Abstract
This study is unique in that it examines the evolution of white matter injury very early and at 12 months post-injury in pediatric patients following traumatic brain injury (TBI). Diffusion tensor imaging (DTI) was acquired at two time-points: acutely at 6–17 days and 12 months following a complicated mild (cMild)/moderate (mod) or severe TBI. Regional measures of anisotropy and diffusivity were compared between TBI groups and against a group of age-matched healthy controls and used to predict performance on measures of attention, memory, and intellectual functioning at 12-months post-injury. Analysis of the acute DTI data using tract based spatial statistics revealed a small number of regional decreases in fractional anisotropy (FA) in both the cMild/mod and severe TBI groups compared with controls. These changes were observed in the occipital white matter, anterior limb of the internal capsule (ALIC)/basal ganglia, and corpus callosum. The severe TBI group showed regional differences in axial diffusivity (AD) in the brainstem and corpus callosum that were not seen in the cMild/mod TBI group. By 12-months, widespread decreases in FA and increases in apparent diffusion coefficient (ADC) and radial diffusivity (RD) were observed in both TBI groups compared with controls, with the overall number of regions with abnormal DTI metrics increasing over time. The early changes in regional DTI metrics were associated with 12-month performance IQ scores. These findings suggest that there may be regional differences in the brain's reparative processes or that mechanisms associated with the brain's plasticity to recover may also be region based.
Keywords: diffusion tensor imaging, factor analysis, longitudinal, neurocognitive outcome, pediatric
Introduction
Traumatic brain injury (TBI) is a significant cause of death and disability in children with approximately half a million children seen each year in the emergency department.1 Approximately 25% of these injuries are considered moderate to severe1 resulting in prolonged memory, attention, executive function, and emotional impairments.2
The mechanical forces acting on the brain during TBI can shear and/or stretch axons to a breaking point, resulting in primary axotomy or partially damage axons initiating a secondary cascade of molecule events that can cause Wallerian degeneration.3 The acute axonal injury can progress and develop into delayed and secondary axonal degeneration over hours, days, and months following the TBI in response to persistently altered Ca2+ fluxes, mitochondrial dysfunction, and reactive oxygen species generation triggered by the initial mechanical forces.4–7 A number of key molecular pathways that initiate and trigger this process have been identified, including the depletion of nicotinamide mononucleotide adenylyltransferase and the activation of sterile α and Toll/interleukin-1 receptor motif containing 1 and mitogen-activated protein kinases (MAPK).8 Importantly, the time course for the development of axonal pathological changes suggests a window of opportunity for intervention.8
Diffusion tensor imaging (DTI) has been used to measure axonal integrity of whole brain, regional, or individual white matter tracts using such metrics as fractional anisotropy (FA), apparent diffusion coefficient (ADC)/mean diffusivity (MD), axial diffusivity (AD), or radial diffusivity (RD). FA represents the degree to which diffusion is anisotropic and is influenced by both AD (which denotes the axial vector of water diffusion), and RD (which is an average of the radial vectors orthogonal to the axial vector). ADC and MD measure the amount of diffusion within tissue, which is averaged over three orthogonal directions.9 In normal white matter, higher FA and lower ADC/MD values are thought to reflect intact, dense axons and greater myelination.10 The loss of white matter integrity following traumatic axonal injury is particularly important, as these changes are thought to contribute to longstanding functional impairments.11,12
Recent meta-analyses of DTI studies of pediatric (< 18 years of age) mild, moderate, and severe TBI patients in the medium to long term (> 4 weeks to 5 years) have shown decreased FA and concomitant increases in ADC/MD in multiple white matter tracts that suggest evolving axonal and myelin injury in the first few months post-injury.13,14 Studies included in these meta-analyses reported significant changes in the corpus callosum, internal capsule, cingulum bundle, uncinate fasciculus, longitudinal fasciculus, and the frontal and temporal lobe white matter. In the small number of studies performed in the acute stage (< 4 weeks), all were in mild adolescent (10–17 years of age) TBI patients where the opposite pattern is observed; moderate to large increases in FA and concurrent decreases in ADC/MD were reported in regions including the whole brain, corpus callosum, corona radiata, fornix, left cingulum, internal capsule, anterior thalamic radiation, right inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, right thalamus, and left white matter.15–17 Of these studies, only two studies reported regional increased RD and/or reduced AD, supporting the idea that axonal injury and demyelination is part of the post-TBI pathophysiological spectrum.16,18
While there is emerging evidence supporting the relation between DTI and cognitive outcome; variations between when the data were acquired, the regions evaluated, subject age, and the measures used to assess cognition have led to diverse findings.19 A study by Kurowski and colleagues in chronic (> 12 months) moderate–severe pediatric TBI patients (mean age 7.8 years) reported a significant correlation between left and right frontal white matter FA with measures of executive function and attention with no correlation between corpus callosum FA and cognitive measures.20 Adamson and colleagues showed a significant correlation between FA in the genu of the corpus callosum and word reading scores in a population of complicated mild to severe adolescent TBI patients (mean age 15.3 years) 1–2 years after injury.21 In a longitudinal study of pediatric complicated mild-severe TBI patients age 7–17 years, Wu and colleagues showed decreased FA in the corpus callosum which was correlated to processing speed at 3 and 12 months.22
To date, very few studies have examined longitudinal white matter integrity after pediatric TBI, with acute studies being collected on average more than 1 month post injury.22–26 Given the progressive nature of axonal neuropathology and that time post-injury significantly affects the relation between DTI and cognitive measures,27 shorter time windows for acute studies are necessary to obtain a more thorough understanding of the evolution of white matter damage following pediatric TBI. This study is unique in that it examines white matter integrity at both acute (6–17 days) and later (12 month) post-injury time-points in pediatric patients with complicated mild (cMild)/moderate (mod) and severe TBI. In addition, we sought to examine the relation between the early post-injury measures of white matter disruption to 12-month neurocognitive scores to determine if the early DTI findings would predict neurocognitive outcome. We hypothesized that early DTI findings would predict performance on measures of attention, memory, and intellectual functioning—broad neurocognitive modalities that, regardless of age at injury are sensitive to TBI across the lifespan.2
Methods
Participants
All procedures were reviewed and approved by the Loma Linda University Health System (LLUHS) Institutional Review Board. Informed written consent was obtained from all subjects' parents or legal guardians if below the age of 11 and from the subject if over 11 years of age. An assent form was signed by subjects between the ages of 7–11 years.
Male and female TBI participants between the ages of 5 and 18 years were enrolled consecutively following hospital admission from 2009–2013 if they met the following criteria: 1) absence of previous brain injury, neurologic disorders, drug or alcohol abuse or magnetic resonance imaging (MRI) contraindications including dental braces and 2) moderate to severe TBI with Glasgow Coma Scale (GCS) scores between 3–12 or cMild (GCS 13–15) if hemorrhage was detected on an initial brain imaging (computed tomography [CT]) examination. Based on the GCS score, TBI patients were dichotomized into severe (GCS <8) or cMild/mod (GCS 9–15) TBI groups. Participants with cMild TBI were included in the modTBI group as the presence of cerebral lesions on CT has been shown to be predictive of poor outcome at 12-months.28 Control participants between the ages of 5 and 18 years who met criteria 1 were recruited from LLUHS pediatric clinics.
