Abstract
Aims
People with cardiovascular disease or risk factors are at increased risk when exposed to SARS-CoV-2. Most are treated with statins, but the impact of these drugs on clinical outcomes of COVID-19 remains unclear. This report is therefore based on meta-analyses of retrospective observational studies aimed at investigating the impact of previous statin therapy in patients hospitalized for COVID-19.
Methods
In studies reporting on the clinical outcomes of COVID-19 in statin users vs non-users, two endpoints have been used—in-hospital death rates, and disease severity as assessed by admission to intensive care units (ICUs)—with a special focus on patients with diabetes.
Results
Regarding mortality, 13 studies were included in the meta-analysis for a total of 10,829 statin users (2517 deaths) and 31,893 non-users (7516 deaths): univariate analysis showed no statistically significant reduction in deaths (OR: 0.97, 95% CI: 0.92–1.03), although between-study heterogeneity was high (I² = 97%). As for disease severity, 11 studies were selected for a total of 3462 statin users (724 endpoints) and 10,560 non-users (1763 endpoints): here again, univariate analysis showed no reduction in severity (OR: 1.09, 95% CI: 0.99–1.22; I² = 93%). Collectively, in 10 studies using multivariable analysis adjusted for the more prevalent baseline risk factors among statin users, lower OR values were reported than with univariate analyses (0.73 ± 0.31 vs 1.44 ± 0.84, respectively; P = 0.0028; adjusted OR: P = 0.0237 vs non-users). Limited but conflicting findings were observed for diabetes patients.
Conclusion
Although no significant reductions in either in-hospital mortality or COVID-19 severity were reported among statin users compared with non-users after univariate comparisons, such reductions were observed after adjusting for confounding factors. These highly heterogeneous observational findings now require confirmation by ongoing randomized clinical trials.
Keywords: COVID-19, Intensive care unit, Mortality, SARS-CoV-2, Statin, Type 2 diabetes
Introduction
Several risk factors for more severe coronavirus disease 2019 (COVID-19) infection have been identified in the general population [1], [2] as well as in the diabetes population [3], [4]. In addition to older age, male gender and obesity as risk factors, patients with comorbidities such as diabetes mellitus (DM) [5], arterial hypertension [6] and established cardiovascular (CV) disease have been identified as being more prone to progress to more severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection requiring admission to intensive care units (ICUs) because of acute respiratory distress syndrome (ARDS) and leading to premature death [7]. Indeed, according to international guidelines, most patients with CV risk factors and/or CV disease are treated with statins to mitigate their overall CV risk [8]. Remarkably, however, in an extensive review of COVID-19 and CV disease covering the topic from basic mechanisms to clinical perspectives, statin therapy was not considered at all [9]. In fact, it is still unclear as to whether statins can positively or negatively influence prognoses of COVID-19 [10], [11].
Two previous meta-analyses of observational studies compared clinical outcomes in statin users vs non-users and gave conflicting results: one demonstrated a positive impact [12], whereas the other failed to show any significant differences in prognosis [13]. Nevertheless, these meta-analyses were performed using a rather limited number of studies (some only available as preprints). Furthermore, a recent analysis of the Coronavirus SARS-CoV-2 and Diabetes Outcomes (CORONADO) study concluded that the routine use of statins is associated with an increased in-hospital mortality related to COVID-19 in patients with type 2 DM [14], findings that are in contrast to the reduction in death rate found in a US study of diabetes patients treated with statins [15]. Thus, the available reported data are divergent, preventing any definite conclusions to be drawn on the effects of statin therapy on COVID-19 clinical outcomes.
For this reason, the aim of the present meta-analyses of retrospective observational studies was to investigate the impact of statin therapy on two major clinical outcomes—in-hospital mortality and disease severity requiring ICU admission, mainly for invasive mechanical ventilation (IMV)—in patients hospitalized with COVID-19. Also, in light of the recent published report by CORONADO investigators [14], a particular focus has also been placed on other studies that recruited patients with diabetes.
Materials and methods
Data sources and search strategy
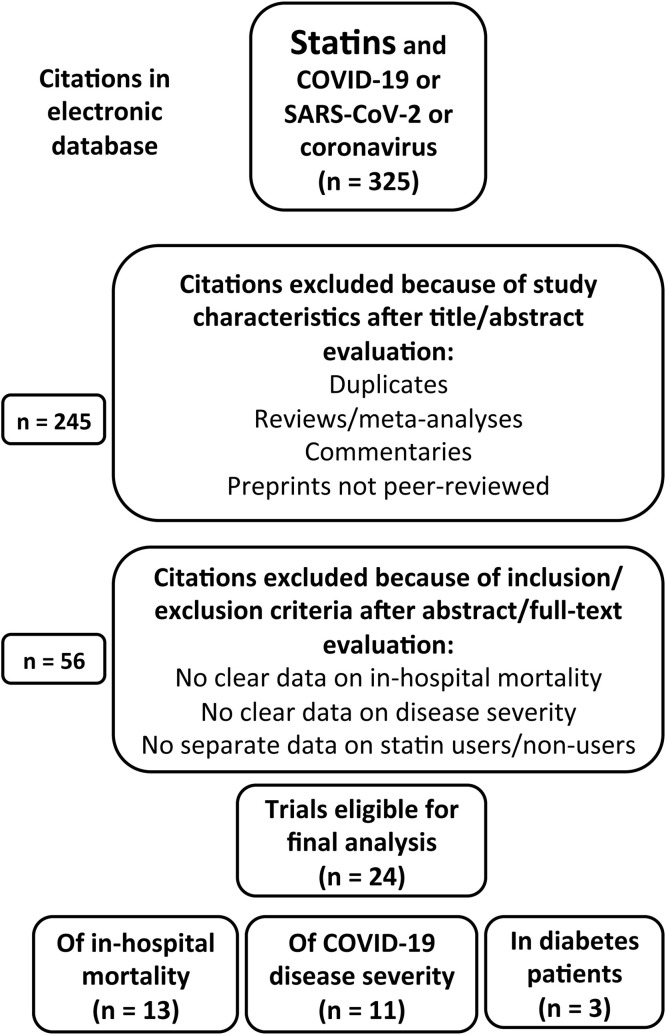
Electronic searches were performed in PubMed and Scopus from December 2019 to December 2020 using the following search terms: ‘statin’ combined with ‘COVID-19’ or ‘SARS-CoV-2’ or ‘coronavirus’ (Fig. 1 ). Two independent researchers (A.S., M.M.) screened the literature, analyzed the selected studies and summarized the search results, using the same inclusion and exclusion criteria (see below). Any resulting discrepancies were resolved by mutual discussion. The reference lists of previous systematic reviews and meta-analyses [12], [13] and of any related narrative reviews or commentaries [16], especially those involving diabetes populations [3], [4], were manually examined to identify any additional publications relevant to the present study. A search for duplicates was done manually.
Fig. 1.
Flow chart of the study selection process: some studies reported data on both in-hospital mortality and severe coronavirus disease 2019 (COVID-19) infection.
