Abstract
Aims
To generate different larval stages of Strongylus vulgaris and to study cytokine responses in cultures of eqPBMC exposed to defined larval stages of S. vulgaris and cyathostomins with the aim to understand the early immune reaction to these parasites.
Methods and results
EqPBMC were exposed to S. vulgaris larvae (L3, exsheated L3 and L4) and cyathostomin L3 and analysed for cytokine gene expression. Procedures for decontamination, culturing and attenuation of larvae were established. Transcription of IL‐4, IL‐5 and IL‐13 was induced by both S. vulgaris and cyathostomin L3. Moulting of S. vulgaris from L3 to L4 stage was accompanied by a shift to high expression of IL‐5 and IL‐9 (exsheated L3 and L4) and IFN‐γ (L4 only). In parallel, the adjuvant G3 modified the cytokine profile induced by both parasites by reducing the expression of IL‐4, IL‐5 and IL‐10 while concomitantly enhancing the expression of IFN‐γ.
Conclusion
The L4 stage of S. vulgaris generated a cytokine profile different from that induced by the earlier L3 stage of S. vulgaris and cyathostomins. This diversity depending on the life cycle stage will have implications for the choice of antigen and adjuvant in future vaccine design.
Keywords: adjuvant, cytokine, gene expression, horse, parasite, peripheral blood mononuclear cells
1. INTRODUCTION
Strongylus vulgaris is considered to be the most pathogenic parasite infecting horses. The control of S. vulgaris has during the last decades relied on regular treatments with anthelminthic drugs. However, an emerging anthelminthic resistance observed in other equine nematodes such as the closely related cyathostomins 1 , 2 has resulted in medical treatment restrictions issued in 2007 by the European Union (2001/82/EG). In the Nordic countries, these restrictions are reported to have contributed to an increased prevalence of S. vulgaris, 3 , 4 urging for development of alternative methods to control equine parasites.
Formulation of vaccines against parasites is a great challenge because of the parasite's complex life cycle and their ability to modulate the host immune response. The life cycle of S. vulgaris involves migration through host tissues by different larval stages. Horses are infected by ingesting infective third stage (L3) larvae on pasture. At this point, the L3s are still encapsulated in their protective second stage (L2) cuticle. In the gastrointestinal tract, the L3 larvae exsheat its L2 cuticle and, approximately two days post‐ingestion, penetrate the mucosa and submucosa of the intestine where they moult into the fourth stage (L4). After two to three days, the L4s penetrate the arterioles of the submucosa and start to migrate through the mesenteric arteries, against the blood flow, until reaching the cranial mesenteric artery about 14 days post‐infection. There, the L4s continue to grow for three to four months before they moult into fifth stage (L5) that finally migrate back to the large intestine where they require six to eight more weeks to sexually mature. 5 The pathology of S. vulgaris infection is related to the larval migration causing lesions, thickening of the arterial wall, clot formation and tissue necrosis 6 which potentially can end up with life‐threatening nonstrangulating intestinal infarction. 7
Early attempts to immunize ponies with radiation‐attenuated S. vulgaris L3 resulted in reduction of larval burdens and reduced clinical signs at challenge infections. 8 , 9 , 10 The only sign of pathological damage in the intestines of these immunized ponies was fibrosis in the submucosa indicating that the protective immune response was generated within the intestinal submucosa and directed against the late stage of L3 and/or early L4 stage of larval development. 9 The protective immune response was characterized by production of S. vulgaris‐specific antibodies and activation of eosinophils that kill L3 larvae by antibody‐dependent cytotoxicity. 9 , 11 , 12 , 13 In addition, vaccination with attenuated S. vulgaris L3 increased the production of IgG(T), IL‐4 and IL‐5 but not IFN‐γ, IL‐2 and IL‐10 measured 14 days after challenge infection. 10 Out of these parameters, IgG and IL‐4 as well as TNF‐α have been estimated in donkeys naturally infected with S. vulgaris and cyathostomins 14 Thus, infection with S. vulgaris and cyathostomins generate a cytokine response in the host but these profiles are not yet fully characterized.
The present study was undertaken to establish methods to generate different larval stages of S. vulgaris and cyathostomins and to study immune reactions to these preparations in cultures of equine PBMC (eqPBMC). The cytokine responses were profiled with techniques previously applied to evaluate the immune stimulatory effects of a novel nanoparticle adjuvant G3. 15 To evaluate the applicability of this quillaja saponin adjuvant in formulations with parasite antigens, the immunomodulatory effects by G3 on selected S. vulgaris and cyathostomin preparations were explored in vitro using eqPBMC.
2. MATERIALS AND METHODS
2.1. Blood donor horses
Blood was collected from 12 Swedish warmblood horses housed at the Department of Clinical Sciences, SLU, Uppsala. These horses, mares and geldings 8‐21 years old, are clinically examined, vaccinated for tetanus and influenza on a regular basis. Parasite diagnostics (National Veterinary Institute, Uppsala) are performed every spring and include tapeworm and strongyle egg counts with larval cultures for detection of S. vulgaris. Horses with >200 eggs per gram faeces (EPG) are treated with Ivomec® vet (oral paste 0.2 mg/kg; Boehringer Ingelheim Animal Health A/S, Copenhagen, Denmark). All horses included in the study were negative for S. vulgaris at culture. The blood sampling was approved by the regional committee for animal experimentation in Uppsala, Sweden (5.8.18‐15533/2018).
2.2. Parasite material
Faecal samples were collected from private owned horses diagnosed with S. vulgaris and cyathostomin infections. The faecal samples were mixed 1:1 with vermiculite (Weibull, Sweden), moistened with tap water and incubated in a jar for 14 days at room temperature. L3 larvae were recovered after 12 hours sedimentation using the inverted petri‐dish method. 16 The liquid phase was collected and centrifuged for 3 minutes at 248 x g, leaving approximately 2‐3 mL water containing larvae. Cyathostomin and S. vulgaris L3 were identified morphologically, based on size and number of intestinal cells. 17 The samples were stored in water at +4°C until use.
2.3. Decontamination of larval preparations
In our specimens, S. vulgaris always occurred in co‐infection with cyathostomins. To obtain single species samples, S. vulgaris and cyathostomin L3 were isolated by collecting larvae with a 10 µL pipette under the microscope. Between 80 and 100 L3 of each species were washed two times, by 1‐2 hours incubation in 10 mL PBS containing 400 IU/mL penicillin, 200 µg/mL streptomycin and 1 µg/mL amphotericin B (PBS + antimicrobials) followed by, two incubations for 24 hours in PBS + antimicrobials with 30 µg/mL polymyxin B (Sigma‐Aldrich, USA). All centrifugation steps were performed for 5 minutes at 50 × g.
