Abstract
Objective
The aim of the study was to assess current management of patients with atrial fibrillation (AF) and acute coronary syndrome (ACS) undergoing coronary stenting.
Design
Non-interventional, prospective, nationwide study.
Setting
76 private or public cardiology centres in Italy.
Participants
Patients with ACS with concomitant AF undergoing percutaneous coronary intervention (PCI).
Primary and secondary outcome measures
To obtain accurate and up-to-date information on pharmacological management of patients with AF admitted for an ACS and undergoing PCI with stent implantation.
Results
Over a 12-month period, 598 consecutive patients were enrolled: 48.8% with AF at hospital admission and 51.2% developing AF during hospitalisation. At discharge, a triple antithrombotic therapy (TAT) was prescribed in 64.8%, dual antiplatelet therapy (DAPT) in 25.7% and dual antithrombotic therapy (DAT) in 8.8% of patients. Among patients with AF at admission, TAT and DAT were more frequently prescribed compared with patients with new-onset AF (76.3% vs 53.8% and 12.5% vs 5.3%, respectively; both p<0.0001), while a DAPT was less often used (11.2% vs 39.5%; p<0.0001). At multivariable analysis, a major bleeding event (OR: 5.40; 95% CI: 2.42 to 12.05; p<0.0001) and malignancy (OR: 5.11; 95% CI: 1.77 to 14.78; p=0.003) resulted the most important independent predictors of DAT prescription.
Conclusions
In this contemporary registry of patients with ACS with AF treated with coronary stents, TAT still resulted as the antithrombotic strategy of choice, DAT was reserved for high bleeding risk and DAPT was mainly prescribed in those developing AF during hospitalisation.
Trial registration number
Keywords: adult cardiology, coronary heart disease, cardiac epidemiology, coronary intervention, myocardial infarction
Strengths and limitations of this study.
Prospective, nationwide observational study.
Contemporary community-based registry evaluating the antithrombotic management of patients with acute coronary syndrome and atrial fibrillation undergoing percutaneous coronary intervention.
Data limited to the hospitalisation period.
Introduction
Approximately 10% of patients with acute coronary syndrome (ACS) requiring percutaneous coronary intervention (PCI) with stent implantation presents a concomitant atrial fibrillation (AF).1–11 Such patients theoretically need oral anticoagulation (OAC) and dual antiplatelet therapy (DAPT), a combination known as triple antithrombotic therapy (TAT), in order to decrease both the risk of thromboembolism due to AF and the risk of thrombosis and recurrent ischaemic events due to ACS and coronary stents.1–7 Unsurprisingly, TAT is associated with a high rate of major and fatal bleeding events.12
Recently, several randomised trials demonstrated the favourable safety profile of a double antithrombotic therapy (DAT), which combines OAC with a P2Y12 receptor inhibitor, as compared with TAT.13–17
After the validation of these novel antithrombotic strategies and the dissemination of direct oral anticoagulants (DOACs) in clinical practice, no nationwide or community-based data describing contemporary pharmacological management of patients with AF and ACS treated with PCI are available. In this regard, the Italian National Association of Hospital Cardiologist (ANMCO) designed the MATADOR-PCI (Management of Antithrombotic TherApy in Patients with Chronic or DevelOping AtRial Fibrillation During Hospitalisation for PCI) Study, aimed to obtain accurate and up-to-date information concerning management and outcome of patients with AF admitted in cardiology intensive care units (CCUs) for an ACS undergoing PCI with stent implantation.
Methods
The MATADOR-PCI was a prospective, observational, nationwide registry of consecutive patients with a confirmed diagnosis of ACS treated with PCI and concomitant AF conducted in Italy during a 1-year period.
All consecutive patients with ACS (non-ST elevation-ACS (NSTE-ACS) or ST-elevation myocardial infarction (STEMI)) undergoing PCI and with AF at the time of hospital admission, either paroxysmal, persistent or permanent, or developing during the index hospitalisation were included. Patients admitted with a diagnosis of ACS at the time of enrolment but not confirmed during hospitalisation, ACS treated medically, with surgical revascularisation or with percutaneous coronary balloon angioplasty without stent implantation and those not giving informed consent, were excluded from the survey.
ANMCO invited to participate in this study all Italian cardiology centres with a CCU and a catheterisation laboratory performing at least 400 PCIs per year (medium–high volume according to Italian standards), including university teaching hospitals, general and regional hospitals, and private clinics. No specific protocols or recommendations for evaluation, management and/or treatment have been put forth during this observational study. However, current guidelines for the management of patients with AF, myocardial revascularisation and ACS have been discussed during the investigator meetings.
Data collection and data quality
Data on demographics, cardiovascular and non-cardiovascular medical history, previous interventional procedures, type of ACS, type of AF, the timing of AF onset (if AF occurred during hospitalisation), in-hospital management, pharmacological treatment, timing of PCI, severity and extension of coronary artery disease, number and type of stent, laboratory values, ECG characteristics, haemodynamic parameters and in-hospital major clinical events were collected.
