Abstract
Articular cartilage has restricted self-regenerative capacity; therefore, treatment of cartilage lesions is a great challenge in the field of orthopedics. In the present study, we evaluate the enhancing effect of a transforming growth factor-beta 1 (TGF-β1)-immobilized scaffold, fabricated by incorporating TGF-β1-loaded gelatin microspheres into PLGA framework, on the differentiation of adipose-derived stem cells (ASCs) into chondrocytes. Significant increase in cell proliferation was observed in the TGF-β1-immobilized PLGA-gelatin scaffold, as compared with the ASC-seeded non-TGF-β1-immobilized PLGA-gelatin scaffold. When chondrogenic differentiation of ASCs was evaluated for both constructs, sulfated glycosaminoglycan (sGAG) content was significantly higher in the TGF-β1-immobilized scaffold. This study showed that ASCs containing the TGF-β1-immobilized scaffold better promoted cartilage regeneration in defective articular cartilage, which is assessed by histological observation. Based on the above results, we conclude that TGF-β1-immobilized PLGA-gelatin scaffold seeded with ASCs considerably enhances the quality of the tissue-engineered cartilage, therefore, advancing the field of cartilage tissue engineering.
Keywords: Chondrogenesis, Adipose-derived stem cells, PLGA, Gelatin microspheres, Transforming growth factor-beta 1
Introduction
Articular cartilage is a special connective tissue that functions not only as a frictionless articulating surface in diarthrodial joints, but also as a shock absorber. However, due to very slow cellular and molecular turnover, articular cartilage has limited capacity for self-repair when damaged by aging, developmental disorders, trauma, excessive weight or a combination of these. Consequently, the regeneration of cartilage in damaged tissue repair is a major concern. Over the past several decades, various methods have been used to restore the normal function of injured cartilage such as abrasion, drilling, and microfracture [1–3]. Although all of these approaches have been proven beneficial, none is optimal for repairing cartilage lesions. Cell-based tissue engineering is an alternative to the conventional treatment techniques and provides a more promising approach to repairing damaged cartilage.
In cartilage tissue engineering, chondrocytes and stem cells are commonly used to regenerate cartilage. Previous studies have demonstrated that autologous cells were preferable, but the proliferative capacity of differentiated autologous chondrocytes decreased and cells dedifferentiate when passaged for cell expansion, and the autologous chondrocytes displayed donor site limitations and increased morbidity [4]. Due to the drawbacks associated with chondrocyte-based cell therapy, recent research has focused on the usage of stem cells such as bone marrow-derived stem cells for cartilage tissue engineering. More recently, studies have indicated that adipose tissue is an attractive cell source that contains multipotent progenitor cells, which have the potential to differentiate into chondrogenic, osteogenic, neurogenic, and myogenic cells when induced by the appropriate biological factors in vitro [5, 6]. In addition, a small amount of adipose tissue may provide a large quantity of autologous stem cells and adipose-derived stem cells (ASCs) and can be maintained in a stable and an undifferentiated status during in vitro expansion. Therefore, ASCs represent a promising cell source for regenerative medicine, and will open new avenues for therapeutic approaches [7–10].
The use of ASCs in cartilage tissue engineering requires further development of cell-seeded scaffolds. At present, biodegradable scaffolds are mainly divided into synthetic and natural materials. Synthetic scaffolds, such as poly(lactic-co-glycolic acid) (PLGA), exhibit valuable physiochemical properties and an appropriate shape and surface morphology. However, due to their hydrophobic surface properties and lack of specific cell-accepted signals, synthetic scaffolds may not present a favorable surface for cellular attachment and proliferation, thereby limiting cellular differentiation [11]. Natural scaffolds, such as collagen II, are well-characterized extracellular matrix (ECM) proteins that play a central role in cell-cell and cell-ECM interactions [12]. However, natural scaffolds are often limited by their rapid biodegradation. To overcome this drawback and promote cellular differentiation, one alternative is to create a composite scaffold.
