Abstract
Objective
The coronavirus disease of 2019 (COVID-19) due to SARS-CoV-2 infection has been found to cause an increased risk of venous thrombo-embolism (VTE). The aims of the study were to determine the frequency of VTE in critically ill patients with COVID-19 and its correlation with D dimer levels and pharmacological prophylaxis.
Methods
This was a cohort study of critically ill patients due to COVID-19. All patients admitted to the intensive care unit on the same day of April 2020 were selected, regardless of length of stay, and a single bilateral venous duplex ultrasound in the lower extremities was performed up to 72 hours later. Pulmonary embolism (PE) was diagnosed by computed tomography angiography. Asymptomatic and symptomatic VTE were registered, including pre-screening in hospital VTE. Characteristics of patients, blood test results, doses of thromboprophylaxis received, VTE events, and mortality after seven day follow up were recorded.
Results
A total of 230 critically ill patients were studied. The median intensive care unit stay of these patients was 12 days (interquartile range [IQR] 5 – 19 days). After seven days follow up, the frequency of patients with VTE, both symptomatic and asymptomatic, was 26.5% (95% confidence interval [CI] 21% – 32%) (69 events in 61 patients): 45 with DVT and 16 with PE (eight of them with concomitant DVT). The cumulative frequency of symptomatic VTE was 8.3% (95% CI 4.7% – 11.8%). D dimer values ≥ 1 500 ng/mL were diagnostic of VTE, with a sensitivity of 80% and a specificity of 42%. During follow up after screening, six patients developed new VTE. Three of them developed a recurrence after a DVT diagnosed at screening, despite receiving therapeutic doses of heparin. Mortality rates at seven day follow up were the same for those with (6.6%) and without (5.3%) VTE.
Conclusion
Patients with severe COVID-19 infection are at high risk of VTE, and further new symptomatic VTE events and recurrence can occur despite anticoagulation. The prophylactic anticoagulant dose may need to be increased in patients with a low risk of bleeding.
Keywords: COVID-19, Deep vein thrombosis, SARS-CoV-2 infection, Pulmonary embolism, Venous thromboembolism
What this paper adds.
In a large series of hospitalised patients with COVID-19, it is confirmed that they are at a high risk of venous thrombo-embolism (VTE). Routine screening and early anticoagulation for deep vein thrombosis did not prevent further symptomatic VTE events. The data suggest a potential benefit from increasing the dose of thromboprophylactic anticoagulation for these patients.
Introduction
The coronavirus disease of 2019 (COVID-19) is a viral illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2), now deemed a pandemic by the World Health Organization.1 Preliminary reports suggest that haemostatic abnormalities, including disseminated intravascular coagulation, may occur in patients infected by COVID-19.2, 3, 4 Additionally, critical illness and immobilisation may predispose hospitalised patients with COVID-19 to develop venous thrombo-embolism (VTE).5, 6, 7 In two recent studies, one in every four patients (25% and 27%) with proven COVID-19 pneumonia admitted to intensive care units (ICUs) developed symptomatic, confirmed VTE.8 , 9 In another study, 26 consecutive patients with severe COVID-19 were screened for deep vein thrombosis (DVT) by duplex ultrasound (DUS), and 69% were positive.10 The authors suggested considering systematic screening for VTE and using high dose VTE prophylaxis in severe ICU COVID-19 patients.
It is hypothesised that a single bilateral DUS looking for DVT signs in hospitalised patients with proven COVID-19 pneumonia might detect DVT before the development of pulmonary embolism (PE). Thus, in this study all patients were screened for DVT who had been admitted on a specific day to the ICU with COVID-19 pneumonia with the aim of assessing (1) the frequency of DVT and its correlation with D dimer levels; (2) the influence of pharmacological prophylaxis after ICU admission on the frequency of VTE; and (3) the impact on outcome of a single ultrasound screening to detect asymptomatic DVT.
