Abstract
Objective
Microcirculatory perfusion disturbances following hemorrhagic shock and fluid resuscitation contribute to multiple organ dysfunction and mortality. Standard fluid resuscitation is insufficient to restore microcirculatory perfusion; however, additional therapies are lacking. We conducted a systematic search to provide an overview of potential non‐fluid‐based therapeutic interventions to restore microcirculatory perfusion following hemorrhagic shock.
Methods
A structured search of PubMed, EMBASE, and Cochrane Library was performed in March 2020. Animal studies needed to report at least one parameter of microcirculatory flow (perfusion, red blood cell velocity, functional capillary density).
Results
The search identified 1269 records of which 48 fulfilled all eligibility criteria. In total, 62 drugs were tested of which 29 were able to restore microcirculatory perfusion. Particularly, complement inhibitors (75% of drugs tested successfully restored blood flow), endothelial barrier modulators (100% successful), antioxidants (66% successful), drugs targeting cell metabolism (83% successful), and sex hormones (75% successful) restored microcirculatory perfusion. Other drugs consisted of attenuation of inflammation (100% not successful), vasoactive agents (68% not successful), and steroid hormones (75% not successful).
Conclusion
Improving mitochondrial function, inhibition of complement inhibition, and reducing microvascular leakage via restoration of endothelial barrier function seem beneficial to restore microcirculatory perfusion following hemorrhagic shock and fluid resuscitation.
Keywords: animal models, capillary perfusion, fluid resuscitation, hemorrhagic shock
Abbreviations
- ALM
adenosine, lidocaine, and magnesium
- ATP
adenosine triphosphate
- eNOS
endothelial NO synthase
- HB/M
a combination of beta‐hydroxybutyrate and melatonin
- NO
nitric oxide
- PARP
poly(ADP‐ribose) polymerase
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RBC
red blood cell
- ROS
reactive oxygen species
- SYRCLE
Systematic Review Centre for Laboratory Animal Experimentation
- TNF‐α
tumor necrosis factor‐α
1. INTRODUCTION
Microcirculatory perfusion disturbances following hemorrhagic shock and fluid resuscitation are a major complication and associated with the development of multiple organ dysfunction and increased mortality. 1 Standard treatment of hemorrhagic shock involves control of bleeding followed by fluid resuscitation. A combination of crystalloids and blood products is given to improve reperfusion and oxygenation of ischemic tissue. 2 Microcirculatory perfusion is impaired early following hemorrhagic shock 1 , 3 ; however, fluid resuscitation fails to restore microcirculatory perfusion. 3 , 4 As microcirculatory perfusion is essential for tissue delivery of oxygen and nutrients, its persistent impairment 3 , 4 , 5 is detrimental for organ function. Therefore, additional therapeutic strategies to restore microcirculatory perfusion are warranted.
The pathophysiology of hemorrhagic shock and fluid resuscitation is complex and involves a systemic inflammatory response, coagulation disturbances, mitochondrial dysfunction, and endothelial activation. 6 The vascular endothelium plays a key role in the pathophysiology of hemorrhagic shock via the regulation of coagulation, inflammation, leukocyte trafficking, and vascular tone and permeability. 7 Under normal circumstances, endothelial cells are tightly bound and leakage of fluids to the interstitium is relatively low. 8 However, during inflammation, as seen during hemorrhagic shock, the endothelium is activated by proinflammatory mediators. This leads to increased endothelial permeability, 7 , 9 with progressive leakage of fluids to the interstitium and, eventually, tissue edema. To enable sufficient blood flow, restoration of the circulating volume is necessary; however, fluid resuscitation can also further aggravate fluid leakage and tissue edema. 7 , 10 Tissue edema increases diffusion distances between capillaries 11 leading to impaired tissue perfusion, reduced ATP production, and mitochondrial dysfunction. 12 , 13 Blood transfusion, hypothermia, and acidosis are other factors evoking the development of coagulopathy, complement activation, 14 and systemic inflammation. 7 , 15 The systemic inflammatory response is accompanied by the formation of ROS, which are normally neutralized by antioxidants. 12 However, hemorrhagic shock‐induced mitochondrial dysfunction causes a shift of the balance toward increased ROS production over antioxidant neutralization capacity, which leads to cell apoptosis and tissue damage. 12
Additional therapeutic interventions are warranted to improve the success rate of the current treatment strategies to restore microcirculatory perfusion following hemorrhagic shock and fluid resuscitation. Hence, the aim of this review was to provide an overview of the effect of non‐fluid‐based therapeutic interventions, given in addition to fluid resuscitation, on microcirculatory perfusion following experimental hemorrhagic shock compared to untreated, with fluid resuscitated controls.
2. METHODS
2.1. Protocol and registration
This systematic review conforms to the standards of reporting according to the PRISMA reporting guideling 16 (PRISMA checklist: Table S1) and registered at the International Prospective Register of Systematic Reviews (PROSPERO; CRD42018095432). 17 During analysis, it appeared that by following the PROSPERO protocol, the extent of extracted data was very large and heterogenic, which made it impossible to give a clear overview. Therefore, in this review only the results of non‐fluid‐based therapeutic agents on microcirculatory perfusion were reported, which deviates from the PROSPERO protocol that includes microcirculatory perfusion as primary outcome and microvascular leakage as secondary outcome.
2.2. Eligibility criteria
This systematic review included animal studies with any type, depth, and duration of experimental hemorrhagic shock; however, exchange transfusion was excluded. Any form, volume, and duration of fluid resuscitation with either shed blood, crystalloids, or colloids were included. Any type, dose, and timing of non‐fluid‐based drug administration were included; however, drugs had to be administrated in addition to standard fluid resuscitation. The control population consisted of animals with hemorrhagic shock and solely fluid resuscitation with either shed blood, crystalloids, or colloids. Study protocols with trauma were included, but additional sepsis, pregnancy, aneurysms, or alcohol intoxication was excluded. All outcome parameters reflecting microcirculatory flow dynamics were eligible (eg, flow rate, RBC velocity, or functional capillary density), independent of unit of measures. These parameters reflect the efficiency of microcirculatory perfusion via either the flow rate of RBC per tissue weight (blood flow), flow rate of blood cells solely (RBC velocity), or the distribution of perfused vessels in a certain organ (functional capillary density, number of perfused vessels).
2.3. Information sources and search
To identify eligible studies, PubMed, EMBASE.com, and The Cochrane Library (Wiley) electronic bibliographic databases were searched in collaboration with a medical information specialist (EJ and AvL). The first search was run in February 2019. The search was re‐run on March 6, 2020, before final analysis to retrieve most recent studies for inclusion. The full search strategy (Appendix S1) was based on the combination of the following search components: ‘hemorrhagic shock’, ‘capillaries’ or ‘microvasculature’ and ‘perfusion’ or ‘flow velocity’. Reviews, meeting abstracts, conference reports, letters, or editorials were excluded.
2.4. Study selection
Screening was performed in two phases: initial screening based on title and abstract followed by full‐text screening of the eligible articles for final inclusion. Titles and abstracts of studies retrieved using the search strategy and those from additional sources were screened independently by two observers (AvL and CvdB). Duplicates were identified and removed using EndNote™ (EndNote X7.4, Thomson Reuters), and studies that potentially met the inclusion criteria were identified according to the above‐mentioned inclusion and exclusion criteria. The screening results were organized in EndNote. The full texts of these potentially eligible studies were retrieved and independently assessed for eligibility by two observers. Discrepancies were resolved through discussion and consensus. The reference lists of included studies were screened for additional eligible studies not retrieved by our search (snowball search).
