Summary
Psoriasis is an incurable chronic inflammatory skin disorder. The imiquimod (IMQ)-induced mouse model of psoriasis is the most widely used model for drug discovery and pre-clinical studies of psoriasis. The inflamed and thickened skin frequently compromises the quality of single-cell suspensions generated from IMQ-induced skin lesions, which has an impact on subsequent analyses by flow cytometry. This protocol details the complete procedure for the establishment of a mouse model of psoriasis and flow cytometric detection of immune cells in the inflamed epidermis and dermis.
For complete details on the use and execution of this protocol, please refer to Lou et al. (2020).
Graphical Abstract
Highlights
-
•
Appropriate strains, ages, and anatomic sites for IMQ-induced mouse model of psoriasis
-
•
Easy and effective procedures to process mouse skin after IMQ treatment
-
•
High-quality single-cell suspensions generated from inflamed mouse epidermis and dermis
-
•
Expected immune cell percentages in mouse epidermis and dermis treated with IMQ or not
Psoriasis is an incurable chronic inflammatory skin disorder. The imiquimod (IMQ)-induced mouse model of psoriasis is the most widely used model for drug discovery and pre-clinical studies of psoriasis. The inflamed and thickened skin frequently compromises the quality of single-cell suspensions generated from IMQ-induced skin lesions, which has an impact on subsequent analyses by flow cytometry. This protocol details the complete procedure for the establishment of a mouse model of psoriasis and flow cytometric detection of immune cells in the inflamed epidermis and dermis.
Before You Begin
-
1.
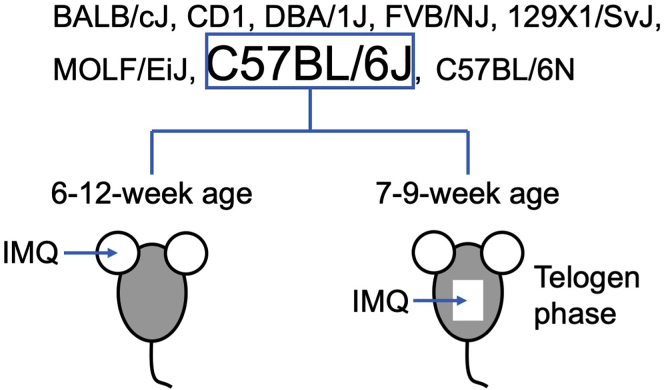
Before working with this protocol, ensure that the appropriate mouse strain is chosen. According to a study comparing gene expression profiles of psoriasiform phenotype induced by IMQ in different mouse strains, C57BL/6J (B6) mice outweighed other strains including BALB/cJ, CD1, DBA/1J, FVB/NJ, 129X1/SvJ, and MOLF/EiJ mice, to better mirror the characteristics of human psoriasis (Swindell et al., 2017). We recommend using mice with B6 background for studies involving IMQ-induced mouse model of psoriasis. This protocol is optimized for mice with B6 background and has also been tested on mice with BALB/c background. Compared to C57BL/6J strain, C57BL/6N strain was protected from IMQ-induced psoriasis-like skin inflammation, with the macroscopic and histological alterations markedly reduced (Bezdek et al., 2017). To guarantee a pronounced psoriasis-like phenotype, we recommend using C57BL/6J mouse strain.
-
2.
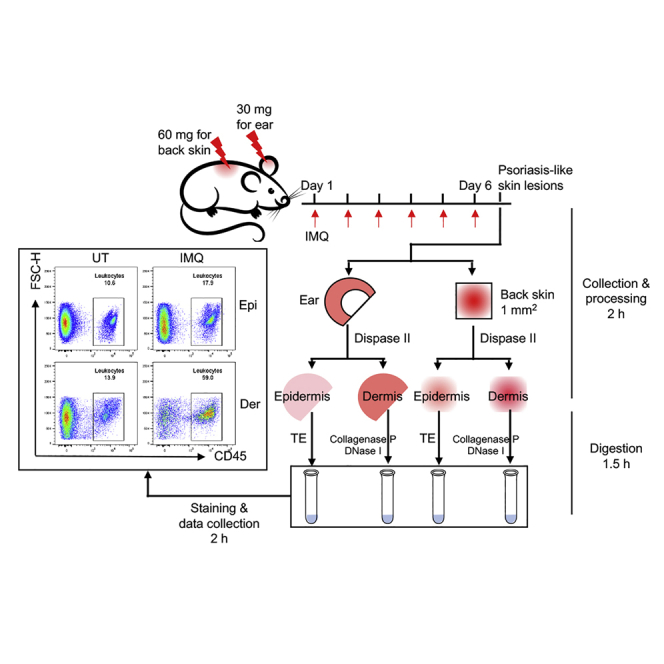
Choose the appropriate anatomic site of skin for further experiments. We routinely use back skin or ears for IMQ induction, and the processing procedures are slightly different. This protocol includes the procedures for the processing of both back skin and ears. 6–12-week-old mice can be used for IMQ application on the ear; 7–9-week-old mice can be used for IMQ application on the back skin when the hair cycle is in the telogen phase (Figure 1). Both males and females can be used.
-
3.
Set up a flow panel for cells of interest. Following is the panel we use in this protocol (Table 1).
