Summary
Here, we present a modified protocol for a mouse heart failure (HF) model using minimally invasive transverse aortic constriction (miTAC). miTAC is a more effective method in mice than the standard open-chest transverse aortic constriction (TAC) to generate an HF model. miTAC does not require the cutting of the ribs or tracheal intubation with artificial ventilation; it also has a higher survival rate. The successful outcome of the HF model can be verified using transthoracic echocardiography and histology.
For complete details on the use and execution of this protocol, please refer to Hu et al. (2003) and Richards et al. (2019).
Subject Areas: Cell Biology, Model Organisms
Graphical Abstract
Highlights
-
•
miTAC is an effective method to generate a mouse heart failure model
-
•
miTAC does not need tracheal intubation with artificial ventilation
-
•
The survival rate of miTAC is higher than that of open-chest TAC
Here, we present a modified protocol for a mouse heart failure (HF) model using minimally invasive transverse aortic constriction (miTAC). miTAC is a more effective method in mice than the standard open-chest transverse aortic constriction (TAC) to generate an HF model. miTAC does not require the cutting of the ribs or tracheal intubation with artificial ventilation; it also has a higher survival rate. The successful outcome of the HF model can be verified using transthoracic echocardiography and histology.
Before You Begin
Male C57BL/6J mice (8–10 weeks, 25–27 g) were used in this protocol. Animal experiments were conducted following the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments at Tongji University. Mice were housed under a 12 h light/dark cycle and provided with food and water ad libitum.
Surgical Instruments Preparation
-
1.
Prepare a 24-gauge cannula needle ( ∼2.6 cm length), a 27-gauge needle, and two 26-gauge needles, and bend them into corresponding shapes (Figure 1).
CRITICAL: It is necessary to blunt the sharp point of the 27-gauge needle (Rockman et al., 1991) to avoid damaging the aorta.
Alternatives: An alternative strategy can be chosen, using a retractor inserted between the cut sterna to gain visibility of the aortic arch.
-
2.
Set up a Hot Bead Sterilizer to 250°C for sterilizing surgical instruments.
Note: During the establishment of animal models, the autoclaved surgical instruments should be changed by each mouse. When using large quantities of mice, the Hot Bead sterilization method can rapidly sterilize surgical instruments after surgery. 20 s is enough for the sterilization of surgical instruments at 250°C.
-
3.
Set up the heating pad to 37°C for maintenance of intra-operative body temperature.
Note: The heating pad is used for keeping the body temperature of the mouse during surgery.
-
4.
Disinfect the surgical platform with 75% alcohol.
-
5.
Set up a warmed (31°C–35°C) cage with a heating lamp.
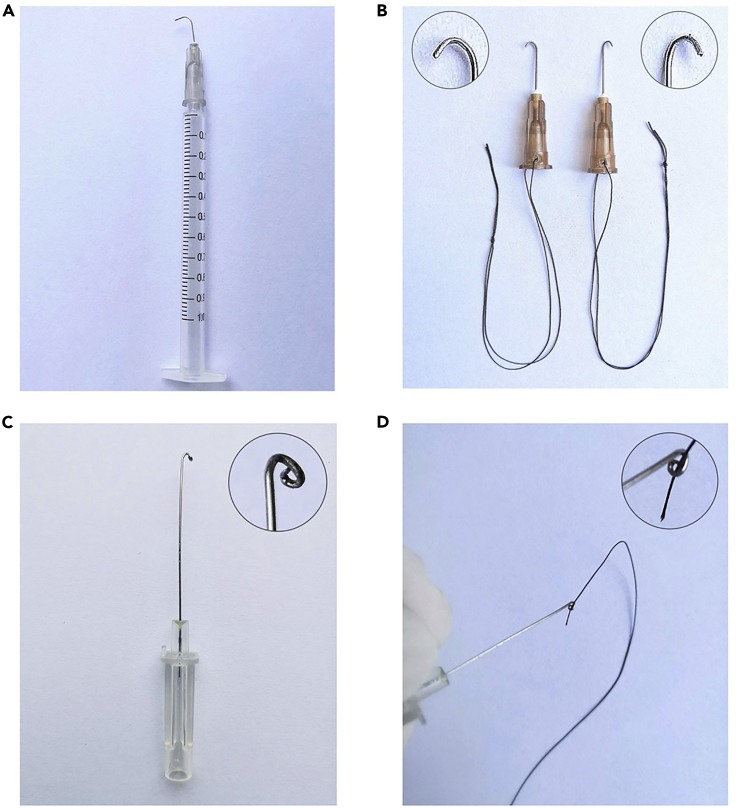
Figure 1.
