Abstract
Long non-coding RNA (lncRNA) forkhead box P4 antisense RNA 1 (FOXP4-AS1) has been determined to function as an oncogene in various types of cancer. However, the biological function and the underlying mechanisms of FOXP4-AS1 in mantle cell lymphoma (MCL) remain to be uncovered. The expression and the associated clinicopathological characteristics and prognostic significance of FOXP4-AS1 were explored in MCL clinical samples. The effects of FOXP4-AS1 on MCL cellular behaviors, including proliferation, migration and invasion were analyzed using CCK-8, crystal violet and Transwell assays. The downstream molecules of FOXP4-AS1 were explored using bioinformatics analysis and dual luciferase assay. Our results showed that FOXP4-AS1 expression was upregulated in MCL patients, and that the high expression of FOXP4-AS1 was correlated with the unfavorable prognosis of patients. Functionally, while FOXP4-AS1 downregulation inhibited proliferation, migration and invasion of MCL cells, FOXP4-AS1 overexpression had promotive effects on these cellular processes. Mechanistically, FOXP4-AS1 was found to act as a competing endogenous (ce)RNA for miR-423-5p to regulate the expression of nucleus accumbens-associated 1 (NACC1). The negative regulation of FOXP4-AS1 on miR-423-5p compared to that of miR-423-5p on NACC1 was determined at the mRNA or protein levels in MCL cells. Moreover, an inverse expression correlation between FOXP4-AS1 and miR-423-5p, and that between miR-423-5p and NACC1 was confirmed in MCL clinical samples. In addition, rescue assay showed that miR-423-5p upregulation or NACC1 knockdown abolished the promoting effects of FOXP4-AS1 on MCL cell proliferation, migration and invasion. In conclusion, FOXP4-AS1 promotes MCL progression through the upregulation of NACC1 expression by inhibiting miR-423-5p. FOXP4-AS1 may serve as a novel therapeutic target for patients with MCL.
Keywords: mantle cell lymphoma, FOXP4-AS1, microR-423-5p, NACC1, progression
Introduction
Mantle cell lymphoma (MCL) is a relatively rare subtype of B cell non-Hodgkin's lymphoma that only affects 1 out of 200,000 individuals worldwide (1). Due to the aggressive characteristics and continuous relapse pattern, the prognosis of MCL patients remains relatively poor, with a median survival time of only 3-5 years (2). In addition, the survival outcomes of MCL patients are even worse when the disease develops into advanced stages (3). Currently, following an improved understanding of the molecular pathogenesis of MCL, the application of a series of novel treatment approaches, including immunotherapy agents and cellular therapies have improved the survival rates of MCL patients (4). However, the treatment of MCL remains greatly challenging. Thus, identification of the potential mechanisms that contribute to the disease is vitally important in order to enable early diagnosis or treatment for MCL patients.
Long non-coding RNA (lncRNA) is a type of non-coding RNA with a length of more than 200 nucleotides that possesses limited or no ability to encode proteins (5). In recent years, a vast number of lncRNAs have been reported to play crucial regulatory roles in the initiation and progression of cancer behaviors (6). The oncogenic or tumor-suppressive role of various lncRNAs, such as MALAT1 (7), HAGLROS (8) and GATA6-AS (9) in the tumorigenesis of MCL has been well documented. During tumorigenesis, lncRNAs have been reported to act as competing endogenous RNAs (ceRNAs) through competitive binding with miRNA response elements to upregulate mRNAs (10). Hence, it is crucial to identify and to investigate the function and mechanism of novel lncRNAs during the progression of MCL. lncRNA forkhead box P4 antisense RNA 1 (FOXP4-AS1) has been reported as an oncogene in multiple solid cancers, including osteosarcoma (11), prostate cancer (12), colorectal cancer (13), esophageal squamous cell carcinoma (14) and cervical cancer (15). Nevertheless, the biological function and related mechanisms of FOXP4-AS1 in MCL remain to be uncovered.
As a member of the bric-a-brac tramtrack broad complex, nucleus accumbens-associated protein 1 (NACC1) exerts a vital regulatory role in several biological processes, including cell proliferation, apoptosis and transcription regulation (16). Moreover, the oncogenic role of NACC1 has been determined in carcinomas of many organs, such as colon/rectum (17,18), prostate (19), ovary (20) as well as hematological neoplasms (21).
In this study, FOXP4-AS1 was reported to be upregulated in human MCL tissues. High expression of FOXP4-AS1 was found to be closely associated with poor prognosis and some clinicopathologic parameters. We also demonstrated that FOXP4-AS1 promoted the proliferation, migration and invasion of MCL cells. Furthermore, our data revealed that FOXP4-AS1 promoted MCL development through the upregulation of NACC1 expression by interacting with miR-423-5p. Thus, FOXP4-AS1 may be a novel biomarker that can be used as a therapeutic candidate for MCL patients.
Materials and methods
Human clinical samples and cell lines
A total of 60 patients with MCL and 53 healthy volunteers (control group) at the First Affiliated Hospital of Shantou University Medical College (Shantou, China) between May 2016 and January 2018 were enrolled in this study. Blood samples were taken from each participant 1 day after admission. Plasma samples were prepared using a conventional method (22). B lymphocytes were isolated from the same blood samples using anti-CD19 magnetic beads and the cells were released using DETACHaBEAD CD19 Kit (Invitrogen; Thermo Fisher Scientific, Inc.). Samples with the purity and/or viability above 90% were selected for downstream assays. All samples were stored in liquid nitrogen until being used for RNA extraction. All patients were confirmed to have MCL based on the criteria of the World Health Organization classification of hematological neoplasms (1). All MCL patients were diagnosed and treated for the first time, and received chemotherapy. Some clinical parameters, including age, sex, white blood cell count (WBC), clinical stage, Eastern Cooperative Oncology Group (ECOG) score, serum lactate dehydrogenase (LDH) level and Mantle Cell Lymphoma International Prognostic Index (MIPI) score were collected. All MCL patients were followed up regularly, and the overall survival (OS) and the disease-free survival (DFS) rates were recorded. The OS was calculated from the time of treatment to the time of death or last follow-up. DFS was calculated from the time of treatment to the first time of recurrence diagnosis, death or last follow-up. Written informed contents were obtained from all the patients enrolled in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College and conducted in compliance with the Declaration of Helsinki.
Human MCL cell lines JVM-2 and Z138, and normal B lymphocytes IM-9 were provided by the American Type Culture Collection (ATCC). All the cells were authenticated using a STR profiling method. Cells were cultured and maintained in RPMI-1640 culture medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Invitrogen; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin and 100 mg/ml streptomycin at 37°C with 5% CO2 in a humidified incubator.
