Summary
Dynamic microtubules are essential for many processes in the lives of eukaryotic cells. To study and understand the mechanisms of microtubule dynamics and regulation, in vitro reconstitution with purified components has proven a vital approach. Imaging microtubule dynamics can be instructive for a given species, isoform composition, or biochemical modification. Here, we describe two methods that visualize microtubule dynamics at high speed and high contrast: (1) total internal reflection fluorescence microscopy and (2) label-free interference reflection microscopy.
For complete details on the use and execution of this protocol, please refer to Hirst et al. (2020).
Subject Areas: Biophysics, Cell Biology, Microscopy
Graphical Abstract

Highlights
-
•
In vitro reconstitution of microtubule dynamics
-
•
Label-free imaging by IRM when tubulin amounts are limiting
-
•
Labeling tubulin protocol
-
•
Imaging of fluorescent tubulin by TIRFM
Dynamic microtubules are essential for many processes in the lives of eukaryotic cells. To study and understand the mechanisms of microtubule dynamics and regulation, in vitro reconstitution with purified components has proven a vital approach. Imaging microtubule dynamics can be instructive for a given species, isoform composition, or biochemical modification. Here, we describe two methods that visualize microtubule dynamics at high speed and high contrast: (1) total internal reflection fluorescence microscopy and (2) label-free interference reflection microscopy.
Before You Begin
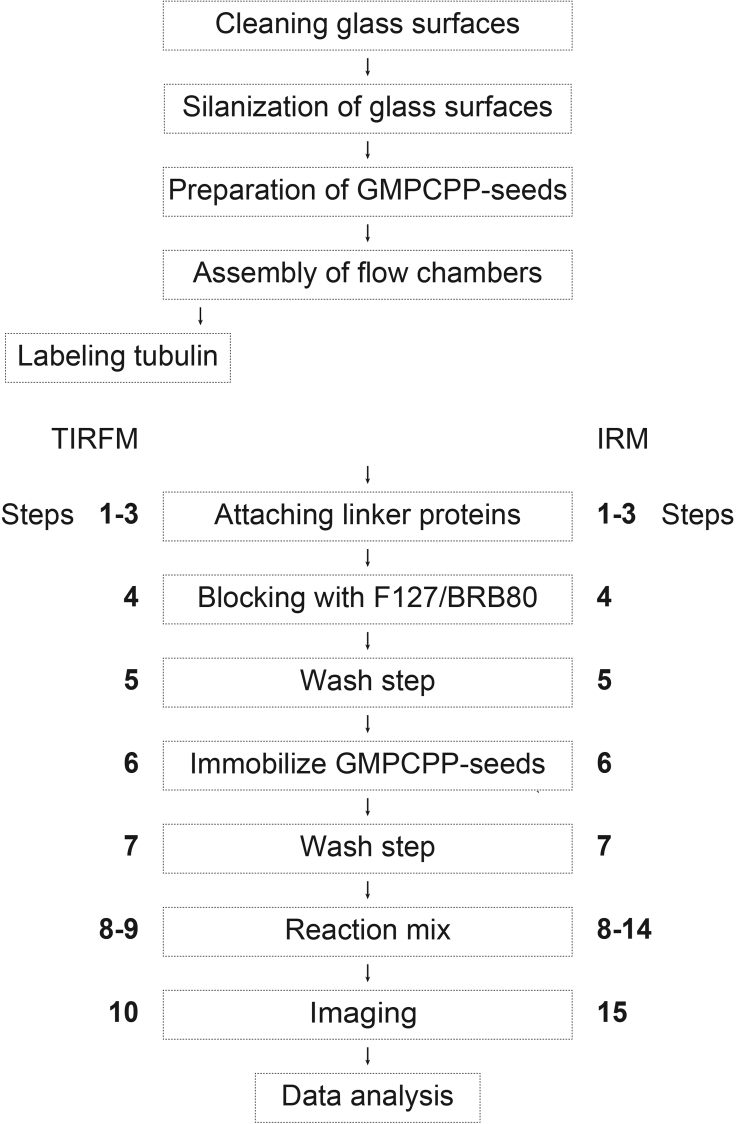
The assays described here can be used to simply test whether the purified tubulin is assembly competent. These methods can be further used to characterize all parameters of microtubule dynamic instability (Mitchison and Kirschner 1984) intrinsic to your purified tubulin. These parameters can be specific and thus instructive for a given species (Chaaban et al., 2018, Hirst et al., 2020), isoform composition (Ti et al., 2018, Vemu et al., 2017) or biochemical modification (Ori-McKenney et al., 2016). The assays use stabilized microtubule seeds bound to functionalized glass surfaces in flow chambers. Microtubule seeds can be immobilized on the surface using biotinylated or rhodamine-labeled microtubules and neutravidin or anti-rhodamine antibody, respectively. Both alternatives are described below. In the TIRF assay, microtubules are visualized by the addition of fluorescently labeled tubulin while the alternative approach, IRM, includes label-free tubulin for imaging by interference reflection microscopy (IRM) (Curtis 1964, Simmert et al., 2018, Mahamdeh et al., 2018). An overview of the workflow is shown in Figure 1.
Figure 1.
Overall Schematic Workflow
For each stage (dotted box), steps are annotated.
Preparation of Materials for TIRFM and IRM
Cleaning and Silanization of Glass Coverslips
Both TIRFM and IRM combine the advantage of low background and high signal-to-noise ratio. Both techniques require thoroughly cleaned glass surfaces to avoid non-specific binding of (fluorescently labeled) proteins, as this will lower the signal-to-noise ratio as well as the effective working concentrations. Moreover, both techniques require the immobilization of microtubules onto the glass surface via antibodies or linker proteins such as neutravidin (Figure 2). Thus, the glass surfaces need to be chemically functionalized by silanization. Silanization renders the hydrophilic glass surface hydrophobic. Antibodies or linker proteins adsorb more stably onto hydrophobic surfaces via hydrophobic interactions, which are often stronger than hydrophilic or electrostatic interactions. Here, we use trimethylchlorosilane (TMCS, also known as chlorotrimethylsilane, MTS), which compared to other silanization coatings, has two main advantages: First, TMCS can be covalently bound to the glass surface via the gas phase, thus reducing aggregation effects. Second, the level of surface hydrophobicity can be adjusted. We here first describe a simple silanization protocol and then a more elaborate TMCS coating protocol via vacuum deposition, where hydrophobicity can be adjusted to individual experimental needs by varying the amount of TMCS and incubation times.
Figure 2.
Schematics and Representative Images of In Vitro Reconstituted Microtubules Imaged by TIRFM and IRM
(A) Schematic of a TIRFM microtubule assembly assay using biotinylated GMPCPP-stabilized seeds (red) attached to the coverslip via neutravidin (blue) as nucleation templates for dynamic microtubules (green).
(B) Dynamic Cy3-labeled Xenopus microtubules (green) nucleated from GMPCPP-stabilized Cy5-labeled tubulin seeds (red) imaged by TIRFM.
(C) Schematic of dynamic microtubules (green) nucleated from rhodamine-labeled GMPCPP-stabilized tubulin seeds (red) immobilized via anti-rhodamine antibodies and imaged by IRM.
(D) Xenopus microtubules nucleated from stabilized seeds as in (B) and imaged by IRM. Scale bars, 10 μm.
For the entire cleaning and silanization process, microscope coverslips (22 × 22 mm, with 0.13–0.17 mm thickness) and coverslips (18 × 18 mm) are held in racks composed of inert material to space them apart and allow full exposure to cleaning solutions and reagents. Cleaning and silanization steps are performed in glass beakers that allow the glass to be completely immersed in a minimal volume of solution. Care should be taken to minimize exposure to dust. For example, if the glass must be moved between rooms for the plasma cleaning step, the container should be sealed. To prevent scratching the glass surfaces, coverslips should be held by their edges preferentially using plastic tweezers.
Cleaning of Glass Coverslips for TIRFM
Timing: 1.5 h
The glass surfaces are pre-cleaned in alkali and solvents, then air plasma is used for the final cleaning (Nguyen et al., 2015).
-
1.
Place coverslips in a rack in a beaker containing 3 M KOH (168.3 g/L) and sonicate continuously in a sonication bath for 20 min
-
2.
Rinse 5× with ultra-pure water.
-
3.
Sonicate in ultra-pure water for 20 min.
-
4.
Transfer coverslips to a new beaker filled with 100% ethanol.
-
5.
Sonicate in 100% ethanol for 20 min.
-
6.
Transfer coverslips to a new beaker filled with 100% ethanol.
-
7.
Remove from ethanol and dry using clean compressed air, compressed nitrogen, or allow to air-dry in a dust-free environment.
-
8.
Place coverslips in a plasma oven and evacuate for 10 min.
-
9.
Switch on the plasma oven at maximum intensity for 5–10 min.
Note: It is important that the coverslips and the rack are completely dry before proceeding to the silanization step. The glass can be checked for cleanliness by ensuring that a drop of water spreads on it.
Cleaning of Glass Coverslips for IRM
Timing: 1.5 h
Since IRM is also sensitive to non-fluorescent contamination and insufficient attachment of microtubules, we use a more thorough cleaning protocol for the coverslips prior to silanization for IRM imaging.
-
10.
Load the coverslips into racks using cleaned tweezers.
