Abstract
Objectives
Assessment of the adaptive immune response against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is crucial for studying long‐term immunity and vaccine strategies. We quantified IFNγ‐secreting T cells reactive against the main viral SARS‐CoV‐2 antigens using a standardised enzyme‐linked immunospot assay (ELISpot).
Methods
Overlapping peptide pools built from the sequences of M, N and S viral proteins and a mix (MNS) were used as antigens. Using IFNγ T‐CoV‐Spot assay, we assessed T‐cell and antibody responses in mild, moderate and severe SARS‐CoV‐2 patients and in control samples collected before the outbreak.
Results
Specific T cells were assessed in 60 consecutive patients (mild, n = 26; moderate, n = 10; and severe patients, n = 24) during their follow‐up (median time from symptom onset [interquartile range]: 36 days [28;53]). T cells against M, N and S peptide pools were detected in n = 60 (100%), n = 56 (93.3%), n = 55 patients (91.7%), respectively. Using the MNS mix, IFNγ T‐CoV‐Spot assay showed a specificity of 96.7% (95% CI, 88.5–99.6%) and a specificity of 90.3% (75.2–98.0%). The frequency of reactive T cells observed with M, S and MNS mix pools correlated with severity and with levels of anti‐S1 and anti‐RBD serum antibodies.
Conclusion
IFNγ T‐CoV‐Spot assay is a reliable method to explore specific T cells in large cohorts of patients. This test may become a useful tool to assess the long‐lived memory T‐cell response after vaccination. Our study demonstrates that SARS‐CoV‐2 patients developing a severe disease achieve a higher adaptive immune response.
Keywords: ELISpot, SARS‐CoV‐2, T cells
We quantified IFNg‐secreting T cells reactive against the M, N and S viral SARS‐CoV‐2 proteins using a standardized enzyme‐linked immunospot assay in 60 patients. The frequency of reactive T cells correlated with severity, and with levels of anti‐S1 and anti‐RBD (receptor binding domain) serum antibodies, demonstrating a higher adaptive immune response after developing a more severe disease. IFNg T‐CoV‐Spot assay may also become a useful tool to assess the long‐lived memory T cell response after vaccination.

Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has spread rapidly worldwide. A large majority of SARS‐CoV‐2 patients, reportedly up to 60% in a highly confined population, remain asymptomatic. 1 A few patients will need hospitalisation for moderate symptoms related to pneumonia but their requirement for oxygen therapy is low. 2 The most severely affected patients, who require intensive care unit admission, develop acute respiratory distress syndrome at about 7–10 days after symptom onset and multiple organ failure. 3 Severe lung damage can be induced by heterogeneous patterns ranging from diffuse alveolar damage to acute fibrinous and organising pneumonia, with frequent pulmonary thrombosis. 4 , 5 , 6 Several convergent reports describe an immune dysregulation in these severe patients, associating an impaired type I interferon production, 7 , 8 and a dramatic increase in plasma levels of other cytokines, such as interleukin‐6 (IL‐6), TNFα, IL‐2 and the soluble IL‐2 receptor (sIL‐2R), IFNγ and IL‐10. 2 , 9 , 10 , 11 , 12 Peripheral lymphopenia is also reported in more than 80% of patients, and severe patients presented the lowest lymphocyte counts, 9 , 13 , 14 , 15 and exhausted and reduced functional T cells. 14 , 16 Blood lymphopenia was also associated with lymphocyte depletion in spleen and lymph nodes, decreased or absent germinal centres, 5 , 17 and with aggressive migration to the lungs. 14 All these features could affect specific immunisation in these patients. Earlier seroconversion and/or higher titres of specific antibodies were detected in the patients with a severe outcome, 18 , 19 , 20 suggesting that an adaptive response may be involved in severe patients. Some patients also remain RT‐PCR‐positive for weeks despite the presence of antibodies, and, conversely, some patients recover without generating high titres of specific antibodies: these discordant data suggest that cellular‐specific immunity may be critical for clinical resolution of SARS‐CoV‐2 infection. 21 , 22 Given that SARS‐CoV‐2 is an emerging virus potentially responsible for severe disease with a high mortality rate, it is crucial to understand the role of the immune adaptive response, that is after primo‐infection, and at the earliest opportunity, to explore the immune response after induced immunisation, that is after vaccination. Among the major viral structural proteins, spike (S), nucleocapsid (N) and membrane (M) proteins bear the immunodominant B‐cell epitopes, but also T‐cell epitopes. 23 Currently, the anti‐S adaptive response is the one mainly being investigated, but anti‐M and anti‐N responses may be of interest to better apprehend naturally acquired specific immunity. Understanding the cellular response may also be important regarding the suspected cross‐reactivity with human coronaviruses that cause the common cold: indeed, anti‐SARS‐CoV‐2‐reactive CD4+ T cells were found in 34% to 60% of unexposed subjects. 24 , 25
To address the major issue of the cellular response in SARS‐CoV‐2 patients, and especially in severe patients, we quantified IFNγ‐secreting T cells reactive against the main viral SARS‐CoV‐2 antigens using a standardised enzyme‐linked immunospot (ELISpot) assay (IFNγ T‐CoV‐Spot assay) and compared T‐cell and antibody responses in mild, moderate and severe SARS‐CoV‐2 patients.
Results
Quantification of M, N, S and MNS mix‐specific reactive T cells in SARS‐CoV‐2 patients
IFNγ T‐CoV‐Spot assay was first performed in 60 consecutive SARS‐CoV‐2 patients using the M, N, S peptide pools and the MNS mix. Main characteristics of the patients are detailed in Table 1. The median (range) time from symptom onset (day 0, D0) to sampling was 36 (10–70) days and only 5 patients were sampled before D21. The patients were classified as mild (n = 26), moderate (n = 10) or severe (n = 24). Severe patients were older, more likely to be male and were sampled later compared to mild and moderate patients (Table 1). Variations in numbers of IFNγ‐Spot‐forming cells (SFCs) were observed from one peptide pool to another in a given patient (Figure 1a), a higher magnitude of response was detected with M peptide pool, and the MNS mix induced the highest in vitro T‐cell response compared with each pool of peptides alone (Figure 1b). Despite these differences in reactivity from one protein to another, there was a large correlation between the numbers of IFNγ‐SFCs detected with M, N and S pools in this population of patients (r > 0.6 for each M/N, M/S and N/S correlation), suggesting that a given patient could have a high frequency of reactive T cells against the three proteins or, conversely, a low frequency of reactive T cells against the three proteins (Figure 1c–e).
Table 1.
