Abstract
Antitumour necrosis factor alpha agents are important treatments in many inflammatory conditions including rheumatoid arthritis, psoriatic arthritis and the inflammatory bowel diseases. However, there have been case reports of optic neuritis and other demyelinating diseases as complications of these agents. This case report presents a patient with ulcerative colitis on infliximab who presented with sudden onset mono-ocular visual field loss and highlights the diagnosis and management of infliximab-induced optic neuritis.
Keywords: inflammatory bowel disease, visual pathway, neuroopthalmology, biological agents, drugs: CNS (not psychiatric)
Background
Optic neuritis is an inflammatory demyelinating condition effecting the optic nerve, which causes acute monocular vision loss.1 There are various potential causes including infections, autoimmune and inflammatory conditions, metabolic abnormalities, as well as adverse reactions to drugs. One of the classes of drugs reported to cause optic neuritis is the monoclonal antibody that targets tumour necrosis factor alpha (TNF-α).1–5
Anti-TNFα agents are important treatments in many inflammatory conditions, such as rheumatoid arthritis, psoriatic arthritis and inflammatory bowel diseases.2 3 These block TNFα, an inflammatory cytokine that is produced by activated macrophages to induce inflammation and apoptotic cell death.2 Anti-TNFα medications are associated with several adverse effects including infections and malignancy and have also been identified as a rare of cause demyelinating diseases with several case reports of optic neuritis as well as multiple sclerosis (MS).4 This report presents a case of optic neuritis likely attributable to infliximab therapy.
Case presentation
A 59-year-old man presented with a 2-day history of insidious onset mono-ocular vision loss in the left inferomedial quadrant. This was not associated with headache, orbital tenderness, jaw claudication, fever, hearing loss and dizziness. The contralateral eye was not affected.
He has a background history of ulcerative colitis (UC) diagnosed 2 years prior, which was initially managed with mesalazine 4.8 g daily and mercaptopurine 100 mg daily. He subsequently had a severe flare of ulcerative colitis with sigmoidoscopy demonstrating an ulcerative colitis endoscopic index of severity (UCEIS) of 5. He was then commenced on infliximab (Remicade) at 5 mg/kg (induction at weeks 0, 2 and 6, and then every 8 weeks thereafter). Ten days following his fourth dose of infliximab (at weeks 0, 2, 6 and 14), he developed his left mono-ocular vision loss. Therefore, the infliximab was discontinued. In addition, his ulcerative colitis remained active despite commencement of infliximab, with persistent loose watery stool with mucous and frequency of up to 10 times a day.
His other medical history of significance is hypertension, managed with olmesartan 20 mg daily. There was no family history of demyelinating disease such as MS or optic neuritis.
Clinical examination revealed a corrected visual acuity is 6/9.5 bilaterally. His pupils were both equal and reactive to light, but there was a left-sided relative afferent pupillary defect. Funduscopy examination showed anormal optic disc on the right and optic disc swelling on the left. He had normal visual field on the right, with a left inferomedial quadrantopia. His eye movement was normal with smooth pursuit and normal saccades, and there was no diplopia. The rest of his cranial nerve examination was unremarkable. His upper limb and lower limb neurological examination were both normal.
Investigations
His laboratory parameters (box 1) demonstrated an elevated CRP and ESR in keeping with his active inflammatory bowel disease. A vasculitis screen was normal.
Box 1. Laboratory parameters.
Full blood count
Haemoglobin 149 g/L.
Mean cell volume 96 fL.
White cell count 4.73×109/L.
Neutrophil count 1.86×109/L.
Platelet 252×109/L.
Urea and electrolytes
Sodium 143 mmol/L.
Potassium 4.1 mmol/L.
Bicarbonate 25 mmol/L.
Urea 6.7 mmol/L.
Creatinine 80 µmol/L.
Liver function test
Bilirubin 9 µmol/L.
Alanine Transaminase (ALT) 25 U/L.
Alkaline Phosphatase (ALP) 94 U/L.
Gamma-Glutamyl Transferase (GGT) 15 U/L.
Albumin 40 g/L.
C reactive protein 22 mg/L.
Erythrocyte sedimentation rate 9 mm/hour.
Anti-nuclear Antibody (ANA), Extractable Nuclear Antibodies (ENA), Antineutrophil cytoplasmic antibody (ANCA), anti-dsDNA, Anti-Sjögren Syndrome A (anti-SSA) and Anti-Sjögren Syndrome B (anti-SSB) negative.