To assess the level of bacterial contamination in these L3 preparations, supernatants were collected after each 24‐hour incubation. Approximately 200 µL supernatants were plated on bovine blood agar and fastidious anaerobic agar (SVA, Uppsala, Sweden), and CFU was counted after 24 and 48 hours. Endotoxin levels in the supernatants were estimated using the Limulus amoebocyte lysate (LAL) assay (Pierce™ LAL Chromogenic Endotoxin Quantification Kit, Thermo Fisher Scientific).
2.4. Exsheatment and in vitro culture of S. vulgaris larvae
A published protocol for exsheatment of the S. vulgaris L2 cuticle was optimized. 18 In brief, washed L3 were incubated for 2‐10 minutes in 5 mL pre‐warmed (37°C) sodium hypochlorite solution (Milton 2%, SVA, Uppsala, Sweden) diluted to various concentrations ranging from 1.0% to 0.1%. To stop exsheatment and reduce toxic effects, 5 mL PBS was added and the rate of exsheatment was verified under the microscope. These larvae, devoid of their L2 cuticle, are referred to as “exsheated S. vulgaris L3”.
To trigger moulting into the L4 stage, exsheated L3 were cultured in cKW2 medium 19 until shedding of the L3 cuticle was observed. Larvae in cKW2 medium (NTCT‐135 medium (Gibco), yeast extract (2.25 g/L), peptone (2.813 g/L) and dextrose (2.813 g/L), an equal volume of FCS (Invitrogen, Life Technologies, Carlsbad, CA) and supplemented with 400 IU/mL penicillin, 200 µg/mL streptomycin and 1 µg/mL amphotericin B) were transferred to a cell culture flask (Nunclon; Nunc, Roskilde, Denmark) and gently bubbled for 2 minutes with a 10% CO2, 5% O2 and 85% N2 gas mixture via a sterile plugged pipette. The cultures were incubated at 37 ⁰C, and two‐thirds of cKW2 medium were changed and bubbled every 3‐4 days. Larval size, morphology and time of moulting were monitored in an inverted microscope (Nikon Eclipse TS100). Moulted larvae, devoid of both L2 and L3 cuticle, are referred to as “S. vulgaris L4” in accordance with previous definitions. 9 , 19 , 20
Both exsheated L3 and L4 larvae were washed three times in RPMI 1640 medium (BioWhittaker, Cambrex Bioscience, Verviers, Belgium) containing 400 IU/mL penicillin, 200 µg/mL streptomycin and 1 µg/mL amphotericin B before UV irradiation.
2.5. UV irradiation of larvae
The various larval preparations (cyathostomin L3, S. vulgaris L3, exsheated S. vulgaris L3 and S. vulgaris L4) were centrifuged for 5 minutes at 50 x g and suspended in 1 mL cell culture medium, that is RPMI 1640 medium supplemented with 20 mM HEPES, 2 mM L‐glutamine, 200 IU/mL penicillin, 100 µg/mL streptomycin, 50 µmol/L 2‐mercaptoethanol and 5% FCS. The larvae were distributed into 100 µL droplets containing 12 larvae on a petri‐dish and UV‐irradiated (GS Gene LinkerTM UV Chamber, Bio‐Rad) at various times (3‐15 minutes) and doses (30‐125 MJ). The effect was evaluated as movement and disintegration of larvae. The optimal irradiation conditions were set as the minimum time and dose giving 100% nonmotile but fully intact larvae.
2.6. Cell culture conditions
Collection of equine blood, isolation of PBMC and culture set‐ups were performed as previously described. 15 One mL cell culture medium containing 5‐6 × 106 eqPBMC was seeded in 6‐well plates (Nunclon; Nunc, Roskilde, Denmark) and incubated for 30‐60 minutes at 37°C in 7% CO2. Thereafter, one mL cell culture medium containing twelve UV‐irradiated larvae (cyathostomin L3,S. vulgaris L3, exsheated S. vulgaris L3 or S. vulgaris L4) were added. When indicated, chitin (Chitin from crab shells, Sigma) or endotoxin (1 EU/mL; Thermo Fisher Scientific) were used as controls. Chitin was dissolved by sonication in DMSO (Sigma‐Aldrich, USA), brought up in PBS and used at a final concentration of 50 µg/mL. To control for possible effects of cKW2 medium ingredients or effector molecules deriving from the L4 larvae, such as excretory/secretory proteins, 200 µL medium supernatants were collected before and after culture and added to parallel cultures of eqPBMC. After 18 hours, eqPBMC were harvested in TRIzol reagent (Invitrogen).
2.7. In vitro adjuvant effects
The adjuvant G3 (NanoQuilR Research Reagent; CRODA Denmark A/S) was used at a final concentration of 5 µg/mL containing <0.0035 EU endotoxin/mL. The adjuvant was used alone or together with twelve UV‐irradiated cyathostomin L3 or S. vulgaris L3 in 2 mL cultures containing 5‐6x106 eqPBMC in 6‐well plates. EqPBMC cultured in plain growth medium were used as negative control.
2.8. RNA isolation and cDNA synthesis
RNA was extracted using a combined TRIzol and column‐based protocol (EZNA total RNA kit, Omega Bio‐Tek, Norcross, GA). 15 The quantity and purity of the extracted RNA were measured by spectrophotometry (NanoDrop ND‐1000, NanoDrop Technologies, Montchanin, DE), and RNA quality index (RQI) was estimated to ≥9.3 using capillary gel electrophoresis (Experion RNA StdSense Analysis Kit, Bio‐Rad, Sweden). cDNA was synthesized from 1.2 μg of RNA (GoScript Reverse transcription system; Promega). To control for genomic DNA contamination, each RNA was treated with RQ1 RNAse‐free DNAse (Promega, Madison, WI, USA) and a –RT control was run in parallel. The samples were diluted 1:5 and stored at −20°C until use.
