Abstract
Context
We previously reported that inorganic iodine therapy in lactating women with Graves disease (GD) did not affect the thyroid function in 25 of 26 infants despite their exposure to excess iodine via breast milk.
Objective
To further assess thyroid function in infants nursed by mothers with GD treated with inorganic iodine.
Design
Case series
Setting
Tajiri Thyroid Clinic, Japan
Participants
One hundred infants of lactating mothers with GD treated with potassium iodide (KI) for thyrotoxicosis
Main Outcome Measures
Infant blood thyrotropin (TSH) and free thyroxine (FT4) levels were measured by the filter paper method. Subclinical hypothyroidism was defined as TSH ≥10 μIU/mL and ≥5 μIU/mL in infants aged <6 and ≥6 months, respectively.
Results
Overall, 210 blood samples were obtained from 100 infants. The median infant age was 5 (range, 0-23) months; median maternal KI dose, 50 (4-100) mg/day; median blood TSH level, 2.7 (0.1-12.3) μIU/mL; and median blood FT4 level, 1.04 (0.58-1.94) ng/dL. Blood TSH level was normal in 88/100 infants. Twelve infants had subclinical hypothyroidism; among them, blood TSH levels normalized after maternal KI withdrawal or stopping breastfeeding in 3 infants. In 7 infants, blood TSH levels normalized during KI administration without stopping breastfeeding. Two infants could not be followed up.
Conclusion
In Japan, inorganic iodine therapy for lactating women with GD did not affect thyroid function in most of the infants. Approximately 10% of infants had mild subclinical hypothyroidism, but blood TSH level normalized during continued or after discontinuing iodine exposure in all followed up infants.
Keywords: breastfeeding, inorganic iodine, infants, thyroid function, Graves disease
Drug treatment for Graves disease (GD) in lactating women should consider transferability of the drug to the breast milk and the effect of the transferred drug on the infant’s thyroid function. Although antithyroid drugs (ATDs) are known to transfer to the breast milk [1-3], ATDs in small to moderate doses for lactating women with GD have been reported to have no adverse effect on the infant’s thyroid function [4, 5]. As such, small to moderate amounts of ATDs are also given to breastfeeding mothers with GD [6].
In Japan, inorganic iodine is sometimes used to treat hyperthyroidism in GD patients with mild hyperthyroidism or those who experience ATD-related side effects [7, 8]. Inorganic iodine therapy is also a treatment option for lactating women with GD who cannot tolerate the side effects of ATDs. However, inorganic iodine is concentrated in the mammary gland and excreted in breast milk [9], thus exposing the infants to excess iodine. Because excess iodine can cause hypothyroidism in infants, breastfeeding should generally be avoided during inorganic iodine treatment of lactating mothers. However, many mothers with GD hope for breastfeeding due to its benefits over formula feeding or because infants reject the formula. Therefore, it is important to clarify the effects of inorganic iodine administration to lactating mothers with GD on the thyroid function of their infants.
Large doses of iodine in lactating women have been reported to cause hypothyroidism in their infants [10-13]. However, the mothers in these studies did not have thyroid disease, and the subjects were premature or newborn infants. In our previous study, inorganic iodine therapy in lactating women with GD in Japan, which is an iodine-sufficient country, did not affect the thyroid function in 25 of 26 infants despite their exposure to excess iodine via breast milk [14]. This study aimed to further evaluate the thyroid functions of infants breastfed by mothers with GD treated with inorganic iodine.
Materials and methods
Subjects
We evaluated 100 infants of 81 lactating mothers with GD who were treated with potassium iodide (KI) alone for thyrotoxicosis between February 2008 and February 2017. The 100 infants included the 26 infants evaluated in our previous study [14]. There were 55 infants whose mothers were already receiving KI treatment before childbirth and 45 infants whose mothers resumed or started KI due to the appearance or exacerbation of thyrotoxicosis after childbirth. KI was administered to patients with mild hyperthyroidism (serum free thyroxine [FT4] level below 4.0 ng/dL and mild symptoms), those with side effects due to ATDs, those who wanted to avoid side effects of ATDs, those who became pregnant while taking methimazole, and those with postpartum thyrotoxicosis in whom it was difficult to differentiate between GD and painless thyroiditis. The KI dose was adjusted every 1 to 3 months to maintain normal maternal thyroid function. Some infants were fully breastfed, whereas others received mixed feeding (ie, breast milk and formula). Informed consent was obtained from all mothers. This case series was approved by our clinical Institutional Review Board.