Image acquisition and processing
TBI subjects were scanned acutely (range: 6–17 days) and at 12 months after injury. Controls were scanned initially and 1 year later using the same protocol. Conventional whole–brain three-dimensional (3D) T1 weighted (T1WI, MPRAGE, repetition time [TR] and echo time [TE] = 1950 msec and 2.26 msec, 1.0 × 1.0 × 1.0 pixel size) and 30 direction DTI (TR/TE = 5700/1022 msec, 1.2 × 1.22 resolution, 3 mm slice thickness, 0.9 mm gap, b = 0 and 1000 sec/mm2) were acquired using a 3T Siemens Tim Trio MR scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12 channel receive-only head coil.
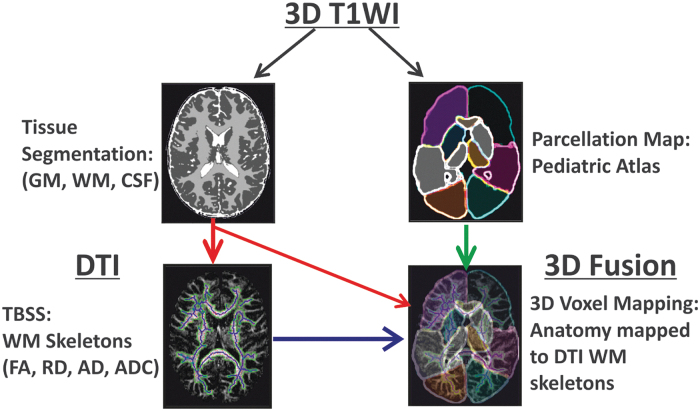
DTI data were processed using our previously published battery of computational tools (pre-processing, tract based spatial statistics (TBSS), image co-registration, and brain anatomy parsing) created using an in house pipeline developed in MATLAB (version R2014a, Mathworks, Natick MA) and incorporating image segmentation, registration and brain parsing routines from SPM5 (The Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, London, England), FSL (FLIRT image registration routine; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT), and the Laboratory of Neuroimaging (LONI) Brain Parser software catalog (University of Southern California, CA; www.pipeline.loni.usc.edu; Fig. 1).29
FIG. 1.
Overall scheme of image processing used in this study. The subject's high resolution T1 data were input into an atlas-based brain parcellation algorithm and segmented into tissue classes (white matter, gray matter, and cerebrospinal fluid) as well as 17 brain anatomic regions in T1/T2 space. The subject's diffusion data was co-aligned with its own T1/T2 data to transfer tissue and anatomy information to diffusion tensor imaging (DTI) spaces. Finally, tract-based statistics from DTI were fused for individual brain anatomy and tissue regions to estimate normative developmental trends in a pediatric dataset. Color image is available online.
Briefly, the proprietary Siemens MRI scanner generated tensor file, which contains b0 and directional tensor matrices (Dxx, Dxy, Dxz, Dyy, Dyz, Dzz) were used to compute DTI tensor images, which were then interpolated to generate continuous 3D volumes matching the resolution of the T1WI (1.2 × 1.2 × 3.0 mm) volume using a 3D cubic interpolation routine. Binary brain masks of the b0 images were used to remove the background noise and remove the skull in the DTI tensor images. Skull stripped tensor images were then used to compute the FA, ADC, AD and RD maps based on standard formulas.30,31 Cerebrospinal fluid (CSF) masking was performed using ADC images to produce a final binary brain mask, which was applied to the other DTI maps to improve the TBSS analysis. ADC values of >500 × 10-5 mm2/sec were used to detect CSF and voxels containing CSF outside the brain were eliminated from the binary mask while voxels within the ventricles were allowed to remain. If needed, additional skull stripping was performed during this step using an ADC threshold of <5 × 10-5 mm2/sec. All reconstructed b0, FA, and diffusivity data sets were reviewed for motion artifacts and no studies were removed from further analysis.
In our TBSS method, the physical locations of the skeleton are the central voxels within a white matter tract. However, we replaced the values in the skeleton voxels with several specific statistical measures (mean, median, maximum, and mode) of the voxel values on the line perpendicular to the skeleton for each DTI index. Therefore, all voxel values up to and including the gray matter-white matter junction that are found within the white matter mask are considered statistically as part of the mean, medium, maximum, and mode calculation in the value of the skeleton voxel.29
White matter brain tracts were extracted and a FA skeleton was computed using a FA threshold >0.2 based on our previous work, which showed that smaller FA thresholds overestimated while larger FA thresholds underestimated white matter regions.29 Region-based connected component analysis was used to detect small nonconnected outlier regions <20 voxels in size and removed using area-based iterative filtering to produce a single connected skeleton for each white matter region. A MATLAB routine for medial axis computation (“bwmorph [BinaryMask, ‘skel’, inf]”) was used to create a skeleton of the white matter mask for each FA map by iteratively removing boundary pixels of the white matter binary mask to produce a single connected skeleton for each white matter region. The white matter binary mask and skeleton derived from the FA maps were superimposed onto the RD, AD, and ADC maps. Skeletons underwent non-linear transformation to MNI152 space using FSL's FLIRT image registration routine (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). The resultant images were visually inspected for misalignment as result of distortion from the transformation of severely damaged brain tissue. Data from 12 subjects were discarded and removed from further analysis.
Anatomic regions were delineated from the co-registered T1WI and DTI skeleton data using a pediatric brain parsing pipeline from the LONI Brain Parser software catalog (University of Southern California, CA; www.pipeline.loni.usc.edu; Fig. 1) Following TBSS analysis and brain parsing, the mean values for FA, RD, and AD were extracted for the: frontal, parietal, occipital, and temporal white matter. Other regions included the corpus callosum, thalamus (which included the posterior limb of the internal capsule; PLIC/thalamus), basal ganglia (which included the anterior limb of the internal capsule; ALIC/basal ganglia), brainstem, and cerebellum.
Neurological and neurocognitive testing
Neurologic outcome was assessed by a pediatric neurologist using the Pediatric Cerebral Performance Category Scale (PCPCS). Serial neurological examinations were performed on all TBI patients at study entry, initial MRI, hospital discharge, 3, 6, and 12 months post-injury, and initially and at 1 year for controls. Clinical information and surrogates of acute neurological injury (duration of loss of consciousness, days in coma, presence of seizures, days on a ventilator, days in hospital) were recorded for each TBI subject during their hospitalization.