Inclusion and exclusion criteria
In the absence of randomized clinical trials (RCTs), only observational studies could be included in the analyses. The inclusion criterion was observational studies in patients with COVID-19, from which the following relevant information was collected: (i) number of patients fulfilling criteria for severe illness (mainly assessed as admission to ICU or a need for IMV) and/or in-hospital death; and (ii) number of patients treated with any statin before admission (‘statin users’) or not (‘non-users’). All types of observational studies were taken into account (retrospective, single-centre, multicentre, cohort study, case series …) in the initial literature search whatever the number of subjects involved. Also, despite the present study being mainly focused on hospitalized patients, other studies of outpatients were also considered for a separate complementary investigation.
Exclusion criteria were: (i) commentaries or hypothetical mechanistic papers; (ii) narrative or comprehensive reviews; (iii) preprint papers not yet peer-reviewed or officially published; and (iv) observational studies with no reporting of the number of statin users and non-users who developed either of the two study outcomes (in-hospital mortality or severe COVID-19 infection). If important data were not clearly reported in the original papers, their corresponding authors were contacted to obtain further valuable information if available.
Data extraction
The following data were extracted from all included studies: (i) total number of patients involved in the study; (ii) number of statin users and non-users; (iii) number of patients who progressed to more severe forms of COVID-19 (ICU admission and/or IMV) or who died within either 7 or 28 days of hospitalization (if this information was available); (iv) mean age of the overall study population (including statin users and non-users if this information was available); and (v) mode of comparison used in the reported findings (univariate, multivariate, propensity score-matching, logistic regression modelling). Studies including patients with diabetes were also identified along with type of DM and, finally, studies of outpatients rather than in-hospital patients were collected to be analyzed separately.
Statistical analysis
Differences between statin users vs non-users were examined by odds ratio (OR) and 95% confidence interval (CI), with P < 0.05 considered statistically significant. Interstudy heterogeneity was assessed using Cochran’s Q test statistic and the Higgins and Thompson I² index; if the latter value was > 50%, this indicated a substantial degree of heterogeneity. Meta-analyses were performed using Review Manager (RevMan) 5.3 software (The Nordic Cochrane Centre, Copenhagen, Denmark). When separate analyses were available in some studies, comparisons between univariate vs multivariate/adjusted results were performed using paired t test.
Results
Study characteristics
The study selection process is depicted in Fig. 1. A total of 325 references were identified by the initial electronic search using the MeSH (Medical Subject Headings) terms ‘statin’ combined with ‘COVID-19’ or ‘SARS-CoV-2’ or ‘coronavirus’. As the first step, duplicates and citations based on study characteristics were excluded after title and abstract evaluation (n = 245). The second step was to exclude other citations based on the inclusion/exclusion criteria after abstract/full-text evaluation.
Initially, 24 eligible observational studies were identified that reported valuable data on in-hospital mortality and/or COVID-19 disease severity, including three studies of patients with diabetes [14], [15], [17]. Of these selected studies, 14 provided data on in-hospital mortality [15], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30] (Table 1 )—although one reported only adjusted hazard ratios (HRs) with no crude numbers of deaths in statin users vs non-users and, therefore, could not be included in the meta-analysis (authors were contacted, but did not communicate their results) [26]—while 11 studies reported data on COVID-19 severity (as determined by ICU, IMV; Table 2 ) [18], [22], [23], [27], [28], [30], [31], [32], [33], [34], [35]. Also, a study by Tan et al. [36] collected very few events in a Chinese population (personal communication from the authors), thereby preventing the use of this study in the final analysis. In addition, a Japanese study by Higuchi et al. [37] included only seven patients treated with statins; however, as the very high reported HR could be considered an outlier compared with the other studies, this study was likewise not included in the meta-analysis.
Table 1.
Statin use and in-hospital mortality in patients with coronavirus disease 2019 (COVID-19).
| Reference | Country | Type of study | Patients (total n) |
Mean agea (years) |
Statin (n/N) |
No statin (n/N) |
HR (95% CI)b |
HR (95% CI)c P |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. [18] |
China | Retrospective, multicentre | 4305 | 58 (66 vs 57), 65 vs 65 after 4:1 PSM |
45/861 (5.2%) |
325/3444 (9.4%) |
0.53 (0.38–0.73) |
0.58 (0.43–0.80), univariate after 4:1 PSM, P = 0.001 |
28-day mortality |
| Mallow et al. [19] | USA | Retrospective, multicentre | 21,676 | 65 | 1039/5313 (19.6%) |
3896/ 16,363 (23.8%) |
0.78 (0.72–0.84) |
0.54 (0.49–0.60), logistic regression, P < 0.001 |
In-hospital mortality |
| Krishnan et al. [20] | USA | Retrospective, single-centre | 152 | 66 | 57/92 (62.0%) |
35/71 (49.3%) |
1.68 (0.89–3.14) |
NA | ICU mortality |
| Rodriguez-Nava et al. [21] |
USA | Retrospective, single-centre | 87 | 68 | 23/47 (48.9%) |
25/40 (62.5%) |
0.57 (0.24–1.36 |
0.38 (0.18–0.77), multivariable Cox regression, P = 0.008 |
In-hospital (ICU) mortality |
| Saeed et al. [15] |
USA | Retrospective, single-centre | 4252 | 65 | 312/1355 (23.0%) |
782/2897 (27.0%) |
0.81 (0.70–0.94) |
0.88 (0.83–0.94), PSM, P < 0.01 |
In-hospital mortality |
| Gupta et al. [22] |
USA | Retrospective cohort (2 centres) |
All cohort: 2626, PSM: 1296 |
70/62, (69/71) |
NA/951, 96/648 (14.8%) |
NA/1675, 172/648 (26.5%) |
0.48 (0.36–0.64), univariate after 1:1 PSM |
0.59 (0.38–0.63), multivariable, adjusted, all cohort, P < 0.001 |
30-day mortality |
| Song et al. [23] | USA | Retrospective, single-centre | 249 |
62 (71 vs 54) |
27/123 (22.0%) |
15/126 (11.9%) |
2.08 (1.05–4.14) |
0.88 (0.37–2.08), fully adjusted, P = 0.781 |
In-hospital mortality |
| Grasselli et al. [24] | Italy | Retrospective, multicentre | 3988 | 63 (66 vs 61) |
479/741 (64.6%) |
1411/3165 (44.6%) |
2.27 (1.92–2.68) |
0.98 (0.81–1.20), multivariable, P = 0.87 |
In-hospital mortalityd |
| Rossi et al. [25] | Italy | Retrospective, single-centre | 71 | 72 (71 vs 73) |
9/42 (21.4%) |
10/29 (34.5%) |
0.52 (0.18–1.50) |
NA P < 0.05 |
In-hospital mortality |
| Bifulco et al. [26] | Italy | Retrospective, single-centre | 541 (123 deaths) |
65 (73 vs 63) |
NA/117 | NA/424 | NA | 0.75 (0.26–2.17), adjusted P = 0.593 |
In-hospital mortality [15] |
| Masana et al. [27] | Spain | Retrospective, multicentre | 1162 (after genetic matching) | 67 (73 vs 62) |
115/581 (19.8%) |
148/581 (25.4%) |
0.72 (0.55–0.95), univariate (after genetic matching) |
0.60 (0.39–0.92), competing-risks, P = 0.02 |
In-hospital mortality |
| Butt et al. [28] | Denmark | Cohort | 4842 | (73 vs 50) | 292/843 (34.6%) |
589/3999 (14.7%) |
2.57 (2.34–2.96), unadjusted Cox regression |
1.05 (0.89–1.23), fully adjusted | All-cause mortality |
| Alamdari et al. [29] | Iran | Retrospective, single-centre | 459 | 62 | 6/117 (5.1%) |
57/342 (16.7%) |
0.27 (0.11–0.64) |
NA P = 0.002 |
In-hospital mortality |
| Soleimani et al. [30] | Iran | Retrospective, single-centre | 254 | 66 | 17/66 (25.8%) |
51/188 (27.1%) |
0.93 (0.49–1.76) |
NA | In-hospital mortality |
n/N, number of deaths/number of patients; HR, hazard ratio; CI, confidence interval; PSM, propensity score-matching; NA, not available; ICU, intensive care unit.
