Abstract
Background
There is an unmet need for a validated, test‐specific symptom questionnaire to evaluate carbohydrate perception during breath tests. Our aim was to develop and validate a questionnaire for the assessment of symptoms after a provocative carbohydrate load.
Methods
After a literature search and initial focus group‐style interviews, five relevant complaints were identified. Responses were given on a Likert‐type faces scale with a language children use and understand. Reliability, validity and responsiveness to change were established by the implementation of the questionnaire during breath tests in 215 pediatric subjects. Correlation between the questionnaire and a medical interview by a pediatrician who was blinded to the results of the questionnaire (n = 19) was determined.
Key Results
The questionnaire had good face and content validity (Lawshe ratio = 1). Intraclass correlation coefficients for test‐retest reliability (n = 116) demonstrated good repeatability (P < .001), and effect sizes were small (Cohen's d < 0.15 for all symptoms). Convergent validity and discriminant validity were supported according to the multitrait‐multimethod matrix method. The results obtained by the questionnaire correlated highly with the result of the medical interview (P < .001; Fisher's exact test). Cronbach's alpha was 0.81. Responsiveness was verified for the whole patient group and subgroups with medium to high effect sizes.
Conclusions and Inferences
The paediatric Carbohydrate Perception Questionnaire (pCPQ) is a simple, test‐specific questionnaire for a pediatric population. It is a valid instrument with excellent psychometric properties to assess gastrointestinal symptoms after carbohydrate ingestion. The pCPQ can replace non‐validated symptom assessment during carbohydrate breath tests and allows a standardized diagnosis of carbohydrate intolerance.
Keywords: breath tests, carbohydrates, intolerance, questionnaire
The unbiased diagnosis of carbohydrate intolerance requires a valid recording of carbohydrate induced symptoms. The paediatric Carbohydrate Perception Questionnaire overcomes the current lack of standardized and validated symptom assessment during carbohydrate breath tests, minimizes bias and can be the basis to standardize the assessment of carbohydrate intolerance.

Key Points.
The paediatric Carbohydrate Perception Questionnaire (pCPQ) allows standardized symptom assessment during carbohydrate breath tests and a valid diagnosis of carbohydrate intolerance, thereby providing an unbiased basis for treatment decisions.
We aimed to develop a symptom questionnaire for a paediatric population for use in carbohydrate hydrogen breath tests.
After basic development of the questionnaire validity of the questionnaire was established by the implementation of the questionnaire during breath tests in 215 paediatric subjects.
A symptom questionnaire for a paediatric population has been developed for use in carbohydrate hydrogen breath tests.
The paediatric Carbohydrate Perception Questionnaire (pCPQ) has excellent psychometric properties for the assessment of gastrointestinal symptoms after carbohydrate ingestion.
1. INTRODUCTION
The ingestion of carbohydrates in an amount that exceeds enteric absorptive capabilities can cause clinical symptoms such as gastrointestinal pain, bloating, or diarrhea. Malabsorbed carbohydrates reach the colon where bacterial metabolism converts them into gas and short‐chain fatty acids. These metabolites may be the cause of the clinical symptoms of carbohydrate malabsorption, although carbohydrate‐induced symptoms can also arise without detectable malabsorption. 1 , 2 Breath tests are clinical tools for the diagnosis of malabsorption by measuring the presence of hydrogen and methane in the exhaled air after the ingestion of provocative doses of poorly absorbable carbohydrates such as lactose or fructose. Breath tests are inexpensive, simple, and safe procedures and are widely used in pediatric practice for the evaluation of children or adolescents with abdominal symptoms that might be related to carbohydrate ingestion, although the clinical relevance of malabsorption determined by breath tests is disputed. 3 , 4
Apart from the determination of malabsorption, a carbohydrate challenge also allows the assessment of symptoms after carbohydrate ingestion, that is, the determination of intolerance to the carbohydrate in question. 5 Intolerance after a carbohydrate challenge rather than malabsorption corresponds to a history of clinical symptoms. 1 Although carbohydrate malabsorption and intolerance are not equivalent, 1 , 6 the terms ‘carbohydrate malabsorption’ and ‘carbohydrate intolerance’ are often confused or used interchangeably. 7 This has led to conflicting results of therapeutic studies of carbohydrate restriction diets. 8 Since it is intolerance that is of clinical relevance rather than malabsorption, the treatment of carbohydrate‐related symptoms, for example, by diet or enzyme replacement, should not be primarily aimed at altering malabsorption but rather at improving abdominal symptoms. 9 The unbiased diagnosis of intolerance requires a valid recording of carbohydrate‐induced symptoms.