Figure 1.
Flowchart of Mouse Strain and Age Selection
Table 1.
Flow Panel for Basic Analysis of Major Immune Cell Types in Mouse Skin
| Fluorophore | Marker | Final Dilution | Volume Per Sample |
|---|---|---|---|
| Alexa Fluor 700 | CD45 | 1:200 | 0.5 μL |
| FITC | CD3ε | 1:100 | 1 μL |
| APC | CD11b | 1:200 | 0.5 μL |
| PE | F4/80 | 1:200 | 0.5 μL |
| BV510 | CD11c | 1:100 | 1 μL |
| PerCP-Cy5.5 | Ly6G | 1:200 | 0.5 μL |
| DAPI | n/a | 1:1,000 | 0.2 μL |
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD45 (clone 30-F11) | eBioscience | Cat#56-0451-82 |
| Anti-mouse CD3 (clone 145-2C11) | Biolegend | Cat#100305 |
| Anti-mouse CD11c (clone N418) | Biolegend | Cat#117353 |
| Anti-mouse F4/80 (clone T45-2342) | BD Pharmingen | Cat#585410 |
| Anti-mouse Ly6G (clone RB6-8C5) | eBioscience | Cat#45-5931-80 |
| Anti-mouse CD11b (clone M1/70) | eBioscience | Cat#17-0112-82 |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™) | BD Pharmingen | Cat#553141 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| IMQ cream | MedShine | Cat#120503 |
| Pentobarbital sodium | Sigma-Aldrich | Cat#P3761 |
| Chloral hydrate | Sangon Biotech | Cat#A600288 |
| DAPI Solution | BD Pharmingen | Cat#564907 |
| 20× PBS | Sangon Biotech | Cat#B548117 |
| HBSS, no calcium, no magnesium, no phenol red | Gibco | Cat#14175079 |
| Dispase II | Sigma-Aldrich | Cat#D4693-1G |
| Trypsin-EDTA (0.25%) | Gibco | Cat#25200072 |
| Fetal Bovine Serum (FBS) | Gibco | Cat#10270-106 |
| DNase I | Roche | Cat#10104159001 |
| Collagenase P from Clostridium histolyticum | Roche | Cat#11213857001 |
| HyClone Dulbecco's Modified Eagle Medium (DMEM)/high glucose with L-Glutamine; without sodium pyruvate | Cytiva | Cat#SH30022.01 |
| EDTA | Sigma-Aldrich | Cat#ED2SS-100G |
| Bovine Serum Albumin (BSA) | MP Biomedicals | Cat#232-936-2 |
| Critical Commercial Assays | ||
| UltraComp eBeads™ Compensation Beads | Invitrogen | Cat#01-2222-42 |
| LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit | Invitrogen | Cat#L23105 |
| Fixation/Permeabilization Solution Kit | BD Cytofix/Cytoperm | Cat#554714 |
| Foxp3 / Transcription Factor Staining Buffer Set | eBioscience | Cat#00-5523-00 |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6J | Shanghai SLAC Laboratory Animal Co., Ltd. | n/a |
| Software and Algorithms | ||
| BD FACSDiva™ Software v8.0 | BD Biosciences | https://www.bdbiosciences.com/cn/instruments/research/software/flow-cytometry-acquisition/bd-facsdiva-software/m/111112/resourcestools |
| FlowJo v10.4 | Tree Star | https://www.flowjo.com/ |
| Other | ||
| Curved forceps | Shanghai Jinzhong | Cat#JD1060 |
| Curved scissors | Shanghai Jinzhong | Cat#Y00040 |
| EP tube | Axygen | Cat#MCT-150-A |
| 2-mL microfuge tube | Ambion | Cat#AM12425 |
| 15-mL conical centrifuge tube | NEST | Cat#601002 |
| 50-mL centrifuge tube | NEST | Cat#602002 |
| 5-mL round-bottom flow test tube | Falcon | Cat#38055 |
| Centrifuge | Thermo Scientific | Multifuge 3SR+ |
| Cell culture CO2 incubator | Thermo Scientific | Series 8000 DH |
| Countess II | Thermo Scientific | AMQAX1000 |
| BD LSRFortessa™ cell analyzer | BD Biosciences | n/a |
Materials and Equipment
Pentobarbital Sodium Solution
Dissolve 0.45 g pentobarbital sodium in 50 mL PBS to make a 0.9% working solution. Use 10 μL per g body weight for a mouse.
Dispase II Solution
Dissolve 100 mg Dispase II in 10 mL HBSS to make a 10 mg/mL Dispase II stock. Filter and store at −20°C in 1 mL aliquots for up to 6 months. Dilute the stock with HBSS to 5 mg/mL as working solution. The working solution should be prepared on the day for sample processing and pre-chilled at 4°C. Use 1 mL for one sample.
100× Collagenase P Stock
Dissolve 100 mg Collagenase P in 1 mL DMEM/high glucose (not supplemented with antibiotics or FBS) to make a 100 mg/mL stock. Store at −20°C in 100 μL aliquots for up to 6 months.
1,000× DNase I Stock
Dissolve 100 mg DNase I in 1 mL ddH2O to make a 100 mg/mL stock. Store at −20°C in 100 μL aliquots for up to 6 months.