2.9. qPCR analysis
Expression of the genes encoding IFN‐γ, IL‐1β, IL‐4, IL‐5, IL‐6, IL‐8, IL‐9, IL‐10, IL‐12p40, IL‐13, IL‐17A, IL‐23p19, TGF‐β and TNF‐α was determined relative to that of the reference genes RPL32 and SDHA. Optimized protocols for all cytokines but IL‐5 and IL‐9 are previously described. 15 IL‐5 21 and IL‐9 (F:5′‐CCGATTGTTTGTGTCTGGTT‐3′ and R:5′‐TGTGACAGACCCTCCTGGAA‐3′) were optimized to a primer concentration of 500 nmol/L and annealing temperatures of 58°C (efficiency 99% and r 2 .99) and 59°C (efficiency 93.3% and r 2 .99), respectively.
All samples were run in 25 µL duplicate reactions, consisting of 2 µL cDNA in 23 µL Quantitect SYBR Green PCR mix (Qiagen) in a CFX96 Touch PCR machine (Bio‐Rad). The run protocol was an initial cycle of 95°C for 15 minutes followed by 40 cycles of 95°C for 15 seconds, the assay specific annealing temperature for 30 seconds and 72°C for 30 seconds ending with a melt curve analysis to verify the PCR product. The Cq values for each cytokine gene were normalized to the geometric mean of the reference genes and calibrated to that in the medium controls. Genes with fold change (FC) values <0.5 or >2 were considered as down‐regulated or up‐regulated, respectively. In the case when the gene of interest was not detectable in the qPCR (14 out of 852 samples), a FC = 1 was assigned to that sample.
2.10. Statistical analysis
Statistical analysis was performed using the software Prism 7.0 (GraphPad Software, Inc). Differences between stimulations were calculated on ∆∆Ct‐values using the repeated measures ANOVA followed by Tukey's multiple comparison test where P‐values <.05 were regarded as significant.
3. RESULTS
3.1. Preparation of larvae
Various preparations of larvae, (a) cyathostomin L3 (b) S. vulgaris L3 (c) exsheated S. vulgaris L3 and (d) S. vulgaris L4, were established from feacal cultures. Bacterial cultures from both L3 preparations showed initially a sparse growth of bacteria but after 24‐hour incubation in PBS + antimicrobials no microbial growth was recorded. Supernatants from the cyathostomin larvae contained 0.79 and 0.66 IU/mL endotoxin after the first and second incubations, respectively. The corresponding figures for S. vulgaris were 0.90 and 0.70 IU/mL of endotoxin, respectively. The optimal dose for attenuation by UV irradiation of both these preparations was found to be 5 minutes at 50 MJ (Figure 1A,B).
FIGURE 1.
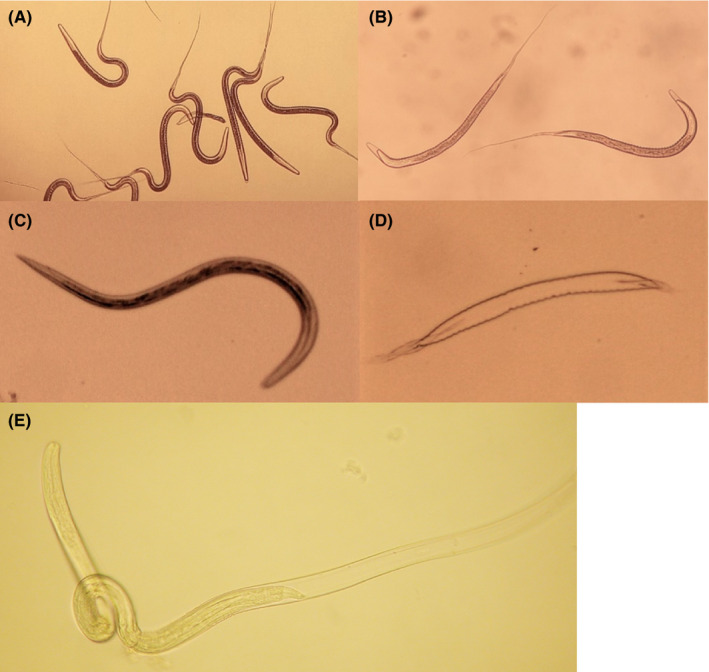
Various preparations of S. vulgaris larvae. (A) S. vulgaris L3 before UV irradiation, (B) UV‐irradiated S. vulgaris L3, (C) S. vulgaris L3 exsheated in 0.1% sodium hydrochlorite, (D) L2 cuticle from exsheated S. vulgaris L3, and (E) S. vulgaris moulting to L4 after five days of culture in cKW2 medium (Photo: Nikon Eclipse TS100/Nikon Coolpix 990)
3.2. Exsheatment and culture of S. vulgaris
The optimal condition for exsheatment of the L2 cuticle was determined to incubation for 4 minutes in 0.1% sodium hypochlorite. A longer incubation time (>5 minutes) and higher concentration of hypochlorite (>0.2%) damaged the larvae. At the optimal conditions, more than 95% of the larvae exsheated through a breach in the anterior end, leaving cast‐off cuticles and actively motile larvae (Figure 1C,B). Without their protective L2 cuticle, these exsheated larvae were more sticky and fragile, demanding low speed centrifugation (<50 x g) and gentle pipetting.
To trigger L4 moulting, exsheated L3 were cultured in cKW2 medium. In accordance with previous observations, 19 more than 90% of the larvae moulted at day 5 when at a size between 0.6 and 0.7 mm. Upon moulting, the larvae escaped through a breach in the anterior end (Figure 1E), leaving intact cast‐off cuticles in the culture medium. This is in accordance with previous observations 20 , 22 although it is not possible to distinguish between the late L3 and the newly moulted L4 based on morphology. 20 These exsheated S. vulgaris L3 and L4 preparations only resisted a lower dose of UV irradiation, 3 minutes at 30 MJ, to remain intact.
3.3. Cyathostomin and S. vulgaris L3 induce Th2 polarizing cytokines
Twelve UV‐irradiated cyathostomins or S. vulgaris L3 were added to cultures of eqPBMC and incubated for 18 hours prior to transcription analysis. Throughout the incubation time, the PBMCs were accumulated in close proximity to the cyathostomin larvae whereas the S. vulgaris larvae seemed to repel the PBMC (Figure 2A,B). IL‐4 and IL‐13 were induced in PBMC by both parasite species whereas IL‐5 was induced for four out of six horses by S. vulgaris and for two out of six horses by the cyathostomins. The gene expression of IL‐1β, IL‐6, IL‐8, IL‐9, IL‐12p40, IL‐17A, IL‐23p19 and TGF‐β varied slightly between horses exposed to cyathostomins or S. vulgaris L3 but was consistently low. The G3 adjuvant up‐regulated the genes encoding IFN‐γ, IL‐1β, IL‐6, IL‐12p40, IL‐17A and IL‐23p19 but not IL‐4, IL‐5, IL‐10, IL‐13 or TGF‐β. EqPBMC exposed to chitin up‐regulated IL‐5, but none of the other cytokines tested. Exposure to endotoxin induced IL‐10, IL‐1β, IL‐6 and IL‐17A (Figure 3; Table 1).