Users vs non-users.
Univariate models (see Fig. 2).
Multivariate or adjusted models.
From ICU admission to hospital discharge.
Table 2.
Statins and severe disease in hospitalized patients with coronavirus disease 2019 (COVID-19).
| Reference | Country | Type of study | Patients (total n) |
Mean age, yearsa | Statin (n/N) |
No statin (n/N) |
HR (95% CI)b |
HR (95% CI)c P |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. [18] |
China | Retrospective, multicentre | 4305 | 58 (66 vs 57), 65 vs 65 after 4:1 PSM |
64/861, 24/861 |
353/3444, 192/3444 |
0.80 (0.62–1.05), 0.51 (0.34–0.78) |
P = 0.110, P = 0.002 |
ICU, IMV |
| Yan et al. [31] | China | Retrospective, multicentre | 619 | 49 | 5/16 (31.3%) |
123/594 (20.7%) |
1.74 (0.59–5.10) | 0.98 (0.32–2.99), P = 0.97 |
Severe or critical disease |
| Gupta et al. [22] | USA | Retrospective cohort (2 centres) |
All cohort: 2626, PSM: 1296 |
70/62, 70 (69/71) |
NA/951, 179/648 (27.6%) |
NA/1675, 269/648 (41.5%) |
NA, 0.54 (0.43–0.68), univariate (after 1:1 PSM) |
0.54 (0.44–0.67), multivariable, adjusted, all cohort, P < 0.001 |
IMV or mortality |
| Song et al. [23] | USA | Retrospective, single-centre | 249 | 62 | 19/123 (15.4%) |
26/126 (20.6%) |
0.70 (0.37–1.35) |
0.45 (0.20–0.99), fully adjusted, P = 0.048 |
Tracheal intubation |
| Argenziano et al. [32] | USA |
Retrospective, single-centre, case series |
1000 (850 with statin data) | 63 | 62/218 (28.4%) |
174/632 (27.5%) |
1.05 (0.74–1.47) |
NA | ICU |
| Daniels et al. [33] | USA | Retrospective, single-centre | 170 | 59 | 20/46 (43.5%) |
70/124 (56.5%) |
0.59 (0.30–1.17) |
0.29 (0.11–0.71), multivariable logistic regression, P = 0.009 |
Death or ICU |
| Dreher et al. [34] | Germany | Retrospective, single-centre | 50 | 65 | 9/18 (50.0%) |
15/32 (46.9%) |
1.13 (0.36–3.60) | NA | ARDS |
| Masana et al. [27] | Spain | Retrospective, multicentre | 1162 | 67 (73 vs 62) |
84/581 (14.5%) |
96/581 (16.6%) |
0.85 (0.52–1.17), univariate (after genetic matching) |
P = 0.36 | IMV |
| Butt et al. [28] | Denmark | Cohort | 4842 | 73 vs 50 | 204/843 (24.2%) |
419/3999 (10.5%) |
2.41 (2.04–2.85), unadjusted Cox regression |
1.16 (0.95–1.41), fully adjusted | Severe disease |
| Meunier et al. [35] | France | Retrospective, single-centre | 234 | 67 | 26/42 (61.9%) |
88/192 (45.8%) |
1.92 (0.97–3.81) |
NA | Severe disease |
| Soleimani et al. [30] | Iran | Retrospective, single-centre | 254 | 66 | 52/66 (78.8%) |
130/188 (69.1%) |
1.66 (0.85–3.23) | NA | Severe disease |
n/N, number of deaths/number of patients; HR, hazard ratio; CI, confidence interval; PSM, propensity score-matching; ICU, intensive care unit; IMV, invasive mechanical ventilation; NA, not available; ARDS, acute respiratory distress syndrome.
Users vs non-users.
Univariate model (see Fig. 3).
Multivariate or adjusted model.
Three studies that specifically focused on patients with DM were also identified [14], [15], [17] (Table 3 ) as well as some studies carried out in outpatients exposed to SARS-CoV-2 (see Discussion section below). However, these studies were not included in the two main meta-analyses, but were instead analyzed separately.
Table 3.
Statin use and clinical outcomes (tracheal intubation or mortality) in diabetes patients with coronavirus disease 2019 (COVID-19).
| Reference | Country | Type of study | Patients (total n) | Mean age (years) |
Statin (n/N) |
No statin (n/N) |
HR (95% CI) | P | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Cariou et al. [14] (type 2 diabetes patients) | France | Retrospective, multicentre, hospitalized patients | 2449 | 70.9 | 355/1192 (29.8%), 431/1192 (36.2%) |
339/1257 (27.0%), 425/1257 (33.8%) |
1.38 (1.04–1.83) after IPTW, 1.22 (0.98–1.58) after IPTW |
P = 0.1338, P = 0.2191 |
7-day tracheal intubation and/or death (primary outcome), 28-day primary outcome |
| 220/1192 (18.5%), 229/1192 (19.2%) |
235/1257 (18.7%), 248/1257 (19.7%) |
1.18 (0.86–1.61) after IPTW, 1.13 (0.83–1.53) after IPTW |
P = NA, P = NA |
7-day tracheal intubation, 28-day tracheal intubation |
|||||
| 153/1192 (12.8%), 285/1192 (23.9%) |
123/1257 (9.8%), 229/1257 (18.2%) |
1.74 (1.13–2.65) after IPTW, 1.46 (1.08–1.95) after IPTW |
P = 0.02, P < 0.001 |
7-day mortality, 28-day mortality |
|||||
| Saeed et al. [15] (diabetes patients) | USA | Retrospective, single-centre, hospitalized patients | 2266 | 68.0 | 236/983 (24%) |
500/1283 (39%) |
0.51 (0.43–0.61), multivariable, adjusted, 0.88 (0.84–0.91) after IPTW |
P < 0.001, P < 0.001 |
In-hospital mortality |
| Holman et al. [17] (type 2 diabetes patients) | UK | Population-based cohort | 2,874,020 | 67.5 | 7355/ 2,099,505 (3.5%) |
3086/752,245 (4.1%) |
0.72 (0.69–0.75) adjusted for demographic/clinical characteristics |
P < 0.0001 | Covid-19-related death (no focus on hospital) |
| Holman et al. [17] (type 1 diabetes patients) | UK | Population-based cohort | 264,390 | 46.6 | 338/118,995 (2.7%) |
120/142,710 (0.8%) |
0.82 (0.65–1.03) adjusted for demographic/clinical characteristics |
P = 0.081 | Covid-19-related death (no focus on hospital) |
n/N, number of deaths/number of patients; HR, hazard ratio; CI: confidence interval; IPTW, inverse probability of treatment weighting (with logistic regression analysis after propensity score-matching); NA, not available.