2.5. Data collection
Data extraction was conducted by one reviewer (AvL) and confirmed by another (CvdB). The following data were extracted and assembled in Microsoft Excel:
General study characteristics (author, year, country),
Animal model (species, age, sex, experimental groups, body weight),
Experimental model (hemorrhagic shock protocol: shock induction, target blood pressure, duration of shock; fluid resuscitation protocol: type of resuscitation fluid, volume, duration of follow‐up period),
Type of intervention (timing, dosage, route of administration, vehicle),
Outcome measurement (technique, organ, and time point of measurement) and outcome measures (quantitative results).
2.6. Risk of bias in individual studies
Risk of bias was determined by two independent reviewers (AvL and CvdB), based on the SYRCLE Risk of Bias tool. 18 Risks of bias were scored for the ten entries as described by Hooijmans et al 18 and supplemented with two questions addressing treatment of a sham/control group and inclusion of a power calculation. “Yes,” “no,” or “unclear” was used to indicate a low, high, or unclear risk of bias, respectively. Any statement of randomization or blinding was scored with “yes,” and absence of a certain statement was reported as “no.” Disagreements were resolved through consensus‐oriented discussion. Final statements of randomization and blinding at any point were formulated based on the risk assessment tool. The possibility of publication bias was assessed by evaluating a funnel plot of the trial standardized mean differences for asymmetry.
2.7. Summary measures and analysis
The intention of the current study is to give an overview of the effect of non‐fluid‐based therapeutic agents on microcirculatory perfusion. We did not limit the target organ or type of measurement, as this would influence outcome. Consequently, the results of the retrieved studies varied widely with regard to outcome parameter, techniques, unit measures, and organ. Due to this, additional quantitative analysis such as a meta‐analysis was not feasible. Our summary measures therefore take the form of a qualitative interpretation and a narrative analysis. Results of individual studies are reported in table format to combine experimental details with outcome measures. Significant differences in means as reported by the authors were categorized as “effective” when a significant increase was reported and “non‐effective” when no statistical effect or a significant decrease in blood flow was reported. Subsequently, studies were classified based on working mechanism. To provide 95% confidence intervals, individual studies are plotted in forest plots (Review Manager 5.3, Cochrane Centre, The Cochrane Collaboration). In case of multiple outcome measurements per study, only blood flow or RBC velocity was used in the descriptive results, with blood flow determined as most valuable. The results regarding characteristics and details of experimental protocol are reported as percentage of the total amount of studies. For the results regarding the microvascular flow assessment, the results are reported as number per working mechanism and percentage of effective studies per working mechanism.
3. RESULTS
3.1. Study selection
The search strategy is presented in a PRISMA diagram (Figure 1). The systematic literature search yielded 1269 records. After removal of duplicates (n = 465), 804 records were screened from which 73 full texts were subsequently examined for eligibility. Two additional records were identified through a snowball search. Finally, 48 studies were included, published between 1969 and 2019, by 35 individual authors. Countries of origin are listed as follows: Austria (n = 1), Brazil (n = 3), Canada (n = 3), China (n = 1), Germany (n = 11), Hungary (n = 1), Italy (n = 2), Japan (n = 1), the Netherlands (n = 1), the UK (n = 1), and the United States (n = 23).
Figure 1.
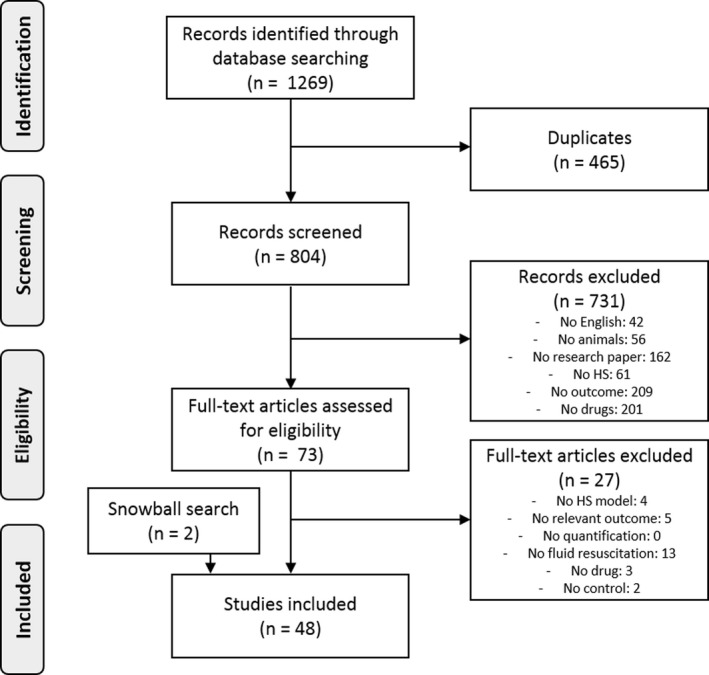
PRISMA diagram representing the flowchart of study selection. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
3.2. Study characteristics
Study characteristics of all included studies are presented in Table 1. Studies were performed in four different species, with rats being mainly used (77%). Other species used were hamsters (13%), dogs (8%), and pigs (2%). Most studies (71%) used male animals, seven studies (15%) used female animals, six studies (12%) did not report the sex, and one study (2%) explicitly stated the use of both sexes. The number of used animals varied from 4 to 26 animals per group.
Table 1.
Study characteristics and hemorrhagic shock protocols
| Species | Studies (number) | Sex (male/ female/ both/ ND) | Group size (number) | Weight range (g) | Hemorrhagic shock protocol | Resuscitation protocol | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pressure/ volume controlled | Target MAP (mm Hg) | Duration HS (min) | Trauma (number) | Resuscitation fluid (number) | Follow‐up (min) | ||||||
| Rat | 37 | 28/ 6/ 0/ 3 | 4‐26 | 150‐390 | 35/ 2 | 25‐65 | 15‐120 | 18 | Blood (4), crystalloid (17), Blood + crystalloid (16) | 30‐24 h | (13, 19, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 45, 49, 50, 51, 52, 53, 55, 58, 61, 62, 63, 64, 65 |
| Dog | 4 | 0/ 1/ 1/ 2 | 5‐15 | 15 500‐36000 | 3/ 1 | 30‐50 | 60‐210 | 2 | Blood (2), colloid (1), Blood + crystalloid (1) | 60‐180 | (46, 47, 48, 59 |
| Hamster | 6 | 6/ 0/ 0/ 0 | 5‐10 | 55‐140 | 3/ 3 | 30‐60 | 45‐60 | 1 | Blood (3), crystalloid (2), colloid (1) | 60‐120 | (29, 44, 54, 56, 57, 60 |
| Pig | 1 | 0/ 0/ 0/ 1 | 10 | 8000‐12 000 | 0/ 1 | 50 | ND | 0 | Crystalloid (1) | ND | (20) |
Study characteristics of included studies.
Abbreviations: HS, hemorrhagic shock; MAP, mean arterial pressure; ND, not determined.
3.3. Experimental protocol
Details regarding the used experimental models are presented in Table 1. Most studies (85%) used a fixed‐pressure model to induce hemorrhage. Target mean arterial pressure varied from 25 to 65 mm Hg. The duration of the shock period ranged from 15 minutes to 3.5 hours. Three studies did not report the duration of the shock period. 19 , 20 , 21 In approximately half the studies, trauma was induced (44%), mainly by a midline laparotomy.
