Abstract
Background
Intestinal resident macrophages play a crucial role in homeostasis and have been implicated in numerous gastrointestinal diseases. While historically believed to be largely of hematopoietic origin, recent advances in fate‐mapping technology have unveiled the existence of long‐lived, self‐maintaining populations located in specific niches throughout the gut wall. Furthermore, the advent of single‐cell technology has enabled an unprecedented characterization of the functional specialization of tissue‐resident macrophages throughout the gastrointestinal tract.
Purpose
The purpose of this review was to provide a panorama on intestinal resident macrophages, with particular focus to the recent advances in the field. Here, we discuss the functions and phenotype of intestinal resident macrophages and, where possible, the functional specialization of these cells in response to the niche they occupy. Furthermore, we will discuss their role in gastrointestinal diseases.
Keywords: enteric nervous system, gastrointestinal tract, lamina propria, muscularis externa, neuroimmune interaction, resident macrophages
Key Points.
Resident intestinal macrophages carry out essential house‐keeping functions and are essential for intestinal homeostasis.
Recent advances in single‐cell transcriptomics have unveiled a hitherto unknown heterogeneity within the macrophage pool, suggesting a niche‐specific functional specialization of tissue resident macrophages.
Functional specialization of macrophages is crucial for intestinal homeostasis and the maintenance of other cell types, including neurons and blood vessels. Dysfunction of intestinal macrophages perturbs the niche in which they reside and may underlie pathologies of the gastrointestinal tract.
1. INTRODUCTION
Tissue‐resident macrophages are highly specialized phagocytes that actively contribute to tissue homeostasis. This task relies on their ability to sense and respond to challenges including metabolic changes, tissue damage, and microbial insults, while performing tissue‐specific functions to support surrounding cells and structures. 1 Depending on the tissue in which they reside, macrophages may have to fulfill completely different tasks. For example, lung alveolar macrophages are specialized in the removal and recycling of surfactant molecules produced by alveolar epithelial cells, while in the intestinal lamina propria, macrophages contribute to the local tolerogenic milieu. 2 , 3 In the brain, resident macrophages, that is microglia, assist in synaptic pruning and provide neurotrophic factors such as brain‐derived neurotrophic factors. 4 Thus, tissue‐resident macrophages, while generally maintaining “housekeeping” functions such as phagocytosis across tissues, are further characterized and specialized based on the specific requirements of their environment. Furthermore, the advent of single‐cell RNA sequencing technology has enabled a deeper understanding of the functional differentiation of macrophage subtypes within tissues and the importance of niche‐specific imprinting of these cells.
Traditionally, macrophages have been described as phenotypically either M1, classically activated or pro‐inflammatory macrophages, or M2, alternatively activated or anti‐inflammatory macrophages. The definition of M1 and M2 macrophage polarity derives from the pregenomic era, when expression of a specific set of surface markers, in addition to the production of cytokines in response to stimuli, was considered to establish and define two subtypes of macrophages in vitro. While this initial subdivision proved to be extremely useful to begin to unravel macrophage polarization, considerable advances in transcriptomic and proteomic analysis have revealed substantial heterogeneity not only between macrophages located in different tissues, but also within the tissue itself, suggesting a crucial role of niche‐specific signals in establishing tissue‐resident macrophage subsets and their phenotype. Indeed, single‐cell RNA sequencing technology enables the unbiased exploration of differing cell types and has led to the discovery of novel subpopulations implicated in physiological and pathophysiological mechanisms. These findings include the identification of subpopulations whose functions extend well the generation of a pro‐ or anti‐inflammatory setting in response to stimuli, such as a population that regulates arterial stiffness and collagen deposition in the aorta, or a subpopulation that regulates adipocyte size and lipid homeostasis in adipose tissue, to name a few. 5 , 6 As such, the original M1 and M2 nomenclature, while useful, appears simplistic in the age of single‐cell technology and will thus not be employed in this review. Instead, attention will be given to the unique characteristics of the different niches populated by intestinal resident macrophages and the consequent specialization of these cells.
2. ANATOMICAL DISTRIBUTION OF INTESTINAL RESIDENT MACROPHAGES
Intestinal resident macrophages can be found throughout the different layers of the gastrointestinal tract, as illustrated in Figure 1. A distinct population of macrophages is located in close proximity to the intestinal epithelium and crypt base, which consists of specialized epithelial cells including stem cells and Paneth cells. 7 However, the largest population of macrophages within the intestine can be found randomly displaced within the villi. Additionally, a distinct macrophage population is positioned within the submucosal plexus, which together with the myenteric plexus forms an integrated circuitry that efficiently modulates intestinal motility, secretion across the mucosal surface and blood flow. 8 , 9 Deeper within the tissue, macrophages in the muscularis externa are located distant from the intestinal lumen and are found in a dense network closely located to the myenteric plexus, part of the enteric nervous system (ENS). 9 , 10 In addition, macrophages are distributed adjacent to interstitial cells of Cajal (ICC), the pacemaker cells of the gut, and fibroblast‐like cells within the muscularis externa. 11 Macrophages are present in lower numbers within the circular and longitudinal muscle layers of the muscularis externa, and within the serosal layer that separates the intestine from the peritoneum. Finally, macrophages can be detected within intestinal lymphoid tissue, including Peyers’ Patches (PP) and within mesenteric lymph nodes. 12 , 13
Figure 1.