Cell transfection
FOXP4-AS1 small interfering RNA (siRNA) and the corresponding negative control (si-NC) were synthesized by RiboBio (Guangzhou, China). The pcDNA3.1 vector containing the coding sequence of FOXP4-AS1 (pcDNA-FOXP4-AS1) and the empty pcDNA3.1 vector were purchased from Genechem Co. (Shanghai, China). The empty pcDNA3.1 vector was used as negative control. While miR-423-5p mimics, miR-423-5p inhibitors and the corresponding negative controls were sourced from RiboBio (Guangzhou, China); NACC1 siRNA (siNACC1) was purchased from Sigma-Aldrich; Merck KGaA. Cell transfections were performed using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific, Inc.) in accordance to the standard method recommended by the manufacturer. Cells were extracted 48 h after transfection, for subsequent experiments.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from clinical samples or cell lines using TRIZOL regent (Invitrogen; Thermo Fisher Scientific, Inc.) or miRNeasy Mini kit (Qiagen) and subsequently reverse-transcribed into cDNA using SuperScript IV Reverse Transcriptase (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. The reactions for qPCR were conducted on ABI QuantStudio 6 (Thermo Fisher Scientific, Inc.) with SYBR® Premix Ex Taq (Takara Bio, Inc.) for the detection of FOXP4-AS1 and NACC1, or with TaqMan MicroRNA Assay Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.) for the detection of miR-423-5p. The following thermal cycling conditions were used for the PCR: 95°C for 1 min, followed by 40 cycles of 95°C for 20 sec and 56°C for 30 sec. Using the 2−ΔΔCq method (23), the relative expression levels of FOXP4-AS1 and NACC1 were normalized to that of GAPDH; while the relative expression level of miR-423-5p was normalized to that of U6. The sequences of PCR primers used in this study are shown in Table SI.
Western blotting
JVM-2 and Z138 cells were lysed in RIPA solution (Thermo Fisher Scientific, Inc.) for total protein extraction. Protein concentration was determined using Bradford reagent (Sigma-Aldrich; Merck KGaA) according to the manufacturer's instructions. Protein samples (15 µg) were separated by 10% SDS-PAGE before being transferred onto polyvinylidene fluoride membranes, which were then blocked with 5% fat-free milk followed by incubation with anti-NACC1 (at 1:1,000 dilution; cat. no. Ag6096; ProteinTech Group, Inc.) or anti-GAPDH (at 1:1,000 dilution; cat. no. 60004-1-Ig; ProteinTech Group, Inc.) primary antibodies at 4°C overnight. The membranes were then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (at 1:2,000 dilution; cat. no. 7074; Sangon Biotech Co., Ltd.) for 1 h at room temperature. The immunoreactive protein bands were visualized by chemiluminescence.
CCK-8 assay
Transfected cells were seeded into a 96-well plate at a density of 1×104 cells per well. After that, 10 µl of CCK-8 solution (Dojindo, Kumamoto, Japan) was added into the medium of each well at 24, 48, 72 and 96 h before the optical density (OD) was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Crystal violet assay
Control and transfected cells seeded into 6-well plates at a density of 1,000 cells per well were cultured with the medium changed every three days. Two weeks later, the culture medium was removed and cells were stained with 0.5% crystal violet for 10 min at room temperature. Subsequently, the cells were washed with PBS before the cell images were captured using a digital camera. The OD was measured at 570 nm with a microplate reader (Bio-Rad Laboratories, Inc.).
Transwell assays
A 24-well Transwell assay (8.0 µm, Corning) was used to examine the migration and invasion abilities of the MCL cells. While 100 µl of cell suspension containing 2×105 cells was added into the upper chamber; 600 µl of culture medium containing 10% FBS was added into the lower chamber. For the invasion assay, the upper chamber was precoated with 100 µl of Matrigel (BD Biosciences). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2 for 24 h. Then, invasive cells were fixed with methanol at 4°C for 15 min, washed with PBS twice and stained with 1% crystal violet at 4°C for 30 min. Finally, 5 randomly selected fields were captured using a light microscope (Olympus Corp.) at ×200 magnification for cell counting.
Bioinformatics analysis
Starbase v2.0 (http://starbase.sysu.edu.cn/starbase2/) and DIANA tools (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted) were used to predict the miRNAs that had complementary base paring with FOXP4-AS1.
Subcellular fractionation assay
Subcellular fractionation assay was conducted using PARIS™ Kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to the recommended protocols. JVM-2 and Z138 cells were washed in pre-chilled PBS before being lysed in cell fractionation buffer. Cell fractionation buffer was then added to the cell supernatant before centrifugation. Cell disruption buffer was used to lyse cell nuclei. The isolated RNA was examined by RT-qPCR. GAPDH and U6 were used respectively as the cytoplasmic and nuclear fractionation indicators.
RNA immunoprecipitation (RIP) assay
RIP assay was performed using Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore). JVM-2 and Z138 cells were washed in pre-chilled PBS before being lysed in RIP buffer at 4°C for 30 min. Magnetic beads conjugated to human anti-Ago2 antibodies (Millipore) or normal mouse immunoglobulin G (IgG; Millipore) were used to capture the RNAs used for RT-qPCR analysis. Then, the expression levels of FOXP4-AS1 or miR-423-5p were measured by RT-qPCR.
Luciferase reporter assay
Amplified FOXP4-AS1 and 3′-UTR (untranslated region) of NACC1 were individually subcloned into firefly plasmid pmirGLO luciferase vector (GeneChem). Wild-type FOXP4-AS1 and NACC1 3′-UTR (FOXP4-AS1-WT or NACC1-WT) were constructed. Site-directed mutation of miR-423-5p binding sites in FOXP4-AS1 and NACC1 3′-UTR (FOXP4-AS1-MUT or NACC1-MUT) was generated using GeneTailor™ Site-Directed Mutagenesis System (Invitrogen; Thermo Fisher Scientific, Inc.). JVM-2 and Z138 cells were co-transfected with the plasmid constructs and miRNAs (miR-423-5p mimic or mi-NC) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Cells were lysed after 48 h of incubation before the reporter activities were determined using a dual-luciferase reporter assay system (Promega Corp.).
Statistical analysis
All statistical analyses were processed using SPSS 19.0 software package (SPSS Inc.) and GraphPad Prism 5.0 (GraphPad Software Inc.). All experimental results were independently repeated at least three times. Data are expressed as mean ± standard deviation (SD). Categorical data were compared using Chi-square test. The correlations between the expression of FOXP4-AS1, miR-423-5p and NACC1 were analyzed using Pearson's correlation method. The OS and DFS rate of patients with MCL were estimated using Kaplan-Meier analysis. Independent predictors of OS and DFS were detected using univariate analysis and multivariate Cox regression analysis with step-wise selection. The significant prognostic factors upon univariate analysis (P<0.05) were subjected to multivariate analysis using Cox proportional hazards regression model. Differences between groups were compared using Student's t-test or the ANOVA followed by the Tukey post hoc test. Differences were considered to be significant at P<0.05.