Assessment of infant thyroid function
The infant’s blood levels of thyrotropin (TSH) and FT4 were measured when the mother visited the clinic throughout the period when the mother was taking KI and breastfeeding. In all measurements, blood samples were collected from the outer edge of the footpad using the less invasive and simple filter paper method. Blood TSH and FT4 levels were measured via enzyme-linked immunosorbent assay using dried blotted blood samples. Blood TSH levels were measured using Cretin TSH ELISA II (Eiken Chemical Co., Ltd, Tokyo, Japan) [15] and blood FT4 levels were measured using ENZAPLATE N-FT4 (Siemens Healthcare Diagnostics KK, Tokyo, Japan; normal range 0.65-1.49 ng/dL) [16]. TSH concentrations were expressed as serum concentrations, which were calculated by multiplying the whole blood values by 1.6 [14]. With reference to the expert opinion of congenital hypothyroidism mass screening guidelines (2014 revised edition), subclinical hypothyroidism was defined as a blood TSH level of ≥10 μIU/mL and ≥5 μIU/mL in infants aged <6 and ≥6 months, respectively [17].
Results
Gestational age at birth (weeks) and mass screening test
The 100 infants included 46 boys and 54 girls. The data of gestational age at birth were not available for 12 infants. The median gestational age at birth of the 88 infants was 39 (range, 35-41) weeks. There were 9 preterm infants. Among them, there were 6 and 3 infants who were born at 35 and 36 weeks of gestational age, respectively. All infants underwent mass screening for congenital hypothyroidism 4 to 7 days after birth, and none of them presented with hypothyroidism according to interviews with mothers. Four infants had transient mild neonatal hyperthyroidism.
Infant thyroid function
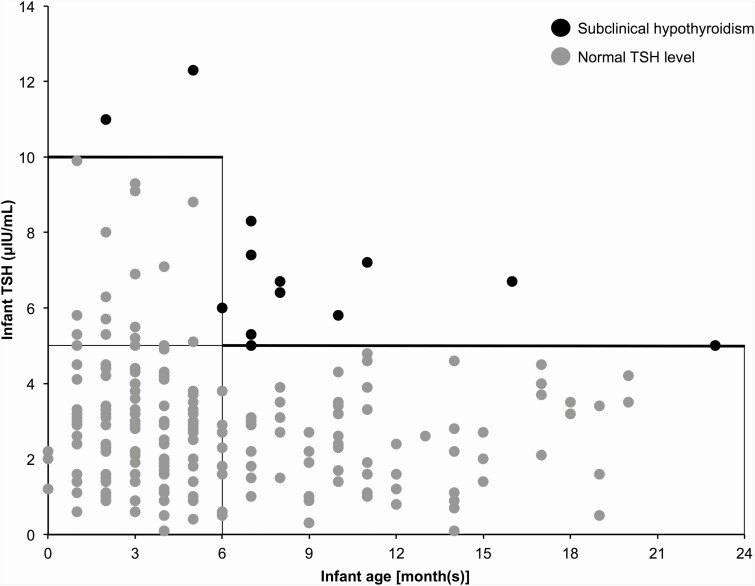
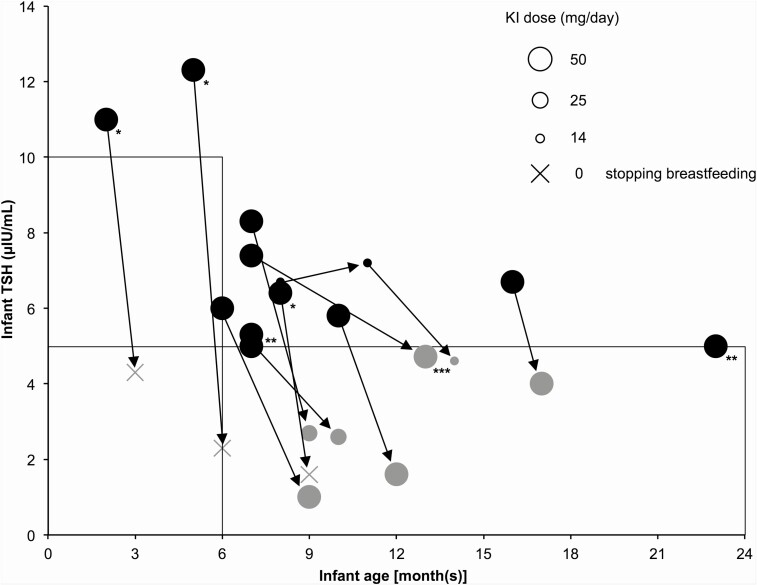
In total, 210 blood samples were obtained from the 100 infants. The median age of the infants was 5 (range, 0-23) months, the median maternal KI dose was 50 (4-100) mg/day, the median maternal duration of KI administration from before until after delivery was 298 (13-3082) days, the median infant blood TSH level was 2.7 (0.1-12.3) μIU/mL, and the median infant blood FT4 level was 1.04 (0.58-1.94) ng/dL. Figure 1 shows the relationship between infant age and blood TSH levels for the 210 samples. Overall, 88 infants (197 samples) had normal TSH levels. In the infant with the highest blood TSH level, the blood TSH level was 12.3 μIU/mL, and the blood FT4 level was 0.87 ng/dL. There were 12 infants (13 samples) who had subclinical hypothyroidism, and the course of blood TSH levels in these infants is shown in Fig. 2. In 3 infants, blood TSH levels normalized within 2 months after maternal KI discontinuation or termination of breastfeeding. In 7 infants, blood TSH levels normalized during KI administration without cessation of breastfeeding (among them, in 3 infants, their mothers reduced breastfeeding frequency because we encouraged them to stop breastfeeding). We could not follow up the course in 2 infants. In one of these infants, the effect of KI monotherapy on thyroid function could not be followed up because the mother used methimazole in addition to KI during treatment.
