Abstract
Mast cells are tissue resident allergic effector cells that drive IgE-mediated food allergies. There are several steps leading to mast cell activation in the context of allergic disease that can be targeted to prevent mast cell activation and degranulation. These include blocking IgE-FcεRI crosslinking and type 2 cytokine receptor activation; modulating cell-surface neural chemical receptors; stabilizing mast cell membranes to prevent co-localization of activating receptors; impeding intracellular signaling; and engaging cell surface inhibitory receptors. This review highlights several ITIM-containing inhibitory mast cell surface receptors that could serve as pharmaceutical targets to prevent mast cell activation and degranulation in the context of food allergy. When activated, these ITIM-containing inhibitory receptors recruit the phosphatases SHP-1, SHP-2, and/or SHIP to dephosphorylate the tyrosine kinases responsible for activation signals downstream of the IgE-FcεRI complex. We describe several members of the Ig and Ig-like inhibitory receptor and C-type lectin inhibitory receptor superfamilies. Fundamental studies exploring the behavior of these receptors within the context of experimental food allergy models are needed. A deeper understanding of how these receptors modulate mast cell-driven food allergic responses will shape future strategies to harness these inhibitory receptors to treat food allergy.
Keywords: mast cell, inhibitory receptor, ITIM, food allergy, peanut
Introduction
Mast cells are one of the critical, primary allergic effector cell types that drive IgE-mediated food allergies. These tissue resident cells contain cytoplasmic secretory granules packed with pre-formed mediators that include histamine, tumor necrosis factor (TNF)-alpha, serotonin, and multiple serine proteases including tryptase and chymase. Activated mast cells can release these granules into the surrounding tissue microenvironment in addition to synthesizing several pro-inflammatory mediators including leukotrienes, prostaglandins, chemokines, and cytokines [1]. Human mast cells (MCs) are commonly separated into two subsets, tryptase-producing mucosal MC (MCT) and connective tissue MC that express chymase and tryptase (MCCT) [2]. There is increasing recognition of MCC which express chymase, but not tryptase [3], and play a role in tumor biology [4]. Murine MCs are typically divided into connective tissue MCs or mucosal MCs depending on their anatomic location and granule contents [3]. Transcriptional profiling techniques have revealed that mast cells have unique transcriptional profiles compared to other allergic effector cells, i.e. basophils, and that the striking heterogeneity across tissue mast cell populations is driven by their tissue microenvironment [1]. Despite this, all mast cells express the high affinity IgE receptor, FcεRI, and can be involved in the development of allergic disease [5].
During sensitization to a food antigen (or allergen), B cells produce food allergen-specific IgE that later binds FcεRI on both basophils and mast cells. After subsequent exposure to that particular food, allergenic food proteins crosslink the food allergen-specific IgE bound to the FcεRI, activating sensitized basophils and mast cells, resulting in the release of pre-formed and newly synthesized mediators that drive allergic inflammation and its associated symptoms [6]. Despite decades of research, a cure for food allergies remains elusive. However, treatments for food allergy, for instance, epicutaneous, sublingual, and oral immunotherapy (OIT), are under active investigation [7,8]. Investigational OIT targeting the top eight food allergens in the US [9] and a recent, US Food and Drug Administration (FDA)-approved OIT to treat peanut allergy [10] consistently promote robust desensitization to these foods [8]. In a subset of patients with egg and peanut allergy, OIT even leads to sustained unresponsiveness, or temporary resolution of food allergy, although the durability of this limited remission is unclear [8].
Moreover, immunotherapy still carries a risk of allergic reactions due to allergen exposure, and to date, FDA-approved peanut OIT only provides protection against accidental peanut exposure. It does not cure peanut, or any other food allergy. Thus, there remains a critical need for the development and deployment of therapies that treat food allergies in addition to and aside from peanut allergy. Ideally, these therapies should target multiple food allergies at once, since 30% of those with food allergy are allergic to more than one food [11]. Furthermore, simultaneous treatment of multiple food allergies improves the quality of life for the caregivers of allergic individuals [12]. FDA-approved biologics like omalizumab and dupilumab used to treat allergic diseases like asthma, atopic dermatitis, and nasal polyposis, show promise in the mitigation of food allergy because they target critical components of Type 2 allergic inflammation, including IgE (omalizumab) and IL-4/IL-13 receptor signaling (dupilumab) [6]. But while studies are ongoing to examine a role for these biologics in treating food allergy [11,13,14], there is increasing interest in developing therapies that target the final common pathway leading to symptom development and anaphylaxis in food allergy – MC activation, degranulation, and allergic inflammatory mediator release. There are several components leading to MC activation in the context of allergic disease that can be targeted to prevent MC activation and degranulation. These include blocking IgE-FcεRI crosslinking as well as cell-surface neural chemical receptor activation and type 2 cytokine receptors; stabilizing MC membranes to prevent co-localization of activating receptors; impeding intracellular signaling; and engaging cell surface inhibitory receptors. This review will focus on engaging inhibitory cell surface receptors as a strategy to prevent MC activation and degranulation in the context of food allergy. Since rodent MC lines and murine models of experimental food allergy are valuable tools to study potential therapies, we will review inhibitory receptors on mouse and rat as well as human MCs.
Signaling Downstream of the High Affinity IgE Receptor, FcεRI
Both the human and rodent FcεRI are tetrameric immunoreceptor family members, containing alpha (α), beta (β), and 2 gamma (ɣ2) subunits [15-17]. The alpha chain binds IgE and the beta and gamma chains have immunoreceptor tyrosine activation motifs (ITAMs) that enable activation of the receptor [17]. Though beta chains are always present in murine FcεRI, they may be absent in human FcεRI. When the beta subunits are present in human FcεRI, they serve as amplifiers of activation signals [17,18].
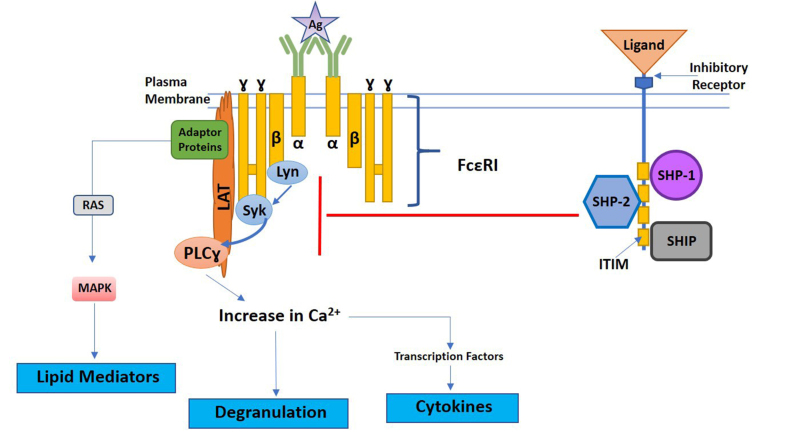
During conventional IgE-FcεRI downstream signaling, cross-linking of IgE bound to the FcεRI by antigen leads to the activation of the Src tyrosine kinase, Lyn [17,18]. Lyn phosphorylates ITAMs on beta and gamma chains of the FcεRI leading to the recruitment of the cytosolic signal transduction protein Syk to the ITAMs [17]. Syk is then phosphorylated by Lyn and via autophosphorylation, starting the downstream cascade of signaling molecules that increase intracellular calcium, activate transcription factors responsible for cytokine production, induce lipid mediator production, and cause mast cell degranulation (Figure 1) [17,18]. In addition to Lyn, downstream FcεRI signaling can be initiated by other Src kinases like Fyn, which then activates the phosphatidylinositol 3-kinase (PI3K) signaling pathway.
Figure 1.
Figure 1. Simplified diagram of FcεRI downstream signaling and inhibitory receptor phosphatase recruitment. Antigen-mediated crosslinking of IgE bound to FcεRI induces activation of FcεRI ITAMs. Activation of FcεRI ITAMs is driven by protein tyrosine kinase, Lyn, promoting the recruitment and phosphorylation of Syk. Phosphorylation of Syk induces downstream signaling responsible for mast cell degranulation, lipid mediator, and cytokine production. Engagement of inhibitory receptors by ligands inhibit FcεRI signaling through the recruitment of the phosphatases SHP-1, SHP-2, and/or SHIP. Phosphatases are recruited to inhibitory receptor ITIMs, leading to the inhibition of FcεRI downstream signaling proteins. The specific proteins inhibited can vary depending on the specific inhibitory receptor engaged. A generic inhibitory receptor is depicted above.