C3, C4 normal.
The patient had a CT brain that showed a small left frontal parafalcine meningioma with no other acute pathology. An MRI head with gadolinium contrast (figure 1) showed left posterior globe flattening and optic disc prominence suggestive of optic neuritis. There was no orbital mass lesion. There was no leptomeningeal enhancement. There was no white matter lesion to suggest MS (figures 2 and 3). The cerebrospinal fluid (CSF) spaces and basal cisterns are preserved.
Figure 1.
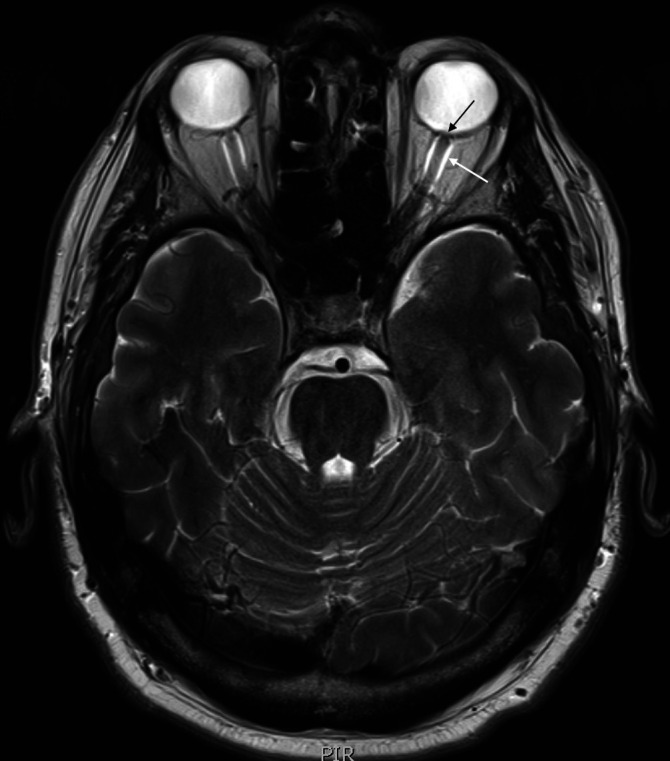
MRI head and optic nerve. T2 axial image of MRI head (black arrow): bulging of the left optic nerve head. White arrow: fluid around the optic nerve sheath bilaterally, left more than right.
Figure 2.
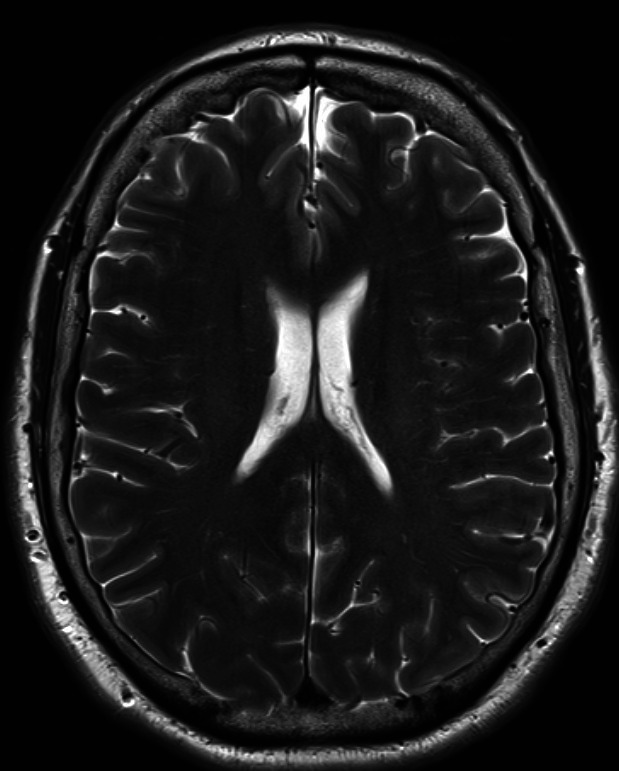
MRI head. An axial view of his MRI head.
Figure 3.
