Summary
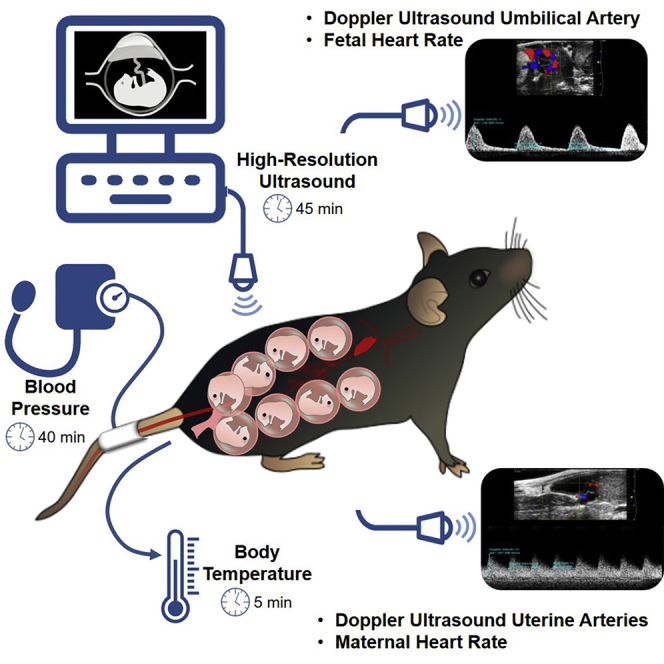
Pregnancy is a unique physiological state in which two individuals coexist: the mother and the fetus. Disruption of maternal-fetal crosstalk leads to pregnancy and neonatal pathologies. Therefore, assessing maternal-fetal well-being is essential for understanding the physiological and pathological processes occurring during pregnancy. Herein, we provide a protocol that allows for the determination of body temperature, blood pressure, and the evaluation of uterine and umbilical arteries as well as maternal and fetal heart rate using high-resolution ultrasound in pregnant mice.
For complete details on the use and execution of this protocol, please refer to Gomez-Lopez et al. (2020).
Graphical Abstract
Highlights
-
•
Assessing maternal-fetal well-being is critical for studying pregnancy in mice
-
•
Evaluation of maternal-fetal parameters improves translational value
-
•
High-resolution ultrasound of uterine and umbilical arteries can predict pathology
-
•
This protocol is suitable for evaluating pregnancy disease and treatments in mice
Pregnancy is a unique physiological state in which two individuals coexist: the mother and the fetus. Disruption of maternal-fetal crosstalk leads to pregnancy and neonatal pathologies. Therefore, assessing maternal-fetal well-being is essential for understanding the physiological and pathological processes occurring during pregnancy. Herein, we provide a protocol that allows for the determination of body temperature, blood pressure, and the evaluation of uterine and umbilical arteries as well as maternal and fetal heart rate using high-resolution ultrasound in pregnant mice.
Before You Begin
Pregnancy is a unique physiological state in which two individuals coexist: the mother and the fetus. Both individuals undergo physiological changes throughout pregnancy that require close surveillance. The disruption of maternal-fetal parameters can result in the development of pregnancy complications, which have been termed “great obstetrical syndromes” (Romero, 2009; Di Renzo, 2009; Brosens et al., 2019; Harris et al., 2019). Therefore, to improve the translational value of our research focused on the study of the immunobiology of pregnancy in mice, it is essential to perform an exhaustive maternal-fetal assessment by evaluating vital signs as well as maternal-fetal well-being using high-resolution ultrasound. Specifically, herein we present a complete and non-invasive protocol for evaluating the following parameters: a) body temperature using a rectal probe, b) non-invasive blood pressure measurement using a tail cuff, and c) umbilical and uterine artery blood flow as well as maternal and fetal heart rate using high-resolution Doppler ultrasound.
CRITICAL: All procedures must be approved by the Institutional Review Board according to established regulations and guidelines. In our protocol, all procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Wayne State University under Protocol No. 18-03-0584. Importantly, we recommend that this procedure be performed by a person trained in ultrasound procedures.
Preparation for Body Temperature Measurement
Timing: 1 min
-
1.
Turn on the physiological monitor of the Vevo® Imaging station (Figure 1A).
Figure 1.
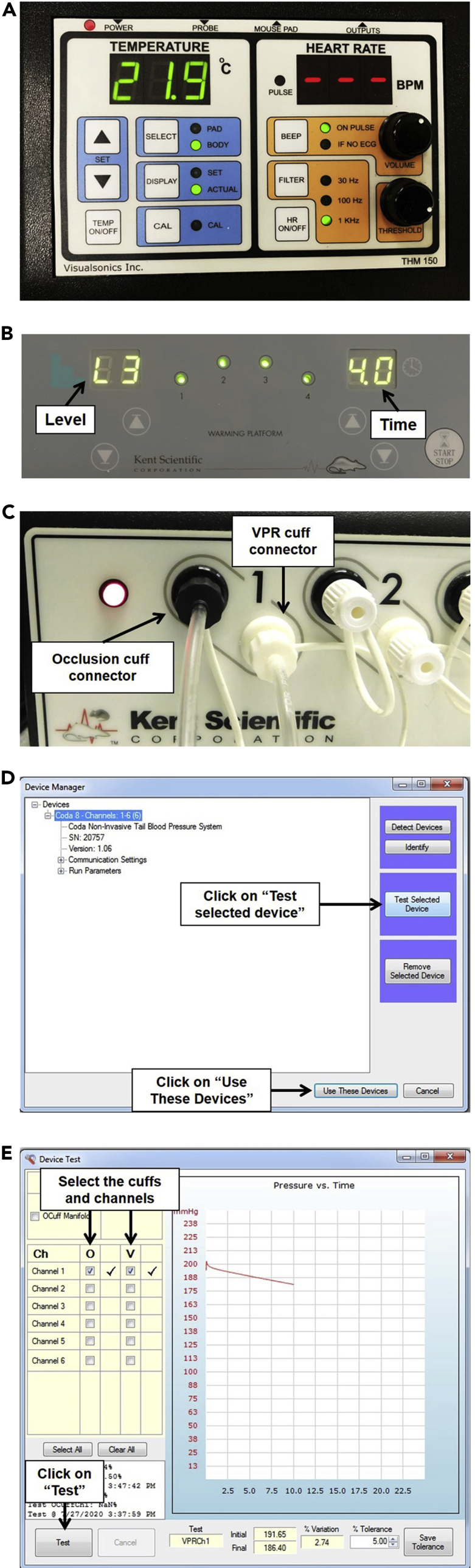
Setup and Parameters for Temperature and Blood Pressure Measurement
(A) Physiological monitor showing the temperature and heart rate readouts.
(B) Animal warming platform of the CODA™ Non-invasive Blood Pressure System showing level and time.
(C) CODA™ High Throughput Controller showing the occlusion cuff connector and VPR cuff connector.
(D) Device Manager window for the CODA™ High Throughput Controller software.
(E) Device Test window showing a representative curve that indicates a passed test.
-
a.Remove the cap from the thermometer (rectal probe).
-
a.
Preparation for Blood Pressure Measurement
Timing: 5 min
-
2.Turn on the animal warming platform.
-
a.Set the level to “3” and the time to “4” (Figure 1B).
-
a.
-
3.
Turn on the computer that is connected to the CODA™ High-Throughput controller.
- 4.
-
5.To test the CODA™ device, open the CODA™ software and configure it as follows:
-
a.Select the CODA™ device (Figure 1D).
-
b.Click on “Test selected device” (Figure 1D).
-
c.Click on “Cuffs and channels” to test them.
-
d.Click “Test.” The cuffs will start inflating and deflating while a curve appears on the screen (Figure 1E).
-
e.Once testing is finished, close the device test window and click on “Use these devices.” If the testing fails, see Troubleshooting: Problem 1.
-
a.
-
6.
Prepare the restrainer that will be used. Due to the larger size of pregnant mice, we recommend using a size #3 restrainer (mouse large).
CRITICAL: The CODA™ High-Throughput system has to be installed in an area of the lab with a room temperature ≥20o C. To avoid stressing the mouse, avoid the use of perfume, over-manipulation, loud noises, and any stimuli that can disturb or irritate the mouse. We recommend preparing several extra occlusion cuffs and VPR cuffs, in case of breakage, so that they can be quickly replaced without spending extra time to reinitiate the experiment.