Dermis Dissociation Buffer
Dilute DNase I Stock to a final concentration of 100 μg/mL and Collagenase P Stock to a final concentration of 1 mg/mL with DMEM/high glucose (not supplemented with antibiotics or FBS). The buffer should be prepared on the day for sample processing and pre-chilled at 4°C. Use 3 mL for one ear sample or 3.5 mL for one back-skin sample.
CRITICAL: Do not use RPMI1640 medium, as the buffer capacity of this medium is not good enough for dermis dissociation and cell viability.
0.05% TE Buffer
Dilute 2 mL Trypsin-EDTA (0.25%) with 8 mL HBSS to a final concentration of 0.05%. Store at 4°C for up to 1 month. Use 1 mL for one sample.
Trypsin Neutralizing Solution
Dilute 2.5 mL FBS with 47.5 mL HBSS to a final concentration of 5%. Store at 4°C for up to 3 months. Use 1 mL for one sample.
Staining Buffer
Dissolve 0.372 g EDTA and to a final concentration of 2 mM and 2.5 g BSA to a final concentration of 0.5% with 500 mL PBS. Filter and store at 4°C for up to 3 months.
DAPI Buffer
Dilute 50 μL DAPI Solution with 50 mL Staining Buffer. Store at 4°C in the dark for up to 3 months. Use 200 μL for one sample.
Step-By-Step Method Details
Establishment of IMQ-Induced Mouse Model of Psoriasis
Timing: 1 week
IMQ cream is applied to mouse skin for 6 consecutive days to induce psoriasis-like skin lesions.
-
1.
Inject 90 mg/kg pentobarbital sodium intraperitoneally (i.p.) to anesthetize the mice.
Optional: 400 mg/kg chloral hydrate (i.p.) can also be used.
Note: We use 7-week-old male C57BL/6J mice in this protocol.
-
2.
Shave a 1.5 cm × 2 cm area on mouse back skin using an electric pet shaver, not required if IMQ is applied to the ear.
CRITICAL: Do not scratch the mouse skin, especially when genetically engineered mice might have vulnerable skin barriers.
-
3.
Weigh 5% IMQ cream using an electronic balance, 60 mg for back skin or 30 mg for ventral and dorsal sides of an ear.
-
4.
Smear the IMQ cream to mouse skin gently and evenly with a pair of curved forceps (Methods Video S1).
-
5.
Repeat steps 1 and 3–4 same time every day for 5 days.
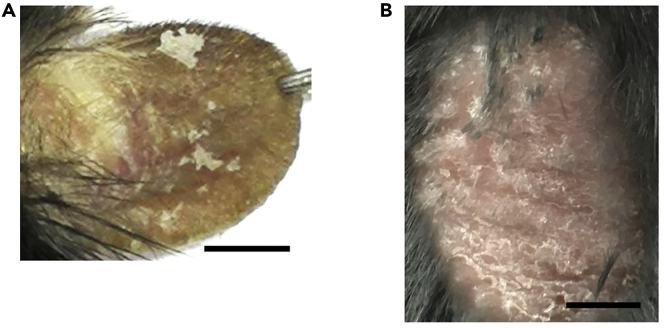
Note: The skin thickens gradually along with daily application of IMQ. After 5 days of IMQ application, obvious erythematous lesions covered with squama will form (Figure 2).
Figure 2.
Typical Psoriasis-like Skin Lesions after IMQ Application
(A) Typical psoriasis-like skin lesions on mouse ear after 5 days of IMQ treatment.
(B) Typical psoriasis-like skin lesions on mouse back skin after 5 days of IMQ treatment.
Scale bars: 0.5 cm.
Skin Collection and Processing
Timing: ∼2 h
After 6 days of IMQ application, collect the skin and separate the epidermis from the dermis. The skin can also be collected at an alternative timepoint (e.g., after 3 days, 5 days or 7 days of IMQ application) and processed in exactly the same way.
-
6.
Euthanize the mice by CO2 inhalation.
-
7.
Collect skin samples.
-
a.
Cut off a 1 cm × 1 cm area from mouse back skin if IMQ was applied.
Note: Such an area is enough for flow cytometric analysis of the epidermis and the dermis. If a larger area is needed, multiply the reagents accordingly.
-
b.
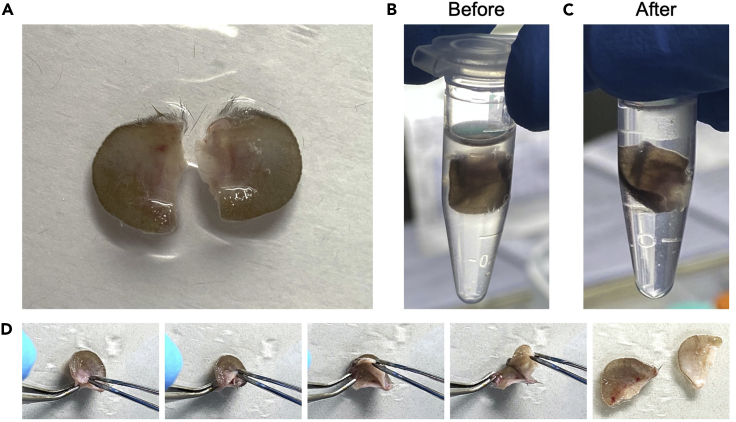
Cut off the whole ear if IMQ was applied (Figure 3A).
