Abstract
Novel quinazolinone compounds have been studied in the field of drug discovery for a long time. Among their broad range of pharmacological effects, certain compounds effectively inhibit cancer cell proliferation. MJ-33 is a quinazolinone derivative with proposed anticancer activities that was synthesized in our laboratory. The present study aimed to evaluate the anticancer activity of MJ-33 in fluorouracil (5FU)-resistant colorectal cancer cells (HT-29/5FUR) and to investigate the underlying molecular mechanisms. The cell viability assay results indicated that HT-29/5FUR cell viability was inhibited by MJ-33 treatment in a concentration-dependent manner compared with the control group. The cellular morphological alterations observed following MJ-33 treatment indicated the occurrence of apoptosis and autophagy, as well as inhibition of cell proliferation in a time-dependent manner compared with the control group. The acridine orange, LysoTracker Red and LC3-green fluorescent protein staining results indicated that MJ-33 treatment significantly induced autophagy compared with the control group. The DAPI/TUNEL dual staining results demonstrated increased nuclear fragmentation and condensation following MJ-33 treatment compared with the control group. The Annexin V apoptosis assay and image cytometry analysis results demonstrated a significant increase in apoptotic cells following MJ-33 treatment compared with the control group. The western blotting results demonstrated markedly decreased Bcl-2, phosphorylated (p)-BAD, pro-caspase-9 and pro-caspase-3 expression levels, and notably increased cytochrome c and apoptotic peptidase activating factor 1 expression levels following MJ-33 treatment compared with the control group. Moreover, the expression levels of autophagy-related proteins, including autophagy related (ATG)-5, ATG-7, ATG-12, ATG-16, p62 and LC3-II, were increased following MJ-33 treatment compared with the control group. Furthermore, MJ-33-treated HT-29/5FUR cells displayed decreased expression levels of p-AKT and p-mTOR compared with control cells. The results suggested that MJ-33-induced apoptosis was mediated by AKT signaling, and subsequently modulated via the mitochondria-dependent signaling pathway. Therefore, the results suggested that suppression of AKT/mTOR activity triggered autophagy in the HT-29/5FUR cell line. In summary, the results indicated that MJ-33 inhibited HT-29/5FUR cell viability, and induced apoptosis and autophagy via the AKT/mTOR signaling pathway. The present study may provide novel insight into the anticancer effects and mechanisms underlying MJ-33 in 5FU-resistant colorectal cancer cells.
Keywords: MJ-33, colorectal cancer, fluorouracil-resistant HT-29 cells, autophagy, apoptosis
Introduction
Colorectal cancer (CRC) is a serious malignant disease, typically initiated by aberrant epithelial cell proliferation in the colon or rectum (1). CRC is the third most frequently diagnosed type of cancer worldwide (2), and is characterized by a high and continuously increasing mortality rate, estimated to reach 60% by 2035 (3). The treatment options for CRC currently include chemotherapy, targeted therapy and immunotherapy (4). The chemotherapeutic agents most widely used for CRC include 5-fluorouracil (5FU), irinotecan and oxaliplatin (4,5). 5FU is a first-line drug that has been extensively used as a monotherapy or as part of a combination regimen for the treatment of CRC (4,5). However, the proportion of patients with advanced CRC who respond to 5FU is limited to 10-15% (6), whereas ~50% of patients with metastatic CRC display resistance to 5FU-based chemotherapies (7,8). Therefore, the development of novel therapeutic strategies for 5FU-resistant CRC is important.
Several quinazolinone compounds have been developed and approved as novel anticancer agents, including nolatrexed, idelalisib and raltitrexed (9–11). In our laboratory, several quinazolinone compounds (such as MJ-29, MJ-33 and LJJ-10) have been designed, synthesized and studied to investigate their anticancer activities (12,13). For example, the inhibitory effect of MJ-33 on AKT-mediated DU145 prostate cancer cell metastasis was previously reported (14). However, the cytotoxicity and mechanisms underlying MJ-33 in chemoresistant cells are not completely understood. Therefore, the present study aimed to investigate the anticancer activities of MJ-33 in a CRC cell line with acquired resistance to 5FU, namely HT-29/5FUR.
AKT is an important cancer-related regulator that is responsible for cancer cell survival, proliferation and migration (15,16). Moreover, AKT overactivation has been reported to serve as a biomarker of tumorigenesis, tumor growth, metastasis and resistance to cancer therapies (17). AKT/mTOR signaling-mediated control of autophagy and apoptosis in chemoresistant cells has been previously reported (18). In addition, inhibiting the PI3K/AKT signaling pathway restores the sensitivity of HT-29 cells to 5FU (19). A previous study also demonstrated the inhibitory effects of MJ-33 on AKT phosphorylation, resulting in antimetastatic activity (14).
The crosstalk between apoptosis and autophagy is a novel therapeutic target in cancer (20). In the later stage, when autophagy no longer serves a cytoprotective role, it leads to apoptosis activation to induce programmed cell death (21). In a previous study, a novel quinazolinone derivative induced autophagy-related apoptotic cell death in lymphoblastic leukemia MOLT-4 cells (22). Therefore, it was hypothesized that the mechanism underlying MJ-33-induced anticancer activity was associated with the induction of apoptosis and autophagy. The present study investigated the cytotoxic effects and mechanisms underlying MJ-33, and explored the involvement of the AKT downstream signaling pathway in HT-29/5FUR cells.
Materials and methods
Chemicals and reagents
MJ-33 was synthesized in our laboratory at the School of Pharmacy, China Medical University. DAPI, 3-Methyladenine (3-MA), chloroquine (CQ), Bafilomycin A1 (Baf.A1), AKT activator (SC-79), Minimum Essential medium and MTT were purchased from Sigma-Aldrich (Merck KGaA). L-glutamine, RPMI-1640 medium, penicillin, streptomycin, trypsin-EDTA, acridine orange (AO), LysoTracker Red, LC3-green fluorescent protein (GFP) and FBS were purchased from Thermo Fisher Scientific, Inc. Pan-caspase inhibitor (z-VAD-fmk), caspase-9 inhibitor (z-LEHD-fmk) and caspase-3 inhibitor (z-DEVD-fmk) were purchased from Merck KGaA. 5FU was obtained from Pharmacia & Upjohn.
Cell lines and cell culture
The human colorectal cancer HT-29 cell line (23) was obtained from the Bioresource Collection and Research Center, Food Industry Research and Development Institute. The cell line was authenticated via STR profiling (Mission Biotech Co., Ltd. Cells were cultured in 75-cm2 tissue culture flasks in RPMI-1640 medium supplemented with 2 mM L-glutamine, 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with 5% CO2. 5FU-resistant colorectal cancer cells (HT-29/5FUR) were established according to the following protocol. Parental HT-29 cells were exposed to an initial dose of 0.5 µg/ml (3.843 µM) at 37°C and surviving cells were cultured to 90% confluence for four passages (4 weeks). Surviving cells were exposed to 1 µg/ml 5FU (7.688 µM) for four passages (4 weeks) and then 1.5 µg/ml 5FU (11.530 µM) for four passages (4 weeks). Finally, surviving cells were exposed to 2 µg/ml 5FU (15.372 µM), the clinically relevant plasma concentration, for four passages (4 weeks). Surviving resistant cells were named 5FU-resistant colorectal cancer cells (HT-29/5FUR) (24,25). The doubling time of parental HT-29 cells was 17 h and the doubling time of HT-29/5FUR cells was 34 h.
Prior to MJ-33 treatment, HT-29/5FUR cells were treated with 10 mM 3-MA, 100 µM CQ or 10 nM Baf.A1 at 37°C for 1 h, 10 µM SC-79 at 37°C for 30 min, or 15 µM z-VAD-fmk, 15 µM z-LEHD-fmk or 15 µM z-DEVD-fmk at 37°C for 2 h.
The normal human colon CCD 841 CoN cell line (CRL-1790; American Type Culture Collection) was cultured in Minimum Essential medium supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C with 5% CO2.
Cell viability assay
HT-29/5FUR and CCD 841 CoN cells were seeded (1×104 cells/well) into 96-well plates and treated with different concentrations of MJ-33 (25, 50, 75 and 100 µM). Control cells were treated with DMSO. Positive control cells were treated with 30 µM oxaliplatin (Sanofi S.A.). Cells were incubated with each treatment at 37°C for 24 or 48 h with 5% CO2. Then, cell viability was assessed by performing the MTT assay as previously described (18,26).
Cell death and morphological changes on microscopic observation
HT-29/5FUR cells (1×104 cells/100 µl) were treated with 25, 50, 75 and 100 µM MJ-33 or DMSO at 37°C for 48 h with 5% CO2. Subsequently, cultured cells were observed using a phase-contrast light microscope (Leica Microsystems GmbH; magnification, ×400) to visualize morphological alterations characteristic of apoptotic or autophagic cell death.