Homemade Surgical Instruments
(A) Creating a ligation spacer: bend a 27-gauge needle 90° with a needle holder.
(B) Making a curved needle: take a 24-gauge cannula needle and curve it with a needle holder to make a loop for use in passing the suture through the aortic arch.
(C) Drag hooks (26-gauge needle) by bending the needle tip to the proper angle, as illustrated above.
(D) The curved needle with suture.
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| Isoflurane | RWD Life Science | Cat# R510-22-16 |
| Sterile saline | Vetec | Cat# 101322517 |
| Povidone-iodine solution (2%) | Hopebio | Cat# HB6266 |
| Ethanol (75%) | LIRCON | Cat# XJJ75 |
| Buprenorphine solution | Sigma-Aldrich | Cat# B7536 |
| Software and Algorithms | ||
| GraphPad Prism | GraphPad | Prism 7 |
| Experimental Models: Organisms/Strains | ||
| Mouse | Shanghai SLAC | Cat# Slac:C57BL/6J |
| Other | ||
| Prolene 6-0 | EIHICON | Cat# W8706 |
| Polyglicolic Acid (PGA) 6-0 | Jinhuan Medical | Cat# R611 |
| Polyglicolic Acid (PGA) 4-0 | Jinhuan Medical | Cat# CR436 |
| Polypropylene 6-0 | Johnson & Johnson | Cat# W08706 |
| 27-gauge needle | HENKE SASS WOLF | Cat# NH2712 |
| Cannula Needle | Juye Forna Medical Instrument | Cat# FN-001 |
| Electric razor | Codos | Cat# CP-8000 |
| Halsey Needle Holders | Fine Science Tools | Cat# 12501-13 |
| Halsey Micro Needle Holder | Fine Science Tools | Cat# 12500-12 |
| Angled dissector Scissors | Fine Science Tools | Cat# 14082-09 |
| Hardened Fine Scissors, straight | Fine Science Tools | Cat# 14090-11 |
| Moria Iris Forceps, straight | Fine Science Tools | Cat# 11370-32 |
| Semken Forceps, straight | Fine Science Tools | Cat# 11008-13 |
| Semken Forceps, curved | Fine Science Tools | Cat# 11008-13 |
| Micro-Mosquito Hemostats | Fine Science Tools | Cat# 13011-12 |
| Ophthalmic gel | Bausch Lomb | Cat# 1989189972 |
| Pressure-sensitive adhesive tape | Qingdao haishihainuo | Cat# SKU4140361 |
| Heating Pad | Yuyan Instruments | Cat# S-100 |
| Heating lamp | Yuyan Instruments | Cat# HR1625 |
| Gemini Cautery System | Yuyan Instruments | Cat# 3011 |
| Anesthesia machine | RWD Life Science | Cat# R540IP |
| Hot Bead Sterilizer | All For Life Science | Cat# AS-07070-00 |
| LED cold light illuminator | Meimei Metering Electricity Technology | Cat# CP201803141310 |
| Animal anesthesia machine | RWD Life Science | Cat# R415-AP |
Step-By-Step Method Details
Animal Preparation
Timing: 5 min
Anesthetize and fix animals for surgery.
-
1.
Mice must be deprived of food and water 6-hour before the surgical operation.
CRITICAL: Food deprivation before surgery is essential to avoid the risk of vomiting or bringing up food to the throat or lungs.
-
2.