Growth factors (GFs) play an important role in regulating chondrocyte metabolism and chondrogenesis. Among the GFs of potential interest is transforming growth factor-beta (TGF-β), a major regulatory factor that drives lineage selection and differentiation progression in mesenchymal cells. TGF-β has been shown to influence cells from the chondrogenic lineage in vivo, promoting initial stages of mesenchymal condensation, prechondrocyte proliferation, production of extracellular matrix and cartilage-specific molecule deposition, while also inhibiting terminal differentiation [13]. TGF-β1, one of TGF-β family members, is known to induce ASCs to differentiate into chondrocytes [6, 14]. Although studies have demonstrated that TGF-β1 induces the chondrogenic differentiation of stem cells in vitro when added directly to the culture media, this method decreased the efficacy of TGF-β1. Additionally, TGF-β1 protein also has a very short biological half-life, so a suitable concentration could not be maintained over an extended period. Thus, the biggest concern regarding TGF-β1 delivery is whether the released protein actually retains its biological activity. To address these various challenges, TGF-β1-immobilized various scaffolds were investigated.
In this study, we hypothesized that TGF-β1-immobilized scaffold, fabricated by incorporating TGF-β1-loaded gelatin microspheres into PLGA framework would enhance the chondrogenesis of ASCs. To test this hypothesis, a TGF-β1-immobilized PLGA-gelatin scaffold was fabricated and seeded with ASCs. Cell proliferation, differentiation, and in vivo implantation were then evaluated in ASC-seeded non-TGF-β1-immobilized scaffold (ASCs/scaffold) and TGF-β1-immobilized scaffold (ASCs/hybrid scaffold).
Materials and Methods
Preparation of a TGF-β1-Immobilized PLGA-gelatin Scaffold
A PLGA (50:50 wt.% poly lactic acid (PLA): polyglycolic acid (PGA); 12,000–16,500 kDa; Polysciences, Inc.) copolymer scaffold was fabricated via low temperature-deposition manufacturing (LDM) as previously reported [15].
TGF-β1-loaded gelatin microspheres (MS-TGF-β1) were prepared. In short, 1.1 g of acidic gelatin (Sigma Co., St Louis, MO) was dissolved in 7 mL of double-distilled water (ddH2O) at 53 °C. This aqueous gelatin solution was added to 30 mL of olive oil and 0.2 mL of Tween 80 (Sigma Co.) drop by drop with stirring at 500 rpm. The temperature of the emulsion was decreased to 0 °C while constantly stirring. After 12 min, 10 mL of chilled acetone (4 °C) was added to the mixture. To remove residual oil, these microspheres were washed with acetone and then cross-linked with 10 mM glutaraldehyde (GA; Sigma Co.) at 4 °C. The cross-linked microspheres were washed with ddH2O 24 h later and counted. Then, 500 μL of suspension containing 2×104 microspheres was placed in a 1.0-mL microcentrifuge tube for lyophilization. The dried microspheres (2×104) were loaded with 50 ng of TGF-β1 (Protec, USA) by swelling in 5 μL of TGF-β1 solution (10 ng/μL) at pH 7.4. The mixture was gently stirred with a pipette tip and then incubated at 4 °C overnight and then followed by lyophilization.
To construct the TGF-β1-immobilized scaffold, MS-TGF-β1 (0.2 mg) dispersed in 90 % aqueous ethanol (100 μL, pH 7.4) was carefully incorporated into PLGA (cylinder: 3 mm in thickness and 4 mm in diameter) and immediately lyophilized. The total amount of TGF-β1 loaded into the PLGA was 0.8 ng. The non-TGF-β1-immobilized scaffold was constructed by using the same method above and MS without TGF-β1 was loaded.
The morphology of the PLGA and PLGA-gelatin scaffolds was observed by scanning electron microscopy (SEM). Those empty scaffolds were incubated in culture media before fixation.