AO, LysoTracker Red and LC3-GFP staining
HT-29/5FUR cells (1×105 cells/ml) were treated with 75 µM MJ-33 or DMSO for 24 h at 37°C. After harvesting using trypsin-EDTA, cells were fixed in 4% paraformaldehyde on ice for 15 min. Subsequently, cells were stained with 1 µg/ml AO, LysoTracker Red or LC3-GFP for 20 min at room temperature. Stained cells were visualized using ImageXpress Micro Confocal High-Content Image System (Molecular Devices, LLC; magnification, ×400) and analyzed using MetaXpress (version 5.3.0.4; Molecular Devices, LLC) to detect acidic vesicular organelles (for AO staining), lysosomal function (for LysoTracker Red staining) or typical punctate pattern (for LC3-GFP staining).
DAPI and TUNEL dual staining
HT-29/5FUR cells were seeded (1×105 cells/ml) into 12-well plates and treated with 75 µM MJ-33 or DMSO for 48 h at 37°C with 5% CO2. Cells were harvested and fixed with absolute ethanol at room temperature for 10 min. Subsequently, cells were stained with 1 µg/ml DAPI solution for 30 min at room temperature as previously described (27). To detect DNA breaks, the In Situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics GmbH) was used according to the manufacturer's protocol. After applying mounting medium (10% glycerol in PBS), stained samples were observed using a fluorescence microscope (Leica Microsystems GmbH; magnification, ×400) as previously described (28). For each coverslip, 3 fields of view with similar numbers of cells were photographed for counting and further analysis.
Caspase-3 and caspase-7 activity assays
The fluorochrome-labeled inhibitor of caspases assay (FLICA) method was applied using the FAM-FLICA® Caspases 3 & 7 Assay Kit (ImmunoChemistry Technologies, LLC; cat. no. 93) and the NucleoCounter NC-3000 (ChemoMetec A/S) according to the manufacturer's protocol. All samples were analyzed using NucleoView NC-3000 software (version 1.4; ChemoMetec A/S). Briefly, HT-29/5FUR cells were seeded (1×106 cells/well) into 6-well plates, treated with 75 µM MJ-33 or DMSO for 12 h at 37°C. Cells were resuspended in each well using 0.5 ml PBS. Samples were incubated with diluted FLICA reagent and Hoechst 33342 for 1 h at 37°C. Following washing twice with apoptosis wash buffer, cells were resuspended in 100 µl apoptosis wash buffer supplemented with PI. Subsequently, 30 µl cell suspension was immediately loaded into a 2-chamber slide (NC-Slide A2; ChemoMetec A/S) for analysis on the NucleoCounter NC-3000 using the built-in caspase assay program.
Annexin assay and image cytometry analysis
Experiments were conducted using the Annexin V-FITC Apoptosis Detection Kit (cat. no. AVK050; Strong Biotech Corp.) and the Counter NC-3000 cytometer (ChemoMetec A/S) according to the manufacturer's protocol and the previously described protocol (29). Briefly, HT-29/5FUR cells were seeded (total 1×106 cells/well) into 6-well plates. Samples were treated with 75 µM MJ-33 or DMSO at 37°C for 6 h. Cells were resuspended in each well with 0.5 ml PBS. Subsequently, samples were incubated with 100 µl Annexin V binding buffer as previously described (29). Then, samples were incubated with 2 µl Annexin V-CF 488A conjugate and 2 µl Hoechst 33342 (10 µg/ml) at 37°C for 15 min. Following centrifugation at 200 × g at 37°C for 5 min, samples were washed with Annexin V binding buffer. Cell pellets were resuspended using 100 µl Annexin V binding buffer supplemented with PI at room temperature for 15 min. All samples were immediately analyzed using NucleoView NC-3000 software (version 1.4; ChemoMetec A/S). Subpopulations of stained cells were determined using scatterplots: Healthy cells (Annexin V−/PI−); early apoptosis (Annexin V+/PI−); late apoptosis (Annexin V+/PI+); and necrosis (Annexin V−/PI+).
Cell confluence assay
The IncuCyte S3 ZOOM System instrument (Essen BioScience) was used to conduct the cell confluence assay. HT-29/5FUR cells were seeded (1×104 cells/well) into a 96-well plate with 50 µM MJ-33 or DMSO for 48 h at 37°C. Cells were visualized and photographed every 2 h as previously described (30).
Western blotting
HT-29/5FUR cells were lysed with Trident RIPA Lysis Buffer (GeneTex, Inc.). Protein concentrations were determined using a Bio-Rad protein assay system (Bio-Rad Laboratories, Inc.). Proteins were separated as previously described (18,31,32). Briefly, proteins (35 µg) were separated via 10-12% SDS-PAGE and transferred using the iBlot Dry Blotting System (Invitrogen; Thermo Fisher Scientific, Inc.) to PVDF membranes. The membranes were blocked with PBS containing 0.1% Tween-20 and 5% skimmed dry milk for 2 h at room temperature. Subsequently, the membranes were incubated overnight at 4°C with primary antibodies (all purchased from Cell Signaling Technology, Inc.) targeted against: Bcl-2 (cat. no. 4223; 1:1,000), Bax (cat. no. 5023; 1:1,000), BAD (cat. no. 9292; 1:1,000), phosphorylated (p)-BAD (cat. no. 5284; 1:1,000), cytochrome c (cat. no. 4280; 1:1,000), apoptotic peptidase activating factor-1 (Apaf-1; cat. no. 8969; 1:1,000), caspase-9 (cat. no. 9508; 1:1,000), caspase-3 (cat. no. 9662; 1:1,000), autophagy related (ATG)-5 (cat. no. 12994; 1:1,000), ATG-7 (cat. no. 8558; 1:1,000), ATG-12 (cat. no. 4180; 1:1,000), ATG-16 (cat. no. 8089; 1:1,000), p62 (cat. no. 23214; 1:1,000), LC3 (cat. no. 12741; 1:1,000), AKT (cat. no. 9272; 1:1,000), p-AKT (cat. no. 4060; 1:1,000), mTOR (cat. no. 2972; 1:1,000), p-mTOR (cat. no. 5536; 1:1,000) and β-actin (cat. no. 8457; 1:1,000). Following primary incubation, the membranes were incubated for 4 h at room temperature with anti-rabbit IgG (cat. no. 7074; 1:10,000; Cell Signaling Technology, Inc.) and anti-mouse IgG (cat. no. 7076; 1:10,000; Cell Signaling Technology, Inc.) HRP-conjugated secondary antibodies. Protein bands were visualized using Immobilon Western HRP Substrate (Merck KGaA) for 1 h.
Statistical analysis
Data are presented as the mean ± SD (n=3). Comparisons among multiple groups were analyzed using one-way ANOVA followed by Dunnett's or Tukey's post hoc test. Statistical analyses were performed using SPSS software (version 16.0; SPSS, Inc.). P<0.001 was considered to indicate a statistically significant difference.
Results
MJ-33 selectively exerts cytotoxic effects on HT-29/5FUR cells, which are associated with apoptosis and autophagy
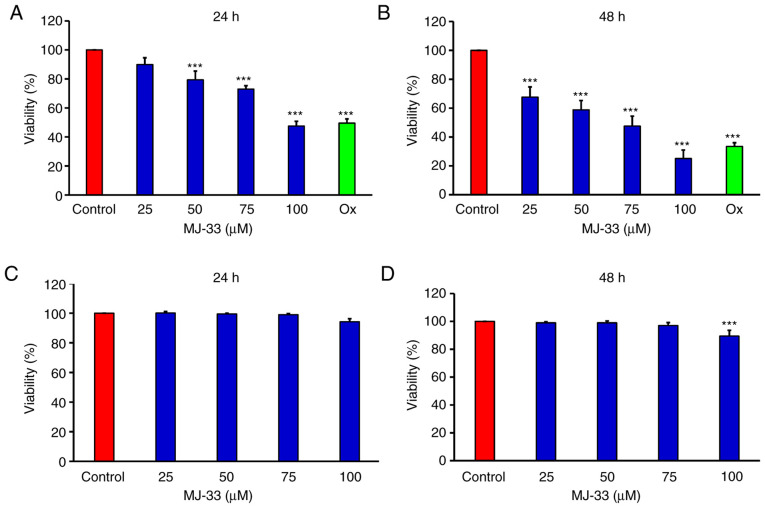
The MTT assay was performed to examine the antiproliferative effects of MJ-33 on HT-29/5FUR cells in vitro. The results demonstrated that cell viability was significantly inhibited by MJ-33 in a concentration-dependent manner compared with the control group (Fig. 1A and B). As the positive control, oxaliplatin also significantly inhibited HT-29/5FUR cell viability compared with the control group. In CCD 841 CoN cells, cell viability was not significantly altered by MJ-33 treatment, except for in the 100 µM MJ-33 for 48 h group, compared with the control group (Fig. 1C and D).