Anesthetize the mouse using 2% isoflurane and oxygen with a flow rate of 0.5 L/min in an induction chamber connected with an animal anesthesia machine.
-
3.
Quickly shave the fur from the neckline to the xiphoid process level using an electric razor.
-
4.
Apply ophthalmic gel to the eyes to avoid eye dryness, settle the animal on a heating pad in a supine position with the head facing the operator, and fix the paws with medical pressure-sensitive adhesive tape.
Note: The head of the mouse faces the operator giving a more clear view and facilitates handle, and it can be operated clearly without a Stereo Microscope in the next step.
-
5.
Maintain the anesthesia with 1.5%–2% isoflurane with an airflow of 0.1–0.3 L/min (Brede et al., 2002).
Note: Maintenance of anesthesia with an oxygen flow rate of around 0.3 L/min provides several benefits, including decreased use of inhaled agents, improved body temperature and humidity homeostasis, and reduced environmental pollution (Adelsperger et al., 2016; Brattwall et al., 2012).
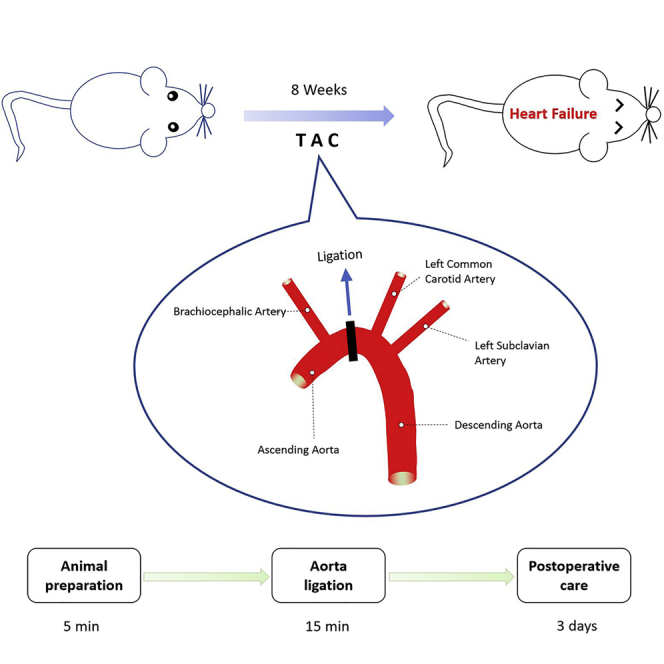
Surgical Procedure for Ligation
Timing: 15 min
Bind the aortic arch using Prolene 6-0 suture with a curved 27-gauge needle help.
-
6.
Sterilize the skin using 2% povidone-iodine solution and 75% alcohol, separately.
-
7.
Confirm sufficient level of anesthesia by performing a toe pinch with a straight forceps. The anesthetized mouse should not respond to the stimulation.
Note: Increase isoflurane vol % if the mouse in response to the toe pinch.
-
8.
Begin with partial thoracotomy by creating a 1.5–2 cm incision (Figure 2A) in the skin to the second rib with fine scissors (Hu et al., 2003).
-
9.
Pull the salivary glands toward the head with Semken Forceps (straight) to reveal the sternal notch, and try to maintain the integrity of the salivary glands.
-
10.
Cut the sternum about 4–5 mm using a dissector scissor (Figure 2B).
CRITICAL: Pay special attention to this step; do not cut over 6 mm to avoid damaging the parietal pleura and to prevent developing the pneumothorax.
-
11.
Gently separate the muscle tissue on the trachea with two Iris forceps.
-
12.
Pull the sternum apart on both sides with two manual made drag hooks (Figure 2D), and fix the middle of string threaded in the drag hook to the surgical pad with an adhesive tape (Figure 2C).
Note: The power of adhesive tape is enough to fix the drag hook. You can also easy to adjust the pull strength of the drag hook by pulling the end of the string thread and push the tape to fix.
-
13.
Carefully separate the connective tissue and thymus lobes with two pairs of Iris Forceps (straight) up and to both sides. At the same time, the brachiocephalic artery, the left and the right common carotid arteries, and the aortic arch will be visible (Figure 2E).