Resuscitation fluid consisted of crystalloids (42%), a combination of crystalloids and blood (35%), blood only (19%), or colloids (4%). Overall, the volume of fluids was based on the volume withdrawn blood to induce hemorrhagic shock and ranged from 25% to 500% of the volume blood withdrawn. Follow‐up time after start of fluid resuscitation ranged from 30 minutes to 24 hours. Two studies did not report their follow‐up time. 20 , 22
3.4. Quality assessment
None of the studies met all SYRCLE criteria, indicating a risk of bias for all studies. The majority of the studies (58%) reported randomization at any point and only 25% reported blinding at any point. Half of the studies reported similar baseline values; the remaining studies showed differences at baseline or did not report baseline values. Individual results of the quality assessment per study (Table S2) and a summary of the total quality assessment (Figure S1) are provided as supplemental data. There was a potential risk of publication bias as the funnel plot of all studies revealed asymmetry (Figure S2).
3.5. Microvascular flow assessment
The non‐fluid‐based therapeutic interventions were grouped based on their general working mechanisms, resulting in the following groups: antioxidants (n = 7), drugs targeting cell metabolism (n = 6), coagulation (n = 4), complement inhibitors (n = 2), hormones (n = 9), direct attenuation of inflammation (n = 6), endothelial barrier modulators (n = 2), vasoactive agents (n = 19), or other agents (n = 6) involved in homeostasis. A total of 62 drugs were tested, of which seven were tested in multiple studies. An overview of all drugs and the dosage and timing of administration is presented in Table S3. The results as organized by working mechanism are presented below. In Table S4‐S7, the data are presented in table format, together with details of each individual experimental protocol.
3.5.1. Antioxidants (n = 7)
Six studies tested the effect of antioxidants on blood flow, 23 , 24 , 25 , 26 , 27 , 28 and 50% reported an increase in blood flow compared to untreated controls (Figure 2, Table S4). 23 , 24 , 25 , 27 In total, seven antioxidants were tested. Blood flow was mainly measured in the intestinal or hepatic microvascular bed. The remaining studies reported no effect of the tested treatment. 26 , 28 Contrasting results were found by two different groups testing the effect of pentoxifylline, 25 , 28 as one group reported a restoration of intestinal blood flow following treatment, 25 while the other group found no effect of pentoxifylline on RBC velocity in the scrotum. 28 Two studies did not report the used group size and could therefore not be shown in the forest plot. 23 , 24
Figure 2.
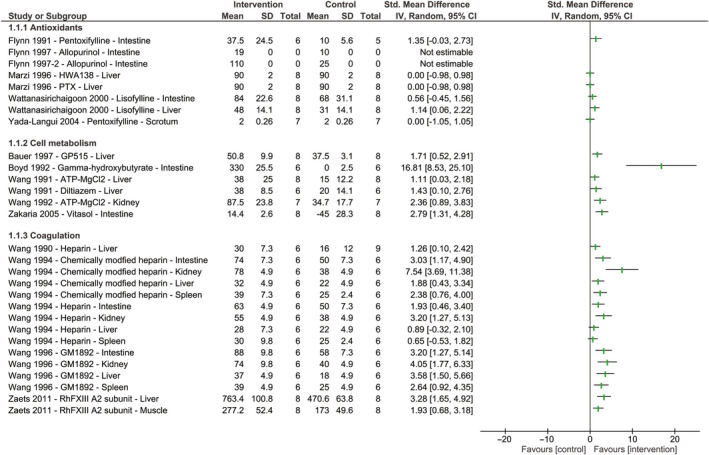
The effect of antioxidants and therapeutic agents targeting cell metabolism and coagulation on blood flow or red blood cell velocity following hemorrhagic shock and fluid resuscitation. Forest plots represent standardized mean differences accompanying 95% confidence intervals. Study names are reported as author, year of publication, name of therapeutic agent, and organ of measurement. Studies that did not report group sizes or standard deviations are shown as “not estimable.” No meta‐analysis was performed due to heterogeneity; therefore, no pooled effect is shown
3.5.2. Cell metabolism (n = 6)
Of the six studies targeting cell metabolism, five (83%) reported a restoration of blood flow following treatment with the drug (Figure 2, Table S4). 21 , 29 , 30 , 31 , 32 Two of these studies tested the same drug, ATP‐MgCl2, on different organs, liver, and kidney, and reported a restoration of blood flow in both organs. 30 , 32 The remaining study reported a nonsignificant increase in hepatic blood flow following treatment with an adenosine kinase inhibitor. 33 Boyd et al 29 reported a complete stasis of blood flow in untreated controls, whereas in gamma‐hydroxybutyrate‐treated animals, intestinal blood flow was well maintained, leading to a large 95% confidence interval.
3.5.3. Coagulation (n = 4)
Three studies tested the effect of heparin and chemically modified heparin on hepatic, intestinal, renal, and splenic blood flow (Figure 2, Table S4). 34 , 35 , 36 Overall, they reported that the chemically modified variant of heparin was primarily effective in restoring blood flow following hemorrhagic shock and fluid resuscitation. The fourth study reported a restoration in blood flow after treatment with recombinant human FXIII, measured in both hepatic and muscular microcirculation. 37
3.5.4. Complement system (n = 2)
Two studies investigated targeting the complement system to restore blood flow and showed promising effects (Figure 3, Table S5). 38 , 39 Administration of recombinant human soluble complement receptor‐1 restored intestinal blood flow, 38 and administration of a C1‐esterase inhibitor restored RBC velocity, but only slightly. 39
Figure 3.
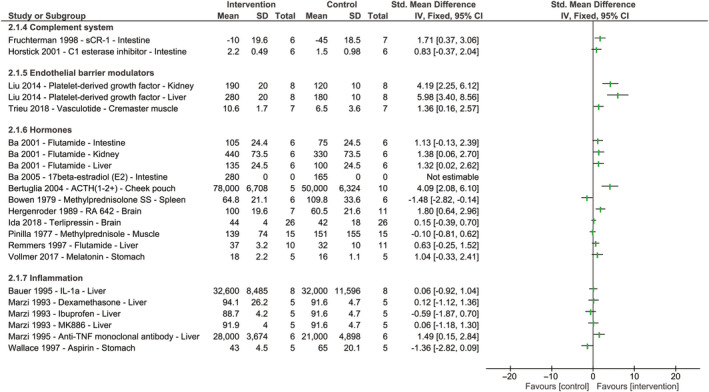
The effect of therapeutics targeting the complement system or systemic inflammation, endothelial barrier modulators, and hormones on blood flow or red blood cell velocity following hemorrhagic shock and fluid resuscitation. Forest plots represent standardized mean differences accompanying 95% confidence intervals. Study names are reported as author, year of publication, name of therapeutic agent, and organ of measurement. Studies that did not report group sizes or standard deviations are shown as “not estimable.” No meta‐analysis was performed due to heterogeneity; therefore, no pooled effect is shown
3.5.5. Endothelial barrier function (n = 2)
Both studies targeting the endothelial barrier with either the angiopoietin‐1 mimetic vasculotide or platelet‐derived growth factor showed a restoration in blood flow and/or perfusion following administration (Figure 3, Table S5). 40 , 41
3.5.6. Hormones (n = 9)
Nine different hormones were reported, of which four (44%) restored blood flow following hemorrhagic shock and fluid resuscitation (Figure 3, Table S5). 42 , 43 , 44 , 45 Three of these effective treatment strategies targeted sex hormones with the use of testosterone receptor blocker flutamide 43 , 45 or by addition of 17B‐estradiol. 42 The remaining studies either reported no effect (44%) 13 , 19 , 46 , 47 or a decrease in blood flow (11%) following treatment with hormones. 48 One study did not report the used group size and could therefore not be shown in the forest plot. 42
3.5.7. Inflammation (n = 6)
Four studies tested the effect of six agents attenuating inflammation (Figure 3, Table S5). None of the therapeutic agents restored blood flow following hemorrhagic shock and fluid resuscitation. 22 , 49 , 50 , 51
3.5.8. Vasoactive agents (n = 19)
A total of 19 vasoactive agents were tested, of which six (32%) restored blood flow following hemorrhagic shock and fluid resuscitation (Figure 4, Table S6). 19 , 42 , 52 , 53 , 54 , 55 Eleven vasoactive agents (58%) did not affect blood flow. 19 , 22 , 56 , 57 , 58 , 59 , 60 The remaining two studies (10%) reported a decrease in blood flow, 61 or results varied markedly between organs. 20 Vasopressin showed contrasting results with a decrease in intestinal blood flow, 61 but no effect on dorsal blood flow. 57 This may be because measurements were performed in different organs and different concentrations were given, and high concentrations of vasopressin are known to have adverse effects on microcirculatory perfusion. One study reported the effect of vasoactive agent metaraminol on blood flow under conscious and unconscious conditions in nine different organs. 20 However, the reported results varied markedly and the experimental protocol is largely unclear which led to a high risk of bias. For this study, only the results of the clinically most relevant model, namely the conscious model, were shown in the forest plot. Two studies (10%) did not report the used group size and could therefore not be shown in the forest plot. 42 , 54
Figure 4.