Main characteristics of included SARS‐CoV‐2 patients (at the time of IFNγ T‐CoV‐Spot assay)
| All patients (n = 60) | Mild disease (n = 26) | Moderate disease (n = 10) | Severe disease (n = 24) | P‐value | |
|---|---|---|---|---|---|
| Age, years | 49 (20;92) | 35 (28.7;47) | 53 (37;64) | 63 (51;72) | < 0.0001 |
| Sex (male) | 29 (48.3) | 5 (19.2) | 3 (30) | 21 (87.5) | < 0.0001 |
| Time since symptom onset, days | |||||
| Median (IQR) | 36 (28;53) | 32 (25;37) | 34 (28;57) | 52 (36;56) | 0.001 |
| Range | 10–70 | 10–59 | 15–70 | 13–64 | |
| Time since diagnosis by RT‐PCR, days | |||||
| Median (IQR) | 31 (20;41) | 26 (8;31) | 35 (24;53) | 40 (28;48) | 0.003 |
| Range | 3–64 | 5–57 | 3–64 | 8–52 | |
| Patient management | |||||
| Outpatients | 25 (41.7) | 25 (96.1) | 0 | 0 | na |
| Hospitalised patients | |||||
| Discharged at the time of sample | 28 (46.7) | 1 (3.8) | 10 (100) | 17 (70.8) | na |
| Still hospitalised at the time of sample | 7 (11.7) | 0 | 0 | 7 (29.2) | na |
| T‐cell counts a | |||||
| CD3+ T cells (×109 L−1) | 1.2 (0.6;2.7) | 1.2 (0.8;1.8) | 1.0 (0.7;1.4) | 1.3 (1.0;1.8) | 0.4 |
| CD3+CD4+ T cells (×109 L−1) | 0.8 (0.2;1.5) | 0.7 (0.4;1.0) | 0.7 (0.4;0.9) | 0.7 (0.6;1.1) | 0.64 |
| CD3+CD8+ T cells (×109 L−1) | 0.5 (0.1;1.2) | 0.5 (0.3;0.7) | 0.3 (0.2;0.5) | 0.5 (0.4;0.6) | 0.35 |
Data are median (IQR) or n (%). P‐values were calculated using the Kruskal–Wallis test or chi‐square test, as appropriate. IQR, interquartile range; na, not applicable; RT‐PCR, real‐time reverse transcription PCR assay; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
T‐cell counts were available in 38 of 60 patients and in 17, 6 and 15 mild, moderate and severe patients, respectively.
Figure 1.
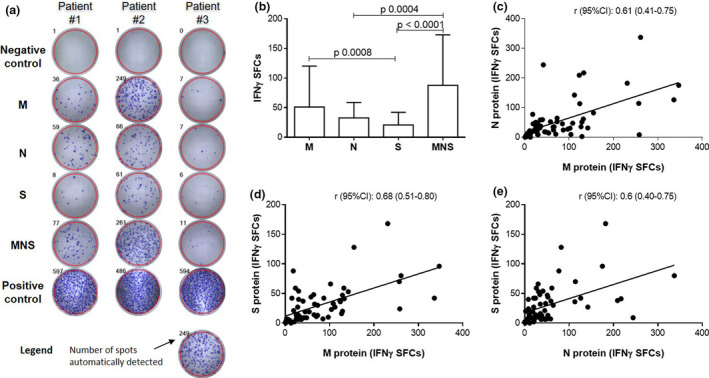
M, N, S and MNS mix‐reactive T cells in 60 SARS‐CoV‐2 patients. (a) Automated detection of IFNγ‐SFCs in wells after 16–20 h of stimulation. Negative and positive control wells allow quality control assessment; reactive T cells are counted in ‘M’, ‘N’, ‘S’ and ‘MNS’ wells. Three representative patterns of response are illustrated. Negative control: well without antigen/mitogen. Positive control: well with phytohaemagglutinin (PHA). (b) Comparisons of IFNγ‐SFCs according to the tested antigens. Data are presented as the median + IQR and P‐values comparing the tested antigens from Dunn’s tests (post hoc for the Kruskal–Wallis test). (c–e) Comparisons of IFNγ‐SFCs according to the tested antigens. Correlations were assessed using Spearman’s rank correlation coefficients (r).
Assessment of sensitivity and specificity of the IFNγ T‐CoV‐Spot assay
Among the 60 patients, reactive T cells were detected against M, N, S and the MNS mix of peptides in n = 60 (100%), n = 56 (93.3%), n = 55 (91.7%) and n = 58 (96.7%), respectively. Considering the high correlations between IFNγ‐SFCs detected with each of M, N and S proteins and with the MNS mix (r ≥ 0.7) (Figure 2a), we tested 31 cryopreserved pairs of sera and PBMCs collected before the outbreak (control samples) using the MNS mix (Figure 2b). Three of the PBMC samples (9.7%) tested positive for reactive T cells against the MNS mix. From the ROC curve, we estimated that a number of IFNγ‐SFCs ≥ 4 (optimal cut‐off value) had a sensitivity (95% CI) of 96.7% (88.5–99.6%) and a specificity of 90.3% (95% CI, 75.2–98.0%) (Figure 2c). An additional cohort of 112 SARS‐CoV‐2 patients was tested with the MNS mix using an entirely automated procedure including PBMC isolation and spot detection. Detailed characteristics of both cohorts of patients are shown in Table 2. ROC curve applied to this confirmation cohort gave a similar sensitivity of 95.5% (95% CI, 89.9–98.5%) for a cut‐off ≥ 4 IFNγ‐SFCs.
Figure 2.
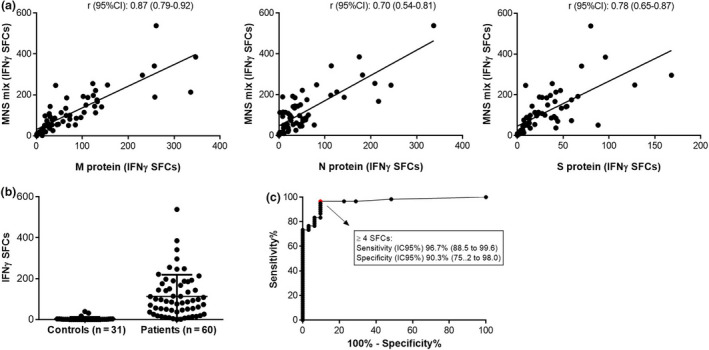
Characteristics of IFNγ T‐CoV‐Spot assay using the MNS mix as antigenic preparation. (a) Correlation (Spearman’s rank correlation) between IFNγ‐SFC counts with MNS and with M, N and S alone in the first 60 patients. (b) IFNγ‐SFC counts with MNS in 31 controls and 60 SARS‐CoV‐2 patients. (c) ROC curve for T‐CoV‐Spot using the MNS mix with an optimal cut‐off value at 4 IFNγ‐SFCs.
Table 2.