Myocardial infarction (MI) was defined according to the third universal definition of MI.18 Stroke was identified as an acute neurological deficit lasting >24 hours and affecting the ability to perform daily activities with or without confirmation by imaging techniques. Stent thrombosis was defined according to the Academic Research Consortium recommendations.19 Bleeding events were defined according to the Bleeding Academic Research Consortium (BARC) criteria.20 A major bleeding was defined as BARC ≥3.
At each site, the principal investigator was responsible for screening eligible consecutive patients. Data were collected using a web-based, electronic case report forms with the central database located at the ANMCO Research Center. By using a validation plan, integrated in the data entry software, data were checked for missing or contradictory entries and values out of the normal range.
Statistical analysis
Considering the explorative and observational nature of the study, no formal sample size calculation has been performed. However, considering the number of patients with ACS with AF at the time of hospital admission or developing AF during the index hospitalisation enrolled in previous snapshots performed in Italy and endorsed by ANMCO in the last 15 years,21 it was estimated to include approximately 500 patients (8% of patients with ACS undergoing PCI in 1 year in about 100 centres) to allow for a representative national cohort in terms of geographical distribution and well balanced in terms of complexity (eg, PCI volume, cardiac surgery).
Normally distributed variables were expressed as mean±SD, and compared using the Student’s t-test, whereas non-normally distributed variables as median and IQR and compared with the Mann-Whitney U test. Categorical variables were reported as numbers and percentages and compared using the Χ2 test or Fisher exact tests, as appropriate.
The study cohort was stratified according to the two prespecified groups of patients: (1) those with AF at the time of hospital admission and (2) those developing AF after hospital admission for an ACS.
Clinically relevant variables which were significant at univariate analysis were included in a multivariable model (logistic regression) in order to identify the independent predictors of DAT and TAT prescription at discharge, compared with other antithrombotic strategies. The variables included in the logistic model for DAT were: age (<65 reference group, 65–74, ≥75 years), gender, onset of AF (at admission vs during hospitalisation), type of ACS (STEMI vs NSTE-ACS), diabetes mellitus, malignancy, major bleeding (history or occurred during hospitalisation). Variables included in the logistic model for TAT were the following: age (<65 reference group, 65–74, ≥75 years), gender, onset of AF (at admission vs during hospitalisation), type of ACS (STEMI vs NSTE-ACS), hypertension, history of heart failure, previous revascularisation, prior acute MI, stroke/transient ischaemic attack, malignancy, major bleeding (history or occurred during hospitalisation). When more than two categories were present, dummy variables were introduced to define a reference group.
A p value of <0.05 was considered statistically significant. All tests were two-sided. Analyses were performed with SAS system software, V.9.4.
Results
Each site started patient enrolment after local Institutional Review Board approval. Therefore, data were collected in different periods of consecutive 12 months in each site between August 2018 and December 2019. The study has been carried out in 76 cardiology centres (68 (89.5%) with a 24-hour/7-day primary PCI service and 19 (25.0%) with also a cardiac surgery onsite), well representing the Italian cardiology reality in terms of geographical distribution and level of hospital technology. Five hundred ninety-eight consecutive patients have been enrolled: 292 (48.8%) with AF at hospital admission and 306 (51.2%) developing AF during the index hospitalisation. Among this latter group, 131 (42.8%) developed AF before and 175 (57.2%) after PCI; the median time from admission to AF onset was 18.0 (IQR 1.0–49.0) hours. Among the 211 patients with AF at admission and a history of AF, 116 (55.0%) had a permanent AF.
Baseline characteristics of the study population are shown in table 1. The mean age of enrolled patients was 73±10 years, 70% were men, 33% diabetics and 26% had prior coronary revascularisation. Patients with AF at admission presented more frequently a diagnosis of NSTE-ACS and were older, with a higher incidence of prior episodes of AF and major risk factors compared with patients developing AF during hospitalisation (table 1). The mean CHA2DS2-VASc was 3.7±1.6 and 2.9±1.7 (p<0.0001), while the HAS-BLEED was 2.6±1.1 and 2.1±1.1 (p<0.0001), in patients with AF at admission or developing AF during the hospitalisation, respectively.
Table 1.