Material and methods
Patients
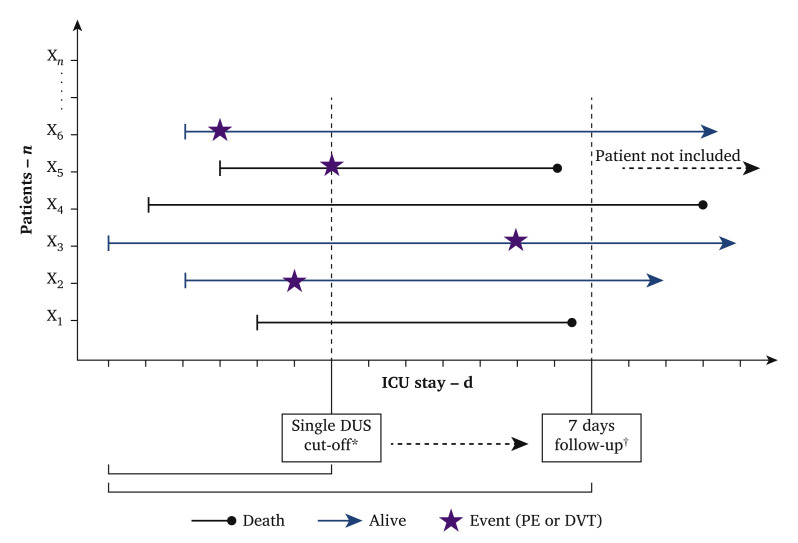
This was a cohort study of patients with COVID-19 admitted to the ICUs of two Spanish university hospitals: Hospital Universitari Vall d’Hebron (HUVH), in Barcelona, and Hospital Universitari Germans Trias i Pujol (HUGTiP), in Badalona. All patients had confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection confirmed by a positive result on polymerase chain reaction testing of a nasopharyngeal sample. The study consisted of a single ultrasound scan for all hospitalised patients in the ICU on the same day of April 2020, regardless of the length of their stay. Due to the complexity of the examinations in patients admitted to the ICU, ultrasound scans were distributed between the day of selection and up to 72 hours later. The screening ultrasound was not repeated in order to avoid unnecessary exposure to the investigators due to the unknown risk of infection and, furthermore, personal protective equipment for research could not be used much at a time when it was scarce for medical staff. Exclusion criteria were lack of a confirmed diagnosis of COVID-19, current therapy with extracorporeal membrane oxygenation, pregnancy, or postpartum, and age younger than 18 years old. The protocol was approved by the Ethics Committee of Hospital Vall d’Hebron (number PR(AG)213/2020) on 6 April 2020, and due to the design of the study and the emergency created by the disease, informed consent was considered not necessary. This study was registered in Clinicaltrials.gov (NCT: 04374617). Fig. 1 represents a diagram of the study design with an explanatory example.
Figure 1.
Outline of study design and explanatory example of critically ill patients due to COVID-19 treated in the intensive care unit (ICU) and studied for deep venous thrombosis (DVT), pulmonary embolism (PE) and mortality. ∗Single duplex ultrasound (DUS) cut off frequency = proportion of patients hospitalised in ICU and diagnosed with venous thrombo-embolism (VTE) (i.e., DVT or PE) on the day of the single DUS cut off or before (in this example three events/six patients = 50%). †Seven day follow up frequency = proportion of patients hospitalised in ICU and diagnosed with VTE at or before seven days after the single DUS cut off day.
Study design
Four certified vascular surgeons (two in each centre) performed a single bilateral lower limb DUS, including the femoral, popliteal, and distal veins of all patients. Femoral veins of patients placed in the prone position could not be evaluated. A DVT diagnosis was considered when it was not possible to fully compress a venous segment, in the absence of flow augmentation with calf squeeze or hyperechoic intraluminal defect partially or fully occluding the venous segment. The iliac veins were evaluated in the same way whenever possible. When patient characteristics did not allow it (due to obesity or gas), the iliac veins were evaluated indirectly, interpreting venous Doppler flow phasicity in the common femoral vein bilaterally.
Limb DVT signs (asymmetric swelling between limbs greater than 10 mm or limb pain in patients awake without mechanical ventilation), mechanical prophylaxis (compressive stockings and intermittent pneumatic compression therapy) and presence of femoral catheters were recorded. Pharmacological prophylaxis was part of the standard of care, with different protocols in each hospital (Table 1 ). For the current study, the doses received during the 72 – 96 hours prior to ultrasound scan or symptomatic VTE event were considered, to control possible protocol deviations and to study the relationship between prophylaxis and events. In patients in whom sudden respiratory or cardiovascular deterioration occurred (mainly manifested by hypoxaemia or hypotension not explained by other causes), clinical suspicion of PE was established and transthoracic echocardiography was performed. If there were signs of pulmonary hypertension, right ventricular dilatation or dysfunction, a computed tomography pulmonary angiogram (CTPA) was performed. Patients with symptomatic PE confirmed on the CTPA and those with a swollen limb and confirmed DVT on DUS were considered to have “symptomatic VTE”. The remaining patients with positive DUS or CTPA were considered to have “asymptomatic VTE”. “VTE frequency” for the proportion of patients with symptomatic or asymptomatic VTE detected on the day of screening, including pre-screening in hospital VTE, was considered. “VTE cumulative frequency” for the proportion of patients with symptomatic or asymptomatic VTE, including the events registered the day of the screening, pre-screening in hospital VTE and post-screening seven day follow up, was considered.