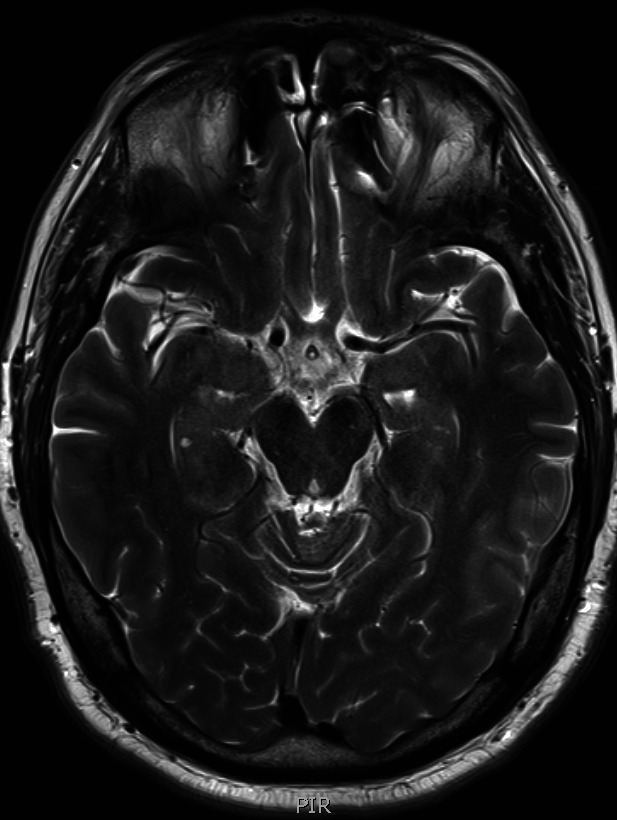
MRI head. Another axial view of his MRI head.
He was reviewed by ophthalmology department who performed visual field testing and optical coherence tomography (OCT) test. Visual field testing of his right eye was normal (figure 4); but on the left eye, it showed visual field defect in the inferomedial quadrant (figure 5). Funduscopy of the right eye was normal (figure 6), but there was papilloedema on the left eye (figure 7). OCT showed his right eye was normal (figure 8) but suggested optic nerve swelling on the left (figure 9). Clinical examination and these tests were consistent with a diagnosis of infliximab-induced optic neuritis.
Figure 4.
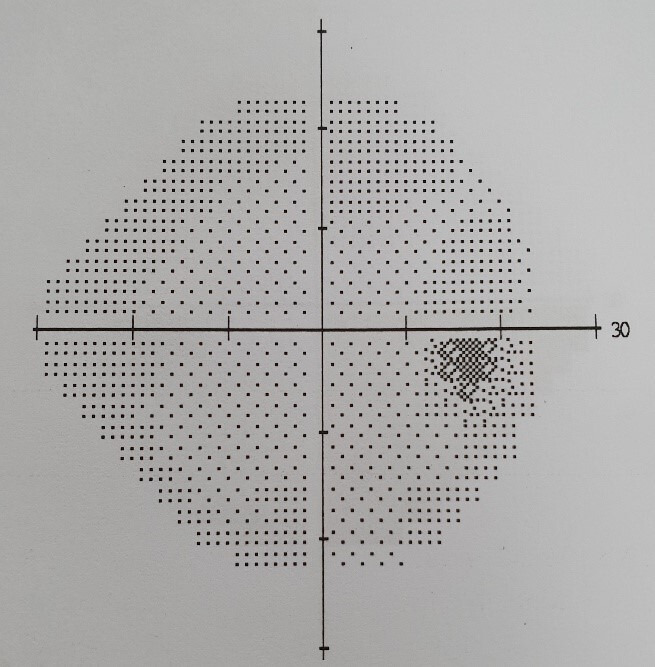
Right eye visual field.
Figure 5.
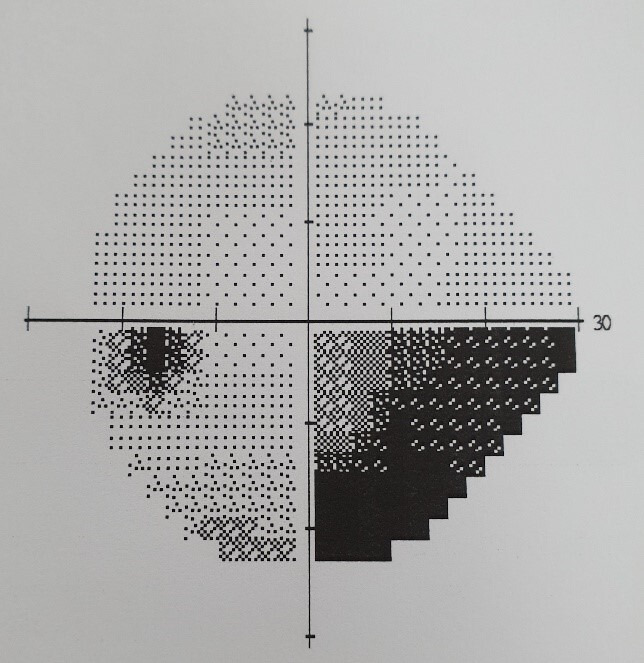
Left eye visual field.
