Abstract
Intra-abdominal thromboses are a poorly characterised thrombotic complication of COVID-19 and are illustrated in this case. A 42-year-old man with chronic hepatitis B (undetectable viral load, FibroScan 7.4 kPa) developed fever and cough in March 2020. 14 days later, he developed right upper quadrant pain. After being discharged with reassurance, he re-presented with worsening pain on symptom day 25. Subsequent abdominal ultrasound suggested portal vein thrombosis. CT of the abdomen confirmed portal and mid-superior mesenteric vein thromboses. Concurrent CT of the chest suggested COVID-19 infection. While reverse transcription PCR was negative, subsequent antibody serology was positive. Thrombophilia screen excluded inherited and acquired thrombophilia. Having been commenced on apixaban 5 mg two times per day, he is currently asymptomatic. This is the first case of COVID-19-related portomesenteric thrombosis described in the UK. A recent meta-analysis suggests 9.2% of COVID-19 cases develop abdominal pain. Threshold for performing abdominal imaging must be lower to avoid this reversible complication.
Keywords: liver disease, hepatitis B, portal hypertension, radiology
Background
Clinicians have had to learn and adapt swiftly as the COVID-19 pandemic has spread across the world. Since the SARS-CoV-2 was first described in Wuhan City, Hubei province, China, in December 2019,1 it has become abundantly clear this novel coronavirus generates a markedly hypercoagulable state.
Thrombotic events are driven by a severe proinflammatory response to COVID-19, generating fibrin deposition and potentially disseminated intravascular coagulation, which confers a much poorer prognosis for patients.2 This thrombotic risk is compounded by hypoxia that typically manifests in severe respiratory COVID-19 illness. Pulmonary embolism, myocardial infarction and deep vein thrombosis remain as the most common thrombotic events associated with COVID-19.3 Incidence of arterial and venothromboembolic events in the setting of COVID-19 infection overall is not known. However, in the intensive care setting with critically ill patients, thrombotic complications related to COVID-19 are reported in 25%–31% of patients.4 5
By contrast, development of intra-abdominal thromboses related to COVID-19 infection is sparsely reported in the literature but is illustrated in this case.
Case presentation
A 42-year-old Eastern European man was under our care since 2006 with eAg negative chronic hepatitis B, having been diagnosed incidentally during a routine sexual health screen. His hepatitis B was well controlled, with an undetectable viral load since 2013 on entecavir 500mcg/day. He was non-cirrhotic, as evidenced by a FibroScan score of 7.4 kPa (IQR 0.9 kPa) in November 2019. His alanine transaminase (ALT) was 34 IU/L in October 2019. His only relevant medical history included a previous trauma-related splenectomy in 1998, for which he had previously taken phenoxymethylpenicillin prophylaxis, but stopped this in 2013. He had also received treatment for peptic ulcer disease secondary to aspirin use in 1999. He had never smoked and rarely consumed alcohol.
On 23 March 2020, the day the UK entered national lockdown for the COVID-19 pandemic, he developed fever and a dry cough. He denied any history of diarrhoea. He was advised to self-isolate, along with his household, and was provided with a 5-day course of amoxicillin 500 mg three times a day. The national guidance at that time was not to routinely undertake swab tests for SARS-CoV-2 reverse transcription PCR (RT-PCR) in symptomatic, non-hospitalised patients. Six days later, he remained feverish (temperature>39°C) with an ongoing cough, which had become productive. He continued to self-isolate and was issued a further course of Doxycycline 100 mg OD for 7 days.
His fever resolved on symptom day-10. On symptom day-14, he woke with constant, non-radiating right hypochondrial pain. The following day, he presented to his local emergency department. His liver function test (LFT) panel demonstrated a bilirubin of 23µmol/l, ALT 55 IU/L, alkaline phosphatase (ALP) 66 IU/L and albumin 31 g/L, and no imaging was undertaken (see table 1). He was thought to have probable biliary colic and was managed conservatively. He was discharged with simple analgesia, in accordance with the advice across the NHS at that time to avoid admission of patients who were deemed low risk and clinically stable.
Table 1.