The quality of carbohydrate intolerance measurement in the clinical setting and in the published literature has been limited: authors tend to incorrectly equate malabsorption with intolerance, the definition of intolerance is not standardized and, most notably, the assessment of symptoms has not been validated and hence is prone to multiple types of bias. 10 In the pediatric literature, the difficulty in data interpretation due to a lack of a validated symptom assessment tool has been acknowledged. 11 Symptoms following a carbohydrate challenge have been assessed by inviting the patient and/or their caregivers to report any undesignated symptom as it may arise, 12 , 13 , 14 by recall of specific symptoms, 15 or by use of a generic pain questionnaire13 or a questionnaire that was not validated for the pediatric population. 16 A wide array of abdominal symptoms, such as abdominal pain, cramping, bloating, distention, nausea, flatulence, and loose stools or diarrhea, has been considered relevant symptoms in children and adolescents. 11 , 12 , 15
Here, we describe the development and validation of a self‐administered symptom measurement questionnaire to assess the severity and the type of abdominal symptoms after the ingestion of a carbohydrate load in the pediatric population, the paediatric Carbohydrate Perception Questionnaire (pCPQ). A preliminary validation of this instrument has been reported elsewhere. 1 The questionnaire has undergone further analysis for complete validation, and this is reported in this manuscript. Our aim was to overcome the obvious lack of standardized and validated symptom assessment during the carbohydrate breath test 17 to minimize bias and to develop a standard tool for the assessment of carbohydrate intolerance.
2. METHODS
2.1. Questionnaire development
After a literature search and initial focus group‐style interviews given to parents and children who underwent breath hydrogen testing and to three experienced pediatric gastroenterologists, five relevant complaints were identified, and a Likert‐type faces questionnaire were constructed.
2.2. paediatric Carbohydrate Perception Questionnaire (pCPQ)
The symptoms evaluated were pain, nausea, meteorism, flatulence, and diarrhea in child‐oriented German language (Figure 1). Responses were given on a 6‐faces scale with a happy face and the words ‘not at all’ on the left, and a sad face and the words ‘particularly bad’ at the right. On the patients’ and parents’ request, half points between faces were added; thus, the final version that underwent further validation was an eleven‐point scale. The time frame of symptoms was given as ‘current’ (for baseline symptom assessment) and ‘since filling out the last questionnaire’.
FIGURE 1.
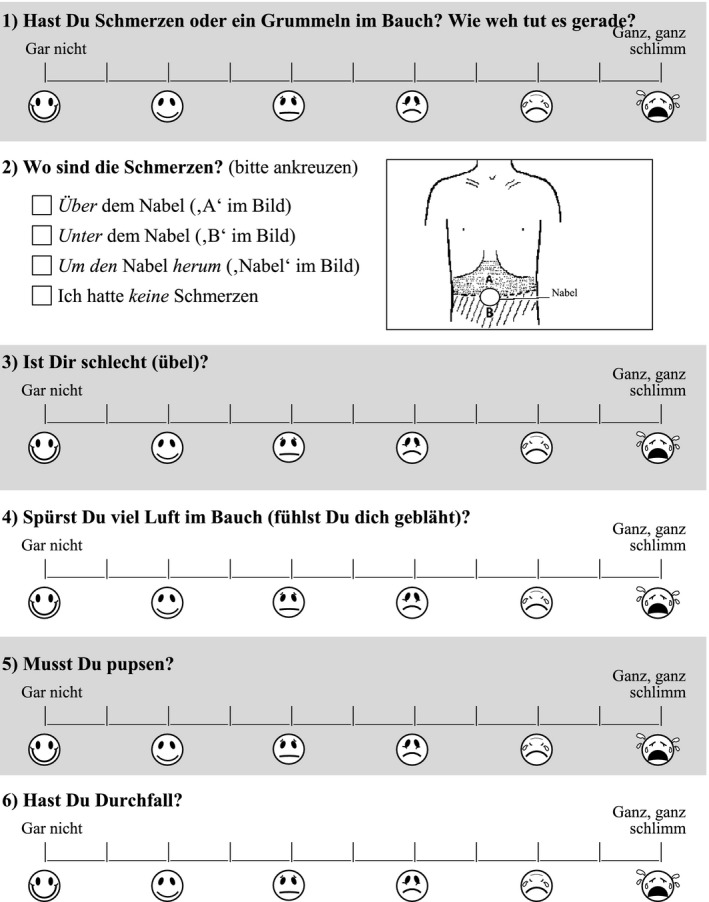
Example of the paediatric Carbohydrate Perception Questionnaire (pCPQ) (first page, to be filled out at time 0, before carbohydrate ingestion). The questionnaire is given in its original language (German); it has to undergo a standard translation process before its valid use in other languages and cultures! 27
2.3. Scale administration
The population cohort consisted of 215 consecutive pediatric patients who underwent carbohydrate (fructose: n = 99 or lactose: n = 116) breath hydrogen testing for diagnostic workup of functional (ie, non‐organic) abdominal pain disorders defined by recurrent or continuous abdominal pain of at least 2 months' duration. Patients were required to complete the pCPQ with or without their caregivers’ assistance (at the patients' discretion) at baseline and every 30 minutes concomitantly with the collection of breath samples for 3 hours. Patients/their caregivers were blinded as to the result of the concurrent breath test.