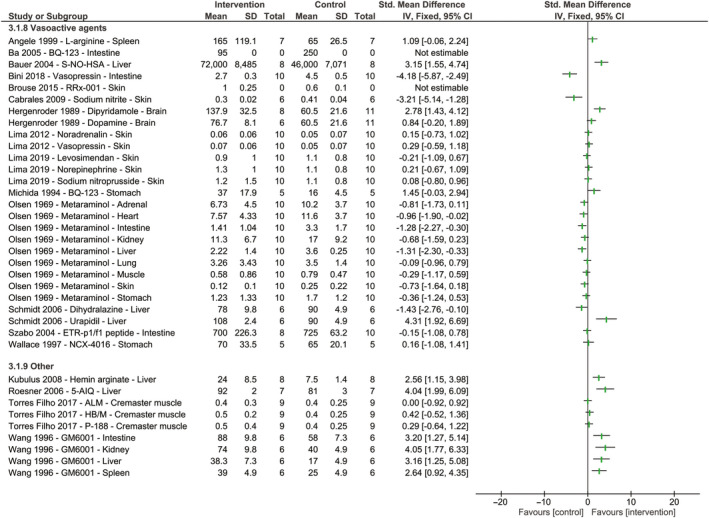
The effect of vasoactive agents or other therapeutics targeting homeostasis on blood flow or red blood cell velocity following hemorrhagic shock and fluid resuscitation. Forest plots represent standardized mean differences accompanying 95% confidence intervals. Study names are reported as author, year of publication, name of therapeutic agent, and organ of measurement. Studies that did not report group sizes or standard deviations are shown as “not estimable.” No meta‐analysis was performed due to heterogeneity; therefore, no pooled effect is shown. For Olsen et al, 20 only results of the most clinically relevant model are shown, namely the conscious model
3.5.9. Others (n = 6)
The remaining studies tested the effect of several other non‐fluid‐based therapeutic interventions involved in homeostasis (Figure 4, Table S7). Three of these studies reported a restoration in blood flow following treatment. 62 , 63 , 64 These effective non‐fluid‐based treatment strategies consisted of hemin arginate, a compound inducing heme oxygenase 1, 62 a PARP inhibitor, 63 and GM6001, a matrix metalloproteinase inhibitor. 64 The remaining three non‐fluid‐based therapeutic agents were not capable of restoring blood flow following hemorrhagic shock and fluid resuscitation. 65 These compounds consisted of a combination of ALM, HB/M and poloxamer‐188 (P‐188). These therapeutics were tested based on previously reported promising results; however, their working mechanism remains unclear.
4. DISCUSSION
This systematic review demonstrates that non‐fluid‐based therapeutic interventions targeting in particular mitochondrial dysfunction, complement activation, and direct modulators of the endothelial barrier were able to restore microcirculatory perfusion following experimental hemorrhagic shock, in addition to fluid resuscitation. Non‐fluid‐based therapeutic treatments consisting of vasoactive agents, steroid hormones, or attenuation of systemic inflammation were less frequently able to restore microcirculatory perfusion. The evidence for these non‐fluid‐based therapeutic interventions comes from 48 preclinical studies and was mainly quantified in the hepatic and intestinal microvascular bed. Future studies should focus on confirming these mechanisms as target and eventually test these drugs in the clinical setting.
4.1. Complement system
The complement system is activated immediately following traumatic injury and continues following fluid resuscitation, 66 and contributes to trauma‐related and ischemic tissue damage. 66 , 67 In this systematic search, two promising inhibitors of complement activation were described. 38 , 39 Inhibition via complement receptor‐1 restored mesenteric blood flow and endothelial cell function, 38 while inhibition of the release of components C3a, C4a, and C5a via a C1‐esterase inhibitor reduced leukocyte adhesion which only slightly restored mesenteric blood flow. 39 A recent review by Karasu et al summarized the current knowledge regarding targeting the complement system in critical illness. They reported that based on preclinical evidence, the complement system appears as an interesting targeting pathway. 68 In the clinical setting, the use of a high‐dose C1‐esterase inhibitor improved survival rates for critically ill patients 69 and although microcirculatory perfusion was not an outcome parameter, this confirms a potential promising use of complement inhibitors.
4.2. Coagulation
Coagulopathy following hemorrhagic shock and fluid resuscitation can lead to endothelial dysfunction. 6 , 70 Non‐fluid‐based therapeutic treatment targeting coagulation via fibrin stabilizing factor FXIII was effective in restoring hepatic microvascular blood flow and reducing pulmonary edema formation. 37 However, targeting coagulopathy in critically ill remains debatable. Despite effectiveness of this treatment strategy, treatment of patients with a high risk of bleeding using anticoagulant therapeutics seems counterintuitive. 71
The majority of experimental studies performed before 2000 were executed in pre‐heparinized animals, leaving the question unresolved whether heparin itself may be beneficial in restoring microcirculatory perfusion following hemorrhagic shock and fluid resuscitation. 34 Although this was confirmed by Wang et al, 34 the anticoagulant properties of heparin exclude its use in the clinical setting of hemorrhage and trauma. In this context, the same group investigated the effect of chemically modified heparin with reduced anticoagulant properties. 35 , 36 Treatment with this compound also restored renal, splenic, and intestinal microvascular blood flow and is proposed to function via inhibition of complement activation. 72 Although both heparin and FXIII were effective in restoring microcirculatory perfusion, the usage of a treatment without anticoagulant properties, such as chemically modified heparin, is preferred when treating patients with an increased risk of bleeding. Chemically modified heparin is proposed to work via complement inhibition. Therefore, the use of complement inhibitors may be favored over heparin treatment strategy to restore microcirculatory perfusion following hemorrhagic shock and fluid resuscitation.