Figure 3.
The Processing of Mouse Ear Skin
(A) A pair of IMQ-treated ears cut off from a C57BL/6J mouse.
(B) The HBSS is turbid due to the presence of IMQ cream on the ear.
(C) After washing the ear rigorously for three times, the HBSS is transparent.
(D) Splitting the dorsal and ventral sections of the ear.
-
8.
Transfer the skin to an EP tube containing 1 mL HBSS and wash rigorously by quickly shaking up and down by hand for 15 s.
-
9.
Repeat step 8 by transferring the skin to a new EP tube and refreshed HBSS for two times to wash off remaining IMQ cream (Figures 3B, 3C, 4A, and 4B).
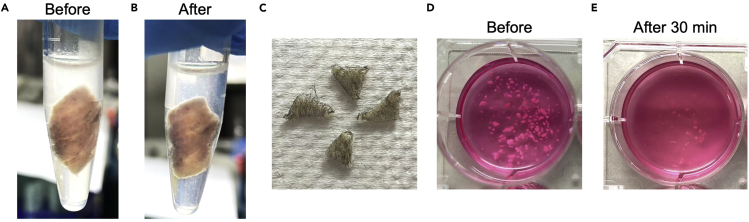
Figure 4.
The Processing of Mouse Back Skin
(A) The HBSS is turbid due to the presence of IMQ cream on the skin.
(B) After washing the skin rigorously for three times, the HBSS is transparent.
(C) 1 cm2-area of back skin is cut into four triangular pieces for subsequent digestion with Dispase II.
(D) Back-skin tissue fragments after mechanical disruption and before enzymatic digestion.
(E) Back-skin tissue suspension after 30 min of enzymatic digestion and pipetting.
CRITICAL: The presence of IMQ cream affects the viability of cells released from enzyme digested tissues in the later steps. Ensure that HBSS is transparent after 3 times of washing, each for 15 s. Note that scales from the epidermis can flow in HBSS after 3 times of washing, which is normal.
-
10.
If processing back skin, cut the back-skin sample with scissors into two pieces along the diagonal, then to four pieces along the diagonal (Figure 4C).
Note: Cut the back skin into triangular pieces to ensure efficient digestion with Dispase II.
-
11.
If processing ears, split the dorsal and ventral sections of the ear with forceps (Figure 3D).
-
12.
Digest the skin (4 pieces of back skin or two sections of an ear) with 1 mL of Dispase II working solution in one well of a 12-well plate at 37°C for 1 h.
Note: Pre-chill HBSS at 4°C for later steps.
-
13.
Transfer the skin to a petri dish with 10 mL of cold HBSS and separate the epidermis from the dermis using curved forceps (Methods Videos S2 and S3).
-
14.
Wash the epidermis and the dermis with cold HBSS for three times.
-
15.
Transfer the epidermis to one well of a 12-well plate containing 1 mL cold HBSS and place the plate at 4°C.
Note: Dermis requires longer time for digestion, so we leave the epidermis in HBSS at 4°C until the processing of dermis is almost finished. This is to ensure all samples ready for antibody staining immediately after single-cell suspensions are prepared. The epidermis can be left at 4°C for 6 h without affecting cell viability.
Dermis Digestion
Timing: ∼1.5 h
Mouse dermis samples are digested to generate single-cell suspensions.
-
16.
Transfer the dermis to one well of a 6-well plate placed on ice containing 1 mL of cold Dermis Dissociation Buffer.
-
17.
Quickly cut the dermis into pieces as small as possible (< 0.5 mm in size) with curved scissors in 1 mL of cold Dermis Dissociation Buffer in the 6-well plate placed on ice.
CRITICAL: The tissue from one ear is regarded as one sample; the 1 cm × 1 cm area from back skin is regarded as one sample. Because the cutting process can be time-consuming if many samples are to be processed, proceed this step with the 6-well plate placed on ice to minimize digestion time differences between samples processed earlier or later. If there are > 4 samples, we recommend having ≥ 2 people working together in this step, to minimize digestion time differences between samples.
-
18.
Add another 2 mL of Dermis Dissociation Buffer to an ear sample or 2.5 mL of Dermis Dissociation Buffer to a back-skin sample (Figure 4D).
-
19.
Incubate the sample in a 37°C cell culture incubator for 30 min.
-
20.
Cut the 5 mm-long top end of a 1-mL pipet tip with scissors, and gently mix the tissue suspension by pipetting. Incubate the sample in a 37°C cell culture incubator for another 30 min.
Note: The tissue fragments become smaller visibly upon pipetting (Figure 4E).
-
21.
Cut the 5-mm-long top end of a 1-mL pipet tip with scissors, and gently mix the tissue suspension by pipetting.
CRITICAL: There should leave no obvious debris by this time. If not, continue the 37°C incubation for another 10–30 min until the debris disappears after pipetting. Check the sample status every 10 min. Longer than 1.5 h of collagenase digestion at 37°C affects cell viability.
-
22.
Pass the suspension through a 40–μm cell strainer into a 50-mL tube.
-
23.