Figure 6.
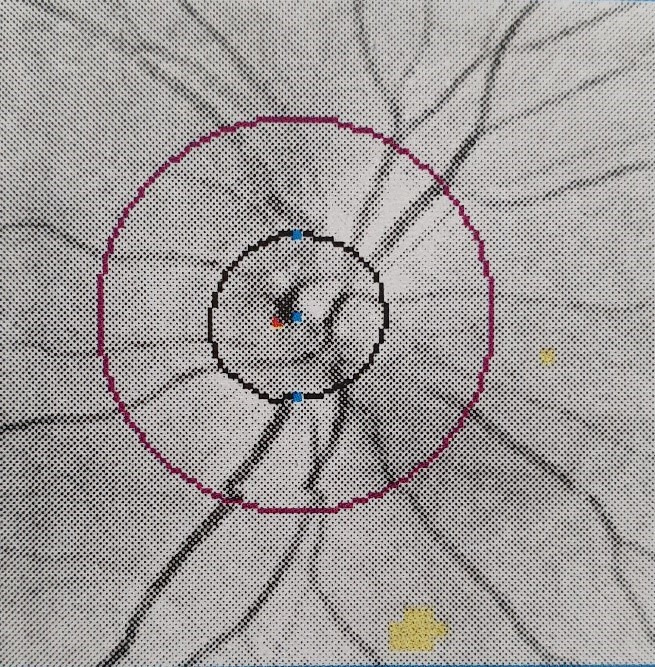
Right eye funduscopy.
Figure 7.
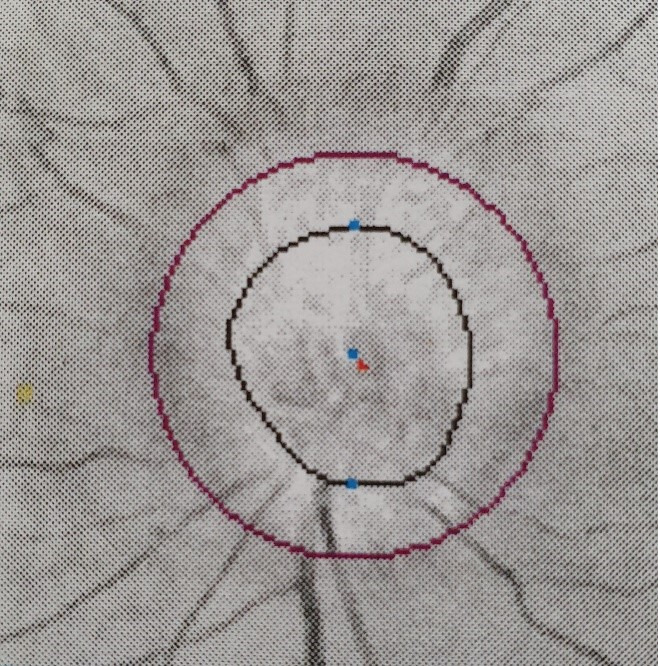
Left eye funduscopy.
Figure 8.