Preparation for High-Resolution Doppler Ultrasound Measurement
Timing: 5 min
-
7.Turn on the oven to warm up the Aquasonic® ultrasound gel.
-
a.Turn on the oven 30 min before starting the ultrasound procedure to allow time for the gel to reach the desired temperature.
-
b.Set the temperature to 37oC.
-
a.
-
8.Turn on the Vevo® ultrasound machine.
-
a.Check that the MS550D transducer is connected to the ultrasound equipment.
-
a.
-
9.
Check that there is enough oxygen in the oxygen tank to perform the procedure.
-
10.
Check the level of isoflurane in the anesthesia vaporizer. If the level is low, refill with isoflurane before starting the procedure.
-
11.
Check that the tubing for the anesthesia is connected to the “Induction Chamber.”
-
12.
Prepare five pieces of tape of approximately 4 cm in length, each. Tape will be used to attach the mouse and the rectal probe firmly on the heating pad during the ultrasound evaluation.
-
13.
Take approximately 12 non-woven gauze pads and dampen them with sterile deionized water. These will be used to clean the removed fur and the Nair™ cream from the mouse’s abdomen.
CRITICAL: Keeping the Aquasonic® gel warm is critical. When the gel is cold, bubbles can form and interfere with the ultrasound wave, thereby disturbing the image. In addition, the use of warm gel maintains the mouse’s body temperature. As 3D ultrasound is not being performed, we do not recommend using the 3D motor.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6, 8 to 12 weeks old; experiments were performed on 17.5 days post coitum (dpc) | The Jackson laboratory | RRID:IMSR_JAX:000664 |
| Other | ||
| Vevo®2100 Imaging system | VisualSonics Inc. | RRID:SCR_015816 |
| Advanced Physiological Monitoring Unit | VisualSonics Inc. | TMH-150 |
| CODA™ Non-invasive blood pressure system | Kent Scientific | RRID:SCR_018585 |
| Isotec 4 vaporizer | EVAD Co. | N/A |
| Large Mouse Holder | Kent Scientific | HLD-ML |
| Fluriso™ (Isoflurane, USP) | Vetone® | 502017 |
| Aquasonic® clear Ultrasound gel | Parker laboratories, Inc. | BT-025-0039N |
| Aquagel® lubricating gel | Parker laboratories, Inc. | TB-142-0000G |
| Systane® nighttime eye ointment | Alcon Canada INC | DIN 02444062 |
| Nair™ depilatory cream | Church & Dwight Co., Inc. | N/A |
| Gauze (Clinisorb non-woven sponges) | Dukal | 2102 |
| Q-tips® cotton swabs | Unilever | NRSL-2239-06 70506572 |
Alternatives: This protocol has been used with different mouse strains as well as with syngeneic and allogeneic matings. Moreover, we have used this protocol on pregnant mice at gestational ages ranging from 10.5–18.5 dpc; therefore, it can be used throughout pregnancy in different types of mice.
Step-By-Step Method Details
Body Temperature Measurement
Timing: 5 min
Body temperature, one of the vital signs measured in the clinical setting, allows for evaluation of the presence of fever (i.e., elevated body temperature) or hypothermia (i.e., low body temperature). Interestingly, mice do not develop fever when they have systemic infection/inflammation; in fact, their body temperature decreases during these conditions (Copeland et al., 2005). Therefore, by measuring the body temperature we can evaluate the response of mice to different stimuli, indirectly distinguishing between a local and a systemic inflammatory response. In line with this concept, we recently investigated the changes in body temperature among different mouse models of lipopolysaccharide (LPS)-induced preterm birth. We showed that the intra-amniotic injection of LPS induced preterm birth without changing the body temperature, resembling the sub-clinical nature of this condition in pregnant women. However, the intra-peritoneal administration of LPS induced preterm birth accompanied by maternal hypothermia, showing that these models resemble a systemic inflammatory response (Gomez-Lopez et al., 2018). A summary video of the procedure is shown in Methods Video S1.
-
1.
Transport the mouse to the Vevo® imaging station.
-
2.
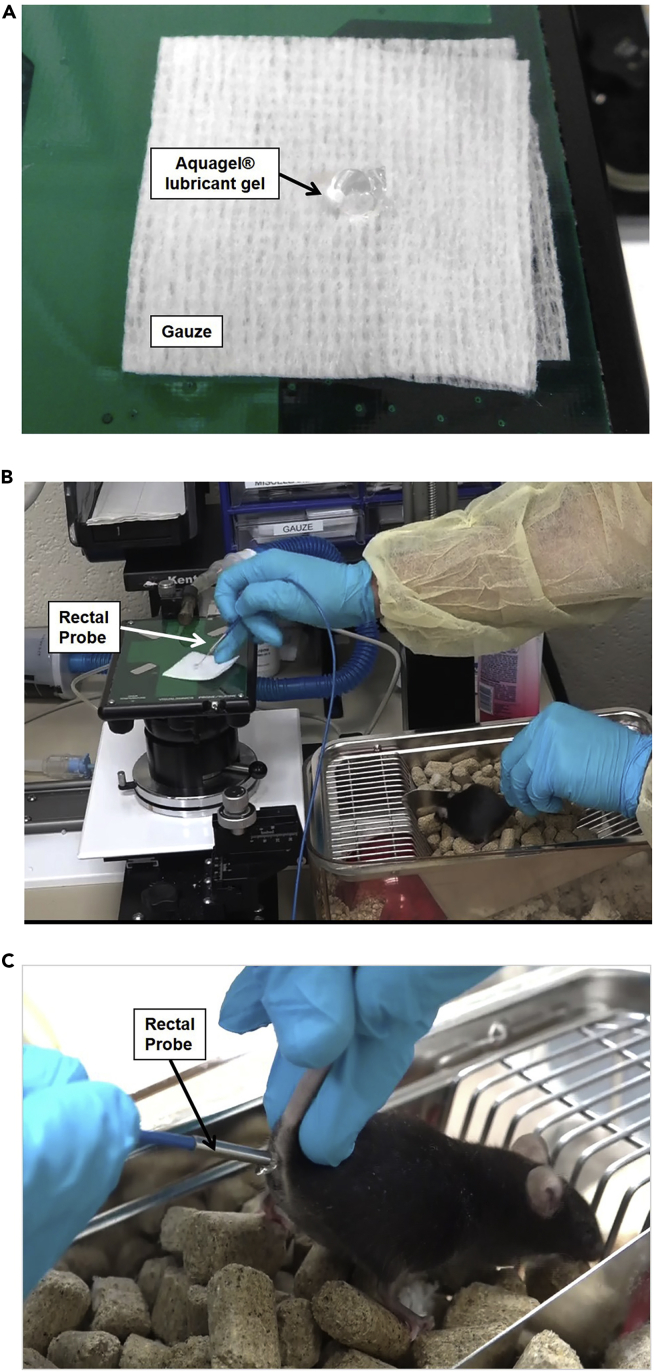
Place a small amount of Aquagel® lubricant gel on a piece of gauze in preparation for the body temperature measurement. The gel will be used once you are holding the mouse (Figure 2A).
Figure 2.
Temperature Measurement Procedure
(A) Application of Aquagel® lubricant gel to the gauze pad.
(B) Dipping the rectal probe in Aquagel® lubricant gel.
(C) Insertion of the rectal probe into the mouse’s anus.
See also Methods Video S1.
-
3.
Take the mouse out of the cage and place it on the wire lid.
-
4.Hold the mouse by the base of its tail, exposing the anus.
-
a.If the mouse begins to defecate (a natural stress response), gently remove any adherent feces with tweezers if needed.
-
a.
-
5.
Take the rectal probe (thermometer) and dip the tip of the probe into the Aquagel® lubricant gel that was previously prepared (Figure 2B).
-
6.
Stimulate the anal sphincter with the tip of the rectal probe and insert the rectal probe into the anus while holding the mouse firmly to avoid movement (Figure 2C).
-
7.
Look at the physiological monitor and record the temperature once it has stabilized.
-
8.
Take the probe out carefully to avoid any damage to the rectum or anus.