-
14.
Separate the connective tissue cautiously and gently just under the aortic arch using two pairs of Iris forceps so that the curved needle with silk suture can pass through the aortic arch smoothly (Figure 2F).
CRITICAL: Do not separate the tissue down toward the thoracic cavity too deep and broad, and just under the aortic arch; otherwise, it is easy to lead to the formation of pneumothorax. Excessive tissues around the aortic arch should be excluded or it will affect the degree of coarctation of the aortic arch.
-
15.
Place the curved needle with Prolene 6-0 suture under the aortic arch, then pull the curved needle toward the head a little, and then prick the tissue covering the head of the curved needle with a pair of Iris forceps (Figures 2G and 2H).
Note: The forceps can only prick the head of the curved needle to avoid damaging the aorta.
Note: The advantage of threading the curved needle with silk suture before the operation is that it can save the time of threading during operating.
-
16.
Clip the suture out slowly and gently with forceps and gently retrieve the curved needle (Figures 2I and 2J).
-
17.
Place the blunted spacer (curved 27-gauge needle, diameter: approximately 0.410 mm) into the suture loop (Figure 2K).
-
18.
Tie a loose double knot, then fasten the fist knot and second knot in turn, and finally, tie a knot again; Immediately, remove the spacer quickly but softly.
CRITICAL: The degree of tightness of the ligation is critical to the success and reproducibility of the procedure: too tight a ligation can result in high mortality, but too loose will delay the formation of the HF model.
-
19.
Cut the redundant suture, and check for bleeding around the aortic arch, sternal edges, and other soft tissue. If there is bleeding, stanch the bleeding site using the electrocoagulation hemostatic device (LEIYEA, 19-5002).
-
20.
Release the string thread and remove the drag hooks from sternal edges on each side.
-
21.
Close the chest muscle wall using 6/0 Polyglicolic Acid (PGA) suture with a simple interrupted suture pattern.
-
22.
Close the skin using 4/0 PGA suture in a continuous suture pattern.
-
23.
Disinfect the suture site with povidone-iodine solution, remove adhesive tape from limbs, and return the mouse to the warmed cage.
Note: For trained operators, the entire modified transverse aortic constriction (TAC) surgical procedure usually takes 7–12 min, not including preparation time. For beginners, it might take even longer.
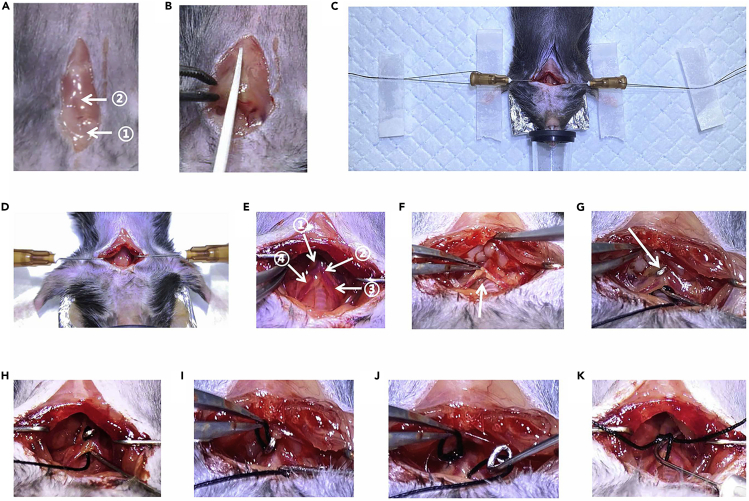
Figure 2.
Some of the Details in the Procedure
(A) A 1.5–2 cm incision. ① Salivary glands; ② sternum.
(B) Cut the sternum about 5 mm.
(C) The string threaded in the drag hook was fixed to the surgical pad with adhesive tape.
(D) Drag hooks.
(E) ① The aortic arch; ② the brachiocephalic artery; ③ the right common carotid arteries; ④ the left common carotid arteries.
(F) The tissue under the aortic arch was separated (arrow).