Table 1.
Pharmacological venous thrombo-embolism (VTE) prophylaxis protocols at Hospital Universitari Germans Trias I Pujol [HUGTiP] and Hospital Universitari Vall d’Hebron [HUVH] in patients with severe COVID-19 admitted to the intensive care unit
| Prophylaxis dose | HUGTiP | HUVH |
|---|---|---|
| Standard∗ | BMI <35 and D dimer <2 000 ng/mL | Medical criteria |
| Intermediate† | BMI >35 or D dimer >2 000 ng/mL | |
| Therapeutic‡ | Confirmed VTE | D dimer >1 500 ng/mL and severe acute respiratory failure (PaO2/FiO2 <150) and raised inflammatory markers or confirmed VTE |
BMI = body mass index; VTE = venous thrombo-embolism.
Enoxaparin 40 mg once daily or 0.5 mg/kg once daily.
Enoxaparin 60 mg once daily or 1 mg/kg once daily.
Enoxaparin 1 mg/kg twice daily (bid).
All patients with symptomatic or asymptomatic VTE were treated with therapeutic doses of low molecular weight heparin (LMWH). All patients were followed up during the first seven days after screening. The impact of VTE on mortality, risk of subsequent VTE events, and length of hospital and ICU stay was evaluated.
Variables of interest
Key data elements included demographics, VTE risk factors, comorbidities, concomitant medications, and use of VTE prophylaxis. Risk factors and co-morbidities were registered according to previous medical records, from primary care and records of drug use, clinical interview in the emergency room, and interview with close family members in some patients. The following blood tests were also included on hospital admission and at the time of DUS: leucocyte count, lymphocyte count, platelet count, prothrombin time, D dimer, fibrinogen, interleukin-6, creatinine, and ferritin levels. D dimer concentration in citrated plasma was measured using a reagent based on latex particles coated by Fab monoclonal fragments with specificity towards D dimer (HemosIL D dimer HS, Instrumentation Laboratory, Lexington, MA, USA). Assays were performed on ACLTOP LAS 750 coagulometers (Instrumentation Laboratory, Bedford, MA, USA). Finally, peripheral arterial thrombo-embolic events and bleeding complications associated with anticoagulation were recorded. Major bleeding and non-major but clinically relevant bleeding were defined according to the International Society on Thrombosis and Haemostasis classification.11
Statistical methods and sample size
Categorical variables were compared using the chi squared test (two sided) and Fisher's exact test (two sided). Continuous variables were compared using Student’s t test or Mann–Whitney U test when the variables were not normally distributed. In order to assess the relationship between D dimer levels on the day of DUS and VTE during the seven day follow up, the receiver operating characteristic area under the curve (ROC-AUC) was calculated. In addition, a D dimer level of 1 500 ng/mL was selected,8 its sensitivity, specificity, predictive values and odds ratios (OR) and corresponding 95% confidence intervals (CIs) were calculated. Statistical analyses were conducted with SPSS for Windows (version 20; SPSS Inc. Chicago, IL, USA).
Recent studies reported a 25% rate of VTE in these patients.8 , 9 Based on this information, a sample size of 201 subjects would be sufficient to estimate this percentage of events with 95% confidence and an accuracy of ± 6% of the proportion estimate.12
Results
Two hundred and thirty COVID-19 positive patients underwent bilateral DUS screening (118 in Vall d’Hebron hospital, 112 in Germans Trias i Pujol hospital). One of the hospitals selected all patients admitted to the ICU on April 7th and performed all ultrasounds on those patients between 7 and 9 April. The other hospital selected all patients admitted to the ICU on 15 April and scanned those patients between 15 and 16 April. The clinical characteristics and co-morbidities were similar in both hospitals, the only difference being the proportion of patients with hypertension (58% vs. 38%; p < .001). The clinical characteristics and blood tests are shown in Table 2 . Most patients (80%) were mechanically ventilated, 13% were in prone position, and 3.0% had a swollen limb. Four of the 230 patients (1.7%) had suffered a peripheral arterial thrombo-embolic event before being included in the study, three with acute leg ischaemia and one with acute arm ischaemia. All underwent surgery attempting to restore circulation, but one patient needed lower limb amputation.