Results
Clinical significance of FOXP4-AS1 expression in patients with MCL
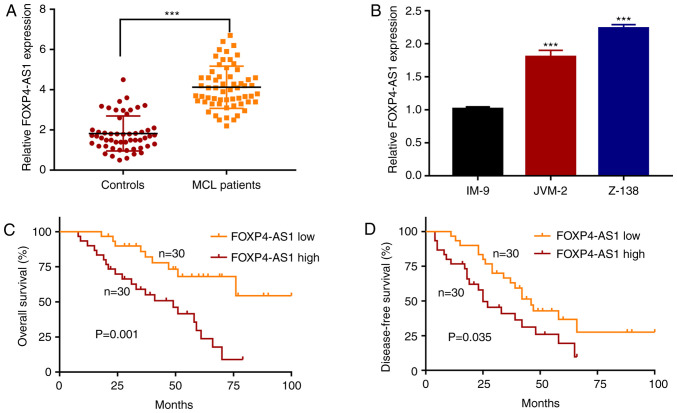
Firstly, the expression levels of FOXP4-AS1 in the plasma samples of 60 MCL and 53 healthy patients (controls) were determined by RT-qPCR. As shown in Fig. 1A, the results showed that the expression of FOXP4-AS1 was significantly upregulated in MCL tissues compared to that in the control groups (P<0.001). Then, the expression levels of FOXP4-AS1 in human MCL cell lines JVM-2 and Z138, and in normal B lymphocytes IM-9 were examined. The expression of FOXP4-AS1 was found to be significantly higher in the two MCL cell lines than that in the IM-9 cells (P<0.001; Fig. 1B). Using the median value of FOXP4-AS1 expression as the cut-off value, MCL patients were divided into two groups: FOXP4-AS1 low (30 patients) and FOXP4-AS1 high (30 patients). As shown in Fig. 1C and D, Kaplan-Meier analysis revealed that MCL patients with high FOXP4-AS1 expression had worse OS and DFS than those with low FOXP4-AS1 expression (P=0.001 and P=0.035, respectively). Taken together, the results indicated that FOXP4-AS1 is upregulated in the serum of MCL patients, and that the high expression of FOXP4-AS1 is associated with unfavorable prognosis of MCL patients.
Figure 1.
FOXP4-AS1 expression is upregulated in MCL plasma samples and is correlated with clinical prognosis. (A) Relative expression of FOXP4-AS1 in plasma samples of MCL patients (n=60) and healthy controls (n=53) evaluated by qPCR. ***P<0.001. (B) Relative expression of FOXP4-AS1 in MCL cell lines JVM-2 and Z-138 and normal B lymphocytes IM-9. ***P<0.001, compared with the IM-9 cell line. Kaplan-Meier curves for overall survival (C) and disease-free survival (D) of patients with low and high expression of FOXP4-AS1 (FOXP4-AS1 high, n=30; FOXP4-AS1 low, n=30). Data are presented as mean ± SD of three independent experiments. MCL, mantle cell lymphoma; FOXP4-AS1, forkhead box P4 antisense RNA 1.
Next, the association between FOXP4-AS1 expression and clinicopathologic parameters in MCL patients was analyzed. As shown in Table I, the number of patients in clinical stages III–IV was higher in the high FOXP4-AS1 expression group than that in the low FOXP4-AS1 expression group (P=0.028). The high FOXP4-AS1 expression group had more patients with an elevated level of LDH (P=0.035). The proportion of high risk MIPI in the high FOXP4-AS1 expression group was higher compared with that in the low FOXP4-AS1 expression group (P=0.038). Moreover, multivariate analysis showed that FOXP4-AS1 expression was a significant prognostic factor, both for OS (P=0.012; Table II) and DFS (P=0.032; Table III).
Table I.
FOXP4-AS1 expression and clinicopathologic parameters in patients with MCL.
| FOXP4-AS1, n (%) | ||||
|---|---|---|---|---|
| Parameters | Total (n=60) | High expression (n=30) | Low expression (n=30) | P-value |
| Age, years | ||||
| <60 | 18 | 10 (33.3) | 8 (26.7) | 0.573 |
| ≥60 | 42 | 20 (66.7) | 22 (73.3) | |
| Sex | ||||
| Male | 44 | 23 (76.7) | 21 (70.0) | 0.559 |
| Female | 16 | 7 (23.3) | 9 (30.0) | |
| WBC | ||||
| >10×109/l | 20 | 11 (36.7) | 9 (30.0) | 0.584 |
| ≤10×109/l | 40 | 19 (63.3) | 21 (70.0) | |
| Stages | ||||
| I–II | 20 | 6 (20.0) | 14 (46.7) | 0.028 |
| III–IV | 40 | 24 (80.0) | 16 (53.3) | |
| ECOG | ||||
| <2 | 29 | 13 (43.3) | 16 (53.3) | 0.438 |
| ≥2 | 31 | 17 (56.7) | 14 (46.7) | |
| Serum LDH | ||||
| Normal | 36 | 14 (46.7) | 22 (73.3) | 0.035 |
| Elevated | 24 | 16 (53.3) | 8 (26.7) | |
| MIPI | ||||
| Low/medium risk | 32 | 12 (40.0) | 20 (66.7) | 0.038 |
| High risk | 28 | 18 (60.0) | 10 (33.3) | |
FOXP4-AS1, forkhead box P4 antisense RNA 1; MCL, mantle cell lymphoma; WBC, white blood cell; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index. Significant P-values (P<0.05) are in bold print.
Table II.
Univariate and multivariate analysis for overall survival of the patients with MCL.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (years), ≥60 vs. <60 | 1.102 (0.873-1.541) | 0.641 | ||
| Sex, male vs. female | 1.009 (0.699-1.765) | 0.113 | ||
| WBC, >10×109/l vs. ≤10×109/l | 1.256 (0.887-1.941) | 0.174 | ||
| Stages, III–IV vs. I–II | 1.875 (1.534-2.213) | <0.001 | 1.762 (1.412-2.012) | 0.001 |
| ECOG, ≥2 vs. <2 | 0.892 (0.645-1.321) | 0.099 | ||
| Serum LDH, elevated vs. normal | 1.382 (0.801-1.738) | 0.087 | ||
| MIPI score, high risk vs. low/medium risk | 1.631 (1.231-2.012) | 0.009 | 1.598 (1.112-1.851) | 0.030 |
| FOXP4-AS1 expression, high vs. low | 1.578 (1.270-1.980) | 0.003 | 1.496 (1.119-1.831) | 0.012 |
MCL, mantle cell lymphoma; WBC, white blood cell; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index; HR, hazard ratio; CI, confidence interval. Significant P-values (P<0.05) are in bold print.
Table III.