Breath tests were performed by experienced technical assistants. The preparation for the breath test and the method used has been described in detail1. In short, patients received standard instructions before the test (fructose (1 g/kg bodyweight, maximum of 25 g) or lactose (2 g/kg; maximum of 50 g); Kwizda Pharma). End‐expiratory breath samples were taken at 30‐minute intervals for 3 hours. Hydrogen levels of end‐expiratory breath samples were recorded.
2.4. Data and statistical analysis
An increase in the production of hydrogen ≥20 parts per million (ppm) over baseline was considered positive, that is, an indicator of carbohydrate malabsorption. A diagnosis of intolerance was established if during the 3 hours of breath testing an increase in the symptom score of more than 1 point over baseline was observed after carbohydrate ingestion, provided that the resulting absolute score was two or higher. 1
2.5. Reliability
Test‐retest reliability of the measure was assessed in all 116 patients who underwent the lactose challenge through the use of the first questionnaire (filled out before lactose ingestion) and the second questionnaire (filled out 30 minutes after lactose ingestion). Test‐retest reliability was established through two methods. The first was evaluating the average within‐patient change of each symptom score over the 30‐minute interval with statistical inference via the Wilcoxon signed‐rank test. Reliability was also assessed by intraclass correlation coefficients (ICC (3,1): two‐way mixed effects, consistency, single rater/measurement) for each item. Cohen's d was calculated as a measure of effect size.
Cronbach's alpha was calculated as a measure of internal consistency after calculating the sum of each item score over the observation period.
2.6. Questionnaire validation
Face validity was determined by four children and five parents, and content validity was assessed by four pediatricians and one gastroenterologist. The numbers were deemed appropriate due to uniform responses, and all participants were native German speakers.
2.6.1. Construct validity
The sample was used to suggest a grouping of symptom items into domains of symptom burden. A data‐driven approach was adopted using principal component analysis followed by varimax rotation. Components were extracted based on a fixed number of 3. Based on these findings, convergent and discriminant validity was determined by the multitrait‐multimethod matrix method described by Campbell and Fiske. 18
2.6.2. Concurrent validity
The questionnaire was administered to 19 consecutive patients undergoing lactose or fructose hydrogen breath tests. A pediatrician who was blinded to the result of the questionnaire ascertained the presence of symptoms by medical interview after the breath test. Fisher's exact test was used to determine the correlation between the questionnaire (symptom score <2 vs ≥2 during the breath test) and the physician interview.
2.7. Responsiveness
The difference between baseline symptom scores and the peak symptom scores during the test was assessed for each individual symptom and a global symptom score that was calculated for each participant by summing individual symptom scores at given time points. The Wilcoxon signed‐rank test was used for statistical inference, and effect sizes were determined as Cohen's d.
Statistical analysis was performed using SPSS 24 (IBM SPSS Statistics for Windows, Version 24.0, released 2016, IBM Corp).
The study was approved by the institutional Ethics Committee of the Medical University of Vienna (EK Nr 1149/2012). Date of approval granted by the ethical board: 6th June 2012. No written, informed consent was obtained in this retrospective study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
3. RESULTS
The characteristics of the patients are shown in Table 1. A total of 215 patients participated in the breath and intolerance tests, and 9 patients dropped out due to incomplete data. Forty‐one out of 77 patients with malabsorption (53.3%) were sensitive to the respective carbohydrate (‘intolerance’) (Table 2), while 44 out of 85 patients with intolerance (51.8%) had no H2‐increase ≥20 ppm during the breath test (no detectable malabsorption).
TABLE 1.
