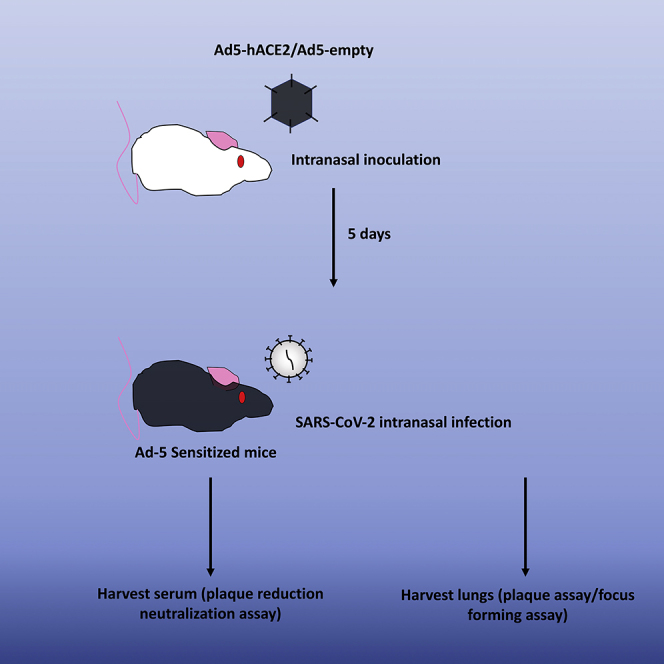
Summary
Common laboratory mice such as BALB/c and C57BL/6 mice are not permissive to SARS-CoV2 infection. Sensitization of laboratory mice with Adenovirus expressing human ACE2 (Ad5-hACE2) provides a rapid model for testing viral intervention in vivo. Despite the lack of lethal outcome, Ad5-hACE2-sensitized mice show 20% weight loss on average upon viral challenge with infectious virus being detected at the site of sensitization. This protocol describes the sensitization and subsequent infection of common laboratory mice for use in testing anti-viral interventions.
For complete details on the use and execution of this protocol, please refer to Sun et al. (2020).
Subject Areas: Immunology, Model Organisms
Graphical Abstract
Highlights
-
•
Sensitization of non-permissive laboratory mice to SARS-CoV-2 infection by Ad5-hACE2
-
•
A mouse model for SARS-CoV-2 infection for testing anti-viral interventions
-
•
Rapid model applicable to mice with different genetic backgrounds
Common laboratory mice such as BALB/c and C57BL/6 mice are not permissive to SARS-CoV-2 infection. Sensitization of laboratory mice with Adenovirus expressing human ACE2 (Ad5-hACE2) provides a rapid model for testing viral intervention in vivo. Despite the lack of lethal outcome, Ad5-hACE2-sensitized mice show 20% weight loss on average upon viral challenge with infectious virus being detected at the site of sensitization. This protocol describes the sensitization and subsequent infection of common laboratory mice for use in testing anti-viral interventions.
Before You Begin
Second-generation E1/E3-deleted Adenovirus is used to minimize wild-type outgrowth and immunogenicity in mice. The Adenovirus used in this protocol was generated by the University of Iowa Gene Transfer Vector Core as previously described (Anderson et al., 2000).
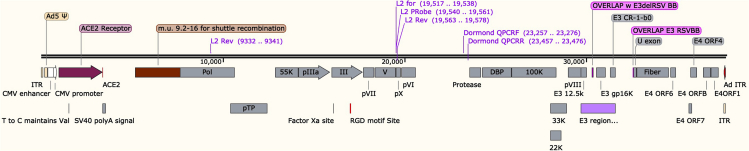
Adenovirus (Ad5-hACE2 and AD5-empty) were prepared and tittered by the University of Iowa Gene Transfer Vector Core (https://medicine.uiowa.edu/vectorcore/). The viruses are available from the University of Iowa Gene Transfer Vector Core upon request. A map of Ad5-hACE2 vector is shown below (Figure 1).