(G) The pricking point (arrow).
(H) The curved needle with silk suture under the aortic arch.
(I) The suture was clipped out.
(J) The retrieved curved needle.
(K) The blunted spacer in the suture loop.
Postoperative Care
Timing: 3 days
Use the pain reliever to minimize animal pain.
-
24.
For postoperative analgesia, inject an opiate drug buprenorphine (0.1 μg/g) intraperitoneally after the surgery.
Note: The postoperative analgesia is indispensable for mice after surgery. According to Animal Welfare, laboratory animals need to be given analgesia during and after an operation, and every effort should be made to minimize animal pain, suffering, and distress.
-
25.
Put the mouse in a warmed (30°C–35°C) cage, and the mouse will regain consciousness approximately 2 min later.
-
26.
Keep the mouse in the warmed cage for 1 h for completely recover from the surgical operation, then move to the housing environment (25°C).
Note: To reduce pain, mice are injected with buprenorphine every 8 h for three days. Buprenorphine is administered intraperitoneally in this protocol. Using subcutaneous injection can extend the time to 12 h.
Alternatives: ibuprofen (Motrin) can be administered in drinking water (0.2 mg/mL).
CRITICAL: Postoperative care has a positive effect on the speedy recovery of the animals. Poor postoperative care may cause the animal to die.
Expected Outcomes
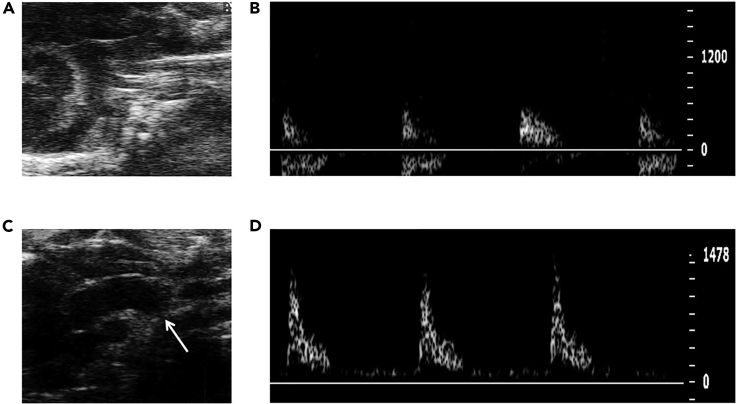
We verified the pressure overload after TAC. The aortic peak flow velocity significantly increased in TAC mice (Figure 3). Based on the result of a series of experiments, the average operative survival was more than 95%. When done correctly, the survival following TAC will over 98% before 1 week (Figure 4A), which is higher than the previous report (deAlmeida et al., 2010). In addition, the most common reasons for mice death are the aortic hemorrhage, pneumothorax, and too tight ligation of the aortic arch.
Figure 3.
Pulsed-Wave Doppler Ultrasound Imaging from the Aortic Arch
(A) The aortic arch of a sham mouse.
(B) Aortic peak flow velocity of a sham mouse.
(C) The aortic arch with constriction site of a TAC mouse.
(D) Aortic peak flow velocity of a TAC mouse.
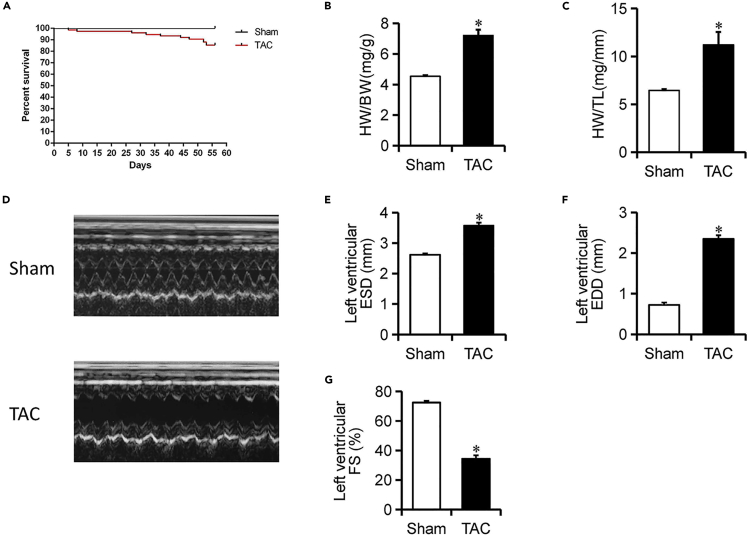
Figure 4.