Table 2.
Clinical characteristics and blood test results on admission to the intensive care unit of included patients with severe COVID-19
| Clinical characteristics | Patients (n = 230) |
|---|---|
| Male sex | 177 (77.0) |
| Age – y | 61.8 (55–67) |
| Body mass index – kg/m2 | 30.3 (27.5–33.2) |
| Arterial hypertension | 110 (47.8) |
| Non-insulin dependent diabetes mellitus | 51 (22.2) |
| Dyslipidaemia | 77 (33.5) |
| Chronic kidney disease | 21 (9.1) |
| Atrial fibrillation | 4 (1.7) |
| Prior coronary disease | 9 (3.9) |
| Prior stroke | 8 (3.5) |
| Peripheral artery disease | 5 (2.2) |
| VTE prior to COVID-19 infection | 3 (1.3) |
| Prior treatment with antiplatelets | 24 (10.4) |
| Prior treatment with anticoagulants | 6 (2.6) |
| Intubated | 183 (79.6) |
| Acute peripheral arterial thrombo-embolism | 4 (1.7) |
| Hospital and ICU length of stay | |
| Hospital stay – d | 15 (9–21) |
| ICU stay – d | 12 (5–19) |
| Blood tests | |
| D dimer – ng/mL | 2 135 (1 051–4 610) |
| D dimer >500 ng/mL | 210 (91) |
| D dimer >1 000 ng/mL | 174 (76) |
| D dimer >1 500 ng/mL | 147 (64) |
| Platelet count – ×109/L | 272.5 (205.5–374.0) |
| Prothrombin time – s | 13.1 (12.2–13.8) |
| Fibrinogen – g/L | 5.1 (4.0–6.6) |
| Lymphocyte count – ×109/L | 1.1 (0.7–1.5) |
| Glomerular filtration – mL/min/1.73 m2 | 90.0 (79.3–90.0) |
| Lactate dehydrogenase – IU/L | 363.5 (294.3–445.8) |
| C reactive protein – mg/dL | 4.3 (0.9–10.9) |
| Ferritin – ng/mL | 817 (510–1 347) |
| Interleukin 6 – pg/mL | 163 (38.3–674.2) |
Data are presented as n (%) or median (interquartile range). ICU = intensive care unit; VTE = venous thrombo-embolism.
Venous thrombo-embolism frequency
On screening ultrasound, there were 63 VTEs in 58 patients (frequency 25.2%; 95% CI 20% – 31%), with no differences between hospitals: 47 patients had DVT (symptomatic in five patients), six had symptomatic PE, and five had PE with DVT (Table 3 ). DVT affected the iliofemoral veins in 22 patients (two bilateral), femoropopliteal in 16 (four bilateral) and distal veins in 20 (three bilateral). The frequency of symptomatic VTE events was 7.0% (95% CI 3.7% – 10.2%). Symptomatic PE was suspected in 38 patients (16.5%), but only 16 such patients (42%) had confirmed PE on CTPA. Nine of the 16 patients (56%) with confirmed PE had lower limb DVT. As for the location of the DVTs on the screening programme, 36 were proximal (including 4 distal) and 16 distal only. They were bilateral in six patients (proximal three patients and distal three patients).
Table 3.
Venous thrombo-embolism (VTE) outcomes: screening based frequency and after seven day follow up of patients admitted to the intensive care unit with COVID-19
| Patients included (n = 230) |
||
|---|---|---|
| Screening | 7 day follow up | |
| Patients with VTE | 58 (25.2) | 61 (26.5) |
| Asymptomatic DVT | 39 (67.2) | 38 (62.3)§ |
| Symptomatic DVT | 8 (13.8) | 7 (11.5)‖ |
| PE∗ | 6 (11.3) | 8 (13.1) |
| PE with DVT∗ | 5 (8.6) | 8 (13.1) |
| VTE frequency† | 25.2 (20–31) | 26.5 (21–32) |
| Symptomatic VTE frequency‡ | 7.0 (3.7–10.2) | 8.3 (4.7–11.8) |
Data are presented as n (%) or frequency (95% confidence interval). DVT = deep venous thrombosis; PE = pulmonary embolism.