Figure 1.
Schematic Diagram Showing the Organization of the Ad5-hACE2 Vector for Generation of Ad5-hACE2
Also, propagate SARS-CoV-2 to high titers (∼107 PFU/mL) as follows at biosafety level 3 containment with appropriate personal protective equipment as specified by institutional rules.
-
1.
Prepare a T75 flask of Calu-3 cells with DMEM supplemented with 10% FBS at 80%–90% confluence.
-
2.
Wash cells with DPBS.
-
3.
Remove DPBS and mix the appropriate amount of stock virus (see Note) obtained from BEI with 5 mL serum-free DMEM to give the infecting inoculum.
-
4.
Infect cells by adding the infecting inoculum to the cells.
-
5.
Incubate flask at 37°C incubator with 5% CO2 for 1 h and rotate the plate every 15 min to keep cells from dessicating.
-
6.
Remove the infecting inoculum after 1 h and add 10 mL of DMEM with 10% FBS.
-
7.
Incubate the flask at 37°C incubator with 5% CO2 and monitor daily under microscope for cytopathic effect (CPE).
-
8.
Freeze the flask at −80°C when 50% CPE is observed.
-
9.
On the next day, thaw the flask at 23°C. Aliquot the 10 mL medium into 10 × 1 mL vial and store the vials at a −80°C freezer as stock virus.
-
10.
Thaw one vial of 1 mL stock virus and further make 100 μL aliquots as working vials. Store the working vials in a −80°C freezer.
Note: Titer the 100 μL working vial to obtain the virus titers as indicated below. For every experiment, thaw a new working vial for use and discard the working vial after each experiment. This will ensure the same virus titers of all vials as all vials are frozen and thawed for the same number of times. We routinely grow virus at 0.01 MOI and freeze flasks at 48 h post infection. The typical titers we obtain using this protocol are around 107 PFU/mL.
We used Venezuelan Equine Encephalitis Replicon Particles (VRPs) generated as previously described (Deming et al., 2006) (Agnihothram et al., 2018)
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-SARS-CoV-2 nucleocapsid protein polyclonal antibody | Sino Biological | Cat.# 40143-T62 |
| HRP-labeled goat anti-rabbit secondary antibody | Jackson ImmunoResearch Laboratories | Cat.# 111-035-144; AB_2307391 |
| Bacterial and Virus Strains | ||
| SARS-CoV-2/human/USA/USA-WA1/2020 (GenBank: MN985325.1) | N/A | |
| Ad5-empty | (Sun et al., 2020) | N/A |
| Ad5-hACE2 | (Sun et al., 2020) | N/A |
| Venezuelan Equine Encephalitis Replicon particles expressing SARS-CoV-2 Spike protein | (Sun et al., 2020) | N/A |
| Venezuelan Equine Encephalitis Replicon particles expressing SARS-CoV-2 Nucleocapsid protein | (Sun et al., 2020) | N/A |
| Venezuelan Equine Encephalitis Replicon particles expressing SARS-CoV-2 Membrane protein | (Sun et al., 2020) | N/A |
| Venezuelan Equine Encephalitis Replicon particles expressing SARS-CoV-2 Envelop protein | (Sun et al., 2020) | N/A |
| Venezuelan Equine Encephalitis Replicon particles expressing GFP | (Sun et al., 2020) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| VetaKet® CIII (ketamine hydrochloride injection, USP) | Akron, Inc. | Cat.# 59399-114-10 |
| AnaSed® (xylazine sterile solution) Injection | Akron, Inc. | Cat.# 59399-111-50 |