In-hospital mortality
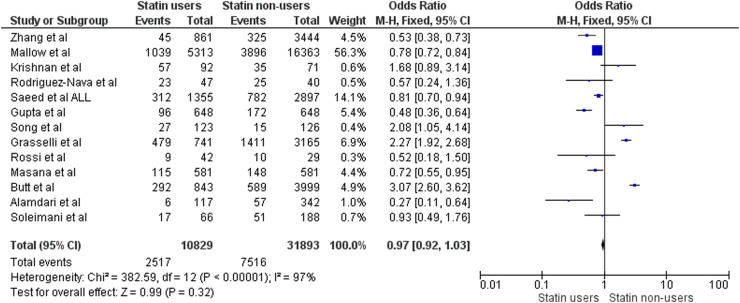
The included observational studies reporting data on in-hospital mortality are listed in Table 1 [15], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. Results of the corresponding meta-analysis are illustrated in Fig. 2 (which includes all studies except for Bifulco et al. [26]). A total of 13 studies were included in the meta-analysis for in-hospital mortality, involving a total of 10,829 statin users (2517 deaths) and 31,893 non-users (7516 deaths). Univariate analysis found no statistically significant reduction in death rate (OR: 0.97, 95% CI: 0.92–1.03) on comparing statin users with non-users, although there was substantial between-study heterogeneity (I² = 97%).
Fig. 2.
Meta-analysis of studies comparing in-hospital mortality in statin users vs non-users with COVID-19 infection. M-H, Mantel–Haenszel method.
Clinical outcomes of severity
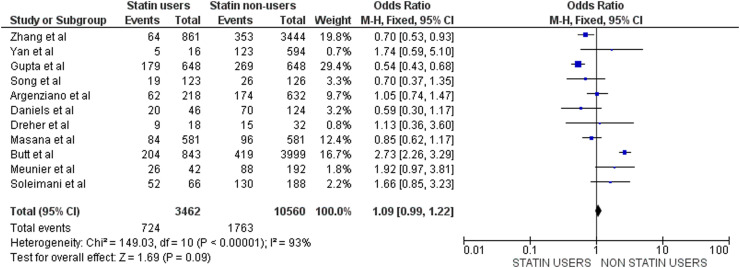
The list of included observational studies reporting data on disease severity (ICU, IMV) is presented in Table 2 [18], [22], [23], [27], [28], [30], [31], [32], [33], [34], [35], while results of the corresponding meta-analysis are illustrated in Fig. 3 . A total of 11 studies were included in this meta-analysis for a total of 3462 statin users (724 severity endpoints) and 10,560 non-users (1763 severity endpoints). Univariate analysis could find no reduction in the incidence of severe COVID-19 among statin users (OR: 1.09, 95% CI: 0.99–1.22) and, once again, high heterogeneity (I² = 93%) was noted.
Fig. 3.
Meta-analysis of studies comparing outcomes according to severity of COVID-19 infection in statin users vs non-users. M-H, Mantel–Haenszel method.
Multivariable analysis and adjusted comparisons
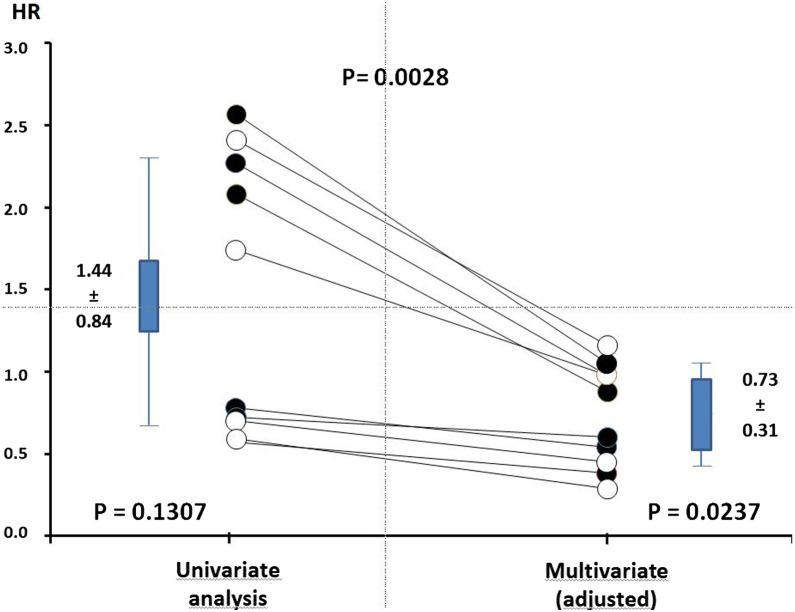
As statin users have different clinical characteristics from non-users, which might expose them to a greater risk of developing more severe COVID-19 infection, it is important to take into account the potentially confounding factors that could influence the final results. In 10 series of data—six considering in-hospital mortality (Table 1) and four considering COVID-19 severity (Table 2) [19], [21], [23], [24], [27], [28], [31], [33]—multivariable analyses or those adjusted for covariates, if available, resulted in more favourable results than univariate analyses in patients treated with statins compared with patients not treated with statins at admission to hospital: adjusted OR (mean ± SD) 0.73 ± 0.31 vs non-adjusted OR 1.44 ± 0.84; P = 0.0028; Fig. 4 ). Thus, the adjusted OR revealed a statistically significant reduction by 27% of hard clinical outcomes in statin users vs non-users (P = 0.0237). Furthermore, all studies that used propensity score matching for their comparison of in-hospital mortality reported hazard ratios below one in favour of statin users, respectively, 0.58 (P = 0.001) [18], 0.88 (P < 0.01) [15] and 0.48 (P < 0.001) [22] (Table 1). One well-recognized risk factor that can easily be identified is older age in statin users vs non-users, especially in studies reporting higher HRs (> 2) on univariate analyses [23], [24], [28] (Table 1, Table 2). However, it was not possible to identify any other risk factors for more severe COVID-19 and its associated increased death rate, although some studies reported more male patients, more diabetics, more hypertensives and/or more people with previous CV disease in statin users than in non-users, a finding in agreement with international guidelines for optimal management of such at-risk patients [8].
Fig. 4.
Comparison of hazard ratios (HRs) in statin users vs non-users using multivariate (adjusted) analysis compared with univariate analysis based on 10 sets of data, including six on in-hospital mortality (solid circles) and four on disease severity (open circles). For more detailed information, see Table 1, Table 2. Data are from references [[19], [21], [23], [24], [27], [28], [31], and 33].