While Lyn functions, in part, as an initiating signal proximal to FcεRI coaggregation, it also has regulatory function in response to high or supra-optimal antigen stimulation [19,20] In addition, Lyn-deficient bone marrow derived mast cells exhibit over-exuberant degranulation when activated, and Lyn-/- mice have increased serum IgE levels, increased histamine levels, increased mast cell numbers, increased expression of FcεRI, and increased occupancy of FcεRI by IgE [21]. These are all features that predispose to allergic inflammation in murine models, suggesting that Lyn signaling can negatively regulate FcεRI signaling in the context of allergic inflammation [21].
Inhibitory Receptors on Mast Cells: Adrenergic Receptors
The classic means of inhibiting MC activation and degranulation hinges on engaging cell surface alpha and beta-adrenergic receptors which bind the catecholamine neurotransmitter epinephrine (adrenaline). For example, epinephrine bound to beta-2 adrenoreceptors block human lung MC activation in vitro [22]. This inhibitory effect can be attenuated in the presence of the mast cell growth and survival factor stem cell factor (SCF) [22] or if human lung MC are co-cultured with airway smooth muscle cells [23]. Genetic polymorphisms in the gene encoding the beta-2 adrenoreceptor can also impact the inhibitory activity of beta-2 adrenergic agonists [24]. Studies in rat peritoneal mast cells have shown that epinephrine engaging alpha-adrenergic receptors suppresses mast cell exocytosis, a critical component needed for mast cell degranulation, in a dose-dependent fashion [25]. This is the rationale behind using epinephrine autoinjectors to treat anaphylaxis and short and long acting beta-agonists in the management of allergic asthma and anaphylaxis involving bronchospasm [26]. Although epinephrine, in particular, can attenuate mast cell degranulation irrespective of whether the MC was activated via FcεRI-dependent or independent mechanisms, its effects are short-lived, given its short half-life and route of administration typically requires an injection [26]. Thus, a role for longer acting therapeutics that can engage alternative inhibitory MC surface receptors remains.
ITIM-containing Inhibitory Immune Receptors on Mast Cells
Catecholamine neurotransmitter receptors are not the only inhibitory receptors present on MCs. Constitutively expressed cell surface inhibitory immunoreceptors can also block MC activation and degranulation by negatively regulating FcεRI signaling. Inhibitory receptors have immunoreceptor tyrosine-based inhibition motifs (ITIMs) that, when activated, recruit the phosphatases SHP-1, SHP-2, and/or SHIP to dephosphorylate the tyrosine kinases responsible for downstream activation signals (Figure 1) [20,27]. Inhibitory receptors can either be engaged alone or, in some cases, coaggregated with FcεRI to block signaling downstream of FcεRI and inhibit degranulation [27]. The direct impact of ITIM-containing inhibitory receptors on allergen-induced FcεRI signaling make these molecules attractive targets for potential therapeutics for food allergy.
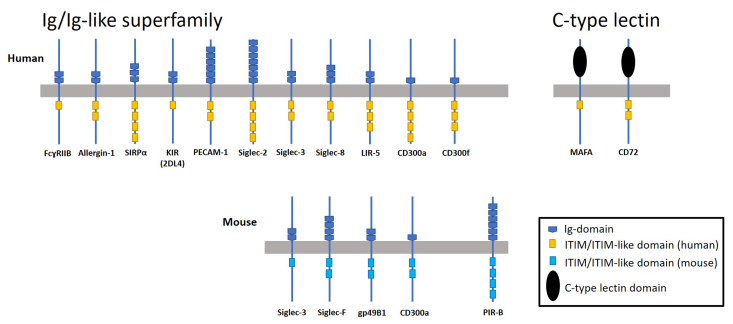
Inhibitory immunoreceptors found on human and mouse mast cells are frequently divided into two groups based on protein structure, the Ig and Ig-like receptor superfamily and the C-type lectin superfamily. Most of the inhibitory immune receptors belong to the Ig and Ig-like receptor family, including FcɣRIIB, Allergin-1, gp49B1, leukocyte Ig-like receptor (LIR)-5, paired Ig-like receptor (PIR)-B, signal regulatory protein (SIRP)α, Sialic acid binding Ig-like lectins (Siglecs), the CD300 family, Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1), and killer inhibitory receptors (KIR). The C-type lectin superfamily includes mast cell function-associated antigen (MAFA) and CD72 (Figure 2 and [27-29]). Selected members of these inhibitory receptor superfamilies on both rodent and human MCs are summarized in Table 1 and described in detail below.
Figure 2.
Figure 2. Schematic representation of ITIM and ITIM-like domains on Ig/Ig-like superfamily and C-type lectin mast cell inhibitory receptors. Human and mouse ITIM-containing receptors depicted are FcɣRIIB, Allergin-1, SIRPα, KIR2DL4, PECAM-1, Siglec -2, -3, -8/F, LIR-5/gp49B1, CD300a, CD300f, PIR-B, MAFA, and CD72. The number of ITIM and Ig/or C-type lectin domains are specifically depicted if different between humans and mice.
Table 1. Summary of human and mouse mast cell ITIM-containing receptors.
| Receptors | Species | ITIM or ITIM-like domains | Phosphatases critical for inhibitory signaling | Ligands | References |
| FcɣRIIB | H, M | 1 | SHIP | IgG | [30-34] |
| Allergin-1 | H, M | 2 | SHP-1 | Unknown | [47] |
| Gp49B1 | H (LIR-5), M | 3, 2* | SHP-1 | Integrin αvβ3 | [52] |
| PIR-B | M | 4* | SHP/SHIP independent | MHC-I | [58] |
| SIRPα | H, M | 4 | SHP-2 | CD47 | [60,61] |
| Siglec-2 | H, M | 4 | SHP-1 | Sialic acid | [64] |
| Siglec-3 | H, M | 2, 1* | SHP-1 | Sialic acid | [63] |
| Siglec-8 | H, M (Siglec-F) | 2 | SHP-1 | Sialic acid | [63] |
| CD300a | H, M | 4, 2* | SHP-1, SHIP | Phosphatidylethanolamine and Phosphatidylserine | [76,77] |
| CD300f | H, M | 3 | SHP-1, SHP-2 | Ceramide and sphingomyelin | [83,84] |
| PECAM-1 | H, M | 2 | SHP-2 | PECAM-1, Integrin αvβ3, CD38, and CD177 | [89,92] |
| KIR2DL4 | H | 1 | SHP-2 | HLA-G | [33] |
| CD72 | H, M | 2 | SHP-1, Cbl-b** | CD100/Semaphorin 4D | [96,97] |
| MAFA | H | 1 | SHP-2, SHIP | Unknown | [98] |
*indicative of number of ITIM or ITIM-like domains for mouse receptor if different from human. **Cbl-b is a ubiquitin ligase also important for inhibitory signaling of CD72. H=Human, M=Mouse
Ig Receptor FcɣRIIB
FcɣRIIB is a low affinity receptor for IgG studied extensively in the context of mast cell inhibition. FcɣRIIB driven inhibition of MC activation occurs through the recruitment of SHIP to the FcɣRIIB ITIM [30-34]. Crosslinking of FcεRI with FcɣRIIB leads to inhibition of MC activation in RBL-2H3 cells transfected with murine FcɣRIIB and in murine MCs [35]. Multiple studies have shown that interactions between antigen-specific IgG antibodies and FcɣRIIB can inhibit MC degranulation [36,37]. Fusion proteins and bispecific antibodies have been created to coaggregate FcεRI and FcɣRIIB for inhibition of MCs [16,38-40]. For instance, Zhu et al. created a bifunctional human fusion protein (GE2) targeted toward Fcɣ and Fcε receptors, to examine effects of simultaneously targeting and juxtaposing FcɣRIIB and FcεRI in human basophils and mouse MCs from transgenic mice engineered to express the human FcεRI [16]. Their chimeric protein complexed with both FcɣRIIB and FcεRI, blunting MC and basophil function. It showed dose and time-dependent inhibition of antigen-specific IgE-driven histamine production with modified Syk signaling in human basophils and muted antigen-specific IgE-mediated passive cutaneous anaphylaxis (PCA) in human FcεRI transgenic mice [16]. They later showed that even when the fusion protein was deployed after human blood basophils and human lung mast cells had been sensitized, it could inhibit IgE-mediated responses in these cells [41].