| TrueBlue™ Peroxidase Substrate (KPL, Gaithersburg, MD) | SeraCare | Cat.# 510-0030 |
| DAB+ Substrate Chromogen System (Dako Omnis) | Dako | Cat.# GV825 |
| Fetal Bovine Sera | GIBCO | Cat.# 10270-106 |
| Penicillin-Streptomycin | Invitrogen | Cat.# 60106-1 |
| L-Glutamine, 100× | Invitrogen | Cat.# 2503081 |
| DPBS | GIBCO | Cat.# 14190235 |
| DMEM | Life | Cat.# C11965500BT |
| Crystal Violet | Millipore Sigma | Cat.# C0775 |
| Agarose | RPI | Cat.# 9002-18-0 |
| Formaldehyde solution | Millipore Sigma | Cat.# 252549 |
| Carboxymethylcellulose powder | Millipore Sigma | Cat.# C4888 |
| Triton™ X-100 | Millipore Sigma | Cat.# T8787 |
| Isoflurane, USP | Millipore Sigma | Cat.# 1349003 |
| Experimental Models: Cell Lines | ||
| Vero E6 | ATCC | CRL-1586 |
| Calu-3 2B4 | ATCC | HTB-55 |
| Experimental Models: Organisms/Strains | ||
| BALB/c mice | Charles River Laboratories (USA) | N/A |
| C57BL/6 mice | Charles River Laboratories (USA) | N/A |
| Other | ||
| Fisherbrand™ Micro Blood Collecting Tubes | Fisherscientific | Cat.# 02-668-15 |
| BD insulin syringes (12.7 mm × 28G needle) | BD | Cat.# 329410 |
| Fisherbrand™ Disposable Tissue Grinders, 35 mL | Fisherscientific | Cat.# 0254208 |
Step-By-Step Method Details
Transduction of Mice with Adenovirus Expressing hACE2 – Day 0
Timing: ∼1 h
-
1.
Thaw aliquots of Ad5-hACE2 and Adenovirus without hACE2 expression (Ad5-empty) stock by slowly swirling in water at 23°C.
-
2.
Store the thawed virus on ice during the procedure.
-
3.
Anesthetize mice with ketamine/xylazine (17.5 mg/mL ketamine; 2.5 mg/mL xylazine) at a dose of 100 μL/20 g body weight of mouse through intraperitoneal injection.
-
4.
Ensure the mouse is completely sedated before proceeding to the next step.
-
5.
Transduce mice intranasally using a P200 micropipette with 2.5 × 108 PFU of Ad5-hACE2 or Ad5-empty (negative control) in 75 μL DMEM per mouse.
-
6.
Dispense the virus dropwise on the nostril of the mouse. Allow the mouse to inhale the drop of virus before applying the next drop (Methods Video S1).
-
7.
Observe the mouse for normal breathing, light chest massage can be given if the mouse exhibit difficulty breathing.
-
8.
Transfer transduced mice to a separate container and repeat steps 4–7 until all mice are transduced.
-
9.
Return transduced mice to animal cages. Check to make sure mice are conscious before returning them to animal facility. It usually takes around 30–45 min for the mice to recover from anesthesia. Allow mice to recover for 5 days before proceeding to step 10.
Intranasal Infection of SARS-CoV-2 – Day 5
Timing: ∼1 h
-
10.
Repeat steps 1–8 on day 5 post transduction for intranasal infection, but replace Ad5-hACE2 or Ad5-empty with SARS-CoV-2 and dilute the virus stock to achieve 1 × 105 PFU of SARS-CoV-2 in 50 μL of DMEM for each mouse.
Note: Infected mice should be monitored daily for weight change and disease progression. Try to weigh mice at the same time every day to avoid intraday fluctuation. (Sun et al., 2020). Also, we took the day of infection with SARS-CoV-2 as day 0 and did not weigh mice before day 0. All steps involving SARS-CoV-2 should be performed in a BSL3 laboratory with appropriate PPE.