Note: To prevent scratching the glass surface, coverslips should be held by their edges preferentially using plastic tweezers.
-
11.
Rinse two glass boxes with ultra-pure water.
-
12.
Load the racks filled with coverslips into one of the cleaned glass boxes.
-
13.
Fill the glass box with ultra-pure water until the coverslips/slides are fully immersed.
Note: To prevent the coverslips/slides from falling out of the racks, it is important to slowly fill in the water at the corner of the glass box.
-
14.
Transfer the racks with coverslips to the second glass box using tweezers.
-
15.
Fill the second glass box with 20% (v/v) Mucasol-water solution completely covering the coverslips.
Note: To prevent dust contaminations, it is important to always close the lid of the glass box.
-
16.
Put the glass box holding the racks in Mucasol-water into a sonicator.
Note: To reduce extensive heating during the sonication process, we recommend filling the sonicator with pre-cooled water. The water level in the sonicator surrounding the glass box should be at least as high as the Mucasol-water level.
-
17.
Sonicate for 15 min at 100% sonication power.
-
18.
Transfer the racks holding the coverslips into an empty glass box.
-
19.
Fill the glass box with ultra-pure water, close the lid, and wash for a minimum of 2 min while slowly shaking the box.
-
20.
Transfer the racks holding the coverslips/slides into an empty glass box.
-
21.
Fill the glass box with 100% ethanol and close the lid.
-
22.
Put the glass box with the coverslips in ethanol into a sonicator.
-
23.
Sonicate for 10 min at 100% sonication power.
-
24.
Transfer the racks holding the coverslips into an empty glass box.
-
25.
Fill the glass box with ultra-pure water and wash for at least 2 min with closed lid while slowly shaking the box.
-
26.
Transfer the racks holding the coverslips/slides into an empty glass box
-
27.
Start the next cleaning cycle with a Mucasol-water solution as from step 15 onward and proceed until step 25.
-
28.
Repeat this complete cleaning cycle once more (three times in total).
Note: We recommend changing gloves at least every cycle.
-
29.
After the cleaning, directly proceed with the silanization protocol.
Note: To avoid impurities settling on the glass surface, it is best to directly proceed with the silanization protocol. It is possible, however, to store the cleaned coverslips in ultra-clean water overnight in a sealed glassed box.
Simple Silanization of Glass Coverslips
Timing: 4–5 h
This protocol for glass surface silanization is according to Szkop et al., 2018.
-
30.
Submerge coverslips in a solution of 0.54 M anhydrous imidazole in acetonitrile.
-
31.
Stir with a stir bar small enough to avoid contact with the submerged rack and add TMCS (see handling notes in Materials and Equipment) to a final concentration of 3.3% v/v using a syringe with the tip submerged in the acetonitrile solution. Avoid the introduction of air bubbles.
-
32.
Seal the beaker and incubate at 45°C for 3 h.
-
33.
Submerge the rack in 100% methanol in a clean beaker and sonicate for 10 min.
-
34.
Sonicate again for 15 min in fresh 100% methanol.
-
35.
Remove the rack and rinse with ultra-pure water twice.
-
36.
Dry in a dust-free environment.
-
37.
Place the coverslips in a clean container and seal with Parafilm.
Note: In such conditions, silanized glass can be stored for up to one month at room temperature.
Note: After silanization, it is important to validate the hydrophobicity of the slides. To do so, take out one coverslip, apply a water droplet of 10 μL onto the coverslip, and observe the water contact angle of the droplet. The hydrophobicity is sufficient when the angle between the air-water surface and the glass-water surface is >90° (Figure 3).
Figure 3.
Hydrophobicity Test of Cleaned and Silanized Glass Surfaces
Schematic showing the behavior of a 10 μL water droplet on a glass surface with increasing hydrophobicity (left to right). The contact angle θ, which increases with hydrophobicity, is the angle between the glass surface and the tangent to the surface of the droplet where it meets the glass.
Silanization of Glass Coverslips Using Vacuum Deposition
Timing: 3–3.5 h
-
38.
Immerse the coverslips/slides in 1 M HCl and close the glass container with a lid.
-
39.
Sonicate for 90 min at maximum sonication power at 75°C.
Note: Be careful after the incubation while handling the hot acid.
-
40.
Transfer the rack into a fresh glass box and wash 3× with ultra-pure water.
-
41.
Dry the coverslips thoroughly using filtered and de-humidified pressured air or nitrogen.
-
42.
Dry the coverslips a second time inside a clean desiccator by applying vacuum for 10–20 min.
Note: The coverslips have to be dried thoroughly to ensure successful TMCS coating.
-
43.
Remove the vacuum inside the desiccator while filling the desiccator with filtered and de-humidified air at the vacuum valve.
Note: Open the vacuum valve slowly to avoid sudden airflow. Using filtered and de-humidified air instead of ambient air is important to ensure dust-free and non-humid conditions.
-
44.
Evaporate 800 μL TMCS onto the coverslips inside the desiccator: place 800 μL TMCS in an Eppendorf tube at the bottom of the desiccator, apply vacuum (∼20 mbar) for 5 min to fill the chamber with TMCS, close the desiccator chamber, and incubate for 5 min, apply again vacuum for 5 min, close the desiccator chamber, and incubate for 5 min, repeat these cycles of vacuum/incubation until the 800 μL TMCS are completely evaporated. Typically, this will take 30–40 min.
-
45.
Store the silanized slides in a closed container, which has been cleaned and dried using pressured air. The silanized slides can be stored for up to one month. For best results, we suggest using the slides within 2–3 weeks as the TMCS coating degrades over time and becomes less homogeneous.
Note: After the silanization procedure, it is important to validate the hydrophobicity of the slides (Figure 3) as described above. If the coverslips are not sufficiently hydrophobic, repeat the evaporation of TMCS as described in step 44.
Preparation of Stabilized Microtubule Seeds
TIRFM and IRM rely on stabilized microtubule seeds that are immobilized on the glass surface and that serve as nucleation templates for dynamic microtubules. This step produces stabilized microtubules by polymerizing tubulin in the presence of GMPCCP, a slowly hydrolysable GTP analog (Hyman et al., 1992). In case highly stable microtubule seeds are needed, for example in long-term measurements or microtubule depolymerization assays (Caplow and Shanks 1996; Helenius et al., 2006), the stability of microtubules can be enhanced by two to three cycles of polymerization in GMPCPP. This results in an increased proportion of GMPCPP-tubulin in the microtubule lattice (Gell et al., 2010). In addition, with each centrifugation, the sample is separated from non-functional tubulin dimers. Biotinylated tubulin can be incorporated to allow the seeds to bind a neutravidin-coated glass surface (Figure 2A), or rhodamine-labeled tubulin to bind an anti-rhodamine antibody-coated glass surface (Figure 2C). This protocol is optimized for tubulin purified from bovine and porcine brains. For other tubulin sources, this protocol might require empirical optimization. For example, room temperature is sufficient to allow growth of microtubule seeds using Xenopus laevis tubulin (Hirst et al., 2020, Reusch et al., 2020).
Single-Cycled GMPCPP-Stabilized Microtubule Seeds
Timing: 3–4 h
-
46.
Quickly thaw tubulin in your hand or in a 37°C water bath for larger volumes and place it on ice.
-
47.
Prepare the GMPCPP seed reaction by mixing tubulin (final concentration 2.8 μM), fluorescently labeled tubulin (final concentration 0.4 μM), biotin-labeled tubulin (final concentration 0.8 μM), MgCl2 (final concentration 2 mM) and GMPCPP (final concentration 1 mM) in 1× BRB80 (80 mM PIPES, 1 mM MgCl2, 1 mM EGTA pH 6.9 with KOH).
-
48.
Incubate on ice for 10 min to allow for efficient nucleotide exchange, as well as depolymerization of residual microtubules.
-
49.
Transfer to 37°C and incubate for 1 h.
CRITICAL: Use a cut-off pipette tip when pipetting polymerized microtubules to avoid shearing microtubules into smaller seeds.
Note: The distribution of microtubule lengths can be tailored by altering the tubulin concentration and polymerization time. Lower tubulin concentrations with longer incubation time will lead to longer microtubules (Gell et al., 2010).
-
50.
Gently layer the reaction mixture over 100 μL of prewarmed (37°C) 60% glycerol/1× BRB80 cushion solution supplemented with 0.5 mM GMPCPP in an appropriate centrifuge tube (e.g., 230 μL thick-walled centrifuge tubes from Beckman Coulter). Two distinct liquid phases should be visible in the tube.
-
51.
Using a prewarmed rotor (e.g., TLA-100), centrifuge at 70,000 rpm (220,000 × g) for 10 min at 35°C.
-
52.
Taking care not to disturb the separated phases, gently remove the supernatant layer.
-
53.
Rinse the top of the cushion and centrifuge tube walls twice with 50 μL warm 1× BRB80 to remove unpolymerized tubulin.
-
54.
Taking care not to disturb the pellet, remove the cushion layer.
-
55.
Gently rinse the pellet and centrifuge tube walls twice with warm 1× BRB80 to remove residual glycerol.
Note: The microtubule pellet attaches to the tube wall and can be visible as an opaque pellet. Care should be taken to avoid touching the pellet and allowing the pellet to fall dry.
-
56.