Clinical characteristics, haemodynamic variables, laboratory parameters and antithrombotic therapy at baseline
| Overall (n=598) | AF at admission (n=292) | New-onset AF (n=306) | P value | |
| Age, years (mean±SD) | 73±10 | 76±10 | 72±10 | <0.0001 |
| Males, n (%) | 417 (69.7) | 203 (69.5) | 214 (69.9) | 0.91 |
| Body mass index, kg/m2 (mean±SD) | 27.3±4.3 | 27.2±4.2 | 27.3±4.5 | 0.92 |
| Final diagnosis, n (%) | <0.0001 | |||
| STEMI | 273 (45.7) | 101 (34.6) | 172 (56.2) | |
| NSTE-ACS | 325 (54.3) | 191 (65.4) | 134 (43.8) | |
| Clinical history and risk factors, n (%) | ||||
| Prior episodes of AF | 253 (42.3) | 211 (72.3) | 42 (13.7) | <0.0001 |
| Active smokers | 119 (19.9) | 46 (15.8) | 73 (23.9) | 0.01 |
| Diabetes mellitus | 198 (33.1) | 109 (37.3) | 89 (29.1) | 0.03 |
| Hypertension | 467 (78.1) | 245 (83.9) | 222 (72.6) | 0.0008 |
| Hypercholesterolaemia | 310 (51.8) | 155 (53.1) | 155 (50.7) | 0.55 |
| Peripheral artery disease | 51 (8.5) | 33 (11.3) | 18 (5.9) | 0.02 |
| Previous stroke/TIA | 66 (11.0) | 43 (14.7) | 23 (7.5) | 0.005 |
| History of angina | 177 (29.6) | 114 (39.0) | 63 (20.6) | <0.0001 |
| History of heart failure | 72 (12.0) | 51 (17.5) | 21 (6.9) | <0.0001 |
| Previous MI | 135 (22.6) | 82 (28.1) | 53 (17.3) | 0.002 |
| Prior PCI | 143 (23.9) | 87 (29.8) | 56 (18.3) | 0.001 |
| Prior CABG | 28 (4.7) | 21 (7.2) | 7 (2.3) | 0.005 |
| History of major bleeding | 16 (2.7) | 11 (3.8) | 5 (1.6) | 0.11 |
| Chronic kidney disease | 121 (20.2) | 82 (28.1) | 39 (12.8) | <0.0001 |
| COPD | 79 (13.2) | 43 (14.7) | 36 (11.8) | 0.29 |
| Cancer | 23 (3.9) | 15 (5.1) | 8 (2.6) | 0.11 |
| Haemodynamic variables | ||||
| Killip III–IV, n (%) | 76 (12.7) | 27 (9.3) | 49 (16.0) | 0.13 |
| Electrical instability, n (%) | 55 (9.2) | 14 (4.8) | 41 (13.4) | 0.0003 |
| SBP, mm Hg (mean±SD) | 132±26 | 132±25 | 132±27 | 0.85 |
| HR, bpm (mean±SD) | 87±26 | 88±28 | 86±25 | 0.22 |
| Ejection fraction, % (mean±SD) | 46.8±10.4 | 47.0±10.3 | 46.5±10.4 | 0.56 |
| Laboratory parameters | ||||
| Haemoglobin, g/L (mean±SD) | 133±19 | 132±19 | 134±19 | 0.12 |
| Creatinine, mg/dL (mean±SD) | 1.2±1.0 | 1.2±0.8 | 1.2±1.1 | 0.03 |
| LDL cholesterol, mg/dL (mean±SD) | 104±38 | 100±36 | 107±40 | 0.05 |
| Triglycerides, mg/dL (median (IQR)) | 104 (78–144) | 104 (78–148) | 105 (77–140) | 0.84 |
| Platelets, 109/L (mean±SD) | 223±82 | 211±76 | 235±86 | 0.0003 |
| INR (mean±SD) | 1.3±0.6 | 1.4±0.7 | 1.1±0.2 | <0.0001 |
| Antithrombotic therapy, n (%) | ||||
| ASA only | 146 (24.4) | 66 (22.6) | 80 (26.1) | 0.31 |
| P2Y12 inhibitors only | 21 (3.5) | 11 (3.8) | 10 (3.3) | 0.74 |
| DAPT | 32 (5.4) | 14 (4.8) | 18 (5.9) | 0.55 |
| LMWH | 15 (2.5) | 7 (2.4) | 8 (2.6) | 0.87 |
| VKA | 73 (12.2) | 65 (22.3) | 8 (2.6) | <0.0001 |
| DOAC | 137 (22.9) | 119 (40.8) | 18 (5.9) | <0.0001 |
AF, atrial fibrillation; ASA, acetylsalicylic acid; bpm, beats per minute; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet therapy; DOACs, direct oral anticoagulants; HR, heart rate; INR, international normalised ratio; LDL, low-density lipoprotein; LMWH, low-molecular weight heparin; MI, myocardial infarction; NSTE-ACS, non-ST elevation acute coronary syndrome; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction; TIA, transient ischaemic attack; VKA, vitamin-K antagonist.
At the time of admission, 178 (29.8%) were receiving acetylsalicylic acid, 32 (5.4%) a DAPT and 210 (35%) an OAC (this latter more frequently used in patients with AF at admission compared with the other group) (table 1).
Antithrombotic therapy in the periprocedural period
A pretreatment with DAPT was employed in 345 (57.8%) patients, without differences between the two groups. Among the 210 patients on chronic OAC, it was interrupted before PCI in 163 (77.6%).
Table 2 shows the angiographic and procedural variables of enrolled patients. A radial approach was used in 86%, a multivessel disease was present in 51% and a drug-eluting stent (DES) was implanted in 98% of patients. A complete revascularisation was obtained in 70% of cases.
Table 2.