In Vitro TGF-β1 Release Study
For analysis of TGF-β1 controlled release, the TGF-β1-immobilized scaffold was immersed in 5 mL of phosphate buffered saline (PBS) and placed in a shaking water bath at 37 °C. After 0.5, 1, 3, 5, 7, 10, 14, 21, and 28 days, the supernatant was collected and centrifuged at 13,500 rpm. Additional PBS was added to maintain a constant suspension volume after supernatant collection. Analysis of TGF-β1 protein expression was performed using an enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Isolation of Adipose-Derived Stem Cells and Culture
ASCs were isolated from the cervical adipose tissue of adult rabbits as per protocol used in a previous report [14]. In short, the extracted tissue was washed with PBS and minced finely using surgical scissors. The minced tissue was digested for 1 h at 37 °C with 0.15 % collagenase (type I, Sigma, USA), and then enzyme activity was neutralized with basal culture medium containing Dulbecco’s modified eagle’s medium (DMEM, Invitrogen, USA), 10 % FBS (Invitrogen, USA), 100 U/mL penicillin, and 100 μg/mL streptomycin. To remove tissue debris, samples were then filtered through a 500 μm mesh filter. The cell suspension was centrifuged at 800 g and resuspended in culture medium. The cells were seeded in 25-cm2 flasks (Corning-Costar, USA) at density of 4×105 cells/cm3 and incubated at 37 °C and 5 % CO2. The medium was exchanged after 24 h and three times a week thereafter. Once the cells reached more that 80 % confluence, cells were detached with 0.25 % trypsin and 0.1 % EDTA.
To seed the cells into the non-immobilized scaffolds or immobilized scaffolds (3 mm thickness, 5 mm diameter), 40 μL of cell suspension containing 1×106 cells was added into the upper side of each pre-wetted scaffold. Each sample was then shifted into a 24-well tissue culture plate and incubated at 37 °C and 5 % CO2 for 4 h. The ASC-seeded TGF-β1-immobilized scaffold (ASCs/hybrid scaffold) were incubated in basal culture media consisting of 1 % FBS, 1× ITS (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenious acid, 1.25 μg/mL bovine serum albumin, and 5.35 μg/mL linoleic acid; BD Biosciences, NJ), 100 U/mL penicillin, and 100 μg/mL streptomycin. The ASC-seeded non-TGF-β1-immobilized scaffold (ASCs/scaffold) was incubated in chondrogenic media containing 10 ng/mL TGF-β1 and the basal culture media. The two groups were cultured for 14 days respectively.
Sulfated Glycosaminoglycan (sGAG) and DNA Content
The total sGAG content of the scaffolds was detected at 1, 7, and 14 days using a previous report [16]. In short, the scaffolds were washed with PBS and digested with 1 mL of papain solution (1 mg/mL in 50 mM sodium phosphate, pH 6.5, which contains 2 mM N-acetyl cysteine and 2 mM EDTA) for 20 h at 65 °C. They were then measured by their reaction with 1, 9-dimethylmethylene blue (DMMB, Sigma, USA) at 525 nm, using shark chondroitin sulfate (Sigma, USA) as standard.
To measure the total amount of DNA in each scaffold, the same papain-implant digest, in conjunction with the Hoechst 33258 fluorescent dye, was assayed [17]. A 100-μl aliquot of digest was diluted in 1 mL of dye/buffer solution and the fluorescence of the samples was evaluated for excitation at 365 nm and emission at 458 nm using known concentrations of herring sperm as a standard curve (Sigma, USA).
SEM Analysis of Cells and Composite Scaffold
For SEM examination, samples were fixed in 2 % phosphate buffered glutaraldehyde solution, dehydrated with a graded isopropanol series, and finally air-dried. The dried samples were mounted on aluminum supports and sputter-coated with gold before analysis.
In Vivo Study
Fifteen New Zealand white rabbits were classified into the following three groups: the defect group, the ASC-seeded non-TGF-β1-immobilized PLGA-gelatin scaffold group (ASCs/scaffold), and the ASC-seeded TGF-β1-immobilized PLGA-gelatin scaffold group (ASCs/hybrid scaffold). A cylindrical, full-thickness defect (diameter 5 mm) was created in the patellar groove of each distal femur using a hand drill. All the rabbits were allowed to move freely after surgery without immobilization. The present study was approved by the research ethics committee of the institution. Twelve weeks postoperatively, samples were harvested, fixed, decalcified, embedded and sectioned, and stained with safranin O.
Statistical Analysis
Sample values were expressed as the mean±standard deviation (SD). The values were analyzed by analysis of variance (ANOVA) using SPSS 11.0 statistical software. P-values less than 0.05 were considered significant.