4.3. Mitochondrial function
Mitochondrial dysfunction following hemorrhagic shock and fluid resuscitation is characterized by increased ROS formation 73 and decreased ATP production, 12 , 74 partly due to increased calcium content during ischemia. 75 Several studies focused on improving ATP content, 21 , 30 , 31 , 32 , 33 or reducing calcium levels with the use of a calcium antagonist, 21 and all restored microcirculatory blood flow while using comparable models of moderate hemorrhagic shock. In addition, reducing ROS formation with a xanthine oxidase inhibitor, an antioxidant, restored mesenteric blood flow following hemorrhagic shock and fluid resuscitation. 23 , 24 Pentoxifylline, a methylxanthine derivate, was investigated in two different studies. One study reported an increase in blood flow following treatment, 25 whereas the other study reported no effect on blood flow. 28 Interestingly, the study that reported no effect of pentoxifylline used a more severe model of hemorrhagic shock compared to the study that reported an increase in blood flow, suggesting that the effectiveness of pentoxifylline is affected by the severity of hemorrhagic shock. In patients with septic shock, reducing calcium levels proved to be effective in restoring microcirculatory perfusion, 76 confirming its clinical relevance as possible treatment strategy.
4.4. Endothelial barrier modulators
Improving endothelial barrier function appeared as a promising target to restore blood flow following hemorrhagic shock and fluid resuscitation. 40 , 41 Although no clinical studies have targeted endothelial barrier function yet, markers for endothelial cell activation and injury were upregulated in critically ill patients 77 , 78 and associated with multiple organ failure and unfavorable outcome. 77 Consequently, restoring endothelial barrier function may be beneficial in improving microvascular blood flow following hemorrhagic shock and fluid resuscitation. 79
4.5. Vasoactive agents
During hemorrhagic shock, the production of NO via eNOS 38 is reduced due to diminished vascular endothelial shear stress and hypoxia, 80 leading to vasoconstriction. As described in this review, restoration of NO levels via direct NO supplementation or administration of NO precursors restored blood flow, 52 , 53 , 54 which even lasted up to 24 hours as reported by Bauer et al 53 However, increasing NO levels via the NOS‐independent pathway by administration of nitrite only restored blood flow temporarily, 56 suggesting that NO supplementation via eNOS is favorable. Other non‐fluid‐based agents with vasoactive properties showed conflicting results. Neither vasopressors 20 , 57 , 60 , 61 nor vasodilators 42 , 55 , 59 , 60 were effective in the preclinical setting. One of the studies investigating the effect of vasopressin reported a decrease in blood flow and an increase in capillary leak as a result of the given treatment, 61 while another study reported no effect of vasopressin on blood flow. 57 The use of vasopressin in the emergency setting remains under debate, as it has been associated with increased mortality in hemorrhagic shock patients. 81 Overall, clinical trials targeting microcirculatory perfusion in critically ill patients remain inconclusive with regard to the use of vasoactive agents. 82 Using vasoactive drugs seems mainly effective as hemodynamic support, however, exerts a range of unwarranted effects. 83
4.6. Inflammation
The inflammatory response is characterized by the release of proinflammatory cytokines such as interleukin‐1β and TNF‐α and leukocyte adhesion. 51 , 84 , 85 Interestingly, attenuation of these cytokines did not restore hepatic blood flow following experimental hemorrhagic shock and fluid resuscitation. 49 , 51 In parallel, direct attenuation of inflammation with dexamethasone, ibuprofen, a 5‐lipoxygenase inhibitor, or aspirin did not affect microcirculatory blood flow 22 , 50 in animals. The experimental models used in these studies were relatively homogenous as the majority used a moderate hemorrhagic shock model with a target blood pressure of 40 mm Hg and shock duration of one hour. Collectively, direct attenuation of inflammation does not appear to be effective in restoring microcirculatory perfusion following experimental hemorrhagic shock and fluid resuscitation. Similar results were reported by several clinical trials in patients with sepsis and septic shock, where treatment with anti‐inflammatory agents did not improve outcome of these patients. 86 Accordingly, current trials focus on stimulating the immune response rather than inhibiting specific components of the immune system. 86 However, future studies should elaborate on the efficiency of this approach.
4.7. Hormones
Gender differences have been a subject of interest for the past decade, revealing that females show improved microcirculatory function and reduced inflammatory response compared to males following trauma and hemorrhage. 42 , 87 , 88 All hormonal treatments including either blocking the testosterone receptor 43 , 45 or the addition of estradiol steroid hormone 17B‐estradiol 42 restored blood flow following experimental hemorrhagic shock and fluid resuscitation. However, conflicting results were found when administrating steroid hormones in animals. 44 , 46 , 48 One study even reported a decrease in splenic blood flow following treatment with a steroid hormone. 48 Important to note, however, is the rigorous hemorrhagic shock model used in this study, as the animals were kept in shock for 3.5 hours, which limits the translation of this model to the clinical setting. The use of steroid hormones as treatment strategy to restore blood flow is based on the possible attenuation of systemic inflammation. The lack of the capability to restore blood flow is therefore in line with previously discussed anti‐inflammatory agents. Comparable results were reported by Martino et al, 89 where the authors discussed conflicting results of corticosteroids on survival, tested in major clinical trials with critically ill patients.
Collectively, improving microcirculatory blood flow following hemorrhagic shock and fluid resuscitation by blocking or stimulating sex hormones appears effective. A clinical trial showing a lower in‐hospital mortality rate in female patients confirms the importance of investigating gender differences in severely injured patients. 90 As also shown in the current review, the majority of the therapeutic agents is tested in male animals, limiting translation to the female population due to hormonal differences. Further research is therefore not only necessary to confirm the efficiency of these therapeutics in females, but should also elaborate on the role of sex hormones as treatment strategy.
5. LIMITATIONS
The quality and translational value of the included studies varied markedly. As only 24% of the studies reported blinding of the investigators or outcome assessors at any point, a cautious approach to their interpretation is necessary. Most of the studies were performed in rodents rather than in large animals, and only a few studies reported a power calculation. Moreover, most of the microcirculatory perfusion measurements were performed in either the liver or the intestinal bed. Although more difficult to access, one would prefer to measure the effect of therapeutic agents on pulmonary and renal function in order to increase translatability, 6 as these organs are particularly susceptible to microvascular failure in the earliest phase following hemorrhagic shock. 91 Nonetheless, as the splanchnic circulation is particularly susceptible to hemorrhagic shock and fluid resuscitation, these results are of additional value. Due to the heterogeneity of studies, meta‐analysis was unwarranted, limiting the ability to assess the relative effect of a specific targeting pathway. Therefore, this study was unable to identify the most promising treatment strategy. Nevertheless, this systematic review provides an overview of published literature on non‐fluid‐based therapeutic treatment interventions to restore hemorrhagic shock and fluid resuscitation‐related microcirculatory perfusion deficits in animals. It should, however, be noted that the current review summarizes preclinical evidence. Translation of the current results into the clinical setting, with ultimately improvement of patient outcome, still takes several steps. At this moment, clinical evidence to strengthen the evidence of potential treatment strategies is rare, due to the complex origin of current patient population.