FIGURE 2.
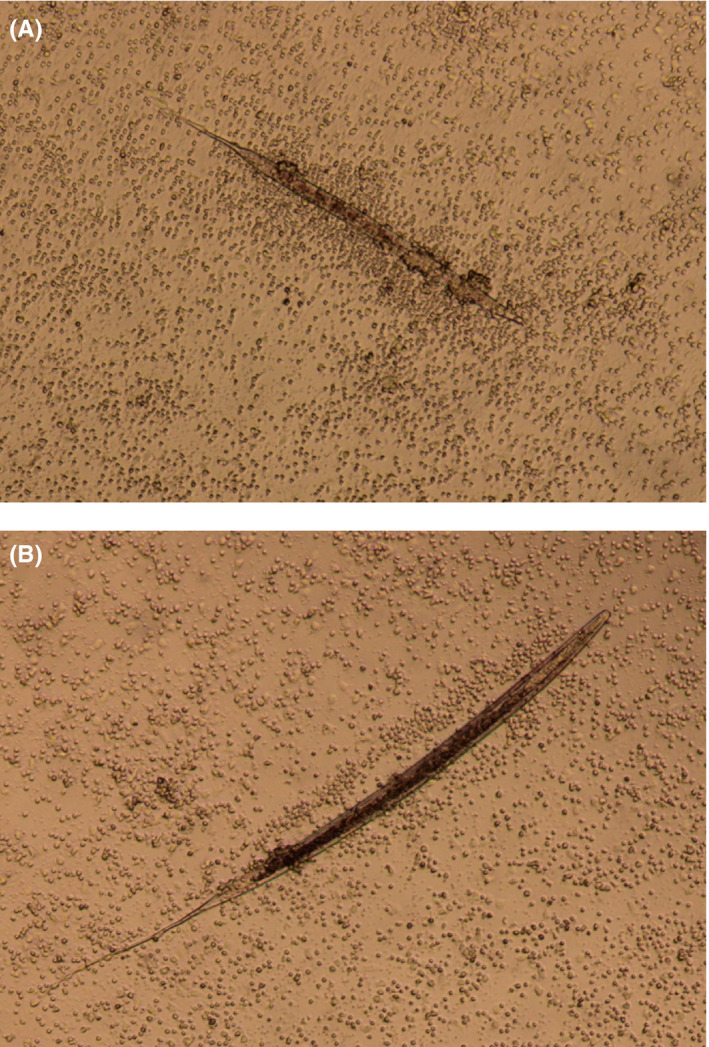
Cultures of eqPBMC isolated from the same horse and exposed to UV‐irradiated cyathostomin L3 (A) or S. vulgaris L3 (B) for 4 hours. (Photo: Leitz Labvert/Canon EOS Rebel T3i)
FIGURE 3.
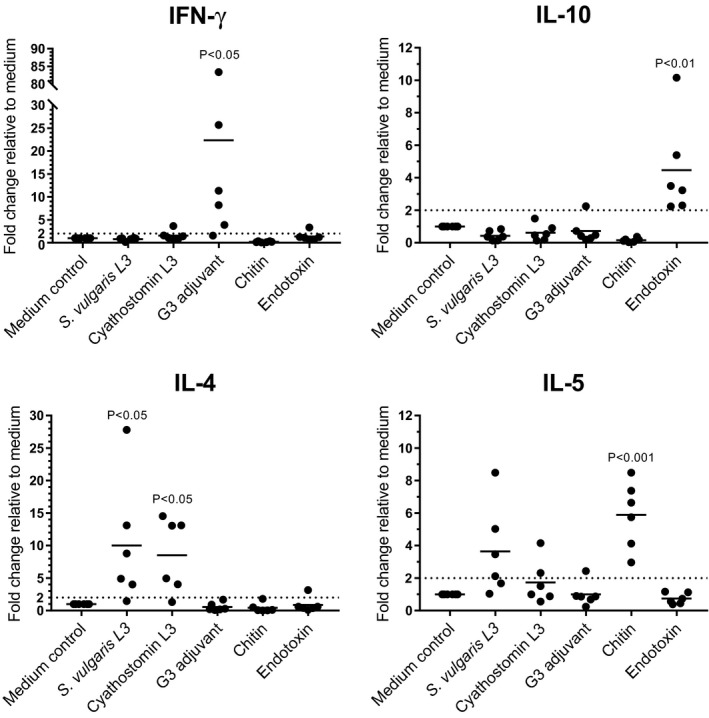
Relative expression of cytokine genes in eqPBMC cultured in plain growth medium (medium control) or in the presence of UV‐irradiated S. vulgaris L3, cyathostomin L3, the G3 adjuvant, chitin or endotoxin. The cytokine gene expression was normalized to the geometric mean for the reference genes (SDHA and RPL32) and calibrated to that in the medium control. FC > 2 (indicated by dashed line) is considered as up‐regulated, and significant differences against the medium control are indicated in the figure by P < .05, P < .01, P < .001
TABLE 1.