Studies using murine bone marrow-derived MCs have shown that supra-optimal antigen stimulation of MCs, with associated supra-optimal FcεRI cross-linking, actually triggers antigen/IgE-dependent and independent co-localization of FcɣRIIB and FcεRI, dampening MC activation [30]. In addition, a candidate vaccine engineered to induce protective immune responses against peanut allergy in a mouse model of peanut allergy was found to protect against peanut challenge in sensitized mice by generating peanut allergen-specific IgG that engaged FcɣRIIB [42]. Notably, expression of FcɣRIIB is tissue specific; primary human skin MCs do not express FcɣRIIB while gastrointestinal MCs do [43]. This may mean that therapeutic targeting of FcɣRIIB could have differential impact on symptom development and resolution in food allergy, depending on the organ most significantly affected.
Ig-like Superfamily Inhibitory Receptors
Allergin-1. Allergy-inhibitory receptor (Allergin)-1 is expressed in both human and mouse mast cells [44]. Unlike mice, humans express 3 isoforms of Allergin-1: Allergin-1L, Allergin-1S1, and Allergin-1S2, with Allergin-1S1 being the main form expressed on human mast cells [44]. Co-ligation of FcεRI with Allergin-1 on primary human mast cells [44] or with transfected Allergin-1 expressed in a rat basophil leukemia mast cell line (RBL-2H3) [45] inhibits IgE-mediated degranulation. Mice lacking Allergin-1 have increased allergic responses in both PCA and passive systemic anaphylaxis (PSA) models [45]. Recent studies have shown the importance of Allergin-1, not only on MCs, but also basophils, in the inhibition of IgE-mediated food allergic responses—with specific influence on anaphylaxis [46]. In fact, Allergin-1 on bone marrow derived dendritic cells has been shown to suppress a mouse model of house dust mite-induced allergic airway inflammation through inhibition of Syk via SHP-1 [47]. Extending these studies to explore the role of Allergin-1 on lung resident mast cells in the house dust mite-induced airway inflammation model and in MCs within different murine food allergy models (peanut, egg, milk, etc.) will provide important data on Allergin-1 action within MCs that could shape future therapeutics targeting this receptor.
Gp49B1. Gp49B1 is a transmembrane mouse receptor within the gp49 family that contains two ITIMs [48]. Co-ligation of gp49B1with FcεRI inhibited degranulation of mouse MCs [49]. Castells et al. later identified αvβ3 as a ligand for gp49B1 and demonstrated that binding of αvβ3 to gp49B1 also led to inhibition of antigen-induced IgE mediated MC degranulation [50]. Gp49B1 also suppressed SCF-induced MC degranulation and associated tissue swelling. Intradermal injection of SCF into gp49B1-deficient mice resulted in four times more degranulating MCs and twice the amount of tissue swelling than in wildtype mice [51]. Gp49B1 recruits SHP-1 phosphatase, modulating downstream calcium release to induce inhibitory effects [52]. The human homologue of gp49B1 appears to be Leukocyte Ig-like receptor (LIR)-5 (also known as HM18, ILT3, LILRB4, CD85k, and LILB1-5) [53]. LIR-5 is expressed intracellularly in human MC, migrating to the cell surface after FcεRI crosslinking [54]. LIR-5 expression in dendritic cells marks them as tolerogenic in healthy individuals [55]. Its expression in myeloid-derived suppressor cells helps drive immune tolerance in cancer [55,56]. To date, however, the impact of MC-expressed LIR-5 in the severity of symptoms and development of anaphylaxis in food allergy remains unknown.
PIR-B. Paired immunoglobulin (Ig)-like receptor (PIR)-B is a glycoprotein with 4 ITIMS expressed on the cell surface of mouse mast cells, and closely related to gp49B1 and the human LIR family of receptors [57]. PIR-B is constitutively tyrosine phosphorylated and predominantly expressed on murine mast cells compared to its activating isoform, PIR-A [57,58]. Co-ligation of FcεRI and PIR-B leads to inhibition of IgE-mediated serotonin release [58], while co-ligation of c-kit and PIR-B inhibits calcium mobilization [57]. Interestingly, mouse bone marrow-derived MC PIR-B did not require SHP-1 for inhibitory function, setting it apart from PIR-B inhibitory receptors observed in other cell types [57,58]. Uehara et al. found that one of the 4 ITIMs responsible for PIR-B inhibitory function did not bind to the SHP-1, SHP-2, or SHIP phosphatases traditionally associated with the inhibitory effects of ITIMs. Thus, MC PIR-B has the potential to inhibit MC activation in a SHP/SHIP-independent fashion [58]. Similar inhibition through PIR-B and FcεRI co-ligation was also observed in RBL-2H3 mast cells with chimeric receptors, where the 3rd and 4th ITIMs were critical for inhibitory function of IgE mediated calcium mobilization and degranulation [59].
SIRPα. Signal regulatory protein α (SIRPα), a Src homology-containing receptor phosphotyrosine phosphatase found on human and rodent MCs and other innate immune cells, possesses two conventional ITIMs and two ITIM-like domains [60]. Coaggregation of SIRPα with FcεRI on RBL-2H3 MCs leads to inhibition of IgE-mediated responses, through a reduction in calcium, decreased phosphorylation of FcεRI ITAMs, and loss of MAPK activation through recruitment of SHP-1 and SHP-2 [60]. Pan and colleagues found that murine MC activation reduced expression of SIRPα. Knocking down SIRPα in these cells in vitro enhanced IgE- FcεRI-mediated allergic (IL-4, IL-13) and inflammatory (IL-6, TNF-alpha) cytokine production. Intradermal ear injection of SIRPα-deficient MCs sensitized with dinitrophenyl (DNP)-specific IgE into MC-deficient mice followed by intravenous challenge with DNP heightened the allergic response in this PCA murine model compared to intradermal injection of sensitized SIRPα-wildtype MCs [61]. SIRPα negatively regulated FcεRI signaling by sequestering SHP-2 phosphatase from a PI3K regulatory subunit, resulting in impaired PI3K activation and blunted IgE-FcεRI-mediated responses [61].
Siglecs. Sialic acid-binding immunoglobulin-like lectins (Siglecs) are type 1 transmembrane proteins expressed on multiple immune cells that contain an N-terminal sialic acid binding domain [62,63]. There are 15 known Siglecs in human and nine in mice [62]. In humans, Siglecs-2, 3 (also known as CD22 and CD33, respectively) and 5-12 are considered inhibitory as they contain ITIMs. When Siglecs bind to sialic acid moieties on the ends of glycoproteins and glycolipids of the same cell or different cells, this alters the spatial location of Siglecs relative to transmembrane immune receptors, facilitating the immunomodulatory effects of these receptors [64]. CD22 (Siglec-2), CD33 (Siglec-3), and Siglecs-5 through 10 have been observed at varying levels of expression on human mast cells [64-68], with Siglec-7 and 8 most consistently associated with human mast cells [64].
When inhibitory Siglecs are brought in close proximity to the IgE-FcεRI signaling complex on MCs, they can also reduce MC activation. When Siglec-7 and Siglec-9 stably transfected into the MC RBL-2H3 cell line were crosslinked to the FcεRI, this inhibited serotonin release by RBL-2H3 cells [69]. Recently, Duan et al. highlighted the role of CD33 in inhibiting IgE-mediated activation of human and murine MCs. Using liposomes containing the ligand for CD33 and the model allergen trinitrophenyl (TNP), Duan and colleagues targeted TNP-specific IgE-FcεRI complexes on MCs, forcing proximity between the inhibitory CD33 and FcεRI to promote inhibition [68].
Unlike its companion Siglecs described above, Siglec-8 does not require co-ligation or other means of forced ligation with FcεRI to exert its inhibitory effects on MC activation [63]. Yokoi et al. showed that while Siglec-8 engagement on eosinophils resulted in eosinophil apoptosis, Siglec-8 engagement on human mast cells did not induce apoptosis. Rather, it blocked FcεRI-dependent histamine and prostaglandin release as well as calcium flux in MCs, and prevented anti-IgE-induced human bronchial ring contraction in a model of allergic asthma [63]. Experiments employing RBL-2H3 cells transfected with human Siglec-8 confirmed that Siglec-8-induced inhibition of calcium flux and MC degranulation depended on a functional membrane-proximal ITIM domain [63]. Kerr and colleagues used gene expression profiling and flow cytometry to demonstrate that AK002, a humanized monoclonal antibody specifically targeting Siglec-8, could decrease sputum eosinophil counts and inhibit FcεRI-activated lung mast cells [70]. This antibody also blocked MC signaling pathways and MC activation in a model of non-allergic airway inflammation [71]. Anti-Siglec-8 monoclonal antibody also effectively blocked MC activation during passive systemic anaphylaxis in a humanized mouse strain that produces mature Siglec-8-expressing MC populations [72].