All PEs were symptomatic.
Patients with symptomatic and asymptomatic VTE.
Patients with symptomatic VTE.
Two patients with DVT developed and additional PE (they were classified as “PE with DVT”) and one patient developed a new asymptomatic DVT detected while inserting a femoral catheter.
One patient with DVT developed an additional PE and was classified as “PE with DVT”.
Follow up at seven days
During the seven day follow up, six patients developed new VTE: new asymptomatic DVT one patient (incidentally diagnosed by ultrasound trying to insert a femoral catheter); symptomatic PE in patients with prior negative DUS two patients; and symptomatic PE despite receiving therapeutic doses of LMWH because of a confirmed DVT at baseline three patients. Thus, there were 69 VTEs in 61 patients, for a VTE cumulative frequency of 26.5% (95% CI 21% – 32%). When only symptomatic events were considered (n = 20), the frequency was 8.3% (95% CI 4.7% – 11.8%) (Table 3).
In the seven days of follow up, there were 11 patients (4.7%) who had bleeding while being anticoagulated, five considered to be major and six non-major but clinically relevant.11 Eight of these were receiving anticoagulation for recently diagnosed VTE (1 PE and 7 DVT) while the other three were receiving therapeutic dose prophylactic anticoagulation due to high D dimer values.
Patients with VTE had a longer stay in the ICU (median, 22 days [IQR 15 – 28]), than those without VTE (median, 17 days [IQR 12 – 26]). There were no differences in mortality between subgroups: four patients (6.6%) with VTE died, compared with nine patients (5.3%) without VTE (p = .70). However, there were more discharges from the ICU in non-VTE patients in seven days (58, 34.3%) than in patients with VTE (12, 19.6%) (p = .034).
Prophylaxis and VTE
Overall, 127 patients (56%) were prescribed standard prophylactic doses of LMWH, 33 (14.5%) intermediate doses and 67 patients (29.5%) received therapeutic doses. The proportion of patients who developed a first VTE (symptomatic or asymptomatic) was similar in each subgroup: 27%, 21%, and 30%, respectively (Table 4 ). When D dimer levels were <1 500 ng/dL, the rate of VTE was 15% (12 of 80 patients), irrespective of the LMWH doses received (p = .68). When D dimer levels were >1 500 ng/mL, the rate was 33% (28 of 84) in patients with standard prophylactic doses and 32% (20 of 63) in those with intermediate or therapeutic doses (p = 1.0). In patients with D dimer levels >1 500 ng/mL receiving therapeutic doses of LMWH the VTE rate was 37% (17 of 46 patients), the highest rate in all of the subgroups.
Table 4.
Venous thrombo-embolism (VTE) frequency related to anticoagulation dose given to patients admitted to the intensive care unit due to COVID-19
| Symptomatic VTE (n = 23; 10.1%) | Asymptomatic VTE (n = 38; 16.7%) | Total VTE (n = 61 (26.9%) | No VTE (n = 166; 73.1%) | All patients (n = 227; 98.7%)†, ∗ | p | p value (vs. intermediate dose) | p value (vs. therapeutic doses) | |
|---|---|---|---|---|---|---|---|---|
| VTE prophylaxis | .58‡ | |||||||
| Standard doses | 12 (52.2) | 22 (57.9) | 34 (55.7) | 93 (56.0) | 127 (55.9) | .51§ | .65‖ | |
| Intermediate doses | 1 (4.3) | 6 (15.8) | 7 (11.5) | 26 (15.7) | 33 (14.5) | .36¶ | ||
| Therapeutic doses | 10 (43.5) | 10 (26.3) | 20 (32.8) | 47 (28.3) | 67 (29.5) | .36¶ |
Three patients without prophylaxis.
Three patients only with mechanical prophylaxis.
Comparing patients with and without VTE between all prophylaxis subgroups.
Patients with and without VTE in the standard dose subgroup compared with the intermediate dose subgroup.