Univariate and multivariate analysis for disease-free survival of the patients with MCL.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age (years), ≥60 vs. <60 | 1.084 (0.581-1.890) | 0.876 | ||
| Sex, male vs. female | 1.054 (0.643-1.723) | 0.234 | ||
| WBC, >10×109/l vs. ≤10×109/l | 1.172 (0.617-2.210) | 0.506 | ||
| Stages, III–IV vs. I–II | 1.875 (1.534-2.213) | 0.001 | 1.762 (1.412-2.012) | 0.012 |
| ECOG, ≥2 vs. <2 | 0.831 (0.483-1.701) | 0.320 | ||
| Serum LDH, elevated vs. normal | 1.382 (0.801-1.738) | 0.087 | ||
| MIPI score, high risk vs. low/medium risk | 1.471 (0.790-3.010) | 0.291 | ||
| FOXP4-AS1 expression, high vs. low | 1.578 (1.270-1.980) | 0.004 | 1.496 (1.119-1.831) | 0.032 |
MCL, mantle cell lymphoma; WBC, white blood cell; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index; HR, hazard ratio; CI, confidence interval. Significant P-values (P<0.05) are in bold print.
Knockdown of FOXP4-AS1 inhibits the proliferation, migration and invasion of MCL cells
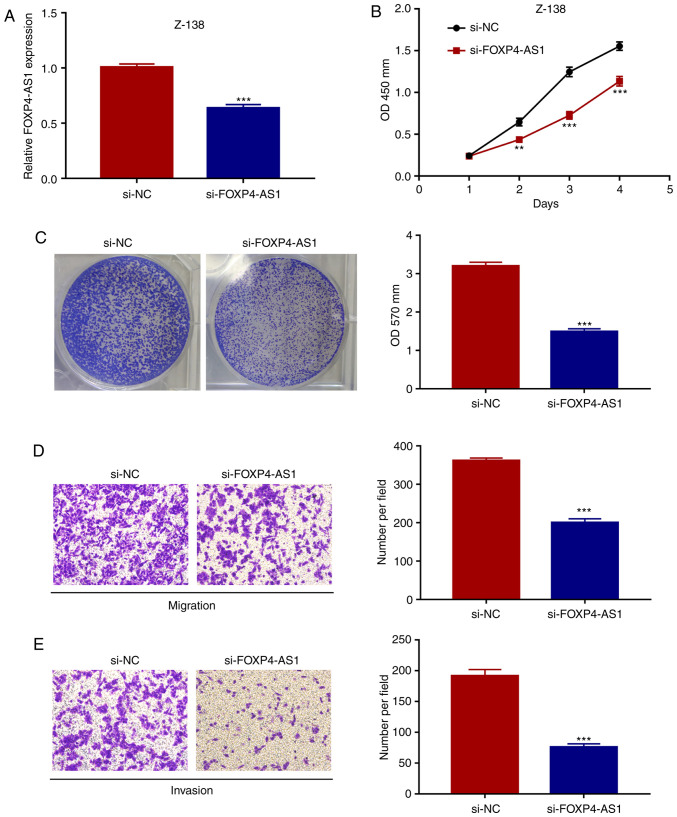
The biological function of FOXP4-AS1 in MCL was assessed using two different MCL cell lines. As shown in Fig. 1B, while Z-138 cells exhibited a relatively higher level of FOXP4-AS1 expression level, JVM-2 cells presented a lower level of FOXP4-AS1 expression. Thus, FOXP4-AS1 was knocked down in Z-138 cells but overexpressed in the JVM-2 cells. The efficiency of FOXP4-AS1 knockdown was evaluated by RT-qPCR (P<0.001; Fig. 2A). Subsequently, the effects of FOXP4-AS1 on the proliferation of MCL cells were assessed by CCK-8 and crystal violet assays. As shown in Fig. 2B and C, the results showed that FOXP4-AS1 knockdown led to significantly decreased cell viability of the Z-138 cells (all P<0.001). Then, Transwell assays showed that the migration and invasion abilities of Z-138 cells were significantly inhibited following FOXP4-AS1 downregulation (all P<0.001; Fig. 2D and E). Overall, these observations demonstrated that knockdown of FOXP4-AS1 inhibits the proliferation, migration and invasion abilities of MCL cells.
Figure 2.
FOXP4-AS1 knockdown inhibits the proliferation, migration and invasion of MCL cells. (A) Relative expression of FOXP4-AS1 in Z-138 cells transfected with siRNA plasmid evaluated by qPCR. Effects of FOXP4-AS1 knockdown on the viability of Z-138 cells as assessed by CCK-8 (B) and crystal violet (C) assays. Effects of FOXP4-AS1 knockdown on the migration (D) and the invasion (E) of Z-138 cells as assessed by Transwell assays. Data are presented as mean ± SD of three independent experiments. **P<0.01, ***P<0.001, compared with the si-NC group. MCL, mantle cell lymphoma; FOXP4-AS1, forkhead box P4 antisense RNA 1.
FOXP4-AS1 overexpression promotes the proliferation, migration and invasion of MCL cells
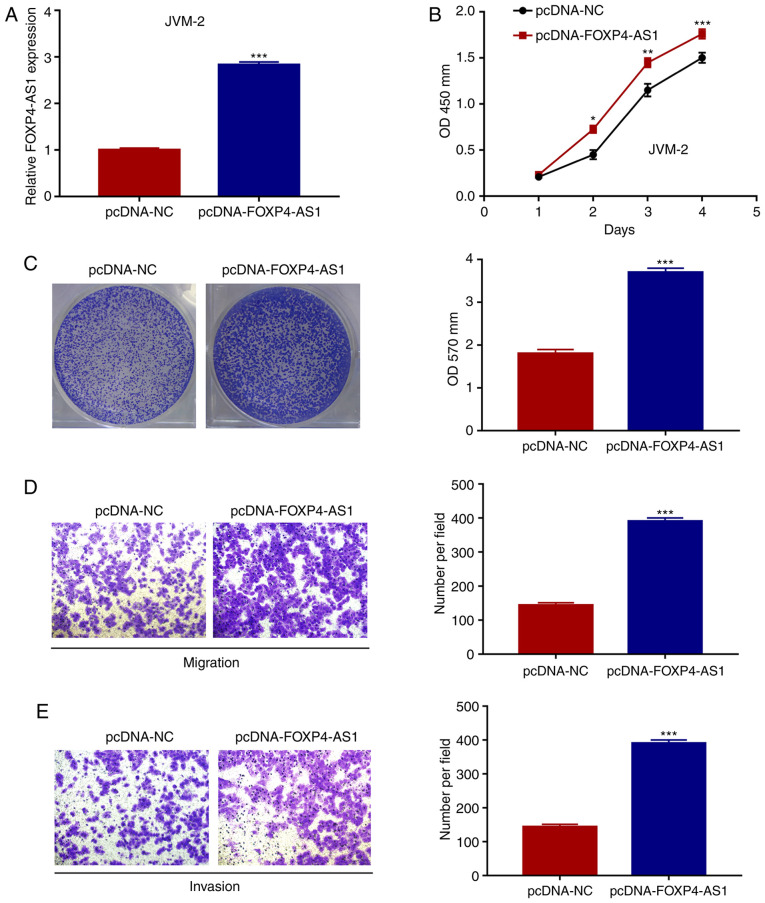
As shown in Fig. 3A, the overexpression efficiency of FOXP4-AS1 in JVM-2 cells was firstly confirmed by RT-qPCR (P<0.001). CCK-8 and crystal violet assays demonstrated that FOXP4-AS1 overexpression significantly promoted the proliferation abilities of the JVM-2 cells (all P<0.001; Fig. 3B and C). Moreover, Transwell assays demonstrated that FOXP4-AS1 overexpression markedly promoted the migration and invasion capacities of JVM-2 cells (all P<0.001; Fig. 3D and E). Taken together, these results demonstrated that FOXP4-AS1 overexpression promotes the growth, migration and invasion of MCL cells in vitro.