Figure 1.
Relationship between infant age and blood TSH levels in infants breastfed by mothers with Graves disease treated with KI. Black and gray circles indicate subclinical hypothyroidism and normal TSH level, respectively. The blood TSH level was normal in 88 infants (197 samples), whereas subclinical hypothyroidism was observed in 12 infants (13 samples).
Figure 2.
Course of blood TSH levels in the 12 infants with subclinical hypothyroidism. Each circle indicates the KI dose administered to a mother. Arrows indicate data from the same cases. Black and gray circles indicate subclinical hypothyroidism and normal TSH level, respectively. In 3 infants (*), the blood TSH levels normalized due to discontinuation of KI or stopping breastfeeding, whereas the blood TSH levels normalized during KI administration without cessation of breastfeeding in 7 infants. Two infants (**) could not be followed. One blood TSH level test (***) was performed at another hospital; thus the examination method is unknown.
Thyroid function in infants with lactating mothers with Graves disease who started or resumed taking KI after childbirth
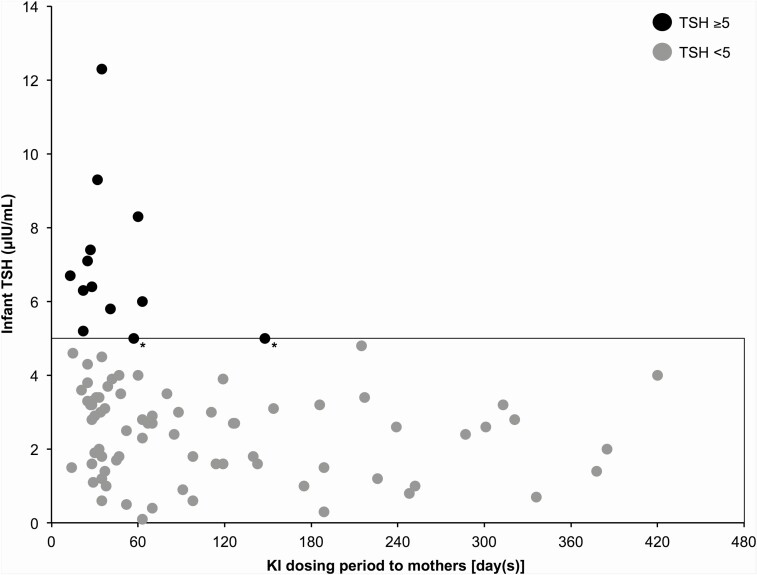
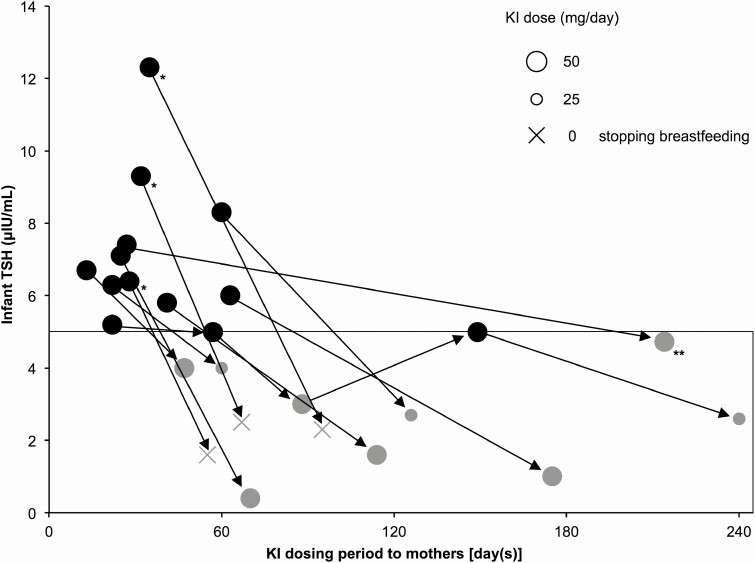
To examine the short-term effects of iodine overload on infants, we examined the relationship between the period of maternal KI administration and infant blood TSH levels in 45 infants (87 samples) of lactating mothers with GD who started or resumed KI after childbirth (Fig. 3). Among them, 11 infants (13 samples) had blood TSH levels ≥5 μIU/mL, and most of these samples (11 of the 13 samples) had been collected immediately after the mother started taking KI. In 3 of these 11 infants, the blood TSH levels decreased to <5 μIU/mL within 2 months after maternal KI withdrawal or stopping breastfeeding. In the remaining 8 infants, the blood TSH level decreased to <5 μIU/mL during KI administration without cessation of breastfeeding (among them, in 3 infants, their mothers reduced breastfeeding frequency because we encouraged them to stop breastfeeding) (Fig. 4).
Figure 3.
Relationship between the period of maternal KI administration and infant blood TSH levels in infants of lactating mothers with Graves disease who started or resumed KI after childbirth. Black circles indicate blood TSH levels ≥5 μIU/mL, and gray circles indicate blood TSH levels <5 μIU/mL. Among the 45 infants (87 samples), 11 infants (13 samples) had blood TSH levels ≥5 μIU/mL. All samples with blood TSH levels ≥5 μIU/mL, except 2 samples (*), were obtained immediately after the mother started KI.
Figure 4.