Engaging and activating Siglecs expressed on immune cells involved in food allergy, aside from MCs, has shown promise as a potential means of treating type-2 allergic inflammation driven by food. In a mouse model of oral egg allergen-induced eosinophilic intestinal inflammation, Dong et al. showed that activating Siglec-F (the mouse equivalent of human Siglec-8) with an anti-Siglec-F antibody blunted intestinal eosinophilic inflammation, thus improving the integrity of the intestinal barrier [73]. It also reduced Th2 cytokine and IgE levels in mice with egg ovalbumin-induced eosinophilic inflammation, reducing their concomitant diarrhea and weight loss [73]. Siglec-engaging Tolerance-inducing Antigenic Liposomes (STALs) created with a high affinity inhibitory Siglec ligand juxtaposed to an antigen or immune-modulating therapeutic of choice can be used to induce tolerance in murine models of allergy and autoimmune diseases by enhancing the co-localization of the inhibitory Siglec with the specific antigen-receptor or drug-binding protein [74,75]. Orgel and colleagues deployed STALs that incorporated a ligand for B-cell expressed CD22 (Siglec-2) adjacent to Ara h2, the predominant allergic epitope in peanut allergy. In a murine model of peanut allergy, Orgel et al. showed that targeting both a Siglec and a food-antigen specific receptor prevented sensitization to Ara h2 and significantly blunted the severity of allergic reactions to whole peanut extract [74].
Targeting Siglecs on B cells and eosinophils has shown promise as a therapeutic approach in mouse models of food-induced allergic inflammation, while targeting Siglecs on MCs has proven effective in reducing MC activation in both allergic and non-allergic airway inflammation models. Given the variety of tools under active investigation, from liposomes to monoclonal antibodies, engineered to engage Siglecs, this particular class of ITIM-containing inhibitory receptors appears to have the greatest promise as a potential target in treating food allergy. Future studies should target Siglecs highly expressed on MCs, especially Siglec-8, in the context of in vivo food allergy models, taking advantage of both STALs technology and the Siglec-8-specific monoclonal antibody.
CD300 family. CD300 multigene family members are type I transmembrane proteins belonging to the Ig-superfamily inhibitory receptors. Genes encoding for these receptors are located on chromosome 17 in humans and chromosome 11 in mice. CD300a and CD300f possess long cytoplasmic tails containing ITIMs making them the only inhibitory receptors within the family [76]. As with other ITIM-containing inhibitory receptors, the suppressive functions of CD300a and CD300f are linked to the recruitment of SHP-1 and SHIP to their ITIMs. Both receptors are expressed on several immune cells, including allergic effector cells like MCs, basophils, and eosinophils [76]. Bachelet et al. showed that CD300a is constitutively expressed on human MCs; its expression could be decreased in vitro with exposure to eosinophil-derived major basic protein and eosinophil-derived neurotoxin [77]. Immune complex-mediated crosslinking of CD300a on human MCs inhibited IgE-mediated activation. Neutralization of the murine homolog of CD300a enhanced allergic inflammatory mediator release in a model of allergic peritonitis [77]. Co-ligation of CD300a and FcεRI through simultaneous engagement of CD300a and IgE also inhibited MC activation and associated kinase phosphorylation in human and mouse MCs [78]. Since the identification of CD300a natural ligands, phosphatidylserine (PS) and phosphatidylethanolamine (PE), studies have examined the impact of these natural CD300a ligands on mast cell regulation. Both PS and PE are present on the outer portion of cell plasma membranes of infected, transformed, apoptotic, or activated cells [76]. Using mouse bone marrow derived mast cell cultures, Wang et al. showed that cis binding of PS exposed on the MC outer membrane during degranulation with CD300a is important for self-regulation of murine MC degranulation [79]. Moreover, mice deficient in CD300a took longer to recover from anaphylaxis in a PSA model [79].
CD300a shares 80% homology extracellularly with family member CD300c, an activating receptor. Both receptors also bind PS and PE [76]. Thus, future investigations should explore whether or not cross-reactive binding occurs between the two receptors. Some studies have linked upregulation of CD300a expression with clinical allergy. Sabato et al. showed that CD300a expression is upregulated after crosslinking of the FcεRI and is increased in basophils of birch pollen allergic individuals compared to healthy controls [80]. Interestingly, investigations of the ratios of CD300a compared to CD300c expressed on human basophils also highlight potential use of these receptors as biomarkers for allergy. Zenarruzabeitia et al. found that lower ratios of CD300a to CD300c correlated with increased hypersensitivity in cow’s milk allergy in children [81]. However, Larsen and colleagues found no difference in CD300a expression in peripheral blood derived mast cells from peanut allergic individuals compared to controls. These authors did not report on CD300c expression [82]. Thus, whether similar expression patterns in the ratio of CD300a to CD300c exist in mast cells from food allergic patients remains unknown. Future studies should harness the existing murine models of food allergy to characterize CD300a and CD300c expression patterns on MCs across different tissue sites and across different food allergens.
CD300f has two ITIMs and one immunoreceptor tyrosine-based switch motif that can recruit SHP-1 to induce inhibitory effects in mouse [83] and human [84] MCs. The natural ligand for mouse CD300f is ceramide while both ceramide and sphingomyelin serve as ligands for human CD300f [85]. These lipid ligands can inhibit IgE-mediated activation of MCs upon binding to the receptor in the presence of FcεRI engagement [83,84]. Mice deficient in CD300f show increased allergic responses in PSA and PCA models [83]. Moreover, when a mouse model of ovalbumin-induced IgE- and MC-dependent food allergy was established in CD300f-deficient mice and compared to wildtype mice, these CD300f-deficient mice developed higher levels of total and ovalbumin-specific IgE, and higher levels of mucosal mast cell protease-1, a marker of mucosal MC activation [86]. Using a different food allergy model in the CD300f-deficient mice, the authors were able to show enhanced clinical food-allergic responses, represented by larger drops in core body temperature upon allergen challenge [86]. When ceramide-CD300f binding was disrupted in wildtype mice used in the ovalbumin-food allergy model, the frequency of ovalbumin-induced allergic diarrhea increased [86]. In sum, CD300f appears to be critical in the regulation of MC-driven allergic responses in this mouse model. This suggests that pharmacologic engagement of CD300f could be used to blunt allergic symptoms in food allergy. Manipulation of the CD300f inhibitory pathway must proceed with caution, however, since CD300f has been shown to transmit activating signals via PI3K-binding motifs and the growth factor receptor-bound protein 2 (Grb2) [76,87].
PECAM-1. Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) is a cell adhesion molecule linked to the transmigration of cultured bone marrow derived MCs across skin endothelial cells lining skin vessel walls [88]. Its natural ligands include PECAM-1 itself, integrin αvβ3, CD38, and CD177 [89]. The ability of PECAM-1 to modulate cell motility depends on the interaction of its ITIM domain with SHP-2 [90]. The presence of this ITIM also explains its status as an inhibitory receptor. Polymorphisms in the gene encoding PECAM-1 have been associated with increased risk of asthma [91], although it is not clear whether MCs or other leukocyte populations are differentially activated. The absence of PECAM-1 in mice increased IgE-mediated allergic responses in PSA and PCA models. MCs from PECAM-1-deficient mice also showed increased MC mediator release in comparison to MCs from wild type mice after FcεRI activation [92]. However, since PECAM-1 is used to facilitate the trafficking of multiple leukocyte populations [93], it may prove a challenging target to inhibit MC activation in food allergy.
KIRs. Killer cell inhibitory receptors (KIR) for MHC Class I are type I transmembrane proteins expressed primarily by innate natural killer (NK) and adaptive T lymphocytes. However, when Blery and colleagues reconstituted KIR function in the non-lymphoid MC RBL-2H3 cell line through transfection, they demonstrated that KIR required co-ligation with the ITAM-dependent activating FcεRI receptor in order to exert its inhibitory effect. Moreover, it used an inhibitory pathway distinct from FcɣRIIB, blocking not only extracellular calcium entry, but also extracellular calcium release from the endoplasmic reticulum [34]. Recently, others have shown endogenous expression of KIR2DL4 receptor in the LAD2 human MC line, on cultured human MCs derived from the peripheral blood of healthy individuals, and on human tissue MCs [33,94]. Ueshima et al. found that KIR2DL4 inhibits IgE-mediated responses in human mast cells through the recruitment of SHP-2 [33]. While the interaction of KIR2DL4 with its natural ligand human leukocyte antigen (HLA)-G has been explored in the context of anti-tumor immunity and pregnancy [95], a possible role for the HLA-G-KIR2DL4 interaction in mitigating MC activation in food allergy remains to be examined.