Figure 1.
Effects of MJ-33 on HT-29/5FUR and CCD 841 CoN cell viability. Cells were seeded (1×104 cells/well) into 96-well plates and treated with 0, 25, 50, 75 and 100 µM MJ-33. HT-29/5FUR cell viability following treatment for (A) 24 or (B) 48 h. CCD 841 CoN cell viability following treatment for (C) 24 or (D) 48 h. Data are presented as the mean ± SD from three independent experiments. Data were analyzed using one-way ANOVA followed by Dunnett's post hoc test. ***P<0.001 vs. control. 5FUR, fluorouracil-resistant; Ox, oxaliplatin.
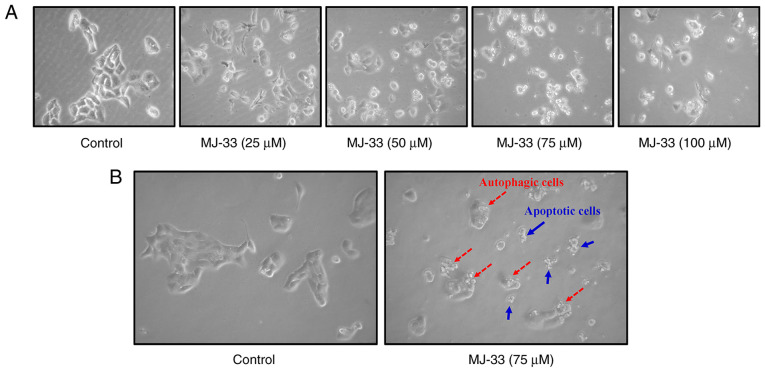
The sensitivity of HT-29/5FUR cells and its parental cell line (HT-29) to MJ-33, 5FU and Ox are presented in Table I. The IC50s of 5FU and Ox in HT-29/5FUR cells were higher compared with in HT-29 cells. Conversely, the IC50 of MJ-33 in HT-29/5FUR cells was lower compared with HT-29 cells. Based on the IC50 values obtained, 75 µM MJ-33 was selected for subsequent experiments. To further examine the effects of MJ-33 treatment on HT-29/5FUR cell morphology, light microscopy and the IncuCyte S3 ZOOM system were used. Compared with the control group, MJ-33 treatment induced morphological alterations, including cell shrinkage, and nuclear fragmentation and condensation, and cell death (Fig. 2A, B and Video S1) suggested apoptotic phenomena. In addition, autophagic cells with increasing volume of autophagic vesicles were observed following MJ-33 treatment (Fig. 2B). The results suggested that MJ-33-induced cell death was mediated via mechanisms involving apoptosis and autophagy.
Table I.
IC50s of MJ-33, 5FU and oxaliplatin in parental HT-29 and HT-29/5FUR cells following treatment for 24 or 48 h.
| A, Parental HT-29 cells | |||
|---|---|---|---|
| Time (h) | MJ-33 (µM) | 5FU (µM) | oxaliplatin (µM) |
| 24 | 96.65±2.96 | 20.14±2.13 | 30.98±1.98 |
| 48 | 70.22±3.56 | 9.68±2.29 | 14.98±2.36 |
| B, HT-29/5FUR cells | |||
| Time (h) | MJ-33 (µM) | 5FU (µM) | oxaliplatin (µM) |
| 24 | 95.67±4.96 | 61.48±2.25 | 50.65±2.33 |
| 48 | 63.21±4.17 | 29.29±3.33 | 22.46±3.69 |
Data are presented as the mean ± SD from at least three independent experiments. 5FU, fluorouracil; 5FUR, 5FU-resistant.
Figure 2.
Effects of MJ-33 on HT-29/5FUR cell morphology. (A) Morphological alterations and cell death in HT-29/5FUR cells following treatment with MJ-33 (magnification, ×200). (B) Apoptotic and autophagic cells were observed. 5FUR, fluorouracil-resistant (magnification, ×200).
MJ-33 induces apoptosis via the mitochondrial intrinsic signaling pathway in HT-29/5FUR cells
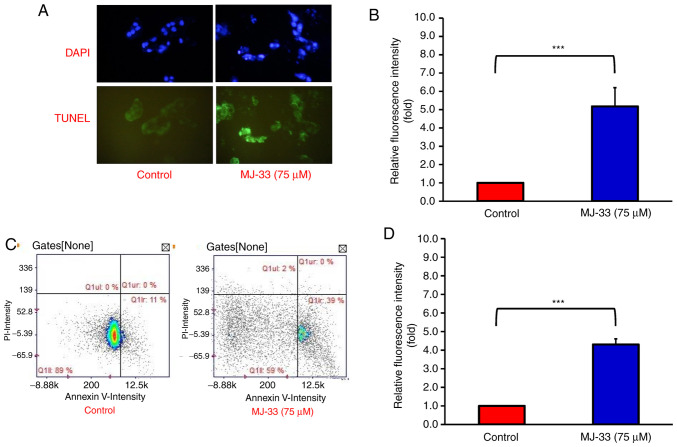
MJ-33-induced cell death was observed in HT-29/5FUR cells, thus the apoptotic bodies were subsequently quantified. By performing DAPI and TUNEL staining on HT-29/5FUR cells, the proportion of apoptotic cells after MJ-33 treatment was examined. Under a fluorescence microscope, the nuclear morphology of dead cells was observed, which displayed chromatin condensation, a well-defined hallmark of apoptosis (Fig. 3A). The TUNEL-stained cells emitted green fluorescence in response to DNA fragmentation, which is an indicator of late-stage apoptosis (33). The results demonstrated that MJ-33 treatment significantly increased TUNEL-positive staining compared with the control group (Fig. 3B). Image cytometry analysis was conducted to determine the apoptotic subpopulations of MJ-33-treated and control cells. The percentage of early apoptotic cells was increased from 11% in control cells to 39% in MJ-33-treated cells (Fig. 3C). Moreover, MJ-33 treatment significantly increased the number of Annexin-V-positive/PI-negative cells compared with the control group (Fig. 3D). Therefore, the results further indicated that HT-29/5FUR cell apoptosis was induced by MJ-33 treatment.
Figure 3.
MJ-33 treatment induces HT-29/5FUR cell apoptosis. (A) DNA condensation and apoptotic DNA fragmentation were observed by performing DAPI and TUNEL staining in MJ-33-treated HT-29/5FUR cells (magnification, ×200). (B) Quantification of TUNEL-positive cells. (C) Cell apoptosis was assessed by performing Annexin V/PI dual staining via image cytometry in MJ-33-treated HT-29/5FUR cells. (D) Quantification of Annexin V+/PI− cells. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. ***P<0.001. 5FUR, fluorouracil-resistant.
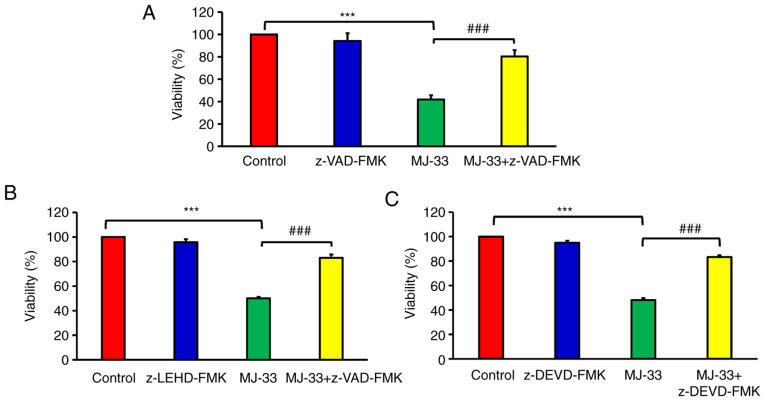
To improve the current understanding of MJ-33-induced apoptotic death in MJ-HT-29/5FUR cells, the possible regulatory signaling pathways were investigated. It was hypothesized that MJ-33 induced apoptotic cell death via the caspase-dependent signaling pathways. Therefore, cell viability was examined following MJ-33 treatment with or without pan-caspase inhibitor (z-VAD-FMK). Cell viability in the MJ-33-treated groups was significantly lower compared with the control group (Fig. 4). Furthermore, cell viability was significantly higher in the MJ-33 + z-VAD-FMK/MJ-33 group compared with the MJ-33 group, suggesting that MJ-33-induced HT-29/5FUR cell apoptosis was mediated via caspase activity. To determine the effect of specific caspase enzymes on MJ-33-induced apoptosis, cells were pretreated with z-LEHD-FMK (to inhibit caspase-9) or z-DEVD-FMK (to inhibit caspase-3). The cell viability assay results demonstrated MJ-33-induced cytotoxicity in HT-29/5FUR cells was significantly inhibited by caspase-9 and caspase-3 inhibitors. The results indicated that MJ-33-induced apoptosis was mediated via caspase-9 and caspase-3 (Fig. 4B and C). The aforementioned results suggested that MJ-33 induced apoptotic cell death via the intrinsic signaling pathway in HT-29/5FUR cells.