Figure 3.
FOXP4-AS1 overexpression promotes the proliferation, migration and invasion of MCL cells. (A) Relative expression of FOXP4-AS1 in JVM-2 cells after transfection with the pcDNA-FOXP4-AS1 plasmid evaluated by qPCR. Effects of FOXP4-AS1 overexpression on the viability of JVM-2 cells as assessed by CCK-8 (B) and crystal violet (C) assays. Effects of FOXP4-AS1 overexpression on the migration (D) and the invasion (E) of JVM-2 cells as assessed by Transwell assays. Data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001, compared with pcDNA-NC group. MCL, mantle cell lymphoma; FOXP4-AS1, forkhead box P4 antisense RNA 1.
FOXP4-AS1 acts as a sponge for miR-423-5p in MCL
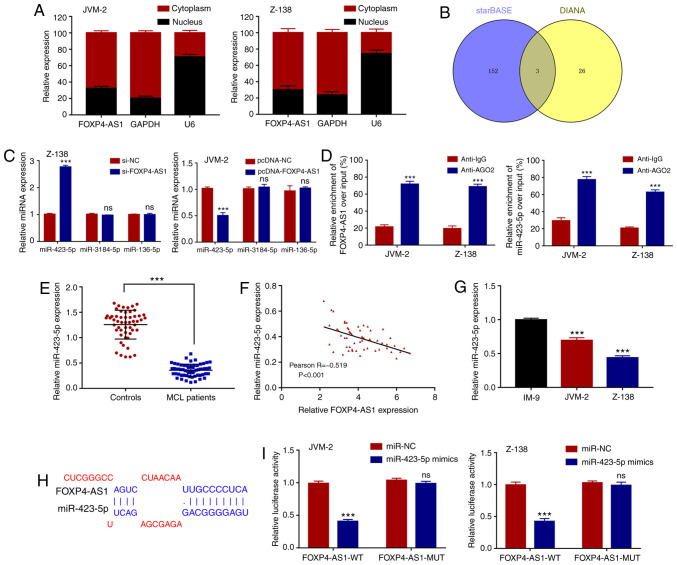
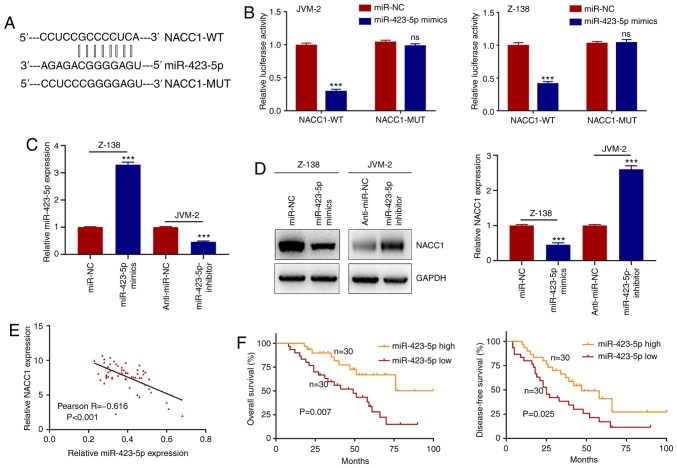
lncRNAs have been reported to exert their functions transcriptionally or post-transcriptionally in regards to the regulation of downstream mRNAs (24). To explore the underlying function of FOXP4-AS1 in MCL, subcellular fractionation assay was utilized to detect its cellular fractionation in two MCL cell lines. As shown in Fig. 4A, FOXP4-AS1 was found to be predominantly localized in the cytoplasm of the MCL cells. Therefore, the identification of post-transcriptional regulation of FOXP4-AS1 in MCL suggests that FOXP4-AS1 may act as a ceRNA in the regulation of downstream genes via the sequestration of miRNAs. Based on our analyses using online software Starbase v2.0 (http://starbase.sysu.edu.cn/starbase2/) and DIANA tools (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2/index-predicted), three most significant miRNAs that had complementary binding with FOXP4-AS1 were identified (Fig. 4B). Then, the expression levels of these three miRNAs in Z-138 and JVM-2 cells transfected with FOXP4-AS1 overexpression vector or shRNAs were examined. As shown in Fig. 4C, only miR-423-5p was significantly upregulated and downregulated, respectively through the silencing and the overexpression of FOXP4-AS1. To further investigate the association between FOXP4-AS1 and miR-423-5p, RIP assays were performed using human anti-Ago2 antibodies in the two MCL cells. As shown in Fig. 4D, a significant enrichment in both FOXP4-AS1 and miR-423-5p was observed in the two MCL cell lines. Clinical sample analysis showed that the expression of miR-423-5p in serum samples had a decreased trend in MCL patients compared with that in the controls (P<0.001; Fig. 4E). Pearson correlation analysis demonstrated that, in serum samples, FOXP4-AS1 expression was negatively correlated with miR-423-5p expression in MCL patients (P<0.001; Fig. 4F). In addition, miR-423-5p had lower expression levels in MCL cells compared with those levels in the IM-9 cells (P<0.001; Fig. 4G). The predicted binding sequence between FOXP4-AS1 and miR-423-5p was obtained using bioinformatics prediction tools Starbase v2.0 (Fig. 4H). To further verify the potential physical interactions between FOXP4-AS1 and miR-423-5p, luciferase reporter assays were conducted in Z-138 and JVM-2 cells that were co-transfected with the WT or MUT FOXP4-AS1 reporters and miR-423-5p mimics or mi-NC. As shown in Fig. 4I, while a significantly decreased WT reporter activity was observed following transfection of miR-423-5p mimics; the mutant reporter activities in all transfected cells showed no obvious changes. These observations indicated that the interaction between FOXP4-AS1 and miR-423-5p is an independent regulatory factor. Overall, these results demonstrated that FOXP4-AS1 directly suppresses the expression of miR-423-5p by sponging miR-423-5p in MCL.
Figure 4.