C-type Lectins
CD72. A 45 kilo Dalton type II transmembrane protein, CD72 (Lyb-2) is an ITIM-containing inhibitory receptor whose natural ligand is CD100 or Semaphorin 4D (Sema4D) [96]. Kataoka and colleagues showed that CD72 is expressed on human MCs generated from CD34+ peripheral blood cells of healthy human volunteers. The authors concurrently stimulated CD72 with CD100 or an agonistic anti-CD72 antibody and the canonical MC receptor KIT (whose signals are critical for MC growth and survival), with stem cell factor (SCF). This resulted in inhibition of KIT-triggered phosphorylation of Src family kinases and extracellular-regulated kinases. KIT-driven mast cell motility, proliferation, and chemokine generation were also significantly impaired [96]. Engaging CD72 on mouse bone marrow derived-MCs suppressed IgE-FcεRI-mediated degranulation in addition to cell surface KIT and FcεRI-expression [97]. By contrast, activation of CD72 on human mast cells did not impair IgE-FcεRI-driven MC degranulation, only KIT-mediated signaling [97]. This species difference suggest that even if future murine food allergy models explore CD72 activation as a strategy to inhibit MC activation and improve symptoms, this may not be directly applicable to IgE-mediated food allergy in humans.
MAFA. Mast cell function-associated antigen (MAFA) is a type II membrane glycoprotein originally discovered on the rat mucosal-type mast cell line RBL-2H3 [98,99]. Like CD72, it has an extracellular C-type lectin domain that, when engaged, can inhibit FcεRI activation via Lyn-mediated phosphorylation of its ITIM domain with subsequent SHIP and SHP phosphatase recruitment to the plasma membrane to disperse the inhibitory signals [98]. Time-resolved phosphorescence anisotropy and fluorescence resonance energy transfer studies suggest that MAFA can associate with isolated or clustered FcεRIs [100]. Interestingly, MAFA does not require co-ligation with FcεRI to prevent activation [98]. Nevertheless, MAFA-FcεRI co-clusters are more effective at inhibiting MC degranulation than MAFA clusters alone [101]. Murine (KLRG1) and human (MAFA-L) homologs of MAFA have been described. However, the murine homolog is actually expressed on NK cells, while the human MAFA-L is expressed on MCs, but is not exclusive to this immune cell type [98]. MAFA is a far more challenging inhibitory receptor target in the development of therapeutics for food allergy. The complete absence of MAFA homolog expression on murine MCs hampers the ability to study the utility of targeting this receptor in the context of a murine food allergy model. Moreover, human MAFA-L expression across different immune cell types could increase off-target effects of any pharmaceutical directed at this receptor.
Conclusions and Outlook
Inhibitory receptors play a role in the natural regulation of FcεRI activation on mast cells and represent attractive targets for the development of therapeutics used to treat pathologic, mast cell-driven, food allergic responses. Indeed, a recent clinical trial demonstrating the safety and efficacy of an anti-Siglec-8 antibody for eosinophilic gastritis and duodenitis, gastrointestinal conditions characterized by elevated mast cell numbers in addition to eosinophilia [102], suggest that engaging this particular ITIM-containing inhibitory receptor may hold the most promise in blocking mast cell activation that promotes food allergic symptoms. Reliable biomarkers for diagnosing and monitoring the evolution of a food allergy may arise from studying changes in the surface expression levels of various inhibitory receptors in the context of food allergy. Engaging inhibitory receptors to treat food allergy could eliminate the need for allergen specific therapy, providing protection for those with multiple allergies. Activating inhibitory receptors in conjunction with oral immunotherapy could enhance the efficacy of this treatment while reducing side effects. For many of these inhibitory receptors, fundamental studies exploring the behavior of these receptors within the context of experimental food allergy models are clearly needed. Clinical and translational studies that characterize shifts in inhibitory receptor expression over the course of allergen-specific immunotherapy could also inform our understanding of the role for these receptors in desensitization or the induction of sustained unresponsiveness in food-allergic individuals. Clarifying how these receptors modulate food allergic responses will shape the strategies used to target them (natural ligands vs. agonistic monoclonal antibodies vs. small molecule agonists). Pharmaceuticals that engage and activate mast cell inhibitory receptors may serve as a novel approach to food allergy management that brings us closer to a cure.
Acknowledgments
We thank Dr. Johanna Smeekens for critical review of this manuscript. Jada Suber is supported by the Initiative for Maximizing Student Development (NIH grant R25GM055336) and the UNC Food Allergy Initiative. Onyinye Iweala is supported by NIH grant K08AI141691 and a 2020 AAAAI Foundation Career Development Award. The funders had no role in the analysis, decision to prepare or publish the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- FcεRI
Fc Epsilon Receptor I, the high affinity IgE receptor
- FDA
US Food and Drug Administration
- Ig
immunoglobulin
- ITAM
immunoreceptor tyrosine activation motifs
- ITIM
immunoreceptor tyrosine-based inhibition motifs
- KIR
Killer cell inhibitory receptors
- LIR
leukocyte Ig-like Receptor
- MAFA
mast cell function-associated antigen
- MC
mast cell
- IgE
immunoglobulin E
- MCT
tryptase-producing mast cell
- MCC
chymase-producing mast cell
- MCCT
chymase- and tryptase-producing mast cell
- NK
natural killer
- OIT
oral immunotherapy
- PCA
passive cutaneous anaphylaxis
- PI3K
phosphatidylinositol 3-kinase
- PECAM-1
Platelet Endothelial Cell Adhesion Molecule-1
- PSA
passive systemic anaphylaxis
- RBL
rat basophil leukemia
- SCF
stem cell factor
- SHP
Src homology 2 domains containing protein tyrosine phosphatase
- SHIP
SH2-contaning inositol 5’ phosphatase
- Siglec
Sialic acid-binding immunoglobulin-like lectins
- TNF
tumor necrosis factor
Author Contributions
JS: Conception and design; Writing; Review; Editing. OII: Conception and design; Writing; Review; Editing.