Laboratory findings on days 14, 25 and 26 of COVID-19 infection
| Lab result (reference range) | Symptoms D14 (presented to GP with abdominal pain) |
Symptoms D25 (D1 hospital admission) |
Symptoms D26 (D2 hospital admission) |
| Bilirubin (<21µmol/L) | 23 | 33 | 30 |
| Bilirubin (<0.24 mg/dL) | 0.26 | 0.37 | 0.34 |
| ALP (30–130 IU/L) | 66 | 74 | 71 |
| ALT (10–60 IU/L) | 55 | 31 | 30 |
| Albumin (35–50 g/L) | 31 | 35 | 34 |
| Lipase (13–60 IU/L | – | 21 | – |
| White cell count (4.0–11×109/L) | 11.15 | 13.84 | 13.3 |
| Haemoglobin (130–170 g/L) | 152 | 147 | 148 |
| Mean cell volume (83–100 fL) | 87.7 | 90.7 | 88.2 |
| Platelets (150–400×109/L | 568 | 364 | 331 |
| Neutrophils (1.5–8.0×109/L) | 6.03 | 8.08 | 8.31 |
| Lymphocytes (1.0–4.0×109/L | 2.51 | 2.92 | 2.06 |
| C reactive protein (<6.0 mg/L) | 29 | 44 | 131 |
| Random glucose (4.0–7.8 mmol/L) | 6.5 | – | – |
| Sodium (133–145 mmol/L) | 138 | 141 | 137 |
| Potassium (3.5–5.3 mmol/L) | 4.3 | 4.0 | 5.4 |
| Urea (2.5–7.8 mmol/L) | 3.3 | 3.6 | 3.6 |
| Creatinine (59–104 µmol/L) | 81 | 69 | 63 |
| eGFR (mL/min) | >90 | >90 | >90 |
| Troponin T (<14 ng/L) | 9 | – | – |
ALP, alkaline phosphatase; ALT, alanine transaminase; eGFR, estimated glomerular filtration rate; GP, general practitioner.
Nine days later (symptom day 25), he re-presented to his general practitioner (GP) with ongoing worsening upper abdominal pain. His GP repeated his LFTs, which revealed his bilirubin was now 33 µmol/L, with an ALT of 31 IU/L, ALP of 74 IU/L and albumin of 35 g/L. His full blood count was largely unremarkable, with haemoglobin of 147 g/L, platelets of 364×109/L, a mild leucocytosis of 13.8×109/L and neutrophils of 8.1×109/L. His C reactive protein was elevated at 44 mg/L. His GP referred him to the general surgical team with abdominal pain. On admission, he was apyrexial (36.9°C), haemodynamically stable (pulse 95 beats/min, blood pressure 131/79 mm Hg) with normal oxygen saturation of 98% on air and normal respiratory rate.
Investigations
An abdominal ultrasound showed absent anterograde flow in the portal vein with collateralisation, consistent with portal vein thrombosis (PVT). This had developed since his last normal ultrasound scan, which had demonstrated anterograde flow in the portal vein 11 months previously.
His subsequent contrast-enhanced CT of the abdomen demonstrated loss of enhancement of the entire length of the portal vein (figure 1) and a smaller thrombus in the mid-superior mesenteric vein, with expansion and surrounding inflammatory stranding, consistent with thrombosis. There was also mural oedema of the distal duodenum, distal small bowel and descending colon. The patient had a concurrent CT of the chest showing bilateral, patchy, ill-defined ground-glass opacities with basal predominance, worse on the right, consistent with COVID-19 infection (figure 2). While his RT-PCR was negative, subsequent SARS-CoV-2 antibody serology was positive. His prothrombin time was normal. A thrombophilia screen excluded inherited and acquired thrombophilia, including antiphospholipid syndrome, myeloproliferative disorders and paroxysmal nocturnal haematuria. No JAK2 mutation was detected. His protein C & S levels and antithrombin activity was normal. His lupus anticoagulant screen was negative.
Figure 1.
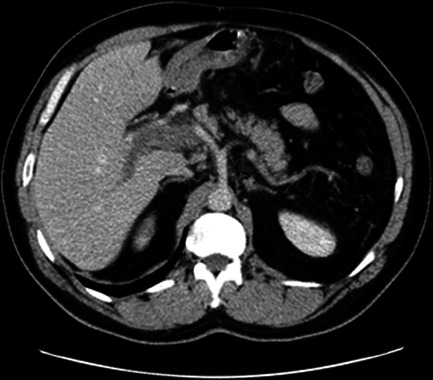
Axial contrast-enhanced CT in the portal venous phase shows loss of enhancement of the portal vein with expansion and surrounding stranding, which is consistent with acute thrombosis.
Figure 2.
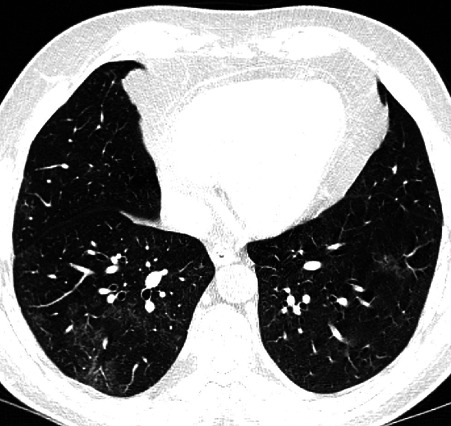
Axial non-contrast CT image demonstrates bilateral patchy ill-defined ground-glass opacities with basal predominance and worse on the right. Clinicoradiological findings were consistent with COVID-19 infection.