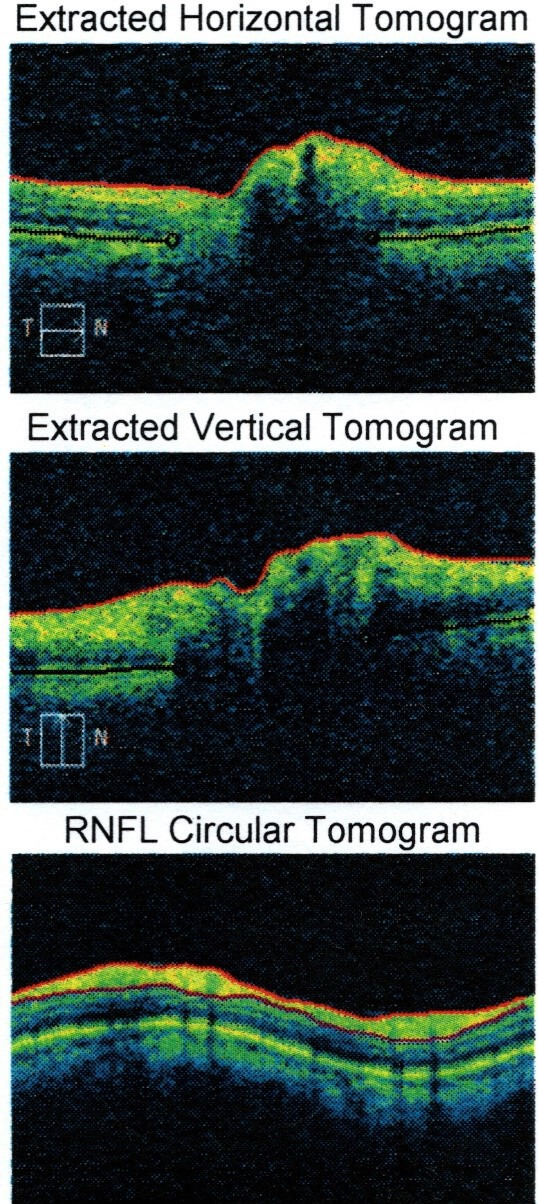
Right eye optical coherence tomography.
Figure 9.
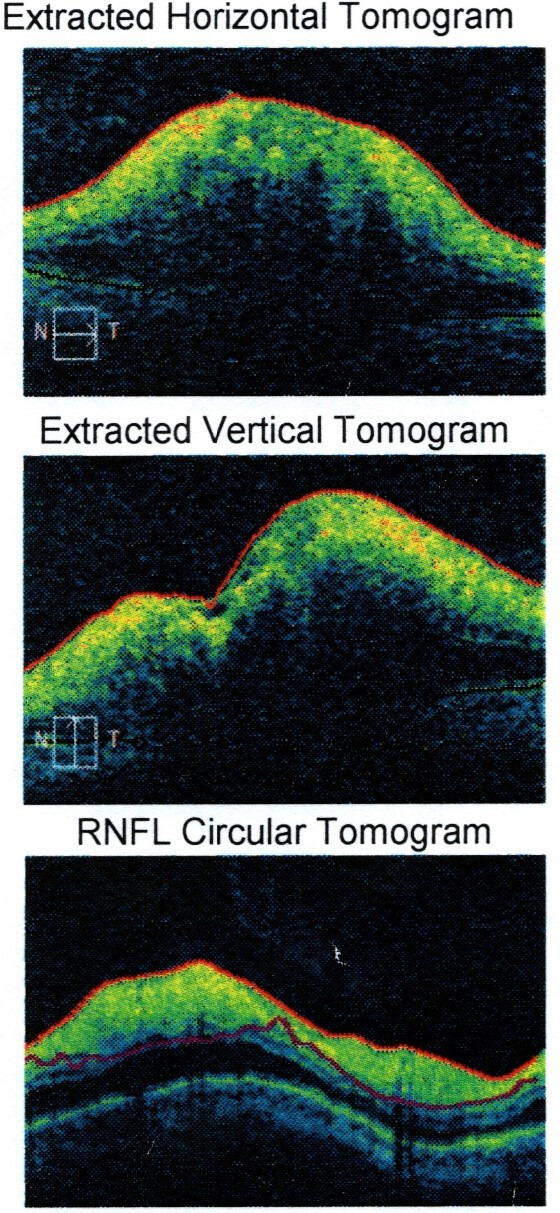
Left eye optical coherence tomography. RNFL, Retinal Nerve Fiber Layer.
He was also reviewed by neurology department who confirmed similar finding of left inferomedial quadrantopia, left RAPD and left optic disc swelling. His MRI finding was discussed in conjunction with the radiologists in the neuroradiology meeting, and the consensus was infliximab induced optic neuritis after considering the timing of onset, his clinical and radiological findings. There was no indication to perform lumbar puncture at the time.
Right eye visual field.
Left eye visual field.
Right eye funduscopy.
Left eye funduscopy.
Right eye OCT.
Left eye OCT.
His stool microscopy, culture and sensitivity (MCS) were negative for bacteria, virus or parasites. He had a colonoscopy that showed ongoing active distal proctosigmoiditis to 50 cm, with spontaneous bleeding, loss of vascularity and superficial ulceration. His UCEIS score was 7—vascular: 2, bleeding: 3 and erosions/ulcers: 2. The more proximal colon was normal. On the biopsy of the sigmoid colon, there was ulceration and crypt dropout in keeping with active chronic colitis.