Angiographic and procedural variables and antithrombotic therapies administered in the cath lab
| Overall (n=598) | AF at admission (n=292) | New-onset AF (n=306) | P value | |
| Radial approach, n (%) | 517 (86.5) | 260 (89.0) | 257 (84.0) | 0.07 |
| Multivessel disease, n (%) | 306 (51.2) | 140 (48.0) | 166 (54.3) | 0.12 |
| Basal TIMI 0/1, n (%) | 226 (38.1) | 88 (30.1) | 138 (45.1) | <0.001 |
| Site of PCI, n (%) | ||||
| Left main | 44 (7.4) | 19 (6.5) | 25 (8.2) | 0.44 |
| Left anterior descending | 326 (54.5) | 155 (53.1) | 171 (55.9) | 0.49 |
| Circumflex | 176 (29.4) | 82 (28.1) | 94 (30.7) | 0.48 |
| Right coronary artery | 229 (38.3) | 116 (39.7) | 113 (36.9) | 0.48 |
| Arterial/venous graft | 9 (1.5) | 7 (2.4) | 2 (0.7) | 0.08 |
| Type of stent, n (%) | ||||
| BMS | 15 (2.5) | 9 (3.1) | 6 (2.0) | 0.38 |
| DES, durable polymer | 363 (60.7) | 162 (55.5) | 201 (65.7) | 0.01 |
| DES, biodegradable polymer | 163 (27.3) | 90 (30.8) | 73 (23.9) | 0.06 |
| DES, polymer-free | 78 (13.0) | 47 (16.1) | 31 (10.1) | 0.03 |
| >2 stents implanted, n (%) | 115 (19.2) | 53 (18.2) | 62 (20.3) | 0.51 |
| Complete revascularisation, n (%) | 421 (70.4) | 206 (70.6) | 215 (70.3) | 0.94 |
| Antithrombotic therapies administered in the cath lab, n (%) | ||||
| ASA | 31 (5.2) | 20 (6.9) | 11 (3.6) | 0.07 |
| DAPT | 101 (16.9) | 63 (21.6) | 38 (12.4) | 0.003 |
| GP IIb/IIIa inhibitors | 69 (11.5) | 14 (4.8) | 55 (18.0) | <0.0001 |
| Cangrelor | 9 (1.5) | $ (1.4) | 5 (1.6) | 0.23 |
| Unfractionated heparin | 333 (55.7) | 187 (64.0) | 146 (47.7) | <0.0001 |
AF, atrial fibrillation; ASA, acetylsalicylic acid; BMS, bare metal stent; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; GP IIb/IIIa, glycoprotein IIb/IIIa; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
In-hospital clinical events
The median duration of hospitalisation in cardiology wards was 8 (IQR 5–12) days (7 (IQR 5–9) vs 9 (IQR 6–13) days for patients with AF at admission or new-onset AF, respectively; p<0.0001). Ten (1.7%) patients died during the hospitalisation (five with AF at admission and five with new-onset AF). Among the remaining 588 (98.3%) patients discharged alive, a sinus rhythm was present in 362 (61.6%) (106 (36.9%) with AF at admission and 256 (85.1%) new-onset AF; p<0.0001). In patients with new-onset AF, the median duration of the arrhythmia was 4 (IQR 1.0–26.0) hours and an electrical cardioversion was performed in 28 (9.2%).
In-hospital clinical events are shown in figure 1. An urgent revascularisation occurred in 6.9%, a thromboembolic or major bleeding event in 3% and a definite stent thrombosis in 0.5% of cases, without differences between the two groups.
Figure 1.
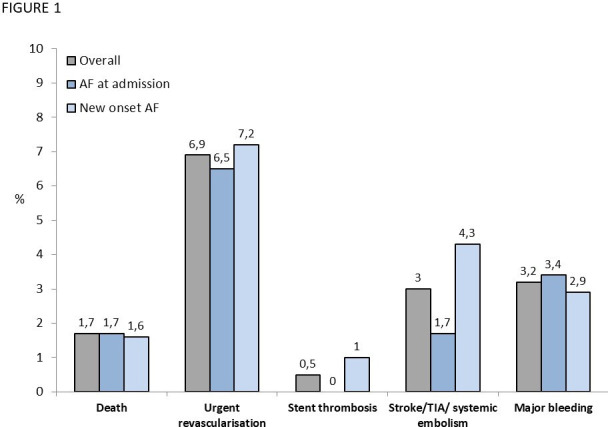
In-hospital clinical events. AF, atrial fibrillation; TIA, transient ischaemic attack.
Antithrombotic therapies at discharge
The single antithrombotic compounds prescribed at discharge are shown in figure 2.
Figure 2.
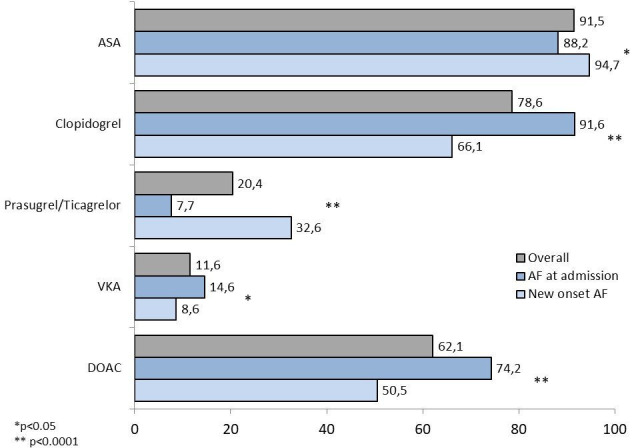
Antithrombotic therapies prescribed at discharge. AF, atrial fibrillation; ASA, acetylsalicylic acid; DOAC, direct oral anticoagulant; VKA, vitamin-K antagonist.