FOXP4-AS1 acts as the molecular sponge of miR-423-5p. (A) Predominant localization of FOXP4-AS1 in the cytoplasm of MCL JVM-2 and Z-138 cells as detected by subcellular fractionation assay. (B) Three miRNAs that show complementary binding with FOXP4-AS1 as identified from starBase and DIANA tools. (C) Expression levels of three miRNAs in cells transfected with FOXP4-AS1 as detected by qPCR. ***P<0.001, compared with the si-NC or pcDNA-NC group; ns, not significant. (D) RNA immunoprecipitation (RIP) experiments for FOXP4-AS1 and miR-423-5p in MCL cells. ***P<0.001, compared with the anti-IgG group. (E) Relative expression of miR-423-5p in plasma samples of MCL patients (n=60) and healthy controls (n=53) evaluated by quantitative real-time PCR. ***P<0.001. (F) Pearson correlation analysis between FOXP4-AS1 and miR-423-5p expression levels in plasma samples of MCL patients. (G) Relative expression of miR-423-5p detected in MCL cell lines JVM-2 and Z-138 and normal B lymphocytes IM-9. ***P<0.001, compared with the IM-9 cell line. (H) Predicted binding site of miR-423-5p in FOXP4-AS1. (I) Luciferase reporter assay of FOXP4-AS1-WT or FOXP4-AS1-MUT co-transfected into MCL cells with miR-423-5p mimics or miR-NC. ***P<0.001, compared with the miR-NC group; ns, not significant. Data are presented as mean ± SD of three independent experiments. MCL, mantle cell lymphoma; FOXP4-AS1, forkhead box P4 antisense RNA 1.
NACC1 is the target of miR-423-5p in MCL
Likewise, the interaction between miR-423-5p and NACC1 was evaluated. Firstly, the binding sequence of miR-423-5p and NACC1 3′-UTR was predicted and obtained (Fig. 5A). Then, the luciferase activity of reporter containing wild-type NACC1 (NACC1-WT) or mutant-type NACC1 (NACC1-MUT) in cells transfected with miR-423-5p mimics was examined. As shown in Fig. 5B, the luciferase activity of NACC1-WT vector, but not NACC1-MUT vector was found to be decreased by miR-423-5p mimics. Then, we overexpressed miR-423-5p expression in Z-138 cells and knocked down its expression in JVM-2 cells (Fig. 5C). Moreover, the protein and mRNA levels of NACC1 were observed to be decreased in the Z-138 cells transfected with the miR-423-5p mimics, and increased in JVM-2 cells transfected with the miR-423-5p inhibitors (Fig. 5D). In addition, the expression levels of miR-423-5p and NACC1 in MCL plasma samples were examined. As shown in Fig. 5E, an inverse expression correlation was found between NACC1 expression and miR-423-5p expression in MCL patients (P<0.001). Kaplan-Meier analysis showed that MCL patients with high miR-423-5p expression had better OS and DFS than those with low miR-423-5p expression (Fig. 5F; P=0.007 and P=0.025, respectively). Taken together, the data demonstrated that FOXP4-AS1 promotes NACC1 expression by interacting with miR-423-5p in MCL.
Figure 5.
NACC1 is a target of miR-423-5p in MCL. (A) Predicted binding site of miR-423-5p in NACC1. (B) Luciferase reporter assay of NACC1-WT or NACC1-MUT co-transfected into MCL JVM-2 and Z-138 cells with miR-423-5p mimics or miR-NC. ***P<0.001, compared with the miR-NC group; ns, not significant. (C) The miR-423-5p expression in MCL cells transfected with miR-3184-5p mimics or inhibitors as assessed by qPCR. ***P<0.001, compared with the miR-NC or anti-miR-NC group. (D) NACC1 at the protein and mRNA levels in MCL JVM-2 and Z-138 cells transfected with miR-3184-5p mimics or inhibitors as assessed by western blotting and qPCR. ***P<0.001, compared with the miR-NC or anti-miR-NC group. (E) Pearson correlation analysis between miR-423-5p and NACC1 expression levels in plasma samples of MCL patients. (F) Kaplan-Meier curves for overall survival and disease-free survival of patients with low and high expression of miR-423-5p (miR-423-5p high, n=30; miR-423-5p low, n=30). Data are presented as mean ± SD of three independent experiments. MCL, mantle cell lymphoma; NACC1, nucleus accumbens-associated 1; WT, wild-type; MUT, mutant.
FOXP4-AS1 facilitates MCL progression via the miR-423-5p/NACC1 pathway
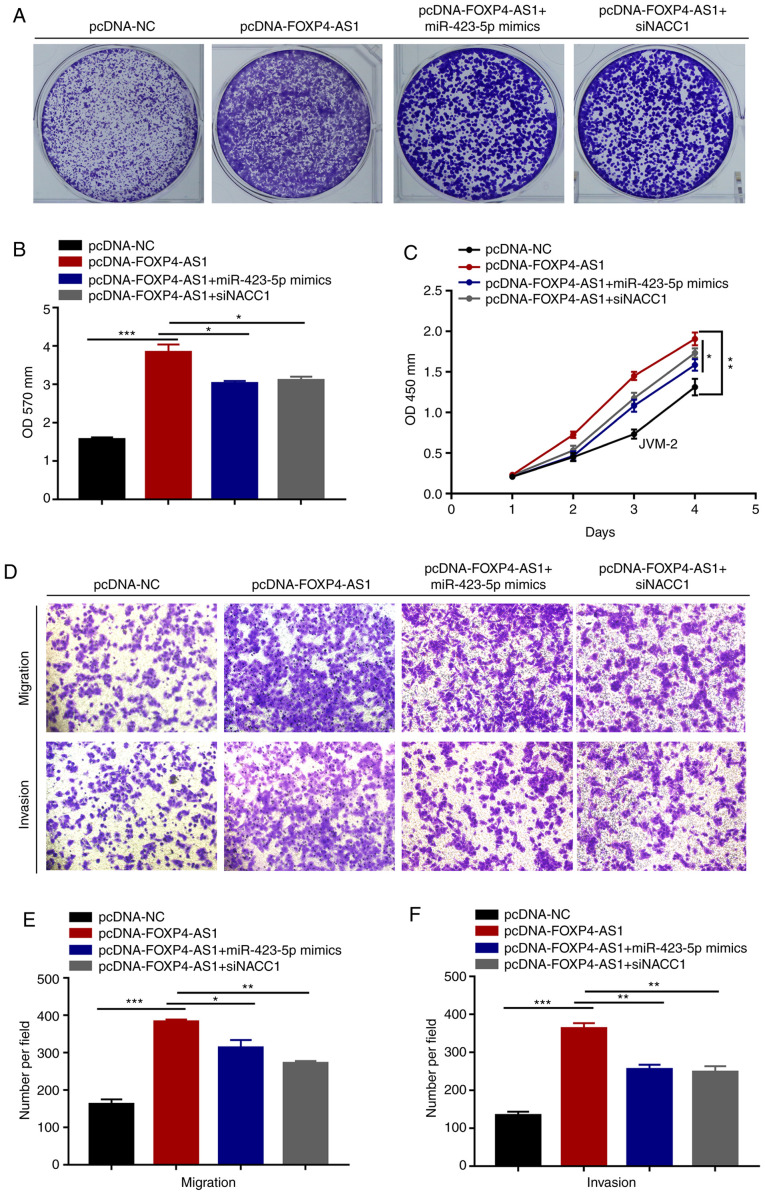
To further determine whether FOXP4-AS1 exerts biological roles through the miR-423-5p/NACC1 pathway, rescue assays were conducted in JVM-2 cells. First, we successfully overexpressed expression of miR-423-4p and downregulated expression of NACC1 (Fig. S1). Crystal violet and CCK-8 assays showed that miR-423-5p mimics or si-NACC1 rescued the promoting effect of FOXP4-AS1 overexpression on JVM-2 cell proliferation (Fig. 6A-C). Meanwhile, Transwell assays demonstrated that miR-423-5p mimics or si-NACC1 reversed the migration and invasion potential of FOXP4-AS1-overexpressing JVM-2 cells (Fig. 6D-F). Overall, these observations indicate that FOXP4-AS1 promotes MCL progression through miR-423-5p/NACC1 pathway.