Treatment
To manage his flare of ulcerative colitis, he was commenced on intravenous hydrocortisone 100 mg four times a day, mesalazine oral 4.8 g daily and mesalazine 2 g/60 mL enema once daily, in addition to his usual mercaptopurine 100 mg daily. His infliximab therapy was ceased and switched to vedolizumab 300 mg 4 weekly, a monoclonal antibody that blocks α4β7 integrin. Within a week, his stool frequency has reduced to three times a day, with formed and solid stool. He was discharged home with a weaning course of oral prednisolone 50 mg daily for 2 weeks, then decrease dose by 5 mg/day with each subsequent week (45 mg/day in week 3, 40 mg/day in week 4 and so on).
Outcome and follow-up
His flare of ulcerative colitis has resolved within 3 weeks of induction therapy. With regards to his visual symptom, he reported some improvement following cessation of infliximab. His left eye visual acuity has improved to 6/6. However, he still has residual visual field defect even after 3 months of cessation of infliximab. He is currently being followed up monthly by gastroenterology, neurology and ophthalmology in outpatient clinic.
Discussion
Biologic medications directed against TNFα are effective for the treatment of several inflammatory diseases, such as rheumatoid arthritis, psoriatic arthritis and the inflammatory bowel diseases that comprise Crohn’s disease and UC.3 Among these, infliximab, adalimumab and golimumab are used in the treatment of inflammatory bowel diseases.5
These biological agents are generally safe; however, they do have some potential side effects including risk of infection and malignancy. In an umbrella review of 10 meta-analysis observing the risk of infection in treating UC with biologicals, eight of them concluded that it is a safe approach that is not associated with statistically significant risk of developing serious or opportunistic infection, tuberculosis or malignancies. However, the other two meta-analysis suggested biological therapy may heighten the likelihood of developing serious infections.5 In another meta-analysis of randomised controlled studies, the incidence of malignancy is similar in Crohn’s disease patients treated with anti-TNFα agents than with placebo. The risk of non-Hodgkin’s lymphoma was found to be higher in combination therapy of anti-TNFα with immunomodulators (thiopurines and methotrexate) but not with anti-TNFα monotherapy.6
Anti-TNFα agents have also been implicated in demyelinating diseases such as MS and optic neuritis.1–9 The mechanism in which this occur remain unknown. One theory is that anti-TNF agents are unable to cross the blood–brain barrier. It has local anti-inflammatory effect on tissues with adequate drug penetration, such as intestines and joints, but may have systemic proinflammatory effect in tissue with low drug level such as central nervous system (CNS).3 In most cases, the symptoms resolve with cessation of the anti-TNFα therapy, and in a few cases reoccurred with rechallenge.3–9 However, not all patients recover completely, and a small minority reported no improvement.7
In a retrospective study on 2017 patients with IBD or joint disorders treated with anti-TNF agents (etanercept, infliximab, adalimumab or golimumab), 12 patients were found to develop various forms of peripheral neuropathy. Five improved with discontinuation of the medication alone, while the rest of the non-responders were given intravenous immunoglobulin (IVIg) treatment. Eight of the patients recovered completely.9 A randomised placebo-controlled trial of anti-TNFα in treatment of MS was discontinued early when early preliminary result showed patients in the treatment group were having more frequent exacerbations of MS than patients in the placebo group.10
Another retrospective cohort study found that optic neuritis occurred in similar frequency among patients with Inflammatory bowel disease who are taking modifying antirheumatic drugs and those exposed to biologicals.11 It is unsure whether optic neuritis occurs as a result of the anti-TNFα therapy or the disease itself. The temporal association with infliximab exposure suggests this as a cause in for our patient’s optic neuritis.