CRITICAL: Before transducing or infecting mice, make sure the mice are completely anesthetized so that the mice remain totally still when virus droplets are applied onto the nose as they inhale. Excessive movement of the mice may result in incomplete inhalation of the virus droplets if mice are not properly sedated. When dispensing the virus on the nostril, do not place the tip inside the nostril. The virus should be applied onto the nose. It is critical to apply the next drop only when the previous drop has been inhaled.
Harvest Virus by Homogenizing Lungs for Validation of Infection
Timing: ∼1 h
-
11.
Anesthetize mice with ketamine/xylazine (17.5 mg/mL ketamine; 2.5 mg/mL xylazine, intraperitoneal injection) at a lethal dose of 300 μL/20 g body weight.
-
12.
Ensure the mouse is completely sedated by observing pedal reflex before proceeding to the next step.
-
13.
Position the mouse on an absorbent bench pad on top of a polystyrene foam board so that the ventral side of the mouse faces upwards.
-
14.
Attach the limbs of the mouse to the foam board with 25 g × 5/8 inch needles.
-
15.
Spray the ventral side of the mouse with 70% ethanol.
-
16.
Lift up the fur/skin close to the urethral opening with forceps. Incise the skin midline from the urethral opening up to the mandible with surgical scissors.
-
17.
Pull the fur/skin on both sides of the mouse sideways with forceps to peel the skin off the underlying tissues.
-
18.
Incise the abdominal wall midline by lifting up with forceps until the base of the thorax. Open the abdominal cavity by making transverse incisions on both sides of the midline.
-
19.
Make an incision on the diaphragm right under the sternum and cut the diaphragm following the costal arch. Expose the lungs and heart by removing the ribcage.
-
20.
Prepare a 10 mL syringe filled with sterile DPBS. Insert a 25 g × 5/8 inch needle to the apex of the right ventricle and tear off the left atrium with forceps.
-
21.
Perfuse the lungs by injecting at least 5 mL of DPBS into the right ventricle.
-
22.
After perfusion, remove the lungs from the thoracic cavity and weigh in a weighing dish.
-
23.
Place the lung into a sterile, disposable tissue homogenizer and add 1 mL DPBS.
-
24.
Homogenize the lungs in DPBS by grinding inside the homogenizer.
-
25.
Transfer the homogenates into a 2 mL screw-top sterile vial.
-
26.
Samples are stored at −80°C.
Note: When harvesting organs other than the lungs, transcardiac perfusion should be performed by inserting needle to the apex of the left ventricle with puncture of the right atrium. Also, lung homogenates can be briefly pulsed down for 10 s after thaw before use. Viral titers of lung homogenate can be quantified by plaque assay or focus-forming assay.
SARS-CoV-2 Plaque Assay – Day 0
Timing: ∼30 min
Plaque assay serves to determine viral titer by revealing individual infectious virion with overlaying media that limit virus spread only to neighboring cells.
-
27.
Seed Vero E6 cells with DMEM supplemented with 10% FBS at approximately 1.25 × 105 cells per well in 12-well plate so that they reach >95% confluency the next day (after 16 h) (day 1).
SARS-CoV-2 Plaque Assay – Day 1
Timing: ∼1 h for processing and 3 days for incubation
-
28.
Prepare a 48-well dilution plate by adding 720 μL serum-free DMEM to each well.
-
29.
Thaw lung homogenates by slowly swirling in water at 23°C. Pulse centrifuge the vial for 10 s to clarify the homogenates.
-
30.
Keep the thawed lung homogenates on ice during the procedure.
-
31.
Serially dilute the samples by 10-fold with serum-free DMEM by transferring 80 μL of virus sample to the first well of the 48-well plate and mix well by pipetting up and down.
-
32.
Repeat the dilution process by adding 80 μL from the current well to the next dilution well.
-
33.
Remove the media from each well of the 12-well plate. Wash with 500 μL of DPBS and remove DPBS from the wells.
-
34.
Add 200 μL of diluted virus from the 48-well plate to each well of the 12-well plate in the order of the highest dilution to the lowest dilution.