Detailed characteristics of SARS‐CoV‐2 patients
| Patients tested with M, N, S peptide pools and MNS mix (n = 60) | Patients tested with MNS mix only, for sensitivity confirmation (n = 112) | Total, n = 172 | |
|---|---|---|---|
| Age, years | 49 (20;92) | 57 (19;94) | 55.5 (19;94) |
| Sex (male) | 29 (48%) | 71 (63%) | 100 (58%) |
| Comorbidities | |||
| Hypertension | 17 (28%) | 43 (38%) | 60 (35%) |
| Chronic heart disease | 4 (7%) | 23 (21%) | 27 (16%) |
| Diabetes | 9 (15%) | 28 (25%) | 37 (22%) |
| Malignancy | 7 (12%) | 8 (7%) | 15 (9%) |
| Chronic lung disease (COPD, asthma and/or sleep apnoea syndrome) | 3 (5%) (0/1/2) | 22 (20%) (3/6/15) | 25 (15%) (3/7/17) |
| Chronic kidney disease | 2 (3%) | 7 (6%) | 9 (5%) |
| Chronic liver disease | 3 (5%) | 2 (2%) | 5 (3%) |
| HIV infection | 0 | 0 | 0 |
| Overweight/obesity | 15/58 (26%) | 50/94 (53%) | 65/152 (43%) |
| Smokers | 0/56 (0%) | 5/84 (6%) | 5 (4%) |
| Systemic steroids | 4 (7%) | 4 (4%) | 8 (5%) |
| Chemotherapy and/or immunosuppressants | 3 (5%) | 8 (7%) | 11 (6%) |
| Signs and symptoms | |||
| General symptoms | |||
| Fever | 48 (80%) | 92 (82%) | 140 (81%) |
| Fatigue | 50 (83%) | 86 (77%) | 136 (79%) |
| Myalgia | 26 (43%) | 56 (50%) | 82 (48%) |
| Respiratory symptoms | |||
| Cough | 41 (68%) | 86 (77%) | 127 (74%) |
| Dyspnoea | 39 (65%) | 86 (77%) | 125 (73%) |
| Pneumonia | 36 (60%) | 86 (77%) | 122 (71%) |
| Ear, nose and throat symptoms | |||
| Rhinorrhea, nasal obstruction | 13 (22%) | 22 (20%) | 35 (20%) |
| Anosmia and/or dysgeusia | 18 (30%) | 39 (35%) | 57 (33%) |
| Digestive symptoms | |||
| Diarrhoea | 11 (18%) | 25 (22%) | 36 (21%) |
| Nausea/vomiting | 6 (10%) | 10 (9%) | 16 (9%) |
| Abdominal pain | 10 (17%) | 9 (8%) | 19 (11%) |
| Headaches | 18 (30%) | 26 (23%) | 44 (26%) |
| Skin symptoms | |||
| Chilblains | 0 | 0 | 0 |
| Rash | 3 (5%) | 2 (2%) | 5 (3%) |
| Positive PCR at diagnosis | |||
| Deep nasal | 46 (63%) | 71 (63%) | 117 (68%) |
| Tracheal | 34 (57%) | 78 (70%) | 112 (65%) |
| Baseline blood tests | |||
| Total white blood cells (×109 L−1) | 6.39 (2.6;17.9) (n = 40) | 6.23 (1.4;22.0) (n = 74) | 6.37 (1.4;22.0) (n = 114) |
| Neutrophils (×109 L−1) | 4.6 (1.8;9.7) (n = 34) | 4.3 (0.64;18) (n = 59) | 4.44 (0.64;18) (n = 93) |
| Lymphocytes (×109 L−1) | 0.92 (0.05;1.4) (n = 34) | 0.90 (0.25;3) (n = 59) | 0.9 (0.05;3) (n = 93) |
| Eosinophils (×109 L−1) | 0 (0;0.3) (n = 34) | 0 (0.0.4) (n = 59) | 0 (0:0.4) |
| Creatinine (µmol L−1) | 70 (44;160) (n = 39) | 79 (44;336) (n = 73) | 71 (44;336) (n = 112) |
| C‐reactive protein (mg L−1) | 58.5 (7;254) (n = 38) | 60 (0;400) (n = 71) | 60 (0;400) |
| Onset of symptoms to T‐CoV‐Spot assay (days) | 36 (10;70) | 38 (8;92) | 37 (8;92) |
| Patient management | |||
| Outpatients | 25 (42%) | 24 (21%) | 49 (28%) |
| Hospitalised, discharged at the time of sample | 28 (47%) | 79 (71%) | 107 (62%) |
| Hospitalised, still hospitalised | 7 (12%) | 9 (8%) | 17 (10%) |
| Severity status (since diagnosis) | |||
| Mild | 25 (42%) | 24 (21%) | 49 (28%) |
| Moderate | 11 (18%) | 49 (44%) | 60 (35%) |
| Severe | 24 (40%) | 39 (35%) | 63 (37%) |
| T‐cell counts (at the time of T‐CoV‐Spot assay) | |||
| CD3+ T cells (×109 L−1) | 1.2 (0.6;2.7) (n = 39) | 1.2 (0.5;2.2) (n = 72) | 1.4 (0.55;2.7) (n = 111) |
| CD3+CD4+ T cells (×109 L−1) | 0.75 (0.25;1.5) (n = 39) | 0.7 (0.3;1.8) (n = 72) | 0.7 (0.25;1.8) (n = 111) |
| CD3+CD8+ T cells (×109 L−1) | 0.5 (0.08;1.2) (n = 39) | 0.4 (0.1;1.2) (n = 72) | 0.4 (0.08;1.2) (n = 111) |
Data are median (range), n (%), or n/N (%), where N is the total number of patients with available data.
Frequency of specific reactive T cells according to disease severity
Using this validated standardised approach, we then compared the number of IFNγ‐SFCs according to severity status in the cohort of 60 patients. The frequency of reactive T cells observed with M, S and MNS mix pools correlated with severity (after adjustment for time from symptom onset to follow‐up with IFNγ T‐CoV‐Spot assay) (Figure 3). We also tested the other secreted cytokines in the well containing the MNS mix (n = 38 available samples, median [IQR] time from symptom onset: 36 [28;53] days) (Figure 4). We detected IL‐2, IL‐4, IL‐5, IL‐13 and IL‐17A secretion in supernatants of stimulated cells in a large majority of patients, with high concentrations for IL‐2 (Figure 4a). Despite the fact that higher levels of IL‐2, IL‐5, IL‐13 and IL‐17A were detected in supernatants of stimulated PBMCs from severe patients, only IL‐2 levels correlated with severity (Figure 4b).
Figure 3.

Assessment of IFNγ‐secreting SARS‐CoV‐2‐reactive T cells according to patients’ severity status (n = 60). P‐values are calculated using partial Spearman’s rank correlation coefficients with severity status, adjusted for time from symptom onset to sampling. Comparisons between severe and mild patients using Dunn’s tests (post hoc for the Kruskal–Wallis test) are indicated as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 or ****P ≤ 0.0001.