Characteristics of patients
| Female | Male | Overall | |
|---|---|---|---|
| Patients [n (%)] | 123 (57%) | 92 (43%) | 215 (100%) |
| Drop outs [n (%)] | 5 (4%) | 4 (4%) | 9 (4%) |
| Lactose challenge/drop out (n) | 68/4 | 48/2 | 116/6 |
| Fructose challenge/drop out (n) | 55/1 | 44/2 | 99/3 |
| Age, years (mean ± SEM) | 11.3 ± 0.31 | 10.7 ± 0.4 | 11.0 ± 0.2 |
| 25th percentile | 8.4 | 8.1 | 8.4 |
| 75th percentile | 13.9 | 12.3 | 13.6 |
| Weight (mean ± SEM) | 37.6 ± 1.3 | 37.6 ± 1.8 | 37.6 ± 1.0 |
| Breath test results | |||
| Lactose malabsorption [n (%)] | 21 (32.8%) | 14 (30.4%) | 35 (31.8%) |
| Lactose intolerance [n (%)] | 30 (46.9%) | 20 (43.5%) | 50 (45.5%) |
| Fructose malabsorption [n (%)] | 22 (40.7%) | 20 (47.6%) | 42 (43.8%) |
| Fructose intolerance [n (%)] | 15 (27.8%) | 20 (47.6%) | 35 (36.5%) |
TABLE 2.
Distribution of patients with carbohydrate malabsorption and carbohydrate intolerance (chi2: 29.7; P < .001)
| No intolerance | Intolerance | ||
|---|---|---|---|
| No malabsorption | 85 (41.3%) | 44 (21.4%) | 129 (62.6%) |
| Malabsorption | 36 (17.5%) | 41 (19.9% | 77 (37.4%) |
| 121 (58.7%) | 85 (41.3) | 206 (100%) |
3.1. Test‐retest reliability
When n = 116 patients were given the pCPQ twice, that is, before and 30 minutes after drinking the lactose solution, their paired scores for the five items did not change significantly (P > .05 for all items), while the correlation of items was highly significant (P < .001 for all items) (Table 3). The results are supportive of good agreement between occasions. 19 Cohen's d is a standardized measure of effect size in which values <0.4 are considered small. All d values were well inside the small range. Overall, the data are supportive of test‐retest reliability.
TABLE 3.
Test‐retest scores for the CPQ items
| Item | n | Mean changea | SDb | P c | ICCd | P e | Cohen's d |
|---|---|---|---|---|---|---|---|
| Pain | 116 | 0.12 | 1.11 | .63 | 0.71 | <.001 | 0.10 |
| Nausea | 116 | 0.004 | 0.89 | 1.0 | 0.75 | <.001 | 0.005 |
| Meteorism | 116 | ‐0.09 | 0.79 | .36 | 0.80 | <.001 | ‐0.11 |
| Flatulence | 116 | ‐0.009 | 0.61 | 1.0 | 0.79 | <.001 | ‐0.01 |
| Diarrhea | 116 | 0.07 | 0.45 | .29 | 0.62 | <.001 | 0.15 |
a,b: average within‐patient change and standard deviation (SD).
c P‐value from the Wilcoxon signed‐rank test.
dICC: intraclass correlation coefficient.
e P‐value from the Pearson correlation.
3.1.1. Internal consistency
Cronbach's alpha was 0.81, indicating good internal consistency.
3.2. Validity
The scale had strong face validity, as it was simple, easy to understand and brief. The content validity ratio according to Lawshe 20 equalled 1.
The three factors obtained from principal component analysis were grouped into (a) intestinal gas (two variables: meteorism and flatulence; average loading: 0.84), (b) pain/nausea (two variables: pain and nausea: average loading 0.82), and (c) diarrhea (one variable; loading: 0.96). The significance according to Bartlett's test of sphericity 21 was calculated to be <0.001. The correlation of items within factors was highly significant (P < .001) for both meteorism and flatulence (correlation coefficient 0.61) as well as for pain and nausea (0.63); hence, convergent validity was supported. None of the items correlated higher with items of other factors than with items of its own factor; hence, discriminant validity was supported. 18
Additionally, concurrent validity was demonstrated by a highly significant correlation of the questionnaire (symptomatic vs not symptomatic) with the results of the physician interviews in 19 pediatric subjects (P < .001; Fisher's exact test).
3.3. Responsiveness
Sensation scores at baseline and maximal differences in symptom scores to baseline (∆Syx) are given in Table 4 for the whole study group. ∆Syx was significant for each symptom, with medium effect sizes. The effect sizes increased when patients with malabsorption were assessed and were highest in patients with intolerance (Table 5).
TABLE 4.
Sensation scores for the whole patient group at baseline and maximal differences in symptom scores to baseline (ΔSyx)
| Sensation scores at baseline | (ΔSyx) | P‐value | ||
|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | Cohen's d | ||
| Pain | 0.82 ± 0.08 | 0.77 ± 0.10 | 0.53 | <.001 |
| Nausea | 0.59 ± 0.07 | 0.42 ± 0.07 | 0.41 | <.001 |
| Meteorism | 0.50 ± 0.06 | 0.60 ± 0.08 | 0.53 | <.001 |
| Flatulence | 0.28 ± 0.04 | 0.59 ± 0.07 | 0.57 | <.001 |
| Diarrhea | 0.10 ± 0.03 | 0.42 ± 0.08 | 0.36 | <.001 |
| Sum of all Symptoms | 2.32 ± 0.19 | 2.13 ± 0.26 | 0.57 | <.001 |
The P‐value is from the Wilcoxon signed‐rank test between the baseline symptom score and the score at the symptom peak.