-
35.
Incubate the 12-well plate at 37°C incubator with 5% CO2 for 1 h and rotate the plate every 15 min to keep cells from dessicating.
-
36.
Melt sterile 1.2% agarose in PBS (in a 50 mL Falcon tube) by heating in a microwave oven for 2 min or until melted. Keep the melted 1.2% agarose in a 65°C water bath.
-
37.
Remove 200 μL inocula from each well after 1 h of incubation. Mix the melted 1.2% agarose at 56°C with 2× DMEM at 37°C (1:1, v/v; i.e., for 3 × 12-well plate, 20 mL of 1.2% agarose + 20 mL of 2× DMEM).
-
38.
Immediately add 1 mL of agarose/growth media mixture to each well by a 10 mL serological pipette.
-
39.
Wait for the agar overlay to solidify, add 500 μL of DMEM with 10% FBS on top of the solid agar and incubate cells at 37°C with 5% CO2 for 3 days.
SARS-CoV-2 Plaque Assay – Day 4
Timing: ∼1 h
-
40.
Remove the residual media on top of the agar overlay from each well.
-
41.
Add 1 mL of 10% formaldehyde (∼37.5 mL PBS + ∼12.5 mL 37% formaldehyde) each well and incubate at 23°C for 20 min to fix cells and inactivate viruses.
-
42.
Remove the liquid and agar overlay using curved forceps.
-
43.
Add 0.1% crystal violet (5 mL of 1% crystal violet + 45 mL PBS) to each well to delineate plaques.
-
44.
Incubate at 23°C until plaques are visible.
-
45.
Remove the crystal violet, rinse wells with PBS, and count plaques.
Note: When preparing the dilution plate, add serum-free DMEM to enough wells so that the intended dilution factor is achieved. The volume of serum free DMEM added to each well of the dilution plate can vary according to the final volume of diluted virus required for infection. In our experiments, we routinely use 200 μL of diluted virus in 12-well plates for plaque assay with technical triplicate for each dilution (4 dilutions can be tested in a 12-well plate). Therefore, we dilute 80 μL of virus into 720 μL of serum-free DMEM serially to achieve serial 10-fold dilution. Also, always pipette at least 10 μL of virus or solution to avoid pipetting errors. When counting plaques, choose a dilution with easily distinguished plaques (10–20 plaques) and calculate titer in plaque-forming units per mL (PFU/mL): PFU/mL = (plaques/well) × (dilution factor)/(mL inoculum), and calculate PFU/g according lung weight.
SARS-CoV-2 Focus-Forming Assay – Day 0
Timing: ∼30 min
The idea of focus-forming assay is similar to that of plaque assay which reveals individual infectious virion. However, instead of observing plaque formation in plaque assay, focus-forming assay visualizes intracellular antigen of the virus by immunostaining which can be done with higher-throughput and in shorter amount of time.
-
46.
Seed Vero E6 cells with DMEM supplemented with 10% FBS (2 x104 cells/well) in a 96-well plate.
SARS-CoV-2 Focus-Forming Assay – Day 1
Timing: ∼2 h
-
47.
Thaw samples and warm 1.6% methylcellulose in DPBS to 37°C in water bath.
-
48.
Serially 10-fold dilute lung homogenates in DMEM containing 2% FBS serum DMEM (20 μL of sample with 180 μL DMEM).
-
49.
Aspirate cell culture medium from the plate.
-
50.
Transfer 50 μL of diluted virus to each well and incubate the cells at 37°C for 1 h. Rotate gently every 15 min to keep cells from drying out.
-
51.
Remove infecting inocula after 1 h.
-
52.
Add 100 μL/well pre-warmed (37°C) 1.6% methylcellulose in DMEM with 2% FBS.
-
53.
Incubate the cells for another 24 h at 37°C.
SARS-CoV-2 Focus-Forming Assay – Day 2
Timing: ∼2 h
-
54.