Figure 4.
MJ-33-induced HT-29/5FUR cell apoptosis via initiation of the caspase cascade. Cell viability following treatment with MJ-33 and/or (A) pan-caspase inhibitor (z-VAD-FMK), (B) caspase-9 inhibitor (z-LEHD-FMK) or (C) caspase-3 inhibitor (z-DEVD-FMK). Data are presented as the mean ± SD from three independent experiments. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. ***P<0.001; ###P<0.001. 5FUR, fluorouracil-resistant.
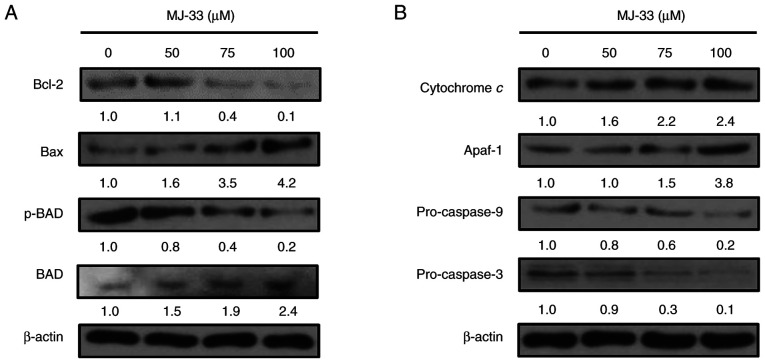
To identify the roles of proapoptotic proteins in the molecular mechanisms underlying MJ-33-induced apoptosis, the expression levels of proapoptotic proteins, including Bax, Bcl-2 and p-BAD, were investigated in MJ-33-treated HT-29/5FUR cells. Compared with the control group, Bax and BAD protein expression levels were markedly increased in a concentration-dependent manner following treatment with MJ-33 (Fig. 5A). By contrast, p-BAD protein expression levels were markedly decreased by MJ-33 treatment compared with the control group, and reversely associated with the protein expression levels of Bax. Compared with the control group, Bcl-2 expression levels were slightly increased following treatment with 50 µM MJ-33, but notably decreased following treatment with 75 and 100 µM MJ-33.
Figure 5.
MJ-33 activated the intrinsic apoptosis signaling pathway. Western blotting was performed to measure the expression levels of (A) proapoptotic and (B) intrinsic apoptosis signaling pathway-related proteins. The density of the bands compared with the control sample (set to 1.0) are presented above each band. p, phosphorylated; Apaf-1, apoptotic peptidase activating factor 1.
Similarly, the protein expression levels of cytochrome c, Apaf-1, pro-caspase-9 and pro-caspase-3 were examined in HT-29/5FUR cells following treatment with MJ-33. The protein expression levels of cytochrome c and Apaf-1 were notably increased by MJ-33 treatment in a concentration-dependent manner compared with the control group (Fig. 5B). Conversely, the protein expression levels of pro-caspase-9 and pro-caspase-3 were decreased by MJ-33 treatment compared with the control group. The results indicated that apoptotic cell death was promoted by MJ-33 treatment via the mitochondria-mediated apoptotic signaling pathway.
MJ-33 activates an autophagic mechanism in HT-29/5FUR cells
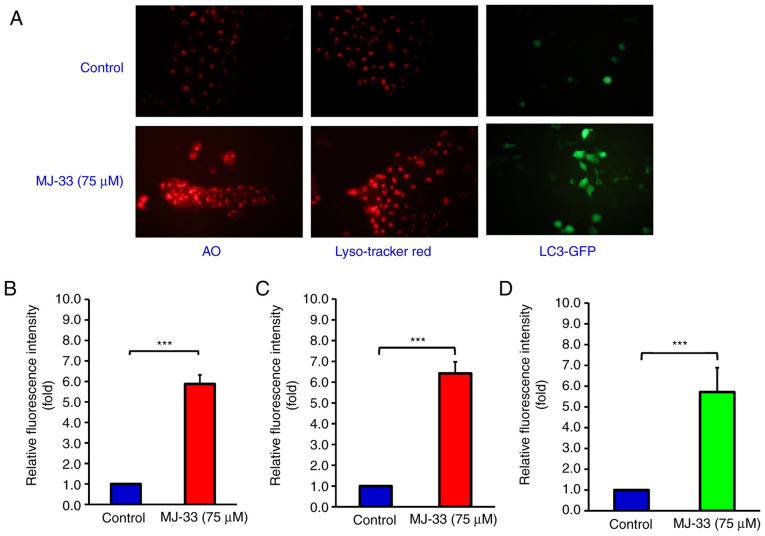
The aforementioned results prompted further investigation into MJ-33-induced autophagy in HT-29/5FUR cells. Cells were treated with 75 µM MJ-33 and then stained with AO, LysoTracker Red or LC3-GFP (Fig. 6A). The relative fluorescence intensity emitted in the red range indicated significantly increased uptake of AO in the MJ-33-treated group compared with the control group, which suggested increased formation of acidic vesicles (Fig. 6B). LysoTracker Red staining results suggested significantly increased lysosomal activity and autophagosome maturation in the MJ-33 treatment group compared with the control group (Fig. 6C). Additionally, the green fluorescence emitted in the LC3-GFP stained group was significantly higher in the MJ-33 treatment group compared with the control group, indicating punctate formation, which is typically observed in autophagic cells (Fig. 6D). Collectively, the aforementioned results suggested that MJ-33 treatment activated an autophagic mechanism in HT-29/5FUR cells.
Figure 6.
MJ-33 induced HT-29/5FUR cell autophagy. (A) AO, LysoTracker Red and LC3-GFP staining of MJ-33-treated HT-29/5FUR cells was observed under a fluorescence microscope (magnification, ×200). Relative fluorescence intensity of (B) AO, (C) LysoTracker Red uptake and (D) LC3-GFP. Data are presented as the mean ± SD from three independent experiments. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. ***P<0.001. 5FUR, fluorouracil-resistant; AO, acridine orange; GFP, green fluorescent protein.
MJ-33 inhibits AKT/mTOR activity and elevates the expression of autophagy-related proteins in HT-29/5FUR cells
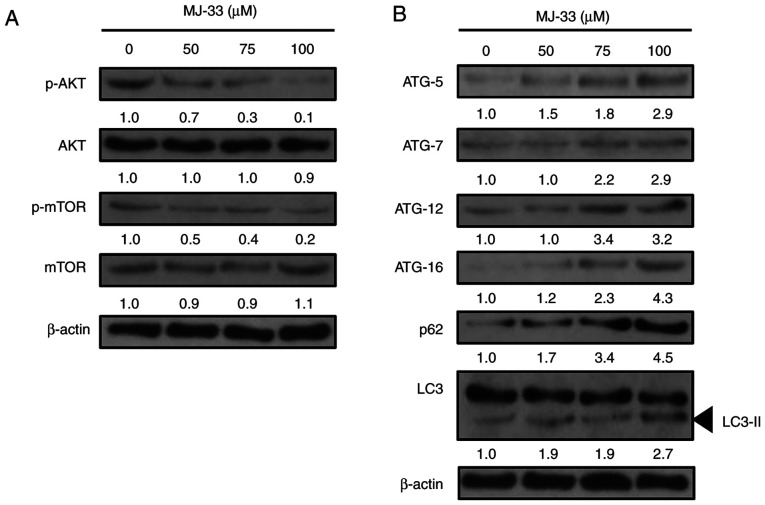
It was previously reported that MJ-33 treatment regulated the MAPK, AKT, NF-κB and activator protein-1 signaling pathways in DU145 cells (14). Since AKT/mTOR signaling may mediate both apoptosis and autophagy (16,34), the effect of MJ-33 treatment on AKT and mTOR protein expression levels in HT-29/5FUR cells was evaluated. The western blotting results demonstrated that the ratios of p-AKT/AKT and p-mTOR/mTOR protein were notably decreased by MJ-33 treatment in a concentration-dependent manner compared with the control group (Fig. 7A). Therefore, the results suggested that the activity of the AKT/mTOR axis was inhibited by MJ-33 treatment in HT-29/5FUR cells.
Figure 7.
Effects of MJ-33 treatment on the expression levels of AKT/mTOR axis- and autophagy-related proteins in HT-29/5FUR cells. Western blotting was performed to measure the expression levels of (A) AKT/mTOR axis- and (B) autophagy-related proteins in HT-29/5FUR cells following MJ-33 treatment. The density of the bands compared with the control sample (set to 1.0) are presented above each band. 5FUR, fluorouracil-resistant; p, phosphorylated; ATG, autophagy related.