Results in patients with diabetes
Only a few studies focused on the impact of statins in patients with diabetes, with divergent results (Table 3). While Saeed et al. [15] reported significant reductions in in-hospital mortality among statin-users compared with non-users in a US study (HR: 0.88, 95% CI: 0.84–0.91), the opposite results were reported by Cariou et al. [14] in the French observational CORONADO study (HR: 1.46, 95% CI: 1.08–1.95). Such differences could not be explained by different modes of comparison, as both studies used inverse probability of treatment weighting approaches for their comparisons. Thus, no clear explanation for such conflicting results have been proposed. Holman et al. [17] reported better prognoses for COVID-19 patients with type 2 DM and, to a lesser extent, with type 1 DM, who were treated with statins; however, these data were obtained in a different outpatient cohort (population-based cohort study; Table 3).
Discussion
Both meta-analyses of retrospective observational studies investigating the effects of previous statin therapy on in-hospital mortality associated with COVID-19 and severity of the infection (admission to ICU, need for IMV) revealed no reductions in statin users compared with non-users, whereas a substantial yet poorly explained heterogeneity was observed between studies. In any case, these results should be interpreted with caution because statin users are generally patients who have additional risk factors, such as older age, male gender, DM, hypertension and CV disease [8], all of which have been reported to worsen COVID-19 prognoses [7], [9], [38]. This difference in patients’ characteristics between statin users and non-users explains why the fully adjusted and multivariate analyses gave more favourable results than univariate analyses (Table 1, Table 2). In fact, the results using adjusted data confirm those reported in the meta-analysis by Kow et al. [12], who reported a 30% reduction in fatal or severe COVID-19 infection. On the other hand, the univariate analyses confirm the lack of significant protection among statin users reported in the meta-analysis by Hariyanto et al. [13].
Previous studies before the COVID-19 outbreak had already reported favourable statin effects on the outcome of severe pulmonary infection [39], especially in the presence of a hyperinflammatory phenotype of ARDS, that were associated with improved survival with simvastatin compared with a placebo [40]. In contrast, other studies have reported disappointing results with the use of statins as a late treatment for ARDS [41], [42]. On the other hand, statins have been shown to improve prognoses and reduce mortality among patients in hospital with influenza virus infections [43], [44], [45]. An association between outpatient statin treatment and reduction of disease severity among patients hospitalized during the 2009 H1N1 pandemic has also been reported [46]. In 2015, it was even suggested, based on theoretical grounds, that statins might be able to decrease the fatality rate of Middle East Respiratory Syndrome (MERS) infection [47]. However, caution is nonetheless required in the absence of RCTs. Indeed, while observational studies have reported improved outcomes in patients with various infections (including community-acquired pneumonia) or sepsis who were taking statins, most RCTs of inpatients with sepsis or pneumonia requiring IMV failed to demonstrate any beneficial effect with statin therapy [48], [49].
Some retrospective studies have suggested the possible detrimental effect of reduced serum low-density lipoprotein (LDL) cholesterol levels on COVID-19 prognoses [50]. However, reverse causality—SARS-CoV-2 infection as a cause of LDL cholesterol reduction—instead of true causality, where LDL cholesterol reduction is a factor promoting the viral infection, might explain the association between low LDL cholesterol and severe COVID-19 manifestations. As previously discussed [51], such an association may distract from refutations of the potential benefits of statin therapy in clinical settings of patients exposed to SARS-CoV-2 infection. In fact, every study included in the present meta-analyses failed to present results on blood lipid levels, and only a few [18], [19], [21] made clear mention of whether or not statin use identified at admission was maintained throughout the stay in hospital [16]. In one study [52] focusing on liver abnormalities associated with COVID-19, statin use was commonplace both before admission (40%) and during hospitalization (80%), with no differences in peak liver biochemistry values between users and non-users.
Cariou et al. [14], using data from the large-scale multicentre CORONADO study carried out in France, reported that routine statin treatment is significantly associated with increased mortality (based on 7-day and 28-day in-hospital death rates) in patients with type 2 DM hospitalized for COVID-19. However, the composite primary outcome, comprising tracheal intubation and/or death within either 7 or 28 days of admission, was not statistically different between statin users and non-users (Table 3). The association between statin use and outcomes was estimated by logistic regression analysis after applying inverse probability of treatment weighting using a propensity score-weighting approach. The CORONADO study included type 2 DM patients who had a higher risk of mortality than in other studies, especially the one by Holman et al. [17], whose population-based cohort study in the UK reported positive results with statins mostly in patients with type 2 DM. Thus, it was proposed that the effect of statins on COVID-19 prognosis might vary according to infection severity [14]. However, this hypothesis is still only speculative and has yet to be confirmed.
In a US study of hospitalized diabetes patients, the mortality rate was even higher than that reported in CORONADO, although statin use was significantly associated with a reduction of in-hospital mortality [15]. More important, this difference was observed despite the use of the same statistical approach in both studies (inverse probability of treatment weighting; Table 3). In addition, it should be noted that this same CORONADO study, using a similar approach to analyze collected data, found that previous metformin therapy reduced both 7-day and 28-day in-hospital mortality rates [53], a finding in agreement with those reported in other studies [54]. Nevertheless, the reason behind such mixed results in the CORONADO study of a protective effect with metformin and deleterious effects with statins remains unclear, especially as both statins [55] and metformin [54] exert pleiotropic effects that could contribute to reducing inflammation and inducing vascular protection.
In addition to the studies of patients hospitalized due to COVID-19 included in the present meta-analyses, two other studies were carried out outside of hospital and reported contrasting results. The first was performed in COVID-19-infected older adults residing in nursing homes in Belgium. This retrospective multicentre cohort study found an association between statin use and the absence of symptoms in COVID-19 that remained statistically significant even after adjusting for covariates (OR: 2.65, 95% CI: 1.13–6.68). However, the effect of statin intakes on serious clinical outcomes was not statistically significant, albeit trending in the same beneficial direction (OR: 0.75, 95% CI: 0.24–1.87) [56]. In the multinational multicentre Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS) cohort study, a significant univariate association between statin intake and increased risk of complicated clinical stages of COVID-19 at diagnosis was found (OR: 1.40, 95% CI: 1.09–1.80; P = 0.009). Unfortunately, statin use was excluded from multivariable analyses (adjusted for age, gender, underlying CV diseases, DM, pulmonary diseases) due to model quality, as admitted by the authors [57]. Differences between the results of these two studies are most likely explained by the adjustment for covariates in the Belgian study [56] compared with only univariate associations in the international one [57]. Finally, in a symptom surveillance study for COVID-19 in Germany, a statistically significant inverse relationship between self-reported typical COVID-19 symptoms and self-reported statin therapy was found [58], although whether or not statin therapy had beneficial effects for combatting COVID-19 could not be deduced from the survey.