Fix the cells with 200 μL/well 4% paraformaldehyde in PBS for 1 h at 23°C.
-
55.
Remove fixative and wash with 200 μL/well PBS three times.
-
56.
Permeabilize cells with 50 μL/well 0.2% Triton X-100 with 1% BSA for 30 min at 23°C.
-
57.
Wash with 200 μL/well PBS three times.
-
58.
Incubate permeabilized cells with 50 μL/well of rabbit anti-SARS-CoV-2 nucleocapsid protein polyclonal antibody (1:1000) in PBS with 1% BSA at 37°C for 1 h.
-
59.
Discard primary antibody and wash three times with 200 μL/well PBST (0.1% Tween) and remove residue liquid completely.
-
60.
Add 50 μL/well goat anti-rabbit IgG HRP (1:5000) in PBS with 1% BSA and incubate at 37°C for 1 h.
-
61.
Discard secondary antibody and wash three times with 200 μL/well PBST (0.1% Tween), remove residue liquid completely.
-
62.
Add 50 μL/well True Blue, shake the plate on a shaker for 5–10 min at 23°C.
-
63.
Remove TrueBlue and Wash with 200 μL/well ddH2O, completely remove residue liquid, and count the foci by ELISPOT reader.
Note: For each sample, choose a dilution with easily distinguished foci (e.g., 20–200 per well) and calculate titer in focus-forming units per mL (FFU/mL): FFU/mL = (foci/well) × (dilution factor)/(mL inoculum), and calculate FFU/g according lung weight.
SARS-CoV-2 Plaque Reduction Neutralization Assay – Day 0
Timing: ∼30 min
Plaque reduction neutralization assay measures the reduction of the number of plaques of a virus standard with known titer so as to infer the neutralizing titers in the sample being tested.
-
64.
Seed Vero E6 cells in 12-well plate that will reach >95% confluency the next day (day 1).
SARS-CoV-2 Plaque Reduction Neutralization Assay – Day 1
Timing: ∼3 h for processing and 3 days for incubation
-
65.
Serially two-fold dilute the mouse serum in 400 μL of serum-free DMEM to reach a 2× concentration of the final concentration to be tested in a 48-well plate. Pipette up and down to mix well.
-
66.
Thaw aliquots of SARS-CoV-2 stock by slowly swirling in water at 23°C.
-
67.
Store the thawed virus on ice during the procedure.
-
68.
Dilute the virus in serum-free DMEM to achieve a concentration of around 80–100 PFU of virus in 400 μL. Pipette up and down to mix well.
-
69.
Transfer 400 μL of the diluted virus to the 400 μL of the diluted sample. Pipette up and down to mix well.
-
70.
Repeat until all the diluted samples of different concentration contain virus.
-
71.
Incubate the virus/sample mix at 37°C with 5% CO2 for 1 h.
-
72.
Remove the media from 12-well plate of Vero E6 cells.
-
73.
Add 200 μL of diluted virus/sample mix to each well in the order of the highest dilution to the lowest dilution.
-
74.
Repeat steps 36–40 in SARS-CoV-2 plaque assay – day 1 with the virus/sample mix.
SARS-CoV-2 Plaque Reduction Neutralization Assay – Day 4
Timing: ∼1 h
-
75.
Repeat steps 41–46 in SARS-CoV-2 plaque assay – day 4.
Note: Equal volume of virus and diluted sample should be used. The sample is diluted into a 2× concentration in step 65. For example, for a 1:20 dilution of the sample, it is diluted to 1:10 in 400 μL of serum-free DMEM so that when mixed with 400 μL of virus, the final concentration of the sample is1:20 (1×). Also, technical triplicates should be performed for each dilution. A total of 800 μL of virus/sample mix is sufficient for a triplicate technical repeat for a single dilution. In a 12-well plate format, four dilutions each with three technical repeats are performed. The total volume of virus therefore to be prepared is 1.6 mL (400 μL × 4). In addition, a triplicate well control can be performed with the virus alone to obtain the actual PFU per well without neutralization.