-
9.
Place the mouse back in its cage.
-
10.
Clean the rectal probe with disinfectant spray.
CRITICAL: We recommend measuring maternal temperature as the first step of the evaluation of physiological parameters because the body temperature of the mouse changes while under anesthesia. Be very careful to insert the rectal probe into the anus instead of the vagina. If you notice that the mouse is nervous, you can cover it with a napkin to keep it calm and avoid the risk of injury of the mouse
Note: Aquagel® can be stored at room temperature.
Blood Pressure Measurement
Timing: 40 min
One of the greatest physiological changes during pregnancy occurs in the maternal cardiovascular system, resulting in reduced maternal blood pressure and an increased resting pulse (Robson et al., 1989; Clark et al., 1989; Mahendru et al., 2012) due to hormonal and anatomical changes (Wilson et al., 1980; Chapman et al., 1998). On the other hand, around 2%–7% of pregnant women present with pre-eclampsia, a pregnancy complication characterized by hypertension and proteinuria that has deleterious effects on both the mother and the fetus (Redman and Sargent, 2005; Chaiworapongsa et al., 2014). Moreover, during severe inflammatory processes in humans, such as sepsis, maternal blood pressure decreases, causing hypotension (Singer et al., 2016). Therefore, measuring blood pressure in pregnant mice is a useful tool to understand the systemic changes induced by any type of treatment. A video demonstrating the mouse preparation for blood pressure measurement is shown in Methods Video S2.
-
11.Software setup for the CODATM Non-invasive blood pressure system
-
a.Personnel Manager
-
i.Go to “Tools,” then select “Manage Personnel.”
-
ii.In the “Researcher” tab, click on the “+” button and add the Researcher’s name or identifying information as preferred. Additional researchers can be added if needed.
-
iii.Click the “save icon” to save the information.
-
iv.Select the “Technicians Tab” to modify or add a new technician, following the same instructions used for adding a new researcher.
-
v.To select the specimens, go to “Specimens Tab” and click on the “+” button.
-
vi.Add a name for the animal, and then select “Mouse” in the column “type.”
-
vii.Click on the “save icon” to save the information, and then close the “Manage Personnel” window.
-
i.
-
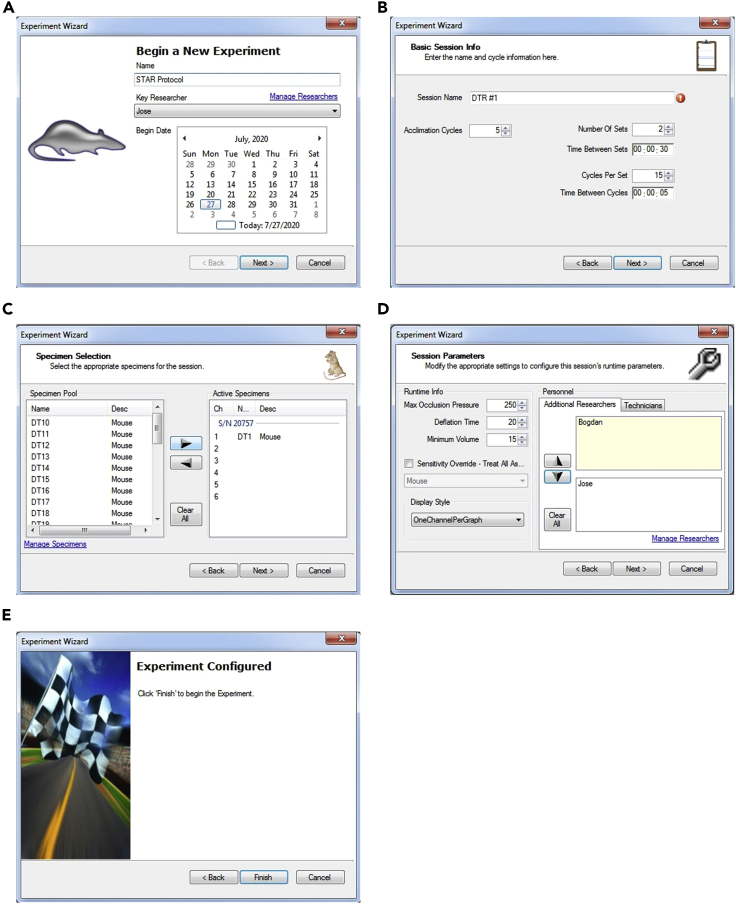
b.Create a new experiment (Figure 3A)
-
a.
Figure 3.
Experiment Setup in CODA™ Software for Blood Pressure Measurement
(A) New Experiment window.
(B) Session Info window.
(C) Specimen Selection window.
(D) Session Parameters window.
(E) Final window showing “Finish” button that will begin the experiment.
-
i.Select “File,” then “New,” then “Experiment.”
-
ii.Enter the name of the experiment.
-
iii.Select a Researcher.
-
iv.Select a Begin Date and click “Next.”
-
i.
-
c.Basic Session Info (Figure 3B)
-
i.Enter a Session name. We recommend using the same name that you are using to identify the mouse of interest. (e.g., DTR #1).
-
ii.Enter the number of acclimation cycles. We recommend selecting 5 acclimation cycles.
-
iii.Enter the number of sets. We recommend selecting 2 sets to improve the chance of successful measurement.
-
iv.Set the time between sets to 30 s.
-
v.Enter the cycles per set. We recommend selecting 15 cycles per set.
-
vi.Set the time between cycles to 5 s.
-
vii.Click “Next” to go to the Specimen Selection.
-
i.
-
d.Specimen Selection (Figure 3C)
-
i.Select the specimen by double-clicking on it, and move it into the “active specimens.” Be careful to add the specimen to the appropriate channel that you are going to use.
-
ii.Click “Next” to go to the Session parameters.
-
i.
-
e.Session Parameters (Figure 3D)
-
i.Select the Max Occlusion Pressure. We recommend selecting “250.”
-
ii.Select the Deflation time. We recommend selecting “20.”
-
iii.Select the Minimum Volume. We recommend selecting “15.”
-
iv.Set the Display Style. We recommend selecting “One Channel Per Graph.”
-
v.If needed, add Additional Researchers or Technicians by double-clicking on them.
-
vi.Click “Next” to go to the Session Script.
-
vii.Check the Session Script to ensure that all the entered parameters are correct, and click “Next.”
-
viii.Do not click “Finish” until the mouse is inside the restrainer and you are ready to perform the experiment (Figure 3E). Once you click “Finish,” the experiment will start.
-
i.
-
12.Animal Setup
-
a.Animal restrainer
-
i.Place the cage containing the mouse next to the CODA™ platform.
-
ii.Open the restrainer, removing the rear hatch. Release the nasal cone in order to have free movement in case the nasal cone needs to be moved while placing the mouse into the restrainer.
-
iii.Hold the mouse by the base of its tail and place it into the restrainer with its head facing toward the nasal cone.
-
iv.Secure the rear hatch, being careful not to pinch the mouse’s tail or any other body parts. Keep its tail in your hand the entire time, do not release it.
-
v.While holding the mouse by the base of its tail, slide the nasal cone backward to limit the mouse’s movements. Secure the nasal cone.
-
vi.Place the restrainer over the warming platform. Keep the mouse on the warming platform for 5 min to acclimate it. We recommend covering the restrainer with a dark cloth to reduce stress to the mouse.
-
i.
