Summary
This protocol is designed to prepare adult axenic Drosophila before monitoring their behavior in a two-choice feeding assay, where flies are confronted with an axenic versus a dead or alive bacteria-contaminated feeding solution. Several aspects of the procedure, including raising and aging flies in axenic conditions, starving adult flies, and composing feeding solutions, are detailed. The bacterium used in this protocol, Erwiniacarotovora carotovora-152141 (Ecc-152141), is commonly used to decipher the mechanisms controlling host-pathogen interactions in the Drosophila model.
For complete details on the use and execution of this protocol, please refer to Charroux et al. (2020).
Graphical Abstract
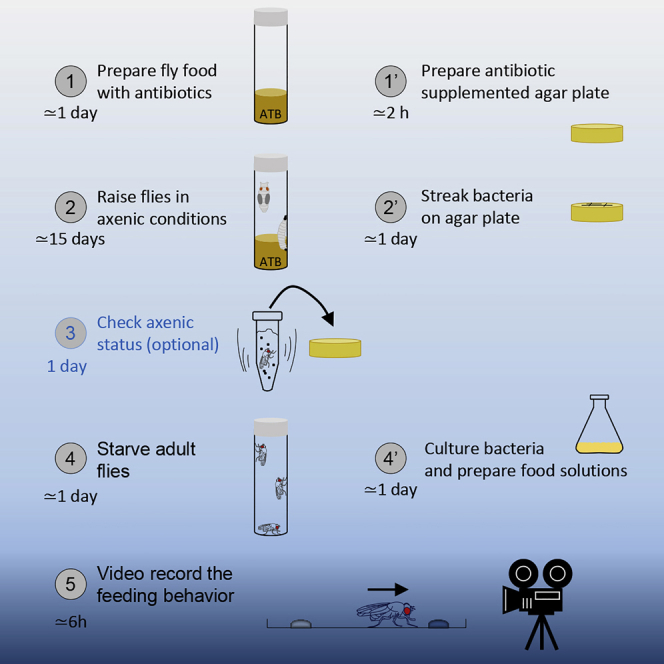
Highlights
-
•
Antibiotics are used to raise flies in axenic conditions
-
•
Flies are starved with a straightforward procedure
-
•
Easy preparation of bacteria-contaminated feeding solutions
-
•
The procedure is suitable for any behavioral assay using axenic flies
This protocol is designed to prepare adult axenic Drosophila before monitoring their behavior in a two-choice feeding assay, where flies are confronted with an axenic versus a dead or alive bacteria-contaminated feeding solution. Several aspects of the procedure, including raising and aging flies in axenic conditions, starving adult flies, and composing feeding solutions, are detailed. The bacterium used in this protocol, Erwiniacarotovora carotovora-152141 (Ecc-152141), is commonly used to decipher the mechanisms controlling host-pathogen interactions in the Drosophila model.
Before You Begin
Prepare Antibiotic Mixture
Timing: ∼1 h
-
1.
Dilute each antibiotic in a 200 mL volume of the indicated solvent. Ampicillin 50 mg/mL (in ultrapure water), Kanamycin 50 mg/mL (in ultrapure water), Tetracyclin 10 mg/mL (in 96% ethanol) and Erythromycin 5 mg/mL (in 96% ethanol) (Bosco-Drayon et al., 2012).
-
2.
Mix well with the use of a vortex and combine 5 mL of each antibiotic solution into a 50 mL plastic vial (Final volume = 20 mL). Store aliquots at −20°C. Each 20 mL aliquot is suitable for 5 L of fly food media.
Prepare Yeast/Cornmeal Fly Food Media
Timing: ∼3 h
-
3.
For 1 L of food, add 8.2 g of agar, 80 g of cornmeal flour and 80 g of yeast extract to 1 L of water at room temperature (19°C–25°C). Stir and heat until water is boiling, then cook for 10 more minutes. Let the solution cool down to ∼50°C then add 5.2 g of methylparaben sodium salt and 4 mL of 99% propionic acid.
-
4.
If required, add 4 mL of antibiotic mixture for 1 L of fly food media.
-
5.
Pour ∼12 mL of fly food to each plastic vial (25 mm width × 90 mm height).
-
6.
Cover the vials with a gauze and let the food dry overnight (∼16–18 h) at room temperature (19°C–25°C).
-
7.
Close the tubes with ultra-dense plugs (25 mm width × 28 mm height).
Note: In case of urgent use, prepare the tubes 24 h before. This ensures proper drying of the fly media.
Note: Fly food vials can be stored at 4°C up to 1 month.
