR. Direct myosin activation by omecamtiv mecarbil for heart failure with reduced ejection fraction. Handb Exp Pharmacol 2017;243:465–490. [DOI] [PubMed] [Google Scholar]
- 2. Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 2011;331:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Psotka MA, Gottlieb SS, Francis GS, Allen LA, Teerlink JR, Adams KF Jr, Rosano GM, Lancellotti P. Cardiac calcitropes, myotropes, and mitotropes: JACC review topic of the week. J Am Coll Cardiol 2019;73:2345–2353. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink JR, Clarke CP, Saikali KG, Lee JH, Chen MM, Escandon RD, Elliott L, Bee R, Habibzadeh MR, Goldman JH, Schiller NB, Malik FI, Wolff AA. Dose‐dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first‐in‐man study. Lancet 2011;378:667–675. [DOI] [PubMed] [Google Scholar]
- 5. Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double‐blind, placebo‐controlled, crossover, dose‐ranging phase 2 trial. Lancet 2011;378:676–683. [DOI] [PubMed] [Google Scholar]
- 6. Teerlink JR, Felker GM, McMurray JJ, Ponikowski P, Metra M, Filippatos GS, Ezekowitz JA, Dickstein K, Cleland JG, Kim JB, Lei L, Knusel B, Wolff AA, Malik FI, Wasserman SM; ATOMIC‐AHF Investigators . Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: the ATOMIC‐AHF study. J Am Coll Cardiol 2016;67:1444–1455. [DOI] [PubMed] [Google Scholar]
- 7. Teerlink JR, Felker GM, McMurray JJ, Solomon SD, Adams KF, Jr , Cleland JG, Ezekowitz JA, Goudev A, Macdonald P, Metra M, Mitrovic V, Ponikowski P, Serpytis P, Spinar J, Tomcsanyi J, Vandekerckhove HJ, Voors AA, Monsalvo ML, Johnston J, Malik FI, Honarpour N; COSMIC‐HF Investigators . Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC‐HF): a phase 2, pharmacokinetic, randomised, placebo‐controlled trial. Lancet 2016;388:2895–2903. [DOI] [PubMed] [Google Scholar]
- 8. Biering‐Sorensen T, Querejeta Roca G, Hegde SM, Shah AM, Claggett B, Mosley TH Jr, Butler KR Jr, Solomon SD. Left ventricular ejection time is an independent predictor of incident heart failure in a community‐based cohort. Eur J Heart Fail 2018;20:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Njoroge JN, Teerlink JR. Systolic time intervals in patients with heart failure: time to teach new dogs old tricks. Eur J Heart Fail 2020;22:1183–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel PA, Ambrosy AP, Phelan M, Alenezi F, Chiswell K, Van Dyke MK, Tomfohr J, Honarpour N, Velazquez EJ. Association between systolic ejection time and outcomes in heart failure by ejection fraction. Eur J Heart Fail 2020;22:1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vaduganathan M, Claggett B, Packer M, McMurray JJV, Rouleau JL, Zile MR, Swedberg K, Solomon SD. Natriuretic peptides as biomarkers of treatment response in clinical trials of heart failure. JACC Heart Fail 2018;6:564–569. [DOI] [PubMed] [Google Scholar]
- 12. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta‐analytic approach. J Am Coll Cardiol 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Teerlink JR, Diaz R, Felker GM, McMurray JJ, Metra M, Solomon SD, Legg JC, Buchele G, Varin C, Kurtz CE, Malik FI, Honarpour N. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC‐HF. JACC Heart Fail 2020;8:329–340. [DOI] [PubMed] [Google Scholar]
- 14. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 15. Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, Narasimhan C, Park SW, Yu CM, Ngarmukos T, Omar R, Reyes EB, Siswanto BB, Hung CL, Ling LH, Yap J, MacDonald M, Richards AM. Regional and ethnic differences among patients with heart failure in Asia: the Asian Sudden Cardiac Death in Heart Failure registry. Eur Heart J 2016;37:3141–3153. [DOI] [PubMed] [Google Scholar]
- 16. Teng TK, Tromp J, Tay WT, Anand I, Ouwerkerk W, Chopra V, Wander GS, Yap JJ, MacDonald MR, Xu CF, Chia YM, Shimizu W, investigators A‐H, Richards AM, Voors A, Lam CS. Prescribing patterns of evidence‐based heart failure pharmacotherapy and outcomes in the ASIAN‐HF registry: a cohort study. Lancet Glob Health 2018;6:e1008–e1018. [DOI] [PubMed] [Google Scholar]
- 17. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, Hill CL, McCague K, Mi X, Patterson JH, Spertus JA, Thomas L, Williams FB, Hernandez AF, Fonarow GC. Medical therapy for heart failure with reduced ejection fraction: the CHAMP‐HF registry. J Am Coll Cardiol 2018;72:351–366. [DOI] [PubMed] [Google Scholar]
- 18. Khariton Y, Hernandez AF, Fonarow GC, Sharma PP, Duffy CI, Thomas L, Mi X, Albert NM, Butler J, McCague K, Nassif ME, Williams FB, DeVore A, Patterson JH, Spertus JA. Health status variation across practices in outpatients with heart failure: insights from the CHAMP‐HF (Change the Management of Patients With Heart Failure) Registry. Circ Cardiovasc Qual Outcomes 2018;11:e004668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 20. McMurray JJV, DeMets DL, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD; DAPA‐HF Committees and Investigators . The Dapagliflozin And Prevention of Adverse‐outcomes in Heart Failure (DAPA‐HF) trial: baseline characteristics. Eur J Heart Fail 2019;21:1402–1411. [DOI] [PubMed] [Google Scholar]
- 21. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 22. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413‐1424. [DOI] [PubMed] [Google Scholar]
- 23. Gheorghiade M, Konstam MA, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Short‐term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status trials. JAMA 2007;297:1332–1343. [DOI] [PubMed] [Google Scholar]
- 24. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators . Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome trial. JAMA 2007;297:1319–1331. [DOI] [PubMed] [Google Scholar]
- 25. Gheorghiade M, Bohm M, Greene SJ, Fonarow GC, Lewis EF, Zannad F, Solomon SD, Baschiera F, Botha J, Hua TA, Gimpelewicz CR, Jaumont X, Lesogor A, Maggioni AP; ASTRONAUT Investigators and Coordinators . Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013;309:1125–1135. [DOI] [PubMed] [Google Scholar]
- 26. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E; PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 27. McMurray JJ, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 28. Jarjour M, Henri C, de Denus S, Fortier A, Bouabdallaoui N, Nigam A, O'Meara E, Ahnadi C, White M, Garceau P, Racine N, Parent MC, Liszkowski M, Giraldeau G, Rouleau JL, Ducharme A. Care gaps in adherence to heart failure guidelines: clinical inertia or physiological limitations? JACC Heart Fail 2020;8:725–738. [DOI] [PubMed] [Google Scholar]
- 29. Tahhan AS, Vaduganathan M, Greene SJ, Fonarow GC, Fiuzat M, Jessup M, Lindenfeld J, O'Connor CM, Butler J. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: a systematic review. JAMA Cardiol 2018;3:1011–1019. [DOI] [PubMed] [Google Scholar]
- 30. Mentzer G, Hsich EM. Heart failure with reduced ejection fraction in women: epidemiology, outcomes, and treatment. Heart Fail Clin 2019;15:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