-
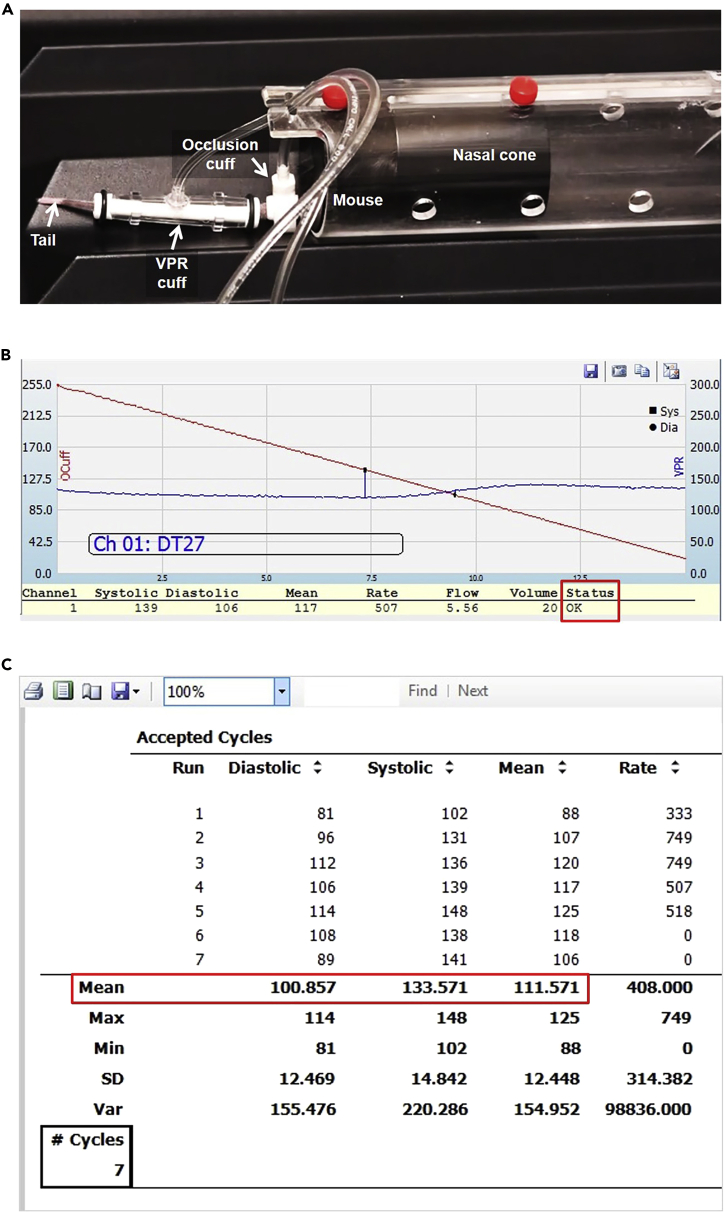
b.Cuff Setting (Figure 4A)
-
a.
Figure 4.
Mouse Setup and Representative Data for Blood Pressure Measurement
(A) Mouse restrainer and cuff setup for blood pressure measurement.
(B) Representative curve indicating an accepted cycle.
(C) Representative report from a successful blood pressure measurement. Red square shows the average Diastolic, Systolic, and Mean measurements.
See also Methods Video S2.
-
i.Place the mouse’s tail into the “Occlusion cuff.” The cuff should be placed as close as possible to the base of the mouse’s tail, without forcing it.
-
ii.Place the mouse’s tail into the “VPR Cuff.” The tail should be placed within 3 mm of the “Occlusion Cuff.”
-
iii.Secure the tubing of both cuffs in the slot located at the top rear of the restrainer.
-
i.
-
13.Experiment
-
a.Start the experiment.
-
i.To start the experiment, click “Finish” in the “Experiment Wizard” window. The experiment will start immediately.
-
ii.Upon starting the experiment, a curve similar to that shown in Figure 4B should appear.
-
i.
-
b.End the experiment
-
i.After ending the experiment, a new window will appear called “Simple session summary report.” This can be closed.
-
ii.Once the experiment is finished, remove the cuffs from the tail and return the mouse to its cage.
-
i.
-
a.
-
14.Data Collection
-
a.After returning the mouse to its cage, click on the “Review an Experiment” icon.
-
b.Select the experiment that you just performed.
-
c.Click on the “Full Report” button.
-
d.Select all of the options and click “Ok.”
-
e.Advance to the summary of the “Accepted Cycles.”
-
f.Record the Mean value of the Diastolic, Systolic, and Mean pressures (Figure 4C).
-
g.Close the software and turn off the machine.
-
a.
CRITICAL: Never leave the mouse unattended. If the cuffs are dislodged, re-adjust them to the correct position. If the mouse is not quiet and is moving its tail inside the restrainer, see Troubleshooting: Problem 2.
High-Resolution Doppler Ultrasound Measurement
Timing: 45 min
High-resolution Doppler ultrasound has emerged as a key tool for evaluating maternal and fetal well-being. In the clinical setting, maternal Doppler ultrasound assessment (i.e., uterine arteries) is used to stratify the risk of developing pre-eclampsia and intra-uterine growth restriction (Martin et al., 2001; Figueras and Gratacos, 2014; Poon et al., 2019; Sotiriadis et al., 2019). Moreover, the umbilical artery Doppler is used to evaluate fetal well-being and to predict deleterious outcomes in a fetus with fetal growth restriction (Yoon et al., 1993; Turan et al., 2008; Tarca et al., 2018; Meyer et al., 2018). In addition, the Doppler wave shape can be used to measure fetal heart rate. A video demonstrating the anesthesia and mouse preparation for ultrasound is shown in Methods Video S3. A video summarizing the high-resolution Doppler ultrasound procedure is shown in Methods Video S4. Representative videos of the maternal and fetal heartbeats are shown in Methods Videos S5 and S6.
-
15.
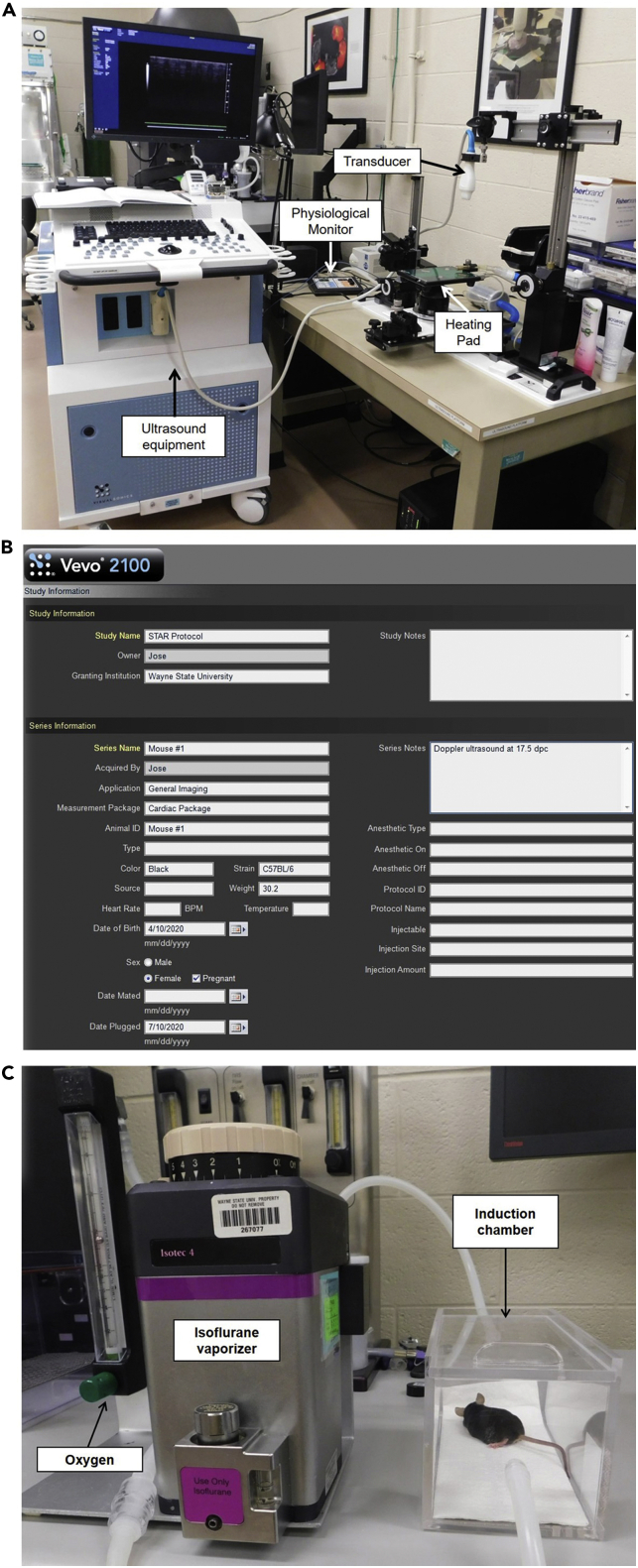
Setting Up the Vevo® Ultrasound software (Figure 5A)
Figure 5.