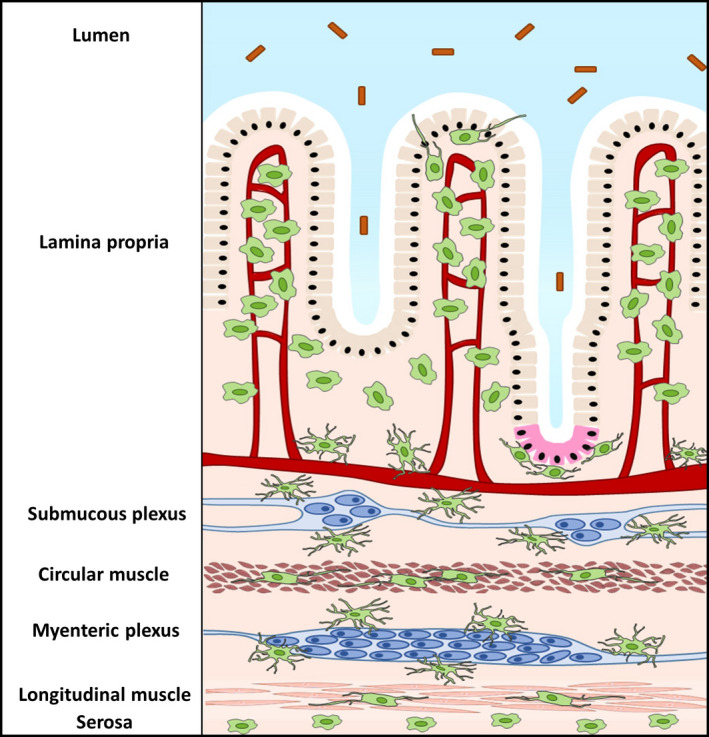
Anatomical distribution of intestinal resident macrophages. Intestinal resident macrophages are located within different layers of the gastrointestinal tract. Macrophages (green) are mostly found randomly displaced within the villi of the lamina propria and can, however, also be found associated to the epithelium and Paneth cells (pink). Deeper within the tissue, macrophages in the submucosa lie closely associated to submucosal neurons and blood vessels. Within the muscularis externa, stellate macrophages can be found in the myenteric plexus, and bipolar macrophages are interspaced between fibers of the circular and the longitudinal muscle. Finally, macrophages are also present in the serosa
3. ORIGIN OF INTESTINAL RESIDENT MACROPHAGES
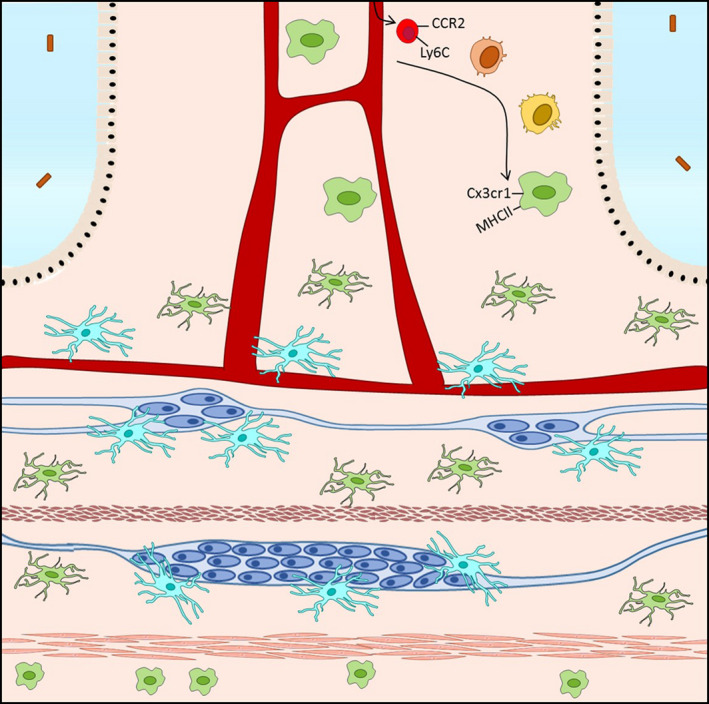
The recent years have seen a major overhaul of our understanding of macrophage ontogeny, mainly due to the continuing development of evermore elegant and sophisticated fate‐mapping models. It was originally proposed that macrophages belong to the mononuclear phagocyte system (MPS), in which they represent the terminal stage of differentiation of bone marrow (BM)‐derived and circulating monocytes. 14 The adult tissue‐resident macrophage pool was thus seen as being constantly replenished by blood monocytes that undergo differentiation upon reaching the final destination tissue through priming by local cues. The first deviation from this paradigm was the tissue‐resident macrophage population of the brain, microglia, which was shown to be seeded prenatally by precursors originating in the embryonic yolk sac, after which these cells are able to self‐maintain by local proliferation throughout life. 15 Over the recent years, it has become clear that most tissues are populated by macrophages derived from the yolk sac or embryonic liver, of which depending of the environment varying proportions will become self‐maintaining and long‐lived or will be replaced by incoming monocytes. Using elegant fate‐mapping models, it has become clear that self‐maintaining long‐lived macrophages and incoming cells coexist and make up the pool of resident macrophages within the heart, 16 , 17 , 18 dermis, 19 liver, 20 lung, 21 and peritoneal cavity 22 , 23 ; and also, the intestine is seeded with embryonic precursors, which in the lamina propria are rapidly replaced by incoming cells of hematopoietic origin and do not persist within the adult intestine. 24 Recently however, we and others were able to identify a long‐lived self‐maintaining population within the intestine, therefore overcoming the notion that the pool of intestinal resident macrophages is solely dependent on replenishment and differentiation of incoming monocytes. 25 , 26 Interestingly, these long‐lived macrophages are located deeper within the gut wall, in close association with blood vessels and enteric neurons of the submucous plexus, and in close proximity to enteric neurons of the myenteric plexus within the muscularis externa (Figure 2). The concept of long‐lived and rapidly replaced incoming macrophages coexisting within the intestinal resident macrophage pool has also been confirmed in humans. Indeed, it was recently shown that the adult small intestinal resident macrophage pool contains 2 subsets that are rapidly replaced by incoming monocytes, and two further distinct subsets that are long‐lived. Strikingly, long‐lived macrophages were found predominantly within the villi and within the submucosa. 27
Figure 2.
Ontogeny of intestinal resident macrophages. Macrophages (green) in the gut consist in a balance of shortlived cells that derive from incoming Ly6Chigh monocytes (red) that differentiate into Cx3cr1+ MHCII+ macrophages (green), and long‐lived self‐maintaining macrophages (light blue) of embryonic origin
The main source of infiltrating cells that give rise to tissue‐resident intestinal macrophages is thought to be circulating Ly6Chigh CCR2+ “classical” monocytes deriving from hematopoietic stem cells in the bone marrow (BM). Both monocyte egression from the BM and infiltration of the lamina propria are heavily dependent on CCL2‐CCR2 signaling. Indeed, Ly6Chigh monocytes are almost completely absent from circulation in CCR2−/− mice, as are CD64+ macrophages in the lamina propria 28 , 29 ; however, in competitive WT:CCR2‐/‐ chimera, wild‐type CD64+ macrophages were able to populate the lamina propria. 30 Conversely, Ly6Chigh monocytes were unable to enter the lamina propria in mice lacking CCL2 expression (Mcp1−/−). 31 Incoming Ly6Chigh monocytes differentiate into mature gut macrophages within the lamina propria, passing through a series of well‐defined intermediates that progressively lose Ly6C expression and begin to express high levels of MHCII and CX3CR1, in a process known as the “monocyte waterfall”. 24 , 30 On one end of the waterfall are Ly6Chigh CX3CR1int MHCII‐ monocytes that appear phenotypically similar to blood monocytes and are characterized by high levels of chemotaxis and extravasation genes such as CCR2, CD62L, VLA‐1, CXCR8, and LFA‐1. 32 As differentiation progresses, these cells first upregulate MHCII expression, before downregulating Ly6C and other extravasation markers. Finally, cells acquire CX3CR1 expression and lose CCR2 expression to give rise to fully matured tissue‐resident macrophages, characterized by expression of F4/80, CD64, CD163, and CD206. Monocyte‐macrophage differentiation is controlled by colony‐stimulating factor (CSF1), and mice lacking this factor (Csf1op/op) or the receptor, CSF1R, are profoundly macrophage deficient. 33 , 34 CSF1 signaling is also required for the maintenance of macrophage populations, as treatment of adult mice with a blocking anti‐CSF1R antibody produces an almost complete depletion of tissue‐resident macrophages, including intestinal macrophages. 35 Differentiation from blood monocyte to intestinal tissue‐resident macrophage requires 5‐6 days and involves major gene expression changes, and produces a cell with increased phagocytic capacity and constitutive IL‐10 production. 28 , 30 , 32 The majority of incoming cells that differentiate to tissue‐resident macrophages within the intestine are thus characterized by a half‐life of around 3‐5 weeks. Similarly as to what is described in mice, a monocyte‐to‐macrophage waterfall has also been uncovered in the human intestinal mucosa, characterized by a progressive maturation from classical CD14high CCR2+ CD11chigh monocytes to CD14lo CCR2‐ CD11chigh mature macrophages. 27 , 28 , 36 Interestingly, while immature macrophage subsets were rapidly replaced by incoming monocytes, mature macrophage subsets persisted and were replaced by incoming cells at a significantly slower rate. 27 The limited data available for the human intestine seem to therefore suggest a balance between rapidly replaced macrophages of hematopoietic origin and long‐lived subsets, analogous as to what has been shown in murine models. However, it should also be noted that in contrast to mature murine macrophages, human macrophages express markers CX3CR1 and CD11c at lower levels. 27