Relative expression (fold change; FC) of cytokine genes in eqPBMC exposed to UV‐irradiated S. vulgaris L3 or cyathostomin L3, the G3 adjuvant, chitin or endotoxin
| Gene | n | S. vulgaris | Cyathostomins | G3 adjuvant | Chitin | Endotoxin |
|---|---|---|---|---|---|---|
| IL‐1β | 6 | 2.01 ± 1.72 | 1.08 ± 0.43 | 1.96 ± 1.03 | 0.13 ± 0.17 | 2.20 ± 1.35 |
| IL‐6 | 6 | 3.62 ± 4.22 | 2.77 ± 2.35 | 2.94 ± 2.81 | 0.43 ± 0.46 | 4.85 ± 4.72 |
| IL‐8 | 6 | 1.23 ± 0.72 | 1.03 ± 0.57 | 1.55 ± 1.57 | 0.07 ± 0.06 | 1.01 ± 0.80 |
| IL‐9 | 6 | 1.27 ± 0.94 | 1.52 ± 1.09 | 1.05 ± 0.49 | 1.59 ± 1.45 | 0.64 ± 0.87 |
| IL‐13 | 6 | 4.6 ± 3.65 | 3.26 ± 2.06 | 1.16 ± 1.09 | 1.61 ± 2.01 | 1.07 ± 3.02 |
| IL‐12p40 | 5 a | 1.22 ± 0.59 | 1.46 ± 0.79 | 4.71 ± 2.17 | 1.37 ± 0.37 | 1.26 ± 0.69 |
| IL‐17A | 5 a | 1.22 ± 0.53 | 1.38 ± 1.16 | 2.59 ± 1.31 | 1.32 ± 0,71 | 1.81 ± 1.44 |
| IL‐23p19 | 6 | 1.22 ± 0.59 | 1.46 ± 0.79 | 4.71 ± 2.11 | 1.37 ± 0.37 | 1.26 ± 0.69 |
| TGF‐β | 6 | 1.30 ± 0.57 | 1.25 ± 0.23 | 1.08 ± 0.43 | 0.98 ± 0.80 | 0.95 ± 0.67 |
Expression of the genes encoding IL‐17A and IL‐12p40 was detected in PBMC from five of the six horses sampled.
3.4. The G3 adjuvant modifies the parasite‐induced cytokine profile
The effect of G3 on the response to parasite antigens was studied by simultaneous exposure of eqPBMC to cyathostomins or S. vulgaris L3 and G3 (Figure 4). The cytokine profile induced by G3, characterized by up‐regulation of IFN‐γ and down‐regulation of IL‐10, persisted also in the presence of cyathostomins or S. vulgaris L3. The up‐regulation of IL‐4 and IL‐5 induced by S. vulgaris L3 was reduced in the presence of G3 while IL‐4 and IL‐5 induced by cyathostomin L3 was only partly reduced by G3.
FIGURE 4.
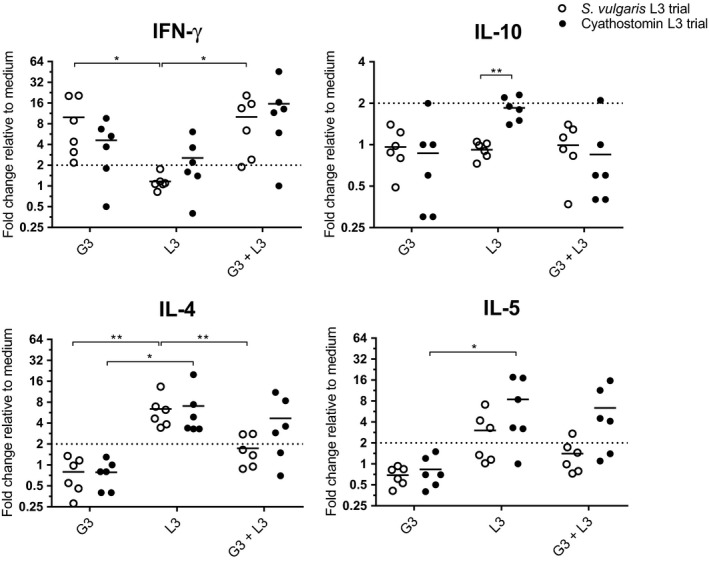
Relative expression of cytokine genes in eqPBMC cultured in the presence of the G3 adjuvant, UV‐irradiated cyathostomin L3 or S. vulgaris L3, alone or combinations thereof. The effect of cyathostomin L3 or S. vulgaris L3 was evaluated in two separate experiments (closed and open circle, respectively). The cytokine gene expression was normalized to the geometric mean for the reference genes (SDHA and RPL32) and calibrated to that in the medium control. FC > 2 (indicated by dashed line) were considered as up‐regulated. Significant differences between stimulations are indicated by *<0.05, **<0.01, ***<0.001
3.5. L3, exsheated L3 and L4 elicit different cytokine profiles
The cytokine gene expression in response to S. vulgaris at different stages of ecdysis was studied by exposing eqPBMC to UV‐irradiated S. vulgaris L3, exsheated S. vulgaris L3 or S. vulgaris L4 (Figure 5). All three preparations induced up‐regulation of IL‐4 and IL‐13 in eqPBMC. Exsheated S. vulgaris L3 and S. vulgaris L4 induced higher levels of IL‐5 and IL‐9 compared to S. vulgaris L3. The L4 stage was the only larval preparation that induced up‐regulation of IFN‐γ. None of the preparations induced TGF‐β (FC for L3 = 0.94 ± 0.37; exsheated L3 = 1.28 ± 0.42; L4 = 1.44 ± 0.96). No conclusive effect on the cytokine gene expression was seen in the pre‐ and post‐cKW2 control inductions.
FIGURE 5.