Prepare Mature Adult Females
Timing: ∼1 week
-
8.
Collect newly emerging adult Drosophila males and females (F0) of your chosen genotype during a 24 h window.
-
9.
In a new vial containing freshly poured fly food, add a few beads of deactivated baking yeast on top of the media. This helps to boost female fecundity.
-
10.
Transfer 20–30 females F0 with 5 males F0 into this vial.
-
11.
Incubate F0 flies at 25°C in a 12 h/12 h light/dark cycle controlled incubator for 5–7 days.
-
12.
Flip the F0 adults in a new vial supplemented with few beads of deactivated baking yeast every 2 days.
CRITICAL: Flipping avoids losing adults that may stick (and die) to the larvae-softened fly food.
Prepare LB Agar Plates ± Rifampicin
Timing: ∼2 h
-
13.
Melt autoclaved LB agar into a microwave oven.
-
14.
Let the melted LB agar cool down on the bench. Move to next step when the temperature is between 50°C and 60°C.
-
15.
Light a Bunsen burner on your bench and wait 1 min before starting manipulating. This will allow the updraft from the heat generated by the Bunsen burner to create a sterile field approximatively 30 cm diameter around the burner. Work in this sterile area.
-
16.
If necessary, add Rifampicin at 100 μg/mL final concentration. Mix well.
-
17.
Pour ∼30 mL of LB agar ± Rifampicin into 90 mm Petri dishes.
-
18.
Cover the plates immediately and let the agar solidify at room temperature (19°C–25°C).
Streak Ecc-15 Bacteria
Timing: ∼10 min
-
19.
Use a Bunsen burner to create a sterile environment as in step 15.
-
20.
Flame sterilize a Pasteur pipette with a narrow tip.
-
21.
Let the pipette cool down, then dip the pipette tip into a thawed glycerol stock of Ecc-152141.
-
22.
Use the Ecc-152141 contaminated tip to streak the LB agar + Rifampicin plate.
-
23.
Incubate the plate for 48 h at 30°C.
-
24.
Seal the plate with Parafilm M and store it at 4°C.
-
25.
Repeat the procedure every 2 weeks.
CRITICAL: we noticed that Ecc-152141 bacterium is sensitive to low temperature (4°C). Storing plates more than 2 weeks at 4°C drastically diminishes Ecc-152141 survival.
Note: Flame sterilization of the Pasteur pipette also seals the pipette tip.
Note:Ecc-152141 is naturally resistant to Rifampicin (Basset et al., 2000).
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Ecc-152141, Erwinia carotovora carotovora-15 (2141) | Basset et al., 2000 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Agar | VWR | Cat # 20768.361 |
| Yeast extract | VWR | Cat # 24979.413 |
| Cornmeal flour Whesthove Maize H1 | Limagrain Ingredients | Cat # WESA16DS |
| Instant yeast deactivated | Confettiperfetti | N/A |
| Methylparaben sodium salt | MERCK | Cat # 106756 |
| Propionic acid | CARLOERBA | Cat # 409553 |
| Ampicillin | Euromedex | Cat # EU0400-B |
| Kanamycin | Fisher | Cat # BP906-5 |
| Tetracyclin | Sigma-Aldrich | Cat # 87128 |
| Erythromycin | Sigma-Aldrich | Cat # E5389 |
| Spectinomycin | Sigma-Aldrich | Cat # S4014 |
| Rifampicin | Sigma-Aldrich | Cat # R3501 |
| Sucrose | Sigma-Aldrich | Cat # S1888 |
| Eriauglaucine blue | Sigma-Aldrich | Cat # 861146 |
| PBS 1× | Eurobio Scientific | Cat # CS0PBS01-08 |
| Luria-Bertani Broth | Sigma-Aldrich | Cat # L3022 |
| Luria-Bertani Broth with agar | Sigma-Aldrich | Cat # L2897 |
| Experimental Models: Organisms/Strains | ||
| D.melanogaster. Canton-S (for instance) | Bloomington Drosophila Stock center | BDSC:64349 |
| Software and Algorithms | ||
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| Yawcam | Magnus Lundvall | https://www.yawcam.com |
| Flybox | Charroux et al., 2020 | N/A |
| Other | ||
| Precellys 24 tissue homogenizer | Bertin Technologies, France | Cat # P000669-PR240-A |
| Colorless transparent Poly (methyl methacrylate) (PMMA) plaques of 4 mm | Vink France | Cat # 101800 |
Materials and Equipment
Equipment
We designed and fabricated in-house the apparatus used for the behavioral assay.