Ultrasound Station and Experiment Setup
(A) Ultrasound station showing the primary components for ultrasound experiments.
(B) New Study window showing the experiment setup.
(C) Isoflurane vaporizer connected to the anesthesia induction chamber containing the mouse.
See also Methods Video S3.
-
a.New Study
-
i.Once the ultrasound is turned on, click on the “New” button located at the top of the screen.
-
ii.Click on “New Study.”
-
iii.Enter the Study name (e.g., “Star Protocol”) (Figure 5B).
-
iv.Select the “Owner.”
-
v.Optional: Enter the “Grant Institution.”
-
vi.Enter the “Series Name.” We recommend keeping the “Series name” the same as the “Animal ID.”
-
vii.In “Acquired By,” select the person who is going to perform the ultrasound experiment.
-
viii.In “Application,” select “General imaging.”
-
ix.In “Measurement Package,” select “Cardiac Package.”
-
x.In “Animal ID,” copy and paste the name chosen as the “Series Name.”
-
xi.Enter the “Color,” “Strain,” “Weight,” “Date of birth,” and “Sex” of the mouse.
-
xii.Select “Pregnant” and add the “date plugged.”
-
xiii.In “Series note” experimental details or notes can be recorded.
-
xiv.Click on the “Ok” button located in the top-right corner of the screen.
-
xv.If the 3D motor stage is connected, there will be a message asking whether you would like to initialize it. Select “No”.
-
i.
-
a.
-
16.Anesthesia (Figure 5C)
-
a.Turn on the oxygen tank and set the oxygen flow to 2 L/min.
-
b.Turn on the isoflurane and set it to 2%.
-
c.Remove the mouse from its cage and place it into the “Induction chamber.”
-
d.Start a timer immediately after placing the mouse in the “Induction chamber.”
-
e.After 3 min, remove the tubing from the “Induction chamber” and connect it to the tubing for the nasal mask on the heating pad at the ultrasound station.
-
f.Carefully remove the mouse from the “Induction chamber” and move it to the heating pad of the ultrasound station.
-
g.Place the mouse in the supine position (i.e., with the face and torso facing up) and connect the mouse’s snout to the nasal mask on the heating pad. If the mouse starts moving, see Troubleshooting: Problem 3.
-
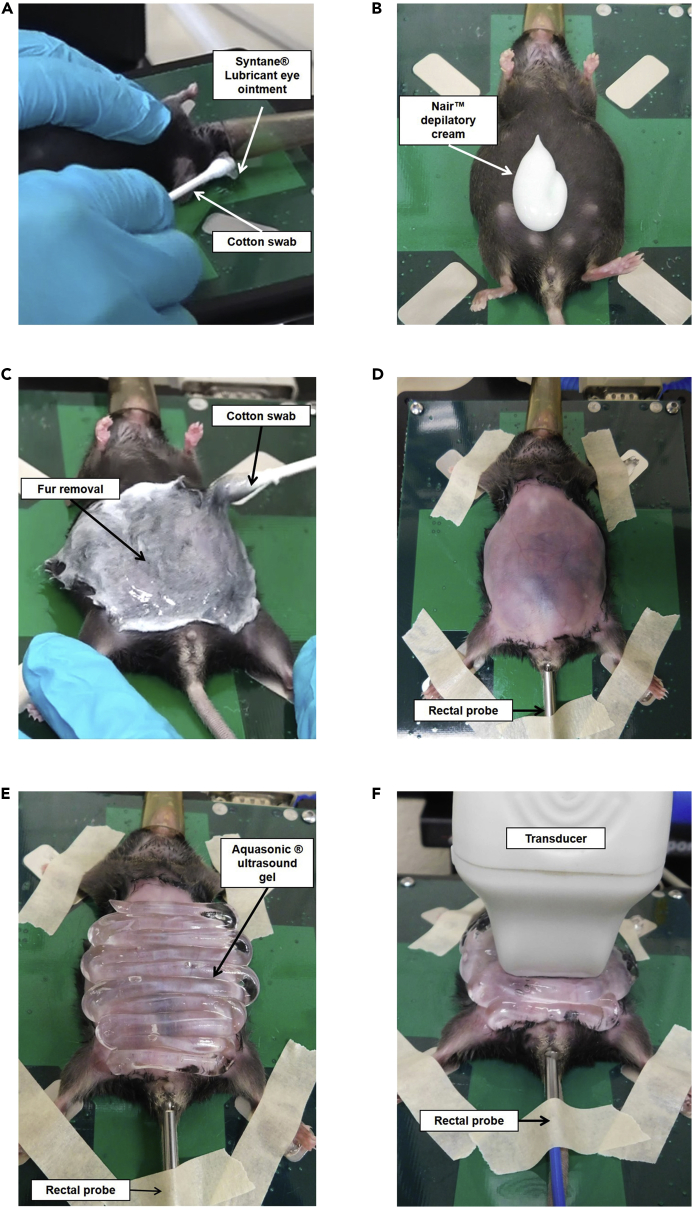
h.Place 1 drop of Syntane® lubricant eye ointment on a cotton swab (approximately 3mm in diameter). Use the swab to place the drop in one of the mouse’s eyes, and then repeat this step for the other eye (Figure 6A).
-
a.
Figure 6.
Mouse Preparation and Transducer Positioning Prior to Ultrasound Measurement
(A) Application of eye lubricant to mouse.
(B) Application of depilatory cream to mouse abdomen.
(C) Spreading of depilatory cream over mouse abdomen.
(D) Mouse abdomen after hair removal with taped paws and rectal probe inserted.
(E) Application of ultrasound gel on mouse abdomen.
(F) Transverse positioning of the transducer over the mouse abdomen prior to ultrasound measurement.
See also Methods Videos S3 and S4.
-
17.Fur Removal
-
a.Apply Nair™ depilatory cream to the mouse’s abdomen (Figure 6B).
-
b.Spread the cream from the pubis to the ribs of the mouse using circular movements with a clean cotton swab (Figure 6C).
-
c.Continue massaging with circular movements for approximately 3 min.
-
d.Using the same cotton swab, remove as much fur as possible.
-
e.Take a piece of dampened gauze and start removing the fur and the cream. Use as many gauze pads as needed to remove all of the Nair™ cream.
-
f.In case there are still areas of fur that were not removed by the initial application of Nair™ cream, more Nair™ cream can be applied locally in these spots. All of the abdominal fur must be removed prior to performing the ultrasound (Figure 6D).
-
a.
CRITICAL: Nair® cream can irritate the skin of the mouse, even causing chemical burns. Therefore, it is imperative to remove the cream completely with the dampened gauze. Avoid applying Nair® cream to the genital area of the mouse because irritation may occur and be a confounding variable for the experiment.
-
18.Fixation of the mouse and insertion of the rectal probe (Figure 6D).
-
a.Remove the Aquasonic® ultrasound gel from the oven and place a drop of gel on each of the electrodes of the heating pad.
-
b.Take each of the mouse’s paws and secure them on the electrodes of the heating pad using the tape that was previously prepared.
-
c.Place a drop of the Aquagel® lubricating gel on a napkin and spread the gel on the tip of the rectal probe (thermometer) before inserting the rectal probe into the anus of the mouse.
-
d.Hold the tail and insert the rectal probe in the anus of the mouse.
-
e.Secure the rectal probe to the heating pad using the last piece of tape that was previously prepared.
-
a.
-
19.General ultrasound scan (B-mode)
-
a.Take the Aquasonic® ultrasound gel out of the oven and shake it vigorously to move all of the gel toward the nozzle to avoid creating bubbles.
-
b.Cover the mouse’s abdomen with the Aquasonic® ultrasound gel (Figure 6E). Avoid creating bubbles because they can distort the image.
-
c.The transducer should be oriented transverse to the abdomen and positioned directly over the lower part of the abdomen (Figure 6F). If the ultrasound image is disturbed/not clear, see Troubleshooting: Problem 4.