Figure 4.
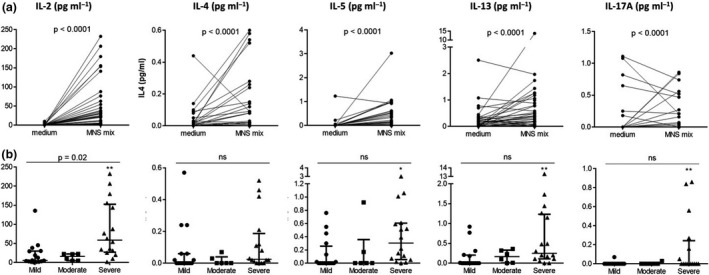
Cytokine levels in the supernatants of PBMCs from SARS‐CoV‐2 patients. (a) Cytokine levels detected after stimulation by the MNS mix or in the negative control (medium). P‐values are from the Wilcoxon test. (b) Cytokine levels detected after stimulation by the MNS mix, according to the severity of the disease in mild (n = 16), moderate (n = 6) and severe patients (n = 16). P‐values are calculated using partial Spearman’s rank correlation coefficients with severity status, adjusted for time from symptom onset to sampling. Comparisons between severe and mild patients using Dunn’s tests (post hoc for the Kruskal–Wallis test) are indicated as *P ≤ 0.05 or **P ≤ 0.01.
Outcome of immunological features from diagnosis to follow‐up, according to disease severity
To explain the higher cellular immune response in severe patients, we retrospectively assessed the main relevant immune characteristics previously reported in severe patients. Data were reported when available among the 172 patients tested by IFNγ T‐CoV‐Spot assay. At SARS‐CoV‐2 diagnosis, lower lymphocyte counts and higher plasma levels of IL‐6, TNFα, sIL‐2Rs and IL‐10 correlated with severity (Figure 5a and b). At follow‐up, lymphocyte counts for each subset returned to normal values and correlation with severity had disappeared (Figure 5a). However, plasma levels of IL‐1β, TNFα, sIL‐2R, IFNγ and IL‐10 remained increased in moderate‐to‐severe patients and, interestingly, IL‐6, TNFα and sIL‐2R levels still correlated with severity (Figure 5b).
Figure 5.

Outcome of main immunological characteristics in mild, moderate and severe SARS‐CoV‐2 patients from diagnosis to follow‐up. (a) Lymphocyte subset counts at diagnosis and during follow‐up. (b) Plasma cytokine levels at diagnosis and at follow‐up (T‐CoV‐Spot assay). For the correlations with severity, P‐values are calculated using partial Spearman’s rank correlation coefficients, adjusted for time from symptom onset to sampling. For comparisons of plasma cytokine levels between plasma controls (n = 10) and SARS‐CoV‐2 patients classified according to severity using Dunn’s tests (post hoc for the Kruskal–Wallis test) are indicated as *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 or ****P ≤ 0.0001. †, number of patients with available data or sample, ‡ time from symptom onset to follow‐up, in days (median [IQR]).
Specific antibody levels and frequency of reactive T cells according to disease severity
We also studied the anti‐N, anti‐S1 and anti‐RBD antibodies in the 49 of the 60 patients for whom a serum sample was available on the same day as the blood sample for IFNγ T‐CoV‐Spot assay. In the whole population, anti‐RBD IgM was detected in 40 (82%) patients and anti‐S1 IgA, IgG and anti‐RBD total antibodies (IgG, IgA, IgM) were detected in 46 (94%), 47 (96%) and 46 (94%) patients, respectively. The antibody ratios of anti‐S1 IgA, anti‐S1 IgG, anti‐RBD IgM and anti‐RBD total antibodies correlated with severity (Figure 6) (no correlation was observed for anti‐N antibodies, data not shown). We then compared the calculated ratio for anti‐S antibody ELISA response with the number of corresponding S‐reactive T cells (IFNγ‐SFCs). A small (anti‐S1 IgG), moderate (anti‐S1 IgA, anti‐RBD IgM) and large correlation (anti‐RBD IgG/IgA/IgM) was observed, respectively (Figure 7) (no correlation was observed for N protein, data not shown).
Figure 6.
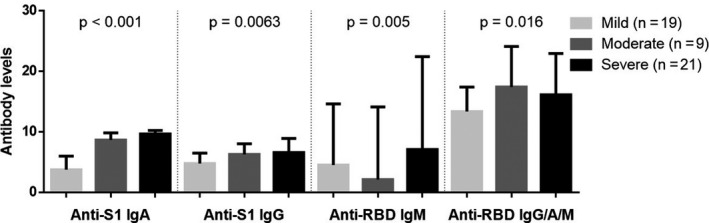
Anti‐S1 and anti‐RBD antibody levels in SARS‐CoV‐2 patients (n = 49), according to patients’ severity status. P‐values were calculated using partial Spearman’s rank correlation coefficients with severity status, adjusted for time from symptom onset to sampling.
Figure 7.
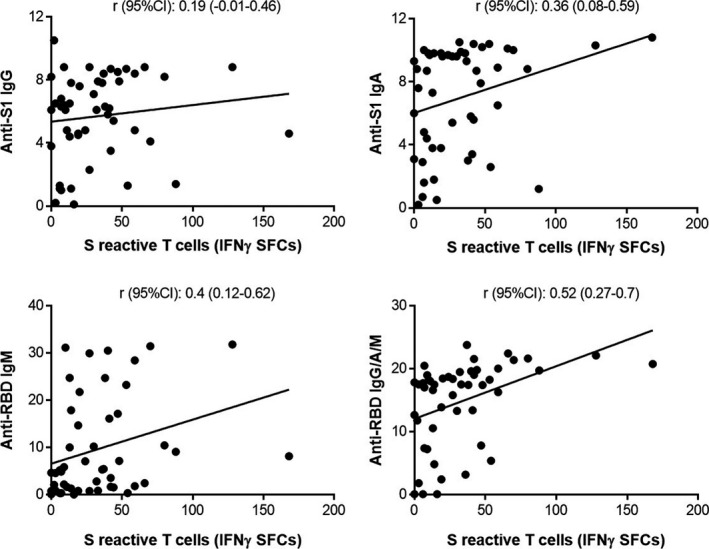
Correlation between anti‐S1 and anti‐RBD antibody ratios and S‐reactive T cells in SARS‐CoV‐2 patients (n = 49). Correlation (Spearman’s rank correlation) between IFNγ‐SFCs obtained with S peptide pool, and anti‐S1 or anti‐RBD antibody index.
Discussion
To improve our understanding of SARS‐CoV‐2 pathophysiology, it is important to comprehend how the T‐cell response develops in relation to disease severity. To this end, we prospectively explored a large group of patients with various levels of severity status. By combining antibody and T‐cell responses, our study demonstrates that SARS‐CoV‐2 patients achieve a higher adaptive immune response after developing a more severe disease.