Neurocognitive testing was performed in an outpatient setting and conducted at 12 months post-injury for TBI subjects and at 1 year for controls. The neurocognitive test battery included measures of general intelligence (Wechsler Abbreviated Scales of Intelligence), attention (Sky Search subtest from the Test of Everyday Attention for Children [Tea-CH; 5–15 years]), and memory (General Memory composite from the Children's Memory Scale [5–17 years] or Wechsler Memory Scale [17–18 years]). All neurocognitive scores are standardized by age based on published norms, with higher scores indicative of better performance. After standardization, memory scores were converted to Z-scores for ease of statistical comparison across differing measures.32,33 The Tea-Ch Sky Search is a brief measure of focused attention, wherein the average time to identify targets is obtained (Tea-Ch C), and then calculated when controlling for the influence of motor speed (Tea-Ch G). Lower standardized scores indicate increased time to identify targets with (Tea-Ch G) and without (Tea-Ch C) controlling for motor speed. The intelligence measure yields three indices: Verbal IQ (VIQ), performance IQ (PIQ), and a Full Scale Intelligence Quotient (FSIQ). The VIQ measures verbal abstract reasoning and the PIQ is a measures fluid reasoning and visual-spatial processing while the FSIQ is an overall composite of general intellectual ability based on both VIQ and PIQ.
Statistical analysis
Group differences in regional DTI metrics at each imaging time-point were determined with linear (mixed effects) regression using age as a covariate and reported as the t statistic. Longitudinal differences in regional DTI metrics were measured within each group using paired t-tests at an alpha level of 0.001 to adjust for multiple comparisons.
Given the large number of measures resulting from regional DTI analysis, we applied a factor analysis to reduce the DTI metrics into a smaller number of underlying factors that could be used in a linear regression model to predict 12-month neurocognitive scores. Factor analysis was performed using principal component analysis of the 36 regional DTI measures to create eigenvectors.34,35 Only eigenvalues greater than 1 were used in subsequent analyses and Varimax rotation with Kaiser normalization was applied to produce several factors that explained the variance. Factor loadings explain the association of each variable with each factor and a factor loading >0.6 indicates a strong association, 0.4-0.6 a moderate association, and <0.4 a weak association. Only strong factor loadings over 0.6 were considered for subsequent analyses. Each subject was assigned a derived score for each of the five rotated factors based on the loadings. Independent sample t-tests were then used to determine whether any of the five factors could differentiate cMild/mod from severe TBI. Mixed linear regression, using age as a covariate, was used to determine if regional DTI metrics and/or factors from the initial imaging time-point could predict 12-month neurocognitive scores. Findings were considered significant if p < 0.001 after Bonferroni correction for multiple comparisons, unless otherwise stated. All statistical analyses were performed in SPSS (version 22; Chicago, IL).
Results
Demographics
A total of 65 pediatric subjects completed the 12-month imaging and neurocognitive assessments and were included in this study. Table 1 summarizes the age, time to initial and follow-up imaging, and injury characteristics of the enrolled participants. Ten participants had sustained a cMild/mod TBI (n = 5 cMild and n = 5 mod) and 18 subjects had sustained a severe TBI. There were 37 participants in the healthy control group.
Table 1.
Subject Demographics
| |
Control (n = 37) |
cMild/mod (n = 10) |
severe (n = 18) |
Control vs. cMild/mod |
Control vs. severe |
cMild/mod vs. severe |
|---|---|---|---|---|---|---|
| mean (SD) | mean (SD) | mean (SD) | p | p | p | |
| Age at injury (years) | 13.5 (3.2) | 12.0 (3.6) | 12.2 (3.6) | ns | ns | ns |
| [range] | [5.5 – 17.4] | [5.8 – 16.9] | [5.0 – 17.8] | |||
| Sex (M/F) | 20/17 | 9/1 | 12/6 | ns | ns | ns |
| Days to MRI (days) | 12.5 (4.1) | 11.1 (3.5) | - | ns | ns | |
| [range] | [8.0 – 18.0] | [6.0 – 17.0] | ||||
| Months to follow-up | 12.9 (1.1) | 12.1 (0.9) | 12.5 (0.7) | ns | ns | ns |
| [range] | [11.7 – 16.7] | [10.8 – 13.8] | [11.3 – 13.9] | |||
| Injury characteristics | ||||||
| LOC >24 h (%) | NA | 0 | 39.1 | |||
| Days in coma | NA | 2.4 (3.4) | 3.8 (4.1) | ns | ns | ns |
| [range] | [0 – 11] | [1 – 12] | ||||
| Days on ventilator | NA | 2.3 (3.1) | 3.7 (4.2) | ns | ns | ns |
| [range] | [0 – 9] | [0 – 14] | ||||
| Days in hospital | NA | 10.5 (6.2) | 14.5 (8.9) | ns | ns | ns |
| [range] | [4 – 23] | [3 – 29] | ||||
| 12-month neurological and neuropsychological testing scores | ||||||
| PCPCS | 1.0 | 1.3 (0.5) | 1.4 (0.7) | < 0.001 | < 0.001 | ns |
| VIQ | 107.4 (13.7) | 94.1 (10.4) | 90.8 (15.2) | 0.025 | < 0.001 | ns |
| PIQ | 107.2 (14.0) | 99.8 (14.1) | 98.5 (10.1) | ns | ns | ns |
| FSIQ | 107.9 (13.4) | 95.7 (10.4) | 94.1 (12.9) | 0.031 | 0.002 | ns |
| Combined memory Z score | 122.1 (15.3) | 101.3 (24.5) | 102.0 (20.1) | 0.022 | 0.005 | ns |
| TEA-CH C attention | 11.1 (3.6) | 9.3 (3.2) | 9.3 (2.8) | ns | ns | ns |
| TEA-CH G attention | 11.3 (3.8) | 9.9 (2.7) | 9.5 (3.4) | ns | ns | ns |
SD, standard deviation; cMild/mod, complicated mild/moderate TBI; ns, nonsignificant ; M, male; F female; MRI, magnetic resonance imaging; LOC, loss of consciousness; PCPCS, pediatric cerebral performance category scale; VIQ, verbal IQ; PIQ, performance IQ; FSIQ, full scale IQ; TEA-Ch, test of everyday attention-for children;
The two TBI groups did not differ significantly in days to initial MRI, loss of consciousness, length of coma, length of time on a ventilator, or length of hospital stay (Table 1). There was no difference between TBI groups on months to imaging follow-up. At 12 months post-injury, both cMild/mod and severe TBI groups showed significantly lower neurocognitive scores, compared with controls, on FSIQ and VIQ but not in PIQ. A significant difference in memory scores (combined memory Z-score), compared with controls, was seen in both TBI groups. Nineteen subjects (n = 5 TBI and n = 14 controls) did not have 12-month attention scores as they were odder than 17 years of age at testing or had incomplete data. There were no between group differences detected on measures of attention (TEA-Ch C and TEA-Ch G).
DTI
Initial DTI: 6–17 days post injury
Both TBI groups showed significant regional differences in white matter integrity compared with controls; however, the number of regions affected, and the magnitude of change was greater in the severe TBI group (Table 2). In the cMild/mod TBI group, the parietal white matter showed significantly lower FA compared with controls. ADC in the occipital white matter was increased compared with controls, although this did not reach significance. There were no significant differences in regional AD or RD in the cMild/mod TBI group compared with the control group.