Resuspend the pellet in an appropriate volume of warm 1× BRB80 with 1 mM DTT using a cut-off pipette tip, aliquot into 5 μL working aliquots, and flash-freeze in liquid nitrogen.
Note: Given the polymerization conditions described, we resuspend the pellet in a volume of 1× BRB80 equal to the volume of the original reaction and make 5 μL aliquots. Once thawed, these aliquots are then diluted 100 - 200-fold for the suspension that will be perfused into the chamber. The dilution, however, will depend on the microtubule seed concentration and the experimental needs. The microtubule seed concentration should be taken into account and kept constant especially for quantitative assays when different concentrations of free tubulin are used.
Triple-Cycled GMPCPP-Stabilized Microtubule Seeds
Timing: 4.5–5 h
-
57.
Thaw tubulin quickly in your hand or in a 37°C water bath for larger volumes and place it on ice.
-
58.
Prepare the GMPCPP seed reaction by mixing purified unlabeled tubulin (final concentration 22.5 μM to ensure a sufficiently high concentration for microtubule growth over multiple cycles), biotin-labeled tubulin or rhodamine-labeled tubulin (final concentration 2.5 μM), MgCl2 (final concentration 2 mM) and GMPCPP (final concentration 1 mM) in 1× BRB80 (80 mM PIPES pH 6.9, 1 mM MgCl2, 1 mM EGTA pH 6.9 with KOH) to a final volume of 20 µL.
Note: Depending on which label is wanted for the microtubule seeds, either biotin- or rhodamine-labeled tubulin should be included in the reaction.
-
59.
Incubate on ice for 10 min to allow for efficient nucleotide exchange, as well as depolymerization of residual microtubules.
-
60.
Incubate at 37°C for 1 h to polymerize microtubules.
-
61.
Centrifuge at 126,000 × g for 5 min (or 22 psi using an Airfuge (Beckman Coulter)) to sediment microtubules.
-
62.
Resuspend the microtubule pellet in 16 μL 1× BRB80.
CRITICAL: Use a cut-off pipette tip when pipetting polymerized microtubules to avoid shearing microtubules into smaller seeds.
-
63.
Incubate the sample on ice for 20 min to depolymerize the microtubules.
-
64.
Add 2 μL of 20 mM MgCl2 to a final concentration of 2 mM and add 2 μL of 10 mM GMPCPP to a final concentration of 1 mM. The final reaction volume is 20 μL.
-
65.
Repeat steps 60 to 64.
-
66.
Repeat steps 60 and 61.
Note: The polymerization incubation time can be prolonged overnight for convenience.
-
67.
Resuspend the microtubule pellet in 100 μL BRB80 prewarmed to 37°C. For resuspension, carefully pipette a volume of 40 μL slowly up and down using a 100 μL cut-off pipette tip.
-
68.
Aliquot microtubule seeds into 5 μL samples, snap freeze, and store them at −80°C.
Note: For experimental use, quickly thaw the aliquot at 37°C. After thawing, the microtubule seeds will have diverse lengths ranging from 2–5 μm.
Labeling Tubulin
Timing: 6 h
The principle of TIRFM is based on the excitement of fluorescent molecules at the interface between a glass surface and an aqueous solution (Axelrod et al., 1984) using an evanescent field generated by the total internal reflection of a laser beam (Figure 2A). Thus, fluorescently labeling tubulin is only required if you wish to image your microtubules via TIRFM. This protocol is a general protocol for coupling moieties with reactive succinimidyl esters to tubulin. We have used it successfully to derivatize tubulin with succinimidyl esters of biotin and a wide range of fluorochromes such as tetramethylrhodamine, X-rhodamine, Cy3, and Cy5. The idea is to label tubulins while in the polymeric state to protect residues essential for microtubule polymerization. The labeling is performed at high pH to optimize the reaction with the succinimidyl esters. Functional tubulin is selected after the labeling reaction by one cycle (or more) of polymerization and depolymerization. In our example, we use a starting amount of 30 mg tubulin, but this amount can be adjusted according to the amount of dye available to avoid freeze/thaw cycles of dye stocks.
Polymerization
-
69.
Thaw 30 mg tubulin in a 37°C water bath and place on ice just before thawing is complete.
-
70.
Add GTP to 1 mM final concentration.
-
71.
Add MgCl2 to 1 mM final concentration.
-
72.
Mix thoroughly.
-
73.
Store on ice for 5 min.
-
74.
Add prewarmed glycerol to 33% (v/v) final concentration.
-
75.
Mix gently but thoroughly.
-
76.
Incubate at 37°C for 30 min.
-
77.
Layer polymerized tubulin onto one volume warm high pH cushion (0.1 M NaHEPES, pH 8.6, 1 mM MgCl2, 1 mM EGTA, 60% v/v Glycerol).
-
78.
Pellet microtubules in a 50.2 Ti rotor at 40,000 rpm (192,000 × g) for 45 min at 35°C.
-
79.
Aspirate the supernatant above the cushion.
-
80.
Rinse the supernatant-cushion interface twice with 37°C labeling buffer (0.1 M NaHEPES, pH 8.6, 1 mM MgCl2, 1 mM EGTA, 40% v/v Glycerol).
-
81.
Aspirate the cushion
-
82.
Resuspend the pellet using a cut-off large pipet tip in 1 mL of warm labeling buffer. Take care to keep the tubulin warm during the resuspension and continue resuspending until no chunks of tubulin are visible.
Labeling of the Microtubule Polymer
-
83.
Estimate the tubulin concentration assuming 70% recovery of the starting tubulin.
-
84.
Add 7- to 10-fold molar excess of the dye (Table 1) to tubulin.
Note: Dye stocks are best prepared fresh from powder that has been stored at −20°C and best dissolved in anhydrous DMSO. Residual dye solution can be stored at −80°C under anhydrous conditions.
-
85.
Label for 30–40 min at 37°C.
-
86.
Gently vortex every 2–3 min during the course of labeling.
-
87.
Add an equal volume of Quench (2× BRB80, 100 mM K-Glutamate, 40% v/v Glycerol) and mix well.
-
88.
Incubate for 15 min at 37°C.
-
89.
Layer the quenched labeling reaction onto 1.5 mL of low pH cushion (1× BRB80 pH 6.9, 60% Glycerol).
-
90.
Spin at 80,000 rpm (300,000 × g) for 20 min at 37°C in a TLA100.3 / TLA100.4 / TLA120.2 rotor.
Table 1.
Monofunctional Dye Characteristics
| Dye | MW (g/mol) | λabs (nm) | λem (nm) | ε (M−1 cm−1) | CF280 |
|---|---|---|---|---|---|
| Cy5 | 855.07 | 651 | 670 | 250,000 | 0.03 |
| Rhodamine / ATTO Rho101 | 787 | 587 | 609 | 120,000 | 0.17 |
| Cy3 | 1,024.94 | 550 | 570 | 150,000 | 0.08 |
| Atto488 | 981 | 500 | 520 | 90,000 | 0.09 |
| ATTO 390 | 440 | 390 | 476 | 24,000 | 0.09 |
Depolymerization
-
91.
Aspirate the supernatant above the cushion and rinse the supernatant-cushion interface twice with warm 1× BRB80.
-
92.
Aspirate the cushion.
-
93.
Resuspend the pellet using a cut-off pipet tip in an appropriate volume of ice-cold 1× BRB80. Resuspend the pellet by gentle pipetting till the suspension is uniform.
-
94.
Keep on ice for at least 60 min.
-
95.
Spin the depolymerized tubulin in a TLA100.2 / TLA100.3 / TLA120.2 rotor at 80,000 rpm (300,000 × g) for 10 min at 2°C.
-
96.
Recover the supernatant from the cold spin.
-
97.
Freeze as single-use aliquots (5–10 μL) in liquid nitrogen and store at −80°C.
Determine Tubulin Concentration and Labeling Stoichiometry
-
98.
Dilute the labeled tubulin 1/50 - 1/100 in 1× BRB80.
-
99.
Determine A280 and correct for the contribution of the dye to the absorbance at A280.
-
100.
Determine Amax (max emission of the fluorophore).
-
101.
Calculate the labeling stoichiometry according to
Note: The molecular extinction coefficient for the tubulin dimer is εtubulin 115,000 M−1 cm−1. Table 1 shows the dyes’ molecular extinction coefficients and the dye correction factors.
Assembly of Flow Chambers
Timing: 10 min
Using the functionalized glass coverslips (22 × 22 mm) and coverslips (18 × 18 mm), we can build simple flow cells, in which we can immobilize microtubules and perfuse reaction mixtures (Figure 4).
-
102.
Cut strips of parafilm approximately 3–4 mm in width and long enough to extend beyond the length of a coverslip.
-
103.
Sandwich parafilm strips between a glass coverslide and a coverslip positioned in the center of the slide to create flow channels 3–4 mm in width using plastic tweezers to avoid scratching the glass. The created flow channels will have an approximate volume of 10–20 μL.
-
104.
Place the assembled slide on a clean hot plate heated to 70°C with the coverslip side facing up. When the parafilm turns clear, gently press on the coverslip using the tweezers, and remove the coverslip from heat.