Results
Morphology of Scaffolds
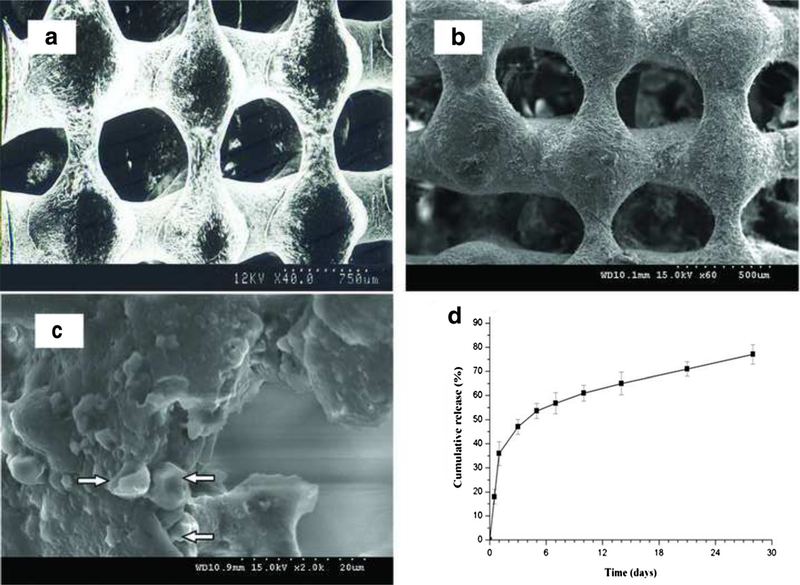
SEM observation showed that the PLGA scaffold surface was smooth (Fig. 1a). The surface of the PLGA-gelatin scaffold was rough (Fig. 1b), and the gelatin microspheres were visibly incorporated into the synthetic PLGA scaffold (Fig. 1c).
Fig. 1.
Scanning electron microscopic images. a. the empty PLGA scaffold. b. the empty PLGA-gelatin scaffold. c. Microspheres in the empty PLGA-gelatin scaffold, note: the arrows point to MS. d. Release profiles of TGF-β1 from the TGF-β1-immobilized scaffold. Data are expressed as mean±SD (n=5). The statistical evaluation of the data is detailed in the text
In Vitro TGF-β1 Release Study
The release kinetics of the TGF-β1-immobilized scaffold was monitored over 28 days. TGF-β1 was expelled in a burst release during the first day. After 5 days, the release of the TGF-β1 was gradually increased in a stable fashion until the end of the release period (4 weeks; Fig. 1d).
DNA Content and sGAG Synthesis
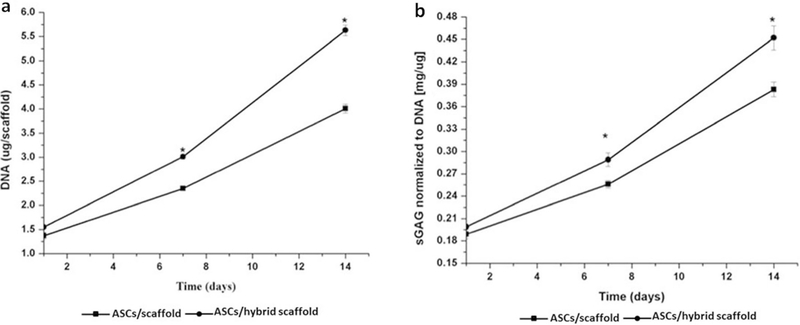
Cell proliferation was determined by a DNA assay, which demonstrated that the DNA content of both the ASCs/scaffold and the ASCs/hybrid scaffold was increased during the culture period. At Day 1, a significant difference was not observed between the ASCs/scaffold (1.37±0.07 μg/scaffold) and the ASCs/hybrid scaffold (1.55 ± 0.03 μg/scaffold) (P>0.05). However, the difference in ASC proliferation was significant between the ASCs/scaffold (2.35±0.04 μg/scaffold, 4.01 ± 0.09 μg/scaffold) and ASCs/hybrid scaffold (3.01±0.05 μg/scaffold, 5.63±0.11 μg/scaffold) at Days 7 and 14 (P<0.05), respectively (Fig. 2a).
Fig. 2.