TABLE 5.
Maximal differences in symptom scores to baseline (ΔSyx) for patients with and without malabsorption and with and without intolerance
| Participants with no carbohydrate malabsorption | Participants with carbohydrate malabsorption | Participants with no carbohydrate intolerance | Participants with carbohydrate intolerance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | P‐value | Cohen's d | Mean ± SEM | P‐value | Cohen's d | Mean ± SEM | P‐value | Cohen's d | Mean ± SEM | P‐value | Cohen's d | |
| Pain | 0.58 ± 0.11 | <.001 | 0.43 | 1.12 ± 0.18 | <.001 | 0.69 | 0.14 ± 0.10 | .05 | 0.14 | 1.72 ± 0.16 | <.001 | 1.20 |
| Nausea | 0.29 ± 0.08 | <.001 | 0.32 | 0.65 ± 0.13 | <.001 | 0.56 | 0.12 ± 0.07 | .03 | 0.15 | 0.92 ± 0.13 | <.001 | 0.78 |
| Meteorism | 0.48 ± 0.10 | <.001 | 0.42 | 0.81 ± 0.12 | <.001 | 0.74 | 0.22 ± 0.09 | .02 | 0.24 | 1.16 ± 0.13 | <.001 | 0.97 |
| Flatulence | 0.36 ± 0.07 | <.001 | 0.45 | 1.02 ± 0.14 | <.001 | 0.81 | 0.30 ± 0.06 | <.001 | 0.42 | 1.07 ± 0.14 | <.001 | 0.83 |
| Diarrhea | 0.21 ± 0.09 | .009 | 0.21 | 0.79 ± 0.16 | <.001 | 0.57 | 0.10 ± 0.06 | .1 | 0.14 | 0.93 ± 0.17 | <.001 | 0.60 |
| Sum of all Symptoms | 1.38 ± 0.26 | <.001 | 0.46 | 3.49 ± 0.52 | <.001 | 0.77 | 0.49 ± 0.23 | .009 | 0.20 | 4.74 ± 0.43 | <.001 | 1.23 |
4. DISCUSSION
The present study validates the pCPQ, a novel instrument developed to assess abdominal symptoms related to the ingestion of poorly absorbable carbohydrates in a standardized manner. The need for this instrument evolved from the limitations of current assessment practices, particularly non‐standardized methods of assessment, which may be subject to doctor‐ and patient‐related biases. At the same time, there is increasing appreciation of the important role of documenting intolerance, that is, the relation between carbohydrate ingestion and the occurrence of symptoms, for clinical decision making, such as suggesting treatment with diets or enzyme supplementation, and research. 9 , 22 Based on our data, the pCPQ has excellent psychometric properties and has a minimal burden on the patient and resources, as it is brief and easy to administer, fill out, score, and interpret.
The key measurement properties of a symptom‐assessment instrument are its reliability and validity in the population to be studied. The study population represented the population of interest, children, and adolescents who were referred for evaluation of the clinical suspicion of carbohydrate intolerance. The youngest patient was 5.0 years old, and 99% were under 18 years of age. The range of age was large but represents the range of age being looked after at a pediatric institution. There was no distinct influence of age on the test results. The proportion of malabsorbers and patients with and without intolerance was comparable among the younger half and the older half of participants. Thirty‐seven percent of patients had malabsorption, of whom 53% had carbohydrate intolerance; on the other hand, 41% of participants had intolerance, and 52% had no H2‐increase ≥20 ppm during the breath test (no detectable malabsorption). We did not measure methane (CH4) in the exhaled air to detect non‐hydrogen producing malabsorbers. 23 Quantification of CH4 in exhaled breath may have increased the number of malabsorbers detected. However, the proportion of patients with isolated elevation of CH4 is small, and a combined measurement of H2 and CH4 showed comparable results in an adult population with respect to a poor association between malabsorption and clinical symptoms. 6 , 22
The reliability of the pCPQ was ascertained by Cronbach's alpha and measurement of reproducibility. Cronbach's alpha, a commonly used measure of internal consistency, was >0.8, generally regarded as good. 24 Test‐retest reliability was established by applying the test twice, before and 30 minutes after the ingestion of lactose, at a time when presumably the consumed carbohydrate had not induced symptoms. 6 Patients who ingested fructose were not included in reliability testing, as fructose ingestion may lead to symptoms within the 30‐minute timeframe, which is earlier than after lactose ingestion. 1 , 25 The time frame was chosen to be short enough to avoid instability of abdominal symptoms caused by the ingestion of the carbohydrate on one hand but at the possible cost of remembering previous answers on the other hand. However, as the patients were not aware of reliability testing and an intervention (lactose ingestion) separated the two tests, we are confident that patients did not intentionally duplicate the questionnaires.