Patients with and without VTE in the standard dose subgroup compared with the therapeutic dose subgroup.
Patients with and without VTE in the intermediate dose subgroup compared with the therapeutic dose subgroup.
D dimer levels and detection of DVT
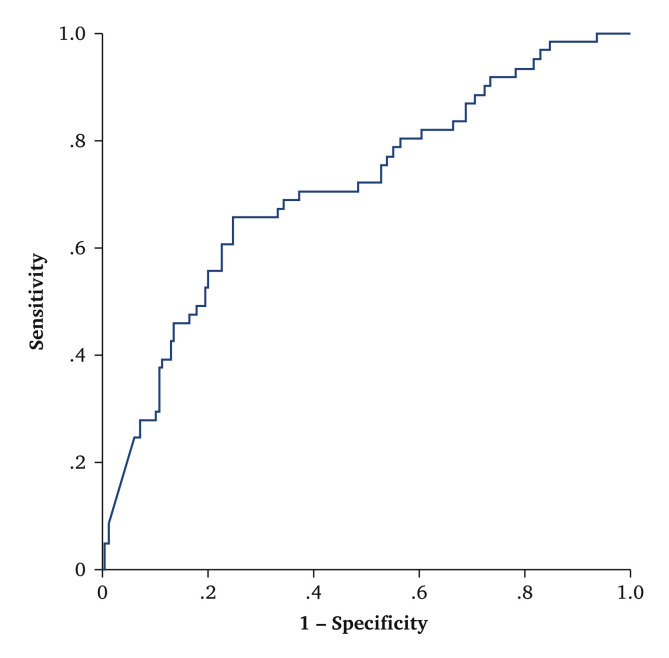
At the seven day follow up, D dimer levels were significantly higher in patients with VTE than in those without VTE. Using a cut off value of 1 500 ng/mL,8 the OR was 2.18 (95% CI 1.1 – 4.3). This D dimer cut off value was more closely related to VTE after one week than to VTE on the day of ultrasound. This is due to the fact that three patients without VTE and elevated D dimer values on the day of the ultrasound developed VTE throughout the following week. The sensitivity was 80% (95% CI 70 – 90), specificity 42% (95% CI 34 – 50), positive predictive value 33% (95% CI 25 – 40), negative predictive value 86% (95% CI 78 – 93). The AUC value was 0.71 (95% CI 0.63 – 0.78; p < .001) (Fig. 2 ).
Figure 2.
Receiver operating characteristic (ROC) curve relating D dimer levels at the seven day follow up and venous thrombo-embolism in 230 critically ill patients with COVID-19.
Discussion
This is the largest reported series screening for VTE in ICU patients with proven COVID-19 pneumonia and reveals a number of findings potentially useful for clinicians. First, one in four screened patients had VTE: 23 patients had symptomatic and 38 asymptomatic VTE. Second, correlation was found between D dimer levels at baseline and the risk of VTE. Unfortunately, a multivariable analysis could not be performed given the small simple size. Third, the LMWH dosage used for VTE prophylaxis was found to have no influence on the VTE frequency. Finally, the usefulness of a screening programme for DVT could not be confirmed in these patients since it did not prevent the development of subsequent symptomatic events.
The frequency of VTE with or without symptoms in the series was 25%, and the frequency of symptomatic VTE was 7%. In similar studies with different designs, prophylaxis, and measured outcomes, the frequency prevalence ranged between 25% and 69%. In a retrospective study on patients not receiving prophylaxis,8 the reported incidence was 25%. In another study,9 there was a 27% rate of symptomatic events per person time of the at risk population. They did not perform routine screening, and most of the events were PEs in patients receiving standard doses of prophylaxis most of the time. The only two studies10 , 13 including systematic screening for DVT had 47% and 69% rates of VTE, but only included 26 and 75 patients, respectively.
This frequency of VTE should be compared with the proportion of events in critically ill non-COVID patients to know the true impact of the virus on the risk of VTE. In a meta-analysis14 of seven studies including 1 783 patients, undergoing DVT screening PE was investigated only when there was a clinical suspicion and patients received pharmacological thromboprophylaxis. The rate of VTE was 12.7% (95% CI 8.7% – 17.5%), half the proportion observed in the study.