Rinse the well with 2 mL DMEM/high glucose containing 10% FBS and pass the suspension through the strainer into the same 50-mL tube.
-
24.
Add 10 mL DMEM/high glucose containing 10% FBS to the strainer to wash the sample.
Note: Filter the sample through the strainer by gravity but no other forces.
-
25.
Transfer the suspension to a 15-mL conical centrifuge tube. Centrifuge at 400 × g for 5 min at 4°C, discard the supernatant, and resuspend the pellet with 2 mL cold Staining Buffer.
-
26.
Centrifuge at 400 × g for 5 min at 4°C, discard the supernatant, and resuspend the pellet with 90 μL cold Staining Buffer.
-
27.
Transfer the sample to a 5-mL round-bottom flow test tube. Keep on ice until staining.
Note: One ear sample generates 5∼8 × 105 live cells; one back-skin sample generates 1∼3 × 105 live cells.
Epidermis Digestion
Timing: 30 min
Mouse epidermis samples are digested to generate single-cell suspensions.
-
28.
Pre-warm 1 mL 0.05% TE Buffer in a 2-mL microfuge tube at 37°C water bath for 10 min.
-
29.
Transfer the epidermis from HBSS to a new well of the 12-well plate that does not contain any buffer.
-
30.
Cut the epidermis into small pieces (∼2 mm in size) with scissors and squeeze the remaining HBSS to avoid unintended dilution of the TE buffer.
-
31.
Transfer the sample to the TE Buffer and incubate at 37°C water bath for 5 min.
-
32.
Shake the sample up and down for five times.
-
33.
Continue incubation at 37°C water bath for 5 min.
Note: The tissue will not completely disappear due to the presence of stratum corneum and hair follicles.
-
34.
Add 1 mL cold trypsin neutralizing solution (4°C) to the sample and pipette rigorously for five times.
-
35.
Pass the suspension through a 40–μm cell strainer into a new 50-mL tube.
-
36.
Rinse the 2-mL tube with 2 mL of cold HBSS and pass the suspension through the strainer into the same 50-mL tube.
-
37.
Use 5 mL of cold HBSS to pass through the strainer to wash the sample.
-
38.
Transfer the suspension to a 15-mL conical centrifuge tube. Centrifuge at 400 × g for 5 min at 4°C, discard the supernatant, and resuspend the pellet with 2 mL cold Staining Buffer.
-
39.
Centrifuge at 400 × g for 5 min at 4°C, discard the supernatant, and resuspend the pellet with 90 μL cold Staining Buffer.
-
40.
Transfer the sample to a 5-mL round-bottom flow test tube. Keep on ice until staining.
Note: One sample generates 1∼2 × 105 live cells, except for untreated back-skin epidermis, which generates 4∼8 × 104 live cells.
Flow Cytometry Antibody Staining
Timing: 1 h
The cell suspensions are stained with indicated antibodies for flow cytometric analysis.
-
41.
Set up an unstained control and seven single-stained controls.
-
a.
Label a tube for each fluorochrome that will be used.
-
b.
Aspirate 20 μL from a dermis sample to a tube labeled “Blank” and add 180 μL Staining Buffer to generate an unstained control. Keep on ice until detection.
-
c.
Aspirate 20 μL from a dermis sample to a tube labeled “DAPI” and add 180 μL DAPI Buffer to generate a DAPI-single-stained control. Keep on ice until detection.
-
d.
Mix UltraComp eBeads™ Compensation Beads by vortex.
-
e.
Add 1 drop of the beads to an empty labeled tube for a fluorochrome and add 0.5 μL of the relevant antibody to the tube.
-
f.
Mix well by flicking and incubate at 4°C for 15 min in the dark.
-
g.
Add 2 mL Staining Buffer, vortex briefly and centrifuge at 400 × g for 5 min at 4°C.
-
h.
Discard the supernatant and resuspend the pellet with 200 μL Staining Buffer. Keep on ice until detection.
-
42.
Adjust the epidermis and dermis sample volumes to 90 μL with Staining Buffer.
-
43.
Dilute Fc block at a dilution of 1:20 with Staining Buffer.
-
44.
Add 10 μL of the diluted Fc block to the sample and incubate at 4°C for 10 min in the dark.
-
45.
Mix the indicated antibodies in an EP tube (Table 1). Add 4 μL of the antibody mix (without DAPI) to the 100-μL sample, vortex briefly, and incubate at 4°C for 30 min in the dark.
-
46.
Add 2 mL Staining Buffer, vortex briefly and centrifuge at 400 × g for 5 min at 4°C.
Optional: After this step, if intracellular cytokines are to be detected, use BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution Kit to process the sample; If transcription factors are to be detected, use eBioscience™ Foxp3 / Transcription Factor Staining Buffer Set to process the sample.
-
47.
Discard the supernatant and resuspend the pellet with 200 μL DAPI Buffer. Keep on ice until detection.
Data Collection
Timing: 1 h
The data are collected using a BD LSRFortessa™ cell analyzer and analyzed using the FlowJo software.
-
48.
Select parameters including FSC-A, FSC-H, FSC-W, SSC-A, SSC-H, SSC-W, Alexa Fluor 700-A (CD45), FITC-A (CD3), APC-A (CD11b), PE-A (F4/80), BV510-A (CD11c), PerCP-Cy5.5-A (Ly6G) and BV421-A (DAPI).