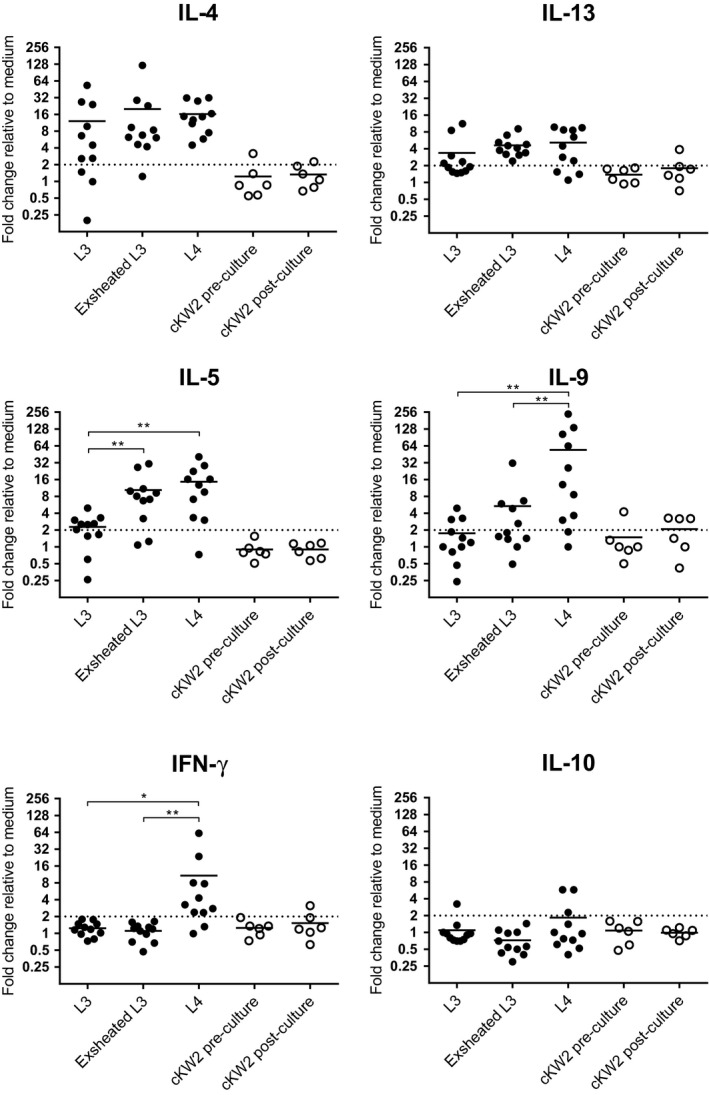
Relative expression of cytokine genes in eqPBMC cultured in the presence of UV‐irradiated S. vulgaris at different stages of ecdysis; S. vulgaris L3, Exsheated S. vulgaris L3 or S. vulgaris L4 (closed circle). As controls, eqPBMC were exposed to plain cKW2 medium (cKW2 pre‐culture) or supernatants collected from the L4 culture (cKW2 post‐culture; open circle). The cytokine gene expression was normalized to the geometric mean for the reference genes (SDHA and RPL32) and calibrated to that in the medium control. FC > 2 (indicated by dashed line) was considered as up‐regulated, and significant differences between larval stages are indicated by *<0.05, **<0.01, ***<0.001
4. DISCUSSION
Alterations in the cytokine response to S. vulgaris third stage (L3) larvae via exsheatment of the L2 cuticle and moulting into L4 were followed in cultures of eqPBMC. The larval development was accompanied by a shift from induction of IL‐4 and IL‐13 (L3, exsheated L3 and L4) via IL‐5 and IL‐9 (exsheated L3 and L4) into IFN‐γ (L4 only). Such a shift in cytokine profile might contribute to the parasite's evasion of the host immune response.
In order to follow cytokine induction accompanying the moulting of the larval cuticles, pure and well‐defined larval preparations are needed. Therefore, cyathostomin L3 were initially used to set up the purification and endotoxin removal procedures. The conditions for exsheating and moulting of the L2 and L3 cuticle, respectively, were elaborated using S. vulgaris and verified by light microscopy. Cytokine mRNA expression levels were then evaluated using short‐term cultures of eqPBMC with appropriate controls as previously elaborated for studies of the G3 adjuvant and TLR agonists. 15 By these precautions, suitable conditions were established to study cytokine responses to defined larval preparations of S. vulgaris in vitro.
All cytokine profiles were characterized using whole UV‐irradiated larvae. What precise larval antigens that interacts with lymphoid cells can only be speculated on as knowledge about the biochemical composition of infective stage nematode larvae is scarce. 23 A possible candidate could be the polysaccharide chitin, which is expressed in most parasite eggshells and at larval moulting. 24 Chitin is known to interact with different PRRs such as TLR‐2, dectin‐1 and mannose‐binding receptors in the lungs and intestine. Depending on its source and structure, chitin induces various types of cytokines, associated with Th1, Th2 or Th17 profiles. 25 In our culture conditions, chitin induced expression of IL‐5 and its contribution to S. vulgaris immune reactions remains to be elucidated.
High levels of IL‐5 mRNA were induced both by the S. vulgaris L4 stage and by exsheated L3. An early onset of IL‐5 followed by eosinophilia has in previous vaccination trials been identified as important to generate a protective immunity against S. vulgaris. 9 , 26 Antibodies from vaccinated horses bound strongly to exsheated L3 that had been cultured for overnight or for 1‐3 days in cKW2 medium. 9 The same culture medium and conditions were used in the present study to generate L4s in six‐day cultures. The increase in IL‐5 and IL‐9 mRNA production induced by exsheated L3 and in particular L4 larvae suggests that the late L3/L4 stage contain surface antigens of importance for immune protection.
Helminth immunity is also suggested to rely on the pleiotropic cytokine IL‐9. 27 By regulating intestinal muscle contractions, mucus production and granulocyte activity, IL‐9 is known to facilitate worm clearance. 28 , 29 However, little is known about the function of IL‐9 at initial infection by larval stages of the parasite. In our study, the L4 but not the L3 stages of S. vulgaris induced high expression of IL‐9, despite the absence of TGF‐β. 30 Thus, these data could suggest that IL‐9 induction occurs after S. vulgaris has penetrated the intestinal wall and is located in the submucosa protected from expulsion.
Unexpectedly, L4 larvae induced remarkably high levels of IFN‐γ mRNA. This was in sharp contrast to L3 and exsheated L3 and might be related to the invasive stage when the parasite penetrates the intestinal wall. If this shift in immune profile reflects immune protection or immune evasion remains to be evaluated. The two horses that expressed the highest levels of IFN‐γ in response to the L4 stage of S. vulgaris also up‐regulated IL‐10. Therefore, the cDNA synthesis and qPCR for IFN‐γ and IL‐10 was repeated for these horses, but showing the same results. In fact, IL‐10 can be produced by most leucocytes and double producing IL‐10 and IFN‐γ capacities has been observed in Th1, Tr1 and dendritic cells and is suggested to be a self‐regulating mechanism to prevent immunopathology. 31 , 32 , 33 The observed simultaneous induction of the Th1 cytokine IFN‐γ and the Th2 cytokines IL‐4, IL‐5 and IL‐13 as well as IL‐9 by S. vulgaris L4 contradicts the original Th1/Th2 paradigm. In the context of helminth infection, the idea of a static relationship between these T‐cell subsets has, however, been challenged. 34 , 35 , 36
As S. vulgaris almost exclusively occurs in co‐infection with the highly prevalent group of species Cyathostominae, 4 , 37 cytokine induction by cyathostomin larvae was evaluated in parallel to S. vulgaris. L3 of both species generated IL‐4 and IL‐13, a Th2 profile corresponding to previous estimations of cytokine responses in horses infected with cyathostomins and S. vulgaris. 10 , 38 However, none of the horses produced IL‐10 or IFN‐γ in response to either cyathostomins or S. vulgaris L3 that would have indicated a recall helper T‐cell profile. Thus, no effect of any previous exposure to these parasites was revealed in the present short‐term cultures.