Survival Curve in Mice (Sham and TAC) and Evaluation in Cardiac Function
(A) Survival curve for an 8-week-long experiment (TAC = 75 and Sham = 75).
(B and C) Heart weight to body weight and tibia length measured 8 weeks after TAC.
(D) Sample 2D echocardiographs from sham and TAC mice.
(E–G) Echocardiography data.
Data presented as means ± SE; n = 5–6; ∗p < 0.05 versus sham.
Compared with the sham-operated mice, both the heart weight/body weight ratio and heart weight/tibial length ratio were dramatically increased at the end of 8 weeks (Figures 4B and C), consistent with the profound dilation seen by 2D echocardiography (Figure 4D). Cardiac function was evaluated in vivo by using 2D echocardiography. 8 weeks after TAC, hearts in TAC mice became markedly dilated, shown here with a tripling of EDD (Figure 4F) and an increased ESD (Figure 4E), accompanied by a significant reduction in contraction (measured as a fractional shortening [FS]; Figure 4G).
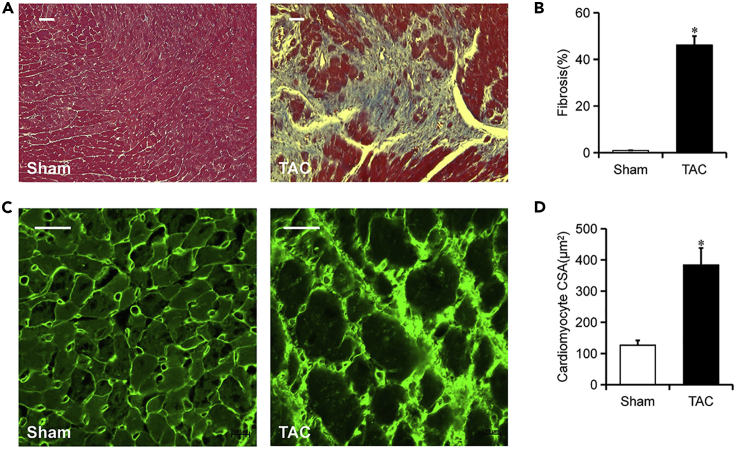
The incomplete ligation to the transverse aorta provoked increased hemodynamic load on the heart. Consequently, greater force must be used by the heart to maintain normal cardiac output, eventually leading to cardiac hypertrophy(Arany et al., 2006). As seen in Figure 5, mice subjected to TAC developed distinctly left ventricular cardiomyocyte hypertrophy (Figure 5C) shown in increased cell cross-sectional area (Figure 5D) and myocardial fibrosis that involved over 40% of the myocardium (Figures 5A and 5B) at terminal harvest ( 8 weeks) (Richards et al., 2019). As mentioned earlier, the cardiac hypertrophy supported the increased heart weight/body weight ratio, and they are associated with each other.
Figure 5.
Mice Subjected to TAC Developed Clinical Heart Failure
(A and B) Representative Masson’s trichrome staining of myocardial fibrosis, as in (B). Blue indicates the accumulation of extracellular matrix or fibrosis; quantitative data (B) is on the right. Magnification: ×20. n = 6. Scale, 20 μm.
(C and D) Representative images of wheat germ agglutinin (WGA) staining for confirming cardiomyocyte dilation; quantitative data (D) is on the right. n = 6. Scale bar, 20 μm. Data presented as means ± SE; ∗p < 0.05 versus sham.