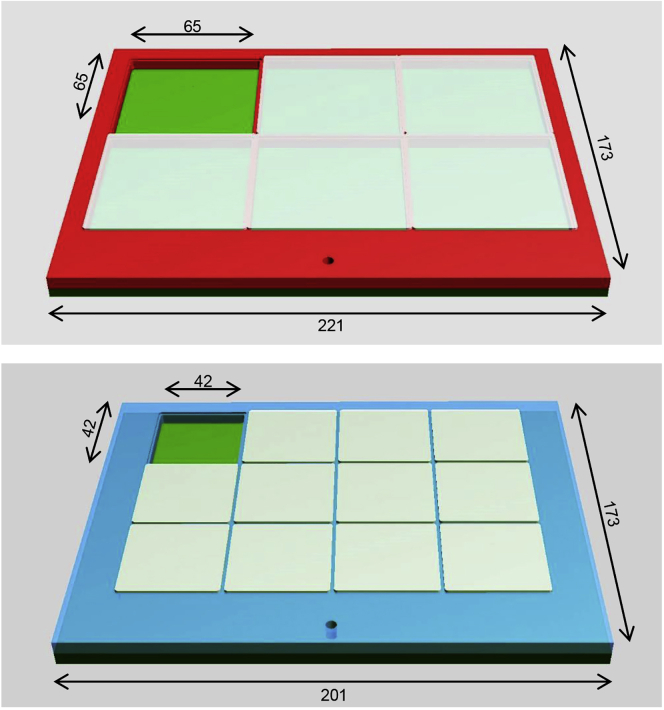
For this, PMMA plaques were sculpted by subtractive machining using carbide milling cutter. Plaques were sealed with chloroform and pressure contact (Figure 1).
Figure 1.
Dimensions of the Apparatus Used for Behavioral Assays
Cartoon of the 6 arenas (top) and the 12 arenas (bottom) apparatus, with the following dimensions (in millimeters): 221 × 173 or 201 × 173 for the main frames of the 6 or 12 arenas apparatus respectively, and 65 × 65 or 42 × 42 for the 6 or 12 arenas, respectively. Each apparatus is composed of three distinct plastic parts, the bottom part (dark green) which is a flat plain slab on top of which is glued the plastic grid containing 6 (red on the top cartoon) or 12 (blue on the bottom cartoon) squared holes, and the 6 (or 12) removable plastic caps (light green) used to cover the arenas. The small hole shown in each cartoons is used to screw the plastic arm (not shown here) design to maintain the camera on top of the apparatus.
Step-By-Step Method Details
Raising Flies in Axenic Condition
Timing: ∼15 days
This procedure aims to grow flies devoid of gut microbiota.
-
1.
Transfer 20 mature adult females F0 (5–7 days old) into a new vial containing fly food supplemented with antibiotics.
-
2.
Let the females lay eggs for approximatively 4 h at 25°C, then remove the flies from the vial. This relatively short time window avoids too many eggs being deposited. We obtain approximatively 100–150 eggs/tube using this procedure.
CRITICAL: It is important to avoid overcrowded larval development, which could otherwise negatively impact larval growth and, as a consequence adult’s fitness.
-
3.
Incubate the vial for ∼10 days in a 25°C incubator.
-
4.
Collect F1 flies during 24 h after the first adult’s emergence from pupal case.
-
5.
Pool and transfer F1 flies into a new vial containing food supplemented with antibiotics.
-
6.
Incubate flies for ∼5–7 days at 25°C in a 12 h/12 h light/dark cycle controlled incubator.
-
7.
Every 2 days, flip the F1 adults in a new vial supplemented with antibiotics and with few beads of deactivated baking yeast.
CRITICAL: Flipping avoids losing adults that may stick (and die) to the larvae-softened fly food.
Check Axenic Status (Optional)
Timing: ∼1 day
This procedure intends to verify that F1 flies are devoid of gut microbiota. This is optional since the antibiotic treatment used in the previous section is very efficient in eliminating extracellular bacteria.
-
8.
In a sterile environment, prepare the homogenizer 1.5 mL microtubes by adding an equivalent volume of 100 μL of 0.75/1 mm glass beads and 800 μL of sterile Luria-Bertani culture medium.
-
9.
Surface-sterilize F1 adult flies by sinking them in 70% EtOH for 20 s.
-
10.
Rinse flies with sterile water.
-
11.
Transfer single individuals to a homogenizer tube.
-
12.
Homogenize the adults using the following cycles on the Precellys tissue homogenizer: 2 cycles of 4,000 rpm for 25 s with a 20 s pause between each cycle.