The adjuvant used in the present study, G3, has when combined with a split influenza virus been shown to balance the Th2 driven immune response towards Th1 with production of IgG2b antibodies and IFN‐γ/IL‐2 double secreting cells in mice. 39 Incorporating a diterpene in G3 (G3‐DT) has been shown to induce a broad immune response by CD8 cytotoxic T cells targeting the internal conserved virus nucleoproteins resulting in cross‐protection to viral flu strains lacking immune compatible haemagglutinin and neuroamidase antigens of the vaccine strain. 40 Previous in vitro studies in the horse 15 also illustrate that G3 when combined with TLR agonists can potentiate or reduce the TLR‐mediated cytokine induction. That was also shown in the present paper using L3 preparations of cyathostomins and S. vulgaris. In this context, the G3 induced up‐regulation of IFN‐γ and down‐regulation of IL‐10 was dominant also in the presence of cyathostomins or S. vulgaris L3. Also, the gene expression of IL‐4 and IL‐5 induced by S. vulgaris L3 was inhibited in the presence of G3 while IL‐4 and IL‐5 induced by cyathostomin L3 was only partly inhibited by G3.
From these results, it is evident that the early immune reaction to S. vulgaris varied with the different larval stages exposed to eqPBMC. In the future, it will be necessary to screen the parasite for both protective antigens and its immunomodulatory properties. Our data indicate that these studies should focus on the late L3 and L4 stages of S. vulgaris. Regardless choice of antigen, an adjuvant with a potent immunomodulatory capacity will be indispensable. In that context, the versatility of the G3 adjuvant makes it an interesting candidate for continued evaluation.
CONFLICT OF INTEREST
BM developed the G3 adjuvant (Patent No. WO 2013/051994 April 2013). KF is employed by CRODA Denmark A/S, supplying the G3 adjuvant for research purposes. Other authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
SH planned the study together with BH, ET and CF, performed the laboratory experiments and analysed the data. ET advised on parasite experiment set‐up. BH contributed to qPCR design and participated in analysing data. FN contributed to the larval preparations. KH prepared the G3 adjuvant provided by CRODA Denmark. BM advised on adjuvant experiment set‐up. CF contributed to evaluate results and work on manuscript. All authors read and approved the final manuscript.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/pim.12794.
ACKNOWLEDGEMENTS
We gratefully acknowledge Lise‐Lotte Fernström, Mari Wallbring and Carola Jansson for technical support and Giulio Grandi for valuable advice. This project was financed by the Swedish‐Norwegian Foundation for Equine Research (H‐16‐47‐193).
Hellman S, Tydén E, Hjertner B, et al. Cytokine responses to various larval stages of equine strongyles and modulatory effects of the adjuvant G3 in vitro. Parasite Immunol 2021;43:e12794 10.1111/pim.12794
Funding information
The project was funded by the Swedish‐Norwegian Foundation for Equine Research (H‐16‐47‐193).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Von Samson‐Himmelstjerna G. Anthelmintic resistance in equine parasites – detection, potential clinical relevance and implications for control. Vet Parasitol. 2012;185:2‐8. [DOI] [PubMed] [Google Scholar]
- 2. Whittaker JH, Carlson SA, Jones DE, Brewer MT. Molecular mechanisms for anthelmintic resistance in strongyle nematode parasites of veterinary importance. J Vet Pharmacol Ther. 2017;40(2):105‐115. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen MK, Vidyashankar AN, Olsen SN, Monrad J, Thamsborg SM. Strongylus vulgaris associated with usage of selective therapy on Danish horse farms‐is it reemerging? Vet. Parasitol. 2012;189:260‐266. [DOI] [PubMed] [Google Scholar]
- 4. Tydén E, Enemark HL, Franko MA, Höglund J, Osterman‐Lind E. Prevalence of Strongylus vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden. Vet Parasitol X. 2019;2:100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McGraw BM, Slocombe JOD. Strongylus vulgaris in the horse: a review. Can Vet Jour. 1976;17(6):150‐157. [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan SJ, Stromberg PC, Storts RW, Sowa BA, Lay JC. Histology and Morphometry of Strongylus vulgaris‐mediated equine mesenteric arteritis. J Comp Pathol. 1991;104:89‐99. [DOI] [PubMed] [Google Scholar]
- 7. Pihl TH, Nielsen MK, Olsen SN, Leifsson PS, Jacobsen S. Nonstrangulating intestinal infarctions associated with Strongylus vulgaris: Clinical presentation and treatment outcomes of 30 horses (2008–2016). Equine Vet J. 2018;4:474‐480. [DOI] [PubMed] [Google Scholar]
- 8. Klei TR, Torbert BJ, Chapman MR, Ochoa R. Irradiated larval vaccination of ponies against Strongylus vulgaris. J Parasitol. 1982;68(4):561‐569. [PubMed] [Google Scholar]
- 9. Monahan CM, Taylor HW, Chapman MR, Klei TR. Experimental immunization of ponies with Strongylus vulgaris radiation‐attenuated larvae or crude soluble somatic extracts from larval or adult stages. J Parasitol. 1994;80(6):911‐923. [PubMed] [Google Scholar]
- 10. Swiderski CE, Klei TR, Folsom RW, et al. Vaccination against Strongylus vulgaris in ponies: comparison of the humoral and cytokine responses of vaccinates and nonvaccinates. Adv vet Med. 1999;41:389‐404. [DOI] [PubMed] [Google Scholar]
- 11. Klei TR, Chapman MR, Torbert BJ, McClure JR. Antibody responses of ponies to initial and challenge infections of Strongylus vulgaris. Vet Parasitol. 1983;12:187‐198. [DOI] [PubMed] [Google Scholar]
- 12. Klei TR, Chapman MR, Dennis VA. Role of the eosinophil in serum‐mediated adherence of equine leukocytes to infective larvae of Strongylus vulgaris. J Parasitol. 1992;78(3):477‐484. [PubMed] [Google Scholar]
- 13. Dennis VA, Klei TR, Chapman MR. Generation and partial characterization of an eosinophil chemotactic cytokine produced by sensitized equine mononuclear cells stimulated with strongylus vulgaris antigen. Vet Immunol and Immunopathol. 1993;37:135‐149. [DOI] [PubMed] [Google Scholar]