Venezuelan Equine Encephalitis Replicon Particle Immunization
Timing: ∼6 weeks
VRPs are replication-competent and propagation-deficient which transduce cells with replicon of Venezuelan Equine Encephalitis virus genome incorporated with gene of interest as a mean to introduce viral antigen for immunization.
Uninfected mice were immunized with 1 × 105 Infectious Units of VRPs expressing the SARS-CoV-2 spike protein (S), nucleocapsid protein (N), membrane protein (M), envelope protein (E) or GFP through footpad injection or intranasally. Mice were primed and boosted once (3 weeks after priming) at the same dose through footpad injection or intranasally. Sera were collected 1–2 weeks after booster. Mice were challenged with SARS-CoV-2, as described in steps 1–10, 3 weeks after booster.
Footpad Injection Immunization
Timing: ∼30 min
-
76.
Dilute VRPs in PBS to achieve the indicated concentration and keep VRPs on ice.
-
77.
Fill syringe (1 mL, hypodermic needle 30 × ½) with appropriate amount of indicated VRPs dilution.
-
78.
Mouse is lightly anesthetized with isoflurane in a drop jar.
-
79.
Wipe the left foot with alcohol prior to injection. Grasp the toes of the extended foot and inject 50 μL subcutaneously into the center of the hind foot. A small bleb will form at the infection site (Methods Video S2). Observe for bleeding after administration.
Intranasal Injection Immunization
Timing: ∼30 min
-
80.
Dilute VRPs in PBS to achieve the indicated concentration and keep VRPs on ice.
-
81.
Fill syringe (1 mL, hypodermic needle 30 × ½) with appropriate amount of indicated VRPs dilution.
-
82.
Mouse is lightly anesthetized with isoflurane in a drop jar.
-
83.
Hold the mouse and carefully pipette 50 μL of diluted VRPs onto the nostrils drop by drop, carefully matching the rate with mouse inhalation (Methods Video S1).
Collection of Sera after Immunization
Timing: ∼1 h
Sera were collected by retro-orbital bleeding at 1–2 weeks after booster.
-
84.
Anesthetize mice with ketamine/xylazine (17.5 mg/mL ketamine; 2.5 mg/mL xylazine) at a dose of 100 μL/20 g body weight of mouse through intraperitoneal injection.
-
85.
Hold the mouse by the back of neck and tight the head with thumb and point finger. Place the sterile capillary tube at the medial canthus of the eye under the nictitating membrane. Slightly puncture the resistant membrane of the sinus (past the eyeball to avoid eyeball injury) (Methods Video S3).
-
86.
Blood will enter the BD Microtainer Gel tube by capillary action. When appropriate amount of blood is collected, withdraw the tube and slightly press on the eyeball with a piece of gauze to stop bleeding.
-
87.
Allow blood to clot for at least 30 min and centrifuge at 4,000 × g for 5 min to separate serum from cells/clot as per the manual of BD Microtainer Gel tube.
Expected Outcomes
hACE2 expression is predominantly found in the alveolar epithelium with occasional positive staining in the airway epithelium on 5 days post intranasal Ad5-hACE2 transduction of mice while mice transduced with Ad5-empty do not show signs of hACE2 staining (Sun et al., 2020).
Ad5-hACE2 transduced mice start to show signs of disease including hunching, ruffled fur, and labored breathing on 2 days post SARS-CoV-2 challenge. Infected BALB/c mice experience up to 20% weight loss during the first 4–6 days post challenge and gradually regain weight typically after 6 days post challenge while infected C57BL/6 mice lost around 10% weight. Ad5-empty transduced mice do not experience signs of disease and weight loss after SARS-CoV-2 challenge. In addition, we mainly used 8- to 10-week-old mice for our experiments, however, we did not find significant differences when 10-month-old mice were used.