References
- Dwyer DF, Barrett NA, Austen KF, Immunological Genome Project Consortium . Expression profiling of constitutive mast cells reveals a unique identity within the immune system. Nat Immunol. 2016. July;17(7):878–87. 10.1038/ni.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN, Wood JG. Mast Cell: A Multi-Functional Master Cell. Front Immunol. 2016. January;6:620. 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res. 2020;9. doi: 10.12688/f1000research.22037.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komi DE, Redegeld FA. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin Rev Allergy Immunol. 2020. June;58(3):313–25. 10.1007/s12016-019-08753-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012. May;18(5):693–704. 10.1038/nm.2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iweala OI, Nagler CR. The Microbiome and Food Allergy. Annu Rev Immunol. 2019. April;37(1):377–403. 10.1146/annurev-immunol-042718-041621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron J, Kim EH. Sublingual and Patch Immunotherapy for Food Allergy. Immunol Allergy Clin North Am. 2020. February;40(1):135–48. 10.1016/j.iac.2019.09.008 [DOI] [PubMed] [Google Scholar]
- Kim EH, Burks AW. Food allergy immunotherapy: oral immunotherapy and epicutaneous immunotherapy. Allergy. 2020. June;75(6):1337–46. 10.1111/all.14220 [DOI] [PubMed] [Google Scholar]
- Eapen AA, Lavery WJ, Siddiqui JS, Lierl MB. Oral immunotherapy for multiple foods in a pediatric allergy clinic setting. Ann Allergy Asthma Immunol. 2019. December;123(6):573–581.e3. 10.1016/J.ANAI.2019.08.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala M, Shih J, Lee G, Vickery B. Peanut Oral Immunotherapy: a Current Perspective. Curr Allergy Asthma Rep. 2020. April;20(5):14. 10.1007/s11882-020-00908-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2018. February;3(2):85–94. 10.1016/S2468-1253(17)30392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani IM, Bégin P, Kearney C, Dominguez TL, Mehrotra A, Bacal LR, et al. Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol. 2014. May;10(1):25. 10.1186/1710-1492-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A, Lavergne MH, Leroux H, Killer K, Azzano P, Paradis L, et al. Protocol for a double-blind, randomized controlled trial on the dose-related efficacy of omalizumab in multi-food oral immunotherapy. Allergy Asthma Clin Immunol. 2020. April;16(1):25. 10.1186/s13223-020-00419-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long A, Borro M, Sampath V, Chinthrajah RS. New Developments in Non-allergen-specific Therapy for the Treatment of Food Allergy. Curr Allergy Asthma Rep. 2020. January;20(1):3. 10.1007/s11882-020-0897-8 [DOI] [PubMed] [Google Scholar]
- Ra C, Jouvin MH, Kinet JP. Complete structure of the mouse mast cell receptor for IgE (Fc epsilon RI) and surface expression of chimeric receptors (rat-mouse-human) on transfected cells. J Biol Chem. 1989. September;264(26):15323–7. [PubMed] [Google Scholar]
- Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002. May;8(5):518–21. 10.1038/nm0502-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010. December;584(24):4933–40. 10.1016/j.febslet.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner H, Kinet JP. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature. 1999. November;402(6760 Suppl):B24–30. 10.1038/35037021 [DOI] [PubMed] [Google Scholar]
- Xiao W, Nishimoto H, Hong H, Kitaura J, Nunomura S, Maeda-Yamamoto M, et al. Positive and negative regulation of mast cell activation by Lyn via the FcepsilonRI. J Immunol. 2005;175:6885-92. doi: 10.4049/jimmunol.175.10.6885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Gibbs BF. SHIP1 and the negative control of mast cell/basophil activation by supra-optimal antigen concentrations. Mol Immunol. 2015. January;63(1):32–7. 10.1016/j.molimm.2014.02.017 [DOI] [PubMed] [Google Scholar]
- Odom S, Gomez G, Kovarova M, Furumoto Y, Ryan JJ, Wright HV, et al. Negative regulation of immunoglobulin E-dependent allergic responses by Lyn kinase. J Exp Med. 2004. June;199(11):1491–502. 10.1084/jem.20040382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse G, Yang W, Duffy SM, Chachi L, Leyland M, Amrani Y, et al. Counterregulation of beta(2)-adrenoceptor function in human mast cells by stem cell factor. J Allergy Clin Immunol. 2010;125(1):257-63 e1-5. doi: 10.1016/j.jaci.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Lewis RJ, Chachi L, Newby C, Amrani Y, Bradding P. Bidirectional Counterregulation of Human Lung Mast Cell and Airway Smooth Muscle β2 Adrenoceptors. J Immunol. 2016. January;196(1):55–63. 10.4049/jimmunol.1402232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LJ, Rostami-Hodjegan A, Suvarna SK, Peachell PT. Influence of beta2-adrenoceptor gene polymorphisms on beta2-adrenoceptor-mediated responses in human lung mast cells. Br J Pharmacol. 2007. October;152(3):323–31. 10.1038/sj.bjp.0707400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe N, Toyama H, Ejima Y, Saito K, Tamada T, Yamauchi M, et al. α1-Adrenergic Receptor Blockade by Prazosin Synergistically Stabilizes Rat Peritoneal Mast Cells. BioMed Res Int. 2020. May;2020:3214186. 10.1155/2020/3214186 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- LoVerde D, Iweala OI, Eginli A, Krishnaswamy G. Anaphylaxis. Chest. 2018. February;153(2):528–43. 10.1016/j.chest.2017.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000. September;106(3):429–40. 10.1067/mai.2000.109428 [DOI] [PubMed] [Google Scholar]
- Katz HR. Inhibitory receptors and allergy. Curr Opin Immunol. 2002. December;14(6):698–704. 10.1016/s0952-7915(02)00400-4 [DOI] [PubMed] [Google Scholar]
- Li L, Yao Z. Mast cell and immune inhibitory receptors. Cell Mol Immunol. 2004. December;1(6):408–15. [PubMed] [Google Scholar]
- Gast M, Preisinger C, Nimmerjahn F, Huber M. IgG-Independent Co-aggregation of FcεRI and FcγRIIB Results in LYN- and SHIP1-Dependent Tyrosine Phosphorylation of FcγRIIB in Murine Bone Marrow-Derived Mast Cells. Front Immunol. 2018. August;9:1937. 10.3389/fimmu.2018.01937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbec O, Fong DC, Turner M, Tybulewicz VL, Cambier JC, Fridman WH, et al. Fc ε receptor I-associated lyn-dependent phosphorylation of Fc γ receptor IIB during negative regulation of mast cell activation. J Immunol. 1998. February;160(4):1647–58. [PubMed] [Google Scholar]
- Kepley CL, Taghavi S, Mackay G, Zhu D, Morel PA, Zhang K, et al. Co-aggregation of FcgammaRII with FcepsilonRI on human mast cells inhibits antigen-induced secretion and involves SHIP-Grb2-Dok complexes. J Biol Chem. 2004. August;279(34):35139–49. 10.1074/jbc.M404318200 [DOI] [PubMed] [Google Scholar]
- Ueshima C, Kataoka TR, Hirata M, Furuhata A, Suzuki E, Toi M, et al. The Killer Cell Ig-like Receptor 2DL4 Expression in Human Mast Cells and Its Potential Role in Breast Cancer Invasion. Cancer Immunol Res. 2015. August;3(8):871–80. 10.1158/2326-6066.CIR-14-0199 [DOI] [PubMed] [Google Scholar]
- Bléry M, Delon J, Trautmann A, Cambiaggi A, Olcese L, Biassoni R, et al. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem. 1997. April;272(14):8989–96. 10.1074/jbc.272.14.8989 [DOI] [PubMed] [Google Scholar]
- Daëron M, Malbec O, Latour S, Arock M, Fridman WH. Regulation of high-affinity IgE receptor-mediated mast cell activation by murine low-affinity IgG receptors. J Clin Invest. 1995. February;95(2):577–85. 10.1172/JCI117701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uermösi C, Zabel F, Manolova V, Bauer M, Beerli RR, Senti G, et al. IgG-mediated down-regulation of IgE bound to mast cells: a potential novel mechanism of allergen-specific desensitization. Allergy. 2014. March;69(3):338–47. 10.1111/all.12327 [DOI] [PubMed] [Google Scholar]
- Burton OT, Tamayo JM, Stranks AJ, Koleoglou KJ, Oettgen HC. Allergen-specific IgG antibody signaling through FcγRIIb promotes food tolerance. J Allergy Clin Immunol. 2018. January;141(1):189–201.e3. 10.1016/J.JACI.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertsching E, Bafetti L, Hess H, Perper S, Giza K, Allen LC, et al. A mouse Fcgamma-Fcepsilon protein that inhibits mast cells through activation of FcgammaRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J Allergy Clin Immunol. 2008. February;121(2):441–447.e5. 10.1016/j.jaci.2007.08.051 [DOI] [PubMed] [Google Scholar]