According to previous studies, inhibition of mTOR activity may induce autophagy via upregulating the expression of the ATG protein family (15,18,35). To further elucidate the molecular mechanisms underlying MJ-33-induced autophagy in HT-29/5FUR cells, the expression levels of autophagy-related proteins, including ATG-5, ATG-7, ATG-12, and ATG-16, p62 and LC3-II, were examined in MJ-33-treated HT-29/5FUR cells. The protein expression levels of all ATG proteins and p62 were notably increased, whereas the ratio of LC3/LC3-II was markedly decreased by MJ-33 treatment in a concentration-dependent manner compared with the control group (Fig. 7B). The results suggested that MJ-33-induced autophagy may be triggered at the vesicle nucleation step, resulting in ATG protein involvement, reductions in the LC3/LC3-II ratio and increased p62 expression, ultimately supporting autophagosome formation and the overall progression of autophagy.
Inhibition of autophagy enhances MJ-33-induced apoptosis in HT-29/5FUR cells
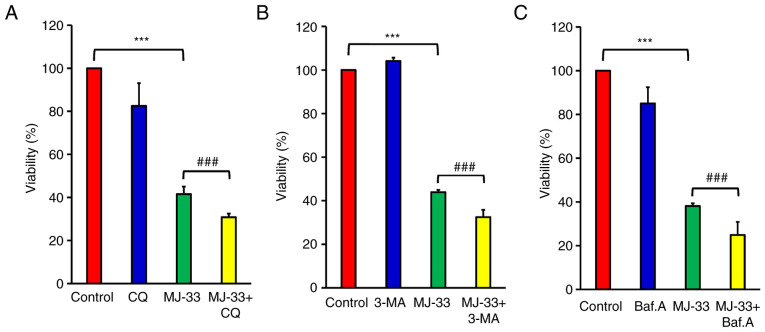
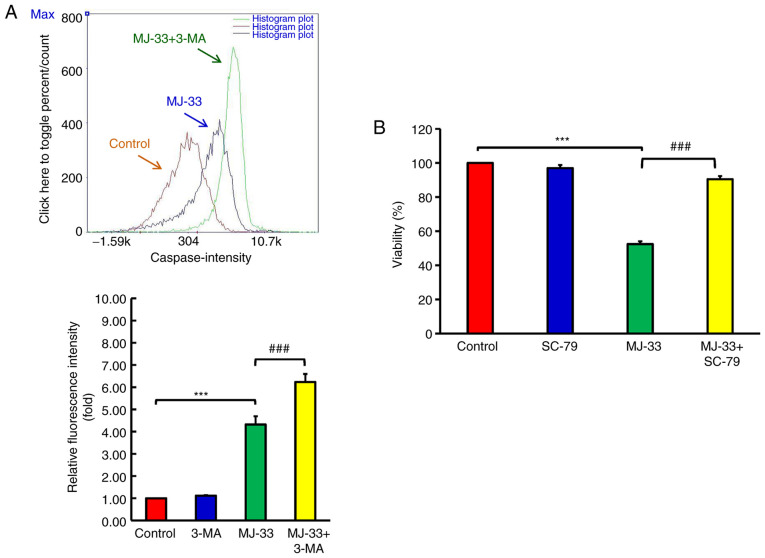
To further examine the contribution of autophagy signaling to MJ-33-induced cell death, HT-29/5FUR cells were treated with different autophagy inhibitors, including CQ, 3-MA and Baf.A1. HT-29/5FUR cells were treated with autophagy inhibitor and/or MJ-33. Combined treatments significantly decreased cell viability compared with treatment with MJ-33 alone (Fig. 8A-C). To clarify the effect of autophagy inhibitors on MJ-33-induced apoptosis, the effects of MJ-33 and 3-MA treatment on caspase-3 and caspase-7 activities were assessed by performing FLICAs. MJ-33 treatment alone significantly elevated caspase-3 and caspase-7 activities compared with the control group (Fig. 9A). However, the combination of MJ-33 and 3-MA treatment induced significantly higher caspase-3 and caspase-7 activities compared with the MJ-33 group. Collectively, the results suggested that the autophagy mechanism in HT-29/5FUR cells was associated with MJ-33-induced cell death, indicating that autophagy may serve a cytoprotective role by inhibiting the apoptosis mechanism.
Figure 8.
Involvement of autophagy in MJ-33-treated HT-29/5FUR cells. Cell viability following treatment with MJ-33 and/or (A) CQ, (B) 3-MA and (C) Baf.A. Data are presented as the mean ± SD from three independent experiments. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. ***P<0.001; ###P<0.001. 5FUR, fluorouracil-resistant; CQ, chloroquine; 3-MA, 3-methyladenine; Baf.A, bafilomycin A1.
Figure 9.
MJ-33-induced apoptosis is regulated via an autophagy mechanism and AKT activity. (A) Effect of 3-MA on caspase-3 and caspase-7 activities in MJ-33-treated HT-29/5FUR cells. (B) Effect of SC-79 on HT-29/5FUR cell viability following treatment with MJ-33. Data are presented as the mean ± SD from three independent experiments. Data were analyzed using one-way ANOVA followed by Tukey's post hoc test. ***P<0.001; ###P<0.001. 3-MA, 3-methyladenine; 5FUR, fluorouracil-resistant.
AKT serves a pivotal role in the mechanism underlying MJ-33-induced cytotoxicity in HT-29/5FUR cells
MJ-33 inhibits AKT activity in a concentration-dependent manner, thus further experiments to determine the role of AKT in MJ-33-induced cytotoxicity were performed. Following treatment with MJ-33 and/or SC-79 (AKT specific activator), HT-29/5FUR cell viability was assessed. SC-79 did not significantly alter cell viability compared with the control group (Fig. 9B). Conversely, SC-79 significantly restored HT-29/5FUR cell viability in MJ-33-treated cells. The results suggested that AKT may serve as a critical regulator of MJ-33-induced cytotoxicity in HT-29/5FUR cells.
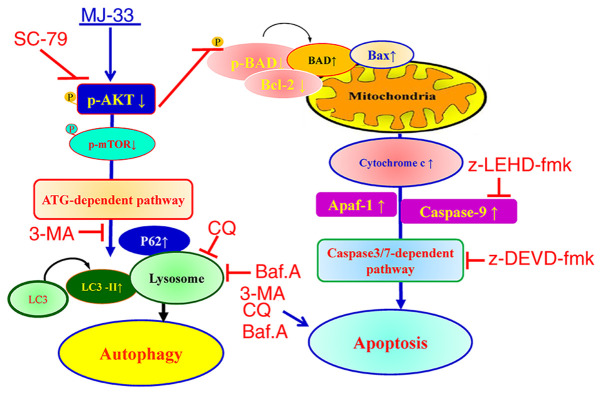
Collectively, the results indicated that MJ-33 selectively induced cytotoxicity in HT-29/5FUR cells via mediating the AKT/mTOR signaling pathway. The proposed molecular mechanism underlying MJ-33 in HT-29/5FUR cells is presented in Fig. 10. The results indicated that MJ-33 inhibited AKT activity, which triggered apoptosis via the caspase-dependent signaling pathway and induced autophagy via the ATG-dependent pathway.
Figure 10.
Schematic diagram of the proposed mechanism underlying MJ-33-induced apoptosis and autophagy in fluorouracil-resistant HT-29 cells. p, phosphorylated; ATG, autophagy related; 3-MA, 3-methyladenine; LC3, microtubule associated protein 1 light chain 3α; CQ, chloroquine; Baf.A, bafilomycin A1; Apaf-1, apoptotic peptidase activating factor 1.
Discussion
Quinazolinone compounds may possess diverse bioactivities, including anticancer properties (22,28,36). The anticancer mechanisms underlying apoptosis induction and antiproliferative activity have been previously reported (28). However, the autophagy-associated mechanisms of action of these compounds, particularly in chemoresistant cancer cells, have not been extensively investigated (36,37). The present study examined the cytotoxic effect of MJ-33 on a chemoresistant CRC cell line. The results indicated that MJ-33-induced cytotoxicity was associated with apoptosis and autophagy. Since the antimetastatic effects of MJ-33 were previously reported (14), the findings of the present study provided further understanding of the effects of MJ-33, contributing to establishing the therapeutic profile of MJ-33, which may be useful for further exploration of the anticancer effects of MJ-33 in 5FU-resistant cancer cells.