Course of blood TSH levels in 11 infants with blood TSH levels ≥5 μIU/mL among infants of mothers with Graves disease who started or resumed KI after childbirth. Each circle indicates the KI dose administered to a mother. Arrows indicate data from the same cases. Black circles indicate blood TSH levels ≥5 μIU/mL, and gray circles indicate blood TSH levels <5 μIU/mL. In 3 infants (*), the blood TSH levels decreased to <5 μIU/mL after maternal KI withdrawal or stopping breastfeeding. In 8 infants, the blood TSH levels decreased to <5 μIU/mL during KI administration without stopping breastfeeding. One blood TSH level test (**) was performed at another hospital; thus the examination method is unknown.
Discussion
In this study, inorganic iodine administration to lactating mothers with GD did not influence thyroid function, evaluated according to blood TSH levels, in approximately 90% of the 100 evaluated infants. About 10% of the infants had mild subclinical hypothyroidism, but none had overt hypothyroidism.
According to World Health Organization, daily iodine intake during lactation should not exceed 500 μg/day due to the lack of added health benefits and concerns about the potential for impaired thyroid function [18]. The American Thyroid Association guidelines recommend that iodine intake during breastfeeding should not exceed 500 to 1100 μg/day because of concerns about the risk of infant hypothyroidism [6]. However, the findings of the present study indicate that inorganic iodine administration in lactating women with GD is less likely to cause overt hypothyroidism in infants past the neonatal period. Therefore, we believe that it is not always necessary to prohibit breastfeeding in women with GD being treated with inorganic iodine if they wish to continue breastfeeding. However, given that breastfeeding during inorganic iodine treatment exposes infants to excess iodine [14] and may cause subclinical hypothyroidism in about 10% of infants, it is important to closely monitor the infant’s thyroid function during inorganic iodine treatment of lactating mothers with GD. Thyroid hormone is essential for normal brain development in infancy. Thus, it is important to immediately take measures such as discontinuation of inorganic iodine therapy for mothers, cessation of breastfeeding, and administration of levothyroxine to breastfed infants, in the event of subclinical or overt hypothyroidism. Because preterm infants are reported to be susceptible to excessive iodine [13], mothers receiving inorganic iodine treatment should avoid breastfeeding preterm infants. In this study, only 1 of 9 preterm infants had subclinical hypothyroidism, and the preterm infants were not susceptible to excess iodine. One possible cause for this finding is the age of gestation at birth. The premature infants in this study were born at ≥35 weeks’ gestation, whereas the premature infants in the previous report were born at ≤34 weeks’ gestation [13].