Of note, studies on the origin of intestinal macrophages have largely focused on the lamina propria macrophages, with very little attention to their muscularis externa counterparts, and also in the muscularis externa, macrophages are seeded embryonically; however, replacement by monocyte‐derived cells appears to occur at a much lower rate than in the lamina propria. 37 Indeed, by employing an inducible fate‐mapping model that labeled all Cx3cr1+ macrophages at 6 weeks of age, we were able to show that while in the lamina propria only 8% of macrophages retained labeling after 35 weeks, in the muscularis externa 28% of macrophages retained labeling. 25 This finding is in line with previous findings showing that in steady‐state conditions, monocytes can be detected in the muscularis externa at only very low numbers. 38 Of note, monocyte‐derived immune cell infiltrates populate the tissue in animal models of inflammation, as will be discussed further on in this review. 38 , 39
4. FUNCTIONAL SPECIALIZATION OF MACROPHAGES WITHIN THE GASTROINTESTINAL TRACT
Macrophages populate the length of the intestine in varying numbers, reaching the highest density in the colon in both humans and rodents. 40 , 41 As in other tissues, intestinal resident macrophages carry out a series of homeostatic functions including the removal of debris or senescent cells and tissue remodeling. 41 , 42 , 43 In addition to housekeeping functions, however, macrophages in the intestine display a functional heterogeneity that can be partially explained by the specific demands of the different compartments in which these cells reside. Macrophages in the lamina propria, close to the epithelial layer, are strategically positioned at the first line of defence against pathogens that occasionally breach the epithelial layer, acting thus primarily as innate immune effector cells. On the other hand, muscularis macrophages are located in close proximity to the ENS to ensure appropriate tissue‐protective reactions to pathogens and inflammatory stimuli, to regulate peristalsis and to protect, support, and nourish enteric neurons. 9 , 25
4.1. Lamina propria macrophages
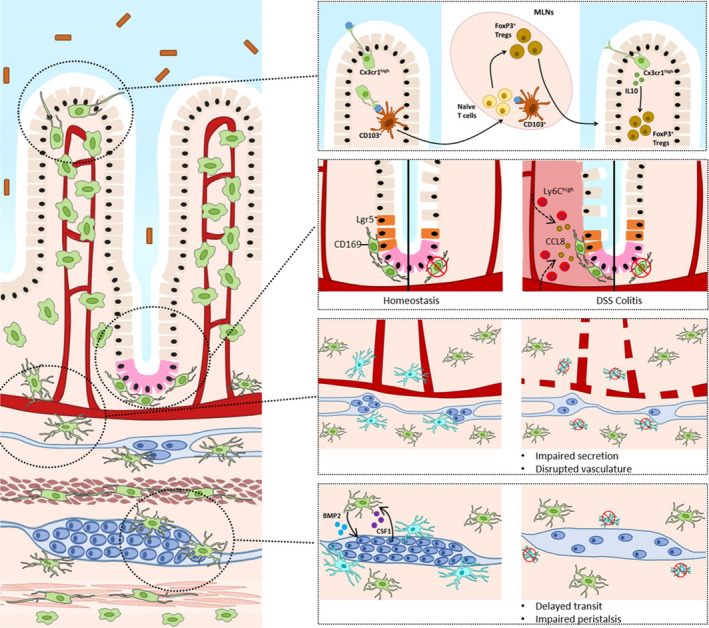
Along the entire length of the GI tract, macrophages are enriched in the lamina propria close to the epithelial layer. 44 These macrophages are first responders to material that breaches the epithelial barrier and are thus highly phagocytic and bactericidal toward pathogens, expressing a series of specialized receptors such as Toll‐like receptors 3‐9, CD36, NOD‐like receptors, and TREM2. 28 , 45 , 46 , 47 , 48 However, despite their highly phagocytic nature, lamina propria macrophages are unique in that their response to commensal microbiota or ingestion of food antigens does not lead to increased pro‐inflammatory responses. 28 , 48 , 49 Indeed, lamina propria macrophages show downregulation of adaptor molecules such as MyD88, CD14, NOD2, and TRAF6 that prevent TLR downstream signaling and translocation of NF‐κB. 47 , 48 , 50 , 51 In addition, these cells constitutively express the anti‐inflammatory cytokine IL‐10 and its receptor IL‐10R. Interestingly, the IL10‐IL10R axis has been implicated in macrophage responsiveness in both humans and in rodents; colonic macrophages in which this axis has been disrupted express higher levels of pro‐inflammatory mediators and display increased responsiveness to TLR stimulation, leading to spontaneous intestinal inflammation. 50 , 52 , 53 , 54 In addition to clearing pathogens that breach the epithelial barrier, CX3CR1high macrophages can extend dendritic projections to sample luminal antigens in a CX3CL1‐dependent manner, without perturbing epithelial integrity (Figure 3A). 55 , 56 This process may explain the role of CX3CR1high macrophages in the development of oral tolerance to dietary antigens in the upper small intestine. 57 The development of oral tolerance implicates the induction of antigen‐specific FoxP3+ Tregs, a process which requires migration of antigen‐presenting cells to the gut‐draining mesenteric lymph nodes (MLNs). 58 , 59 , 60 However, while lamina propria macrophages express high levels of MHCII and are thus capable of antigen presentation, their role in mucosal Treg differentiation remains uncertain. 54 , 61 Lamina propria macrophages lack CCR7 expression and thus cannot migrate to mesenteric lymph nodes to prime naive lymphoid cells after uptake of luminal antigens. 62 , 63 Hence, it has been proposed that CD103+ dendritic cells, which are also more effective T‐cell activators compared to macrophages, prime FoxP3+ regulatory T cells within the MLNs. 54 , 59 , 62 In this respect, it has been demonstrated that CX3CR1high lamina propria macrophages transfer trapped antigens to neighboring CD103+ dendritic cells with a connexin 43‐dependent mechanism.57 Differentiated FoxP3+ regulatory T cells within lymph nodes then return to the lamina propria and are locally maintained by IL‐10 produced by CX3CR1+ macrophages in a microbiota‐dependent manner. 59 , 64 CX3CR1+ macrophages have also been shown to support the generation of CD4+ T‐cell responses and Th17 cell differentiation in response to segmented filamentous bacteria. 40 , 65 , 66 In addition to luminal sampling, lamina propria macrophages in proximity to the epithelium exhibit functions to preserve intestinal epithelial integrity, such as pathogenic clearance and phagocytosis of dead cells.
Figure 3.