Other studies have found an association between raised D dimer levels and worse prognosis.3 , 4 In one study, patients with D dimer levels over six times the upper limit of normal had a higher mortality rate one month later among heparin users than non-users.4 Therefore, the authors suggested using anticoagulation in patients with markedly raised D dimer levels. Moreover, patients with PE presented higher D dimer levels than those with suspected but not confirmed PE.15 Finally, D dimer levels were found to predict VTE in a small study (81 patients),8 with very good sensitivity and specificity. However, using the same cut off value for D dimer, similar sensitivity and lower specificity were obtained.
One in two patients in the cohort (44%) was prescribed higher than standard prophylactic doses of LMWH, and this practice was not associated with a lower frequency of VTE. In the study by Llitjos et al.,10 there was a higher frequency of VTE in patients receiving standard prophylactic doses of LMWH than in those on higher doses. These differences may be explained because the protocol planned to use higher than standard prophylactic doses of LMWH only in patients with raised D dimer levels, and it cannot be ruled out that these patients might already have developed VTE. Even though other authors suggested that standard prophylactic doses may be too low,9 , 10 the ideal LMWH dose to prevent VTE in patients with severe COVID-19 is still unknown and requires randomised trials to confirm whether or not higher than standard prophylactic doses of LMWH might be recommended. It seems reasonable to increase the anticoagulation prophylaxis dose due to the high frequency of VTE and the low proportion of bleeding complications in the study.
Finally, six patients in the series developed symptomatic VTE during the seven day follow up (one DVT and five PE): four patients with negative DUS at baseline and two patients that were receiving therapeutic dose anticoagulation because of asymptomatic DVT during the screening programme. These findings agree with the results reported in one of the studies11 where there were 35 events, 21 of them symptomatic. Seven days later, the incidence of VTE was 26%, as in the series.
The study has a number of potential limitations. First, the circumstances of the pandemic made it impossible to perform a systematic screening programme throughout the whole hospital stay. As a result, only symptomatic VTE events are presented on the seventh day as it was considered inappropriate to perform repeat ultrasound screening to detect asymptomatic DVTs. Although this might affect the validity of the study for isolated patients, the study is considered valid for this group of patients. Second, although this is the largest cohort published to date on this topic, follow up and sample size were small, so more patients are needed to develop a prognostic model and there is a need to extend the surveillance to detect further events as death, new VTE and bleeding.
In conclusion, hospitalised patients with severe COVID-19 are at high risk of VTE, despite the use of high doses of pharmacological prophylaxis in some of them. The data suggest that prophylaxis should be tailored to the patient’s characteristics trying to balance the bleeding risk and clinical severity and to prescribe higher than recommended doses only in patients that would benefit most. Finally, routine ultrasound screening in a pandemic outbreak scenario did not prevent symptomatic VTE. Therefore, strict clinical surveillance is needed to detect new events that can occur despite anticoagulation.
Funding
None.
Conflict of interest
Dr Bellmunt reports personal fees from Sanofi, Rovi, and Bayer, outside the submitted work. Dr Monreal reports unrestricted research grants from Sanofi, Spain and Bayer, Spain during the conduct of the study to sponsor RIETE registry. All other authors have nothing to disclose.
References
- 1.Phelan A.L., Katz R., Gostin L.O. The novel coronavirus originating in Wuhan, China: challenges for global health governance. JAMA. 2020;323:709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Erratum in: Lancet 2020;395:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han H., Yang L., Liu R., Liu F., Wu K.L., Li J. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 4.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., Cuich T., Dreyfuss I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention antithrombotic therapy, and follow-up. JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients in severe SARS-Co-V-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llitjos J.F., Leclerc M., Chochois C., Monsallier J.M., Ramakers M., Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulman S., Kearon C., Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Marrugat J. Sample size and power calculator. Program of Research in inflammatory and cardiovascular disorders. Institut Municipal d'Investigació Mèdica, Barcelona, Spain. Available at: https://www.imim.cat/ofertadeserveis/software-public/granmo/ [Accessed 1 April 2020].
- 13.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malato A., Dentali F., Siragusa S., Fabbiano F., Kagoma Y., Boddi M. The impact of deep vein thrombosis in critically ill patients: a meta-analysis of major clinical outcomes. Blood Transfus. 2015;13:559–568. doi: 10.2450/2015.0277-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard-Lorant I., Delabranche X., Severac F., Helms J., Pauzet C., Collange O. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology. 2020;296:E189–E191. doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]