A DAPT was prescribed in 26%, TAT in 65% and DAT in 9% of patients (figure 3). Among patients with AF at admission, TAT and DAT were more frequently prescribed compared with patients with new-onset AF (76.3% vs 53,8% and 12.5% vs 5.3%, respectively; both p<0.0001), while a DAPT was less often used (11.2% vs 39.5%; p<0.0001) (figure 3). DOACs were largely used in both patients receiving TAT (84.3%) and DAT (84.6%).
Figure 3.
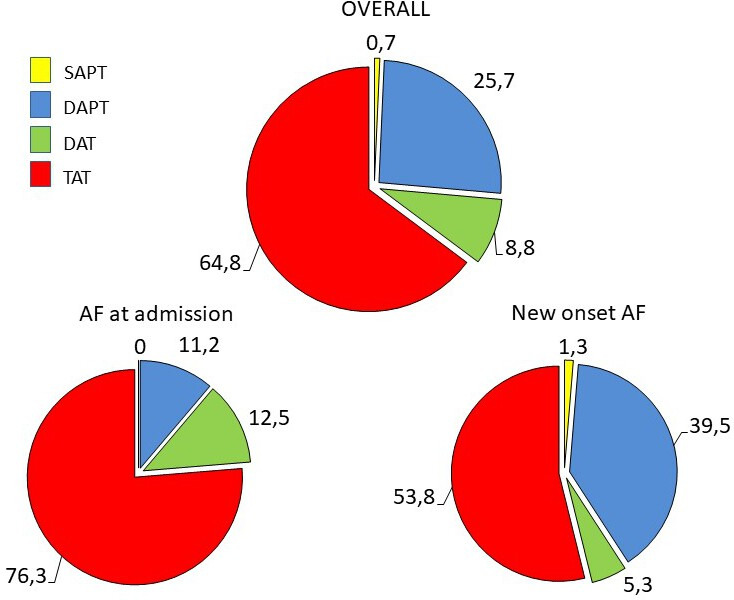
Central illustration. Combination of antithrombotic therapies prescribed at discharge. ACS, acute coronary syndrome; AF, atrial fibrillation; DAPT, dual antiplatelet therapy; DAT, dual antithrombotic therapy; SAPT, single antiplatelet therapy; TAT, triple antithrombotic therapy.
At multivariable analysis, a major bleeding event (OR: 5.40; 95% CIs: 2.42 to 12.05; p<0.0001) and malignancy (OR: 5.11; 95% CI: 1.77 to 14.78; p=0.003) resulted the most important independent predictors of DAT prescription (figure 4). The independent predictors of TAT prescription derived from multivariable analysis are shown in online supplemental table 1.
Figure 4.
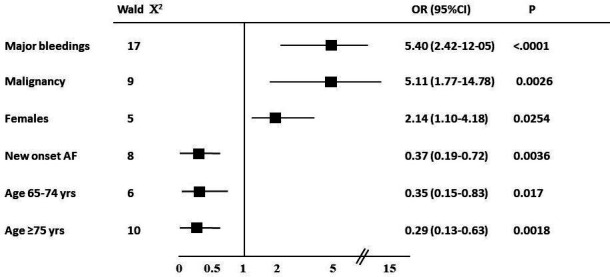
Independent predictors of DAT prescription at multivariable analysis. AF, atrial fibrillation; DAT, dual antithrombotic therapy.
bmjopen-2020-041044supp001.pdf (46KB, pdf)
Discussion
The major findings of this nationwide, contemporary, prospective registry of unselected patients with ACS with concomitant AF undergoing PCI are the following: (1) AF at admission is associated with a high incidence of major risk factors while new onset AF more frequently develops after STEMI; (2) TAT is still the antithrombotic strategy of choice in patients with AF undergoing PCI, especially in those with AF at admission, while DAT is reserved for patients deemed at high bleeding risk; (3) a quarter of patients did not receive any OAC and approximately 40% of patients with new-onset AF have been discharged on DAPT.
It is estimated that 1 of 10 patients with ACS requiring PCI with stent implantation may present AF prior to or occurring during the index hospitalisation.1–3 In this latter group, the relative risk of developing AF is usually highest at the onset of ischaemia, it diminishes over time and is higher in those with greater clinical severity of ACS,22 as confirmed by our data. Despite its relatively frequent occurrence and the many aetiological factors involved in its pathogenetic condition, the short-term or long-term prognostic significance of new-onset AF complicating ACS remains unclear.22–25 In our series of patients with ACS treated with contemporary PCI strategies, as documented by the very high rates of transradial approach and DES implanted, patients with new-onset AF presented a slightly higher, not significant, rate of in-hospital ischaemic events as compared with those with AF at admission. This finding can be related to the more frequent presence of STEMI and haemodynamic instability among patients developing AF during the index hospitalisation.