Apoptosis is a programmed cell death mechanism that serves a key role in the inhibition of tumor growth by limiting the number of cells (33). 5FU is an apoptosis-inducing agent, which is widely utilized for CRC treatment, despite its low reported response rates (6–8). Chemoresistant cancer cells are generally characterized by the absence of response to treatment, maintaining their ability to survive and continuous proliferation under chemotherapy treatment (38). In cancer cells exhibiting resistance to 5FU, the inactivation of apoptosis was reported in several studies (39,40). In the present study, the results suggested that MJ-33 induced cytotoxicity by reactivating the apoptotic mechanism in HT-29/5FUR cells. DNA condensation and fragmentation were observed following MJ-33 treatment, suggesting induction of HT-29/5FUR cell apoptosis. The significantly increased number of early apoptotic cells (Annexin V positive/PI negative) in the MJ-33 treatment group compared with the control group further indicated that MJ-33 induced apoptosis. Moreover, the results suggested that MJ-33-induced apoptosis was mediated via a caspase-dependent signaling pathway. Since AKT activity is responsible for cell survival and apoptosis (15), the effect of MJ-33 on AKT activity and possible downstream signaling pathways were evaluated in HT-29/5FUR cells. Compared with the control group, the p-AKT/AKT ratio was notably decreased following MJ-33 treatment, suggesting that AKT activity was inhibited via downregulation of AKT phosphorylation. Moreover, compared with the control group, AKT activator treatment alone did not enhance cell viability, but in combination with MJ-33 treatment, AKT activator significantly restored cell viability, suggesting that MJ-33-induced apoptosis may be controlled via AKT signaling. In addition, the Bcl-2/BAX and p-BAD/BAD ratios were markedly decreased in the MJ-33 treatment group compared with the control group. The Bcl-2/BAX ratio is a proapoptotic indicator and the activity of BAD is regulated via phosphorylation, both of which control the process of cell apoptosis (13,29). Moreover, compared with the control group, MJ-33 treatment markedly upregulated proapoptotic protein expression, promoting programmed cell death. Furthermore, the notably increased expression levels of cytochrome c and Apaf-1, and markedly decreased expression levels of pro-caspase-9 and pro-caspase-3 in the MJ-33 treatment group compared with the control group suggested that MJ-33-induced apoptosis was triggered and controlled via the intrinsic mitochondria-dependent signaling pathway.
Autophagy is also a mechanism closely associated with cell death and survival (21). The interaction between cytotoxicity and autophagy displays dual roles as dependent on the level of the signal, stimuli or stress can induce autophagy as an offensive or defensive mechanism (41,42). The antiproliferative effect associated with autophagy of several quinazolinone compounds was previously reported (22,43). In the present study, MJ-33-induced HT-29/5FUR cell autophagy was detected by observing morphological alterations via microscopic examination. Furthermore, the formation of acidic vesicles, increasing volume of autophagic vesicles, acidic vesicular organelles and increased lysosomal activity indicated autophagy activation following MJ-33 treatment. Moreover, the punctate patterns suggested autophagosome maturation in MJ-33-treated cells compared with control cells, which also indicated autophagy induction. The ATG12-ATG5 conjugate interacts with ATG16 to form a complex that catalyzes the expanding autophagosome membrane, promoting early-stage autophagy (18,41). The conversion of LC3 to LC3-II via lipidation is required for autophagosome membrane maturation during the process of autophagy (44). In addition, the p62 protein is an autophagy substrate and an indicator for cargo recognition, serving an important role in delivering ubiquitinated proteins to the proteasome for degradation (45). In the present study, compared with the control group, MJ-33 treatment markedly increased p62 protein expression levels, which indicated induction of later-stage autophagy via triggering of autophagy-related protein expression. Collectively, the results indicated that MJ-33-induced HT-29/5FU autophagy was triggered and processed via inhibiting mTOR phosphorylation, and subsequently upregulating the expression of autophagy-related proteins.
Alterations in AKT activity are crucial for cell survival and strongly regulate apoptosis (15). The effect of MJ-33 on the regulation of AKT in HT-29/5FUR cells was investigated. The results demonstrated a markedly reduced p-AKT/AKT ratio in MJ-33-treated cells compared with control cells, indicating that AKT activity was inhibited by suppressing its phosphorylation. It was inferred that MJ-33-induced apoptosis may be controlled via AKT signaling. mTOR is an important molecule downstream of AKT, which serves critical roles in autophagy pre-initiation and progression (15). As the main component of the mTOR complex 1, it inhibits Unc-51 like autophagy activating kinase (46) and also regulates lysosome activity by suppressing transcription factor EB (47). Therefore, the present study investigated the effects of MJ-33 treatment on AKT/mTOR activity in HT-29/5FUR cells, and the association between the effects and MJ-33-induced autophagy. Compared with the control group, MJ-33 treatment inhibited the AKT/mTOR axis, thereby promoting autophagy. In previous studies investigating quinazolinone compounds, the anticancer effects against CRC cells have been reported via a variety of mechanisms, including apoptosis, antiangiogenesis and antimetastasis mechanisms (48,49). The molecular mechanisms associated with the anticancer properties of these compounds have been reported, including thymidylate synthase (TS) inhibition (50) and PI3K inactivation (48,51). The findings of the present study were consistent with previously reported results on the inhibitory effect of MJ-33 on the AKT/mTOR/AMPK axis in DU145 cells (14), which resulted in NF-κB downregulation. Therefore, exploring the relationships among NF-κB activity, the mechanism underlying 5FU resistance and the activity of MJ-33 should be investigated in future studies. Constitutive nuclear NF-κB activity is highly activated in TS inhibitor-resistant cells (52), and inhibiting NF-κB translocation may lower TS levels in CRC (53), causing higher TS expression in 5FU-resistant HT-29 cells (54). Since TS is the primary target of 5FU, the aforementioned studies also indicated that MJ-33 may serve as a potential therapeutic agent among quinazolinone class compounds for developing drugs against 5FU-resistant CRC.
The interplay between autophagy and apoptosis is complicated, with multiple processes, connections and crosstalk (20,21). Autophagy typically occurs before apoptosis in cells, serving an important role in determining cell survival (20,55). However, depending on the stress level, autophagy can result in autophagic cell death or a cytoprotective effect (41,42). In the present study, the association between MJ-33-induced apoptosis and autophagy was examined in HT-29/5FUR cells by using different autophagy inhibitors (3-MA, Baf.A1 and CQ). HT-29/5FUR cells were treated with autophagy inhibitors and/or MJ-33 to evaluate the association between MJ-33-induced autophagy and apoptosis. The three autophagy inhibitors used in the present study interfered with different stages of the autophagic process. At the early stages, 3-MA inhibits class-III-PI3K to block the formation of autophagosomes (56); at later stages, Baf.A1 inhibits vacuolar-type H+-ATPase to prevent fusion of the lysosome with the autophagosome (57), whereas CQ serves as a lysosomal deacidification agent (58). Each autophagy inhibitor combined with MJ-33 treatment significantly decreased cell viability compared with MJ-33 treatment alone. Moreover, 3-MA treatment combined with MJ-33 treatment significantly increased caspase-3 and caspase-7 activities compared with MJ-33 treatment alone, suggesting a cytoprotective role of the autophagy mechanism in HT-29/5FUR cells. The results indicated the protective function of autophagy under these conditions via the ‘recycling’ mechanism, a function of lysosomes to produce amino acids from dysfunctional cellular components. Moreover, the results of the present study were consistent with previous studies reporting that autophagy inhibition may induce apoptosis or enhance chemotherapy-induced apoptosis via several mechanisms involving apoptosis-autophagy crosstalk (59–61). As previously reported, autophagy is a ‘double-edged sword’, resulting in an offensive or defensive mechanism dependent on the stress level (41,42). Based on the results of the present study, it was hypothesized that MJ-33-induced autophagy served a protective role in HT-29/5FUR cells, protecting against stress-induced cell death. MJ-33-induced autophagy-dependent cell death is considered as a result of autophagy-apoptosis interaction, which was recently categorized as ‘autophagy-associated cell death’ (21). Recent views have emerged, suggesting an improved cancer treatment strategy by combining autophagy-inducing anticancer drugs with an autophagy inhibitor compared with monotherapy (57,58,61,62). Future studies should investigate the aspects of MJ-33-induced autophagy, as well as the protective function of MJ-33-induced autophagy in other types of cancer cells, and MJ-33 combination strategies.
The present study had a number of limitations. The proliferative inhibitory concentration of MJ-33 in HT-29/5FUR cells was considerably high, which might be attributed to the chemoresistant properties of the cell line, which could be considered as an immortalized cell line. CRC chemoresistance has been suggested to occur as a result of alterations in drug targets (38,52,54); therefore, higher treatment doses or therapeutic with increased selectivity are required. The insignificant cytotoxicity of MJ-33 on normal colon cells supports further investigation of the anti-CRC effect of MJ-33. Future studies should aim to design suitable combination regimens with possible synergistic effects in order to lower the effective concentration of MJ-33. In the present study, the results suggested that the combination of MJ-33 with an autophagy inhibitor may serve as a useful strategy for improved cytotoxicity with lower treatment doses.