-
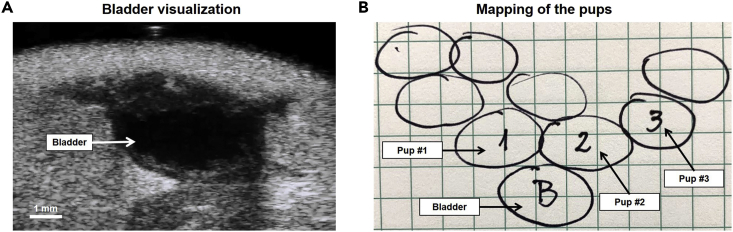
d.Count and determine the localization of the pups (Figures 7A&B).
-
i.First, localize the maternal bladder. It is usually located in the midline and is recognized as a non-echogenic round structure. Its size will vary according to the presence of urine. This organ is going to be our reference point for localizing and mapping the pups (Figure 7A).
-
i.
-
a.
Figure 7.
Representative Maternal Bladder Imaging and Mapping of Pups
(A) Representative ultrasound image showing the maternal bladder from the transverse view.
(B) Example of drawn map showing the location of each pup relative to the maternal bladder.
See also Methods Video S4.
-
ii.Once the bladder is identified, start scanning the abdominal midline of the mouse from the bladder to the sternum. We recommend drawing the position of the pups immediately after identifying them to document the correct number of pups as well as the correct location of each.
-
iii.Once you finish scanning the midline, move the transducer back to the bladder (reference point). Then, using the bar located under the stage, tilt the heating pad to orient the right side of the mouse higher than the left side.
-
iv.With the mouse tilted, start scanning from the bottom to the upper part of the right side, counting and localizing the pups on this side.
-
v.Repeat the previous two steps for the left side of the mouse.
-
vi.Upon finishing the general scan, you should have a map similar to the one shown in Figure 7B showing the distribution of each pup.
-
ii.
-
e.Umbilical Artery Doppler Ultrasound
-
i.To obtain representative measurements and to avoid keeping the mouse under anesthesia for too long, we performed the measurements in three pups per dam. We included the first pup in each uterine horn plus one of the pups that is easier to measure according to its position.
-
ii.After you localize the pup to measure, scan its abdominal wall as well as the placenta to identify the position of the umbilical cord.
-
iii.Press the “Color” button to activate the color Doppler and to better identify the umbilical cord.
-
iv.You can adjust the size of the square frame of the color Doppler to make it larger or smaller by pressing the “Update” button and simultaneously moving the scroll ball.
-
v.Adjust the “Velocity” button to optimize the vessel image.
-
vi.Adjust the “Doppler Gain” button to optimize the vessel image.
-
vii.The umbilical artery is the vessel that has a pulsatile image.
-
viii.You can use the “Image depth” button as well as the “Zoom” button to better localize the umbilical cord. To obtain an optimal measurement, focus on the area of the umbilical cord between the placenta and the abdominal wall of the pup.
-
ix.Press the “PW” button to activate the pulsed-wave Doppler.
-
x.Using the scroll ball, move the cursor to place it at the center of the umbilical cord. Adjust the size of the cursor by moving the “SV/Gate” up or down. The cursor has to be inside of the umbilical artery. If not, the measurement is going to include the umbilical vein, distorting the wave shape.
-
xi.Adjust the angle of insonation by turning the “PW angle” button. The angle has to be as close to 0o as possible. An angle <30o is acceptable.
-
xii.Press the “PW” button again to see the wave of the umbilical artery.
-
xiii.If the entire wave is not seen, the baseline can be moved down using the “baseline” button.
-
xiv.If the wave is inverted, its direction can be modified by pressing the “Invert” button.
-
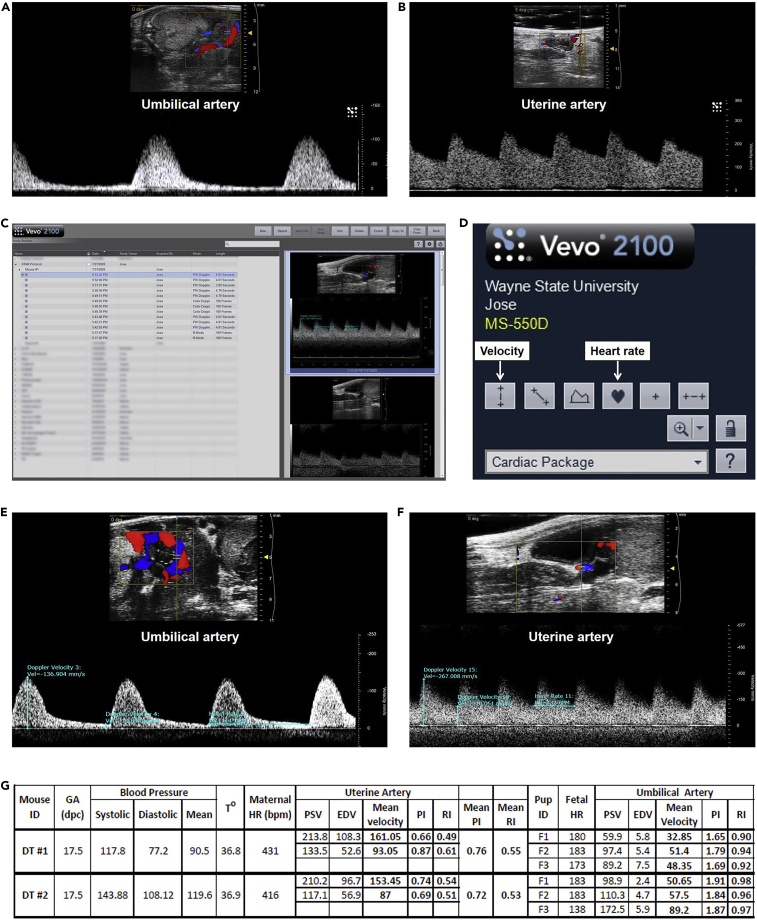
xv.Once an adequate wave shape is obtained, press the “Cine Store” button to save a mini-video of the measurement. The typical wave shape of the umbilical artery is shown in Figure 8A.
-
i.
Figure 8.
Umbilical and Uterine Artery Measurement
(A) Representative image of the typical wave shape of the umbilical artery.
(B) Representative image of the typical wave shape of the uterine artery.
(C) Study Management window for selecting ultrasound videos to measure velocity and heart rate.
(D) User interface showing the icons for measuring velocity and heart rate.
(E) Representative measurement of the umbilical artery peak systolic velocity, end diastolic velocity, and fetal heart rate.
(F) Representative measurement of the uterine artery peak systolic velocity, end diastolic velocity, and maternal heart rate.
(G) Representative data output from maternal-fetal evaluation experiment. GA, Gestational Age; HR, Heart Rate; PSV, Peak Systolic Velocity; EDV, End Diastolic Velocity; PI, Pulsatility Index; RI, Resistance Index.
See also Methods Videos S5 and S6.
-
xvi.Press the “Study Management” button to double check that the video was recorded and tag the video with the number of the pup. Do not perform the data analysis while the mouse is under anesthesia (This step will be performed later).
-
xvii.To tag the video, double click within the video frame.
-
xviii.Press the “Cine Loop Review” button.
-
xix.Press the “Annotate” button.
-
xx.Enter an ID for the pup (e.g., #1) and press “Enter.” To go back to the ultrasound scan screen, click the “Scan/Freeze” button.
-
xxi.Repeat the same procedure for each of the three pups.
-
xvi.
-
f.Uterine artery Doppler ultrasound
-
i.Apply the Aquasonic® ultrasound gel to the lower part of the abdomen, mainly to the suprapubic area.
-
ii.Rotate the ultrasound transducer 90o (oriented toward the sagittal axis of the mouse) and place it on the bladder.
-
iii.To measure both uterine arteries (right and left), first localize the arteries by activating the “color” Doppler.
-
iv.Move the transducer slowly from the middle line to the right side. The uterine artery is a curve-shaped artery that has a high velocity flow; therefore, the color that you will see varies between red, yellow, and blue (i.e., aliasing).
-
v.Once you localize the artery that seems to correspond to the uterine artery, press the “PW” button. The uterine artery is characterized by having a velocity of >100 cm/s. If the artery has a velocity <100 cm/s, it is likely not the uterine artery. Therefore, continue moving the transducer until the uterine artery is found.
-
vi.Once an optimal wave is obtained, press the “Cine Store” button. The typical wave shape of the uterine artery is shown in Figure 8B.