Inorganic iodine treatment may be an option for mothers with GD with adverse reactions to ATDs who wish to breastfeed but do not wish to undergo surgery for GD. If maternal thyroid function is poorly controlled by inorganic iodine therapy, other therapies (eg, radioactive iodine therapy after stopping breastfeeding, thyroidectomy) should be considered. Inorganic iodine therapy is likely to be effective in patients with mild hyperthyroidism [7], but the factors that predict the efficacy of inorganic iodine therapy before treatment are unknown [8].
In total 88 of the 100 infants in this study had normal TSH levels and 7 of 12 infants with subclinical hypothyroidism became euthyroid despite the continuation of maternal KI without cessation of breastfeeding. Accordingly, we speculated that the infant’s thyroid might have escaped from the Wolff–Chaikoff effect in which excess iodine inhibits thyroid hormone synthesis by blocking organification. The Wolff–Chaikoff effect is generally temporary in normal thyroid glands, with the suppression of thyroid hormone synthesis resolved within a few days through the escape phenomenon [19]. In the analysis of infants whose mothers started KI after childbirth (n = 45), most of the samples with blood TSH levels ≥5 μIU/mL were collected immediately after starting KI administration to the mothers. In 8 of the 11 infants whose blood TSH levels ≥5 μIU/mL, the blood TSH levels decreased to <5 μIU/mL despite maternal KI continuation without stopping breastfeeding. Rat data suggest that the ability of human fetuses to escape from the Wolff–Chaikoff effect develops from 36 to 40 weeks of gestation [20, 21]. The results of this study suggest the possibility of the escape phenomenon from the Wolff–Chaikoff effect in infants.
Our result that infant thyroid function was not easily affected by excess iodine may be related to the fact that Japanese people consume a large amount of iodine daily from seaweeds [22]. As discussed in our previous paper [14], infants in iodine-deficient areas may be more strongly affected by excess iodine. The results of this study may also be associated with maternal TSH receptor antibody (TRAb). If maternal TRAb levels in the third trimester of pregnancy are high, maternal TRAbs that cross the placenta may play a role in protecting against the effects of excess iodine exposure on infant thyroid function for 2 to 3 months after birth.
In our previous study, breast milk and infant urine iodine concentrations were evaluated in 26 infants breastfed by mothers with GD taking KI. We found a high iodine concentration in both breast milk (median 15 050 μg/L) and infant urine (median 15 650 μg/L). The KI dose was correlated with infant urinary iodine concentration and breast milk iodine concentration. The infant urinary iodine concentration was also correlated with the breast milk iodine concentration. These results clearly showed that maternal KI administration caused excess iodine in infants via breastfeeding [14]. Therefore, we did not measure breast milk and infant urine iodine concentrations for all mothers and infants enrolled in this study to reduce the burden on mothers and infants, except for mothers and infants in whom these concentrations had been measured for the previous study.
Although the current study targeted breastfed infants, detailed information on lactation status (eg, frequency and type [exclusive breastfeeding or mixed feeding] of feeding) was not completely available. Our previous study involving 26 infants found a positive correlation between the iodine concentration in breast milk and that in the infant’s urine despite variations in the frequency of breastfeeding for each infant [14]. Therefore, it is speculated that the frequency of breastfeeding does not significantly affect the degree of iodine exposure in infants.
In conclusion, treatment with inorganic iodine for lactating women with GD in Japan, an iodine-sufficient country, did not affect the thyroid function in most of the infants. Approximately 10% of infants developed mild subclinical hypothyroidism; however, their blood TSH levels normalized during continued or after discontinuing iodine exposure in all infants, except in 2 infants who were lost to follow-up. These findings indicate that in an iodine-sufficient area, lactating mothers with GD can be treated with inorganic iodine while carefully considering therapeutic indications and monitoring the infant thyroid function.