-
13.
Plate 100 μL of each lysate onto LB agar plates.
-
14.
Incubate the plates at 30°C for 48 h.
-
15.
As a positive control, we suggest to plate lysate obtained from conventionally reared flies (see below).
Note: We recommend to test adults individually for presence of microbiota. This allows more accurate assessment of the efficacy of the antibiotic treatment.
Note: Most Drosophila gut bacteria will grow on LB agar during the incubation time.
Raising Flies in Normal Condition
Timing: ∼15 days
Repeat steps 1–7 from section “Raising flies in axenic conditions” using vials containing fly food media without antibiotics.
Note: This procedure explains how to grow control flies (conventionally reared)
Starving Adult Flies
Timing: ∼1 day
This procedure plans to starve adult flies before using them for the behavioral assay. Be aware that starvation dramatically changes feeding behavior making bitter substance more acceptable, for instance. We thus recommend to systematically use non-starved flies as a reference.
-
16.
Transfer 10 F1 axenic females (5–7 days old) in an empty fly culture vial.
-
17.
Close the vial with an ultra-dense plugs (25 mm width × 28 mm height).
-
18.
With the use of a syringe and a 20G 11/2 0.9 × 40 sterile needle, pierce the plug to add 500 μl of pure water inside the vial. Rapidly flip the tube upside down to allow humidification of the plug, which avoids drowning the flies.
Note: Move slightly the plug to the side to allow airflow and ease humidification, if necessary.
-
19.
Incubate the vial for ∼16 h in a 25°C incubator.
-
20.
Anesthetize the flies by directly sinking the plastic vial in ice. If necessary, gently tap the vial to drop flies to the bottom. Leave flies on ice for no more than 3 min.
-
21.
Drop the flies directly into one apparatus arena.
Note: In one tube, starve the exact number of flies that will be deposit in one arena of the behavior apparatus. This will ease the handling of flies.
Preparation of Axenic and Bacteria Feeding Solution
Timing: ∼1 day
This procedure aims to prepare the feeding solutions used in the behavioral assay, either axenic, or contaminated by alive or heat-inactivated Ecc-152141 bacteria.
-
22.
Use a Bunsen burner to create a sterile environment in the working area.
-
23.
Pour 50 mL of Luria-Bertani media pre-warm to room temperature (19°C–25°C) in a 125 mL Erlenmeyer flask.
-
24.
Flame sterilize a Pasteur pipette with a narrow tip.
-
25.
Let the pipette cool down, then collect Ecc-152141 colonies by gently passing the pipette tip onto an Ecc-152141 streak.
-
26.
Dip the contaminated pipette Pasteur tip into the Luria-Bertani media and gently agitate the pipette. This helps releasing colonies from the tip.
-
27.
Cover the Erlenmeyer with a sterile aluminum foil.
-
28.
Grow bacteria by incubating the Erlenmeyer in a 30°C shaking incubator for ∼16 h.
-
29.
Precipitate bacterial cells by a 20 min centrifugation at 4,000 × g, room temperature (19°C–25°C).
CRITICAL: Do not exceed 4,000 × g during bacterial centrifugation, and do not centrifuge cells at 4°C but rather at 20°C–25°C. This will otherwise affect both the attractive and the aversive properties of Ecc-152141 for adult flies (for more explanation see Charroux et al., 2020).
-
30.
Remove the supernatant and wash the bacterial pellet with 20 mL of sterile 1× PBS.
-
31.
Pellet the bacteria by centrifugation at 4,000 × g, room temperature (19°C–25°C) for 15 min.
-
32.
Eliminate the supernatant and re-suspend the pellet with 400 μl of sterile 1× PBS.
-
33.
Dilute an aliquot of the bacterial pellet 400× as follow: add 50 μl from step 32 to 950 μl of 1× PBS, mix well and transfer 50 μl of this solution to 950 μl of 1× PBS.
-
34.
Measure the optical density at 600 nm (OD600) of the 400× dilution from step 33.
Note: An optical density ranging from ∼0.875 to 1.25 is expected for the solution step 33, which corresponds to an optical density ranging from ∼350 to 500 for the solution step 32.
Note: For the heat-inactivated bacterial solution, remove an aliquot (in general 100 μl) of the concentrated bacteria from step 32 and incubate it at 96°C for 10 min, using an Eppendorf 1.5 mL microtube placed into a dry block heater.
-
35.
Prepare a 150 mM sucrose solution in ultrapure sterile water.
-
36.