- Cemerski S, Chu SY, Moore GL, Muchhal US, Desjarlais JR, Szymkowski DE. Suppression of mast cell degranulation through a dual-targeting tandem IgE-IgG Fc domain biologic engineered to bind with high affinity to FcγRIIb. Immunol Lett. 2012. March;143(1):34–43. 10.1016/J.IMLET.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Tam SW, Demissie S, Thomas D, Daëron M. A bispecific antibody against human IgE and human FcgammaRII that inhibits antigen-induced histamine release by human mast cells and basophils. Allergy. 2004. July;59(7):772–80. 10.1111/j.1398-9995.2004.00332.x [DOI] [PubMed] [Google Scholar]
- Zhang K, Kepley CL, Terada T, Zhu D, Perez H, Saxon A. Inhibition of allergen-specific IgE reactivity by a human Ig Fcgamma-Fcepsilon bifunctional fusion protein. J Allergy Clin Immunol. 2004. August;114(2):321–7. 10.1016/j.jaci.2004.03.058 [DOI] [PubMed] [Google Scholar]
- Storni F, Zeltins A, Balke I, Heath MD, Kramer MF, Skinner MA, et al. Vaccine against peanut allergy based on engineered virus-like particles displaying single major peanut allergens. J Allergy Clin Immunol. 2020. April;145(4):1240–1253.e3. 10.1016/j.jaci.2019.12.007 [DOI] [PubMed] [Google Scholar]
- Burton OT, Epp A, Fanny ME, Miller SJ, Stranks AJ, Teague JE, et al. Tissue-Specific Expression of the Low-Affinity IgG Receptor, FcγRIIb, on Human Mast Cells. Front Immunol. 2018. June;9:1244. 10.3389/fimmu.2018.01244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Tahara-Hanaoka S, Morishima Y, Tokunaga T, Imoto Y, Noguchi E, et al. Expression and function of Allergin-1 on human primary mast cells. PLoS One. 2013. October;8(10):e76160. 10.1371/journal.pone.0076160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi K, Tahara-Hanaoka S, Someya S, Fujiki A, Tada H, Sugiyama T, et al. An immunoglobulin-like receptor, Allergin-1, inhibits immunoglobulin E-mediated immediate hypersensitivity reactions. Nat Immunol. 2010. July;11(7):601–7. 10.1038/ni.1886 [DOI] [PubMed] [Google Scholar]
- Lin YH, Tahara-Hanaoka S, Nagai K, Yoshikawa S, Kubo M, Shibayama S, et al. Selective suppression of oral allergen-induced anaphylaxis by Allergin-1 on basophils in mice. Int Immunol. 2020. March;32(3):213–9. 10.1093/intimm/dxz075 [DOI] [PubMed] [Google Scholar]
- Miki H, Tahara-Hanaoka S, Almeida MS, Hitomi K, Shibagaki S, Kanemaru K, et al. Allergin-1 Immunoreceptor Suppresses House Dust Mite-Induced Allergic Airway Inflammation. J Immunol. 2020. February;204(4):753–62. 10.4049/jimmunol.1900180 [DOI] [PubMed] [Google Scholar]
- McCormick MJ, Castells MC, Austen KF, Katz HR. The gp49A gene has extensive sequence conservation with the gp49B gene and provides gp49A protein, a unique member of a large family of activating and inhibitory receptors of the immunoglobulin superfamily. Immunogenetics. 1999. December;50(5-6):286–94. 10.1007/s002510050604 [DOI] [PubMed] [Google Scholar]
- Katz HR, Vivier E, Castells MC, McCormick MJ, Chambers JM, Austen KF. Mouse mast cell gp49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when coligated with the high-affinity Fc receptor for IgE. Proc Natl Acad Sci USA. 1996. October;93(20):10809–14. 10.1073/pnas.93.20.10809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells MC, Klickstein LB, Hassani K, Cumplido JA, Lacouture ME, Austen KF, et al. gp49B1-α(v)β3 interaction inhibits antigen-induced mast cell activation. Nat Immunol. 2001. May;2(5):436–42. 10.1038/87749 [DOI] [PubMed] [Google Scholar]
- Feldweg AM, Friend DS, Zhou JS, Kanaoka Y, Daheshia M, Li L, et al. gp49B1 suppresses stem cell factor-induced mast cell activation-secretion and attendant inflammation in vivo. Eur J Immunol. 2003. August;33(8):2262–8. 10.1002/eji.200323978 [DOI] [PubMed] [Google Scholar]
- Lu-Kuo JM, Joyal DM, Austen KF, Katz HR. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J Biol Chem. 1999. February;274(9):5791–6. 10.1074/JBC.274.9.5791 [DOI] [PubMed] [Google Scholar]
- Arm JP, Nwankwo C, Austen KF. Molecular identification of a novel family of human Ig superfamily members that possess immunoreceptor tyrosine-based inhibition motifs and homology to the mouse gp49B1 inhibitory receptor. J Immunol. 1997;159:2342-9. [PubMed] [Google Scholar]
- Tedla N, Lee CW, Borges L, Geczy CL, Arm JP. Differential expression of leukocyte immunoglobulin-like receptors on cord-blood-derived human mast cell progenitors and mature mast cells. J Leukoc Biol. 2008. February;83(2):334–43. 10.1189/jlb.0507314 [DOI] [PubMed] [Google Scholar]
- Cheng H, Mohammed F, Nam G, Chen Y, Qi J, Garner LI, et al. Crystal structure of leukocyte Ig-like receptor LILRB4 (ILT3/LIR-5/CD85k): a myeloid inhibitory receptor involved in immune tolerance. J Biol Chem. 2011. May;286(20):18013–25. 10.1074/jbc.M111.221028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeje PL, Bezemer K, Heuvers ME, Dingemans AC, Groen HJ, Smit EF, et al. Immunoglobulin-like transcript 3 is expressed by myeloid-derived suppressor cells and correlates with survival in patients with non-small cell lung cancer. OncoImmunology. 2015. March;4(7):e1014242. 10.1080/2162402X.2015.1014242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Kong DW, Cooper MD, Kubagawa H. Mast cell regulation via paired immunoglobulin-like receptor PIR-B. Immunol Res. 2002;26(1-3):191–7. 10.1385/IR:26:1-3:191 [DOI] [PubMed] [Google Scholar]
- Uehara T, Bléry M, Kang DW, Chen CC, Ho LH, Gartland GL, et al. Inhibition of IgE-mediated mast cell activation by the paired Ig-like receptor PIR-B. J Clin Invest. 2001. October;108(7):1041–50. 10.1172/JCI12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Ono M, Takai T. Inhibitory and stimulatory functions of paired Ig-like receptor (PIR) family in RBL-2H3 cells. J Immunol. 1998. October;161(8):4042–7. [PubMed] [Google Scholar]
- Liénard H, Bruhns P, Malbec O, Fridman WH, Daëron M. Signal regulatory proteins negatively regulate immunoreceptor-dependent cell activation. J Biol Chem. 1999. November;274(45):32493–9. 10.1074/JBC.274.45.32493 [DOI] [PubMed] [Google Scholar]
- Pan YF, Dong LW, Wang M, Yang GZ, Zhang J, Li SX, et al. Signal regulatory protein α negatively regulates mast-cell activation following FcεRI aggregation. Eur J Immunol. 2013. June;43(6):1598–607. 10.1002/eji.201243031 [DOI] [PubMed] [Google Scholar]
- Movsisyan LD, Macauley MS. Structural advances of Siglecs: insight into synthetic glycan ligands for immunomodulation. Org Biomol Chem. 2020. August;18(30):5784–97. 10.1039/d0ob01116a [DOI] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, et al. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008. February;121(2):499–505.e1. 10.1016/j.jaci.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014. October;14(10):653–66. 10.1038/nri3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Myers A, Matsumoto K, Crocker PR, Saito H, Bochner BS. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006. June;61(6):769–76. 10.1111/j.1398-9995.2006.01133.x [DOI] [PubMed] [Google Scholar]
- Park CS, Bochner BS. Potential targeting of siglecs, mast cell inhibitory receptors, in interstitial cystitis. Int Neurourol J. 2011. June;15(2):61–3. 10.5213/inj.2011.15.2.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi S, Gibbs BF, Karra L, Ben-Zimra M, Levi-Schaffer F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J Allergy Clin Immunol. 2014. July;134(1):230–3. 10.1016/j.jaci.2014.03.031 [DOI] [PubMed] [Google Scholar]
- Duan S, Koziol-White CJ, Jester WF, Jr, Nycholat CM, Macauley MS, Panettieri RA, Jr, et al. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J Clin Invest. 2019. March;129(3):1387–401. 10.1172/JCI125456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril T, Floyd H, Lopez F, Vivier E, Crocker PR. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J Immunol. 2004;173:6841-9. doi: 10.4049/jimmunol.173.11.6841. [DOI] [PubMed] [Google Scholar]