6. PERSPECTIVES
Microcirculatory perfusion disturbances following hemorrhagic shock and fluid resuscitation are associated with multiple organ failure and increased mortality. As standard fluid resuscitation only restores macrohemodynamics, additional treatment with a non‐fluid‐based therapeutic agent is essential to restore microcirculatory perfusion. As reported in the current review, targeting mitochondrial function, complement activation, or endothelial barrier function, the majority of the tested agents contributed to a restoration of microcirculatory perfusion following hemorrhagic shock. We emphasize that these therapeutic agents were given in addition to standard fluid resuscitation and do not replace a transfusion protocol. Future studies should reveal whether these therapeutics are also effective in restoring microcirculatory perfusion in the clinical setting. As gender differences play a role in the microcirculatory and inflammatory response to hemorrhagic shock, further research is necessary to clarify the effect of treatment interventions focusing on sex hormones.
Supporting information
Supinfo
van Leeuwen ALI, Dekker NAM, Jansma EP, Boer C, van den Brom CE. Therapeutic interventions to restore microcirculatory perfusion following experimental hemorrhagic shock and fluid resuscitation: A systematic review. Microcirculation. 2020;27:e12650 10.1111/micc.12650
Funding information
Nederlandse Vereniging voor Anesthesiologie: Research Project Grant 2016; European Society of Anaesthesiology: Young Investigator Grant 2017; European Society of Intensive Care Medicine: Levi‐Montalcini Award 2017; Hartstichting: 2016T064; CSL Behring: Prof Heimburger Award 2019; ZonMw: VENI Grant 2019
REFERENCES
- 1. Hutchings SD, Naumann DN, Hopkins P, et al. Microcirculatory impairment is associated with multiple organ dysfunction following traumatic hemorrhagic shock: the MICROSHOCK study. Crit Care Med. 2018;46:e889‐e896. [DOI] [PubMed] [Google Scholar]
- 2. Cannon JW. Hemorrhagic shock. N Engl J Med. 2018;378:370‐379. [DOI] [PubMed] [Google Scholar]
- 3. Tachon G, Harrois A, Tanaka S, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. 2014;42:1433‐1441. [DOI] [PubMed] [Google Scholar]
- 4. Naumann DN, Hazeldine J, Midwinter MJ, Hutchings SD, Harrison P. Poor microcirculatory flow dynamics are associated with endothelial cell damage and glycocalyx shedding after traumatic hemorrhagic shock. J Trauma Acute Care Surg. 2018;84:81‐88. [DOI] [PubMed] [Google Scholar]
- 5. Kara A, Akin S, Ince C. Monitoring microcirculation in critical illness. Curr Opin Crit Care. 2016;22:444‐452. [DOI] [PubMed] [Google Scholar]
- 6. Torres FI. Hemorrhagic shock and the microvasculature. Compr Physiol. 2017;8:61‐101. [DOI] [PubMed] [Google Scholar]
- 7. Gulati A. Vascular endothelium and hypovolemic shock. Curr Vasc Pharmacol. 2016;14:187‐195. [DOI] [PubMed] [Google Scholar]
- 8. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279‐367. [DOI] [PubMed] [Google Scholar]
- 9. van Leeuwen ALI, Naumann DN, Dekker NAM, et al. In vitro endothelial hyperpermeability occurs early following traumatic hemorrhagic shock. Clin Hemorheol Microcirc. 2020. 10.3233/CH-190642 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naumann DN, Beaven A, Dretzke J, Hutchings S, Midwinter MJ. Searching for the optimal fluid to restore microcirculatory flow dynamics after haemorrhagic shock: a systematic review of preclinical studies. Shock. 2016;46:609‐622. [DOI] [PubMed] [Google Scholar]
- 11. Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. 2015;19(Suppl 3):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warren M, Subramani K, Schwartz R, Raju R. Mitochondrial dysfunction in rat splenocytes following hemorrhagic shock. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2526‐2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ida KK, Chisholm KI, Malbouisson LMS, et al. Protection of cerebral microcirculation, mitochondrial function, and electrocortical activity by small‐volume resuscitation with terlipressin in a rat model of haemorrhagic shock. Br J Anaesth. 2018;120:1245‐1254. [DOI] [PubMed] [Google Scholar]
- 14. Younger JG, Sasaki N, Waite MD, et al. Detrimental effects of complement activation in hemorrhagic shock. J Appl Physiol. 1985;2001(90):441‐446. [DOI] [PubMed] [Google Scholar]
- 15. Liu H, Xiao X, Sun C, Sun D, Li Y, Yang M. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Front Biosci (Landmark Ed). 2015;20:927‐933. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Brom CE, Tian S, Borgdorff M.A review of new therapeutic targets for improvement of microvascular leakage and microcirculatory perfusion following hemorrhagic shock PROSPERO 2018 CRD42018095432. 2018.
- 18. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes‐Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hergenroder S, Reichl R. Pharmacological effects of RA 642 on cerebrocortical perfusion in acute hemorrhagic shock in rats. Prog Clin Biol Res. 1989;308:1091‐1095. [PubMed] [Google Scholar]
- 20. Olsen WR. Capillary flow in hemorrhagic shock. 3. Metaraminol and capillary flow in the nonanesthetized and anesthetized pig. Arch Surg. 1969;99:637‐640. [DOI] [PubMed] [Google Scholar]
- 21. Wang P, Ba ZF, Dean RE, Chaudry IH. Diltiazem administration after crystalloid resuscitation restores active hepatocellular function and hepatic blood flow after severe hemorrhagic shock. Surgery. 1991;110:390‐396; discussion 396–397. [PubMed] [Google Scholar]
- 22. Wallace JL, McKnight W, Wilson TL, Del Soldato P, Cirino G. Reduction of shock‐induced gastric damage by a nitric oxide‐releasing aspirin derivative: role of neutrophils. Am J Physiol. 1997;273:G1246‐G1251. [DOI] [PubMed] [Google Scholar]
- 23. Flynn WJ Jr, Pilati D, Hoover EL. Xanthine oxidase inhibition after resuscitated hemorrhagic shock restores mesenteric blood flow without vasodilation. Shock (Augusta, Ga.). 1997;8:300‐304. [DOI] [PubMed] [Google Scholar]
- 24. Flynn WJ Jr, Pilati D, Hoover EL. Xanthine oxidase inhibition prevents mesenteric blood flow deficits after resuscitated hemorrhagic shock by preserving endothelial function. J Surg Res. 1997;68:175‐180. [DOI] [PubMed] [Google Scholar]
- 25. Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline restores intestinal microvascular blood flow during resuscitated hemorrhagic shock. Surgery. 1991;110:350‐356. [PubMed] [Google Scholar]
- 26. Marzi I, Maier M, Herzog C, Bauer M. Influence of pentoxifylline and albifylline on liver microcirculation and leukocyte adhesion after hemorrhagic shock in the rat. J Trauma. 1996;40:90‐96. [DOI] [PubMed] [Google Scholar]
- 27. Wattanasirichaigoon S, Menconi MJ, Fink MP. Lisofylline ameliorates intestinal and hepatic injury induced by hemorrhage and resuscitation in rats. Crit Care Med. 2000;28:1540‐1549. [DOI] [PubMed] [Google Scholar]