The time of onset of visual symptoms varies across case reports found on multiple literatures. One case study reported onset of bilateral optic neuritis 6 months following initiation of infliximab for psoriatic arthritis, with a total of five infusions.7 In another case report, a 55-year-old woman developed left optic neuritis after her ninth dose of 8 weekly infliximab 240 mg infusion for her rheumatoid arthritis.12 A randomised, placebo-controlled study of 168 patients with MS treated with lenercept, another TNF-α agonist, found an increase in frequency of exacerbation at both 24 and 48 weeks after onset of treatment, when compared with placebo.12 13
In general, optic neuritis is a clinical diagnosis based on history and examination finding.14 An MRI study of the brain and orbits with gadolinium contrast provides confirmatory diagnosis of acute optic neuritis and important prognostications regarding future risk of MS.15 Optic nerve inflammation on MRI with gadolinium contrast can be seen in 95% of patients with optic neuritis.16 Lumbar puncture is not an essential diagnostic test in optic neuritis but should be considered in atypical cases (those with bilateral presentation, <15 years of age or symptoms suggesting infection).14 17 Approximately 40%–60% of patients with optic neuritis have non-specific abnormalities in their CSF, including lymphocytosis and elevated protein.14 18 CSF oligoclonal bands are present in 56%–69% of cases, which implies higher risk of developing MS.14 19 Fluorescein angiography and visual evoked potential can also be done but are not routinely performed due to low sensitivity.14 15 18
The mainstay treatment for acute optic neuritis is intravenous corticosteroids, as there is evidence that it might hasten visual recovery and delay onset of MS.20 The Optic Neuritis Treatment Trial (ONTT) is a randomised controlled trial comparing 14 days of oral prednisolone (1 mg/kg/day) versus 3 days of intravenous methylprednisolone (1 g daily) with 11 days of oral prednisolone (1 mg/kg/day) versus 14 days of oral placebo in patients with optic neuritis. Intravenous methylprednisolone group was found to have accelerated recovery but no difference in outcome at 6 months to 1 year compared with the placebo group. It did, however, reduce the risk of conversion to MS within 2 years compared with oral prednisolone or placebo, but there was no difference in MS rates at 5 years.11 The ONTT suggests treatment with 3 days of intravenous methylprednisolone for episodes of optic neuritis.11
Alternative therapies include IVIg and plasma exchange.21–24 Two randomised trials studying the potential benefit of IVIg showed no difference in outcome at 6 months.21 22 In another non-randomised study of IVIg with oral corticosteroid, versus corticosteroid therapy alone, showed significant improvement in the IVIg group.23 In another case report of plasma exchange treatment in corticosteroid-refractory optic neuritis, 7 out of 10 patients showed improvement in visual acuity.24
Conclusion
There have been several cases reported in the literature with anti-TNFα therapy and the development of optic neuritis. Most cases have a favourable outcome when symptoms are reported early, and anti-TNFα discontinuation is prompt.1 Acute treatment of infliximab-induced optic neuritis is primarily intravenous corticosteroids, and in steroid-refractory cases, IVIG and plasma exchange can be considered. In addition, the challenge is to treat the development of optic neuritis and controlling the underlying inflammatory bowel disease, that is, to find an alternative agent. In this setting, a multidisciplinary approach between gastroenterologists, neurologists and ophthalmologists is recommended.
Learning points.
Antitumour necrosis factor alpha agents are associated with demyelinating conditions such as optic neuritis. These should be recognised early when symptoms arise.
A multidisciplinary approach between different specialties is recommended in managing both the demyelinating disease and the inflammatory bowel disease.
Treatment options include intravenous corticosteroids, intravenous immunoglobulin and plasma exchange.
Footnotes
Contributors: AD wrote and submitted the first draft of this paper and sourced the relevant details and images of this case. KS contributed with the diagnosis of this case and requested for the tests. KV contributed with the discussions section of the paper. SP is the supervisor and editor of this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Alexandre B, Vandermeeren Y, Dewit O, et al. Optic neuritis associated or not with TNF antagonists in patients with inflammatory bowel disease. J Crohns Colitis 2016;10:541–8. 10.1093/ecco-jcc/jjw003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Jagow B, Kohnen T. Anterior optic neuropathy associated with adalimumab. Ophthalmologica 2008;222:292–4. 10.1159/000140257 [DOI] [PubMed] [Google Scholar]