The proposed molecular mechanism underlying MJ-33 in HT-29/5FUR cells that was identified in the present study is presented in Fig. 10. Consistent with a previous study that investigated the antimetastasis effects of MJ-33 (14), the interaction of MJ-33 and the AKT/mTOR pathway was further indicated in the present study. The results of the present study may provide novel insights into the cytotoxicity of MJ-33, MJ-33-induced autophagy and apoptosis, as well as the underlying molecular mechanisms. Additional effects of MJ-33 on different hallmarks of cancer, such as angiogenesis, metastasis and molecular targets, require further investigation.
In conclusion, the present study demonstrated that MJ-33 treatment significantly inhibited HT-29/5FUR cell viability compared with the control group in vitro. Moreover, the results indicated that MJ-33-induced cytotoxicity in HT-29/5FUR cells was mediated via the inhibitory effect of MJ-33 on the AKT/mTOR signaling pathway, which subsequently triggered apoptosis and autophagy. Therefore, MJ-33 may serve as a promising anticancer drug for 5FU-resistant CRC.
Supplementary Material
Acknowledgements
The authors would like to thank Mr Lai-Hsiang Chang and Mr Chin-Chen Lin (Tekon Scientific Corp.) for providing assistance and equipment for the present study.
Funding
The present study was supported by the Ministry of Science and Technology, Taiwan (grant no. MOST 109-2320-B-039-041) and China Medical University Hospital (grant no. DMR-108-106).
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MJH, HAH and JSY contributed to designing the study. JHC, DTB, YNJ and YHL performed the experiments. FJT and JSY analyzed the data. HAH, JSY and FJT wrote and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
References
- 1.Pan P, Yu J, Wang LS. Colon cancer: What we eat. Surg Oncol Clin N Am. 2018;27:243–267. doi: 10.1016/j.soc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F, Arnold M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int J Cancer. 2019;144:2992–3000. doi: 10.1002/ijc.32055. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Chen Z, Li J. The current status of treatment for colorectal cancer in China: A systematic review. Medicine (Baltimore) 2017;96:e8242. doi: 10.1097/MD.0000000000008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 6.Pardini B, Kumar R, Naccarati A, Novotny J, Prasad RB, Forsti A, Hemminki K, Vodicka P, Bermejo JL. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br J Clin Pharmacol. 2011;72:162–163. doi: 10.1111/j.1365-2125.2010.03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 8.Giacchetti S, Perpoint B, Zidani R, Le Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y, Coudert B, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136–147. doi: 10.1200/JCO.2000.18.1.136. [DOI] [PubMed] [Google Scholar]
- 9.Khan M, Saif A, Sandler S, Mirrakhimov AE. Idelalisib for the treatment of chronic lymphocytic leukemia. ISRN Oncol. 2014;2014:931858. doi: 10.1155/2014/931858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao H, Zhang Y, Sun J, Zhan C, Zhao L. Raltitrexed inhibits HepG2 cell proliferation via G0/G1 cell cycle arrest. Oncol Res. 2016;23:237–248. doi: 10.3727/096504016X14562725373671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pivot X, Wadler S, Kelly C, Ruxer R, Tortochaux J, Stern J, Belpomme D, Humblet Y, Domenge C, Clendeninn N, et al. Result of two randomized trials comparing nolatrexed (Thymitaq™) versus methotrexate in patients with recurrent head and neck cancer. Ann Oncol. 2001;12:1595–1599. doi: 10.1023/A:1013185402896. [DOI] [PubMed] [Google Scholar]
- 12.Chen KT, Hour MJ, Tsai SC, Chung JG, Kuo SC, Lu CC, Chiu YJ, Chuang YH, Yang JS. The novel synthesized 6-fluoro-(3-fluorophenyl)-4-(3-methoxyanilino)quinazoline (LJJ-10) compound exhibits anti-metastatic effects in human osteosarcoma U-2 OS cells through targeting insulin-like growth factor-I receptor. Int J Oncol. 2011;39:611–619. doi: 10.3892/ijo.2011.1071. [DOI] [PubMed] [Google Scholar]
- 13.Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC, Chung JG. MJ-29 inhibits tubulin polymerization, induces mitotic arrest, and triggers apoptosis via cyclin-dependent kinase 1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J Pharmacol Exp Ther. 2010;334:477–488. doi: 10.1124/jpet.109.165415. [DOI] [PubMed] [Google Scholar]
- 14.Hour MJ, Tsai SC, Wu HC, Lin MW, Chung JG, Wu JB, Chiang JH, Tsuzuki M, Yang JS. Antitumor effects of the novel quinazolinone MJ-33: Inhibition of metastasis through the MAPK, AKT, NF-κB and AP-1 signaling pathways in DU145 human prostate cancer cells. Int J Oncol. 2012;41:1513–1519. doi: 10.3892/ijo.2012.1560. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Nitulescu GM, Van De Venter M, Nitulescu G, Ungurianu A, Juzenas P, Peng Q, Olaru OT, Grădinaru D, Tsatsakis A, Tsoukalas D, et al. The Akt pathway in oncology therapy and beyond (Review) Int J Oncol. 2018;53:2319–2331. doi: 10.3892/ijo.2018.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–248. doi: 10.1016/S1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM, Tsao JW, Juan YN, Chiu HY, Yang JS, Wang CC. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol. 2017;50:873–882. doi: 10.3892/ijo.2017.3866. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Zhang S, Wang R, Wu X, Zeng L, Fu Z. Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by suppressing the PI3K/AKT signaling pathway. Biosci Rep. 2017;37:BSR20160447. doi: 10.1042/BSR20160447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: The interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Guru SK, Pathania AS, Mupparapu N, Kumar A, Malik F, Bharate SB, Ahmed QN, Vishwakarma RA, Bhushan S. A novel quinazolinone derivative induces cytochrome c interdependent apoptosis and autophagy in human leukemia MOLT-4 cells. Toxicol Rep. 2014;1:1013–1025. doi: 10.1016/j.toxrep.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai K, Viars C, Arden K, Tarin D, Urquidi V, Goodison S. Comprehensive karyotyping of the HT-29 colon adenocarcinoma cell line. Genes Chromosomes Cancer. 2002;34:1–8. doi: 10.1002/gcc.10003. [DOI] [PubMed] [Google Scholar]
- 24.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 25.Plasencia C, Abad A, Martinez-Balibrea E, Taron M. Antiproliferative effects of ZD0473 (AMD473) in combination with 5-Fluorouracil or SN38 in human colorectal cancer cell lines. Invest New Drugs. 2004;22:399–409. doi: 10.1023/B:DRUG.0000036682.99818.71. [DOI] [PubMed] [Google Scholar]
- 26.Lee HP, Chen PC, Wang SW, Fong YC, Tsai CH, Tsai FJ, Chung JG, Huang CY, Yang JS, Hsu YM, et al. Plumbagin suppresses endothelial progenitor cell-related angiogenesis in vitro and in vivo. J Functional Foods. 2019;52:537–544. doi: 10.1016/j.jff.2018.11.040. [DOI] [Google Scholar]
- 27.Huang TY, Peng SF, Huang YP, Tsai CH, Tsai FJ, Huang CY, Tang CH, Yang JS, Hsu YM, Yin MC, et al. Combinational treatment of all-trans retinoic acid (ATRA) and bisdemethoxycurcumin (BDMC)-induced apoptosis in liver cancer Hep3B cells. J Food Biochem. 2020;44:e13122. doi: 10.1111/jfbc.13122. [DOI] [PubMed] [Google Scholar]
- 28.Lu CC, Yang JS, Chiang JH, Hour MJ, Lin KL, Lee TH, Chung JG. Cell death caused by quinazolinone HMJ-38 challenge in oral carcinoma CAL 27 cells: Dissections of endoplasmic reticulum stress, mitochondrial dysfunction and tumor xenografts. Biochim Biophys Acta. 2014;1840:2310–2320. doi: 10.1016/j.bbagen.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Beberok A, Wrzesniok D, Rok J, Rzepka Z, Respondek M, Buszman E. Ciprofloxacin triggers the apoptosis of human triple-negative breast cancer MDA-MB-231 cells via the p53/Bax/Bcl-2 signaling pathway. Int J Oncol. 2018;52:1727–1737. doi: 10.3892/ijo.2018.4310. [DOI] [PubMed] [Google Scholar]