Figure 6.
FOXP4-AS1 promotes MCL progression via the miR-423-5p/NACC1 pathway. Crystal violet (A and B) and CCK-8 (C) assays showing reversal of the promoting effect of FOXP4-AS1 overexpression on JVM-2 cell proliferation by miR-423-5p mimics or si-NACC1. (D-F) Transwell assays showing reversion of the migration and invasion potential in FOXP4-AS1-overexpressing JVM-2 cells by miR-423-5p mimics or si-NACC1. Data are presented as mean ± SD of three independent experiments. *P<0.05, **P<0.01, ***P<0.001. MCL, mantle cell lymphoma; FOXP4-AS1, forkhead box P4 antisense RNA 1; NACC1, nucleus accumbens-associated 1.
Discussion
Several studies have demonstrated that long non-coding RNA (lncRNA) forkhead box P4 antisense RNA 1 (FOXP4-AS1) plays a significant oncogenic role in various types of cancer. Specifically, FOXP4-AS1 is an unfavorable prognostic factor and plays a fundamental role in the regulation of colorectal cancer progression (13). Additionally, Wu and colleagues reported that FOXP4-AS1 can regulate FOXP4 by sponging miR-3184-5p, in prostate cancer (12) and also in esophageal squamous cell carcinoma (14). Moreover, FOXP4-AS1 has been shown to facilitate the progression of osteosarcoma by downregulating LATS1 via binding to LSD1 and EZH2 (11). However, the potential biological function of FOXP4-AS1 in MCL progression is unknown.
This study revealed that the expression of FOXP4-AS1 was increased in MCL cell lines, and that high FOXP4-AS1 expression was correlated with unfavorable patient prognosis. Knockdown and overexpression of FOXP4-AS1 were observed to respectively exhibit an inhibitory and promoting effect on the proliferative, migratory and invasive capacities of MCL cells. Moreover, the impacts of FOXP4-AS1 on MCL cell progression were found to be regulated by the miR-423-5p/NACC1 pathway. These data indicate that the FOXP4-AS1/miR-423-5p/NACC1 axis is an oncogenic pathway in promoting the progression of MCL.
In line with the classic ceRNAs regulating mechanism, our results also revealed that miR-423-5p is a target of FOXP4-AS1. Previously, miR-423-5p was demonstrated to act as a tumor suppressor or oncogene in the development of many types of cancer. For example, Lin and colleagues reported that inhibition of miR-423-5p can suppress the proliferation and promote the apoptosis of prostate cancer (25). Tang and colleagues demonstrated that overexpression of miR-423-5p can inhibit cell proliferation, colony formation and invasion of ovarian cancer cells (26). Wang and colleagues demonstrated that miR-423-5p can act as a tumor suppressor gene in osteosarcoma through the regulation of STMN1 expression (27). Moreover, plasma miR-423-5p levels have been reported to serve as a potential novel biomarker for the early detection of colorectal cancer (28). However, the role of miR-423-5p in MCL has not been demonstrated. In this study, miR-423-5p was firstly determined to act as a tumor suppressor for MCL progression and the high expression miR-423-5p was found to be capable of predicting favorable prognosis for MCL patients. In addition, emerging data have shown that miRNAs are pivotal in tumorigenesis through gene targeting (29–31). Our findings demonstrated that NACC1 is the direct target of miR-423-5p and that silencing of NACC1 expression inhibited MCL progression. However, there exists a limitation in the present study that the determination of FOXP4-AS1 expression is lacking in MCL tissues.
In conclusion, this study showed that FOXP4-AS1 plays a role in MCL progression through the upregulation of NACC1 by inhibiting miR-423-5p. FOXP4-AS1 expression predicts poor clinical outcome and serves as a novel oncogene in MCL, which may be a novel therapeutic target for patients with MCL.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was funded by the Medical Research Fund Projects of Guangdong Province (no. A2016503) and the National Natural Science Foundation of China (no. 30901629).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author's contributions
HFT and JLF conducted the experiments, JLF designed the research and JXS, ZWH, and SYC collected and analyzed the clinical samples. YZS, HFT and JLF analyzed the data, and the manuscript was drafted by HFT and JLF. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Written informed contents were obtained from all the patients enrolled in this study. This study was approved by the Ethics Committee of the First Affiliated Hospital of Shantou University Medical College and conducted in compliance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Vose JM. Mantle cell lymphoma: 2017 update on diagnosis, risk-stratification, and clinical management. Am J Hematol. 2017;92:806–813. doi: 10.1002/ajh.24797. [DOI] [PubMed] [Google Scholar]
- 2.Eskelund CW, Kolstad A, Jerkeman M, Räty R, Laurell A, Eloranta S, Smedby KE, Husby S, Pedersen LB, Andersen NS, et al. 15-Year follow-up of the second nordic mantle cell lymphoma trial (MCL2): Prolonged remissions without survival plateau. Br J Haematol. 2016;175:410–418. doi: 10.1111/bjh.14241. [DOI] [PubMed] [Google Scholar]
- 3.Chihara D, Asano N, Ohmachi K, Kinoshita T, Okamoto M, Maeda Y, Mizuno I, Matsue K, Uchida T, Nagai H, et al. Prognostic model for mantle cell lymphoma in the rituximab era: A nationwide study in Japan. Br J Haematol. 2015;170:657–668. doi: 10.1111/bjh.13486. [DOI] [PubMed] [Google Scholar]
- 4.Dreyling M, Amador V, Callanan M, Jerkeman M, Le Gouill S, Pott C, Rule S, Zaja F, European Mantle Cell Lymphoma Network Update on the molecular pathogenesis and targeted approaches of mantle cell lymphoma: Summary of the 12th annual conference of the European mantle cell lymphoma network. Leuk Lymphoma. 2015;56:866–876. doi: 10.3109/10428194.2014.940584. [DOI] [PubMed] [Google Scholar]