Dilute bacteria either alive (from step 32) or heat-inactivated at a final OD600 of 50 into a 50 mM sucrose solution (final concentration), using the 150 mM sucrose solution and ultrapure sterile water.
Note: For the axenic feeding solution, replace the equivalent volume of bacteria with sterile 1× PBS.
-
37.
Color the feeding solutions by adding 1:100 (volume:volume) of Eriauglaucine blue at 1.25 μg/mL.
Note: Flame sterilization of the Pasteur pipette also seals the pipette tip.
Monitoring Behavior during the Two-Choice Feeding Assay
Timing: 6 h
This procedure explains how to deposit flies in the behavior apparatus as well as how to create a movie from image acquisition. An example of such movie is shown in Methods Videos S1, S2, and S3.
-
38.
In each arena, dispense one drop of axenic solution and one drop of bacteria-contaminated solution at precise distance (see legend Figure 2 for more details) from each side of the arena. Use the following volumes for each drop of feeding solution: 35 μl or 60 μl, when using respectively the 12 or 6 arenas apparatus, respectively.
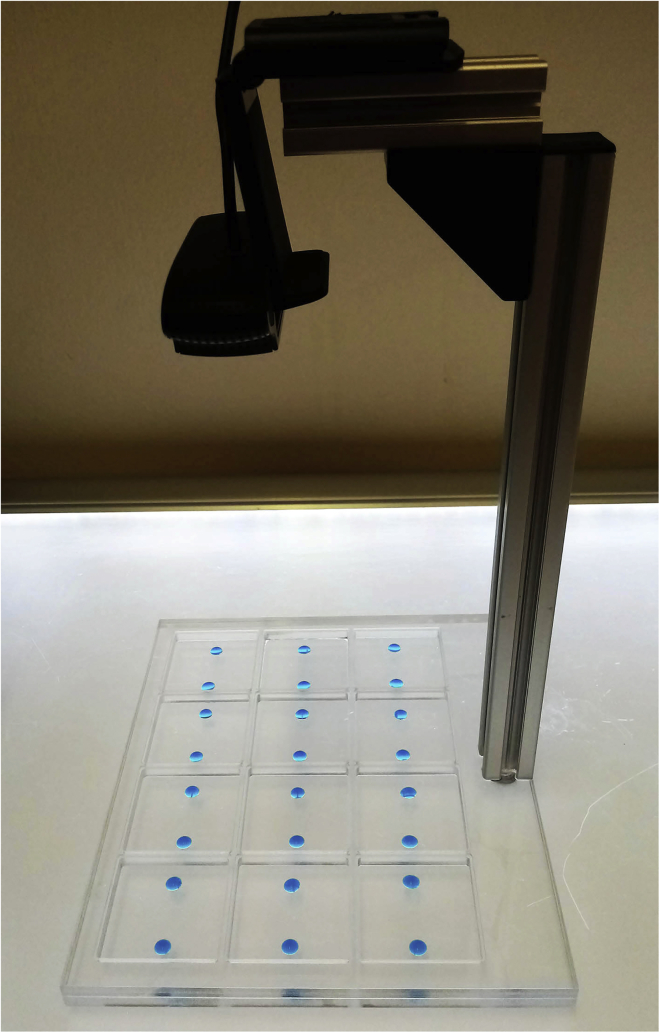
Figure 2.
This Picture Shows a 12 Arenas Apparatus with Two Drops of 35 μL of Feeding Solutions (Colored with Eriauglaucine Blue) Freshly Dispensed at Precise Location within Each Arena: 8 mm from One Side and 21 mm from the Other
Adult Canton-S flies are first attracted but then repulsed by an Ecc-152141-contaminated solution. Video recording of an experiment with 12 arenas, each containing around 10–11 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of alive Ecc-152141 + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The 6 arenas on the left contain wild-type starved Canton-S flies and the 6 arenas on the right contain non-starved Canton-S flies. The recording was performed for a total duration of 6 h. Methods Video S1 corresponds to the first ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
Methods Video S2 corresponds to the middle ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
Methods Video S3 corresponds to the last ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
-
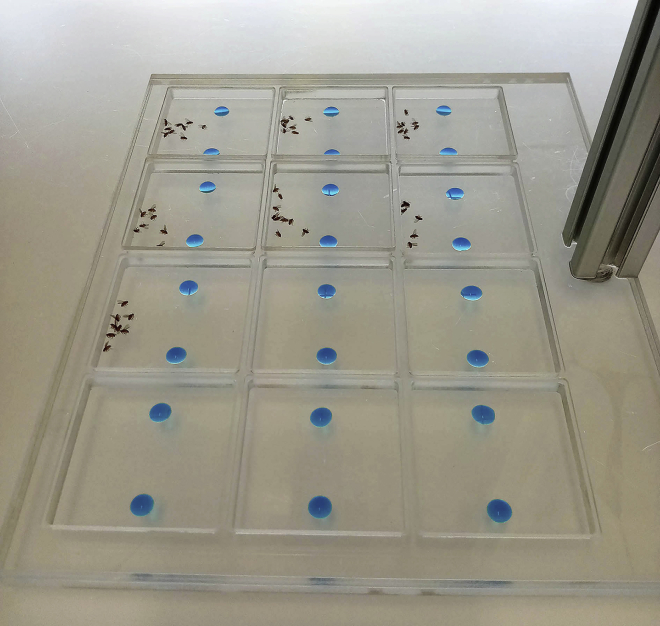
39.