- 14. Abo‐Aziza FAM, Hendawy SHM, Namaky AHE, Ashry HM. Th1/Th2 balance and humoral immune response to potential antigens as early diagnostic method of equine Strongylus nematode infection. Vet World. 2007;10(6):679‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hellman S, Hjertner B, Morein B, Fossum C. The adjuvant G3 promotes a Th1 polarizing innate immune response in equine PBMC. Vet Res. 2018;49:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Wyk JA, Mayhew E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: a practical lab guide. Onderstepoort J Vet Res. 2013;80:E1‐E14. [DOI] [PubMed] [Google Scholar]
- 17. Bevilaqua CML, Rodrigues ML, Concordet D. Identification of infective larvae of some common nematode strongylids of horses [Strongylus vulgaris, S. equinus, S. edentatus, Triodontophorus spp., Poteriostomum spp., Gyalocephalus capitatus, Cylicocyclus radiatus, C. nassatus, C. minutus, C. poculatus]. Rev Med Vet (France). 1993;12:989‐995. [Google Scholar]
- 18. Titoy GAP, Van Rensburg LJ. Cryopreservation of third‐stage larvae of Strongylus vulgaris (large strongyle of horses). Onderstepoort J Vet Res. 1997;64:159‐160. [PubMed] [Google Scholar]
- 19. Chapman MR, Hutchinson GW, Cenac MJ, Klei TR. In vitro culture of equine strongylidae to the fourth larval stage in a cell‐free medium. J Parasitol. 1994;80(2):225‐231. [PubMed] [Google Scholar]
- 20. Farrar RG, Klei TR. In vitro development of Strongylus edentatus to the fourth larval stage with notes on Strongylus vulgaris and Strongylus equinus. J Parasitol. 1985;71(4):489‐499. [PubMed] [Google Scholar]
- 21. Beekman L, Tohver T, Léguillette R. Comparison of cytokine mRNA expression in the bronchoalveolar lavage fluid of horses with inflammatory airway disease and bronchoalveolar lavage mastocytosis or neutrophilia using REST software analysis. J Vet Med. 2012;26:153‐161. [DOI] [PubMed] [Google Scholar]
- 22. Douvres FW, Tromba FG, Doran DJ. The influence of NCTC 109, serum, swine kidney cell cultures on the morphogenesis of Stephanurus dentatus to fourth stage, in vitro. J Parasitol. 1966;52(5):875‐889. [PubMed] [Google Scholar]
- 23. Rogers WP, Sommerville RI. The infective stage of nematode parasites and its significance in parasitism. Adv Parasitol. 1963;1:109‐177. [DOI] [PubMed] [Google Scholar]
- 24. Foster JM, Zhang Y, Kumar S, Carlow CK. Parasitic nematodes have two distinct chitin synthases. Mol Biochem Parasitol. 2005;142(1):126‐132. [DOI] [PubMed] [Google Scholar]
- 25. Komi DEA, Sharma L, Dela Cruz CS. Chitin and its effect on inflammatory and immune responses. Clin rev Allergy Immunol. 2018;54(2):213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klei TR. Equine immunity to parasites. Vet Clin North Am Equine Pract. 2000;16(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan MH, Hufford MM, Olsson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15(5):295‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan WI, Richard M, Akiho H, et al. Modulation of intestinal muscle contraction by interleukin‐9 (IL‐9) or IL‐9 neutralization: correlation with worm expulsion in murine nematode infections. Infect Immun. 2003;71(5):2430‐2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Licona‐Limón P, Henao‐Mejía J, Temann AU, et al. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity. 2013;39(4):744‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitt E, Germann T, Goedert S, et al. IL‐9 production of naive CD4+ T cells depends on IL‐2, is synergistically enhanced by a combination of TGF‐beta and IL‐4, and is inhibited by IFN‐gamma. J Immunol. 1994;153:3989‐3996. [PubMed] [Google Scholar]
- 31. Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10‐producing anti‐inflammatory T cells. Nat Immunol. 2007;8(12):1380‐1389. [DOI] [PubMed] [Google Scholar]
- 32. O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin‐10. Nat Rev Immunol. 2007;7(6):425‐428. [DOI] [PubMed] [Google Scholar]
- 33. Yanagawa Y, Iwabuchi K, Onoé K. Co‐operative action of interleukin‐10 and interferon‐gamma to regulate dendritic cell functions. Immunology. 2009;127(3):345‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peine M, Rausch S, Helmstetter C, et al. Stable T‐bet(+)GATA‐3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 2013;11(8):e1001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bouchery T, Kyle R, Ronchese F, Le Gros G. The differentiation of CD4(+) T‐helper cell subsets in the context of helminth parasite infection. Front Immunol. 2014;5(15):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Deaton AM, Cook PC, De Sousa D, Phythian‐Adams AT, Bird A, MacDonald AS. A unique DNA methylation signature defines a population of IFN‐γ/IL‐4 double‐positive T cells during helminth infection. Eur J Immunol. 2014;44(6):1835‐1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Osterman Lind E, Höglund J, Ljungström BL, Nilsson O, Uggla A. A field survey on the distribution of strongyle infections of horses in Sweden and factors affecting faecal egg counts. Equine Vet J. 1999;31(1):68‐72. [DOI] [PubMed] [Google Scholar]
- 38. Davidson AJ, Hodgkinson JE, Proudman CJ, Matthews JB. Cytokine reponses to Cyathostominae larvae in the equine large intestinal wall. Res Vet Sci. 2005;78:169‐176. [DOI] [PubMed] [Google Scholar]
- 39. Hjertner B, Bengtsson T, Morein B, Paulie S, Fossum C. A novel adjuvant G3 induces both Th1 and Th2 related immune responses in mice after immunization with a trivalent inactivated split‐virion influenza vaccine. Vaccine. 2018;36:3340‐3344. [DOI] [PubMed] [Google Scholar]
- 40. Van de Sandt CE, Kreijtz JH, Geelhoed‐Mieras MM, et al. Novel G3/DT adjuvant promotes the induction of protective T cells responses after vaccination with a seasonal trivalent inactivated split‐virion influenza vaccine. Vaccine. 2014;32:5614‐5623. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