Among the available methods to quantify specific T‐cell response, we chose to develop an ELISpot assay, a very sensitive technique to detect low‐frequency antigen‐specific T cells that secrete effector molecules. 26 Therefore, measuring T‐cell IFNγ release after stimulation with SARS‐CoV‐2‐specific antigens is an important tool to assess both T CD4+ and T CD8+ responses.
We first set up our method using the main SARS‐CoV‐2 antigens, and we identified some variations in the frequency of reactivity against M, N and S peptide pools. This difference could be mainly explained by the different nature of the selected antigens: complete sequence of the protein for M and N overlapping peptide pools, selected domains for the S peptide pool. Distinct in vivo antigen uptake and presentation by dendritic cells (DCs) could also explain these variations, considering the nature of post‐translational modifications, including phosphorylation (N protein) or glycosylation (M and S proteins).
Despite the disparity in terms of frequencies of reactive T cells in response to SARS‐CoV‐2 peptide pools, we observed a large correlation between the three peptide pools. Similar results were published in a preliminary report on 57 samples collected in 28 patients within 21 days following SARS‐CoV‐2 diagnosis. 27 Combined with our data, these results suggest that a given patient may acquire and maintain a similar cellular in vivo response against the three proteins, at least during the first weeks following the infection (median [IQR] from symptoms onset: 36 [28;53] days in our study). In addition, our results highlight that, even if S protein may be a major target for vaccine strategies, M and N are also of interest in anti‐SARS‐CoV‐2 immunisation follow‐up. Indeed, M and N‐reactive T cells were detected in 100% and 93.3% of patients, respectively, versus 91.7% for S‐reactive T cells. A large majority of our patients were convalescent when they were sampled (n = 53/60, 88%). Li et al. 28 observed that convalescent SARS‐CoV patients sampled 12 months after diagnosis had developed a higher S‐reactive T CD4+ response. Considering the large correlations between IFNγ‐SFCs detected in response to each M, N and S protein and with the MNS mix (r ≥ 0.7), and the practical interest of using a single antigenic well (rather than three), assessment of sensitivity and specificity was performed with the MNS mix for the monitoring of SARS‐CoV‐2 patients cellular response in future routine practice.
The presence of pre‐existing cross‐reactive T cells in SARS‐CoV‐2 patients is reported in several studies. 24 , 25 , 29 , 30 This cross‐reactivity with other human coronaviruses could also help to protect individuals against SARS‐CoV‐2 infection. Braun et al. 24 reported the detection of S‐reactive CD4+ T cells in 23 of 68 (35%) healthy donors and mostly against C‐terminal epitopes. However, the cut‐off defining reactive or unreactive T cells was not provided. These control subjects were sampled during the epidemic and were defined as asymptomatic seronegative subjects: SARS‐CoV‐2 infection was ruled out using RT‐PCR in 10 healthy donors and by repeated serological testing at least 28 days after the first sample, which is important considering the low sensitivity of RT‐PCR and delayed antibody detection in asymptomatic patients. 19 , 31 Grifoni et al. 25 also detected S‐, M‐ and ORF‐reactive T cells in up to 60% of healthy donors. Cryopreserved PBMCs collected before the epidemic were chosen as controls, as in our work and others, 29 considering that they may be more relevant and representative than asymptomatic seronegative subjects collected during the epidemic. According to the definition of detection threshold, up to two and up to four of 10 healthy donors had M‐ and S‐reactive T cells, respectively, but no N‐reactive T cells. Our data are quite in line with these results. We preferred to assess the presence of anti‐SARS‐CoV‐2 in control samples using a mix of the three viral antigens. All these control subjects were seronegative for anti‐S1, anti‐N and anti‐RBD antibodies. With this approach, we detected reactive T cells in only three of 31 control samples (9.7%) and a second analysis showed that T cells were reactive to M and S (n = 3/3) and to N (n = 1/3) with low spot counts in each case (data not shown). Finally, the interpretation of a positive or negative result, defining sensibility and specificity, must be discussed. As there are no consensual criteria to define a positive response for an ELISpot assay, we considered several methods to determine a cut‐off. We first considered that a result was ‘positive’ when the number of IFNγ‐SFCs in the stimulated well was at least twice the number of IFNγ‐SFCs observed in the negative well (unstimulated cells). 29 , 32 We also considered a result as ‘positive’ when the number of IFNγ‐SFCs in the stimulated well was greater than the mean of all negative controls + three standard deviations (data not shown). Lastly, we used an ROC curve to define the optimal cut‐off for sensitivity and specificity. Based on those three methods, the specificity was comparable and only three control samples (9.7%) were considered as positive, with different reactive T‐cell subsets in two samples. Considering these results and the absence of anti‐N, anti‐S1 and anti‐RBD antibodies in control subjects, our data may also suggest a cross‐reactivity with other coronaviruses. However, its potential protective properties remain to be demonstrated.
Preliminary data about the earlier stages of the disease suggest a peripheral and lymph node lymphocyte decrease, 5 , 9 , 10 , 13 , 15 , 17 type I IFN deficiency 7 and a diminished proportion of functional and reactive T cells in the most severe patients. 16 When studying cellular response according to disease severity in our cohort of SARS‐CoV‐2 patients, the most severe patients were more likely to be male and more likely to be older (Table 1), and they had lower lymphocyte counts (Figure 7a) and higher plasma cytokine levels (Figure 7b).