Treatment
Our patient was commenced on the factor Xa inhibitor apixiban 5 mg two times per day. He will remain anticoagulated for a minimum of 6 months and then reassessed.
Outcome and follow-up
His repeat triple-phase CT of the abdomen 6 weeks later confirmed an established PVT, retracted in size, with collateralisation extending into the upper abdomen, and resolution of the intestinal oedema. He is currently asymptomatic, remains anticoagulated and is awaiting a screening gastroscopy.
Discussion
To the best of our knowledge, this is the first COVID-19-related portomesenteric thrombosis to be described in the UK, although similar cases have been described in France and Italy in non-cirrhotic patients.
Franco-Moreno et al presented a 27-year-old man from the Dominican Republic, normally fit and well, who presented with epigastric pain following 3 weeks of typical COVID-19 respiratory symptoms, similar to our patient.6 Like our patient he was RT-PCR negative, but had a thoracoabdominal CT suggestive of SARS-CoV-2 infection and PVT. He was subsequently found to be SARS-CoV-2 antibody positive. de Barry et al described a 79-year-old woman, with no medical history but symptoms of epigastric pain and diarrhoea for 8 days.7 The diagnosis of COVID-19 was made on CT of the thorax. This patient was also RT-PCR negative, and there is no mention of antibody status. Their patient also had a concurrent superior mesenteric and jejunal artery thrombosis and required an extended right hemicolectomy and laparotomy. The results of her thrombophilia screen are not known. Finally, La Mura et al published the case of a 72-year-old man with Parkinson’s disease and mild vascular dementia.8 It is unclear what the duration of symptoms was, but he presented with fever and jaundice (bilirubin 126 µmol/L, ALT 257 IU/L) and had an Escherichia coli bacteraemia. Two days later, he was found to be SARS-CoV-2 RT-PCR positive and, despite having a normal abdominal ultrasound on admission, developed abdominal pain 6 days into admission. Subsequent CT revealed a PVT. Interestingly, this was despite 6 days of prophylactic anticoagulation, suggesting the PVT was probably present on admission, given the presence of jaundice. The varying intervals between initial symptoms of COVID-19 infection and development of abdominal pain secondary to PVT reflect the heterogeneity of this presentation. While venothromboembolism risk markedly increases among patients with severe COVID-19, that is, requiring non-invasive or invasive ventilatory support, this does not apply to our patient. In fact, only the patient reported in de Barry et al’s case report was invasively ventilated, having required a laparotomy for ischaemic bowel.7
The PVT in our patient was confirmed as acute related to multiple factors. Firstly, normal portal vein flow and patency had been confirmed 11 months previously on a routine ultrasound. Secondly, corroborating with a clinical presentation of new abdominal pain, the patient’s initial CT of the abdomen demonstrated the portal vein diameter had expanded to 22 mm secondary to an obstructing thrombus with perivascular stranding. Finally, while some subhepatic collateral vessels were also seen, they were small calibre, favouring an acute PVT. In a subsequent CT of his abdomen 6 weeks later, our patient’s portal vein diameter had fallen to approximately 10 mm. Perifocal fat stranding had largely resolved, but the calibre of the venous collaterals had significantly increased, indicating an established thrombus.
Non-malignant PVTs are well recognised in the setting of cirrhosis, in which the estimated prevalence is between 10% and 25%.9 However, our patient had well-controlled chronic hepatitis B with only F1 fibrosis on his FibroScan 4 months prior to his presentation with PVT. This is consistent with the aforementioned case reports, all of which describe non-cirrhotic patients. It remains unclear why these patients developed PVTs and not venous thromboembolism in more conventional sites, such as the pulmonary vasculature or deep veins of the legs. SARS-CoV-2 has been demonstrated to bind to the ACE2 receptor, which is expressed in cholangiocytes.10 11 However, our patient’s ALP was always normal. Furthermore, patients with cirrhosis secondary to biliary disease are not at greater risk of PVTs (pretransplant) compared with patients with hepatitis B or alcohol-related cirrhosis.9
Deranged LFTs in concurrent COVID-19 infection are well recognised,12 occurring in approximately 23% of patients at presentation, most commonly as elevated transaminases.13 The largest meta-analysis of gastrointestinal symptoms associated with COVID-19 infection found 18% of patients presented with gastrointestinal symptoms, with 9.2% developing abdominal pain.14 What our case highlights, reflecting the experience of Franco-Moreno and colleagues, is the insidious presentation of PVT in some patients with COVID-19. Our patient was not jaundiced, did not have deranged LFTs and had relatively mild respiratory symptoms only. Public Health England guidance was for such patients to avoid coming to the hospital where at all possible in March and April 2020. In that context, the concern is that this case reflects a large undiagnosed disease burden due to COVID-19. It also raises questions on how we should risk stratify patients to consider outpatient prophylactic anticoagulation in those with confirmed COVID-19 infection, particularly moving forward as the pandemic continues to spread across the Americas, Africa and Asia.