- 30.Gelles JD, Chipuk JE. Robust high-throughput kinetic analysis of apoptosis with real-time high-content live-cell imaging. Cell Death Dis. 2016;7:e2493. doi: 10.1038/cddis.2016.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee CF, Chiang NN, Lu YH, Huang YS, Yang JS, Tsai SC, Lu CC, Chen FA. Benzyl isothiocyanate (BITC) triggers mitochondria-mediated apoptotic machinery in human cisplatin-resistant oral cancer CAR cells. Biomedicine (Taipei) 2018;8:15. doi: 10.1051/bmdcn/2018080315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CC, Chen KB, Tsai CH, Tsai FJ, Huang CY, Tang CH, Yang JS, Hsu YM, Peng SF, Chung JG. Casticin inhibits human prostate cancer DU 145 cell migration and invasion via Ras/Akt/NF-κB signaling pathways. J Food Biochem. 2019;43:e12902. doi: 10.1111/jfbc.12902. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JH, Xu M. DNA fragmentation in apoptosis. Cell Res. 2000;10:205–211. doi: 10.1038/sj.cr.7290049. [DOI] [PubMed] [Google Scholar]
- 34.Nitulescu GM, Margina D, Juzenas P, Peng Q, Olaru OT, Saloustros E, Fenga C, Spandidos DA, Libra M, Tsatsakis AM. Akt inhibitors in cancer treatment: The long journey from drug discovery to clinical use (Review) Int J Oncol. 2016;48:869–885. doi: 10.3892/ijo.2015.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CC, Chiang JH, Tsai FJ, Hsu YM, Juan YN, Yang JS, Chiu HY. Metformin triggers the intrinsic apoptotic response in human AGS gastric adenocarcinoma cells by activating AMPK and suppressing mTOR/AKT signaling. Int J Oncol. 2019;54:1271–1281. doi: 10.3892/ijo.2019.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohle W, Jourdan FL, Menchon G, Prota AE, Foster PA, Mannion P, Hamel E, Thomas MP, Kasprzyk PG, Ferrandis E, et al. Quinazolinone-based anticancer agents: Synthesis, antiproliferative SAR, antitubulin activity, and tubulin co-crystal structure. J Med Chem. 2018;61:1031–1044. doi: 10.1021/acs.jmedchem.7b01474. [DOI] [PubMed] [Google Scholar]
- 37.Rahman MU, Jeyabalan G, Saraswat P, Parveen G, Khan S, Yar MS. Quinazolines and anticancer activity: A current perspectives. Synthetic Communications. 2017;47:379–408. doi: 10.1080/00397911.2016.1269926. [DOI] [Google Scholar]
- 38.Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: An overview. Cancers (Basel) 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He L, Zhu H, Zhou S, Wu T, Wu H, Yang H, Mao H, SekharKathera C, Janardhan A, Edick AM, et al. Wnt pathway is involved in 5-FU drug resistance of colorectal cancer cells. Exp Mol Med. 2018;50:101. doi: 10.1038/s12276-018-0128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiuthed A, Ninsontia C, Erlenbach-Wuensch K, Ndreshkjana B, Muenzner JK, Caliskan A, Husayn AP, Chaotham C, Hartmann A, Roehe AV, et al. Cytoplasmic p21 mediates 5-fluorouracil resistance by inhibiting pro-apoptotic Chk2. Cancers (Basel) 2018;10:373. doi: 10.3390/cancers10100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravanan P, Srikumar IF, Talwar P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017;188:53–67. doi: 10.1016/j.lfs.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Sever ON, Demir OG. Autophagy: Cell death or survive mechanism. J Oncol Sci. 2017;3:37–44. doi: 10.1016/j.jons.2017.07.001. [DOI] [Google Scholar]
- 43.Xia X, Wang L, Zhang X, Wang S, Lei L, Cheng L, Xu Y, Sun Y, Hang B, Zhang G, et al. Halofuginone-induced autophagy suppresses the migration and invasion of MCF-7 cells via regulation of STMN1 and p53. J Cell Biochem. 2018;119:4009–4020. doi: 10.1002/jcb.26559. [DOI] [PubMed] [Google Scholar]
- 44.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 45.Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett. 2016;21:29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang JS, Lu CC, Kuo SC, Hsu YM, Tsai SC, Chen SY, Chen YT, Lin YJ, Huang YC, Chen CJ, et al. Autophagy and its link to type II diabetes mellitus. Biomedicine (Taipei) 2017;7:8. doi: 10.1051/bmdcn/2017070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao E, Czaja MJ. Transcription factor EB: A central regulator of both the autophagosome and lysosome. Hepatology. 2012;55:1632–1634. doi: 10.1002/hep.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussain A, Qazi AK, Mupparapu N, Guru SK, Kumar A, Sharma PR, Singh SK, Singh P, Dar MJ, Bharate SB, et al. Modulation of glycolysis and lipogenesis by novel PI3K selective molecule represses tumor angiogenesis and decreases colorectal cancer growth. Cancer Lett. 2016;374:250–260. doi: 10.1016/j.canlet.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Jackman AL, Kimbell R, Brown M, Brunton L, Harrap KR, Wardleworth JM, Boyle FT. The antitumour activity of ZD9331, a non-polyglutamatable quinazoline thymidylate synthase inhibitor. Adv Exp Med Biol. 1994;370:185–188. doi: 10.1007/978-1-4615-2584-4_40. [DOI] [PubMed] [Google Scholar]
- 50.Benepal TS, Judson I. ZD9331: Discovery to clinical development. Anticancer Drugs. 2005;16:1–9. doi: 10.1097/00001813-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Hussain A, Qazi AK, Mupparapu N, Kumar A, Mintoo MJ, Mahajan G, Sharma PR, Singh SK, Bharate SB, Zargar MA, et al. A novel PI3K axis selective molecule exhibits potent tumor inhibition in colorectal carcinogenesis. Mol Carcinog. 2016;55:2135–2155. doi: 10.1002/mc.22457. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Cassidy J. Constitutive nuclear factor-kappa B mRNA, protein overexpression and enhanced DNA-binding activity in thymidylate synthase inhibitor-resistant tumour cells. Br J Cancer. 2003;88:624–629. doi: 10.1038/sj.bjc.6600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajitha B, Belalcazar A, Nagaraju GP, Shaib WL, Snyder JP, Shoji M, Pattnaik S, Alam A, El-Rayes BF. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016;373:227–233. doi: 10.1016/j.canlet.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 54.Qiu T, Xiang X, Lei W, Hao C, Zhang L, Feng M, Yu F, Li J, Xiong J. Establishment and biological characterization of 5-fluorouracil-resistant human colon cancer HT-29/5-FU cell line. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31:328–332. (In Chinese) [PubMed] [Google Scholar]
- 55.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–1906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 56.Ito S, Koshikawa N, Mochizuki S, Takenaga K. 3-Methyladenine suppresses cell migration and invasion of HT1080 fibrosarcoma cells through inhibiting phosphoinositide 3-kinases independently of autophagy inhibition. Int J Oncol. 2007;31:261–268. [PubMed] [Google Scholar]
- 57.Kawaguchi T, Miyazawa K, Moriya S, Ohtomo T, Che XF, Naito M, Itoh M, Tomoda A. Combined treatment with bortezomib plus bafilomycin A1 enhances the cytocidal effect and induces endoplasmic reticulum stress in U266 myeloma cells: Crosstalk among proteasome, autophagy-lysosome and ER stress. Int J Oncol. 2011;38:643–654. doi: 10.3892/ijo.2010.882. [DOI] [PubMed] [Google Scholar]
- 58.Liu X, Sun K, Wang H, Dai Y. Inhibition of autophagy by chloroquine enhances the antitumor efficacy of sorafenib in glioblastoma. Cell Mol Neurobiol. 2016;36:1197–1208. doi: 10.1007/s10571-015-0318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng X, Jin X, Li F, Liu X, Liu Y, Ye F, Li P, Zhao T, Li Q. Inhibiting autophagy with chloroquine enhances the anti-tumor effect of high-LET carbon ions via ER stress-related apoptosis. Med Oncol. 2017;34:25. doi: 10.1007/s12032-017-0883-8. [DOI] [PubMed] [Google Scholar]
- 60.Masud Alam M, Kariya R, Kawaguchi A, Matsuda K, Kudo E, Okada S. Inhibition of autophagy by chloroquine induces apoptosis in primary effusion lymphoma in vitro and in vivo through induction of endoplasmic reticulum stress. Apoptosis. 2016;21:1191–1201. doi: 10.1007/s10495-016-1277-7. [DOI] [PubMed] [Google Scholar]
- 61.Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H. Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol. 2009;16:761–771. doi: 10.1245/s10434-008-0260-0. [DOI] [PubMed] [Google Scholar]
- 62.Liu T, Zhang J, Li K, Deng L, Wang H. Combination of an autophagy inducer and an autophagy inhibitor: A smarter strategy emerging in cancer therapy. Front Pharmacol. 2020;11:408. doi: 10.3389/fphar.2020.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.