Plaques and foci are observed after staining steps in plaque assay and focus-forming assay. In theory, the number of plaques or foci in consecutive dilutions should be correlated by a 10-fold difference. Depending on the day of mice harvest, titers of SARS-CoV-2 may range from 102–108 PFU(FFU)/g of lung tissues.
Limitations
hACE2 expression is driven by CMV promoter in the airway only. SARS-CoV-2 induced disease is only localized in the lungs with a lack of extrapulmonary manifestations. This model is limited to studying the virus growth and histological changes in the lungs. Study of virus tropism which requires extrapulmonary expression of hACE2 is facilitated by this approach. Ad5-hACE2 transduced mice do not develop lethality or severe disease. Studies of systemic pathogenesis would require infection model that involves mild and severe COVID-19.
Troubleshooting
Problem 1
No weight loss is observed in mice transduced with Ad5-hACE2 after SARS-CoV-2 challenge
Potential Solution
Make sure both Ad5-hACE2 and SARS-CoV-2 are kept in ice during transduction and infection. Also, re-titer SARS-CoV-2 to ensure the titer is correct. We used 105 PFU of SARS-CoV-2 per mouse and obtained the described weight changes. In our experience, SARS-CoV-2 cultured in Vero E6 does not induce weight loss in Ad5-hACE2 transduced mice at 105 PFU per mouse. Calu-3 cultured SARS-CoV-2 lead to the described weight loss.
Also, it is recommended that immunohistochemical staining of ACE2 be performed in the lungs of transduced mice 5 days post transduction to assess transduction efficiency when this system is first used.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Stanley Perlman, stanley-perlman@uiowa.edu.
Materials Availability
Ad5-hACE2 may be obtained from BEI Resources or the University of Iowa.
Data and Code Availability
No datasets were generated using this protocol.
Acknowledgments
This work is supported by the grants from the National Key Research and Development Program of China (2018YFC1200100) (J.Z.), National Science and Technology Major Project (2018ZX10301403) (J.Z.), emergency grants for the prevention and control of SARS-CoV-2 of Ministry of Science and Technology (2020YFC0841400) (J.Z.) and Guangdong Province (2020B111108001, 2018B020207013) (J.Z.), the National Institutes of Health USA (NIH) (P01 AI060699 [S.P., P.B.M.] and RO1 AI129269 [S.P.]), and the Pathology Core, which is partially supported by the Center for Gene Therapy for Cystic Fibrosis (NIH grant P30 DK-54759) and the Cystic Fibrosis Foundation. P.B.M. is supported by the Roy J. Carver Charitable Trust. We thank Jian Zheng and Alan Sariol for their help in recording the method videos.
Author Contributions
All of the authors contributed to the writing of these protocols.
Declaration of Interests
The authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xpro.2020.100169.
References
- Agnihothram S., Menachery V.D., Yount B.L., Jr., Lindesmith L.C., Scobey T., Whitmore A., Schäfer A., Heise M.T., Baric R.S. Development of a broadly accessible venezuelan equine encephalitis virus replicon particle vaccine platform. J. Virol. 2018;92:e00027-18. doi: 10.1128/JVI.00027-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R.D., Haskell R.E., Xia H., Roessler B.J., Davidson B.L. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- Deming D., Sheahan T., Heise M., Yount B., Davis N., Sims A., Suthar M., Harkema J., Whitmore A., Pickles R. Vaccine efficacy in senescent mice challenged with recombinant SARS-CoV bearing epidemic and zoonotic spike variants. PLoS Med. 2006;3:e525. doi: 10.1371/journal.pmed.0030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhuang Z., Zheng J., Li K., Wong R.L.-Y., Liu D., Huang J., He J., Zhu A., Zhao J. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell. 2020;182:734–743.e5. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated using this protocol.