Indeed, new-onset AF occurs more frequently in critically unwell patients and its incidence increases with greater severity of illness.26 27
The pharmacological management of patients with AF undergoing PCI requires a careful balance of the risk of thromboembolic and atherothrombotic events against the increased chance of bleeding, since most patients with AF are likely to receive TAT for the prevention of stroke, stent thrombosis or recurrent cardiac events.28 In recent years, several randomised controlled trials, including an overall population of more than 10 000 patients, assessed the safety of replacing TAT with DAT in patients with AF treated with PCI.15–18 28 Meta-analyses of these trials showed that DAT is associated with reduced risk for major bleeding compared with TAT, regardless of several features including clinical risk profile and PCI complexity.29 30 However, low-certainty evidence showed inconclusive effects of DAT versus TAT on risks for mortality, stroke and stent thrombosis.29 30
The recent 2019 American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines for AF31 recommended DAT with DOACs as an alternative to TAT to reduce bleeding, while, in the European Society of Cardiology (ESC) guidelines released in 2016,1 this indication was restricted to patients at baseline high bleeding risk. Based on the North American expert consensus document,7 the default approach was DAT, and short-term TAT could be considered in patients who have high thrombotic risk and low bleeding risk. Accordingly, recent 2020 ESC guidelines on the management of AF recommend early cessation (≤1 week) of aspirin and continuation of DAT for up to 12 months in patients with AF with ACS undergoing an uncomplicated PCI if the risk of stent thrombosis is low or if concerns about bleeding risk prevail over concerns about risk of stent thrombosis.32 This appears in accordance with the recent observation of an increased early stent thrombosis with DAT as compared with TAT with DOAC33 supporting an initial course of TAT in all patients with ACS with AF.34 35 Our data suggest that, although DOACs nearly replaced vitamin-K antagonists, TAT is still largely used in contemporary clinical practice. These findings may be related to 2016 ESC guidelines recommendations1 that were available during the conduction of our registry and did not consider all the evidence coming from recent trials, to the lack of hospital protocols updating or to the issues in changing therapeutic habits, as confirmed by previous nationwide surveys conducted in Europe before the availability of newer evidence in this field.36 37 All these data emphasise the need for educational campaigns in order to translate recent evidence and guidelines recommendation into clinical practice.
The antithrombotic strategy is particularly challenging in patients who develop AF during an ACS episode, especially those with paroxysmal episodes of AF.22 38 Indeed, although it is unclear whether new-onset AF associated with ACS has the same thromboembolic risk as a history of AF, substantial risk of AF recurrence following acute ischaemia exists in these subjects.22 In this regard, a consensus document by the European Heart Rhythm Association6 suggests that OAC should be generally prescribed in new-onset AF, according to the individual risk of stroke, in combination with antiplatelet agents. In our registry, a quarter of the overall cohort was treated with DAPT and 40% of patients developing AF during hospitalisation was discharged without any OAC prescription, probably because the AF episode has been considered a transient epiphenomenon triggered by the acute myocardial ischaemia. The high prescription of DAPT and the concomitant low use of OAC could justify the greater prescription of potent oral P2Y12 inhibitors observed in our cohort of patients with new-onset AF compared with those with AF at admission. The low utilisation of OAC in this population is consistent with large retrospective analyses of critically ill patients with sepsis39 40 and a Swedish registry41 and other retrospective studies42–44 on ACS. In a recent analysis of 149 patients developing AF during hospitalisation for ACS and treated by PCI, DAT was strongly associated with mortality at long-term follow-up, suggesting that an intensified antithrombotic regimen should be considered also in this high-risk patient population.44 Studies specific to new-onset AF following ACS are needed in order to better identify those requiring anticoagulation and its optimal duration.
Study limitations
Our study must be evaluated in the light of the known limitations of observational, cross-sectional studies. In addition, the data reported in the present analysis are limited to the time of hospitalisation. However, a clinical follow-up at 6 months from enrolment is ongoing and will assess clinical outcomes and the adherence to prescribed antithrombotic strategy. Finally, even though the participating centres were asked to include in the registry all consecutive patients with ACS with AF requiring coronary stents, we were not able to verify the enrolment process due to the absence of administrative auditing. However, based on the number of patients with AF enrolled in previous nationwide registry of ACS, we believe that the rate of patients enrolled is reliable and it is unlikely that a selective enrolment in a few sites may have substantially changed the study results.
Conclusions
This nationwide registry provides unique insights into the current antithrombotic management of patients with ACS and concomitant AF undergoing coronary stenting. Although recent evidence showed the safety of DAT in this population, our data demonstrate that TAT is still largely prescribed while DAT is reserved for patients deemed at high bleeding risk. At discharge, an OAC was not prescribed in 25% of the overall population and in 40% of patients developing AF during hospitalisation.
Supplementary Material
Footnotes
Contributors: The Steering Committee designed the study. All authors participated in the conduct of the study and contributed to the interpretation of the results. LDL, DG, ADL and MMG drafted, planned and conducted the manuscript. LG and DL analysed the data. AR, LB, SU, AM, FSdU, FF, FL and PC read, revised and approved the final version of the article.