Functional heterogeneity of intestinal resident macrophages. Distinct subpopulations of macrophages carry out specialized functions in relation to the anatomical niche they occupy. A, Cx3cr1high macrophages (green) located below the intestinal epithelium sample the lumen and may participate in the development through the transfer of trapped antigens to CD103+ dendritic cells (orange) that then migrate to the mesenteric lymph nodes (MLNs) to instruct naive T cells. FoxP3+ regulatory T cells then migrate to the intestinal lamina propria to promote tolerance, where they are maintained by IL‐10 produced by resident macrophages. B, CD169+ macrophages are associated to the base of the intestinal crypt where they promote epithelial proliferation and Paneth cell (pink) differentiation. Depletion of macrophages leads to reduced epithelial proliferation and loss of LGR5+ intestinal stem cells. In DSS colitis, macrophages produce CCL8 to attract pro‐inflammatory monocytes from the blood stream. C, Depletion of long‐lived self‐maintaining macrophages leads to enteric neurodegeneration and disruption of blood vessel architecture in the submucosa. D, Macrophages within the myenteric plexus are maintained by CSF1 produced by enteric neurons. Conversely, enteric macrophages produce BMP2 which regulates intestinal peristalsis. Depletion of long‐lived self‐maintaining macrophages leads to loss of enteric neurons, impaired peristalsis and delayed transit
Lamina propria macrophages can also be found closely associated to the crypt base of the villi, relatively distant from the boundary of the lumen and mucosa (Figure 3B). These cells are CD169+ and promote wound regeneration and epithelial proliferation in a MyD88‐dependent fashion, likely through the expression of extracellular matrix proteins such as Mmp13 and Adam9. 46 , 67 , 68 Indeed, mice that are deficient for Csf1 show impaired epithelial differentiation and renewal. 41 , 69 , 70 In addition, it was demonstrated that macrophage depletion via CSF1‐R blockade impairs the differentiation of Paneth cells, specialized epithelial cells in the crypt base that provide niche signals for LGR5+ intestinal stem cells. 71 Consistently, CSF1‐R treatment reduced the expression of trophic factors by Paneth cells and decreased epithelial proliferation. Interestingly, we were recently able to identify a subset of long‐lived self‐maintaining macrophages in close proximity to Paneth cells, making it tantalizing to speculate that these cells consist in a specialized subset primed by local signals to support and protect Paneth cell differentiation. 25 However, such niche‐specific signals have yet to be characterized.
The submucosa is a heavily vascularized layer of connective tissue containing the submucosal plexus that constitutes part of the ENS and regulates intestinal secretion. 72 We recently showed that the majority of self‐maintaining macrophages detected within the lamina propria is located in close association with submucosal neurons and blood vessels. 25 Strikingly, depletion of self‐maintaining macrophages led to loss of submucosal neurons and blood vessel disruption and increased vascular leakage (Figure 3C). Single‐cell RNA sequencing revealed distinct populations associated to blood vessels and neurons, respectively, and each characterized by a unique transcriptome, suggesting that subsets of macrophages functionally specialize to support enteric neurons and blood vessels. These findings prove a hitherto unknown heterogeneity within the intestinal macrophage pool, which begs for further in‐depth characterization of these subsets and their role in gastrointestinal disorders.
4.2. Muscularis macrophages
Muscularis macrophages are located within the muscularis externa, where they constitute the main immune cell population and lie in close proximity to neurons within the myenteric plexus, the circular and longitudinal smooth muscle layers and ICCs, which function together to regulate gastrointestinal motility. 73 , 74 Reflecting the distinct nature of the muscularis niche, its macrophages have been shown to be transcriptionally and morphologically different to their lamina propria counterparts, characterized by upregulation of tissue‐protective genes such as Retnla, Mrc1, and CD163 9 , 25 ; and also within the muscularis niche, subpopulations of macrophages can be identified at least on a morphological level, as cells lying within the myenteric plexus and thus in close contact with enteric ganglia appear stellate, with a “microglia‐like appearance,” while macrophages lying within the muscle layer exhibit a bipolar morphology. 9 Whether this morphological heterogeneity extends to transcriptional and functional heterogeneity, however, has yet to be explored.
At the level of the myenteric plexus, muscularis macrophages lie in close proximity to enteric neurons, where they carry out a series of specialized functions specific to this niche. The priming of macrophages in the myenteric plexus appears to depend mainly on neuron‐derived signals, as muscularis macrophages express a variety of neurotransmitter receptors that can modulate their phenotype, including neurokinin receptors, glycine receptors, nicotinic α7 and β2 acetylcholine receptors, adrenergic β2 receptor, and P2 purine receptors. 9 , 75 , 76 , 77 , 78 In addition, enteric neurons have been shown to produce CSF1, crucial for the maintenance of the macrophage compartment. 79 Conversely, macrophages produce mediators crucial for neuronal function including bone morphogenic protein 2 (BMP2), which has been shown to be important for smooth muscle contractility and peristalsis (Figure 3D). 79 Intriguingly, while macrophages colonize the muscularis externa in mice as early as 8.5 days post‐conception, before onset of neurogenesis and the formation of a complete functional myenteric plexus, and have also been observed in animal models that lack enteric neurons, it appears that enteric neurons rely heavily on the presence of macrophages for their function and survival (own unpublished results). 80 Indeed, we have shown that depletion of the self‐maintaining subpopulation of macrophages leads to a loss of over 50% of enteric neurons in the myenteric plexus, impaired intestinal contractility and prolonged intestinal transit. 25 Moreover, the constitutive absence of muscularis macrophages in Csf1op/op mice is associated with an increased density of enteric neurons and a less organized architecture of the myenteric plexus. 79 This is in line with recent data showing phagocytosis of neuronal debris by muscularis macrophages during steady‐state, suggesting that muscularis macrophages actively shape the ENS in a similar manner as to how microglia shape the CNS through removal of apoptotic debris in development and adulthood. 81 Taken these examples into account, it is plausible that the functional mechanisms employed by microglia to regulate neuronal development in the CNS can be extrapolated to muscularis macrophages in the ENS.
In addition to their interaction with neurons, it was recently demonstrated that muscularis macrophages can directly interact with smooth muscle cells via TRPV4‐mediated release of prostaglandin E2, affecting colonic motility. 82 Indeed, Trpv4‐floxed mice showed significant reduction in colonic contraction upon treatment with GSK101, a TRPV4 channel agonist, an effect which was independent from neuronal input. These findings highlight that muscularis macrophages can also directly influence smooth muscle cells without input from the ENS. Furthermore, muscularis macrophages have also been shown to be closely associated to ICC in both human and mouse; however, the functional implications of this interaction have yet to be elucidated. 83
5. RESIDENT MACROPHAGES IN GASTROINTESTINAL DISEASE
5.1. Lamina propria macrophages in intestinal inflammation and infection
Inflammation in the intestine is associated to considerable influx of Ly6Chigh monocytes that rapidly differentiate into pro‐inflammatory effector cells, a process that has been extensively studied in animal models of colitis. 19 , 28 , 84 , 85 Characteristic of the inflammatory setting is the arrest of differentiation of incoming monocytes, which thus do not fully differentiate into Cx3cr1high macrophages and instead retain a Cx3cr1int signal and features resembling the monocytes they derive from. 28 , 85 This immature state of the macrophage is characterized by failure of IL‐10 upregulation and the excessive production of inflammatory cytokines such as IL‐1β, TNF‐α, IL‐6, IL‐12, and chemokines that further drive the influx of Ly6Chigh monocytes. 28 , 30 , 86 , 87 Arrested differentiation and accumulation of CD14high CD11chigh immature macrophages have also been widely described in patients with inflammatory bowel disease. In line with findings murine models, these immature CD14high cells produce pro‐inflammatory cytokines, display respiratory burst activity and express markers that further drive the influx of pro‐inflammatory cells. 28 , 88 , 89 , 90 , 91 , 92 , 93 The mechanisms underlying incomplete differentiation of incoming monocytes to macrophages in the context of intestinal inflammation have not fully been elucidated; however, a reduction of factors that promote differentiation, such as IL‐10 and TGFβ, in addition to increased pro‐inflammatory signals such as IFNγ may disrupt the pathway required for macrophage differentiation. 28 , 90 , 94 It has been proposed that recruited inflammatory cells play a pathological role in murine models of colitis, and in line, pharmacological or genetic depletion of CCR2‐ameliorated inflammation in DSS‐induced colitis. 85 However, as the role of recruited inflammatory cells in gastrointestinal disorders has been extensively described elsewhere and is not the focus of this review, we kindly refer the interested reader to these excellent reviews. 95 , 96
Resident Cx3cr1high macrophages persist in inflammatory settings and maintain their anti‐inflammatory phenotype and immunological unresponsiveness, a feature that may be crucial to promote mucosal healing. 28 , 87 , 97 In line, depletion of resident macrophages leads to aggravation of experimental colitis. 98 Interestingly, hemoglobin released from hemorrhaging tissue in experimental colitis limits pro‐inflammatory gene expression in Cx3cr1high macrophages, thus limiting intestinal inflammation in DSS‐induced colitis. 99 As in the healthy gut, however, resident macrophages may contribute to the recruitment of monocytes through release of CCR2 ligands. This has been demonstrated in the case of the specialized niche of CD169+ macrophages located preferentially around the base of intestinal crypts, adjacent to blood vessels and lymphatics, that have been shown to produce CCL8 in response to inflammation or injury to attract inflammatory Ly6Chigh monocytes (Figure 3B). 10 These findings highlight the complex and dynamic role of monocytes, macrophages and their intermediates in the context of intestinal inflammation.