Fill all the arenas with cold anesthetized F1 flies. Make sure to deposit flies away from the feeding solutions. See Figure 3.
Figure 3.
Cold Anesthetized Drosophila Are Successively Deposited, away from the Feeding Solutions, in Each of the 12 Arenas
-
40.
Rapidly cover each arena with the lid. See Figure 3.
-
41.
Let the flies recover 10 min before recording images every 5 s.
-
42.
Record for 6–8 h using the camera configuration shown in Figures 2 and 4A.
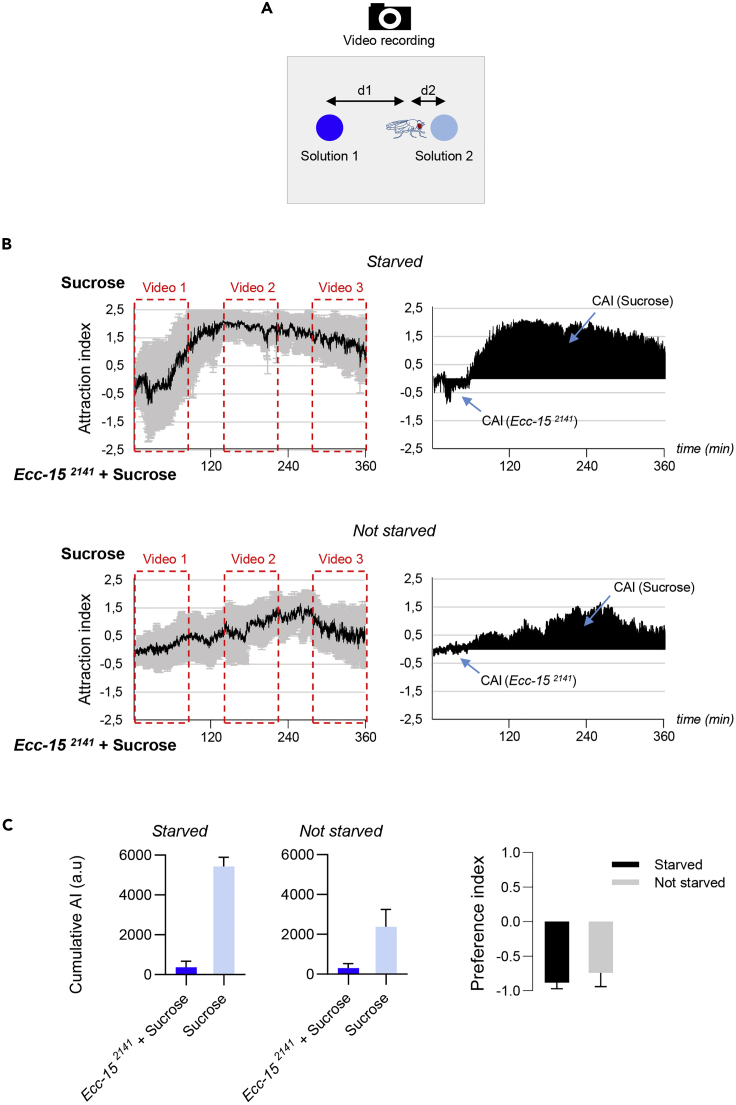
Figure 4.
Analysis of the Behavior of Starved and Non-starved Canton-S Flies Showing that Non-starved Flies Displayed Reduced Attraction and Aversion to Ecc-152141
(A) The drawing illustrates the two distances (d1 and d2) measured at every time frame of the video.