DNA content and sGAG synthesis. a. Change in DNA content of the ASC-seeded non-TGF-β1-immobilized scaffold and the ASC-seeded TGF-β1-immobilized scaffold. Data are expressed as mean±SD (n=5). *statistically significant relative to the ASC-seeded non-TGF-β1-immobilized scaffold (P<0.05). b. Synthesis of sulfated GAG from the ASC-seeded non-TGF-β1-immobilized scaffold and the ASC-seeded TGF-β1-immobilized scaffold. Data are expressed as mean±SD (n=5). *statistically significant relative to the non-releasing ASC-seeded scaffold (P<0.05)
After being normalized against DNA content, measured sGAG levels indicated that both constructs accumulated ECM over the 14-day culture period and exhibited a continual increase in sGAG production. At Day 1, both the ASCs/scaffold and ASCs/hybrid scaffold exhibited no significant change in sGAG levels (0.1891 ± 0.006 and 0.199 ± 0.004 mg/μg, respectively) (P>0.05). At Days 7 and 14, the GAG values for the ASCs/scaffold were 0.256±0.005 and 0.383±0.01 mg/μg, respectively, while the GAG levels in the ASCs/hybrid scaffold were 0.289 ± 0.009 and 0.452 ± 1.16 mg/μg. Significant difference was shown between the two scaffolds at both Days 7 and 14 (P<0.05) (Fig. 2b).
SEM Observation of Composites
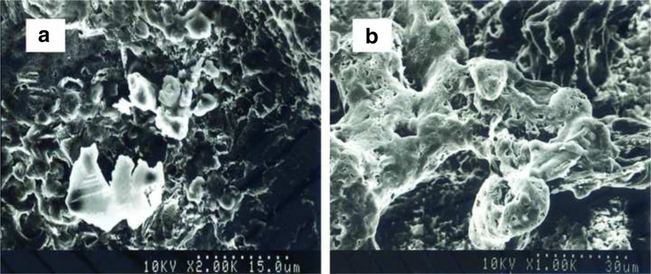
SEM analysis revealed the unique surface characteristics of the ASCs/hybrid scaffold. After culture for 14 days, SEM images revealed that differentiated ASCs distributed in the TGF-β1-immobilized scaffold produced a large amount of ECM (Fig. 3b) in comparison with those distributed in the non-TGF-β1-immobilized scaffold (Fig. 3a).
Fig. 3.
Scanning electron microscopic photomicrographs of the ASC-seeded non-TGF-β1-immobilized scaffold and the ASC-seeded TGF-β1-immobilized scaffold at day 14
In Vivo Study
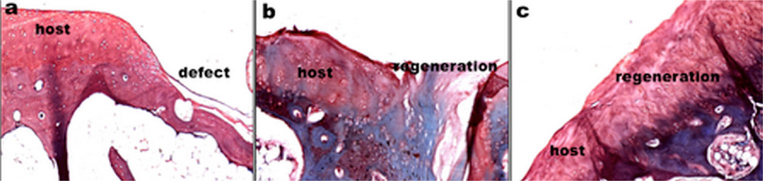
Cartilage regeneration was observed at 12 weeks post-implantation in the model of rabbit cartilage defect (Fig. 4). Histological study revealed that the defect of ASCs/hybrid scaffold group was completely filled with reparative tissue and the surface of the regenerated cartilage was smooth, and the newly formed tissue was well integrated with the host cartilage. Metachromatic staining was extensive in the ASCs/hybrid scaffold group. In addition, the tidemark was clearly recognized in the engineered cartilage tissue. However, in the ASCs/scaffold group, the defect was partly filled with the regenerated tissue, the surface was irregular, and the formed tissue was not well integrated with host cartilage. The metachromatic staining was weak. In the model group, the defect was filled with fibrous tissue.
Fig. 4.
Histological analysis (safranin O staining) of cartilage regeneration after 12-week implantation. a. the defect group (the model group). b. the ASC-seeded non-TGF-β1-immobilized scaffold. c the ASC-seeded TGF-β1-immobilized scaffold
Discussion
Production of engineered hyaline cartilage is crucial for cartilage tissue engineering, which involves a wide variety of considerations including GFs, cell sources, and scaffold biomaterials [18]. These materials should mimic the functional and mechanical properties of the native ECM. The main characteristics of an ideal scaffold include sterility, biocompatibility, biodegradability, proper mechanical properties, and appropriate surface properties for cell adhesion, proliferation, and differentiation.