In the setting of helminths infection, lamina propria macrophages initiate a Th2‐driven immune response to ensure protective responses by production of IL‐4 and IL‐13. 101 , 102 Moreover, during worm infections, these cells produce arginase‐1, resistin‐like alpha and attract eosinophils, favouring worm expulsion but also improving tissue repair after clearance of infection. Furthermore, lamina propria macrophages inhibit the Th1 cell response toward invasive pathogens such as Salmonella typhimurium. Indeed, depletion of CX3CR1+ macrophages led to enhanced Th1 cell response upon oral infection with Salmonella typhimurium or Helicobacter hepaticus, although it should be noted that in this model, both incoming as also resident macrophages were depleted. 64 Of note, this protective function appears to be dependent on the presence of an intact microbiome.
Finally, in the Peyer's patches of the small intestine, which are considered to be the main entry gates for pathogens within the intestine, macrophages are implicated in the initiation of proper inflammatory responses. 103 , 104 They produce less IL‐10 compared to their lamina propria counterparts and show upregulation of interferon‐dependent anti‐microbial and antiviral genes. 12 Moreover, these macrophages show enhanced production of IL‐6 and TNFα upon TLR stimulation, in addition to priming naïve T helper cells for IFNγ production. 12 , 105 , 106
5.2. Muscularis macrophages in gastrointestinal disorders
Several studies clearly indicate a key role for muscularis macrophages in gastrointestinal disorders including ileus and gastroparesis, characterized by impaired gastrointestinal motility and/or transit. 11 Ileus is a condition characterized by impaired contractility of the intestine due to inflammation of the muscle layer following abdominal surgery or sepsis. 107 Despite considerable advances in surgical technique, postoperative ileus remains a condition that can considerably prolong hospitalization following abdominal surgery, leading to increased healthcare costs. 108 Animals studies have shown that an initial neuronally mediated inhibition of gut motility is followed by an inflammatory cascade of events in the muscularis externa initiated by muscularis macrophages, including the upregulation of several transcription factors (ie NF‐kB, STAT, p38‐MAPK), induction of pro‐inflammatory gene expression and the release of chemokines and cytokines (ie, IL‐1β, MCP1, IL‐6, and TNFα). 109 , 110 , 111 This inflammatory process favors the upregulation of adhesion molecules and recruitment of circulating leukocytes such as neutrophils and monocytes to the muscularis externa, which eventually leads to impaired intestinal motility mediated by inflammatory effectors on smooth muscle, intrinsic, and extrinsic nerves. 112 , 113 , 114 In particular, resident macrophages upregulate the inducible nitric oxide synthase iNOS and thus promote ileus via NO‐mediated paralysis of intestinal muscle cells. 115 This activation of resident macrophages is mediated by local Irf4‐dependent CD103+ CD11b+ dendritic cells, which stimulate memory Th1 cells to produce IFNγ by secreting the pro‐inflammatory mediator IL‐12. 116 , 117 The pathological role of muscularis macrophages is further underscored by findings showing that Csf1op/op mice are protected against postoperative ileus. 118 , 119 Moreover, prevention of macrophage activation by stimulation of the vagus nerve, an effect mediated by the release of acetylcholine and activation of α7nAChR expressed by muscularis macrophages, reduces intestinal inflammation and improves postoperative intestinal transit. 120 , 121 , 122 In addition, upregulation of heme‐oxygenase (HO‐1) or activation of glycine receptors in muscularis macrophages have both been shown to ameliorate ileus in animal models. 123 , 124
Macrophages located in the muscularis externa have also been implicated in gastroparesis, a condition characterized by delayed emptying of the stomach in the absence of mechanical obstruction, which can arise as a consequence of diabetes or be idiopathic or iatrogenic. Cardinal symptoms of gastroparesis include postprandial fullness, early satiety, nausea, vomiting, and bloating, and it has been estimated to affect up to 2% of the general population. 125 , 126 Loss of CD206+ macrophages has been associated with increased oxidative stress and pro‐inflammatory cytokine expression, the loss of neuronal nitric oxide synthase‐expressing neurons and the development of delayed gastric emptying. 127 , 128 In addition, the loss of CD206+ macrophages in patients with gastroparesis correlated with loss of ICC, while unopposed oxidative stress has been shown to play a role in ICC loss and development of delayed gastric emptying in animal models. 127 , 129 However, the absence of muscularis macrophages in Csf1op/op mice was protective against the development of gastroparesis in diabetic mice, and CSF1‐mediated replenishment of macrophages resulted in development of delayed gastric emptying and ICC damage. 130 It is thus clear that the role of macrophages in gastroparesis and the mechanisms through which they regulate gastric emptying have yet to be elucidated.
Intestinal ischemia reperfusion injury can occur following hemorrhagic shock, cardiac arrest or arterial occlusion, but also as a complication of surgical procedures such as intestinal transplantation or abdominal aortic surgery. Ischemia activates resident macrophages with increased levels of MPO activity, release of pro‐inflammatory IL‐6 and production of TNF α, initiating an inflammatory response. 131 , 132 This results in damage to the intestinal mucosa, increased gut permeability and consequent bacterial translocation, which can lead to sepsis and multiple organ failure, with a high rate of mortality. 133 Conversely, depletion of resident macrophages is protective against mucosal damage. 132 Interestingly, and in line with findings in models of postoperative ileus, stimulation of the vagus nerve improved survival, blunted NF‐KB response, and reduced levels of TNFα and IL‐6 in models of haemorragic shock. 134 , 135 These findings suggest that resident macrophages may drive the inflammatory response to ischemia and may thus represent an interesting therapeutic target to limit ischemia‐reperfusion damage.
6. CONCLUSIONS
The recent advances in our knowledge of intestinal resident macrophage ontogeny and heterogeneity have uncovered a hitherto unknown spectrum of functional specialization of these cells, that extends well beyond mere phagocytosis of bacteria and host defence mechanisms. Our recent work has shed light on these “multitaskers” as cells that maintain vascular integrity and support neuronal survival; however, the complexity of the intestinal tissue suggests that there may be many more macrophage subpopulations with functional specialization, begging a further concentrated effort to further define the niches and signaling involved. 25 Our increased understanding of macrophage heterogeneity further highlights the need to resolve the functional and phenotypical specialization of these cells on a spatial level, which will be possible in the near future thanks to the staggering progress of techniques such as spatial RNA transcriptomics and spatial mass spectrometry. Furthermore, it will be crucial to expand these findings to the human intestine, in both health and disease, to further unravel the complex interactions between macrophages and other cell types, with the prospect of uncovering mechanisms that underlie gastrointestinal diseases.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTION
MFV and GB wrote and revised the manuscript.