During follow‐up (median [IQR] 36 [28;53] days) and despite normal absolute counts with similar T cell, CD4+ and CD8+ T‐cell counts in the three groups, we observed a significant higher frequency of M‐ and S‐reactive T cells according to severity, despite similar total lymphocyte counts in the groups (Figure 3). This correlation was also confirmed with the MNS mix in our cohort of 60 patients. A similar observation was suggested in a preliminary work by Thieme et al. with a live cell activation‐marker technique in 28 recently diagnosed patients (median (range) time since diagnosis: 4 days (1–21) vs 31 days (3–64) in our study), but differences were not significant among hospitalised patients and no mild patients (i.e. outpatients) were included. 27 To better assess the functionality of specific reactive T cells, we further tested other cytokines in culture supernatants after antigenic stimulation. A strong IL‐2 production suggests a primary response with high activation of naive T cells, 33 but low levels of IL‐5, IL‐13 and IL‐17A were also detected in more severe patients. Another major concern is the type of T cells that we detected. Our results in SARS‐CoV‐2 patients in a median (IQR) interval of 31 days (20;41) after diagnosis are consistent with other reports in which specific T cells were also detected up to 21 days following diagnosis with a trend for higher specific T‐cell counts in severe patients. 27 To improve our understanding of the inflammatory status in SARS‐CoV‐2 patients, we also looked at the main cytokines in plasma, at diagnosis and during follow‐up. Almost all the tested cytokine levels decreased over time, but a gradient remained between moderate and severe patients compared with mild patients (except for IL‐6). A significant correlation with disease severity also persisted for sIL‐2R, IL‐6 and TNFα, illustrating both a higher and longer inflammatory response in moderate‐to‐severe patients. IL‐1β remained increased regardless of disease severity, suggesting a persistent activation of monocytes/macrophages. 34 Conversely, IL‐6 dramatically decreased in moderate‐to‐severe patients after diagnosis, but a correlation persisted between plasma levels and severity (Figure 5). Elevated IL‐6 has been shown to be associated with more severe symptoms, lower lymphocyte counts and poor prognosis. 10 IL‐6 may also play an important role in lung fibrosis, polarisation of naive CD4+ T cells into Th17 cells and an increase in B‐cell IgG production. Its late decrease in surviving patients could explain the lymphocyte count adjustment and expansion of IFNγ‐secreting specific T cells in severe patients. TNFα and sIL‐2R remained increased and correlated with disease severity during follow‐up (Figure 5). In addition, TNFα is reported to be related to inflammatory responses mediated by IL‐1β and IL‐6. 35 The soluble sIL‐2R can be released from activated T cells 36 ; therefore, elevated plasma levels at diagnosis and at follow‐up may reflect a T‐cell activation state and, presumably, a specific adaptive response against SARS‐CoV‐2 in this context. Several reports described a higher and/or longer viral load in severe patients 37 , 38 : a tremendous antigenic stimulation could explain an elevated adaptive immune response; the latter may be less effective to clear virus in view of the higher senescence and exhaustion of activated T cells in severe patients. 16 , 39 , 40
Finally, we confirmed previous reports showing that antibody response was higher in patients with severe SARS‐CoV‐2. Consistent with the fact that specific T cells participate in the generation of specific antibodies, we observed that the numbers of reactive T cells and anti‐S or RBD antibodies were correlated. We can conclude that severe SARS‐CoV‐2 patients develop a globally higher adaptive immune response. Exploring specific T cells during the first days following SARS‐CoV‐2 infection in mild, moderate and severe patients may help us to better understand how the T‐cell response becomes insufficient in the majority of severe patients.
Our study has some limitations. Our ELISpot assay did not allow us to discriminate between the CD4+ and CD8+ T‐cell‐specific responses, and we were not able to assess the in vivo activation state of immune cells. However, our priority was to study and follow up the cellular response in a large cohort of SARS‐CoV‐2 patients with a standardised method, using a reliable threshold for positive results. As a monitoring tool, this approach provides a clear and sensitive measure of immune responses in a short time frame and identifies responses that differ according to the severity of COVID‐19 symptoms.
In conclusion, IFNγ ELISpot assay is a reliable method to explore large cohorts of patients and may be relevant for vaccine trials in healthy subjects and in at‐risk groups of patients. It may also be useful in patients for whom specific antibody detection may be difficult (like in primary antibody deficiencies and/or in patients receiving IVIg). We also highlighted that severe patients develop a higher specific T‐cell response against SARS‐CoV‐2 infection. In addition, our study brings some new insights in the adaptive response in SARS‐CoV‐2 patients and further studies are necessary to explain the importance of the adaptive response during the first days following infection and to assess whether a higher cellular immunity could be protective against new infections.
Methods
Study participants
This study was conducted at Lille University Hospital, France, between 21 April and 20 May 2020. Consecutive patients with a recent symptomatic COVID‐19 diagnosis confirmed by RT‐PCR on a respiratory sample (SARS‐CoV‐2 patients) were sampled for the IFNγ T‐CoV‐Spot assay during hospitalisation and at a visit systematically planned 21–30 days after diagnosis (outpatients) or 21–30 days after hospitalisation discharge (follow‐up visit). Epidemiological, clinical, laboratory and radiological characteristics were obtained from patients’ electronic medical records. Patients were classified as follows: mild (outpatients with minimal symptoms), moderate (SARS‐CoV‐2 symptoms requiring hospitalisation ≥ 24 h with oxygen support (O2) ≤ 3 L min−1) or severe (hospitalisation in intensive care unit and/or O2 > 3 L min–1).
Control samples
Cryopreserved peripheral blood mononuclear cells (PBMCs) collected before the outbreak (during 2018–2019) from heparinised blood samples from immunocompetent non‐infectious subjects were used as negative controls. All serum samples were negative for anti‐N, anti‐S1 and anti‐RBD antibodies. These samples were taken from the POMI‐AF study, which included patients scheduled for cardiac surgery (coronary artery bypass graft surgery and/or valvular surgery) or transcatheter valve implantation (ClinicalTrials.gov Identifier: NCT03376165).
Cell preparation
Cell isolation and enumeration were performed using either a manual procedure (when reactivity against several peptide pools was assessed) or an automated procedure (when reactivity against only one peptide pool was assessed for future routine practice purposes). Briefly, PBMCs were isolated from 5 mL of freshly collected heparinised blood samples using Ficoll–Hypaque density gradient centrifugation (manual procedure) or using an automated magnetic bead‐based system to isolate the cells using positive selection (automated procedure, T‐Cell Select cell isolation, Oxford Immunotec, Abingdon, UK, on a Nimbus Presto platform system, Hamilton Robotics, Reno, Nev). Isolated cells were counted using the Guava ViaCount Reagent (Luminex, Austin, TX, USA) and analysed on a Guava easyCyte flow cytometer (Luminex). Cell suspension was normalised at a final concentration of 2.5 × 106 cells mL−1, and plating with SARS‐CoV‐2 antigens was automatically performed on the Nimbus Presto platform system (2–2.5 x 105 PBMCs added per well) or manually performed (2.5 x 105 PBMCs added per well).