Acknowledgments
Financial Support: No grants or fellowships were received in support of this work.
Glossary
Abbreviations
- ATD
antithyroid drug
- FT4
free thyroxine
- GD
Graves disease
- KI
potassium iodide
- TRAb
thyrotropin receptor antibody
- TSH
thyrotropin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Johansen K, Andersen AN, Kampmann JP, Mølholm Hansen JM, Mortensen HB. Excretion of methimazole in human milk. Eur J Clin Pharmacol. 1982;23(4):339-341. [DOI] [PubMed] [Google Scholar]
- 2. Cooper DS, Bode HH, Nath B, Saxe V, Maloof F, Ridgway EC. Methimazole pharmacology in man: studies using a newly developed radioimmunoassay for methimazole. J Clin Endocrinol Metab. 1984;58(3):473-479. [DOI] [PubMed] [Google Scholar]
- 3. Kampmann JP, Johansen K, Hansen JM, Helweg J. Propylthiouracil in human milk. Revision of a dogma. Lancet. 1980;1(8171):736-737. [DOI] [PubMed] [Google Scholar]
- 4. Azizi F, Khoshniat M, Bahrainian M, Hedayati M. Thyroid function and intellectual development of infants nursed by mothers taking methimazole. J Clin Endocrinol Metab. 2000;85(9):3233-3238. [DOI] [PubMed] [Google Scholar]
- 5. Momotani N, Yamashita R, Makino F, Noh JY, Ishikawa N, Ito K. Thyroid function in wholly breast-feeding infants whose mothers take high doses of propylthiouracil. Clin Endocrinol (Oxf). 2000;53(2):177-181. [DOI] [PubMed] [Google Scholar]
- 6. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315-389. [DOI] [PubMed] [Google Scholar]
- 7. Uchida T, Goto H, Kasai T, et al. Therapeutic effectiveness of potassium iodine in drug-naïve patients with Graves’ disease: a single-center experience. Endocrine. 2014;47(2): 506-511. [DOI] [PubMed] [Google Scholar]
- 8. Okamura K, Sato K, Fujikawa M, Bandai S, Ikenoue H, Kitazono T. Remission after potassium iodide therapy in patients with Graves’ hyperthyroidism exhibiting thionamide-associated side effects. J Clin Endocrinol Metab. 2014;99(11):3995-4002. [DOI] [PubMed] [Google Scholar]
- 9. Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chanoine JP, Boulvain M, Bourdoux P, et al. Increased recall rate at screening for congenital hypothyroidism in breast fed infants born to iodine overloaded mothers. Arch Dis Child. 1988;63(10):1207-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koga Y, Sano H, Kikukawa Y, Ishigouoka T, Kawamura M. Effect on neonatal thyroid function of povidone-iodine used on mothers during perinatal period. J Obstet Gynaecol (Tokyo 1995). 1995;21(6):581-585. [DOI] [PubMed] [Google Scholar]
- 12. Casteels K, Pünt S, Brämswig J. Transient neonatal hypothyroidism during breastfeeding after post-natal maternal topical iodine treatment. Eur J Pediatr. 2000;159(9):716-717. [DOI] [PubMed] [Google Scholar]
- 13. Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab. 2009;94(11):4444-4447. [DOI] [PubMed] [Google Scholar]
- 14. Hamada K, Mizokami T, Maruta T, et al. Effects of inorganic iodine therapy administered to lactating mothers with Graves disease on infant thyroid function. J Endocr Soc. 2017;1(10):1293-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RRID:AB_2877100.
- 16. RRID:AB_2877060.
- 17. Nagasaki K, Minamitani K, Anzo M, et al. Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin Pediatr Endocrinol. 2015;24(3):107-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. WHO Secretariat, Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10(12A):1606-1611. [DOI] [PubMed] [Google Scholar]
- 19. Wolff J, Chaikoff IL. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45(5):504-13, illust. [DOI] [PubMed] [Google Scholar]
- 20. Fisher DA, Klein AH. Thyroid development and disorders of thyroid function in the newborn. N Engl J Med. 1981;304(12):702-712. [DOI] [PubMed] [Google Scholar]
- 21. Theodoropoulos T, Braverman LE, Vagenakis AG. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science. 1979;205(4405):502-503. [DOI] [PubMed] [Google Scholar]
- 22. Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid. 2008;18(6):667-668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.