Notes: (1) A single flow cell is typically used for only one imaging condition. (2) Do not place channels too close to the edges as it may not be possible to image them with the microscope objective. (3) The flow profile in the channel is parabolic, thus there is slower movement of the solution near the channel walls. In order to exchange solution in the channels thoroughly, it is necessary to flow several channel volumes of solution. (4) Instead of parafilm, double-sided tape can be used. (5) Flow chambers constructed with two coverslips (22 × 22 mm and 18 × 18 mm) require a specialized microscope stage insert to hold the flow chamber. Alternatively, a microscope slide (26 × 76 mm) and a coverslip (22 × 22 mm) can be used.
Figure 4.
Schematic of a Flow Chamber
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-Tubulin (clone DM1A) | Sigma-Aldrich | Cat #T9026; RRID: AB_477593 |
| Mouse monoclonal anti-rhodamine antibody (clone 11H10) | Thermo Fisher Scientific | Catalog # 200-301-246 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Pluronic F-127 | Sigma-Aldrich | Cat #: P2443 |
| Anhydrous immidazole | Sigma-Aldrich | 56749 |
| Acetonitrile | Carl Roth | 6827.1 |
| Bovine tubulin | Gell et al., 2011 | N/A |
| Xenopus tubulin | Hirst et al., 2020 | N/A |
| Anhydrous DMSO | Life Technologies | D12345 |
| Piperazine-1,4-bis(2-ethanesulfonic acid) (PIPES) | Sigma-Aldrich | Cat #: P1851 |
| Methylcellulose | Sigma-Aldrich | Cat #: 94378 |
| Glutamic Acid | Roth | 3774.2 |
| Mucasol | Schülke | 70001812 |
| NeutrAvidin Protein | Thermo Fisher | Cat #: 31000 |
| k-Casein from bovine milk | Sigma-Aldrich | Cat #: C0406 |
| Chlorotrimethylsilane (TMCS) | Sigma-Aldrich | Cat #: 386529 |
| Chlorotrimethylsilane (TMCS) (∗used for vacuum deposition) | Merck | Cat #: 818737 |
| Guanosine-5′-[(α,β)-methyleno]triphosphate (GMPCPP) | Jena Bioscience | Cat #: NU-405L |
| Protocatechuic Acid (PCA) | Sigma-Aldrich | Cat #: 03930590 |
| Protocatechuate-3,4-dioxygenase (PCD) | Sigma-Aldrich | Cat #: P8279 |
| (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) | Sigma-Aldrich | Cat #: 238813 |
| Cy3 Mono NHS Ester | GE Healthcare | Cat #: PA13101 |
| Cy5 Mono NHS Ester | GE Healthcare | Cat #: PA15101 |
| Guanosine 5′-triphosphate (GTP) | Sigma-Aldrich | Cat #: G8877 |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) | Sigma-Aldrich | Cat #: E3889 |
| Ethylenediaminetetraacetic acid (EDTA) | Carl Roth | Cat #: 8043.1 |
| Sodium hydroxide (NaOH) | Carl Roth | Cat #: 9356.1 |
| Calcium chloride dihydrate (CaCl₂·2H₂O) | Carl Roth | Cat #: HN04.3 |
| Sodium chloride (NaCl) | Carl Roth | Cat #: HN00.3 |
| Potassium chloride (KCl) | Carl Roth | Cat #: HN02.3 |
| N-2-Hydroxyethylpiperazine-N′-2-ethane sulphonic acid (HEPES) | Carl Roth | Cat #: HN77.5 |
| Potassium hydroxide (KOH) | Carl Roth | Cat #: 6751.1 |
| Magnesium chloride hexahydrate (MgCl₂·6H₂O) | Carl Roth | Cat #: HN03.2 |
| Glycerol | Carl Roth | Cat #: 3783.4 |
| DL-Dithiothreitol (DTT) | Sigma-Aldrich | Cat #: 43815 |
| Sodium bicarbonate (NaHCO₃) | Carl Roth | Cat #: HN01.1 |
| Hydrochloric acid (HCl) | Carl Roth | Cat #: 4625.2 |
| D-Glucose | Sigma | G7528 |
| Glucose oxidase | Sigma | G7016 |
| Catalase | Sigma | C9322 |
| β-Mercaptoethanol | Sigma-Aldrich | Cat #: M6250 |
| Other | ||
| Coverslides, 22 × 22 mm | Corning | Cat #: 2850-22 |
| Coverslips, 18 × 18 mm | Thermo Scientific Menzel | Cat #: 15757572 |
| Ultrasonic Bath | Bandelin | Cat #: 311 |
| Airfuge Air-Driven Ultracentrifuge | Beckman Coulter | #340400 |
| Plasma oven | Harrick Plasma | PDC-32G-2 |
Materials and Equipment
Preparing Materials/Equipment for TIRF
Stock Solutions
0.5 M EGTA/0.5 M EDTA
Prepare a stock solution by combining
-
•
19.02 g of EGTA, or
-
•
18.61 g of EDTA
-
•
90 mL of ultra-pure water
Mix with the help of a stir bar. To dissolve either solution, slowly add NaOH to a pH of 8.0. Once the salt has dissolved adjust the final volume to 100 mL using ultra-pure water. Filter-sterilize or autoclave both solutions and store at room temperature.
1 M CaCl2
Prepare stock solution by combining
-
•
14.7 g of CaCl2·2H2O
-
•
90 mL of ultra-pure water
Stir and dissolve with the help of a stir bar. Adjust the final volume to 100 mL using ultra-pure water. Autoclave and store at room temperature.
1 M MgCl2
Prepare stock solution by combining
-
•
20.33 g of MgCl2·6H2O
-
•
90 mL of ultra-pure water. Stir and dissolve with the help of a stir bar. Adjust the final volume to 100 mL using ultra-pure water. Autoclave and store at room temperature.
1 M DTT
-
•
1.54 g DTT
-
•
10 mL ultra-pure water
Store 100 μL aliquots at −20°C.
100 mM GTP
-
•
0.52 g Guanosine 5′-triphosphate (GTP) sodium salt hydrate
-
•
7 mL ultra-pure water
Mix at room temperature and adjust to pH 7.0 with NaOH. Use 1 M NaOH until the pH reaches 6.5, then add 0.1 M NaOH slowly until the pH reaches 7.0. Add ultra-pure water to a final volume of 10 mL, distribute into single-use aliquots and freeze at −80°C.
Note: Nucleotides undergo rapid hydrolysis at acidic pH, therefore monitor pH while dissolving and keep pH close to neutral. To prevent precipitation, the GTP solution should be made without Mg2+. GTP should be kept on ice and added to the buffers only shortly before starting the experiment.
1× BRB80
1× BRB80 buffer contains 80 mM PIPES, 1 mM MgCl2, and 1 mM EGTA, pH 6.9. To prepare 500 mL of 1× BRB80 buffer, combine:
-
•
12.1 g of PIPES
-
•
1 mL of 0.5 M EGTA
-
•
500 μL of 1 M MgCl2
-
•
8 mL of 10 M KOH
-
•
450 mL of ultra-pure water
Stir the PIPES, adjust the pH to 6.9 using 10 M KOH and then adjust the final volume to 500 mL with ultra-pure water (>18 MΩ/m). PIPES will only dissolve while adjusting pH. The buffer is filtered using a 0.22 μm vacuum filter, degassed, and 50 mL aliquots are stored at −20°C. We found that using especially degassed 1× BRB80 helps to avoid air bubbles forming during experiments.
1% Pluronic F-127 in 1× BRB80
Dissolve Pluronic F-127 in 1× BRB80 to a final concentration of 1% w/v overnight. Filter the solution through a 0.22 μm syringe filter. Store small aliquots (50–100 μL) at −20°C for longer periods. When thawing and using an aliquot, it can be stored at 4°C for several weeks.
0.75% Methylcellulose in 1× BRB80
Dissolve methylcellulose to a final concentration of 0.75% w/v in 1× BRB80 overnight with gentle agitation. The solution can be stored at 4°C for several weeks.
TMCS
TMCS is sensitive to water and oxygen, it easily hydrolyzes and forms hydrogen chloride when in contact with air. We therefore keep the TMCS bottle in a zipbag with hygroscopic pellets and store it in a dessicator. TMCS is volatile. To reduce or even stop dripping, pre-wetting the pipette tip can help. TMCS vapor and liquid may cause burns. Thus, when retriving TMCS from the bottle, we open and close the bottle quickly to avoid long exposure to air. The density of TMCS is lower than water thus aspirating with a pipette should be done slowly to obtain an accurate volume and the aspirated liquid inside the pipette tip should be transferred quickly to avoid liquid leaking from the pipette tip.
The Microscopes
TIRFM
For TIRF microscopy, we use a commercial setup, which is an inverted Nikon Eclipse Ti-E microscope with a motorized TIRF angle, a Nikon Plan Apochromat 100×/1.49 NA oil immersion objective lens, and an Andor iXon Ultra X3 987 EMCCD camera (512 × 512 pixels, 16 μm pixel size, 56 fps full frame, QE max. 90%) and a Photometrics Prime 95B sCMOS camera (95% QE, 1,200 × 1,200 pixels, 11 μm pixel size, 41 fps full frame, 82 fps@12-bit). While both cameras are sufficient to detect individual microtubules by TIRF microscopy, the sCMOS has a larger field of view and is thus better suited for visualizing very long microtubules.