IFNγ ELISpot assay – T‐CoV‐Spot assay
Overlapping peptide pools covering the complete sequences of SARS‐CoV‐2M and N proteins and of the immunodominant domain surface S protein and a mix of M, N and S peptides (MNS mix) were used as antigens (PepTivator® SARS‐CoV‐2 Prot_M [ref 130‐126‐703], PepTivator® SARS‐CoV‐2 Prot_N [ref 130‐126‐699], PepTivator® SARS‐CoV‐2 Prot_S [ref 130‐126‐701], Miltenyi Biotec, Bergisch Gladbach, Germany). Peptides consisted of 15‐mer sequences with 11 amino acids overlap. Microtitre plates coated with anti‐IFNγ antibodies (T‐SPOT.TB, Oxford Immunotec) were used. SARS‐CoV‐2M‐protein, N‐protein and S‐protein peptide pools were added at a concentration of 0.5 µg mL–1, and a mix of M, N, and S peptide pools (MNS mix) was used at the same concentration of 0.5 µg mL–1 for each pool, without any mitogenic stimulator. Reactivity against M, N, S proteins and the MNS mix was compared using the manual procedure for cell preparation to obtain the exact same number of cells in each well. When the MNS mix was tested alone, the automated procedure for cell preparation was used. After incubation for 16–20 h at 37°C in a humidified atmosphere containing 5% CO2, wells were washed and incubated with conjugate reagent for 1 h at 2–8°C. After a washing step, wells were developed for 7 min with substrate solution. The reaction was stopped with distilled water. Plates were allowed to dry in an oven at 37°C for 1 hour. Spot‐forming cells (SFCs) were detected using the CTL ImmunoSpot plate reader. Quality controls included a negative control to assess the spontaneous IFNγ release (wells without antigen) and a positive control to assess cell functionality (wells with the mitogenic stimulator phytohaemagglutinin [PHA]). Samples with insufficient isolated PBMCs (less than 2.5 × 105 cells per well) were excluded. In the negative control, an excess of 10 SFCs should be considered as ‘invalid’. For the positive control, the spot count should be ≥ 20 SFCs; a spot count < 20 SFCs was therefore considered ‘invalid’. For frozen PBMC samples, a spot count < 40 SFCs was considered ‘invalid’. A well with stimulated cells was considered as ‘positive’ if the number of SFCs was at least twice the number of SFCs observed in the negative well (unstimulated cells). 29 , 32
Cytokine measurements
Culture supernatants were collected in the negative control well and in the MNS mix well in a subgroup of patients. IL‐2, IL‐4, IL‐5, IL‐13 and IL‐17A concentrations were assessed using a cytometric bead‐based system according to the manufacturer’s instructions (CBA Flex set, Becton Dickinson, Franklin Lakes, NJ, USA). Cytokine concentrations in supernatants were first assessed in the negative control well and in the MNS mix well. The final cytokine concentration was calculated as the concentration measured in the antigen well minus the concentration in the negative control well. Plasma IL‐1β, IL‐6, TNFα, soluble sIL‐2R, IFNγ and IL‐10 concentrations were assessed using the Ella Automated Immunoassay System (ProteinSimple, San Jose, CA) following the manufacturer’s recommendations.
Serological assays
Considering that the S protein may be the privileged target antigen in vaccine developments and considering the importance of analysing both antibody and cellular response, 41 we focused our work on the comparison of both antibody and cellular response against S protein in SARS‐CoV‐2 patients. Anti‐S1 domain IgA and IgG (Euroimmun Medizinische Labordiagnostika, Lübeck, Germany) and IgM and total antibody (IgG/A/M) antireceptor binding domain (RBD) (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China) were assessed by ELISA assay, according to the manufacturer’s instructions on a serum sampled on the same day as the heparinised blood for IFNγ T‐CoV‐Spot assay. Anti‐N total antibody ELISA results (Elecsys Anti‐SARS‐CoV‐2, Roche Diagnostics, Risch‐Rotkreuz, Switzerland) were also compared with reactive anti‐N protein‐specific T‐cell results obtained with IFNγ T‐CoV‐Spot assay. For ELISA assays, a ratio between each sample and the calibrator was calculated and positive results were defined according to the manufacturer’s recommendations.
Statistics
Continuous variables are expressed as median (interquartile range [IQR], or range) and categorical variables as numbers (percentage). Comparisons according to severity of SARS‐CoV‐2 patients were performed using the Kruskal–Wallis test for continuous variables (followed by post hoc Dunn’s tests) and chi‐squared test for categorical variables. We assessed the correlation between M‐, N‐, S‐ and MNS‐reactive T cells (IFNγ‐SFCs) by calculating Spearman’s rank correlation (r) coefficients. We also assessed the association of the number of IFNγ‐SFCs, the antibody ELISA results and the cytokine levels in supernatants and plasma with the severity of SARS‐CoV‐2 patients using Spearman’s rank correlation coefficients. The strength of the correlation was classified as non‐substantial (r < 0.1), small (0.1 ≤ r < 0.3), moderate (0.3 ≤ r < 0.5) or large (r > 0.5). 42 Considering the difference in time from symptom onset to sampling between outpatients and hospitalised patients, associations with severity were adjusted for the time from symptom onset to sampling for IFNγ T‐CoV‐Spot assay by calculating partial Spearman’s rank correlation. The optimal cut‐off value for the number of SFCs in response to MNS mix to differentiate healthy controls from SARS‐CoV‐2 patients was determined using receiver operating characteristic (ROC) curve by maximising the Youden Index; sensitivity and specificity values of the optimal cut‐off value were calculated with their 95% confidence intervals (95% CI) (estimated using the Clopper–Pearson exact method). Statistical testing was performed at the two‐tailed α‐level of 0.05. Data analyses and graphs were performed using GraphPad Prism software version 6.04 (GraphPad Software, La Jolla, CA, USA).
Study approval
This study was performed in accordance with the Declaration of Helsinki principles for ethical research. Blood and sera used for T‐CoV‐Spot and serological assays were sampled during routine blood tests (no specific sampling was performed for this study). The study was approved by the French data protection agency (CNIL: Commission Nationale de l’informatique et des libertés, registration no. DEC20‐086) and by the local ethics committee (ID‐CRB 2020‐A00763‐36).
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
JD, GL, FV, PV and ML had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JD and GL equally contributed to this work. JD, GL, FV, TJ, AD, JL, PV, JP, KF and ML involved in concept and design. JD, GL, FV, TJ, AD, JL, PV, AD, SS, BG, JB, BP, LB, EKA, ML, CY, BM, LD, DL, SD, DM, EW, FM, MB, IT, VE, EJ, AD, SS, TB, JP, KF and ML acquired, analysed or interpreted the data. JD, GL, AD, JL, PV and ML drafted the manuscript. PV, AD, SS, BG, JB, BP, LB, EKA, ML, CY, BM, LD, DL, SD, DM, EW, FM, MB, IT, VE, EJ, AD, SS, TB, JP and KF critically revised the manuscript for important intellectual content. AD and JL made statistical analysis. JT, AD and EW made administrative, technical or material support.
Acknowledgments
We thank Dominique Becuwe, Véronique Betrancourt, Elise Boucly, Pascale Cracco, Virginie Dutriez, Coralie Lefebvre, Véronique Lekeux and Marie‐Thérèse Meleszka for their technical support and Dr Augustin Coisne, Philippine Alphand and Jenny Schryver, Bertrand Accart and Patrick Gelé (Centre de Ressource Biologique CRB/CIC1403, ID: BB‐0033‐00030) for their contribution to the POMI‐AF cohort (# NCT03376165).