Funding: The sponsor of the study was the Heart Care Foundation, a non-profit independent organisation, which also owns the database. Database management, quality control of the data and data analyses were under the responsibility of the ANMCO Research Centre of the Heart Care Foundation. The study was partially supported by an unrestricted grant by Boehringer Ingelheim, Pharma GmbH & CoKG. No compensations were provided to participating sites, investigators, nor members of the Steering Committee.
Disclaimer: The Steering Committee of the study had full access to all the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: LDL and AR received speakers honoraria from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer/BMS outside the submitted work; All other authors have reported that no potential conflicts of interest exist with any companies/organisations whose products or services may be discussed in this article. LG and DL are employees of ANMCO Research Center, Heart Care Foundation, which conducted the study with an unrestricted grant of research from Boehringer Ingelheim, Pharma GmbH and CoKG.
Patient consent for publication: Not required.
Ethics approval: All patients were informed of the nature and aims of the study and asked to sign an informed consent for the anonymous management of their individual data. Local Institutional Review Boards (IRBs) approved the study protocol according to the current Italian rules. The IRB of the coordinator centre (AO San Camillo-Forlanini) approved the study on 24th January 2018 (reference number: 151/CS). The study was conducted in accordance with the Declaration of Helsinki, the Good Clinical Practice guidelines and the applicable local legislations of non-interventional studies.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request to ANMCO Research Center, Florence, Italy.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;2016:2893–962. [DOI] [PubMed] [Google Scholar]
- 2.GYH L, Collet J, Haude M, et al. Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European heart rhythm association (EHRA), European Society of cardiology Working group on thrombosis, European association of percutaneous cardiovascular interventions (EAPCI), and European association of acute cardiac care (ACCA) endorsed by the heart rhythm Society (HRS), Asia-Pacific heart rhythm Society (APHRS), Latin America heart rhythm Society (LAHRS), and cardiac arrhythmia Society of southern Africa (CASSA). Europace 2018;2019:192–3. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC, Feldman TE, Hirshfeld JW, et al. ACC/AHA/SCAI 2005 guideline update for percutaneous coronary Intervention-Summary article: a report of the American College of Cardiology/American heart association Task force on practice guidelines (ACC/AHA/SCAI writing Committee to update the 2001 guidelines for percutaneous coronary intervention). J Am Coll Cardiol 2006;47:216–35. 10.1016/j.jacc.2005.11.025 [DOI] [PubMed] [Google Scholar]
- 4.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro heart survey on atrial fibrillation. Eur Heart J 2005;26:2422–34. 10.1093/eurheartj/ehi505 [DOI] [PubMed] [Google Scholar]
- 5.Valgimigli M, Bueno H, Byrne RA, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur Heart J 2017;2018:213–60. [DOI] [PubMed] [Google Scholar]
- 6.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018;39:1330–93. 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 7.Angiolillo DJ, Goodman SG, Bhatt DL, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective–2018 update. Circulation 2018;138:527–36. 10.1161/CIRCULATIONAHA.118.034722 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RJ, Yarzebski J, Lessard D, et al. Recent trends in the incidence rates of and death rates from atrial fibrillation complicating initial acute myocardial infarction: a community-wide perspective. Am Heart J 2002;143:519–27. 10.1067/mhj.2002.120410 [DOI] [PubMed] [Google Scholar]
- 9.Wong C-K, White HD, Wilcox RG, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J 2000;140:878–85. 10.1067/mhj.2000.111108 [DOI] [PubMed] [Google Scholar]
- 10.Wong C-K, White HD, Wilcox RG, et al. Significance of atrial fibrillation during acute myocardial infarction, and its current management: insights from the GUSTO-3 trial. Card Electrophysiol Rev 2003;7:201–7. 10.1023/B:CEPR.0000012382.81986.47 [DOI] [PubMed] [Google Scholar]
- 11.McManus DD, Huang W, Domakonda KV, et al. Trends in atrial fibrillation in patients hospitalized with an acute coronary syndrome. Am J Med 2012;125:1076–84. 10.1016/j.amjmed.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rein N, Heide-Jørgensen U, Lijfering WM, et al. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation 2019;139:775–86. 10.1161/CIRCULATIONAHA.118.036248 [DOI] [PubMed] [Google Scholar]
- 13.Dewilde WJM, Oirbans T, Verheugt FWA, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107–15. 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 14.Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]
- 15.Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. 10.1056/NEJMoa1708454 [DOI] [PubMed] [Google Scholar]
- 16.Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
- 17.Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3B trial. Lancet 2019;394:1335–43. 10.1016/S0140-6736(19)31872-0 [DOI] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, et al. Joint ESC/ACCF/AHA/WHF Task force for universal definition of myocardial infarction. third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 19.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–51. 10.1161/CIRCULATIONAHA.106.685313 [DOI] [PubMed] [Google Scholar]