In this study, we used LDM (a method based on rapid prototyping (RP) and phase separation technologies), to fabricate a PLGA scaffold. RP technology can efficiently fabricate a scaffold with an individualized profile, highly interconnected macropores, heterogeneous pore morphologies, and specific mechanical properties. It is known that LDM might retain scaffold bioactivity due to its non-heating liquefying processing; however, the PLGA scaffold fabricated by LDM is not ideal for cellular adhesion that could influence cellular proliferation and differentiation [15].
Gelatin, a partial derivative of collagen, contains an Arg-Gly-Asp (RGD)-like sequence promoting cellular adhesion and migration. The benefits of lower immunogenicity, cost, and aqueous solubility make gelatin an excellent choice of base biomaterial for tissue engineering applications [19]. In addition, gelatin can release encapsulated GFs in a controlled fashion, which may augment the ingrowth and biosynthetic ability of seeded cells. However, the use of gelatin for cartilage defect repair has some limitations, including its inherent poor mechanical stability and rapid biodegradation. To overcome the individual drawbacks of PLGA and gelatin scaffolds and effectively enhance chondrogenic differentiation in vitro and tissue repair in vivo, a hybrid scaffold was fabricated from PLGA and TGF-β1-loaded gelatin microspheres. This gelatin scaffold can provide a sustained release of TGF-β1 to promote defect repair [20].
Cell proliferation in the ASCs/scaffold and the ASCs/hybrid scaffold was measured by quantifying DNA content. Our studies demonstrated that the cell number in both constructs increased over the culture period, with a larger increase in cell proliferation in the ASCs/hybrid scaffold. To observe the chondrogenic phenotype, sGAG production was also examined in the ASCs/scaffold and the ASCs/hybrid scaffold. GAG is considered a sensitive metabolic marker for investigating the chondrogenic phenotype, as has been described for chondrocytes isolated from hyaline cartilage tissue. Biochemical analysis clearly showed that the GAG synthetic activity of the ASCs/hybrid scaffold was significantly higher than that in the ASCs/scaffold at each time point. Such studies provide evidence that the TGF-β1-immobilized scaffold significantly increases the proliferation of ASCs and promotes the differentiation of ASCs into chondrocytes. These data were confirmed by SEM imaging, which revealed that ASCs in the TGF-β1-immobilized scaffold exhibited a differentiated phenotype, proper cell-cell contact, and large ECM production at Day 14. Our results are consistent with the following reports. Recently, it has been reported that TGF-β1 microspheres incorporated into scaffolds increased chondrocyte proliferation and differentiation, suggesting that controlled-release scaffolds have the potential to enhance cartilage formation [21]. More recently, it has been demonstrated that such growth factor delivery system, TGF-β1 loaded into gelatin microspheres and incorporated into fibrin hydrogels, was effective in promoting chondrogenesis of ASCs in vitro [22]. Deng et al. [23] also noted the great potential of scaffolds encapsulating GF-releasing microspheres for promoting chondrocyte retention. In addition, in our previous studies, we also suggested that the composite microspheres composed of gelatin TGF-β1-loaded microspheres and chitosan microspheres enhanced the differentiation of ASCs into chondrocytes in pellet culture in vitro [24, 25]. All those results suggested that these released constructs could serve as cartilaginous tissue scaffolds for improving chondrogenesis [26].
To further compare the characteristics of the ASCs/scaffold and the ASCs/hybrid scaffold, the regenerative study of cartilage defect in vivo was performed. Our study suggested that the regenerated cartilage in both the ASCs/hybrid scaffold group and the ASCs/scaffold group showed the positive for safranin O that is specific for sulfated proteogylcans of hyaline cartilage, but the staining of the ASCs/hybrid scaffold was more intensive than that of the ASCs/scaffold. Histological analysis of cartilage regeneration demonstrated the better surface zone repair and subchondral bone reconstitution present in the ASCs/hybrid scaffold group compared to ASCs/scaffold group.