References
- 1. Huff HV, Singh A. Asymptomatic transmission during the COVID‐19 pandemic and implications for public health strategies. Clin Infect Dis 2020. 10.1093/cid/ciaa654. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pedersen SF, Ho YC. SARS‐CoV‐2: a storm is raging. J Clin Invest 2020; 130: 2202–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C et al Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Copin MC, Parmentier E, Duburcq T et al Time to consider histologic pattern of lung injury to treat critically ill patients with COVID‐19 infection. Intensive Care Med 2020; 46: 1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lax SF, Skok K, Zechner P et al Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med 2020; 173: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poissy J, Goutay J, Caplan M et al Pulmonary embolism in patients with COVID‐19: awareness of an increased prevalence. Circulation 2020; 142: 184–186. [DOI] [PubMed] [Google Scholar]
- 7. Blanco‐Melo D, Nilsson‐Payant BE, Liu WC et al Imbalanced host response to SARS‐CoV‐2 drives development of COVID‐19. Cell 2020; 181: 1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hadjadj J, Yatim N, Barnabei L et al Impaired type I interferon activity and inflammatory responses in severe COVID‐19 patients. Science 2020; 369: 718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen G, Wu D, Guo W et al Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen N, Zhou M, Dong X et al Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong J, Dong H, Xia SQ et al Correlation analysis between disease severity and inflammation‐related parameters in patients with COVID‐19 pneumonia. e‐pub ahead of print Available from: http://medrxivorg; 2020. 10.1101/2020.02.25.20025643. [DOI] [PMC free article] [PubMed]
- 12. Liu J, Li S, Liu J et al Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine 2020; 55: 102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin C, Zhou L, Hu Z et al Dysregulation of immune response in patients with Coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis 2020; 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song JW, Zhang C, Fan X et al Immunological and inflammatory profiles in mild and severe cases of COVID‐19. Nat Commun 2020; 11: 3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang F, Nie J, Wang H et al Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis 2020; 221: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zheng HY, Zhang M, Yang CX et al Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID‐19 patients. Cell Mol Immunol 2020; 17: 541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Feng Z, Diao B et al The novel severe acute respiratory syndrome Coronavirus 2 (SARS‐CoV‐2) directly decimates human spleens and lymph nodes. e‐pub ahead of print Available from: http://medrxivorg; 2020. 10.1101/2020.03.27.20045427. [DOI]
- 18. Long Q, Deng H, Chen J et al Antibody responses to SARS‐CoV‐2 in COVID‐19 patients: the perspective application of serological tests in clinical practice. e‐pub ahead of print Available from: http://medrxivorg; 2020. 10.1101/2020.03.18.20038018. [DOI]
- 19. Yongchen Z, Shen H, Wang X et al Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Emerg Microbes Infect 2020; 9: 833–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao R, Li M, Song H et al Early detection of SARS‐CoV‐2 antibodies in COVID‐19 patients as a serologic marker of infection. Clin Infect Dis 2020. 10.1093/cid/ciaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang B, Wang L, Kong X et al Long‐term coexistence of SARS‐CoV‐2 with antibody response in COVID‐19 patients. J Med Virol 2020; 92: 1684–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu F, Wang A, Liu M et al Neutralizing antibody responses to SARS‐CoV‐2 in a COVID‐19 recovered patient cohort and their implications. e‐pub ahead of print 20 April 2020 Available from: http://medrxivorg; 2020. 10.1101/2020.03.30.20047365. [DOI]
- 23. Grifoni A, Sidney J, Zhang Y, Scheuermann RH, Peters B, Sette A. A Sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS‐CoV‐2. Cell Host Microbe 2020; 27: 671–680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Braun J, Loyal L, Frentsch M et al SARS‐CoV‐2‐reactive T cells in healthy donors and patients with COVID‐19. Nature 2020; 587(7833): 270–274. [DOI] [PubMed] [Google Scholar]
- 25. Grifoni A, Weiskopf D, Ramirez SI et al Targets of T cell responses to SARS‐CoV‐2 Coronavirus in humans with COVID‐19 disease and unexposed individuals. Cell 2020; 181: 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol Biol 2012; 792: 3–23. [DOI] [PubMed] [Google Scholar]
- 27. Thieme CJ, Anft M, Paniskaki K et al The SARS‐CoV‐2 T‐cell immunity is directed against the spike, membrane, and nucleocapsid protein and associated with COVID 19 severity. e‐pub ahead of print 16 May 2020; Available from: http://medrxivorg; 2020. 10.1101/2020.05.13.20100636. [DOI]
- 28. Li CK, Wu H, Yan H et al T cell responses to whole SARS coronavirus in humans. J Immunol 2008; 181: 5490–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le Bert N, Tan AT, Kunasegaran K et al SARS‐CoV‐2‐specific T cell immunity in cases of COVID‐19 and SARS, and uninfected controls. Nature 2020; 584: 457–462. [DOI] [PubMed] [Google Scholar]
- 30. Mateus J, Grifoni A, Tarke A et al Selective and cross‐reactive SARS‐CoV‐2 T cell epitopes in unexposed humans. Science 2020; 370: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS‐CoV‐2. JAMA 2020; 323: 2249–2251. [DOI] [PubMed] [Google Scholar]
- 32. Ng OW, Chia A, Tan AT et al Memory T cell responses targeting the SARS coronavirus persist up to 11 years post‐infection. Vaccine 2016; 34: 2008–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hwang ES, Hong JH, Glimcher LH. IL‐2 production in developing Th1 cells is regulated by heterodimerization of RelA and T‐bet and requires T‐bet serine residue 508. J Exp Med 2005; 202: 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Madej MP, Topfer E, Boraschi D, Italiani P. Different regulation of interleukin‐1 production and activity in monocytes and macrophages: innate memory as an endogenous mechanism of IL‐1 inhibition. Front Pharmacol 2017; 8: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodriguez L. SARS‐CoV‐2 infection: the role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev 2020; 54: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boyman O, Sprent J. The role of interleukin‐2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012; 12: 180–190. [DOI] [PubMed] [Google Scholar]
- 37. Liu Y, Yan LM, Wan L et al Viral dynamics in mild and severe cases of COVID‐19. Lancet Infect Dis 2020; 20: 656–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shi D, Wu W, Wang Q et al Clinical characteristics and factors associated with long‐term viral excretion in patients with severe acute respiratory syndrome Coronavirus 2 infection: a single‐center 28‐day study. J Infect Dis 2020; 222: 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Diao B, Wang C, Tan Y et al Reduction and functional exhaustion of T cells in patients with Coronavirus disease 2019 (COVID‐19). Front Immunol 2020; 11: 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Pang Y, Hu Z et al Thymosin alpha 1 (Tα1) reduces the mortality of severe COVID‐19 by restoration of lymphocytopenia and reversion of exhausted T cells. Clin Infect Dis 2020. 10.1093/cid/ciaa630. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amanat F, Krammer F. SARS‐CoV‐2 vaccines: status report. Immunity 2020; 52: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cohen J. Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: L Erlbaum Associates; 1988 567 p. 1988. [Google Scholar]