-
vii.Press the “Study management” button to double-check that the video was recorded and to identify the artery that was measured, following the same steps used for the identification of the umbilical artery.
-
viii.Repeat the same procedure in the left uterine artery.
-
i.
-
g.Completing the animal experiment
-
i.After obtaining the measurement of the left uterine artery, lift the transducer up.
-
ii.Wipe off all of the Aquasonic® ultrasound gel from the mouse with the dampened gauze.
-
iii.Remove the tape from the rectal probe (thermometer) and carefully remove the rectal probe from the rectum.
-
iv.Remove the tape from the paws of the mouse and clean them off.
-
v.Depending on the aim of the study, you can leave the mouse in a recovery cage with a heating lamp. It should be recovered in about 5–10 min after removal from anesthesia. On the other hand, if the mouse will not be used to observe pregnancy or neonatal outcomes, it can be euthanized by cervical dislocation while still unconscious from anesthesia.
-
i.
-
h.Turning off the anesthesia and oxygen and cleaning the machine
-
i.Immediately after removing the mouse from the nasal mask, stop the timer and record the length of the experiment.
-
ii.Turn the Isoflurane to 0% and the oxygen to 0 L/min.
-
iii.Turn off the oxygen tank.
-
iv.While the oxygen tank is off, turn the oxygen to 10 L/min to flush the rest of the oxygen from the system.
-
v.Remove the isoflurane tubing from the nasal mask back and connect it back to the “induction chamber.”
-
vi.Clean all of the gel from the heating pad as well as the ultrasound transducer.
-
vii.Disinfect the thermometer, heating pad, and probe with disinfectant spray.
-
i.
-
i.Analysis of umbilical artery and fetal heart rate
-
i.Go to “Study management” (Figure 8C).
-
ii.Select the video of the first umbilical artery measured by double-clicking on it (Figure 8C).
-
iii.The video will start playing immediately. Stop the video in the desired section by pressing the “Cine Loop Review” button. You can alter the loop by adjusting the same “Cine Loop Review” button.
-
iv.Press the “Measure” button.
-
v.To measure the peak systolic velocity, click on the “Velocity” button (Figure 8D).
-
vi.Place the cursor on the wave crest and left click (select). This number corresponds to the peak systolic velocity of the umbilical artery (Figure 8E).
-
vii.Click on the “Velocity” button again to measure the end diastolic velocity. Place the cursor on the wave trough. This number corresponds to the end diastolic velocity of the umbilical artery (Figure 8E).
-
viii.To measure the fetal heart, click on the “Heart Rate” button. Place the cursor at the beginning of one of the waves and left click (select) (Figure 8D).
-
ix.Move the cursor to the beginning of the next wave and right click “Update.” This number corresponds to the fetal heart rate (Figure 8E).
-
x.Repeat the same procedure for each of the three pups measured.
-
xi.Record all of the data for further analysis.
-
i.
-
j.Analysis of the uterine artery and maternal heart rate
-
i.Go to “Study management.”
-
ii.Select the video of the right uterine artery by double-clicking on it.
-
iii.Repeat the same procedures used to analyze the umbilical artery (Figure 8F).
-
i.
Expected Outcomes
The individual maternal-fetal parameters measured herein are highly variable and can differ with mouse strain, gestational age, treatment, and other factors; thus, each technique should be independently standardized and validated to determine baseline ranges. In our studies, we have observed normal body temperature readings that fall within a range of 36°C–37°C, as observed in Methods Video S1. For blood pressure, we have observed the following ranges in the control pregnant mice: systolic (144.9±14.4 mmHg), diastolic (98.7±14.2 mmHg), and mean (113.7±13.3 mmHg) (Gomez-Lopez et al., 2020), as shown in Figure 4C. The maternal heart rate has been typically observed to be between 400–500 bpm in our studies, and a representative maternal heartbeat is demonstrated in Methods Video S5. We previously reported a mean fetal heart rate of 188.3±27.9 bpm (Gomez-Lopez et al., 2020), and a representative fetal heartbeat is demonstrated in Methods Video S6. The lowest gestational age in which we have measured fetal parameters by high-resolution ultrasound was 10 dpc.
In addition, the results of each physiological parameter will further depend on the molecules or pathologies that were investigated. For example, we have shown that: i) the expansion of activated CD1d-restricted invariant NKT (iNKT) cells by administration of α-galactosylceramide (α-GalCer) in pregnant mice induced late preterm birth and reduced fetal heart rate (i.e., bradycardia) compared to controls. In addition, α-GalCer did not modify the umbilical artery pulsatility index compared to controls (St Louis et al., 2016); ii) the intra-peritoneal administration of human chorionic gonadotrophin (hCG) in pregnant mice induced a reduction in the umbilical artery pulsatility index as well as an increase in the fetal heart rate compared to the control group (Furcron et al., 2016); iii) intra-peritoneal and intra-uterine injection of LPS induced preterm birth with a decrease in body temperature, while intra-amniotic injection of LPS induced preterm birth without altering the body temperature (i.e., hypothermia), resembling the sub-clinical nature of the latter condition in humans (Gomez-Lopez et al., 2018); iv) in vivo T cell activation by intra-peritoneal administration of anti-CD3ε induced preterm birth accompanied by hypothermia and maternal bradycardia. Moreover, consistent with our previous study, intra-peritoneal administration of LPS induced preterm birth and hypothermia without modifying the maternal heart rate. On the other hand, the administration of RU486 (i.e., mifepristone), a non-inflammatory model of preterm birth, induced preterm birth in the absence of changes in the body temperature or maternal heart rate (Arenas-Hernandez et al., 2019); and v) total regulatory T cell (Treg) depletion in pregnant mice induced fetal bradycardia without alterations in the umbilical artery pulsatility index. Moreover, Treg-depleted mice did not undergo changes in body temperature, mean blood pressure, or maternal heart rate. The uterine artery pulsatility index decreased in mice with Treg depletion compared to controls (Gomez-Lopez et al., 2020). Therefore, the determination of maternal-fetal medicine parameters allows for a translational assessment of well-being in the mother and the fetus.
Quantification and Statistical Analysis
To evaluate the umbilical and uterine arteries, use the following parameters:
Note: The “Mean velocity” is the average of the Peak systolic velocity and the End diastolic velocity. In the clinical setting, the average of the measurements from the right and left uterine arteries of the same patient is used. Thus, we report the average from both uterine arteries of the same mouse. An example of the tabulation of the results is shown in Figure 8G. The statistical analyses for showing the results obtained with this protocol will depend on the study groups in which these parameters will be determined.
Limitations
A possible limitation of our approach is the evaluation of only three representative pups instead of all of the pups in each dam. However, our decision to evaluate three pups is based on a representative number, considering that each dam has 6–8 pups. As the litter size could affect the parameters evaluated in this protocol, we recommend not including animals having less than four pups. Moreover, we always measure the first pup in each uterine horn plus one of the nearest pups to avoid possible effects caused by the position of the pups. Furthermore, the effects of prolonged exposure to anesthesia on hemodynamic parameters are unknown, yet could cause stress and deleterious effects to the dam and her pups. In addition, the protocol described herein is specific to the instruments listed above, and new standardization should be performed for different instruments.
Troubleshooting
Problem 1
CODA™ Device testing failed (Before You Begin, step 5).
Potential Solution
Be sure that the cuffs are well connected to the CODA™ controller. Sometimes the rubber gasket of the cuffs is broken. If so, you can replace the whole cuff or change the rubber gasket using the CODA™ High-Throughput Mouse Cuff kit available from Kent Scientific (MSE-CUFFKIT).
Problem 2
The mouse is moving its tail inside the restrainer (Blood pressure measurement, step 12).
Potential Solution
The nasal cone is probably not secure or has been released. Move the nasal cone back and forth until it is tight and secure it to reduce the mouse’s movements. Make sure the mouse is breathing well.
Problem 3
After the induction of anesthesia, the mouse starts moving on the heating pad (High-resolution Doppler ultrasound measurement, step 16).