We speculated that ASCs had better differentiation into chondrocytes in the TGF-β1-immobilized PLGA-gelatin scaffold, promoting the formation of cartilage tissue in the defect. In vivo study further demonstrated that the TGF-β1-immobilized scaffold could effectively enhance the formation of engineered cartilage. Mehlhorn et al. [27] studied the subcutaneous implantation of chondrogenic differentiation of ASCs seeded into PLGA scaffolds and cultured TGF-beta1-containing medium for 3 weeks in vitro. They found that expression of chondrospecific markers such as collagen type II and type X, cartilage oligomeric matrix protein, and aggrecan are increased in vitro and ASC-seeded PLGA scaffolds show a stable chondrogenic phenotype in a heterotopic model of cartilage transplantation. Recently, Lu et al. [28] demonstrated that baculovirus-engineered rabbit ASCs, which consistently express TGF-β3/BMP-6 ameliorate the chondrogenesis, in vitro cartilaginous constructs production as well as in vivo hyaline cartilage regeneration.
Our study suggested that TGF-β1-immobilized scaffolds more effectively stimulate ASC differentiation into chondrocytes than non-TGF-β1-immobilized scaffold. In conclusion, TGF-β1-immobilized PLGA-gelatin scaffold seeded with ASCs significantly enhances the quality of tissue-engineered cartilage, a finding that may help to promote advancement of cartilage tissue engineering [28].
Footnotes
Disclosures The authors indicate no potential conflicts of interest.
Contributor Information
Feng Yin, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Junfeng Cai, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Wen Zen, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Yanhui Wei, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Wei Zhou, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Feng Yuan, Department of Joint and Bone Disease Shanghai East Hospital, Tongji University School of Medicine, Shanghai 200120, People’s Republic of China.
Shree Ram Singh, Basic Research Laboratory, Stem Cell Regulation and Animal Aging Section, National Cancer Institute, Frederick, MD 21702, USA.
Yiyong Wei, Jiaotong University School of Medicine, Shanghai 200025, People’s Republic of China.
References
- 1.Amrami KK, Askari KS, Pagnano MW, & Sundaram M (2002). Sundaram Radiologic case study. Abrasion chondroplasty mimicking avascular necrosis. Orthopedics, 1018, 1107–1018. [DOI] [PubMed] [Google Scholar]
- 2.Muller B, & Kohn D (1999). Indication for and performance of articular cartilage drilling using the Pridie method. Der Orthopäde, 28, 4–10. [DOI] [PubMed] [Google Scholar]
- 3.Steadman JR, Rodkey WG, & Briggs KK (2002). Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. The Journal of Knee Surgery, 15, 170–176. [PubMed] [Google Scholar]
- 4.Han SH, Kim YH, Park MS, et al. (2008). Histological and biomechanical properties of regenerated articular cartilage using chondrogenic bone marrow stromal cells with a PLGA scaffold in vivo. Journal of Biomedial Materials Research Part A, 87, 850–861. [DOI] [PubMed] [Google Scholar]
- 5.Safford KM, Hicok KC, Safford SD, et al. (2002). Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochemical and Biophysical Research Communications, 294, 371–379. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Mizuno H, et al. (2001). Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering, 7, 211–228. [DOI] [PubMed] [Google Scholar]
- 7.Awad HA, Wickham MQ, Leddy HA, Gimble JM, & Guilak F (2004). Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials, 25, 3211–3222. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Luo E, Chen X, et al. (2005). Molecular and cellular characterization during chondrogenic differentiation of adipose tissue-derived stromal cells in vitro and cartilage formation in vivo. Journal of Cellular and Molecular Medicine, 9, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon IS, Chung CW, Sung JH, et al. (2011). Proliferation and chondrogenic differentiation of human adipose-derived mesenchymal stem cells in porous hyaluronic acid scaffold. Journal of Bioscience and Bioengineering, 112, 402–408. [DOI] [PubMed] [Google Scholar]