Different abdominal symptoms begin at varying time points after carbohydrate ingestion and persist for differing lengths of time, and the various carbohydrates induce symptoms at various time points. 1 , 25 The responsiveness of each item was demonstrated in the patient group as a whole with medium effect sizes. As we had initially expected, greater effect sizes were observed in malabsorbers, and even greater effect sizes were found in carbohydrate‐intolerant patients who were diagnosed as such when one of the symptoms increased by ≥1 over baseline (provided that the resulting absolute score was two or higher). The specific symptom that led to the diagnosis of intolerance differed widely among subjects and often more than one symptom increased ≥1 over baseline during the course of the breath test, with pain (61%) and meteorism (40%) being the most frequent symptoms leading to the diagnosis of intolerance.
There is currently no gold standard to assess the correct cutoff point for the diagnosis of intolerance during the carbohydrate challenge breath test. Since the correlation between the results of the breath test (malabsorption) and intolerance is poor, 1 , 6 an ROC‐curve was not considered. Further studies that us the pCPQ during breath tests and correlate the diagnosis of intolerance with the effects of diets may allow to refine the diagnostic cutoff points. However, we have recently shown that the results of a cutoff point of 1 during a fructose hydrogen breath test highly correlate with clinical symptoms in pediatric patients with functional abdominal pain disorders. 1
The questionnaire was developed by pediatric and adult gastroenterologists experienced in the development of questionnaires after focus group‐style interviews with representatives of the target group (children and their caregivers) and a literature search. The language used was adjusted to be easily understandable by children. The final instrument was tested and validated in a large sample of patients. Noteworthy, we did not apply the questionnaire to a healthy control group and did not assess social and school level of the studied population as well as we did not assess whether the questionnaire was filled out by the patient, their caregivers or in cooperation.
We have assessed several types of validity in this study. Construct validity was confirmed by the multitrait‐multimethod matrix method. We also found clear evidence of concurrent validity of the questionnaire, as the test results highly correlated with the results of blinded physicians´ interviews, while providing standardization, a defined validity and reliability, comparability and scalability. In comparison with an interview, the questionnaire avoids interrogator and responder bias and allows easy analysis and visualization. No differences in validity criteria were found between the sample undergoing lactose and fructose tests.
The evaluation of symptoms induced by the carbohydrate load during breath tests has been recommended by consensus. 22 However, symptom recordings during breath tests are not standardized, and validated instruments do not exist to control for bias in symptom recording in the pediatric population. If there is no data on the reliability or validity of a symptom assessment in the setting it is used, then it should not be used, as it is not known if it truly measures what is intended to be measured and if data are obtained in a consistent, uniform manner. 26 Our data show that the pCPQ has good reliability and repeatability and performs well on several aspects of validity. The current questionnaire is in German and has to undergo a standard translation process before its valid use in other languages and cultures. 27
Each symptom included in the pCPQ was thoroughly discussed among experts and patients and their parents. Abdominal pain, meteorism, flatulence, and diarrhea were accepted undisputedly to be included in the questionnaire. Nausea is not regularly considered a symptom of carbohydrate malabsorption, 25 however, is a common symptom in the pediatric population. 28 Pediatricians and patients/parents requested nausea to be incorporated into the symptom questionnaire. Moreover, nausea has been associated with a high burden of somatic symptoms as well as depression 29 , 30 , 31 and significantly impacts gastrointestinal and extra‐gastrointestinal symptoms, psychologic well‐being, and long‐term health outcomes later in life. 32
The pCPQ allows for the determination of both the quality and the severity of abdominal symptoms after a carbohydrate challenge. A Likert‐type faces scale was used for the scaling of symptom severity. Initially, a six‐point scale was designed, but on the patients' and parents' request, half points between faces were added; thus, the final version that underwent further validation was an eleven‐point scale. Despite some advantages of the visual analogue scale (VAS) in the adult population, some populations (eg, children and the elderly) prefer the Likert scale over the VAS and find it easier to complete. 33 , 34 Overall, Likert scales and VAS are of comparable reliability. 33