Limitations
The degree of tightness of ligation is a key determinant of the success and reproducibility of the procedure. This is a limitation that it is difficult for beginners, and even experienced researchers need a certain amount of practice to master it. To avoid this problem, we have adapted to surgical position shown in Figure 2C; the mouse is in the supine position with its head toward the operator, which can expose the aortic arch to a greater extent and is conducive to operators to master the tightness degree of ligation. In addition, if the animal is not at a proper and steady-state level of anesthesia, pneumothorax may result during ligation. Special attention should be paid to the consistency of the tightness degree of ligation, which is of crucial importance for the reproducibility of the procedure.
Troubleshooting
Problem 1
The rupture of the aortic arch (step 18)
Potential Solution
The 27-gauge needle used for ligation can be smoothed on waste paper, e.g., newspaper, A4 paper.
Problem 2
Animals died in the anesthesia recovery period
Potential Solution
Some anesthetics such as sodium pentobarbital causes severe depression of heart rate, blood pressure, and respiration. It is recommended to choose combined anesthesia or inhalational anesthetics like isoflurane. Observe whether the mouse's nose is blocked by bedding materials such as corncob. Control the number (less than 3) of mice in each cage to prevent the mice recovered previously from pressing against the unrecovered mice.
Problem 3
No significant heart failure occurred after 8 weeks
Potential Solution
Make a triple knot instead of double knot during ligation to prevent the knot slip off.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Guohua Gong (guohgong@tongji.edu.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate any unique datasets or code.
Acknowledgments
This work was supported partly by the National Key Research and Development Program of China (nos. 2017YFA0105601 and 2018YFA0107102), the National Natural Science Foundation of China (no. 31901044 to Q.-Y. and nos. 81970333 and 31771524 to G.-G.H.), and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (no. TP2017036 to G.-G.H.).
Author Contributions
G.-G.H. conceived, designed, and supervised the project. L.-B.L. conducted most experiments. L.-B.L., G.-M., L.-A.Q., and Q.-Y. performed partial data analysis. Q.-Y. provided valuable suggestions. G.G. and L.-B.L. wrote the manuscript.
Declaration of Interests
The authors declare no competing interests.
Contributor Information
Yuan Qin, Email: yuan_q19@163.com.
Guohua Gong, Email: guohgong@tongji.edu.cn.
References
- Adelsperger A.R., Bigiarelli-Nogas K.J., Toore I., Goergen C.J. Use of a low-flow digital anesthesia system for mice and rats. J. Vis. Exp. 2016;115:e54436. doi: 10.3791/54436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany Z., Novikov M., Chin S., Ma Y., Rosenzweig A., Spiegelman B.M. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl. Acad. Sci. U S A. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brede M., Wiesmann F., Jahns R., Hadamek K., Arnolt C., Neubauer S., Lohse M.J., Hein L. Feedback inhibition of catecholamine release by two different alpha(2)-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- Brattwall M., Warrén-Stomberg M., Hesselvik F., Jakobsson J. Brief review: theory and practice of minimal fresh gas flow anesthesia. Can. J. Anaesth. 2012;59:785–797. doi: 10.1007/s12630-012-9736-2. [DOI] [PubMed] [Google Scholar]
- deAlmeida A.C., Van Oort R.J., Wehrens X.H. Transverse aortic constriction in mice. J.Vis.Exp. 2010:e1729. doi: 10.3791/1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P., Zhang D., Swenson L., Chakrabarti G., Abel E.D., Litwin S.E. Minimally invasive aortic banding in mice: effects of altered cardiomyocyte insulin signaling during pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- Richards D.A., Aronovitz M.J., Calamaras T.D., Tam K., Martin G.L., Liu P.W., Bowditch H.K., Zhang P., Huggins G.S., Blanton R.M. Distinct Phenotypes Induced by Three Degrees of Transverse Aortic Constriction in Mice. Sci. Rep. 2019;9:5844. doi: 10.1038/s41598-019-42209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockman H.A., Ross R.S., Harris A.N., Knowlton K.U., Steinhelper M.E., Field L.J., Ross J., Jr., Chien K.R. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. U S A. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate any unique datasets or code.