While this is a rare complication of COVID-19 infection, clinical threshold for performing liver imaging must be lower to avoid missing this reversible complication of COVID-19.
Patient perspective.
I first found out that I had a clot or a portal vein thrombosis on 17 April 2020 and I was alarmed immediately to start taking blood thinners to avoid any further potential damage. It was shocking for me for two reasons: first because I was familiar with such an issue as my father had developed deep vein thromboses on his leg at the age of 51, which was not diagnosed and obviously not treated on time, which caused damage to his liver and later on to his heart by giving him heart attacks. That is what I thought immediately. Second, the pain was hard to manage for which I was taking up to eight painkillers a day, two at each time. I started thinking, will I get better again?
It was concluded that all this happened due to the COVID-19 infection I had around 2–3 weeks before I was diagnosed, even though the pain had started since 7 April 2020, and I was admitted in the hospital yet only at my second visit after I insisted to my general practitioner that I am not well at all, then on 17 April 2020, I was admitted again to the hospital where the clot was discovered.
However, I would like to bring to your attention two issues. First of all, I have been complaining about pain at the very same place where I had the pain when the clot was discovered since 3–4 years ago. I had been referred in a couple of occasions to undergo an ultrasound and in one case to even have an endoscopy. Yet nothing was found on these investigations. Second, about 1–2 years ago, I have been complaining about heart issues as when the pain struck me and I felt totally numbed on my left side of my chest near the armpit. I was near Paulton Hospital and I stopped straight there where an ECG was performed on me. Both times I had this severe chest (nearby armpit) while driving. I was wondering if I had developed blood clot even earlier and I had not realised it and if it was the blood clot that was giving me heart issues.
I am at the moment still taking the blood thinner pill apixaban.
I feel like my life has pretty much normalised. However, from time to time, I feel mild pain and discomfort at the same place where the clot was discovered, which worries me. On a scale of 1–10 of my life being back to normal, I would say I am up to a 8–9. The only thing of concern is what will happen next.
Learning points.
COVID-19 is a markedly prothrombotic state.
When reviewing patients with COVID-19 infection presenting with abdominal pain, clinicians should consider portal vein thrombosis (PVT) in their differential diagnosis.
Clinicians should have a low threshold for performing liver imaging in such patients.
Do not expect to see grossly deranged liver function tests with PVT.
Acknowledgments
We thank our patient for consenting to present this unusual case during the COVID-19 pandemic. We are grateful to the hepatitis team at Bristol Royal Infirmary (Mrs Jane Gitahi), the General Surgery and Radiology staff (Ms Alexis Sudlow, Mr Alan Osborne and Dr Richard Flood) at Southmead Hospital for the patient care provided.
Footnotes
Contributors: KWMA performed literature search and wrote the manuscript. HK reviewed the imaging and the manuscript. AC reviewed the thrombophilia screen and the manuscript. FHG oversaw the project and reviewed the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:2950–73. 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18:1421–4. 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145–7. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franco-Moreno A, Piniella-Ruiz E, Montoya-Adarraga J, et al. Portal vein thrombosis in a patient with COVID-19. Thromb Res 2020;194:150–2. 10.1016/j.thromres.2020.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Barry O, Mekki A, Diffre C, et al. Arterial and venous abdominal thrombosis in a 79-year-old woman with COVID-19 pneumonia. Radiol Case Rep 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.La Mura V, Artoni A, Martinelli I, et al. Acute portal vein thrombosis in SARS-CoV-2 infection: a case report. Am J Gastroenterol 2020;115:1140-1142. 10.14309/ajg.0000000000000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsochatzis EA, Senzolo M, Germani G, et al. Systematic review: portal vein thrombosis in cirrhosis. Aliment Pharmacol Ther 2010;31:366–74. 10.1111/j.1365-2036.2009.04182.x [DOI] [PubMed] [Google Scholar]
- 10.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020. [Google Scholar]
- 11.Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428–30. 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther 2020;52:584–99. 10.1111/apt.15916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020;159:81–95. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]