The combination of carbohydrate breath tests with an unbiased symptom assessment allows for the determination of four different entities after a carbohydrate load, the predictive capacity of which are to be determined in future studies by an evaluation to the response to diet: (a) malabsorption plus symptoms, (b) malabsorption only, (c) symptoms only, and (d) none of the above. While in the past the presence of malabsorption has been the main focus of carbohydrate breath tests, recent data suggest that symptoms after a carbohydrate load may be of superior clinical relevance. 1 , 25 Carbohydrate intolerance in the actual meaning of the term, that is the development of symptoms after carbohydrate ingestion, has been neglected in the clinical context. This may have hampered the adequate support of a significant number of patients with carbohydrate‐induced symptoms. 8 It has been suggested that the term ‘carbohydrate intolerance’ should be replaced by ‘sensitivity to the carbohydrate’ or ‘carbohydrate hypersensitivity’, because there is confusion regarding the term ‘carbohydrate intolerance’ since it has often been used indiscriminately in the context of carbohydrate malabsorption, encompassing both malabsorption and carbohydrate‐induced symptoms. As data accumulate that suggest that symptoms induced by carbohydrate ingestion are mainly due to visceral hypersensitivity, 1 ‘carbohydrate hypersensitivity’ may express a link to visceral hypersensitivity, which is an established and well‐defined term in functional gastrointestinal research. 35
In summary, we have developed a novel instrument to evaluate symptoms before and during carbohydrate breath tests in a large pediatric population. The pCPQ allows for standardized, unbiased symptom assessment during carbohydrate breath tests and therefore a valid diagnosis of carbohydrate intolerance. It may set an imperatively needed standard for the diagnosis of carbohydrate intolerance and is suitable for the clinical setting of breath testing for therapeutic trials and research.
CONFLICT OF INTEREST
Johann Hammer: shareholder: Carboception. Provision of questionnaires: For studies without financial support from industrial sponsors, we provide the questionnaires free of charge.
AUTHOR CONTRIBUTIONS
Johann Hammer involved in study concept and design; study supervision; statistical analysis; interpretation of data; drafting of the manuscript and critical revision of the manuscript for important intellectual content; Nima Memaran and Wolf‐Dietrich Huber involved in acquisition of data; administrative, technical, or material support; and revision of the manuscript; Karin Hammer involved study design, acquisition of data, administrative, technical, or material support; revision of the manuscript; and All authors approved the final version of the manuscript.
Hammer J, Memaran N, Huber W‐D, Hammer K. Development and validation of the paediatric Carbohydrate Perception Questionnaire (pCPQ), an instrument for the assessment of carbohydrate‐induced gastrointestinal symptoms in the paediatric population. Neurogastroenterology & Motility. 2020;32:e13934 10.1111/nmo.13934
REFERENCES
- 1. Hammer V, Hammer K, Memaran N, Huber W, Hammer K, Hammer J. Relationship between abdominal symptoms and fructose ingestion in children with chronic abdominal pain. Dig Dis Sci. 2018;63:1270‐1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Major G, Pritchard S, Murray K, et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate‐related symptoms in individuals with irritable bowel syndrome. Gastroenterology. 2017;152:124‐133. [DOI] [PubMed] [Google Scholar]
- 3. Hammer HF, Hammer J. Diarrhea caused by carbohydrate malabsorption. Gastroenterol Clin North Am. 2012;41:611‐627. [DOI] [PubMed] [Google Scholar]
- 4. Wilder‐Smith CH, Olesen SS, Materna A, Drewes AM. Repeatability and effect of blinding of fructose breath tests in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2019;31:e13497. [DOI] [PubMed] [Google Scholar]
- 5. Suchy FJ, Brannon PM, Carpenter TO, et al. National institutes of health consensus development conference: lactose intolerance and health. Ann Intern Med. 2010;152:792‐796. [DOI] [PubMed] [Google Scholar]
- 6. Wilder‐Smith C, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Costanzo M, Berni CR. Lactose intolerance: common misunderstandings. Ann Nutr Metab. 2018;73(Suppl 4):30‐37. [DOI] [PubMed] [Google Scholar]
- 8. Chumpitazi BP, Shulman RJ. dietary carbohydrates and childhood functional abdominal pain. Ann Nutr Metab. 2016;68(Suppl 1):8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7:8020‐8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi BC, Pak AW. A catalog of biases in questionnaires. Prev Chronic Dis. 2005;2:1‐13. [PMC free article] [PubMed] [Google Scholar]