Table 2.
Significant Differences in DTI Metrics between Control and TBI Groups at the Early and 12-Month MRI Time-Points Reported as t-Statistics
| |
|
cMild/mod |
Severe |
||
|---|---|---|---|---|---|
| Region | Early | 12-month | Early | 12-month | |
| FA | ALIC/basal ganglia | ns | -3.794 *** | -3.913 *** | -4.620 *** |
| Brainstem | ns | -2.917 ** | ns | -2.890 ** | |
| Corpus callosum | ns | ns | -4.375 *** | -4.581 *** | |
| Frontal white matter | ns | -3.073 ** | ns | -2.683 ** | |
| Occipital white matter | ns | -3.610 *** | -4.023 *** | -3.904 *** | |
| Parietal white matter | -3.606 *** | -4.414 *** | ns | -4.346 *** | |
| Temporal white matter | ns | ns | ns | ns | |
| ADC | ALIC/basal ganglia | ns | ns | ns | ns |
| Brainstem | ns | ns | ns | ns | |
| Cerebellum | ns | ns | ns | ns | |
| Frontal white matter | ns | ns | ns | 3.860 *** | |
| Occipital white matter | 2.737 ** | 5.025 *** | ns | 4.358 *** | |
| Parietal white matter | ns | 3.806 *** | ns | 4.394 *** | |
| Temporal white matter | ns | 3.268 ** | ns | 4.012 *** | |
| PLIC/thalamus | -2.018 * | ns | ns | ns | |
| AD | ALIC/basal ganglia | ns | ns | -3.013 ** | ns |
| Brainstem | ns | ns | -3.996 *** | ns | |
| Corpus callosum | ns | ns | -3.381 *** | ns | |
| Frontal white matter | ns | ns | ns | 3.717 *** | |
| Occipital white matter | ns | 3.250 ** | ns | ns | |
| Parietal white matter | ns | 2.645 ** | ns | 3.490 *** | |
| Temporal white matter | ns | 2.641 ** | ns | 3.723 *** | |
| PLIC/thalamus | ns | ns | ns | ns | |
| RD | ALIC/basal ganglia | ns | 3.195 ** | ns | 3.947 *** |
| Brainstem | ns | ns | ns | ns | |
| Cerebellum | ns | ns | ns | ns | |
| Corpus callosum | ns | ns | ns | 3.796 *** | |
| Frontal white matter | ns | 2.654 ** | ns | 3.772 *** | |
| Occipital white matter | ns | 5.230 *** | ns | 4.872 *** | |
| Parietal white matter | ns | 4.296 *** | ns | 4.635 *** | |
| Temporal white matter | ns | 3.136 *** | ns | 3.697 *** | |
A negative t statistic indicates an inverse linear relationship between the injury severity and the DTI metric.
Between-group comparisons at the early time point showed that only corpus callosum FA differed significantly between the groups (bold; t = -2.719; p = 0.02)
p < 0.01; ***p < 0.001.
DTI, diffusion tensor imaging; TBI, traumatic brain injury; MRI, magnetic resonance imaging; cMild/mod, complicated mild/moderate TBI; FA, fractional anisotropy; ALIC, anterior limb internal capsule; ns; ADC, apparent diffusion coefficient; AD, axial diffusivity; PLIC, posterior limb internal capsule; RD, radial diffusivity.
In the severe TBI group, significantly lower FA was seen in the ALIC/basal ganglia, corpus callosum, and occipital white matter compared with controls. AD was significantly reduced in the brainstem and corpus callosum. AD in the ALIC/basal ganglia was reduced in severe TBI patients compared with controls, but did not reach statistical significance after correcting for multiple comparisons. Similar to the cMild/mod TBI group, there were no changes in white matter ADC or RD detected in any region examined in the severe TBI group. Comparisons between the two TBI groups revealed significantly lower FA in the corpus callosum of severe TBI patients compared with the cMild/modTBI group (Table 2).
Follow up DTI: 12-months post injury
At 12 months post-injury the occipital and parietal white matter, ALIC, and corpus callosum all showed reduced FA in the cMild/mod TBI and severe TBI groups, with the exception of the temporal white matter (Table 2). FA also was reduced in the brainstem and frontal white matter but did not reach significance. Similarly, significant regional increases in ADC were seen in the occipital and parietal white matter of both groups. In the severe TBI group, there also was increased ADC in the temporal white matter, which did not reach significance in the cMild/mod TBI group. AD was significantly increased in the frontal, parietal, and temporal white matter of the severe TBI groups, with a trend towards increased AD in the occipital, parietal, and temporal white matter of the cMild/mod TBI group. RD was increased in all cortical white matter regions and the ALIC/ basal ganglia of the severe TBI group and in the occipital, parietal, and temporal white matter of the cMild/mod TBI group. There was a trend towards increased RD in the ALIC/basal ganglia and frontal white matter of the cMild/mod TBI group.