IRM
IRM-setups suitable for single-molecule assays are commercially available. Generally, IRM can be implemented in an epifluorescent or TIRF microscope setup by equipping the microscope with a high-power light source, an objective with a numerical aperture less than 1.3 or an aperture iris that reduces the NA to values lower than 1.3 to avoid total internal reflection of the incoming LED light, and a 50/50 beam splitter plate in the illumination/detection light path (Figure 5). Often, the only required additional component is the beamsplitter. Thus, IRM can be well integrated with other microscopy and micro-manipulation techniques (Simmert et al., 2018, Mahamdeh et al., 2018). Our custom-built microscope implementation is shortly described below and can also be found in Simmert et al., 2018.
-
•
The light source
Figure 5.
Schematic of the IRM Light Path
The light path and optical components necessary for IRM.
IRM can be operated with a simple incoherent light source, such as an LED, a light engine, or an Arc lamp. In our custom-built IRM setup, we use a dimmable, high-power blue LED as an efficient light source (Royal-Blue LUXEON Rebel LED, Lumileds, Germany; λ ± Δλ ≈ 450 ± 20 nm with 525 mW at 700 mA). The chosen LED allows for high currents and can be operated at high light intensity. The blue light allows a high resolution due to its short wavelength (Bormuth et al., 2007). To avoid overheating of the LED, it is mounted onto an aluminum heat sink (Fisher Elektronik, Germany).
-
•
The objective
Our IRM setup is equipped with an apochromat 60× oil immersion TIRF objective lens (CFI Apo TIRF 60× oil, NA 1.49, Nikon Instruments, Japan). To reduce the NA to a value lower than 1.3, the setup is complemented with an additional aperture iris to truncate the incoming light and avoid total internal reflection. Additionally, a field iris increases contrast by removing stray light. This iris is adjusted manually for maximum contrast.
-
•
50/50 beam splitter
A 50/50 beam splitter (also referred to as “half mirror”) separates the illumination and detection light path. As IRM uses reflected light from the sample for detection, the detected light and illumination light share the same light path. Thus, the detected light has to be separated from the illumination light using a 50/50 beam splitter before being projected onto a camera.
-
•
The camera
It is recommendable to use a camera with high dynamic range. Our IRM setup is operated using a CCD camera (LU135-M, Lumenera, Canada), a camera suitable for low light conditions with a pixel size of 4.65 × 4.65 μm, a resolution of 1,392 × 1,040 pixels, a full-frame imaging speed of 15 fps and a dynamic range of 60 dB. In front of the camera, our setup accommodates a zoom (S5LPJ7073, Sill Optics, Germany), which is used to magnify the sample image 165× prior to being projected onto the camera.
-
•
Temperature control
As microtubule dynamic assays are temperature-dependent, it is important to ensure stable temperature conditions throughout the measurement. In our IRM setup, we implemented a millikelvin-precision temperature feedback at the objective heater from below and above at the condenser unit (the condenser is part of the integrated optical tweezers detection systems in our setup and not necessary for IRM itself).
-
•
Fine stage and vibration isolation for high-precision long-term measurements
For the measurements, the sample is mounted on a high-precision piezo-driven stage (PIHera620XYZ, Physik Instrumente, Germany). To avoid interference with the measurements from vibrations (i) the walk-in chamber itself, which accommodates the microscope, is vibration-isolated and (ii) all microscope components are assembled onto an optical table (Standa, Lithuania) placed on an active vibration isolation system (VarioBasic-60, Accurion, Germany).
Step-By-Step Method Details
Fluorescence Imaging of In Vitro Reconstituted Microtubules by TIRFM
Timing: 45 min to 1 h for preparation, 1 h to 1 day for imaging
In this assay, microtubules are visualized by the addition and incorporation of fluorescently labeled tubulin. TIRF microscopy uses a collimated laser aimed at the sample at a low angle of incidence such that the difference in refractive indices of the objective oil and aqueous sample causes the laser to reflect back entirely. A resulting evanescent wave illuminates a region extending approximately 100 nm into the sample, thereby restricting the excitation and detection of fluorophores to a thin region.
-
1.
Flow 20 μL of 100 μg/mL neutravidin (or anti-rhodamine antibodies for rhodamine-labeled seeds) in 1× BRB80 into the flow chamber. Neutravidin binds biotinylated tubulin thereby attaching the seeds tightly to the glass surface (Figure 2A).
Note: Adequately prepared silanized glass will be hydrophobic, preventing the initial volume of liquid from entering the chamber by capillary action. Use a vacuum at low power to draw the neutravidin solution through the chamber. Once the chamber is full, all subsequent washes can be drawn through by touching absorbent paper to one end and pipetting from the other side. Take care to avoid spillage, especially onto the surface that will face the microscope objective.
CRITICAL: Avoid introducing air bubbles to the flow chamber. Air bubbles will disrupt the flow of liquid through the chamber and cause oxidation.
-
2.
Incubate for 5 min.
Note: To avoid the reaction channel from drying out, we place the flow chamber inside a humidified container with a lid (e.g., a petri dish equipped with wet tissue) and add droplets of the reaction mix at the two ends of the flow chamber.
-
3.
Wash out neutravidin with 2 x 20 μL of 1× BRB80.
-
4.
Flow 2 x 20 μL 1% Pluronic F-127 in 1× BRB80 through the chamber and incubate for 15 up to 45 min to block unspecific binding.
Note: To avoid contamination, we use small aliquots of filtered 1% Pluronic-F127 in 1× BRB80, which we store at −20°C.
-
5.
Wash the chamber with 2 x 20 μL of wash buffer composed of 1× BRB80 supplemented with 1 mg/mL κ-casein.
Note: From this point, all solutions should contain κ-casein to enhance blocking of the coverslip surface and reduce non-specific protein-protein interactions.
-
6.
Flow in 20 μL of thawed GMPCPP-seeds diluted in wash buffer into the chamber and incubate for 5 min.
Note: At this point you can check the density of your seeds on the microscope.
-
7.
Remove unbound seeds with 2 x 20 μL wash buffer.
-
8.
Prepare the polymerization reaction by mixing GTP (final concentration 2 mM), β mercaptoethanol (final concentration 1% v/v), κ-Casein (final concentration 1 mg/mL), PCA (final concentration 2.5 mM), PCD (final concentration 25 nM), methylcellulose (final concentration 0.15% w/v), Trolox (final concentration 2 mM), labeled and/or unlabeled tubulin in 1× BRB80.
Note: It is advisable to prepare a 2× polymerization mix without the tubulins for multiple reactions. It can be kept on ice for a maximum of 2 h. Tubulin should be kept on dry ice or in liquid nitrogen and thawed only prior to addition to the reaction mixture. The final concentration of tubulin will depend on your source of tubulin, 10 μM final concentration is a good starting point.
Note: κ-Casein further passivates the glass surface. PCA/PCD (protocatechuic acid/protocatechuate-3,4-dioxygenase) is an enzymatic oxygen scavenging system for improved fluorescent dye stability. Trolox reduces photobleaching. Methylcellulose is a crowding agent that reduces thermal fluctuations of long microtubules. Importantly, concentrations below 0.5% methylcellulose have been shown to have no effect on microtubule growth and shortening velocities (Dixit and Ross 2010).
-
9.
Flow the reaction mixture through the chamber and seal the chamber openings with an inert sealant such as silicone vacuum grease.
-
10.Mount sample onto the microscope and start imaging:
-
a)Adjust the TIRF angle to an angle that eliminates as much background signal from the soluble labeled tubulin as possible and thereby allows microtubules to become visible.
-
b)Keep laser power and exposure time to a minimum to avoid photobleaching. For the above setup, for example, excitation at 488 nm of a sample containing Atto488-labled tubulin with a laser power of 0.54 mW and an exposure time of 200 ms produces a signal that falls within the lowest 6% of the dynamic range of the detection system. While noisy, this is sufficient for visualizing microtubules.
-
a)
Label-free Imaging of In Vitro Reconstituted Microtubules by IRM
Timing: 1.5 h for preparation, 1 h to 1 day for imaging
IRM is based on the interference of light reflected from closely adjacent surfaces or a surface and a subdiffraction-limited object of interest such as a microtubule.
-
11.
Flow in 20 μL anti-rhodamine antibody (20–200 μg/mL in 1× PBS) to immobilize rhodamine-labeled seeds (Figure 2C) or flow in neutravidin to immobilize biotinylated seeds.
-
12.
Incubate 15 min at room temperature.
Note: To avoid the reaction channel from drying out, we place the flow chamber inside a humidified container with a lid (e.g., a petri dish equipped with wet tissue) and add droplets of the reaction mix at the two ends of the flow chamber.
-
13.
Wash the flow cell three times with 20 μL 1× BRB80.
-
14.
Flow in 20 μL of 1% Pluronic-F127 in 1× BRB80 of and incubate for 20 min.
Note: To avoid contamination, we use small aliquots of filtered 1% Pluronic-F127 in 1× BRB80, which we store at −20°C.
-
15.
Wash five times with 20 μL 1× BRB80.
Note: Use 1× BRB80 that is warmed at least to room temperature in order to bring the flow chamber to a moderate temperature for the following injection of GMPCPP-stabilized microtubule seeds.
-
16.
Flow 10–15 μL of diluted GMPCPP-stabilized seeds into the flow chamber using a cut-off 20 μL pipette tip and incubate for 7–10 min at room temperature.