Viola MF, Boeckxstaens G. Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol Motil. 2020;32:e13843 10.1111/nmo.13843
REFERENCES
- 1. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue‐resident macrophages. Nat Immunol. 2013;14:986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209(1):139‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue‐specific context. Nat Rev Immunol. 2014;14:81. [DOI] [PubMed] [Google Scholar]
- 4. Parkhurst CN, Yang G, Ninan I, et al. Microglia promote learning‐dependent synapse formation through brain‐derived neurotrophic factor. Cell. 2013;155:1596‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaitin Diego Adhemar, Adlung Lorenz, Thaiss Christoph A, et al. Lipid‐associated macrophages control metabolic homeostasis in a trem2‐dependent manner. Cell. 2019;178:686‐698.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim HY, Lim SY, Tan CK, et al. Hyaluronan receptor LYVE‐1‐expressing macrophages maintain arterial tone through hyaluronan‐mediated regulation of smooth muscle cell collagen. Immunity. 2018;49:326‐341.e7. [DOI] [PubMed] [Google Scholar]
- 7. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141‐153. [DOI] [PubMed] [Google Scholar]
- 8. Furness JB. The enteric nervous system. The Enteric Nervous System. 2006. 10.1002/9780470988756. [DOI]
- 9. Gabanyi Ilana, Muller Paul A, Feighery Linda, et al. Neuro‐immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol. 1995;10:719‐736. [PubMed] [Google Scholar]
- 11. De Schepper S, Stakenborg N, Matteoli G, Verheijden S, Boeckxstaens GEGE. Muscularis macrophages: Key players in intestinal homeostasis and disease. Cell Immunol. 2017;330:142‐150. 10.1016/j.cellimm.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnardel J, Da Silva C, Henri S, et al. Innate and adaptive immune functions of Peyer’s patch monocyte‐derived cells. Cell Rep. 2015;11:770‐784. [DOI] [PubMed] [Google Scholar]
- 13. Baratin M, Simon L, Jorquera A, et al. T cell zone resident macrophages silently dispose of apoptotic cells in the lymph node. Immunity. 2017;47:349‐362. [DOI] [PubMed] [Google Scholar]
- 14. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845‐852. [PMC free article] [PubMed] [Google Scholar]
- 15. Ginhoux F, Greter M, Leboeuf M, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult‐derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heidt T, Courties G, Dutta P, et al. Differential contribution of monocytes to heart macrophages in steady‐state and after myocardial infarction. Circ Res. 2014;115:284‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molawi K, Wolf Y, Kandalla PK, et al. Progressive replacement of embryo‐derived cardiac macrophages with age. J Exp Med. 2014;211:2151‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamoutounour S, Guilliams M, Montanana Sanchis F, et al. Origins and functional specialization of macrophages and of conventional and monocyte‐derived dendritic cells in mouse skin. Immunity. 2013;39:925‐938. [DOI] [PubMed] [Google Scholar]
- 20. Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature. 2015;518:547‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gibbings SL, Thomas SM, Atif SM, et al. Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol. 2017;57:66‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cain DW, O'Koren EG, Kan MJ, et al. Identification of a tissue‐specific, C/EBPbeta‐dependent pathway of differentiation for murine peritoneal macrophages. J Immunol. 2013;191:4665‐4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bain CC, Hawley CA, Garner H, et al. Long‐lived self‐renewing bone marrow‐derived macrophages displace embryo‐derived cells to inhabit adult serous cavities. Nat Commun. 2016;7:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bain CC, Bravo‐Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929‐937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Schepper S, Verheijden S, Aguilera‐Lizarraga J, et al. Self‐Maintaining gut macrophages are essential for intestinal homeostasis. Cell. 2018;175:400‐415.e13. [DOI] [PubMed] [Google Scholar]
- 26. Shaw TN, Houston SA, Wemyss K, et al. Tissue‐resident macrophages in the intestine are long lived and defined by Tim‐4 and CD4 expression. J Exp Med. 2018;215(6):1507‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bujko A, Atlasy N, Landsverk OJB, et al. Transcriptional and functional profiling defines human small intestinal macrophage subsets. J Exp Med. 2017;215(2):441‐458. 10.1084/jem.20170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bain CC, Scott CL, Uronen‐Hansson H, et al. Resident and pro‐inflammatory macrophages in the colon represent alternative context‐dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311‐317. [DOI] [PubMed] [Google Scholar]
- 30. Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1‐inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150‐3166. [DOI] [PubMed] [Google Scholar]
- 31. Takada Y, Hisamatsu T, Kamada N, et al. Monocyte chemoattractant protein‐1 contributes to gut homeostasis and intestinal inflammation by composition of IL‐10–producing regulatory macrophage subset. J. Immunol. 2010;184(5):2671‐2676. [DOI] [PubMed] [Google Scholar]
- 32. Schridde A, Bain CC, Mayer JU, et al. Tissue‐specific differentiation of colonic macrophages requires TGFβ receptor‐mediated signaling. Mucosal Immunol. 2017;10:1387‐1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cecchini MG, Dominguez MG, Mocci S, et al. Role of colony stimulating factor‐1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 120(6):1357‐1372. [DOI] [PubMed] [Google Scholar]
- 34. Dai X‐M, Ryan GR, Hapel AJ, et al. Targeted disruption of the mouse colony‐stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111‐120. [DOI] [PubMed] [Google Scholar]
- 35. MacDonald KPA, Palmer JS, Cronau S, et al. An antibody against the colony‐stimulating factor 1 receptor depletes the resident subset of monocytes and tissue‐ and tumor‐associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955‐3963. [DOI] [PubMed] [Google Scholar]
- 36. Bernardo D, Marin AC, Fernández‐Tomé S, et al. Human intestinal pro‐inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(‐)CCR2(‐)CX3CR1(‐) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol. 2018;11:1114‐1126. [DOI] [PubMed] [Google Scholar]
- 37. Mikkelsen HB, Garbarsch C, Tranum‐Jensen JJ, Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ‐free mice. J Mol Histol. 2004;35:377‐387. [DOI] [PubMed] [Google Scholar]
- 38. Hori M, Nobe H, Horiguchi K, Ozaki H. MCP‐1 targeting inhibits muscularis macrophage recruitment and intestinal smooth muscle dysfunction in colonic inflammation. Am J Physiol Cell Physiol. 2008;294:C391‐401. [DOI] [PubMed] [Google Scholar]
- 39. Linden DR, Couvrette JM, Ciolino A, et al. Indiscriminate loss of myenteric neurones in the TNBS‐inflamed guinea‐pig distal colon. Neurogastroenterol Motil. 2005;17:751‐760. [DOI] [PubMed] [Google Scholar]
- 40. Denning TL, Norris BA, Medina‐Contreras O, et al. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, Source of mouse strain, and regional localization. J Immunol. 2011;187:733‐747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagashima R, Maeda K, Imai Y, Takahashi T. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J Histochem Cytochem. 1996;44(7):721‐731. [DOI] [PubMed] [Google Scholar]
- 42. Rani R, Smulian AG, Greaves DR, Hogan SP, Herbert DR. TGF‐beta limits IL‐33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol. 2011;41:2000‐2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cummings RJ, Barbet G, Bongers G, et al. Different tissue phagocytes sample apoptotic cells to direct distinct homeostasis programs. Nature. 2016;539:565‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hume DA, Perry VH, Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat Rec. 1984;210:503‐512. [DOI] [PubMed] [Google Scholar]