Divergent mechanisms have been discussed regarding the potential impact of statins on COVID-19 infection. On the one hand, negative effects are possible given that statins may increase cellular expression of angiotensin-converting enzyme 2 (ACE2), the primary receptor allowing entry of SARS-CoV-2 into human cells [59], [60]. It has also been reported that lower LDL cholesterol levels are associated with COVID-19 severity [61]. On the other hand, evidence in silico suggests that statins might serve as efficient inhibitors of the main protease of SARS-CoV-2 [62]. In addition, statin-induced decreases of cholesterol levels in plasma membranes could alter the assembly of ACE2 receptors, resulting in failure of SARS-CoV-2 internalization [10], [63]. Moreover, statins may exert anti-inflammatory [55], immunomodulatory [64] and antioxidant properties [65]. As cytokine storms and consecutive bouts of inflammation play crucial roles in the development of poorer outcomes for COVID-19 [66], positive effects may be expected from these pleiotropic effects of statins [11], [51], [67]. It should also be noted that statins have been described to have antiviral properties [68]. Finally, statins, through their antithrombotic properties [69], could also attenuate diffuse thrombosis, a key complication characteristic of SARS-CoV-2 infection that can markedly worsen a poor prognosis [66]. Table 4 summarizes the various hypothetical mechanisms that might either negatively or positively influence COVID-19 outcomes. In general, there are more arguments favouring the continued use of statin therapy rather than its interruption in patients exposed to SARS-CoV-2 [11], [67], [70]. While an in-depth description of these potential mechanisms is beyond the scope of the present report, such details may be found elsewhere [10], [11], [51], [68], [71], [72], [73].
Table 4.
Hypothetical negative and positive statin mechanisms capable of influencing coronavirus disease 2019 (COVID-19) outcomes.
| Negative mechanisms/concerns | Positive mechanisms |
|---|---|
| Enhancement of SARS-CoV-2 entry through increased cellular expression of ACE2 [11], [60] | Reduction of viral cellular entry through decreased cell membrane cholesterol content (antiviral activity) [63], [68] |
| Possible weakened leucocyte function [81] | ACE2-mediated conversion of angiotensin II to angiotensin [1], [2], [3], [4], [5], [6], [7], [60], [73] |
| Drug–drug interactions with antiviral agents [59] | Inhibition of SARS-CoV-2 main protease (Mpro) [62] |
| Increased risk of myopathies and rhabdomyolysis [82] | Modulation of autophagy [71] |
| Potential hepatotoxicity [35] | Anti-inflammatory effects [55] |
| Immunomodulatory effects [64] | |
| Oxidative stress reduction [65] | |
| Antithrombotic and endothelial effects [69] | |
| Cardiovascular protection [8] |
For more information, see Subir et al. [10]; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2.
Among the strengths of the present study is that it has analyzed a much larger set of observational studies than the two previously published meta-analyses of the same topic [12], [13] while including only peer-reviewed papers, with additional information obtained from some other investigators upon request. In addition, the present meta-analysis has looked at the effects of both in-hospital mortality and admission to ICUs (with or without IMV) and produced consistent results.
However, several limitations should also be acknowledged. First, none of the included studies was prospectively designed to test the study hypothesis and all were retrospective observational studies, thereby bringing the possibility of inherited biases. Second, the substantial heterogeneity observed between the selected studies may blunt the robustness of any conclusions drawn. Third, the characteristics of statin users were different from those of non-users because of the lack of randomization and the classic recommendations for treating patients at risk of CV disease with statins [8]. If anything, the identified risk factors for more severe COVID-19 infection should be more prevalent among statin users than non-users (older age, male gender, previous CV disease, hypertension, DM) [7], yet such detailed information is missing from some reports. Fourth, as emphasized by Fedson [16], most of the papers selected for inclusion in these meta-analyses made no clear mention of whether or not statin use identified at admission was continued throughout the hospital stay, thereby allowing some speculation as to whether the maintenace of statins, or not, may have contributed to the wide heterogeneity observed between studies. In addition, certain important information was missing from most studies, such as the duration of previous statin therapy, the type and dosage of statin used, and the indication (primary or secondary CV prevention) for which it was given, all variables that might have influenced the final results. Moreover, detailed information on plasma lipid levels with statin therapy was absent from all of the selected retrospective observational studies. As a final limitation, the statistical significance of a P value of 0.0237, as observed for the statin-associated reduction in hard clinical outcomes related to COVID-19 on multivariate-adjusted analysis, is within the range (from 0.05 to 0.005) where any translation to strong clinical relevance remains a subject of debate [74].
Nevertheless, the present meta-analytical findings still serve to strengthen practical guidelines for the management of patients with COVID-19, especially those at high CV risk being treated with statins. Indeed, as SARS-CoV-2 infection is associated with a heavy inflammatory burden that can induce vascular damage, myocarditis and cardiac arrhythmias [9], these findings suggest that CV risk factors, including dyslipidaemias [8], should be judiciously controlled as per evidence-based guidelines [75]. On the other Hand, as there is still no evidence that lipid-lowering therapy is unsafe in patients with COVID-19, such treatments should not be interrupted just because of the pandemic or in patients at increased risk of COVID-19 infection. Patients exposed to SARS-CoV-2 who are already using statin therapy should continue the treatment if not contraindicated by European Society of Cardiology guidelines [76] and other US recommendations [38]. On the other hand, great care should be taken to avoid any adverse interactions between lipid-lowering medications and drugs that might be used to treat COVID-19 (for instance, some antiviral agents) [59], especially in patients with abnormal liver function tests or myopathies [77]. One caveat is that, while there is no reason to stop statin therapy in patients hospitalized for COVID-19, the level of evidence remains too low—in the absence of RCTs, some of which are ongoing as reported elsewhere [38], [78], [79], although the impact of their results may be attenuated by successful vaccine strategies in the future—to justify initiating statin therapy in statin-free patients in hopes of reducing the burden of disease and improving clinical outcomes [38], [80].
Conclusion
Patients with the usual comorbidities, including hypertension, CV disease and DM (especially type 2), are at greater risk of severe COVID-19 infection and its related ARDS, requiring admission to ICUs for IMV, while also being exposed to an increased risk of mortality, and most of these patients are taking statins routinely as per DM and CV guidelines. Overall, meta-analyses of observational studies have concluded (even though caution is required because of substantial between-study heterogeneity) that statin users are at similar risk of severe illness and in-hospital mortality as are statin non-users, at least according to univariate analyses. However, having such a similar prognosis conflicts with the expected high risk in statin-treated patients, whose different baseline characteristics should confer greater risk in the presence of COVID-19 (older age, more comorbidities and/or CV antecedents). Nevertheless, multivariable adjusted findings for potential confounding factors have indicated improved prognoses among statin users compared with non-users. Thus, continued statin use is advised for such patients exposed to SARS-CoV-2, whereas the initiation of statin therapy to treat COVID-19 is as yet unsubstantiated by the evidence. In addition, the findings of retrospective observational studies still require confirmation by RCTs.
Funding
No sources of funding were used to assist in the preparation of this manuscript.
Conflict of interest statement
The author declares no conflict of interest directly in relation with the content of this paper.
Acknowledgments
The author thanks Monique Marchand (M.M.) for her invaluable help in the literature search and subsequent statistical analyses. Many thanks also to the investigators (Mallow et al., Grasselli et al., Daniels et al., Meunier et al., Tan et al.) who kindly responded to the author’s requests to provide any additional valuable data not initially mentioned in their original publications.