(B) (Left graphs) Kinetic of the Attraction Index (AI) for sucrose when starved flies or non-starved flies were given the choice between an Ecc-152141contaminated sucrose solution versus sucrose only. (Right graphs) Cumulative AI (CAI) area for each specified solution (arrows) and its distribution over time.
(C) Histograms built with the CAI values from (B).
Error bars correspond to standard deviation. The preference indexes for Ecc-152141are calculated with the CAI values from (B). For (B) left graphs, the black lines and the gray lines correspond respectively to the mean and the standard deviation, and for (B) right graphs, sole the mean value of the CAI obtained with the six replicates is shown in black. (A–C), show graphical data obtained from the analysis of the full video (not shown). The time period corresponding to the three short videos (Methods Videos S1, S2, and S3) shown below are indicated with red dotted lines.
Note: The AI for each time frame is calculated as follow. The distance of each of the 10 females from the droplet 1 (d1) and for the droplet 2 (d2) is measured every 5 s and the AI is calculated as the log2 ratio of the average of distances d1 divided by the average of distances d2. The d2 attraction will be translated into a positive index and the d1 by a negative one. We then calculate a CAI corresponding to the area between the AI curve and the abscissa axis for x = 0, which represents the absolute preference of the flies for each of the two feeding solutions. We then could calculate the preference index (PI) for the solution 1 as follow, PI (solution 1) = (CAI solution 1) − (CAI solution 2)/(CAI solution 1) + (CAI solution 2).
-
43.
Import the series of images into ImageJ as an “image sequence.”
-
44.
Save as a “AVI” selecting JPEG compression and 10 frames/s.
-
45.
Analyze the movie using Flybox as described in Charroux et al., 2020.
Note: The apparatus is placed on top of a LED panel (60 cm × 60 cm, 60 watts).
Note: We use a Logitech C920 HD pro webcam.
Note: We perform all experiments in a behavioral room with constant temperature (24°C) and humidity (65%).
Note: We stop recording when one of the two droplets has disappeared (around 6–8 h) consumed by the flies, thus eliminating a two-choice situation.
Note: We use the webcam software Yawcam to save images.
Expected Outcomes
About 40–50 axenic adults are recovered during 24 h after the first adult’s emergence, with a recovery rate of 55% females versus 45% males. We usually replicate the procedure in parallel, in order to reach the required amount of flies that we need for the behavioral test. For instance, 60 females are required for one experiment with the 6 arenas apparatus, which necessitates 2–3 vials for the collection of adults.
We usually obtain a value of ∼350–500 for the OD600 of the concentrated Ecc-152141 bacterial culture.
Starved adult females typically displayed a two-step stereotyped behavior when put in the presence of the two feeding solutions, one containing Ecc-152141 bacteria in 50 mM sucrose and the other 50 mM sucrose only (See Figure 4B). They should be first attracted by the contaminated solution before moving away from it and staying close to the sucrose solution permanently (See Methods Videos S1, S2, and S3, left panels). As shown in Figure 4C the Cumulative Attraction Index for Ecc-152141 is expected to be lower (369 a.u ± 304 SD) than for sucrose (5,428 a.u ± 462 SD), and the preference index for Ecc-152141 has to be negative (-0.88 ± 0.09 SD). This indicates that flies displayed a global aversion toward Ecc-152141. The video tracking helpfully reveals the presence of the two distinct phases throughout the experiment. While flies are first attracted by the Ecc-152141 solution (for approximatively 60 min), they are later preferentially found in the proximity to the sucrose solution.
Non- starved flies, however, are expected to show no clear attraction phase to the Ecc-152141 contaminated solution and an attraction to sucrose that occurs slowly and progressively (See Figure 4B and Methods Videos S1, S2, and S3, right panels). See Charroux et al. 2020 for more explanations.
Limitations
Our protocol is not unique in studying two-choice feeding assay in presence of live bacteria. For instance, the flyPAD assay has been used in the past to compare appetite of flies toward food contaminated with commensal bacteria (Leitão-Gonçalves et al., 2017). However, our procedure allows longer recording of fly behavior; up to 6 h compare to 1 h with the FlyPAD. This could help in uncovering potential multi-step behavior as we recently reported (Charroux et al., 2020). An alternative would be to use the CApillary FEeder assay (CAFE), but the use of bacteria is impossible due to the sedimentation of bacteria in the micro-capillaries and the resulting clogging.
Our procedure includes raising flies in axenic conditions, by the use a cocktail of antibiotics present in the diet. We cannot exclude the possibility that the antibiotics, as xenobiotic, might have deleterious effect on fly physiology, fitness, and ultimately behavior. An alternative to the use of antibiotics would be to surface sterilized Drosophila eggs with bleach, and transfer them into a vial with autoclaved food. This procedure is however time consuming and more fastidious than our protocol.