- 45. Smith PD, Smythies LE, Shen R, Greenwell‐Wild T, Gliozzi M, Wahl SM. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal Immunol. 2012;5(2):194‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smythies LE, Shen R, Bimczok D, et al. Inflammation anergy in human intestinal macrophages is due to Smad‐induced IκBα expression and NF‐κB inactivation. J Biol Chem. 2010;285:19593‐19604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115(1):66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roberts PJ, Riley GP, Morgan K, Miller R, Hunter JO, Middleton SJ. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol. 2001;54(4):293‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zigmond E, Bernshtein B, Friedlander G, et al. Macrophage‐restricted interleukin‐10 receptor deficiency, but not IL‐10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720‐733. [DOI] [PubMed] [Google Scholar]
- 51. Hirotani T, Lee PY, Kuwata H, et al. The nuclear IkappaB protein IkappaBNS selectively inhibits lipopolysaccharide‐induced IL‐6 production in macrophages of the colonic lamina propria. J Immunol. 2005. 174(6):3650‐3657. [DOI] [PubMed] [Google Scholar]
- 52. Shouval D, Biswas A, Goettel J, et al. Interleukin‐10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti‐inflammatory macrophage function. Immunity. 2014;40:706‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ueda Y, Kayama H, Jeon SG, et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL‐10. Int Immunol. 2010;22(12):953‐962. [DOI] [PubMed] [Google Scholar]
- 54. Krause P, Morris V, Greenbaum JA, et al. IL‐10‐producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL‐23 synthesis. Nat Commun. 2015;6:7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Niess JH, Brand S, Gu X, et al. CX3CR1‐mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 307(5707):254‐258. [DOI] [PubMed] [Google Scholar]
- 56. Chieppa M, Rescigno M, Huang AYC, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203(13):2841‐2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity. 2014;40:248‐261. [DOI] [PubMed] [Google Scholar]
- 58. Cerovic V, Houston SA, Scott CL, et al. Intestinal CD103(‐) dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol. 2013;6:104‐113. [DOI] [PubMed] [Google Scholar]
- 59. Hadis U, Wahl B, Schulz O, et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237‐246. [DOI] [PubMed] [Google Scholar]
- 60. Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Denning TL, Wang Y, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17–producing T cell responses. Nat Immunol. 2007;8:1086‐1094. [DOI] [PubMed] [Google Scholar]
- 62. Schulz O, Jaensson E, Persson EK, et al. Intestinal CD103 +, but not CX3CR1 +, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101‐3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bogunovic M, Ginhoux F, Helft J, et al. Origin of the lamina propria dendritic cell networkc access. Immunity. 2009;31:513‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim M, Galan C, Hill AA, et al. Critical role for the microbiota in CX3CR1+intestinal mononuclear phagocyte regulation of intestinal T cell responses. Immunity. 2018;49:151‐163.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim YS, Lee MH, Ju AS, Rhee K‐J. Th17 responses are not induced in dextran sodium sulfate model of acute colitis. Immune Netw. 2011;11:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Panea C, Farkas A, Goto Y, et al. Intestinal monocyte‐derived macrophages control commensal‐specific Th17 responses. Cell Rep. 2015;12(8):1314‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci. 2005;102(1):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hiemstra IH, Beijer MR, Veninga H, et al. The identification and developmental requirements of colonic CD169(+) macrophages. Immunology. 2014;142:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huynh D, Akçora D, Malaterre J, et al. CSF‐1 receptor‐dependent colon development, homeostasis and inflammatory stress response. PLoS ONE. 2013;8(2):e56951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sauter KA, Pridans C, Sehgal A, et al. Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J Leukoc Biol. 2014;96(2):265‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sehgal A, Donaldson DS, Pridans C, et al. The role of CSF1R‐dependent macrophages in control of the intestinal stem‐cell niche. Nat Commun. 2018;9:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xue J, Askwith C, Javed NH, Cooke HJ. Autonomic nervous system and secretion across the intestinal mucosal surface. Auton Neurosci. 2007;133:55‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Takaki M. Gut pacemaker cells: the interstitial cells of cajal (ICC). J Smooth Muscle Res. 2003;39(5):137‐161. [DOI] [PubMed] [Google Scholar]
- 74. Sanders KM, Koh SD, Ro S, Ward SM. Regulation of gastrointestinal motility‐insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012;9(11):633‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine‐gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856‐G863. [DOI] [PubMed] [Google Scholar]
- 76. Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin‐1 receptor. J Immunol. 1997;159:5654‐5660. [PubMed] [Google Scholar]
- 77. Marques‐da‐Silva C, Burnstock G, Ojcius DM, Coutinho‐Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216:1‐11. [DOI] [PubMed] [Google Scholar]
- 78. Nemethova A, Michel K, Gomez‐Pinilla PJ, Boeckxstaens GE, Schemann M. Nicotine attenuates activation of tissue resident macrophages in the mouse stomach through the beta2 nicotinic acetylcholine receptor. PLoS ONE. 2013;8:e79264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Muller P, Koscsó B, Rajani G, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Avetisyan M, Rood JE, Huerta Lopez S, et al. Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci USA. 2018;115(18):4696‐4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kulkarni S, Micci MA, Leser J, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci USA. 2017;114:E3709‐E3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Luo J, Qian A, Oetjen LK, et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity. 2018;49(1):107‐119.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med. 2010;14:818‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grainger JR, Wohlfert EA, Fuss IJ, et al. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19(6):713‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zigmond E, Varol C, Farache J, et al. Ly6Chi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen‐presenting cells. Immunity. 2012;37:1076‐1090. [DOI] [PubMed] [Google Scholar]
- 86. Zigmond E, Jung S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol. 2013;34:162‐168. [DOI] [PubMed] [Google Scholar]
- 87. Weber B, Saurer L, Schenk M, Dickgreber N, Mueller C. CX3CR1 defines functionally distinct intestinal mononuclear phagocyte subsets which maintain their respective functions during homeostatic and inflammatory conditions. Eur J Immunol. 2011;41:773‐779. [DOI] [PubMed] [Google Scholar]
- 88. Rugtveit J, Nilsen EM, Bakka A, et al. Cytokine profiles differ in newly recruited and resident subsets of mucosal macrophages from inflammatory bowel disease. Gastroenterology. 1997;112:1493‐1505. [DOI] [PubMed] [Google Scholar]
- 89. Grimm MC, Pullman WE, Bennett GM, Sullivan PJ, Pavli P, Doe WF. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387‐395. [DOI] [PubMed] [Google Scholar]
- 90. Kamada N, Hisamatsu T, Okamoto S. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL‐23/IFN‐gamma axis. J Clin Invest. 2008;118:2269‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thiesen S, Janciauskiene S, Uronen‐Hansson H, et al. CD14(hi)HLA‐DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn’s disease. J Leukoc Biol. 2014;95:531‐541. [DOI] [PubMed] [Google Scholar]