References
- 1.Li J., Huang D.Q., Zou B., Yang H., Hui W.Z., Rui F., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2020 doi: 10.1002/jmv.26424. Aug 13;10.1002/jmv.26424. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheen A.J., Marre M., Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Targher G., Mantovani A., Wang X.B., Yan H.D., Sun Q.F., Pan K.H., et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020;46:335–337. doi: 10.1016/j.diabet.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuin M., Rigatelli G., Zuliani G., Rigatelli A., Mazza A., Roncon L. Arterial hypertension and risk of death in patients with COVID-19 infection: systematic review and meta-analysis. J Infect. 2020;81:e84–6. doi: 10.1016/j.jinf.2020.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian W., Jiang W., Yao J., Nicholson C.J., Li R.H., Sigurslid H.H., et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. doi: 10.1002/jmv.26050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Subir R., Jagat J.M., Kalyan K.G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19) Diabetes Metab Syndr. 2020;14:1225–1229. doi: 10.1016/j.dsx.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minz M.M., Bansal M., Kasliwal R.R. Statins and SARS-CoV-2 disease: current concepts and possible benefits. Diabetes Metab Syndr. 2020;14:2063–2067. doi: 10.1016/j.dsx.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14:1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cariou B., Goronflot T., Rimbert A., Boullu S., Le May C., Moulin P., et al. Routine use of statins and increased mortality related to COVID-19 in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.001. Oct 19;S1262-3636(20)30153-1. doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saeed O., Castagna F., Agalliu I., Xue X., Patel S.R., Rochlani Y., et al. Statin use and in-hospital mortality in diabetics with COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018475. e018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fedson D.S. Statin treatment of COVID-19. Am J Cardiol. 2020;136:171–173. doi: 10.1016/j.amjcard.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holman N., Knighton P., Kar P., O’Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.06.015. 176-87 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallow P.J., Belk K.W., Topmiller M., Hooker E.A. Outcomes of hospitalized COVID-19 patients by risk factors: results from a United States hospital claims database. J Health Econ Outcomes Res. 2020;7:165–174. doi: 10.36469/jheor.2020.17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan S., Patel K., Desai R., Sule A., Paik P., Miller A., et al. Clinical comorbidities, characteristics, and outcomes of mechanically ventilated patients in the State of Michigan with SARS-CoV-2 pneumonia. J Clin Anesth. 2020;67 doi: 10.1016/j.jclinane.2020.110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Res Sq. 2020 doi: 10.1038/s41467-021-21553-1. Aug 11;rs.3.rs-56210. doi: 10.21203/rs.3.rs-56210/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song S.L., Hays S.B., Panton C.E., Mylona E.K., Kalligeros M., Shehadeh F., et al. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020;9:759. doi: 10.3390/pathogens9090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy. Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi R., Talarico M., Coppi F., Boriani G. Protective role of statins in COVID 19 patients: importance of pharmacokinetic characteristics rather than intensity of action. Intern Emerg Med. 2020;15:1573–1576. doi: 10.1007/s11739-020-02504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bifulco M., Ciccarelli M., Bruzzese D., Dipasquale A., Lania A.G., Mazziotti G., et al. The benefit of statins in SARS-CoV-2 patients: further metabolic and prospective clinical studies are needed. Endocrine. 2020;20:1–3. doi: 10.1007/s12020-020-02550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masana L., Correig E., Rodriguez-Borjabad C., Anoro E., Arroyo J.A., Jerico C., et al. Effect of statin therapy on Sars-Cov-2 infection-related mortality in hospitalized patients. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. Nov 2;pvaa128. doi: 10.1093/ehjcvp/pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butt J.H., Gerds T.A., Schou M., Kragholm K., Phelps M., Havers-Borgersen E., et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alamdari N.M., Afaghi S., Rahimi F.S., Tarki F.E., Tavana S., Zali A., et al. Mortality risk factors among hospitalized COVID-19 patients in a major referral center in Iran. Tohoku J Exp Med. 2020;252:73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 30.Soleimani A., Kazemian S., Karbalai Saleh S., Aminorroaya A., Shajari Z., Hadadi A., et al. Effects of angiotensin receptor blockers (ARBs) on in-hospital outcomes of patients with hypertension and confirmed or clinically suspected COVID-19. Am J Hypertens. 2020 doi: 10.1093/ajh/hpaa149. Sep 12;hpaa149. doi: 10.1093/ajh/hpaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan H., Valdes A.M., Vijay A., Wang S., Liang L., Yang S., et al. Role of drugs used for chronic disease management on susceptibility and severity of COVID-19: a large case-control study. Clin Pharmacol Ther. 2020;108:1185–1194. doi: 10.1002/cpt.2047. [DOI] [PubMed] [Google Scholar]
- 32.Argenziano M.G., Bruce S.L., Slater C.L., Tiao J.R., Baldwin M.R., Barr R.G., et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniels L.B., Sitapati A.M., Zhang J., Zou J., Bui Q.M., Ren J., et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am J Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dreher M., Kersten A., Bickenbach J., Balfanz P., Hartmann B., Cornelissen C., et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int. 2020;117:271–278. doi: 10.3238/arztebl.2020.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meunier L., Meszaros M., Pageaux G.P. Letter to the Editors: statins and COVID-19: efficacy still to be proven. Hepatology. 2020 doi: 10.1002/hep.31511. Aug 7;10.1002/hep.31511. doi: 10.1002/hep.31511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan W.Y.T., Young B.E., Lye D.C., Chew D.E.K., Dalan R. Statin use is associated with lower disease severity in COVID-19 infection. Sci Rep. 2020;10:17458. doi: 10.1038/s41598-020-74492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higuchi T., Nishida T., Iwahashi H., Morimura O., Otani Y., Okauchi Y., et al. Early clinical factors predicting the development of critical disease in Japanese patients with COVID-19: a single-center, retrospective, observational study. J Med Virol. 2020 doi: 10.1002/jmv.26599. Oct 14;10.1002/jmv.26599. doi: 10.1002/jmv.26599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mechanick J.I., Rosenson R.S., Pinney S.P., Mancini D.M., Narula J., Fuster V. Coronavirus and cardiometabolic syndrome: JACC focus seminar. J Am Coll Cardiol. 2020;76:2024–2035. doi: 10.1016/j.jacc.2020.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feng Y. Efficacy of statin therapy in patients with acute respiratory distress syndrome/acute lung injury: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22:3190–3198. doi: 10.26355/eurrev_201805_15080. [DOI] [PubMed] [Google Scholar]
- 40.Calfee C.S., Delucchi K.L., Sinha P., Matthay M.A., Hackett J., Shankar-Hari M., et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6:691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha P., Delucchi K.L., Thompson B.T., McAuley D.F., Matthay M.A., Calfee C.S., et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44:1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiong B., Wang C., Tan J., Cao Y., Zou Y., Yao Y., et al. Statins for the prevention and treatment of acute lung injury and acute respiratory distress syndrome: a systematic review and meta-analysis. Respirology. 2016;21:1026–1033. doi: 10.1111/resp.12820. [DOI] [PubMed] [Google Scholar]