Longitudinal changes in DTI
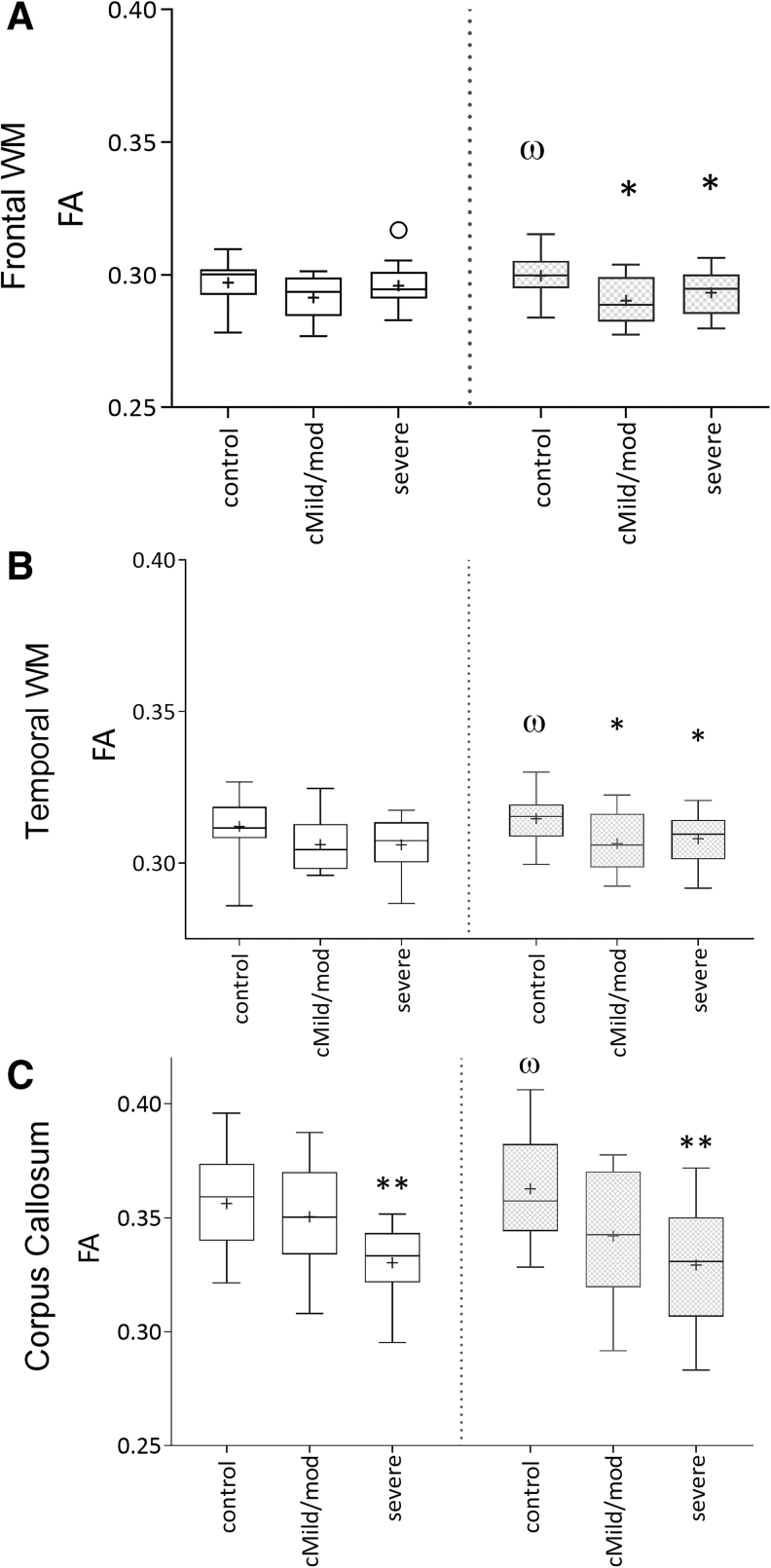
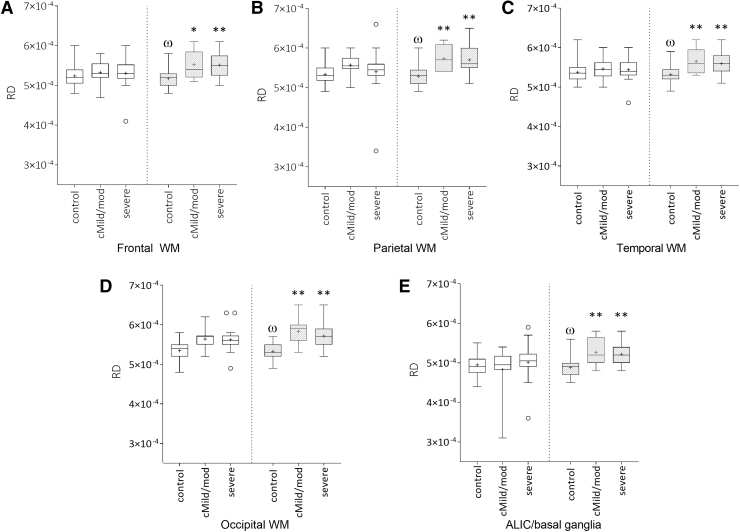
Control subjects showed a significant increase in FA in the frontal and temporal white matter and corpus callosum (Fig. 2) and a significant decrease in RD was seen in the ALIC/ basal ganglia and all cortical white matter regions (Fig. 3) between the two time-points, which was expected with normal myelin maturation. In contrast, RD was increased in the ALIC/ basal ganglia and all cortical white matter regions in both the cMild/mod and severe TBI groups between the two time-points (Fig. 3). The absence of an interval increase in FA and/or decrease in RD in the TBI groups suggests an injury-induced interruption in normal white matter development.
FIG. 2.
Mean regional fractional anisotropy (FA) values in control, complicated mild (cMild)/moderate (mod) traumatic brain injury (TBI), and severe TBI groups showing an interval changes in FA between the initial (3–17 days; white bars) and 12-month (gray bars) imaging time-points. Control subjects showed a significant interval increase in FA in the frontal (A) and temporal (B) white matter, and corpus callosum (C) as expected with normal development (‡p < 0.05). FA was reduced in the frontal and temporal white matter of both the modTBI and severe TBI (sTBI) groups (*p < 0.05) and corpus callosum of the sTBI group (**p < 0.01).
FIG. 3.
Mean regional radial diffusivity (RD) values in control, complicated mild (cMild)/moderate (mod), and severe traumatic brain injury (TBI) groups showing longitudinal change between the initial (3–17 days; white bars) and 12-month (gray bars) imaging time-points. In the control group frontal (A), parietal (B), temporal (C), occipital (D) white matter, and anterior limb of the internal capsule/basal ganglia (E) RD significantly decreased over time (φp < 0.05). In contrast RD was elevated in the same regions of both modTBI and sTBI subjects (*p < 0.05; **p < 0.01).
Factor analysis
Results from the exploratory factor analysis of the initial DTI study are shown in Table 3 and revealed six factors that explained 89.6% of the variance. Factor 1 showed major contributions from the frontal, parietal, occipital, and temporal white matter ADC, AD, and RD, and was termed the “cortical diffusivity factor.” The occipital white matter ADC, AD, and RD were the strongest contributors to this factor. Factor 2 was coined the “subcortical diffusivity factor,” as it showed major contributions from the ADC, AD, and RD of the ALIC/basal ganglia, brainstem and corpus callosum regions. Diffusivity within the corpus callosum was the strongest contributor to this factor. Factor 3 showed major contributions from FA in the ALIC/basal ganglia, frontal, parietal, occipital, and temporal white matter regions and was labeled the “FA factor.”
Table 3.