Note: We recommend diluting 5 μL GMPCPP-stabilized microtubules in 30 μL in 1× BRB80 that is prewarmed to 37°C.
Note: At this point, evaluate at the microscope if the required number of microtubule seeds is immobilized on the glass surface, whether seeds are properly attached, and whether the remaining surface is sufficiently clean (in IRM all contaminants provide contrast).
-
17.
Wash with 60 μL 1× BRB80 that is prewarmed to 37°C.
-
18.
Prepare the polymerization reaction by mixing D-glucose (final concentration 40 mM), glucose oxidase (final concentration 250 nM), catalase (final concentration 65 nM), β-Mercaptoethanol (final concentration 1%), κ-Casein (final concentration 0.1 mg/mL), methylcellulose (final concentration 0.1%) in 1× BRB80.
Note: Instead of using glucose/catalase/oxidase as oxygen scavenger system one can also use Trolox/PCA/PCD. We here describe two of the most common oxygen scavenger systems (1) Trolox/PCA/PCD and (2) Glucose/catalase/oxidase. To increase fluorophore lifetime and protect fluorophores from photophysical bleaching, enzymes - so-called oxygen scavengers - are used. By oxidizing a substrate, they create anaerobic conditions and prevent oxygen-based reactions. Trolox/PCA/PCD can be used for both TIRFM and IRM, but is better suited for assays that use fluorescence, because in addition to the oxygen scavenging activity of PCA/PCD, Trolox also acts as a triplet-state quencher. Glucose/catalase/oxidase can and has historically been used for both, but has the disadvantage of causing a larger drop in pH over time (Selvin and Ha, 2008).
Note: Prepare a final volume of 150–300 μL for one experiment and keep it on ice.
Note: We recommend storing the individual components as stock solutions in small aliquots at 50× or 100× concentration at −20°C. Casein is always centrifuged for 2 min at 13,000 × g before being added to the polymerization reaction, to avoid potential protein clusters being introduced.
-
19.
Supplement the polymerization reaction with fresh GTP.
Note: To induce robust polymerization for a standard microtubule dynamic assay, use a final GTP concentration of 1 mM. The GTP concentration can be adjusted depending on the experimental needs. We usually transport GTP frozen on dry ice to the microscope and add it to the polymerization reaction only directly before starting the experiment at the microscope.
-
20.
Wash the channel with 20 μL polymerization reaction supplemented with GTP.
Note: This wash is important to equilibrate the flow chamber prior to the experiment with polymerization reaction mix and more importantly with the wanted GTP concentration.
-
21.
Thaw label-free tubulin quickly and put it on ice.
-
22.
Spin down the label-free tubulin at 4°C and 13,000 × g for 5 min.
-
23.
Dilute the label-free tubulin to the desired concentration with polymerization reaction mix complemented with GTP.
Note: Prepare this solution always freshly and directly prior to the experiment. For a standard microtubule dynamic assay using porcine tubulin, a final tubulin concentration of 10 μM is sufficient to induce microtubule growth at the ends of the microtubule seeds.
-
24.
Flow the label-free tubulin into the flow cell.
-
25.Start imaging:
-
a)Warm the objective to 34°C before starting the experiment and keep the temperature stable with the implemented temperature control during the experiment.Note: If possible, equilibrate the temperature of the objective and setup overnight. In this manner, drift is minimized. Also, switch on any other heat source overnight to prewarm and equilibrate the whole optical setup and room to a stable temperature. On setups with millikelvin-precision temperature control (Simmert et al., 2018), even the influence of heat from the LED light source can be measured. If necessary, adjust the aperture and field iris for best contrast as pointed out in The Microcopes subsection in Materials and Equipment.
-
b)For imaging, use as much LED power as possible without saturating the camera, we usually use 4% LED power.
-
c)For imaging, we usually average 10 frames in real-time during image acquisition. The resulting final imaging speed of the recorded video with frame averaging is 370 ms/frame. We use 90% of the total dynamic range of greyscale levels to enhance the contrast of the sample.
-
d)To achieve maximal contrast for the microtubules, adjust the focus.
-
e)For a low-noise background image, move the stage laterally sufficiently fast and record and median average 400 images acquired at a frame rate of 25 Hz (Simmert et al., 2018). Images can be recorded while the stage is moving.
-
f)Subtract the background image from any recorded image in real-time (see note below).
-
g)Record videos of microtubule dynamics.
-
a)
Note: The choice of imaging conditions will of course depend on your tubulin source, experimental question, and features of the IRM used. Our custom-written image acquisition software subtracts the background and averages frames in real-time. If background subtraction and image processing cannot be performed in real-time, they can also be done after acquisition of the raw images.
Expected Outcomes
Figure 2 shows representative images of in vitro reconstituted microtubules imaged by TIRFM (Figure 2B) and IRM (Figure 2D). TIRF microscopy uses a collimated laser aimed at the sample at a low angle of incidence such that the difference in refractive indices of the objective oil and aqueous sample causes the laser to reflect back entirely (Figure 2A). A resulting evanescent wave illuminates a region extending approximately 100 nm into the sample, thereby restricting the excitation and detection of fluorophores to a thin region (Axelrod et al., 1984). Elimination of background fluorescence dramatically improves the signal-to-noise ratio and thus allows the spatial resolution of microtubule dynamics using fluorescently labeled tubulin.
IRM is based on the interference of light reflected from closely adjacent surfaces or a surface and a sub-diffraction-limited object of interest, e.g., a microtubule (Curtis 1964; Simmert et al., 2018; Mahamdeh et al., 2018). It creates an image comprising information on the separation and optical properties of these entities. Light is reflected, for example, at the interface between the glass slide and the buffer and at the interface between the buffer and the biological sample (Figure 2C). The reflected light beams interfere and generate a distinct interference pattern that holds information about the distance and, for a sub-diffraction-limited object, information about the molecular mass. For instance, a bundle of two microtubules has twice the contrast compared to a single microtubule. Intriguingly, reflection from an optically denser sample, i.e., one that has a higher refractive index compared to the medium (here the microtubule), results in a 180° phase shift of the reflected light relative to the incoming light. Because this phase shift is not present for the reflection from the glass-water interface, samples that are directly attached to the glass surface appear dark. This dark contrast is a manifestation of destructive interference of the two reflected beams as illustrated in the schematic of Figure 2C and can be seen in the representative image in Figure 2D. In this manner, a phase contrast is converted into an amplitude contrast. In the background areas where no microtubules are attached to the surface, incoming light only gets reflected at the glass-water interface and does not experience an amplitude change. Thanks to the information coming from the reflected interference pattern, even thin and low-contrast diffraction-limited objects such as microtubules can be visualized with enough contrast compared to the background. Microtubules will appear as dark black or light white structures depending on whether the reflected light beams experience positive interference and amplify in amplitude or whether they experience negative interference and cancel each other out. As explained above, they are dark directly at the surface and appear bright with maximum intensity compared to the background when they are approximately 80 nm above the surface (based on blue light with a wavelength of 450 nm). Thus, IRM is more sensitive to height changes in comparison to TIRFM.
Quantification and Statistical Analysis
Microtubule dynamics can be analyzed using kymographs of individual microtubules imaged by TIRFM (Figure 6A) or IRM (Figure 6B). Kymographs are space-time plots, where the image of the length of the microtubule is plotted horizontally along the x-axis and time is plotted vertically along the y-axis. This results in a 2-D representation of microtubule length at all acquired timepoints. The four parameters of dynamic instability (Mitchison and Kirschner 1984) and their distributions can be determined from such kymographs. For convenience, we describe here the analysis of a single microtubule. However, data from many microtubules across several independent experiments need to be obtained to draw robust conclusions.
Figure 6.
Representative Kymographs of Xenopus Microtubules Imaged by TIRFM and IRM to Analyze Parameters of Dynamic Instability
(A and B) Kymograph of a Xenopus microtubule produced from a series of images acquired by (A) TIRFM and (B) IRM.
(C) Example of a single microtubule showing growth, shrinkage, catastrophes, and rescues.
(D) How to calculate the four dynamics parameters of dynamic instability from kymograph data.
Using kymographs, one can measure microtubule polymerization velocity, depolymerization velocity, catastrophe frequency, and rescue frequency (Figure 6C). Microtubule polymerization and depolymerization velocities can be well approximated by a single constant growth rate (Figure 6D). Shrinkage rates, however, are usually an order of magnitude higher than growth rates. Therefore, higher acquisition rates are required for measuring detailed dynamics of the shrinking microtubule end. Catastrophe frequency is determined by dividing the total number of identified shrinkage events by the total time the microtubules were growing (events/time). Alternatively, catastrophes can be plotted as cumulative frequencies of microtubule lifetimes (Gardner et al., 2011). Analogously, rescue frequency is reported as the ratio of the total number of rescues observed over the total amount of time microtubules spent in shrinkage.
Here, we introduce a simple method to analyze microtubule dynamics using ImageJ (Schindelin et al., 2012). Detailed methods for measuring and analyzing microtubule dynamics are described in (Zanic 2016; Asbury 2016; Kapoor et al., 2019).
Generate an Individual Kymograph Using ImageJ
-
1.
Download ImageJ from the National Health Institute server.
-
2.
Download the ImageJ plugin “Multiple Kymograph” (written by J. Rietdorfand and A. Seitz) from the EMBL server to the plugin folder and restart the program.