- 10.Zuk P (2013). Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells, 2013, 713959. [Google Scholar]
- 11.Hao W, Hu YY, Wei Y, et al. (2008). Collagen I gel can facilitate homogenous bone formation of adipose-derived stem cells in PLGA-beta-TCP scaffold. Cells, Tissues, Organs, 187, 89–102. [DOI] [PubMed] [Google Scholar]
- 12.Tseng Q, Duchemin-Pelletier E, Deshiere A, et al. (2012). Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proceedings of the National Academy of Sciences of the United States of America, 109, 1506–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues M, Griffith LG, & Wells A (2010). Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Research & Therapy, 1, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y, Hu Y, Lv R, & Li D (2006). Regulation of adipose-derived adult stem cells differentiating into chondrocytes with the use of rhBMP-2. Cytotherapy, 8, 570–579. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Hu H, Wang H, et al. (2009). Cartilage regeneration of adipose-derived stem cells in a hybrid scaffold from fibrin-modified PLGA. Cell Transplantation, 18(2009), 159–170. [DOI] [PubMed] [Google Scholar]
- 16.Farndale RW, Sayers CA, & Barrett AJ (1982). A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connective Tissue Research, 9, 247–248. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Sah RL, Doong JY, & Grodzinsky AJ (1988). Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Analytical Biochemistry, 174, 168–176. [DOI] [PubMed] [Google Scholar]
- 18.Ochi M, Uchio Y, Tobita M, & Kuriwaka M (2001). Current concepts in tissue engineering technique for repair of cartilage defect. Artificial Organs, 25, 172–179. [DOI] [PubMed] [Google Scholar]
- 19.Ochiya T, Nagahara S, Sano A, Itoh H, & Terada M (2001). Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Current Gene Therapy, 1, 31–52. [DOI] [PubMed] [Google Scholar]
- 20.Ito R, Morimoto N, Pham LH, et al. (2013). Efficacy of the controlled release of concentrated platelet lysate from a collagen/gelatin scaffold for dermis-like tissue regeneration. Tissue Engineering. Part A, 19, 1398–1405. [DOI] [PubMed] [Google Scholar]
- 21.Lee JE, Kim SE, Kwon IC, et al. (2004). Effects of a chitosan scaffold containing TGF-beta1 encapsulated chitosan microspheres on in vitro chondrocyte culture. Artificial Organs, 28, 829–839. [DOI] [PubMed] [Google Scholar]
- 22.Ahearne M, Buckley CT, & Kelly DJ (2011). A growth factor delivery system for chondrogenic induction of infrapatellar fat pad-derived stem cells in fibrin hydrogels. Biotechnolgy and Applied Biochemistry, 58, 345–352. [DOI] [PubMed] [Google Scholar]
- 23.Deng T, Huang S, Zhou S, He L, & Jin Y (2007). Cartilage regeneration using a novel gelatin-chondroitin-hyaluronan hybrid scaffold containing bFGF-impregnated microspheres. Journal of Microencapsulation, 24, 163–174. [DOI] [PubMed] [Google Scholar]
- 24.Han Y, Wei Y, Wang S, & Song Y (2010). Cartilage regeneration using adipose-derived stem cells and the controlled-released hybrid microspheres. Joint, Bone, Spine: Revue du Rhumatisme, 77(2010), 27–31. [DOI] [PubMed] [Google Scholar]
- 25.Han Y, Wei Y, Wang S, & Song Y (2009). Enhanced chondrogenesis of adipose-derived stem cells by the controlled release of transforming growth factor-beta1 from hybrid microspheres. Gerontology, 55, 592–599. [DOI] [PubMed] [Google Scholar]
- 26.Madry H, Rey-Rico A, Venkatesan JK, Johnstone B, & Cucchiarini M (2014). Transforming growth factor Beta-releasing scaffolds for cartilage tissue engineering. Tissue Engineering. Part B, Reviews, 20, 106–125. [DOI] [PubMed] [Google Scholar]
- 27.Mehlhorn AT, Zwingmann J, Finkenzeller G, et al. (2009). Chondrogenesis of adipose-derived adult stem cells in a polylactide-co-glycolide scaffold. Tissue Engineering Part A, 15, 1159–1167. [DOI] [PubMed] [Google Scholar]
- 28.Lu CH, Yeh TS, Yeh CL, et al. (2014). Regnerating cartilages by engineered ASCs: prolonged TGF-β3/BMP-6 expression improved articular cartilage formation and restored zonal structure. Molecular Therapy, 22, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]