- 5.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–361. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Sehgal L, Jain N, Khashab T, Mathur R, Samaniego F. LncRNA MALAT1 promotes development of mantle cell lymphoma by associating with EZH2. J Transl Med. 2016;14:346. doi: 10.1186/s12967-016-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mu G, Liu Q, Wu S, Xia Y, Fang Q. Long noncoding RNA HAGLROS promotes the process of mantle cell lymphoma by regulating miR-100/ATG5 axis and involving in PI3K/AKT/mTOR signal. Artif Cells Nanomed Biotechnol. 2019;47:3649–3656. doi: 10.1080/21691401.2019.1645151. [DOI] [PubMed] [Google Scholar]
- 9.Fan Z, Wang X, Li P, Mei C, Zhang M, Zhao C. Overexpression of lncRNA GATA6-AS inhibits cancer cell proliferation in mantle cell lymphoma by downregulating GLUT1. Oncol Lett. 2019;18:2443–2447. doi: 10.3892/ol.2019.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, et al. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Ge D, Chen X, Qiu J, Yin Z, Zheng S, Jiang C. FOXP4-AS1 participates in the development and progression of osteosarcoma by downregulating LATS1 via binding to LSD1 and EZH2. Biochem Biophys Res Commun. 2018;502:493–500. doi: 10.1016/j.bbrc.2018.05.198. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Xiao Y, Zhou Y, Zhou Z, Yan W. LncRNA FOXP4-AS1 is activated by PAX5 and promotes the growth of prostate cancer by sequestering miR-3184-5p to upregulate FOXP4. Cell Death Dis. 2019;10:472. doi: 10.1038/s41419-019-1699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Lian Y, Yan C, Cai Z, Ding J, Ma Z, Peng P, Wang K. Long non-coding RNA FOXP4-AS1 is an unfavourable prognostic factor and regulates proliferation and apoptosis in colorectal cancer. Cell Prolif. 2017;50:e12312. doi: 10.1111/cpr.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Li T, Yang Y, Kang W, Dong S, Cheng S. YY1-Induced upregulation of FOXP4-AS1 and FOXP4 promote the proliferation of esophageal squamous cell carcinoma cells. Cell Biol Int. 2020;44:1447–1457. doi: 10.1002/cbin.11338. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Yang T, Li L. LncRNA FOXP4-AS1 is involved in cervical cancer progression via regulating miR-136-5p/CBX4 axis. Onco Targets Ther. 2020;13:2347–2355. doi: 10.2147/OTT.S241818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faiola F, Yin N, Fidalgo M, Huang X, Saunders A, Ding J, Guallar D, Dang B, Wang J. NAC1 regulates somatic cell reprogramming by controlling Zeb1 and E-cadherin expression. Stem Cell Reports. 2017;9:913–926. doi: 10.1016/j.stemcr.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju T, Jin H, Ying R, Xie Q, Zhou C, Gao D. Overexpression of NAC1 confers drug resistance via HOXA9 in colorectal carcinoma cells. Mol Med Rep. 2017;16:3194–3200. doi: 10.3892/mmr.2017.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakayama N, Kato H, Sakashita G, Nariai Y, Nakayama K, Kyo S, Urano T. Protein complex formation and intranuclear dynamics of NAC1 in cancer cells. Arch Biochem Biophys. 2016;606:10–15. doi: 10.1016/j.abb.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Fujii T, Shimada K, Tatsumi Y, Tanaka N, Fujimoto K, Konishi N. Syndecan-1 up-regulates microRNA-331-3p and mediates epithelial-to-mesenchymal transition in prostate cancer. Mol Carcinog. 2016;55:1378–1386. doi: 10.1002/mc.22381. [DOI] [PubMed] [Google Scholar]
- 20.Rahman MT, Nakayama K, Rahman M, Katagiri H, Katagiri A, Ishibashi T, Ishikawa M, Iida K, Nakayama N, Otsuki Y, et al. Fatty acid synthase expression associated with NAC1 is a potential therapeutic target in ovarian clear cell carcinomas. Br J Cancer. 2012;107:300–307. doi: 10.1038/bjc.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tatemichi Y, Shibazaki M, Yasuhira S, Kasai S, Tada H, Oikawa H, Suzuki Y, Takikawa Y, Masuda T, Maesawa C. Nucleus accumbens associated 1 is recruited within the promyelocytic leukemia nuclear body through SUMO modification. Cancer Sci. 2015;106:848–856. doi: 10.1111/cas.12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sollier E, Cubizolles M, Fouillet Y, Achard JL. Fast and continuous plasma extraction from whole human blood based on expanding cell-free layer devices. Biomed Microdevices. 2010;12:485–497. doi: 10.1007/s10544-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Cao C, Zhang T, Zhang D, Xie L, Zou X, Lei L, Wu D, Liu L. The long non-coding RNA, SNHG6-003, functions as a competing endogenous RNA to promote the progression of hepatocellular carcinoma. Oncogene. 2017;36:1112–1122. doi: 10.1038/onc.2016.278. [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Lin T, Lin J, Yang M, Shen Z, Liu H, Zou Z, Zheng Z. Inhibition of miR-423-5p suppressed prostate cancer through targeting GRIM-19. Gene. 2019;688:93–97. doi: 10.1016/j.gene.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Tang X, Zeng X, Huang Y, Chen S, Lin F, Yang G, Yang N. MiR-423-5p serves as a diagnostic indicator and inhibits the proliferation and invasion of ovarian cancer. Exp Ther Med. 2018;15:4723–4730. doi: 10.3892/etm.2018.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Peng L, Gong X, Zhang X, Sun R, Du J. MiR-423-5p inhibits osteosarcoma proliferation and invasion through directly targeting STMN1. Cell Physiol Biochem. 2018;50:2249–2259. doi: 10.1159/000495085. [DOI] [PubMed] [Google Scholar]
- 28.Fang Z, Tang J, Bai Y, Lin H, You H, Jin H, Lin L, You P, Li J, Dai Z, et al. Plasma levels of microRNA-24, microRNA-320a, and microRNA-423-5p are potential biomarkers for colorectal carcinoma. J Exp Clin Cancer Res. 2015;34:86. doi: 10.1186/s13046-015-0198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanacore D, Boccellino M, Rossetti S, Cavaliere C, D'Aniello C, Di Franco R, Romano FJ, Montanari M, La Mantia E, Piscitelli R, et al. Micrornas in prostate cancer: An overview. Oncotarget. 2017;8:50240–50251. doi: 10.18632/oncotarget.16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang C, Chen X, Alattar M, Wei J, Liu H. MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis of gastric cancer. Cancer Gene Ther. 2015;22:291–301. doi: 10.1038/cgt.2015.19. [DOI] [PubMed] [Google Scholar]
- 31.Nana-Sinkam SP, Croce CM. MicroRNA regulation of tumorigenesis, cancer progression and interpatient heterogeneity: Towards clinical use. Genome Biol. 2014;15:445. doi: 10.1186/s13059-014-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.