- 92. Lampinen M, Waddell A, Ahrens R, Carlson M, Hogan SP. CD14+CD33+ myeloid cell‐CCL11‐eosinophil signature in ulcerative colitis. J Leukoc Biol. 2013;94:1061‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mahida YR, Patel S, Gionchetti P, Vaux D, Jewell DP. Macrophage subpopulations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989;30:826‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF‐beta1 and Smad7 in the regulation of IBD. Mucosal Immunol. 2008;1(Suppl 1):S50‐S53. [DOI] [PubMed] [Google Scholar]
- 95. Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16(9):531‐543. [DOI] [PubMed] [Google Scholar]
- 96. Grainger JR, Konkel JE, Zangerle‐Murray T, Shaw TN. Macrophages in gastrointestinal homeostasis and inflammation. Pflugers Arch. 2017;469:527‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bain CC, Oliphant CJ, Thomson CA, Kullberg MC, Mowat AM. Proinflammatory role of monocyte‐derived CX3CR1(int) macrophages in helicobacter hepaticus‐induced colitis. Infect Immun. 2018;86:e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802‐815. [DOI] [PubMed] [Google Scholar]
- 99. Kayama H, Kohyama M, Okuzaki D, et al. Heme ameliorates dextran sodium sulfate‐induced colitis through providing intestinal macrophages with noninflammatory profiles. Proc Natl Acad Sci USA. 2018;115:8418‐8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Asano K, Takahashi N, Ushiki M, et al. Intestinal CD169 + macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat Commun. 2015;6:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kreider T, Anthony RM, Urban JF, Gause WC. Alternatively activated macrophages in helminth infections. Curr Opin Immunol. 2007;19(4):448‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Little MC, Hurst RJM, Else KJ. Dynamic changes in macrophage activation and proliferation during the development and resolution of intestinal inflammation. J Immunol. 2014;193:4684‐4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wagner C, Bonnardel J, Da Silva C, et al. Some news from the unknown soldier, the Peyer’s patch macrophage. Cell Immunol. 2018;330:159‐167. [DOI] [PubMed] [Google Scholar]
- 104. Da Silva C, Wagner C, Bonnardel J, Gorvel JP, Lelouard H. The Peyer’s patch mononuclear phagocyte system at steady state and during infection. Front Immunol. 2017;8:1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rochereau Nicolas, Drocourt Daniel, Perouzel Eric, et al. Dectin‐1 Is essential for reverse transcytosis of glycosylated SIgA‐antigen complexes by intestinal M cells. PLoS Biol. 2013;11(9):e1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lelouard H, Lelouard H, Henri S, et al. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer’s patch dendritic cells that express lysozyme. Gastroenterology. 2010;138(1):173‐184.e3. [DOI] [PubMed] [Google Scholar]
- 107. Kalff JC, Schraut WH, Simmons RL, Bauer AJ. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228(5):652‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58(9):1300‐1311. [DOI] [PubMed] [Google Scholar]
- 109. Farro G, Gomez‐Pinilla PJ, Di Giovangiulio M, et al. Smooth muscle and neural dysfunction contribute to different phases of murine postoperative ileus. Neurogastroenterol Motil. 2016;28:934‐947. [DOI] [PubMed] [Google Scholar]
- 110. Türler A, Schwarz NT, Türler E, Kalff JC, Bauer AJ. MCP‐1 causes leukocyte recruitment and subsequently endotoxemic ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282(1):G145‐G155. [DOI] [PubMed] [Google Scholar]
- 111. Wehner S, Schwarz NT, Hundsdoerfer R, et al. Induction of IL‐6 within the rodent intestinal muscularis after intestinal surgical stress. Surgery. 2005;137(4):436‐446. [DOI] [PubMed] [Google Scholar]
- 112. Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20(s1):81‐90. [DOI] [PubMed] [Google Scholar]
- 113. Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117(2):378‐387. [DOI] [PubMed] [Google Scholar]
- 114. Eskandari MK, Kalff JC, Billiar TR, Lee KK, Bauer AJ. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol. 1997;273(3):G727‐G734. [DOI] [PubMed] [Google Scholar]
- 115. Türler A, Kalff JC, Moore BA, et al. Leukocyte‐derived inducible nitric oxide synthase mediates murine postoperative ileus. Ann Surg. 2006;244:220‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Engel DR, Koscielny A, Wehner S, et al. T helper type 1 memory cells disseminate postoperative ileus over the entire intestinal tract. Nat Med. 2010;16:1407‐1413. [DOI] [PubMed] [Google Scholar]
- 117. Pohl J‐M, Gutweiler S, Thiebes S, et al. Irf4‐dependent CD103(+)CD11b(+) dendritic cells and the intestinal microbiome regulate monocyte and macrophage activation and intestinal peristalsis in postoperative ileus. Gut. 2017;66:2110‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Pantelis D, Kabba MS, Kirfel J, et al. Transient perioperative pharmacologic inhibition of muscularis macrophages as a target for prophylaxis of postoperative ileus does not affect anastomotic healing in mice. Surgery. 2010;148(1):59‐70. [DOI] [PubMed] [Google Scholar]
- 119. Wehner S, Behrendt FF, Lyutenski BN, et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56(2):176‐185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stakenborg N, Labeeuw E, Gomez‐Pinilla PJ, et al. Preoperative administration of the 5‐HT4 receptor agonist prucalopride reduces intestinal inflammation and shortens postoperative ileus via cholinergic enteric neurons. Gut. 2019;68:1406‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Matteoli G, Gomez‐Pinilla PJ, Nemethova A, et al. A distinct vagal anti‐inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2013;63:938‐948. [DOI] [PubMed] [Google Scholar]
- 122. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117:289‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Moore BA, Otterbein LE, Turler A, Choi AMK, Bauer AJ. Inhaled carbon monoxide suppresses the development of postoperative ileus in the murine small intestine. Gastroenterology. 2003;124:377‐391. [DOI] [PubMed] [Google Scholar]
- 124. Stoffels B, Türler A, Schmidt J, et al. Anti‐inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil. 2011;23(1):76‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589‐1591. [DOI] [PubMed] [Google Scholar]
- 126. Rey E, Choung RS, Schleck CD, et al. Prevalence of hidden gastroparesis in the community: the gastroparesis ‘iceberg’. J Neurogastroenterol Motil. 2012;18:34‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Choi KM, Kashyap PC, Dutta N, et al. CD206‐positive M2 macrophages that express heme oxygenase‐1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399‐2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Choi KM, Gibbons SJ, Nguyen TV, et al. Heme oxygenase‐1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008;135(6):2055‐2064.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Grover M, Bernard CE, Pasricha PJ, et al. Diabetic and idiopathic gastroparesis is associated with loss of CD206‐positive macrophages in the gastric antrum. Neurogastroenterol Motil. 2017;29(6):e13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cipriani G, Gibbons SJ, Verhulst P‐J, et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. CMGH. 2016;2(1):40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hierholzer C, Kalff JC, Audolfsson G, Billiar TR, Tweardy DJ, Bauer AJ. Molecular and functional contractile sequelae of rat intestinal ischemia/reperfusion injury. Transplantation. 1999;68:1244‐1254. [DOI] [PubMed] [Google Scholar]
- 132. Chen Y, Lui VCH, Rooijen NV, Tam PKH. Depletion of intestinal resident macrophages prevents ischaemia reperfusion injury in gut. Gut. 2004;53:1772‐1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Jrvinen O, Laurikka J, Salenius JP, Tarkka M. Acute intestinal ischaemia. A review of 214 cases. Ann Chir Gynaecol. 1994;83:22‐25. [PubMed] [Google Scholar]
- 134. Guarini S, Altavilla D, Cainazzo MM, et al. Efferent vagal fibre stimulation blunts nuclear factor‐kappaB activation and protects against hypovolemic hemorrhagic shock. Circulation. 2003;107:1189‐1194. [DOI] [PubMed] [Google Scholar]
- 135. Luyer MD, Greve JW, Hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023‐1029. [DOI] [PMC free article] [PubMed] [Google Scholar]