Factor Loadings and Variance Calculated by Factor from the Early Imaging DTI Metrics
| Factor 1 “cortical diffusivity” | Factor 2 “subcortical diffusivity” | Factor 3 “FA” | Factor 4 “thalamus diffusivity” | Factor 5 “corpus callosum” | Factor 6 “thalamus FA” | |
|---|---|---|---|---|---|---|
| TI measure | ||||||
| ALIC/basal ganglia FA | 0.784 | |||||
| ALIC/Basal ganglia ADC | 0.700 | |||||
| ALIC/Basal ganglia AD | 0.694 | |||||
| ALIC/Basal ganglia RD | 0.648 | |||||
| Brainstem FA | ||||||
| Brainstem ADC | 0.754 | |||||
| Brainstem AD | 0.777 | |||||
| Brainstem RD | 0.606 | |||||
| Corpus callosum FA | -0.906 | |||||
| Corpus callosum ADC | 0.877 | |||||
| Corpus callosum AD | 0.849 | |||||
| Corpus callosum RD | 0.840 | |||||
| Frontal white matter FA | 0.849 | |||||
| Frontal white matter ADC | 0.766 | |||||
| Frontal white matter AD | 0.719 | |||||
| Frontal white matter RD | 0.775 | |||||
| Occipital white matter FA | 0.851 | |||||
| Occipital white matter ADC | 0.890 | |||||
| Occipital white matter AD | 0.913 | |||||
| Occipital white matter RD | 0.913 | |||||
| Parietal white matter FA | 0.855 | |||||
| Parietal white matter ADC | 0.880 | |||||
| Parietal white matter AD | 0.865 | |||||
| Parietal white matter RD | 0.872 | |||||
| Temporal white matter FA | 0.785 | |||||
| Temporal white matter ADC | 0.845 | |||||
| Temporal white matter AD | 0.770 | |||||
| Temporal white matter RD | 0.836 | |||||
| PLIC/Thalamus FA | 0.940 | |||||
| PLIC/Thalamus ADC | 0.872 | |||||
| PLIC/Thalamus AD | 0.848 | |||||
| PLIC/Thalamus RD | ||||||
| Variance Explained | ||||||
| Eigenvalue | 10.43 | 6.96 | 4.27 | 2.23 | 1.91 | 1.87 |
| Percentage | 32.60 | 21.74 | 13.34 | 10.08 | 5.97 | 5.83 |
DTI, diffusion tensor imaging; FA, fractional anisotropy; ALIC, anterior limb internal capsule; ADC, apparent diffusion coefficient; AD, axial diffusivity; RD, radial diffusivity; PLIC, posterior limb internal capsule.
Factor 4 showed positive contributions from the ADC and AD of the PLIC/thalamus region and was named the “thalamus diffusivity factor.” Factor 5, the “corpus callosum factor,” had a single negative contribution from the corpus callosum FA. Factor 6 was made up of a single positive contribution from the PLIC/thalamus FA was labeled the “thalamus FA factor.” As ADC is a combination of AD and RD, it was not surprising that these metrics were highly correlated and separated into Factor 1 “cortical diffusivity” and Factor 2 “subcortical diffusivity.” However, a relationship between FA, AD, and RD was not borne out in the factor analysis as these metrics separated out into different factors. When these factors were compared between TBI groups, only the “corpus callosum factor” (Factor 5) was able to distinguish between the two TBI groups (p = 0.008).
Relation between DTI metrics and neurocognitive outcome
Linear regression was used to measure the relation between regional DTI measures and factors from the initial MRI time-point and the 12-month neurocognitive scores. At the initial imaging time-point, the corpus callosum AD was associated with 12-month PIQ (t = 3.67, p = 0.001). A trend towards a relationship between 12-month PIQ scores also was seen with brainstem AD (t = 3.30, p = 0.003), corpus callosum RD (t = 2.84, p = 0.009), and the “subcortical diffusivity factor” (t = 3.23, p = 0.003). A number of white matter regions (ALIC/basal ganglia, cerebellum, frontal, occipital, parietal, and temporal) and the “FA factor” showed a moderate positive linear relationship between FA and 12-month memory; however, they did not reach statistical significance after correction for multiple comparisons. Similarly, FA and RD in the PLIC/thalamus as well as the “thalamus FA factor” were moderately associated with the 12-month Tea-Ch G and C attention scores but did not reach significance.
At the 12-month imaging time-point, the corpus callosum FA (t = 3.14, p = 0.005) showed a positive trend with 12-month PIQ scores. In contrast, the corpus callosum showed a negative trend with PIQ (t = -3.06, p = 0.006). In addition, the temporal white matter ADC (t = -3.18, p = 0.005) and temporal white matter AD (t = -2.93, p = 0.009) showed a trend towards a negative association with 12-month Tea-Ch C scores.
Discussion
DTI has been used for well over a decade to characterize white matter disruption following TBI. In this study, we measured longitudinal changes in white matter anisotropy and diffusivity and related both early and chronic (12-month) imaging findings to 12-month measures of memory, attention, and IQ. Our study was unique in that initial imaging occurred within the first 3 weeks post-injury and included cMild/mod and severe pediatric TBI patients.
At the early imaging time-point, both cMild/mod and severe TBI groups showed reduced FA in occipital, parietal, and/or temporal white matter, brainstem, and PLIC/thalamus white matter regions, with only the severe TBI group showing reduced FA in the ALIC/basal ganglia and corpus callosum. ADC changes were inconsistent, with regions of decreased (PLIC/thalamus, brainstem) or increased (occipital white matter). Regional and tract-specific decreases in FA and/or increases in ADC have been consistently reported following pediatric/adolescent TBI in the middle to late (> 4 week) post-injury period19,21–24,36,37; however, early changes in white matter integrity in the pediatric moderate-severe TBI population are relatively unknown. In the small number of pediatric studies done within 4 weeks of injury, regional increases in FA and reduced ADC/MD have been reported. These discrepancies are likely related to injury severity as our population included complicated mild, moderate, and severe TBI patients, whereas previous studies early after injury focused on mild or concussed pediatric patients.16–18,22 Similar to our results were those reported by Yuh and colleagues who observed early (5–18 days) reductions in white matter FA in a cohort of adult mild TBI patients that were CT/MRI positive for intracranial lesions or skull fractures, as was observed in our cMild/mod TBI group.38
In our study we also performed a separate quantification of RD and AD, which gives additional insight into white matter disruption where RD reflects changes in myelin compaction and AD reflects axon morphology and degradation.39–41 RD changes were only seen in the cMild/mod group where both regions with increased and decreased RD were observed, which would suggest that myelin structure is fluid (perhaps due to increased intramyelin edema) in the early period after TBI. Studies reporting early changes in AD and RD in the pediatric moderate–severe TBI population are limited and outside of the time window used in our study. In the study by Genc and colleagues, whole–brain AD was significantly higher in pediatric TBI patients (mean age 10 ± 2 years) in the subacute (∼5 week post injury) period, primarily in the corpus callosum, middle cerebellar peduncle, and anterior corona radiata.25 In addition, there were significant linear associations between injury severity and whole brain FA (negative), MD and RD (positive).25 Ewing-Cobbs and colleagues and Perez and colleagues reported reduced FA and elevated RD in the corpus callosum of complicated mild, moderate and severe patients scanned at >3 months post injury,19,42 whereas Dennis and colleagues reported no differences in white matter tract RD, MD, or AD at their initial imaging time-point (1–5 months post-injury).26 The discordance between these studies and the findings presented here again highlight the significant role that the time after and evolution of injury plays in the interpretation of DTI findings.43
Like the study by Wilde and colleagues,44 we observed an interval increase in frontal and temporal white matter and corpus callosum FA and decreased cortical white matter RD in normal controls (age 7–17 years), which suggests white matter maturation and ongoing myelination over the 12-months of the study. In our study, any significant interval increase in FA and/or decrease in RD was absent in the TBI groups, suggesting that the developmental trajectory was altered in these patients.44 In addition there was evidence of ongoing white matter injury as evidenced by an increase in the number of regions with reduced FA and widespread interval increases in ADC, AD, and RD. It is highly likely that group differences were more apparent at the 12-month period due in part to the increasing divergence between controls and the TBI groups. In addition, it has been hypothesized that in the acute phase, disruption will be greatest closest to the area of injury and over time, this disruption may spread and become more generalized.36 Regardless, our findings of chronic reductions in FA with chronic increases in ADC, AD, and RD in cortical white matter regions are consistent with previous longitudinal studies in the pediatric and adult populations.19,21,24,26,36
While it is difficult to clearly differentiate the underlying pathophysiological processes responsible for the complex interactions between DTI metrics, elevated MD/ADC is thought to represent ongoing demyelination and axonal death following injury.11,40,45 We also detected increased AD in numerous white matter regions at 12 months post-injury which suggest that the microstructural organization within the axonal elements is also affected. Alternatively, increased AD may reflect gliosis whereas increases in ADC and RD could suggest an absence of neuronal regeneration.21 Several studies have shown alterations in MD and RD that predominate in the chronic phase with early changes in FA and ADC diminishing in size and distribution at 18 months, suggesting some amelioration of the initial deleterious changes.23,36 In our study the early decrease in PLIC/thalamus and brainstem ADC as well as the early decrease in brainstem RD normalized by 12-months post injury, possibly suggestive of recovery.