It is well recognized that behaviors are affected by the circadian rhythms. For this reason, our flies are raised and aged at 25°C in a 12 h/12 h light/dark cycle controlled incubator. Moreover, starvation is systematically performed from 4 pm to 8 am the next day and we repeatedly start our behavioral assay around 8 h 15 min (±15 min) the morning.
Following starvation, Drosophila is known to suppress sleep and enhance locomotion to forage for new food source (Yang et al., 2015, Keene et al., 2010). Consequently, any genetic conditions that will affect such biological response to starvation is expected to impact fly behavior. For that reason, we recommend to systematically assess and compare the behavior of starved versus non-starved animals.
Troubleshooting
Problem
Bacteria culture of Ecc-152141 is too low in density.
Potential Solution
It is very likely that the Ecc-152141 bacterium streak has suffered from low temperature storage (4°C). We recommend to inoculate bacterial culture from a fresh bacterial streak.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Bernard Charroux (bernard.charroux@univ-amu.fr).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The code supporting the current study (Flybox software, Charroux et al., 2020) is available from the corresponding author on request.
Acknowledgments
This work was supported by ANR-11-LABX-0054 (Investissements d’Avenir–Labex INFORM), ANR BACNEURODRO (ANR-17-CE16-0023-01), ANR PEPTIMET(ANR-18-CE15-0018-02), Equipe Fondation pour la Recherche Médicale (EQU201903007783), and l’Institut Universitaire de France to J.R.
Author Contributions
Conceptualization, B.C. and J.R.; Methodology, B.C.; Investigation, B.C.; Formal Analysis, B.C.; Writing – Original Draft, B.C.; Writing – Review & Editing, B.C. and J.R.; Funding Acquisition, J.R.; Supervision, B.C. and J.R.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100117.
Contributor Information
Bernard Charroux, Email: bernard.charroux@univ-amu.fr.
Julien Royet, Email: julien.royet@univ-amu.fr.
References
- Basset A., Khush R.S., Braun A., Gardan L., Boccard F., Hoffmann J.A., Lemaitre B. The phytopathogenic bacteria Erwinia carotovora infects Drosophila and activates an immune response. Proc. Natl. Acad. Sci. U S A. 2000;97:3376–3381. doi: 10.1073/pnas.070357597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Drayon V., Poidevin M., Boneca I.G., Narbonne-Reveau K., Royet J., Charroux B. Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota. Cell Host Microbe. 2012;12:153–165. doi: 10.1016/j.chom.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Charroux B., Daian F., Royet J. Drosophila aversive behavior toward Erwinia carotovora carotovora is mediated by bitter neurons and Leukokinin. iScience. 2020;23:101152. doi: 10.1016/j.isci.2020.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene A.C., Duboué E.R., McDonald D.M., Dus M., Suh G.S., Waddell S., Blau J. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr. Biol. 2010;20:1209–1215. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão-Gonçalves R., Carvalho-Santos Z., Francisco A.P., Fioreze G.T., Anjos M., Baltazar C., Elias A.P., Itskov P.M., Piper M.D.W., Ribeiro C. Commensal bacteria and essential amino-acids control food choice behavior and reproduction. PLoS Biol. 2017;4:e2000862. doi: 10.1371/journal.pbio.2000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Yu Y., Zhang V., Tian Y., Qi W., Wang L. Octopamine mediates starvation-induced hyperactivity in adult Drosophila. Proc. Natl. Acad. Sci. U S A. 2015;112:5219–5224. doi: 10.1073/pnas.1417838112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adult Canton-S flies are first attracted but then repulsed by an Ecc-152141-contaminated solution. Video recording of an experiment with 12 arenas, each containing around 10–11 females and two drops of feeding solution (in blue). Each arena contains one drop of a mixture of alive Ecc-152141 + 50 mM sucrose (left) and one drop of 50 mM sucrose (right). The 6 arenas on the left contain wild-type starved Canton-S flies and the 6 arenas on the right contain non-starved Canton-S flies. The recording was performed for a total duration of 6 h. Methods Video S1 corresponds to the first ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
Methods Video S2 corresponds to the middle ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
Methods Video S3 corresponds to the last ∼90 min of the complete recording (Refer to steps 38–45 in Monitoring behavior during the two-choice feeding assay).
Data Availability Statement
The code supporting the current study (Flybox software, Charroux et al., 2020) is available from the corresponding author on request.