- 28. Yada‐Langui MM, Anjos‐Valotta EA, Sannomiya P, Silva MRE, Coimbra R. Resuscitation affects microcirculatory polymorphonuclear leukocyte behavior after hemorrhagic shock: role of hypertonic saline and pentoxifylline. Exp Biol Med (Maywood). 2004;229:684‐693. [DOI] [PubMed] [Google Scholar]
- 29. Boyd AJ, Sherman IA, Saibil FG. The cardiovascular effects of gamma‐hydroxybutyrate following hemorrhage. Circ Shock. 1992;38:115‐121. [PubMed] [Google Scholar]
- 30. Wang P, Zhou M, Rana MW, et al. ATP‐MgCl2 restores renal microcirculation following trauma and severe hemorrhage. Can J Physiol Pharmacol. 1992;70:349‐357. [DOI] [PubMed] [Google Scholar]
- 31. el Zakaria R, Ehringer WD, Tsakadze N, Li N, Garrison RN. Direct energy delivery improves tissue perfusion after resuscitated shock. Surgery. 2005;138:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang P, Ba ZF, Dean RE, Chaudry IH. ATP‐MgCl2 restores the depressed hepatocellular function and hepatic blood flow following hemorrhage and resuscitation. J Surg Res. 1991;50:368‐374. [DOI] [PubMed] [Google Scholar]
- 33. Bauer C, Bouma MG, Herrmann I, et al. Adenosine kinase inhibitor GP515 attenuates hepatic leukocyte adhesion after hemorrhagic hypotension. Am J Physiol. 1997;273:G1297‐1303. [DOI] [PubMed] [Google Scholar]
- 34. Wang P, Singh G, Rana MW, Ba ZF, Chaudry IH. Preheparinization improves organ function after hemorrhage and resuscitation. Am J Physiol. 1990;259:R645‐R650. [DOI] [PubMed] [Google Scholar]
- 35. Wang P, Ba ZF, Chaudry IH. Chemically modified heparin improves hepatocellular function, cardiac output, and microcirculation after trauma‐hemorrhage and resuscitation. Surgery. 1994;116:169‐175; discussion 175–166. [DOI] [PubMed] [Google Scholar]
- 36. Wang P, Ba ZF, Reich SS, Zhou M, Holme KR, Chaudry IH. Effects of nonanticoagulant heparin on cardiovascular and hepatocellular function after hemorrhagic shock. Am J Physiol. 1996;270:H1294‐H1302. [DOI] [PubMed] [Google Scholar]
- 37. Zaets SB, Xu DZ, Lu Q, et al. Recombinant factor XIII mitigates hemorrhagic shock‐induced organ dysfunction. J Surg Res. 2011;166:e135‐e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782‐791; discussion 791–782. [DOI] [PubMed] [Google Scholar]
- 39. Horstick G, Kempf T, Lauterbach M, et al. C1‐esterase‐inhibitor treatment at early reperfusion of hemorrhagic shock reduces mesentry leukocyte adhesion and rolling. Microcirculation. 2001;8:427‐433. [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Zhang J, Zhu Y, et al. Beneficial effects of platelet‐derived growth factor on hemorrhagic shock in rats and the underlying mechanisms. Am J Physiol Heart Circ Physiol. 2014;307:H1277‐H1287. [DOI] [PubMed] [Google Scholar]
- 41. Trieu M, van Meurs M, van Leeuwen ALI, et al. Vasculotide, an angiopoietin‐1 mimetic, restores microcirculatory perfusion and microvascular leakage and decreases fluid resuscitation requirements in hemorrhagic shock. Anesthesiology. 2018;128:361‐374. [DOI] [PubMed] [Google Scholar]
- 42. Ba ZF, Shimizu T, Szalay L, Bland KI, Chaudry IH. Gender differences in small intestinal perfusion following trauma hemorrhage: the role of endothelin‐1. Am J Physiol Gastrointest Liver Physiol. 2005;288:G860‐G865. [DOI] [PubMed] [Google Scholar]
- 43. Ba ZF, Wang P, Koo DJ, Ornan DA, Bland KI, Chaudry IH. Attenuation of vascular endothelial dysfunction by testosterone receptor blockade after trauma and hemorrhagic shock. Arch Surg. 2001;136:1158‐1163. [DOI] [PubMed] [Google Scholar]
- 44. Bertuglia S, Giusti A. Influence of ACTH‐(1–24) and plasma hyperviscosity on free radical production and capillary perfusion after hemorrhagic shock. Microcirculation. 2004;11:227‐238. [DOI] [PubMed] [Google Scholar]
- 45. Remmers DE, Wang P, Cioffi WG, Bland KI, Chaudry IH. Testosterone receptor blockade after trauma‐hemorrhage improves cardiac and hepatic functions in males. Am J Physiol. 1997;273:H2919‐H2925. [DOI] [PubMed] [Google Scholar]
- 46. Pinilla J, Wright CJ. Steroids and severe hemorrhagic shock. Surgery. 1977;82:489‐494. [PubMed] [Google Scholar]
- 47. Vollmer C, Weber APM, Wallenfang M, et al. Melatonin pretreatment improves gastric mucosal blood flow and maintains intestinal barrier function during hemorrhagic shock in dogs. Microcirculation. 2017;24. [DOI] [PubMed] [Google Scholar]
- 48. Bowen JC. Persistent gastric mucosal hypoxia and interstitial edema after hemorrhagic shock: prevention with steroid therapy. Surgery. 1979;85:268‐274. [PubMed] [Google Scholar]
- 49. Bauer C, Marzi I, Bauer M, Fellger H, Larsen R. Interleukin‐1 receptor antagonist attenuates leukocyte‐endothelial interactions in the liver after hemorrhagic shock in the rat. Crit Care Med. 1995;23:1099‐1105. [DOI] [PubMed] [Google Scholar]
- 50. Marzi I, Bauer C, Hower R, Buhren V. Leukocyte‐endothelial cell interactions in the liver after hemorrhagic shock in the rat. Circ Shock. 1993;40:105‐114. [PubMed] [Google Scholar]
- 51. Marzi I, Bauer M, Secchi A, Bahrami S, Redi H, Schlag G. Effect of anti‐tumor necrosis factor alpha on leukocyte adhesion in the liver after hemorrhagic shock: an intravital microscopic study in the rat. Shock. 1995;3:27‐33. [PubMed] [Google Scholar]
- 52. Angele MK, Smail N, Knöferl MW, Ayala A, Cioffi WG, Chaudry IH. L‐arginine restores splenocyte functions after trauma and hemorrhage potentially by improving splenic blood flow. Am J Physiol Cell Physiol. 1999;276:C145‐C151. [DOI] [PubMed] [Google Scholar]
- 53. Bauer C, Kuntz W, Ohnsmann F, et al. The attenuation of hepatic microcirculatory alterations by exogenous substitution of nitric oxide by s‐nitroso‐human albumin after hemorrhagic shock in the rat. Shock. 2004;21:165‐169. [DOI] [PubMed] [Google Scholar]
- 54. Brouse C, Ortiz D, Su Y, Oronsky B, Scicinski J, Cabrales P. Impact of hemoglobin nitrite to nitric oxide reductase on blood transfusion for resuscitation from hemorrhagic shock. Asian J Transfus Sci. 2015;9:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Michida T, Kawano S, Masuda E, et al. Role of endothelin 1 in hemorrhagic shock‐induced gastric mucosal injury in rats. Gastroenterology. 1994;106:988‐993. [DOI] [PubMed] [Google Scholar]
- 56. Cabrales P. Low dose nitrite enhances perfusion after fluid resuscitation from hemorrhagic shock. Resuscitation. 2009;80:1431‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lima R, Villela NR, Bouskela E. Microcirculatory effects of selective receptor blockade during hemorrhagic shock treatment with vasopressin: experimental study in the hamster dorsal chamber. Shock. 2012;38:493‐498. [DOI] [PubMed] [Google Scholar]
- 58. Schmidt R, Baechle T, Hoetzel A, et al. Dihydralazine treatment limits liver injury after hemorrhagic shock in rats. Crit Care Med. 2006;34:815‐822. [DOI] [PubMed] [Google Scholar]