-
3.
Import the recorded videos into ImageJ (File – Import). Various file formats can be imported, such as stacks, avi, or image sequences (e.g., .avi video file or .tiff sequence).
-
4.
If needed, the brightness and image contrast can be adjusted (Image – Adjust – Brightness/contrast). Adjust the maximum and minimum sliders accordingly to the minimum and maximum values in the histogram. Apply the contrast change to all frames.
-
5.
Make a maximum projection to visualize the microtubule growth path through the entire video (Image – Stacks – Z project). Use the line tool to draw a line along the microtubule and mark the region of interest (ROI) with the “t” key.
Note: We use the segmented line tool with a line width of 3 px. The optimal line width will depend on the individual imaging data, though. To adjust the line thickness, double click on the line tool icon.
-
6.
Make the video the active window by clicking it and highlight the line ROI by choosing it in the ROI window. With the “Multiple Kymograph” tool from the plugin menu, set the line width to 3, and apply with OK.
-
7.
The generated kymograph shows a dynamic microtubule: in the x-direction the microtubule length changes as the microtubule is growing and shrinking, and in the y-direction time is advancing (Figure 6). The units are the pixel size of your sequence and the time interval of the sequence.
-
8.
Save the kymograph as a .tiff file (File - Save As).
Note: While the data analysis via the rectangle tool is simpler, the angle tool might provide more accurate results if nonlinear growth/shrinkage processes are expected.
Angle Tool
-
9.
In order to calculate the speed at which the microtubule is growing, choose the angle tool and draw a line along the growing tip from top to bottom (Figure 6D), measure the angle (Analyze – Measure).
-
10.
Repeat this measuring for all growth events in the kymograph. Save and export the measured angles in a file format that is suitable for the software used to analyse your data (e.g., cvs or excel file).
-
11.
In order to determine the depolymerization velocity, repeat the same measuring procedure with shrinking events (Figure 6D).
-
12.Convert all measured angles to absolute values.
-
-For microtubule growth (1) at the right end of the microtubule, use the absolute value of the measured angle for the following calculations and (2) at the left end of the microtubule, transform the measured angles by 180°- |measured angle|.
-
-For microtubule shrinkage (1) at the right end of the microtubule, transform the measured angles by 180°- |measured angle| and (2) at the left end of the microtubule, use the absolute value of the measured angle for the following calculations.
-
-
-
13.
Transform all resulting angles from degrees to radians (radians = degree x π/180).
-
14.
The speed of microtubule growth (vp) or shrinkage (vd) in μm/s equals the absolute value of the inverted tangent of the angle α in radians multiplied by the pixel size b in μm and by the framerate c in Hz: v = | 1/tan(α) | x b x c.
-
15.
The catastrophe frequency (fcat) and the rescue frequency (fres) are events per total time growing and shrinking, respectively (Figure 6D)
Rectangle Tool
-
16.
Set ImageJ to measure the bounding rectangle of line ROIs under Analyse - Set Measurements. Check the box next to “Bounding Rectangle.”
-
17.
Draw a line along the growing or shrinking tip from the start to the finish of the growth or depolymerization event and measure (Analyse -> Measure).
-
18.
Repeat these measurements for all growth and shrinkage events in the kymograph.
-
19.
Save and export the measured lines in a file format that is suitable for the software used to analyse your data.
Limitations
Microtubule dynamics can be measured using TIRFM or IRM.
TIRFM has the following advantages: (1) Sub-populations of different microtubules or tubulin can be distinguished by labeling with different fluorophores, such as stabilized seeds vs. dynamic microtubules, or sites of tubulin exchange along the microtubule axis (Vemu et al., 2017). (2) Interactions between microtubules and microtubule-associated proteins (MAPs) or motors can be visualized down to the single-molecule level. (3) In case of microtubule dynamics, in particular catastrophe and rescues, one limitation lies in the lack of molecular specificity of IRM. It is not possible to distinguish microtubule seeds and microtubules labeled with different fluorophores (Figure 2D).
While having comparable contrast to fluorescence-based methods, IRM has the following advantages over fluorescence microscopy: (1) Label-free tubulin can be used in the microtubule assays. Since no labeling is needed, the method is more time efficient and the tubulin sample can be studied under native conditions. The label may affect the dynamics or structure of the microtubule itself or interactions of proteins with microtubules. Low amounts of tubulin are sufficient as no protein loss during labeling occurs. (2) Long-term and high-frame rate measurements are possible as the sample does not suffer from bleaching or photodamage (Bugiel et al., 2020). (3) The setup does not require expensive optical components and is thus cost-effective. (4) The sample may experience less drift when using an LED as light source, which induces a minimal amount of local heating (Simmert et al., 2018).
Finally, IRM and TIRFM can also be done simultaneously. For example, in a microtubule-based assay, microtubules and their dynamics can be imaged using IRM while fluorescent imaging can be used to observe additional components, e.g., fluorescently labeled MAPs or motors.
Troubleshooting
Problem
When testing the hydrophobicity of glass surfaces, the water droplet appears too flat (Figure 3).
Potential Solution
Repeat one or multiple cycle of TMCS incubation with 100 μL TMCS.
Problem
The tubulin does not polymerize using standard tubulin polymerization protocols.
Potential Solution
For each tubulin purification, you might need to test a variety of polymerization methods to find the optimal condition promoting microtubule growth. In particular, critical concentration and temperature will influence microtubule polymerization. It might help to assemble microtubules in the presence of a microtubule-stabilizing drug, such as Taxol or GMPCPP. Another problem might be hydrolyzed GTP, in which case a fresh aliquot of GTP should be used.
Problem
Background fluorescence is visible, but microtubules are not.
Potential Solution
Reduce the concentration of labeled tubulin relative to the total tubulin concentration in the reaction. If the concentration of labeled tubulin is too high, the microtubule signal will be too low relative to the background. If adjusting the label concentration does not improve the signal, test whether your tubulin is active using a polymerization assay. Also, the blocking of the flow cell might not have been sufficient and soluble labeled tubulin thus directly attaches to the glass surface.
Problem
Bleaching or photo-induced damage
Potential Solution
Oxidation of dyes leads to photobleaching and oxidation of tubulin can cause microtubules to break apart (Guo et al., 2006). To reduce these effects, we recommend using low laser power (<2 mW), short imaging times (<300 ms), and an enzymatic oxygen scavenger system (Gell et al., 2010). If you still observe significant bleaching or photo-induced damage, check the following points:
-
(1)
Oxygen scavenging enzymes might adsorb onto an incompletely blocked surface, which reduces their effective concentration.
-
(2)
The effectiveness of the oxygen scavenging system might not be optimal for your chosen dye(s) (Aitken et al., 2008; Rasnik et al., 2006).
-
(3)
β-Mercaptoethanol activity decreases with exposure to air. Make sure to only use small aliquots and store them at 4°C in a desiccator. An alternative is using DTT (10 mM final concentration) as a reducing agent although it may affect dye activity (Gell et al., 2010).
-
(4)
Photobleaching may also occur during the focusing step, because the live imaging generally uses a much higher frame rate than the recording step. If this occurs, move the stage to a fresh field of view after focusing the image and adjusting the TIRF angle.
Problem
Interference fringes.
Potential Solution
Laser illumination can produce interference fringes. The relatively low signal strength used in TIRF microscopy sometimes allows minor aberrations in the optical path to cause unwanted artifacts. While not necessarily problematic for manual image analysis, interference fringes can disrupt automated image analysis pipelines. Check first to see if they are eliminated by adjusting the TIRF angle and/or the position of the beam around the periphery of the back focal plane. Interference patterns generally appear at shallow laser angles before illumination is completely eliminated and can be reduced by adjusting slightly toward the epifluorescence position. Particulates on the coverslip or optical components can also cause interference. Be sure to keep components in the optical path clean and take care to avoid exposing the coverslips to dust or reaction buffers, which can evaporate and leave salt deposits on the glass. If strong interference patterns remain even after taking the above measures, they may be a result of damage to the objective or optical fiber, in which case, consult the vendor or manufacturer.
Problem
The surface of the glass slide is not clean enough, and an excessive background signal compromises the imaging quality of the microtubules.
Potential Solution
First, cleaning and silanization of glass surfaces needs to be done thoroughly and, ideally, freshly. Experience shows that if TMCS-coated cover slides are stored for longer time periods (> 4 weeks), the quality of the slides decreases in terms of (i) purity, as dust particles settle even when stored in closed containers and (ii) TMCS becomes less homogenous over time. The latter effect might lead to “hydrophobic islands” on the glass surface, which result in clustering of proteins adsorbed to such patches.
Second, impurities on the glass surface may possibly be introduced by using contaminated solutions during the assay. Thus, we recommend - if possible - to use small aliquots of filtered liquid solutions and to centrifuge solutions containing proteins that tend to form aggregates before introducing them into the assay.
Third, the blocking of the surface is also important to avoid non-specific binding to the surface. If the surface appears dirty, it can also be a sign for insufficient blocking. If the surface appears dirty, it can also be a sign for insufficient blocking.
Fourth, the introduction of air bubbles can compromise the imaging quality. Areas in the flow channel exposed to air bubbles often show clustered protein or salt crystals, which appear as impurity on the glass surface.