- 11. Pawłowska K, Umławska W, Iwańczak B. Prevalence of lactose malabsorption and lactose intolerance in pediatric patients with selected gastrointestinal diseases. Adv Clin Exp Med. 2015;24:863‐871. [DOI] [PubMed] [Google Scholar]
- 12. Pawłowska K, Seredyński R, Umławska W, Iwańczak B. Hydrogen excretion in pediatric lactose malabsorbers: relation to symptoms and the dose of lactose. Arch Med Sci. 2018;14:88‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glatstein M, Reif S, Scolnik D, et al. Lactose breath test in children: relationship between symptoms during the test and test results. Am J Ther. 2018;25:e189‐e193. [DOI] [PubMed] [Google Scholar]
- 14. Däbritz J, Mühlbauer M, Domagk D, et al. Significance of hydrogen breath tests in children with suspected carbohydrate malabsorption. BMC Pediatr. 2014;27(14):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yerushalmy‐Feler A, Soback H, Lubetzky R, et al. One‐third of children with lactose intolerance managed to achieve a regular diet at the three‐year follow‐up point. Acta Paediatr. 2018;107:1389‐1394. [DOI] [PubMed] [Google Scholar]
- 16. Rojo C, Jaime F, Azócar L, et al. Concordance between lactose quick test, hydrogen‐methane breath test and genotyping for the diagnosis of lactose malabsorption in children. Neurogastroenterol Motil. 2018;30:e13271. [DOI] [PubMed] [Google Scholar]
- 17. Hammer J, Hammer HF. There is an unmet need for test‐specific, validated symptom questionnaires for breath tests in adults. Gastroenterology. 2019;156:1220‐1221. [DOI] [PubMed] [Google Scholar]
- 18. Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait‐multimethod matrix. Psychol Bull. 1959;56:81‐105. [PubMed] [Google Scholar]
- 19. Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology". Psychol Assess. 1994;6:284‐290. [Google Scholar]
- 20. Lawshe CH. A quantitative approach to content validity. Pers Psychol. 1975;28:563‐575. [Google Scholar]
- 21. Field A. Discovering Statistics using SPSS for Windows. London – Thousand Oaks –. New Delhi: Sage publications; 2000. [Google Scholar]
- 22. Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2‐breath testing in gastrointestinal diseases: the Rome consensus conference. Aliment Pharmacol Ther. 2009;29(Suppl 1):1‐49. [DOI] [PubMed] [Google Scholar]
- 23. Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane‐based breath testing in gastrointestinal disorders: the North American consensus. Am J Gastroenterol. 2017;112:775‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Streiner DL. Starting at the beginning: an introduction to coefficient alpha and internal consistency. J Pers Assess. 2003;80:99‐103. [DOI] [PubMed] [Google Scholar]
- 25. Wilder‐Smith CH, Olesen SS, Materna A, Drewes AM. Fermentable sugar ingestion, gas production, and gastrointestinal and central nervous system symptoms in patients with functional disorders. Gastroenterology. 2018;155:1034‐1044. [DOI] [PubMed] [Google Scholar]
- 26. Scott I. You can't believe all that you're told: the issue of unvalidated questionnaires. Inj Prev. 1997;3:5‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sperber AD. Translation and validation of study instruments for cross‐cultural research. Gastroenterology. 2004;126(Suppl 1):S124‐128. [DOI] [PubMed] [Google Scholar]
- 28. Kovacic K, Di Lorenzo C. Functional nausea in children. J Pediatr Gastroenterol Nutr. 2016;62:365‐371. [DOI] [PubMed] [Google Scholar]
- 29. Kovacic K, Williams S, Li BUK, Chelimsky G. High prevalence of nausea in children with pain‐associated functional gastrointestinal disorders: are Rome criteria applicable? J Pediatr Gastroenterol Nutr. 2013;57:311‐315. [DOI] [PubMed] [Google Scholar]
- 30. Kovacic K, Li BUK. Childhood chronic nausea: is it just a queasy stomach? Curr Gastroenterol Rep. 2014;16:395‐396. [DOI] [PubMed] [Google Scholar]
- 31. Tarbell SE, Shaltout HA, Wagoner AL, Diz DI, Fortunato JE. Relationship among nausea, anxiety, and orthostatic symptoms in pediatric patients with chronic unexplained nausea. Exp Brain Res. 2014;232:2645‐2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Russell AC, Stone AL, Walker LS. Nausea in children with functional abdominal pain predicts poor health outcomes in young adulthood. Clin Gastroent Hepatol. 2017;15:706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Laerhoven H, van der Zaag‐Loonen HJ, Derkx BH. A comparison of Likert scale and visual analogue scales as response options in children's questionnaires. Acta Paediatr. 2004;93:830‐835. [DOI] [PubMed] [Google Scholar]
- 34. Gorrall BK, Curtis JD, Little TD, Panko P. Innovations in measurement: visual analog scales and retrospective pretest self‐report designs. Actualidades en Psicología. 2016;30:2‐7. [Google Scholar]
- 35. Delgado‐Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194‐S203. [DOI] [PubMed] [Google Scholar]