- 59. Szabo A, Suki B, Csonka E, et al. Flow motion in the intestinal villi during hemorrhagic shock: a new method to characterize the microcirculatory changes. Shock. 2004;21:320‐328. [DOI] [PubMed] [Google Scholar]
- 60. Lima R, Villela N, Castiglione R, de Souza M, Bouskela E. Dissociation between macro‐ and microvascular parameters in the early phase of hemorrhagic shock. Microvasc Res. 2019;126:103909. [DOI] [PubMed] [Google Scholar]
- 61. Bini R, Chiara O, Cimbanassi S, Olivero G, Trombetta A, Cotogni P. Evaluation of capillary leakage after vasopressin resuscitation in a hemorrhagic shock model. World J Emerg Surg. 2018;13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kubulus D, Mathes A, Pradarutti S, et al. Hemin arginate‐induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti‐inflammatory cytokine response after hemorrhagic shock. Shock. 2008;29:583‐590. [DOI] [PubMed] [Google Scholar]
- 63. Roesner JP, Vagts DA, Iber T, Eipel C, Vollmar B, Noldge‐Schomburg GF. Protective effects of PARP inhibition on liver microcirculation and function after haemorrhagic shock and resuscitation in male rats. Intensive Care Med. 2006;32:1649‐1657. [DOI] [PubMed] [Google Scholar]
- 64. Wang P, Ba ZF, Galardy RE, Chaudry IH. Administration of a matrix metalloproteinase inhibitor after hemorrhage improves cardiovascular and hepatocellular function. Shock. 1996;6:377‐382. [DOI] [PubMed] [Google Scholar]
- 65. Torres Filho IP, Torres LN, Salgado C, Dubick MA. novel adjunct drugs reverse endothelial glycocalyx damage after hemorrhagic shock in rats. Shock. 2017;48:583‐589. [DOI] [PubMed] [Google Scholar]
- 66. Hecke F, Schmidt U, Kola A, Bautsch W, Klos A, Kohl J. Circulating complement proteins in multiple trauma patients–correlation with injury severity, development of sepsis, and outcome. Crit Care Med. 1997;25:2015‐2024. [DOI] [PubMed] [Google Scholar]
- 67. Mollnes TE, Fosse E. The complement system in trauma‐related and ischemic tissue damage: a brief review. Shock. 1994;2:301‐310. [DOI] [PubMed] [Google Scholar]
- 68. Karasu E, Nilsson B, Kohl J, Lambris JD, Huber‐Lang M. Targeting complement pathways in polytrauma‐ and sepsis‐induced multiple‐organ dysfunction. Front Immunol. 2019;10:543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Igonin AA, Protsenko DN, Galstyan GM, et al. C1‐esterase inhibitor infusion increases survival rates for patients with sepsis*. Crit Care Med. 2012;40:770‐777. [DOI] [PubMed] [Google Scholar]
- 70. Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73:60‐66. [DOI] [PubMed] [Google Scholar]
- 71. Moore HB, Winfield RD, Aibiki M, Neal MD. Is coagulopathy an appropriate therapeutic target during critical illness such as trauma or sepsis? Shock. 2017;48:159‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weiler JM, Edens RE, Linhardt RJ, Kapelanski DP. Heparin and modified heparin inhibit complement activation in vivo. J Immunol. 1992;148:3210‐3215. [PubMed] [Google Scholar]
- 73. Sims CA, Yuxia G, Singh K, Werlin EC, Reilly PM, Baur JA. Supplemental arginine vasopressin during the resuscitation of severe hemorrhagic shock preserves renal mitochondrial function. PLoS One. 2017;12:e0186339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Paxian M, Bauer I, Rensing H, et al. Recovery of hepatocellular ATP and "pericentral apoptosis" after hemorrhage and resuscitation. FASEB J. 2003;17:993‐1002. [DOI] [PubMed] [Google Scholar]
- 75. Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love‐hate triangle. Am J Physiol Cell Physiol. 2004;287:C817‐833. [DOI] [PubMed] [Google Scholar]
- 76. Morelli A, Donati A, Ertmer C, et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: a randomized controlled study. Crit Care. 2010;14:R232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ganter MT, Cohen MJ, Brohi K, et al. Angiopoietin‐2, marker and mediator of endothelial activation with prognostic significance early after trauma? Ann Surg. 2008;247:320‐326. [DOI] [PubMed] [Google Scholar]
- 78. Jongman RM, van Klarenbosch J, Molema G, Zijlstra JG, de Vries AJ, van Meurs M. Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS One. 2015;10:e0136205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Walley KR. Mitigating microvascular leak during fluid resuscitation of hemorrhagic shock. Anesthesiology. 2018;128:252‐253. [DOI] [PubMed] [Google Scholar]
- 80. Hiratsuka M, Katayama T, Uematsu K, Kiyomura M, Ito M. In vivo visualization of nitric oxide and interactions among platelets, leukocytes, and endothelium following hemorrhagic shock and reperfusion. Inflamm Res. 2009;58:463‐471. [DOI] [PubMed] [Google Scholar]
- 81. Collier B, Dossett L, Mann M, et al. Vasopressin use is associated with death in acute trauma patients with shock. J Crit Care. 2010;25(1):173.e9‐173.e14. [DOI] [PubMed] [Google Scholar]
- 82. Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med. 2010;36:2004‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hollenberg SM. Vasoactive drugs in circulatory shock. Am J Respir Crit Care Med. 2011;183:847‐855. [DOI] [PubMed] [Google Scholar]
- 84. Rajnik M, Salkowski CA, Thomas KE, Li YY, Rollwagen FM, Vogel SN. Induction of early inflammatory gene expression in a murine model of nonresuscitated, fixed‐volume hemorrhage. Shock. 2002;17:322‐328. [DOI] [PubMed] [Google Scholar]
- 85. Barroso‐Aranda J, Zweifach BW, Mathison JC, Schmid‐Schonbein GW. Neutrophil activation, tumor necrosis factor, and survival after endotoxic and hemorrhagic shock. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S23‐S29. [DOI] [PubMed] [Google Scholar]
- 86. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407‐420. [DOI] [PubMed] [Google Scholar]
- 87. Angele MK, Frantz MC, Chaudry IH. Gender and sex hormones influence the response to trauma and sepsis: potential therapeutic approaches. Clinics (Sao Paulo). 2006;61:479‐488. [DOI] [PubMed] [Google Scholar]
- 88. Treiber FA, Kapuku GK, Davis H, Pollock JS, Pollock DM. Plasma endothelin‐1 release during acute stress: role of ethnicity and sex. Psychosom Med. 2002;64:707‐713. [DOI] [PubMed] [Google Scholar]
- 89. Martino EA, Baiardo Redaelli M, Sardo S, et al. Steroids and survival in critically ill adult patients: a meta‐analysis of 135 randomized trials. J Cardiothorac Vasc Anesth. 2018;32:2252‐2260. [DOI] [PubMed] [Google Scholar]
- 90. Pape M, Giannakopoulos GF, Zuidema WP, et al. Is there an association between female gender and outcome in severe trauma? A multi‐center analysis in the Netherlands. Scand J Trauma Resusc Emerg Med. 2019;27:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Regel G, Grotz M, Weltner T, Sturm JA, Tscherne H. Pattern of organ failure following severe trauma. World J Surg. 1996;20:422‐429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