-
49.
Use the unstained control and single-stained controls to set appropriate PMT voltages and adjust compensations on a BD LSRFortessa™ cell analyzer using the BD FACSDiva software.
-
50.
Collect a total of 5,000 events from the unstained control and from each single-stained control.
-
51.
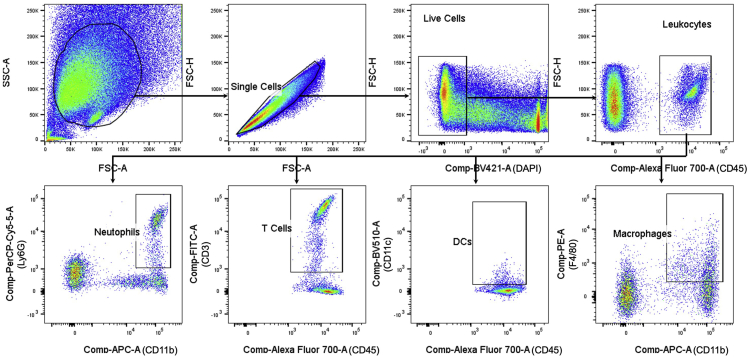
Create gates for the samples (Figure 5), set “Storage Gate” value to “All Events,” “Events to Record” value to 10,000 and “Stopping Gate” value to “P4” (CD45+ cells), and collect data.
Figure 5.
Gating Strategy for Data Collection
“Events to Record” value is set to 10,000 and “Stopping Gate” value is set to “P4” (CD45+ cells). CD45+ cells are gated from DAPI- live cells; CD3+ cells, CD11c+ cells, CD11b+Ly6G+ cells and CD11b+F4/80+ cells are gated from CD45+ cells.
Note: All cells in the epidermis samples and the untreated back-skin dermis will be collected, as the numbers of CD45+ cells in these samples will not reach 10,000.
-
52.
Analyze the data with the FlowJo software.
Note: Dead cells have autofluorescence. Keratinocytes in the epidermis can have autofluorescence detected as FITC. This would not be a problem if dead cells are gated away, a dermis sample is used as the unstained control, and Alexa Fluor 700 is used to stain CD45.
Expected Outcomes
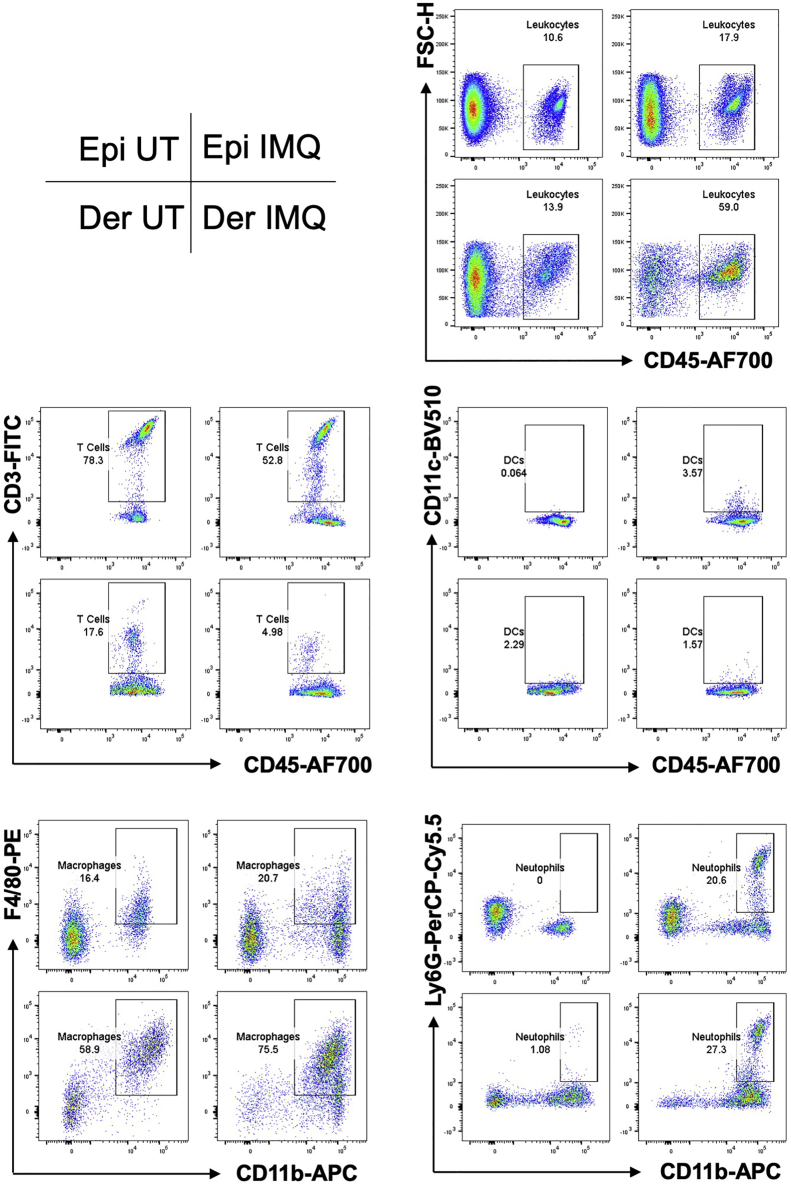
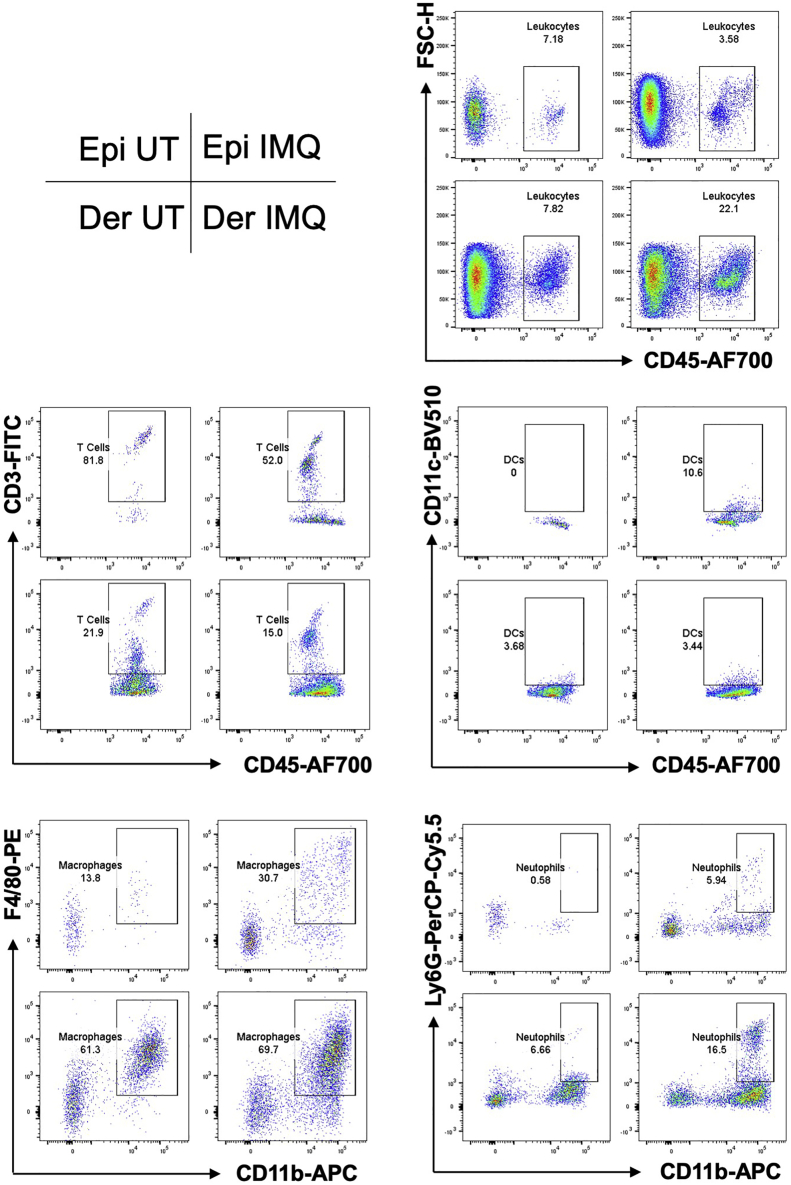