- 43.Frost F.J., Petersen H., Tollestrup K., Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 44.Vandermeer M.L., Thomas A.R., Kamimoto L., Reingold A., Gershman K., Meek J., et al. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 45.Atamna A., Babitch T., Bracha M., Sorek N., Haim B.Z., Elis A., et al. Statins and outcomes of hospitalized patients with laboratory-confirmed 2017-2018 influenza. Eur J Clin Microbiol Infect Dis. 2019;38:2341–2348. doi: 10.1007/s10096-019-03684-y. [DOI] [PubMed] [Google Scholar]
- 46.Fedson D.S. Treating influenza with statins and other immunomodulatory agents. Antiviral Res. 2013;99:417–435. doi: 10.1016/j.antiviral.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Yuan S. Statins may decrease the fatality rate of Middle East Respiratory Syndrome infection. mBio. 2015;6 doi: 10.1128/mBio.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan Y.D., Sun T.W., Kan Q.C., Guan F.X., Zhang S.G. Effect of statin therapy on mortality from infection and sepsis: a meta-analysis of randomized and observational studies. Crit Care. 2014;18:R71. doi: 10.1186/cc13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pertzov B., Eliakim-Raz N., Atamna H., Trestioreanu A.Z., Yahav D., Leibovici L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults - A systematic review and meta-analysis. Clin Microbiol Infect. 2019;25:280–289. doi: 10.1016/j.cmi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Fan J., Wang H., Ye G., Cao X., Xu X., Tan W., et al. Letter to the Editor: low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107 doi: 10.1016/j.metabol.2020.154243. 154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganjali S., Bianconi V., Penson P.E., Pirro M., Banach M., Watts G.F., et al. Commentary: statins, COVID-19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154375. 154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloom P.P., Meyerowitz E.A., Reinus Z., Daidone M., Gustafson J., Kim A.Y., et al. Liver biochemistries in hospitalized patients with COVID-19. Hepatology. 2020 doi: 10.1002/hep.31326. May 16. doi: 10.1002/hep.31326. [DOI] [PubMed] [Google Scholar]
- 53.Lalau J.-D., Al-Salameh A., Hadjadj S., Goronflot T., Wiernsperger N., Pichelin M., et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.101216. 101216. doi: 10.1016/j.diabet.2020.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scheen A.J. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46:423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schonbeck U., Libby P. Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 2004;109 doi: 10.1161/01.CIR.0000129505.34151.23. II18-26. [DOI] [PubMed] [Google Scholar]
- 56.De Spiegeleer A., Bronselaer A., Teo J.T., Byttebier G., De Tre G., Belmans L., et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21 doi: 10.1016/j.jamda.2020.06.018. 909-14 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jakob C.E.M., Borgmann S., Duygu F., Behrends U., Hower M., Merle U., et al. First results of the "Lean European Open Survey on SARS-CoV-2-Infected Patients (LEOSS)". Infection. 2020:1–11. doi: 10.1007/s15010-020-01499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urbach D., Awiszus F., Leiss S., Venton T., Specht A.V., Apfelbacher C. Typical COVID-19 symptoms are inversely associated with statin medication: cross-sectional digital study in Lower Saxony, Germany Results of the first German Symptom Surveillance Study for COVID-19. JMIR Public Health Surveill. 2020 doi: 10.2196/22521. Nov 16. doi: 10.2196/22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dashti-Khavidaki S., Khalili H. Considerations for statin therapy in patients with COVID-19. Pharmacotherapy. 2020;40:484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318 doi: 10.1152/ajpheart.00217.2020. H1084-H90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wei X., Zeng W., Su J., Wan H., Yu X., Cao X., et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reiner Z., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T., et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kocar E., Rezen T., Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1866 doi: 10.1016/j.bbalip.2020.158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeiser R. Immune modulatory effects of statins. Immunology. 2018;154:69–75. doi: 10.1111/imm.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maragaritis M., Channon K.M., Antoniades C. Statins as regulators of redox in the vascular endothelium: beyond lipid lowering. Antioxid Redox Signal. 2014;20:1198–1215. doi: 10.1089/ars.2013.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt N.M., Wing P.A.C., McKeating J.A., Maini M.K. Cholesterol-modifying drugs in COVID-19. Oxf Open Immunol. 2020;1 doi: 10.1093/oxfimm/iqaa001. iqaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorabi A.M., Kiaie N., Bianconi V., Jamialahmadi T., Al-Rasadi K., Johnston T.P., et al. Antiviral effects of statins. Prog Lipid Res. 2020;79 doi: 10.1016/j.plipres.2020.101054. [DOI] [PubMed] [Google Scholar]
- 69.Violi F., Calvieri C., Ferro D., Pignatelli P. Statins as antithrombotic drugs. Circulation. 2013;127:251–257. doi: 10.1161/CIRCULATIONAHA.112.145334. [DOI] [PubMed] [Google Scholar]
- 70.Radenkovic D., Chawla S., Pirro M., Sahebkar A., Banach M. Cholesterol in relation to COVID-19: should we care about It? J Clin Med. 2020;9:1909. doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodrigues-Diez R.R., Tejera-Munoz A., Marquez-Exposito L., Rayego-Mateos S., Santos Sanchez L., Marchant V., et al. Statins: Could an old friend help in the fight against COVID-19? Br J Pharmacol. 2020;177:4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abu-Farha M., Thanaraj T.A., Qaddoumi M.G., Hashem A., Abubaker J., Al-Mulla F. The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci. 2020;21:3544. doi: 10.3390/ijms21103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakur P., Mahajan K., Negi P.C., Ganju N., Asotra S., Kandoria A. Considerations for the use of statin therapy in Coronavirus Disease 2019 Era. Ann Clin Cardiol. 2020;2:55–59. [Google Scholar]
- 74.Monnier L., Bonnet F. Statistical and clinical significances: Are they equivalent? Diabetes Metab. 2020;46:413–414. doi: 10.1016/j.diabet.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 76.European Society of Cardiology (ESC) 2020. ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (latest access December 13, 2020). [Google Scholar]
- 77.Iqbal Z., Ho J.H., Adam S., France M., Syed A., Neely D., et al. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: an expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–136. doi: 10.1016/j.atherosclerosis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagele M.P., Haubner B., Tanner F.C., Ruschitzka F., Flammer A.J. Endothelial dysfunction in COVID-19: current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cadegiani F.A. Repurposing existing drugs for COVID-19: an endocrinology perspective. BMC Endocr Disord. 2020;20:149. doi: 10.1186/s12902-020-00626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fajgenbaum D.C., Rader D.J. Teaching old drugs new tricks: statins for COVID-19? Cell Metab. 2020;32:145–147. doi: 10.1016/j.cmet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minetti G. Mevalonate pathway, selenoproteins, redox balance, immune system, Covid-19: reasoning about connections. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ward N.C., Watts G.F., Eckel R.H. Statin toxicity. Circ Res. 2019;124:328–350. doi: 10.1161/CIRCRESAHA.118.312782. [DOI] [PubMed] [Google Scholar]