- 20.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research Consortium. Circulation 2011;123:2736–47. 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 21.De Luca L, Casella G, Rubboli A, et al. Recent trends in management and outcome of patients with acute coronary syndromes and atrial fibrillation. Int J Cardiol 2017;248:369–75. 10.1016/j.ijcard.2017.08.019 [DOI] [PubMed] [Google Scholar]
- 22.Lau DH, Alasady M, Brooks AG, et al. New-onset atrial fibrillation and acute coronary syndrome. Expert Rev Cardiovasc Ther 2010;8:941–8. 10.1586/erc.10.61 [DOI] [PubMed] [Google Scholar]
- 23.Wong CK, White HD, Wilcox RG, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J 2000;140:878–85. 10.1067/mhj.2000.111108 [DOI] [PubMed] [Google Scholar]
- 24.Pizzetti F, Turazza FM, Franzosi MG, et al. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart 2001;86:527–32. 10.1136/heart.86.5.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeymer U, Annemans L, Danchin N, et al. Impact of known or new-onset atrial fibrillation on 2-year cardiovascular event rate in patients with acute coronary syndromes: results from the prospective EPICOR registry. Eur Heart J Acute Cardiovasc Care 2018. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida T, Fujii T, Uchino S, et al. Epidemiology, prevention, and treatment of new-onset atrial fibrillation in critically ill: a systematic review. J Intensive Care 2015;3:19. 10.1186/s40560-015-0085-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Bryan LJ, Redfern OC, Bedford J, et al. Managing new-onset atrial fibrillation in critically ill patients: a systematic narrative review. BMJ Open 2020;10:e034774. 10.1136/bmjopen-2019-034774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capodanno D, Huber K, Mehran R, et al. Management of antithrombotic therapy in atrial fibrillation patients undergoing PCI. J Am Coll Cardiol 2019;74:83–99. 10.1016/j.jacc.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 29.Khan SU, Osman M, Khan MU, et al. Dual versus triple therapy for atrial fibrillation after percutaneous coronary intervention: a systematic review and meta-analysis. Ann Intern Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes RD, Hong H, Harskamp RE, et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol 2020;5:582. 10.1001/jamacardio.2019.6175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines and the heart rhythm Society. J Am Coll Cardiol 2019;2019:104–32. [DOI] [PubMed] [Google Scholar]
- 32.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for Cardio-Thoracic surgery (EACTS). Eur Heart J 2020;20 10.1093/eurheartj/ehaa612 [DOI] [Google Scholar]
- 33.Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67. 10.1093/eurheartj/ehz732 [DOI] [PubMed] [Google Scholar]
- 34.Alexander JH, Wojdyla D, Vora AN, et al. Risk/Benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation 2020;141:1618–27. 10.1161/CIRCULATIONAHA.120.046534 [DOI] [PubMed] [Google Scholar]
- 35.Rubboli A, GYH L. Algorithm for the management of antithrombotic therapy in atial fibrillation patients undergoing percutaneous coronary intervention: an updated proposal based on efficacy considerations. Eur Heart J Cardiovasc Pharmacother 2020. [DOI] [PubMed] [Google Scholar]
- 36.Lamberts M, Gislason GH, Olesen JB, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol 2013;62:981–9. 10.1016/j.jacc.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 37.Lane DA, Dagres N, Dan G-A, et al. Antithrombotic treatment in patients with atrial fibrillation and acute coronary syndromes: results of the European heart rhythm association survey. Europace 2019;21:1116–25. 10.1093/europace/euz033 [DOI] [PubMed] [Google Scholar]
- 38.Rubboli A, Agewall S, Huber K, et al. New-onset atrial fibrillation after percutaneous coronary intervention with stent: updated proposal of an algorithm for the choice of oral anticoagulant and its dose. Eur J Intern Med 2017;40:e11–12. 10.1016/j.ejim.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 39.Walkey AJ, Quinn EK, Winter MR, et al. Practice patterns and outcomes associated with use of anticoagulation among patients with atrial fibrillation during sepsis. JAMA Cardiol 2016;1:682–90. 10.1001/jamacardio.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quon MJ, Behlouli H, Pilote L. Anticoagulant use and risk of ischemic stroke and bleeding in patients with secondary atrial fibrillation associated with acute coronary syndromes, acute pulmonary disease, or sepsis. JACC Clin Electrophysiol 2018;4:386–93. 10.1016/j.jacep.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 41.Stenestrand U, Lindbäck J, Wallentin L, et al. Anticoagulation therapy in atrial fibrillation in combination with acute myocardial infarction influences long-term outcome: a prospective cohort study from the register of information and knowledge about Swedish heart intensive care admissions (RIKS-HIA). Circulation 2005;112:3225–31. 10.1161/CIRCULATIONAHA.105.552984 [DOI] [PubMed] [Google Scholar]
- 42.Mehta RH, Dabbous OH, Granger CB, et al. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol 2003;92:1031–6. 10.1016/j.amjcard.2003.06.001 [DOI] [PubMed] [Google Scholar]
- 43.Lau DH, Huynh LT, Chew DP, et al. Prognostic impact of types of atrial fibrillation in acute coronary syndromes. Am J Cardiol 2009;104:1317–23. 10.1016/j.amjcard.2009.06.055 [DOI] [PubMed] [Google Scholar]
- 44.Hofer F, Kazem N, Hammer A, et al. Long-term prognosis of de-novo atrial fibrillation during acute myocardial infarction - the impact of anti-thrombotic treatment strategies. Eur Heart J Cardiovasc Pharmacother 2020 10.1093/ehjcvp/pvaa027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041044supp001.pdf (46KB, pdf)