The numbers of collected events from different samples and the expected percentages of live cells and leukocytes are shown (Table 2). Representative data of mouse ear and back-skin samples treated with IMQ or not are shown (Figures 6 and 7). Comparing IMQ-treated to untreated mouse skin, we expect increased percentages of CD45+ leukocytes in the ear epidermis and in the dermis of both ear and back skin. As for CD45+ leukocytes, we expect increased percentages of macrophages and neutrophils in both the epidermis and the dermis; we expect increased percentages of dendritic cells in the epidermis; we expect decreased percentages of T cells in both the epidermis and the dermis.
Table 2.
Expected Percentages of Live Cells and Leukocytes
| Ear |
Back skin |
|||||||
|---|---|---|---|---|---|---|---|---|
| UT |
IMQ |
UT |
IMQ |
|||||
| Epidermis | Dermis | Epidermis | Dermis | Epidermis | Dermis | Epidermis | Dermis | |
| Total collected events | ∼100,000 | ∼200,000 | ∼150,000 | ∼50,000 | ∼10,000 | ∼200,000 | ∼150,000 | ∼300,000 |
| Live cells | 60%–80% | 80%–90% | 50%–70% | 70%–80% | 70%–80% | 80%–90% | 50%–70% | 70%–80% |
| Leukocytes | 5%–12% | 9%–15% | 15%–20% | 45%–60% | 7%–12% | 7%–12% | 3%–5% | 20%–25% |
Figure 6.
Immune Cells in the Epidermis and the Dermis of Mouse Ear
CD45+CD3+ for T cells, CD45+CD11c+ for dendritic cells, CD11b+Ly6G+ for neutrophils, CD11b+F4/80+ for macrophages, gated on CD45+ cells. UT, untreated mouse ear; IMQ, IMQ-treated mouse ear; Epi, epidermis; Der, dermis.
Figure 7.