The second goal of this study was to determine potential relationships between early DTI metrics and measures of memory, IQ, and attention in order to evaluate the prognostic utility of early DTI in predicting functional outcome. Of the three indices of intelligence captured, only the PIQ was associated with any of the early regional DTI metrics. A significant positive association between corpus callosum AD and PIQ, as well as a trend between corpus callosum RD and PIQ, were found, indicating that reduced white matter diffusivity was related to poorer outcome. PIQ is a measure of both fluid reasoning and visual-spatial processing—that is, the ability to solve novel visual problems independent of culture, language, and school-acquired knowledge46—and is more sensitive to acquired brain injury than VIQ due, in part, to visual-motor and processing speed demands on the Block Design subtest.2
The neural substrates that underlie these specific factors of intelligence are just beginning to be discovered with the advancement of imaging capabilities. The size of the corpus callous, in addition to both frontal and parietal lobes have been reported to play a role in intelligence.47–50 Previous studies have reported associations between corpus callosum FA and/or RD with IQ, and our findings both support and extend these observations.19,51 The lack of significant association between DTI and VIQ was not surprising as portions of this measure are often used to estimate premorbid intellectual ability due to its limited vulnerability to injury and high correlation with parental education and income variables, which were not controlled in the current study. Memory deficits are a common consequence of pediatric TBI.52,53 However, we found no significant associations between early DTI measures and memory. Our findings are not in line with reports in studies that included patients with similar injury severity, which we hypothesize, may be related to the earlier imaging time-point used in the present study since the age of subjects were similar.19,54,55
Deficits in attention are also commonly reported following pediatric TBI.56,57 In the present study we did not find significant associations between any early DTI metric and measures of attention, which may also be related to the early imaging time-point used in this study. The present study further extends the literature in pediatric TBI by showing that the disruption of specific white matter regions, when measured within the first 6–17 days of injury, impedes neurocognitive processing mechanisms that underlie fluid reasoning after cMild/mod and severe TBI.
Factor analysis
As an exploratory sub aim, we applied factor analysis to our early DTI metrics to create a set of underlying factors (variables) for each patient that explain the interrelationships among those variables. At the acute imaging time-point the six individual factors clustered according to DTI metric (FA or diffusivity). When these factors were tested to determine if they could differentiate between TBI groups, only Factor 5 (“corpus callosum” FA factor) differentiated between the two injury severities. This was expected as the raw corpus callosum FA was also significantly different between TBI groups. Injury induced changes in the anisotropy and diffusivity of the corpus callosum have been described previously19,36,37,54,58; however, to our knowledge, this is the first time this metric was shown to differentiate between injury severity. Several factors also showed a trend towards a moderate association with 12-month neurocognitive measures. The subcortical diffusivity factor (Factor 2), which included contributions from the ALIC/basal ganglia, brainstem, and corpus callosum ADC, AD, and RD values showed a trend towards a positive association with PIQ.
Limitations
Our study has several limitations and several strengths. First, although our sample size is comparable to other DTI studies in the pediatric population; its small size limits any analysis of age or gender effects. Second, our study examined only two time-points, 6–17 days and 12 months, which does not allow us to determine at what point during the 12-month interval that the expansion in the number of regions with white matter alterations occurred. Third, the DTI data presented here reflects a clinical scanning protocol for now outmoded hardware, compared with the current state of the art. As a result, the DTI acquisition parameters chosen resulted in anisotropic voxels, which allows for whole–brain coverage in shorter scan time, but can underestimate FA in regions of crossing fibers. To account for this, we employed a 3D cubic interpolation scheme to up sample the DTI data to an isotropic pixel size. Dyrby and colleagues demonstrated that interpolating diffusion-weighted imaging datasets produced FA results similar in geometry to those acquired at a higher resolution; however, interpolation can lead to loss of signal, due to partial volume averaging in crossing fibers.59
Fourth, the transformation of severely damaged brain into standardized space can impact the precision of anatomical labelling. In the present study, 12 studies were removed from analysis as a result of misalignment during the atlas mapping step, as a result, our findings are limited to white matter regions in normal-appearing brain anatomy. To counteract local misregistration effects, we employed the widely used, popular TBSS skeletonization approach.60 More recent studies have shown that only 10% of registration misalignments are corrected with this technique61 resulting in adequate separation of adjacent white matter tracts.62 While this could impact the anatomical specificity of our findings, its effects may be limited as we chose a lobar approach to describe our findings. Lastly, aspects of cognitive functions are known to be moderated by family factors such as parental education and parental income level, both independent of injury and also as factors that contribute to recovery following TBI. The current study did not control for the influence of these factors, which may have resulted in weaker associations between DTI metrics and cognitive performance. The strengths of this study are the longitudinal design, the inclusion of multiple injury severities, and the early DTI analysis.
Conclusions
This study confirms previous literature suggesting that white matter injury is a component of the neuropathology seen after pediatric TBI. Our results extend the literature by revealing that early white matter injury, as measured by DTI, can be detected at <4 weeks after and is associated with long term neurocognitive outcome. These findings emphasize the clinical relevance of DTI as a prognostic factor of functional outcome in pediatric TBI patients.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
Funding Information
This work was supported by the National Institutes of Health [NINDS 5R01NS05400].