- Kerr SC, Gonzalez JR, Schanin J, Peters MC, Lambrecht BN, Brock EC, et al. An anti-siglec-8 antibody depletes sputum eosinophils from asthmatic subjects and inhibits lung mast cells. Clin Exp Allergy. 2020. August;50(8):904–14. 10.1111/cea.13681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanin J, Gebremeskel S, Korver W, Falahati R, Butuci M, Haw TJ, et al. A monoclonal antibody to Siglec-8 suppresses non-allergic airway inflammation and inhibits IgE-independent mast cell activation. Mucosal Immunol. 2020. August: 10.1038/s41385-020-00336-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood BA, Brock EC, Leung J, Falahati R, Bryce PJ, Bright J, et al. AK002, a Humanized Sialic Acid-Binding Immunoglobulin-Like Lectin-8 Antibody that Induces Antibody-Dependent Cell-Mediated Cytotoxicity against Human Eosinophils and Inhibits Mast Cell-Mediated Anaphylaxis in Mice. Int Arch Allergy Immunol. 2019;180(2):91–102. 10.1159/000501637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DJ, Cho JY, Miller M, Strangman W, Zhang M, Varki A, et al. Anti-Siglec-F antibody inhibits oral egg allergen induced intestinal eosinophilic inflammation in a mouse model. Clin Immunol. 2009. April;131(1):157–69. 10.1016/j.clim.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel KA, Duan S, Wright BL, Maleki SJ, Wolf JC, Vickery BP, et al. Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2. J Allergy Clin Immunol. 2017. January;139(1):366–369.e2. 10.1016/j.jaci.2016.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang L, Macauley MS, Arlian BM, Nycholat CM, Paulson JC. Encapsulating an Immunosuppressant Enhances Tolerance Induction by Siglec-Engaging Tolerogenic Liposomes. ChemBioChem. 2017. July;18(13):1226–33. 10.1002/cbic.201600702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitallé J, Terrén I, Orrantia A, Bilbao A, Gamboa PM, Borrego F, et al. The Expression and Function of CD300 Molecules in the Main Players of Allergic Responses: Mast Cells, Basophils and Eosinophils. Int J Mol Sci. 2020. April;21(9):E3173. 10.3390/ijms21093173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Moretta A, Moretta L, Levi-Schaffer F, Metcalfe DD. The inhibitory receptor IRp60 (CD300a) is expressed and functional on human mast cells. J Immunol. 2005;175:7989-95. doi: 10.4049/jimmunol.175.12.7989. [DOI] [PubMed] [Google Scholar]
- Bachelet I, Munitz A, Levi-Schaffer F. Abrogation of allergic reactions by a bispecific antibody fragment linking IgE to CD300a. J Allergy Clin Immunol. 2006. June;117(6):1314–20. 10.1016/J.JACI.2006.04.031 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakahashi-Oda C, Okayama Y, Shibuya A. Autonomous regulation of IgE-mediated mast cell degranulation and immediate hypersensitivity reaction by an inhibitory receptor CD300a. J Allergy Clin Immunol. 2019. July;144(1):323–327.e7. 10.1016/j.jaci.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Sabato V, Verweij MM, Bridts CH, Levi-Schaffer F, Gibbs BF, De Clerck LS, et al. CD300a is expressed on human basophils and seems to inhibit IgE/FcεRI-dependent anaphylactic degranulation. Cytometry B Clin Cytom. 2012. May;82(3):132–8. 10.1002/cyto.b.21003 [DOI] [PubMed] [Google Scholar]
- Zenarruzabeitia O, Vitallé J, Terrén I, Orrantia A, Astigarraga I, Dopazo L, et al. CD300c costimulates IgE-mediated basophil activation, and its expression is increased in patients with cow’s milk allergy. J Allergy Clin Immunol. 2019. February;143(2):700–711.e5. 10.1016/j.jaci.2018.05.022 [DOI] [PubMed] [Google Scholar]
- Larsen LF, Juel-Berg N, Hansen A, Hansen KS, Mills EN, van Ree R, et al. No difference in human mast cells derived from peanut allergic versus non-allergic subjects. Immun Inflamm Dis. 2018. December;6(4):416–27. 10.1002/iid3.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa K, Yamanishi Y, Maehara A, Takahashi M, Isobe M, Ito S, et al. The receptor LMIR3 negatively regulates mast cell activation and allergic responses by binding to extracellular ceramide. Immunity. 2012. November;37(5):827–39. 10.1016/J.IMMUNI.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Izawa K, Isobe M, Matsukawa T, Ito S, Maehara A, Takahashi M, et al. Sphingomyelin and ceramide are physiological ligands for human LMIR3/CD300f, inhibiting FcεRI-mediated mast cell activation. J Allergy Clin Immunol. 2014;133:270-3.e1-7. doi: 10.1016/j.jaci.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Izawa K, Kaitani A, Ando T, Maehara A, Nagamine M, Yamada H, et al. Differential Lipid Recognition by Mouse versus Human CD300f, Inhibiting Passive Cutaneous Anaphylaxis, Depends on a Single Amino Acid Substitution in its Immunoglobulin-Like Domain. J Invest Dermatol. 2020. March;140(3):710–713.e3. 10.1016/j.jid.2019.08.439 [DOI] [PubMed] [Google Scholar]
- Uchida S, Izawa K, Ando T, Yamada H, Uchida K, Negishi N, et al. CD300f is a potential therapeutic target for the treatment of food allergy. Allergy. 2020. February;75(2):471–4. 10.1111/all.14034 [DOI] [PubMed] [Google Scholar]
- Alvarez-Errico D, Sayós J, López-Botet M. The IREM-1 (CD300f) inhibitory receptor associates with the p85alpha subunit of phosphoinositide 3-kinase. J Immunol. 2007. January;178(2):808–16. 10.4049/jimmunol.178.2.808 [DOI] [PubMed] [Google Scholar]
- Dudeck A, Leist M, Rubant S, Zimmermann A, Dudeck J, Boehncke WH, et al. Immature mast cells exhibit rolling and adhesion to endothelial cells and subsequent diapedesis triggered by E- and P-selectin, VCAM-1 and PECAM-1. Exp Dermatol. 2010. May;19(5):424–34. 10.1111/j.1600-0625.2010.01073.x [DOI] [PubMed] [Google Scholar]
- Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007. December;27(12):2514–23. 10.1161/ATVBAHA.107.151456 [DOI] [PubMed] [Google Scholar]
- Zhu JX, Cao G, Williams JT, Delisser HM. SHP-2 phosphatase activity is required for PECAM-1-dependent cell motility. Am J Physiol Cell Physiol. 2010;299(4):C854-65. Epub 2010/07/16. doi: 10.1152/ajpcell.00436.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadi E, Hajilooi M, Babakhani D, Rafiei A. Platelet endothelial cell adhesion molecule-1 polymorphism in patients with bronchial asthma. Iran J Allergy Asthma Immunol. 2012. December;11(4):276–81. [PubMed] [Google Scholar]
- Wong MX, Roberts D, Bartley PA, Jackson DE. Absence of platelet endothelial cell adhesion molecule-1 (CD31) leads to increased severity of local and systemic IgE-mediated anaphylaxis and modulation of mast cell activation. J Immunol. 2002. June;168(12):6455–62. 10.4049/JIMMUNOL.168.12.6455 [DOI] [PubMed] [Google Scholar]
- Early M, Schroeder WG, Unnithan R, Gilchrist JM, Muller WA, Schenkel A. Differential effect of Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) on leukocyte infiltration during contact hypersensitivity responses. PeerJ. 2017. July;5:e3555. 10.7717/peerj.3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueshima C, Kataoka TR, Hirata M, Sugimoto A, Iemura Y, Minamiguchi S, et al. Possible Involvement of Human Mast Cells in the Establishment of Pregnancy via Killer Cell Ig-Like Receptor 2DL4. Am J Pathol. 2018. June;188(6):1497–508. 10.1016/j.ajpath.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Kataoka TR, Ueshima C, Hirata M, Minamiguchi S, Haga H. Killer Immunoglobulin-Like Receptor 2DL4 (CD158d) Regulates Human Mast Cells both Positively and Negatively: Possible Roles in Pregnancy and Cancer Metastasis. Int J Mol Sci. 2020. January;21(3):E954. 10.3390/ijms21030954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka TR, Kumanogoh A, Bandara G, Metcalfe DD, Gilfillan AM. CD72 negatively regulates KIT-mediated responses in human mast cells. J Immunol. 2010. March;184(5):2468–75. 10.4049/jimmunol.0902450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka TR, Kumanogoh A, Fukuishi N, Ueshima C, Hirata M, Moriyoshi K, et al. CD72 negatively regulates mouse mast cell functions and down-regulates the expression of KIT and FcεRIα. Int Immunol. 2015. February;27(2):95–103. 10.1093/intimm/dxu087 [DOI] [PubMed] [Google Scholar]
- Abramson J, Xu R, Pecht I. An unusual inhibitory receptor—the mast cell function-associated antigen (MAFA). Mol Immunol. 2002. September;38(16-18):1307–13. 10.1016/S0161-5890(02)00080-9 [DOI] [PubMed] [Google Scholar]
- Ortega Soto E, Pecht I. A monoclonal antibody that inhibits secretion from rat basophilic leukemia cells and binds to a novel membrane component. J Immunol. 1988;141:4324-32. [PubMed] [Google Scholar]
- Song J, Hagen G, Smith SM, Roess DA, Pecht I, Barisas BG. Interactions of the mast cell function-associated antigen with the type I Fcepsilon receptor. Mol Immunol. 2002. September;38(16-18):1315–21. 10.1016/s0161-5890(02)00081-0 [DOI] [PubMed] [Google Scholar]
- Licht A, Pecht I, Schweitzer-Stenner R. Regulation of mast cells’ secretory response by co-clustering the Type 1 Fcepsilon receptor with the mast cell function-associated antigen. Eur J Immunol. 2005. May;35(5):1621–33. 10.1002/eji.200425964 [DOI] [PubMed] [Google Scholar]
- Dellon ES, Peterson KA, Murray JA, Falk GW, Gonsalves N, Chehade M, et al. Anti-Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N Engl J Med. 2020. October;383(17):1624–34. 10.1056/NEJMoa2012047 [DOI] [PMC free article] [PubMed] [Google Scholar]