Immune Cells in the Epidermis and the Dermis of Mouse Back Skin
CD45+CD3+ for T cells, CD45+CD11c+ for dendritic cells, CD11b+Ly6G+ for neutrophils, CD11b+F4/80+ for macrophages, gated on CD45+ cells. UT, untreated mouse back skin; IMQ, IMQ-treated mouse back skin; Epi, epidermis; Der, dermis.
Limitations
The flow panel used in this protocol is for basic analysis of major immune cell types in untreated or IMQ-treated mouse epidermis and dermis. For extended analysis of immune cells using flow cytometry, please refer to “Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition)”(Cossarizza et al., 2019) for further knowledge regarding antibody staining, compensation adjustment, gating strategy, etc.
There is a chance that certain surface marker expression be impaired upon enzymatic digestion.
DAPI is used in this protocol to counter stain dead cells, thus sample fixation for intracellular protein analysis is not expected. To perform intracellular staining, LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit can be used instead of DAPI.
Troubleshooting
Problem 1
Typical psoriasis-like skin lesions do not fully develop.
Potential Solution
A pet shaver cannot make mouse back skin to get rid of fur during the anagen phase in hair cycle, which prevents the penetration of IMQ cream and induction of psoriasis-like skin lesions. Hence, make sure that the fur within the area of interest on the back skin can be thoroughly shaved and do not smear the cream to skin areas covered with fur.
Some anesthesia methods inhibit the development of skin lesions since the inflammation is controlled by the neuro-immuno network. We are sure that Lidocaine and Scopolamine prevent lesion formation induced by IMQ.
Other solutions include:
-
•
Make sure that appropriate mouse strains and mice at the suggested age are chosen for experiment.
-
•
Make sure that the concentration of the IMQ cream is 5% and the cream has not expired.
-
•
Make sure that the IMQ cream is not exposed to air for long and does not dehydrate before applied to the skin.
Problem 2
Low yield of cells from IMQ-treated mouse dermis.
Potential Solution
This is one of the most frequently encountered problems when working with inflamed mouse dermis, which is thickened and hardened due to increased reticular fiber generation. This problem generally originates from rough mechanical disruption or incomplete enzymatic digestion.
To overcome this, we strongly recommend Collagenase P for dermis digestion. As we have tested, Collagenase P is superior to other kinds of collagenases including Collagenase IV and dissociates inflamed dermis efficiently.
Other solutions include:
-
•
Cut the dermis to pieces as small as possible.
-
•
Prepare fresh Dermal Dissociation Buffer.
-
•
Do not stop the digestion incubation until visible debris disappears.
Problem 3
Dissociated cells with low viability.
Potential Solution
Prolonged incubation with enzyme due to inefficient digestion can result in low cell viability. To avoid this:
-
•
Try solutions above to increase digestion efficacy.
-
•
Use DMEM medium to buffer the lowering pH value during digestion.
-
•
Wash off the remaining IMQ cream before processing the skin.
Problem 4
No positive staining of certain markers by flow cytometry.
Potential Solution
-
•
Use unstained and single-stained controls to find appropriate PMT voltages and adjust compensations.
-
•
Try another antibody with a good stain index.
-
•
Try another antibody clone.
Refer to sections II and III [1], “Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition)” for specific solutions (Cossarizza et al., 2019).
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Honglin Wang (honglin.wang@sjtu.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate new datasets or codes.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81725018, 81930088, 81703118, and 81803123) and Shanghai Collaborative Innovation Center for Translational Medicine (TM201925), Innovative Research Team of High-Level Local Universities in Shanghai.
Author Contributions
F.L. and Y.S. conducted the experiments, interpreted the data, and wrote the paper; H.W. polished the paper and supervised the study.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100115.
Contributor Information
Fangzhou Lou, Email: louf@shsmu.edu.cn.
Honglin Wang, Email: honglin.wang@sjtu.edu.cn.
References
- Bezdek S., Hdnah A., Sezin T., Mousavi S., Zillikens D., Ibrahim S., Ludwig R.J., Sadik C.D. The genetic difference between C57Bl/6J and C57Bl/6N mice significantly impacts Aldara-induced psoriasiform dermatitis. Exp. Dermatol. 2017;26:349–351. doi: 10.1111/exd.13131. [DOI] [PubMed] [Google Scholar]
- Cossarizza A., Chang H.D., Radbruch A., Acs A., Adam D., Adam-Klages S., Agace W.W., Aghaeepour N., Akdis M., Allez M. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition) Eur. J. Immunol. 2019;49:1457–1973. doi: 10.1002/eji.201970107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou F., Sun Y., Xu Z., Niu L., Wang Z., Deng S., Liu Z., Zhou H., Bai J., Yin Q. Excessive polyamine generation in keratinocytes promotes self-RNA Sensing by dendritic cells in psoriasis. Immunity. 2020;53:204–216.e10. doi: 10.1016/j.immuni.2020.06.004. [DOI] [PubMed] [Google Scholar]
- Swindell W.R., Michaels K.A., Sutter A.J., Diaconu D., Fritz Y., Xing X., Sarkar M.K., Liang Y., Tsoi A., Gudjonsson J.E., Ward N.L. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med. 2017;9:24. doi: 10.1186/s13073-017-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate new datasets or codes.