- 3.Kaltsonoudis E, Voulgari PV, Konitsiotis S, et al. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev 2014;13:54–8. 10.1016/j.autrev.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Chung JH, Van Stavern GP, Frohman LP, et al. Adalimumab-associated optic neuritis. J Neurol Sci 2006;244:133–6. 10.1016/j.jns.2006.01.012 [DOI] [PubMed] [Google Scholar]
- 5.Bonovas S, Pantavou K, Evripidou D, et al. Safety of biological therapies in ulcerative colitis: an umbrella review of meta-analyses. Best Pract Res Clin Gastroenterol 2018;32-33:43–7. 10.1016/j.bpg.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Papamichael K, Mantzaris GJ, Peyrin-Biroulet L. A safety assessment of anti-tumor necrosis factor alpha therapy for treatment of Crohn's disease. Expert Opin Drug Saf 2016;15:493–501. 10.1517/14740338.2016.1145653 [DOI] [PubMed] [Google Scholar]
- 7.Aasly JO. Bilateral optic neuritis associated with the use of infliximab. Case Rep Ophthalmol Med 2011;2011:1–2. 10.1155/2011/232986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ten Tusscher MPM, Jacobs PJC, Busch MJWM, et al. Bilateral anterior toxic optic neuropathy and the use of infliximab. BMJ 2003;326:579. 10.1136/bmj.326.7389.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsouni P, Bill O, Truffert A, et al. Anti-Tnf alpha medications and neuropathy. J Peripher Nerv Syst 2015;20:397–402. 10.1111/jns.12147 [DOI] [PubMed] [Google Scholar]
- 10.Li SY, Birnbaum AD, Goldstein DA. Optic neuritis associated with adalimumab in the treatment of uveitis. Ocul Immunol Inflamm 2010;18:475–81. 10.3109/09273948.2010.495814 [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Cleary PA, Anderson MM, et al. A randomized, controlled trial of corticosteroids in the treatment of acute optic neuritis. The optic neuritis Study Group. N Engl J Med 1992;326:581. 10.1056/NEJM199202273260901 [DOI] [PubMed] [Google Scholar]
- 12.Foroozan R, Buono LM, Sergott RC, et al. Retrobulbar optic neuritis associated with infliximab. Arch Ophthalmol 2002;120:985–7. [PubMed] [Google Scholar]
- 13.Anon Tnf neutralization in MS: results of a randomized, placebo-controlled multicenter study. The Lenercept multiple sclerosis Study Group and the University of British Columbia MS/MRI analysis group. Neurology 1999;53:457–65. [PubMed] [Google Scholar]
- 14.Osborne B, Balcer L. Optic neuritis: pathophysiology, clinical features and diagnosis. UpToDate, 2018. [Google Scholar]
- 15.Balcer LJ. Clinical practice. Optic neuritis. N Engl J Med 2006;354:1273. 10.1056/NEJMcp053247 [DOI] [PubMed] [Google Scholar]
- 16.Wray SH. Optic neuritis : Albert DM, Jakobiec FA, Principles and practice of ophthalmology. Philadelphia: WB Saunders, 1994: 2539. [Google Scholar]
- 17.Boomer JA, Siatkowski RM. Optic neuritis in adults and children. Semin Ophthalmol 2003;18:174–80. 10.1080/08820530390895172 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs LD, Kaba SE, Miller CM, et al. Correlation of clinical, magnetic resonance imaging, and cerebrospinal fluid findings in optic neuritis. Ann Neurol 1997;41:392–8. 10.1002/ana.410410315 [DOI] [PubMed] [Google Scholar]
- 19.Lightman S, McDonald WI, Bird AC, et al. Retinal venous sheathing in optic neuritis. its significance for the pathogenesis of multiple sclerosis. Brain 1987;110:405. 10.1093/brain/110.2.405 [DOI] [PubMed] [Google Scholar]
- 20.Osborne B, Balcer L. Optic neuritis: prognosis and treatment. UpToDate, 2018. [Google Scholar]
- 21.Noseworthy JH, O'Brien PC, Petterson TM, et al. A randomized trial of intravenous immunoglobulin in inflammatory demyelinating optic neuritis. Neurology 2001;56:1514–22. 10.1212/WNL.56.11.1514 [DOI] [PubMed] [Google Scholar]
- 22.Roed HG, Langkilde A, Sellebjerg F, et al. A double-blind, randomized trial of IV immunoglobulin treatment in acute optic neuritis. Neurology 2005;64:804–10. 10.1212/01.WNL.0000152873.82631.B3 [DOI] [PubMed] [Google Scholar]
- 23.Tselis A, Perumal J, Caon C, et al. Treatment of corticosteroid refractory optic neuritis in multiple sclerosis patients with intravenous immunoglobulin. Eur J Neurol 2008;15:1163–7. 10.1111/j.1468-1331.2008.02258.x [DOI] [PubMed] [Google Scholar]
- 24.Ruprecht K, Klinker E, Dintelmann T, et al. Plasma exchange for severe optic neuritis: treatment of 10 patients. Neurology 2004;63:1081. 10.1212/01.wnl.0000138437.99046.6b [DOI] [PubMed] [Google Scholar]