The problem can also be understood as an advantage though, as different kinds of possible contaminations (also the ones that are non-fluorescent) can be visualized by IRM and thus can be identified early on during an assay.
Problem
Interference pattern along the microtubule that appears as black and white stripes.
Potential Solution
As the signal detected with IRM from the specimen is height sensitive, it can happen that the imaged microtubule appears black and white along its lattice. This is the case when the microtubule is not attached perfectly, i.e., its axis is not parallel to the surface, and is not positioned at equal height in reference to the glass surface. Note that the use of methylcellulose may decrease microtubule fluctuations. As the interference pattern is height sensitive, at one height the interference will result in a black signal (destructive interference) and at a different height the interference will result in a white signal (constructive interference). In our IRM setup we are able to detect small height differences on the order of a few nanometers (based on the following rough estimate: 40 nm—corresponding to the distance between the black signal and the gray background level—divided by the signal-to-noise ratio of about 12 for an average of 10 frames) up to 80 nm if the signal switches from black to white (Simmert et al., 2018). This can cause problems for the image analysis, e.g., when analyzing a kymograph of such data. To avoid such interference patterns along the microtubule, the microtubule seeds have to be well attached to the glass surface and the dynamically growing and shrinking ends of the microtubules have to experience minimal drift during the measurement. This problem can be understood as an advantage though as the data hold additional information in 3D about the height of the microtubule (Simmert et al., 2018).
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Simone Reber (simone.reber@iri-lifesciences.de).
Materials Availability
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Simone Reber (simone.reber@iri-lifesciences.de). In general, plasmid constructs and antibodies are available for sharing.
Data and Code Availability
This study did not generate any unpublished custom code, software, or algorithm.
Acknowledgments
We thank the AMBIO imaging facility (Charité, Berlin) and Nikon at MBL for imaging support. We thank all former and current members of the Reber lab for discussion and helpful advice, in particular Christoph Hentschel and Soma Zsoter for technical assistance. S.R. acknowledges funding by the IRI Life Sciences (Humboldt-Universität zu Berlin, Excellence Initiative/DFG). W.H. was supported by the Alliance Berlin Canberra co-funded by a grant from the Deutsche Forschungsgemeinschaft (DFG) for the International Research Training Group (IRTG) 2290 and the Australian National University. C.K. thanks the Deutsche Forschungsgesellschaft (DFG, JA 2589/1-1). C.K. and M.A. thank Steve Simmert and Tobias Jachowski former and current members of the Schäffer lab.
Author Contributions
Conceptualization, S.R.; Methodology, W.H., C.K., and M.A.; Validation, W.H. and C.K.; Writing – Original Draft, W.H. and C.K.; Writing – Review & Editing, E.S. and S.R.; Funding Acquisition, E.S. and S.R.
Declaration of Interests
The authors declare no competing interests.
References
- Aitken C.E., Marshall R.A., Puglisi J.D. An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys. J. 2008;94:1826–1835. doi: 10.1529/biophysj.107.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbury C.L. Data analysis for total internal reflection fluorescence microscopy. Cold Spring Harb. Protoc. 2016;2016:prot085571. doi: 10.1101/pdb.prot085571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Burghardt T.P., Thompson N.L. Total internal reflection fluorescence. Annu. Rev. Biophys. Bioeng. 1984;13:247–268. doi: 10.1146/annurev.bb.13.060184.001335. [DOI] [PubMed] [Google Scholar]
- Bormuth V., Howard J., Schäffer E. LED illumination for video-enhanced DIC imaging of single microtubules. J. Microsc. 2007;226:1–5. doi: 10.1111/j.1365-2818.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- Bugiel M., Chugh M., Jachowski T.J., Schäffer E., Jannasch A. The kinesin-8 Kip3 depolymerizes microtubules with a collective force-dependent mechanism. Biophys. J. 2020;118:1958–1967. doi: 10.1016/j.bpj.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubules. Mol. Biol. Cell. 1996;7:663–675. doi: 10.1091/mbc.7.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaaban S., Jariwala S., Hsu C.T., Redemann S., Kollman J.M., Müller-Reichert T., Sept D., Bui K.H., Brouhard G.J. The structure and dynamics of C. elegans tubulin reveals the mechanistic basis of microtubule growth. Dev. Cell. 2018;47:191–204. doi: 10.1016/j.devcel.2018.08.023. [DOI] [PubMed] [Google Scholar]
- Curtis A.S.G. The mechanism of adhesion of cells to glass: a study by interference reflection microscopy. J. Cell Biol. 1964;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R., Ross J.L. Studying plus-end tracking at single molecule resolution using TIRF microscopy. Methods Cell Biol. 2010;95:543–554. doi: 10.1016/S0091-679X(10)95027-9. [DOI] [PubMed] [Google Scholar]
- Gardner M.K., Charlebois B.D., Jánosi I.M., Howard J., Hunt A.J., Odde D.J. Rapid microtubule self-assembly kinetics. Cell. 2011;146:582–592. doi: 10.1016/j.cell.2011.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell C., Bormuth V., Brouhard G.J., Cohen D.N., Diez S., Friel C.T., Helenius J., Nitzsche B., Petzold H., Ribbe J. Microtubule dynamics reconstituted in vitro and imaged by single-molecule fluorescence microscopy. Methods Cell Biol. 2010;95:221–245. doi: 10.1016/S0091-679X(10)95013-9. [DOI] [PubMed] [Google Scholar]
- Gell C., Friel C.T., Borgonovo B., Drechsel D.N., Hyman A.A., Howard J. Purification of tubulin from porcine brain. In: Straube A., editor. Microtubule Dynamics. Humana Press; 2011. pp. 15–28. [DOI] [PubMed] [Google Scholar]
- Guo H.L., Xu C.H., Liu C.X., Qu E., Yuan M., Li Z.L., Cheng B.Y., Zhang D.Z. Mechanism and dynamics of breakage of fluorescent microtubules. Biophys. J. 2006;90:2093–2098. doi: 10.1529/biophysj.105.071209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius J., Brouhard G., Kalaidzidis Y., Diez S., Howard J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 2006;441:115–119. doi: 10.1038/nature04736. [DOI] [PubMed] [Google Scholar]
- Hirst W.G., Biswas A., Mahalingan K.K., Reber S. Differences in intrinsic tubulin dynamic properties contribute to spindle length control in xenopus species. Curr. Biol. 2020;30:2184–2190.e5. doi: 10.1016/j.cub.2020.03.067. [DOI] [PubMed] [Google Scholar]
- Hyman A.A., Salser S., Drechsel D.N., Unwin N., Mitchison T.J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol. Biol. Cell. 1992;3:1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V., Hirst W.G., Hentschel C., Preibisch S., Reber S. MTrack: automated detection, tracking, and analysis of dynamic microtubules. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-018-37767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamdeh M., Simmert S., Luchniak A., Schaeffer E., Howard J. Label-free high-speed wide-field imaging of single microtubules using interference reflection microscopy. J. Microsc. 2018;272:60–66. doi: 10.1111/jmi.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Nguyen P.A., Field C.M., Groen A.C., Mitchison T.J., Loose M. Using supported bilayers to study the spatiotemporal organization of membrane-bound proteins. Methods Cell Biol. 2015;128:223–241. doi: 10.1016/bs.mcb.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori-McKenney K.M., McKenney R.J., Huang H.H., Li T., Meltzer S., Jan L.Y., Vale R.D., Wiita A.P., Jan Y.N. Phosphorylation of β-Tubulin by the down syndrome kinase, minibrain/DYRK1a, regulates microtubule dynamics and dendrite morphogenesis. Neuron. 2016;90:551–563. doi: 10.1016/j.neuron.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasnik I., McKinney S.A., Ha T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nat. Methods. 2006;3:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- Reusch S., Biswas A., Hirst W.G., Reber S. Affinity Purification of Label-free Tubulins from Xenopus Egg Extracts. Star Protocols. 2020:100151. doi: 10.1016/j.xpro.2020.100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin P.R., Ha T. Single-molecule Techniques: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- Simmert S., Abdosamadi M.K., Hermsdorf G., Schäffer E. LED-based interference-reflection microscopy combined with optical tweezers for quantitative three-dimensional microtubule imaging. Opt. Express. 2018;26:14499–14513. doi: 10.1364/OE.26.014499. [DOI] [PubMed] [Google Scholar]
- Szkop M., Kliszcz B., Kasprzak A.A. A simple and reproducible protocol of glass surface silanization for TIRF microscopy imaging. Anal. Biochem. 2018;549:119–123. doi: 10.1016/j.ab.2018.03.020. [DOI] [PubMed] [Google Scholar]
- Ti S.C., Alushin G.M., Kapoor T.M. Human β-tubulin isotypes can regulate microtubule protofilament number and stability. Dev. Cell. 2018;47:175–190. doi: 10.1016/j.devcel.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemu A., Atherton J., Spector J.O., Moores C.A., Roll-Mecak A. Tubulin isoform composition tunes microtubule dynamics. Mol. Biol. Cell. 2017;28:3564–3572. doi: 10.1091/mbc.E17-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanic M. Measuring the effects of microtubule-associated proteins on microtubule dynamics in vitro. In: Chang P., Ohi R., editors. The Mitotic Spindle. Humana Press; 2016. pp. 47–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unpublished custom code, software, or algorithm.