Potential Solution
Some mice are less susceptible to anesthesia induction; therefore, be sure to keep the snout inside the nasal cone. However, if the mouse continues moving and wakes up, put it back into the induction chamber for 1 min. Be sure that the tubing is well connected to the induction chamber and, after 1 min of anesthesia induction, change the anesthesia tubing to the nasal cone tubing while simultaneously transferring the mouse back to the heating pad. In addition, it may be possible that the mouse’s snout has dislodged from the nasal mask. Please ensure that the mouse’s snout is properly positioned in the nasal mask and that the tubes are not twisted or bent.
Problem 4
The ultrasound image is disturbed/not clear (High-resolution Doppler ultrasound measurement, step 19).
Potential Solution
The most plausible explanation for this problem is the presence of bubbles between the transducer and the skin. Use a new cotton swab to smooth the gel and remove the bubbles. Remember that there must always be a layer of gel between the part of the skin that you are evaluating and the transducer.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Nardhy Gomez-Lopez (nardhy.gomez-lopez@wayne.edu).
Materials Availability
This protocol does not include any unique reagents.
Data and Code Availability
This protocol does not include any code or dataset.
Acknowledgments
This research was supported in part by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and in part with federal funds from NICHD/NIH/DHHS under contract HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. R.R. contributed to this work as part of his official duties as an employee of the U.S. federal government. The authors gratefully acknowledge Derek Miller and Meyer Gershater for their critical readings of the manuscript.
Author Contributions
Conceptualization, R.R., and N.G.-L.; Methodology, J.G., M.A.-H., and V.G.-F., Validation, J.G., M.A.-H., and B.P.; Investigation, J.G., M.A.-H., B.P., and N.G.-L.; Resources, R.R.; Writing – Original Draft, J.G. and N.G.-L.; Writing – Review & Editing, J.G., R.R., M.A.-H., B.P., V.G.-F., and N.G.-L.; Visualization, J.G., B.P., V.G.-F., and N.G.-L.; Funding Acquisition, R.R. and N.G.-L.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100134.
Contributor Information
Roberto Romero, Email: prbchiefstaff@med.wayne.edu.
Nardhy Gomez-Lopez, Email: nardhy.gomez-lopez@wayne.edu.
References
- Arenas-Hernandez M., Romero R., Xu Y., Panaitescu B., Garcia-Flores V., Miller D., Ahn H., Done B., Hassan S.S., Hsu C.D. Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J. Immunol. 2019;202:2585–2608. doi: 10.4049/jimmunol.1801350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens I., Puttemans P., Benagiano G. Placental bed research: I. The placental bed: from spiral arteries remodeling to the great obstetrical syndromes. Am. J. Obstet. Gynecol. 2019;221:437–456. doi: 10.1016/j.ajog.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Chaiworapongsa T., Chaemsaithong P., Yeo L., Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 2014;10:466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A.B., Abraham W.T., Zamudio S., Coffin C., Merouani A., Young D., Johnson A., Osorio F., Goldberg C., Moore L.G. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- Clark S.L., Cotton D.B., Lee W., Bishop C., Hill T., Southwick J., Pivarnik J., Spillman T., Devore G.R., Phelan J. Central hemodynamic assessment of normal term pregnancy. Am. J. Obstet. Gynecol. 1989;161:1439–1442. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- Copeland S., Warren H.S., Lowry S.F., Calvano S.E., Remick D., Inflammation & The Host Response To Injury Investigartors Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Renzo G.C. The great obstetrical syndromes. J. Matern. Fetal Neonatal. Med. 2009;22:633–635. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- Figueras F., Gratacos E. Update on the diagnosis and classification of fetal growth restriction and proposal of a stage-based management protocol. Fetal Diagn. Ther. 2014;36:86–98. doi: 10.1159/000357592. [DOI] [PubMed] [Google Scholar]
- Furcron A.E., Romero R., Mial T.N., Balancio A., Panaitescu B., Hassan S.S., Sahi A., Nord C., Gomez-Lopez N. Human chorionic gonadotropin has anti-inflammatory effects at the maternal-fetal interface and prevents endotoxin-induced preterm birth, but causes dystocia and fetal compromise in mice. Biol. Reprod. 2016;94:136. doi: 10.1095/biolreprod.116.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N., Arenas-Hernandez M., Romero R., Miller D., Garcia-Flores V., Leng Y., Xu Y., Galaz J., Hassan S.S., Hsu C.D. Regulatory T cells play a role in a subset of idiopathic preterm labor/birth and adverse neonatal outcomes. Cell Rep. 2020;32:107874. doi: 10.1016/j.celrep.2020.107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N., Romero R., Arenas-Hernandez M., Panaitescu B., Garcia-Flores V., Mial T.N., Sahi A., Hassan S.S. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J. Matern. Fetal Neonatal. Med. 2018;31:439–446. doi: 10.1080/14767058.2017.1287894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L.K., Benagiano M., D'elios M.M., Brosens I., Benagiano G. Placental bed research: II. Functional and immunological investigations of the placental bed. Am. J. Obstet. Gynecol. 2019;221:457–469. doi: 10.1016/j.ajog.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Mahendru A.A., Everett T.R., Wilkinson I.B., Lees C.C., McEniery C.M. Maternal cardiovascular changes from pre-pregnancy to very early pregnancy. J. Hypertens. 2012;30:2168–2172. doi: 10.1097/HJH.0b013e3283588189. [DOI] [PubMed] [Google Scholar]
- Martin A.M., Bindra R., Curcio P., Cicero S., Nicolaides K.H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11-14 weeks of gestation. Ultrasound Obstet. Gynecol. 2001;18:583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- Meyer N., Schuler T., Zenclussen A.C. High frequency ultrasound for the analysis of fetal and placental development in vivo. J. Vis. Exp. 2018 doi: 10.3791/58616. [DOI] [PubMed] [Google Scholar]
- Poon L.C., Shennan A., Hyett J.A., Kapur A., Hadar E., Divakar H., Mcauliffe F., Da Silva Costa F., Von Dadelszen P. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019;145(Suppl 1):1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C.W., Sargent I.L. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- Robson S.C., Hunter S., Boys R.J., Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am. J. Physiol. 1989;256:H1060–H1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- Romero R. Prenatal medicine: the child is the father of the man. 1996. J. Matern. Fetal Neonatal. Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriadis A., Hernandez-Andrade E., Da Silva Costa F., Ghi T., Glanc P., Khalil A., Martins W.P., Odibo A.O., Papageorghiou A.T., Salomon L.J. ISUOG Practice Guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet. Gynecol. 2019;53:7–22. doi: 10.1002/uog.20105. [DOI] [PubMed] [Google Scholar]
- St Louis D., Romero R., Plazyo O., Arenas-Hernandez M., Panaitescu B., Xu Y., Milovic T., Xu Z., Bhatti G., Mi Q.S. Invariant NKT cell activation induces late preterm birth that is attenuated by rosiglitazone. J. Immunol. 2016;196:1044–1059. doi: 10.4049/jimmunol.1501962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarca A.L., Romero R., Gudicha D.W., Erez O., Hernandez-Andrade E., Yeo L., Bhatti G., Pacora P., Maymon E., Hassan S.S. A new customized fetal growth standard for African American women: the PRB/NICHD Detroit study. Am. J. Obstet. Gynecol. 2018;218:S679–S691 e4. doi: 10.1016/j.ajog.2017.12.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan O.M., Turan S., Gungor S., Berg C., Moyano D., Gembruch U., Nicolaides K.H., Harman C.R., Baschat A.A. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008;32:160–167. doi: 10.1002/uog.5386. [DOI] [PubMed] [Google Scholar]
- Wilson M., Morganti A.A., Zervoudakis I., Letcher R.L., Romney B.M., Von Oeyon P., Papera S., Sealey J.E., Laragh J.H. Blood pressure, the renin-aldosterone system and sex steroids throughout normal pregnancy. Am. J. Med. 1980;68:97–104. doi: 10.1016/0002-9343(80)90178-3. [DOI] [PubMed] [Google Scholar]
- Yoon B.H., Romero R., Roh C.R., Kim S.H., Ager J.W., Syn H.C., Cotton D., Kim S.W. Relationship between the fetal biophysical profile score, umbilical artery Doppler velocimetry, and fetal blood acid-base status determined by cordocentesis. Am. J. Obstet. Gynecol. 1993;169:1586–1594. doi: 10.1016/0002-9378(93)90441-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This protocol does not include any code or dataset.