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Faul M., Xu L., Wald M., and Coronado V. (2010). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths 2002–2006. Centers for Disease Control and Prevention; National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2. Babikian T. and Asarnow R. (2009). Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropsychology 23, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greer J.E., McGinn M.J., and Povlishock J.T. (2011). Diffuse traumatic axonal injury in the mouse induces atrophy, c-Jun activation, and axonal outgrowth in the axotomized neuronal population. J. Neuroscience 31, 5089–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Büki A., and Povlishock J.T. (2005). All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochir. 148, 181–194 [DOI] [PubMed] [Google Scholar]
- 5. Villegas R., Martinez N.W., Lillo J., Pihan P., Hernandez D., Twiss J.L., and Court F.A. (2014). Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci. 34, 7179–7189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ziogas N.K. and Koliatsos V.E. (2018). Primary traumatic axonopathy in mice subjected to impact acceleration: a reappraisal of pathology and mechanisms with high-resolution anatomical methods. J. Neurosci. 38, 4031–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frati A., Cerretani D., Fiaschi A.I., Frati P., Gatto V., La Russa R., Pesce A.,Pinchi E., Santurro A., Fraschetti F., and Fineschi V. (2017). Diffuse axonal injury and oxidative stress: a comprehensive review. Int. J. Mol Sci. 18, 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koliatsos V.E. and Alexandris A.S. (2019). Wallerian degeneration as a therapeutic target in traumatic brain injury. Current Opinion in Neurology 32, 786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niogi S.N. and Mukherjee P. (2010). Diffusion Tensor Imaging of Mild Traumatic Brain Injury. J. Head Trauma Rehabil. 25, 241–255 [DOI] [PubMed] [Google Scholar]
- 10. Lebel C., Gee M., Camicioli R., Wieler M., Martin W., and Beaulieu C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60, 340–352 [DOI] [PubMed] [Google Scholar]
- 11. Sidaros A, Engberg AW, Sidaros K, Liptrot M.G., Herning M., Petersen P., Paulson O.B., Jernigan T.L., and Rostrup E. (2008). Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 131, 559–572 [DOI] [PubMed] [Google Scholar]
- 12. Faber J., Wilde E.A., Hanten G., Ewing-Cobbs L. A itken M.E., Yallampalli R., MacLeod M.C., Mullins S.H., Chu Z.D., Li X., Hunter J.V., Noble-Haeusslein L. and Levin H.S. (2016). Ten-year outcome of early childhood traumatic brain injury: diffusion tensor imaging of the ventral striatum in relation to executive functioning. Brain Inj. 30, 1635–1641 [DOI] [PubMed] [Google Scholar]
- 13. Roberts R.M., Mathias J.L., and Rose S.E. (2014). Diffusion tensor imaging (DTI) findings following pediatric non-penetrating TBI: a meta-analysis. Dev. Neuropsychol. 39, 600–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roberts R.M., Mathias J.L., and Rose S.E. (2016). Relationship between diffusion tensor imaging (DTI) findings and cognition following pediatric TBI: a meta-analytic review. Dev. Neuropsychol. 41, 176–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardner A., Kay-Lambkin F., Stanwell P., Donnelly J.,W., Huw Williams W., Hiles A., Schofield P., Levi C., and Jones D.K. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma 29, 2521–2538 [DOI] [PubMed] [Google Scholar]
- 16. Mayer A.R., Ling J.M., Yang Z., Pena A., Yeo R.A., and Klimaj S. (2012). Diffusion abnormalities in pediatric mild traumatic brain injury. J. Neurosci. 32, 17961–17969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu T.C., Wilde E.A., Bigler E.D., Yallampalli R., McCauley S.R., Troyanskaya M., Chu Z., Li X., Hanten G., Hunter J.V., and Levin H.S. (2010). Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J. Neurotrauma 27, 303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilde E.A., McCauley S.R., Hunter J.V., Bigler E.D., Chu Z., Wang Z.J., Hanten G.R., Troyanskaya M., Yallampalli R., Li X., Chia J., and Levin H.S. (2008). Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology 70, 948–955 [DOI] [PubMed] [Google Scholar]
- 19. Ewing-Cobbs L., Prasad M.R., Swank P., Kramer L., Cox Jr., C.S., Fletcher J.M., Barnes M., Zhang X., and Hasan K.M. (2008). Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: relation to neurobehavioral outcomes. Neuroimage 42, 1305–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kurowski B., Wade S.L., Cecil K.M., Walz N.C., Yuan W., Rajagopal A., and Holland S.K. (2009). Correlation of diffusion tensor imaging with executive function measures after early childhood traumatic brain injury. J. Pediatr. Rehabil. Med. 2, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adamson C., Yuan W., Babcock L., Leach J.L., Seal M.L., Holland S.K., and Wade S.L. (2013). Diffusion tensor imaging detects white matter abnormalities and associated cognitive deficits in chronic adolescent TBI. Brain Inj. 27, 454–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu T.C., Wilde E.A., Bigler E.D., Li D., Merkley T.L., Yallampalli R., McCauley S.R., Schnelle K.P., Vasquez A.C., Chu Z., Hanten G., Hunter J.V., and Levin H.S. (2010). Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 32, 361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilde E.A., Ayoub K.W., Bigler E.D., Chu Z.D., Hunter J.V., Wu T.C., McCauley S.R., and Levin H.S. (2012). Diffusion tensor imaging in moderate-to-severe pediatric traumatic brain injury: changes within an 18 month post-injury interval. Brain Imaging Behav. 6, 404–416 [DOI] [PubMed] [Google Scholar]
- 24. Ewing-Cobbs L., Johnson C.P., Juranek J., DeMaster D., Prasad M., Duque G., Kramer L., Cox C.S., and Swank P.R. (2016). Longitudinal diffusion tensor imaging after pediatric traumatic brain injury: impact of age at injury and time since injury on pathway integrity. Hum. Brain Mapp. 37, 3929–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genc S., Anderson V., Ryan N.P., Malpas C.B., Catroppa C., Beauchamp M.H., and Silk T.J. (2017). Recovery of white matter following pediatric traumatic brain injury depends on injury severity. J. Neurotrauma 34, 798–806 [DOI] [PubMed] [Google Scholar]
- 26. Dennis E.L., Jin Y., Villalon-Reina J.E., Zhan L., Kernan C.L., Babikian T., R.B., Babbitt C.J., Johnson J.L., Giza C.C., Thompson P.M., and Asarnow R.F. (2015). White matter disruption in moderate/severe pediatric traumatic brain injury: advanced tract-based analyses. Neuroimage Clin. 7, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gardner A., Kay-Lambkin F., Stanwell P., Donnelly J., W Williams W., Hiles A., Schofield P., Levi C., and Jones D.K. (2012). A systematic review of diffusion tensor imaging findings in sports-related concussion. J. Neurotrauma 29, 2521–2538 [DOI] [PubMed] [Google Scholar]
- 28. Levin H.S., Hanten G., Robertson G., Li X., Ewing-Cobbs L., Dennis M., Chapman S., Max J.E., Hunter J., Schachar R., Luerssen T.G., and Swank P. (2008). Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. J. Neurosurg. Pediatr. 1, 461–470 [DOI] [PubMed] [Google Scholar]
- 29. Ghosh N., Holshouser B., Oyoyo U., Barnes S., Tong K., and Ashwal S. (2017). Combined diffusion tensor and magnetic resonance spectroscopic imaging methodology for automated regional brain analysis: application in a normal pediatric population. Dev. Neurosci. 39, 4134–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierpaoli C. and Basser P.J. (1996). Toward a quantitative assessment of diffusion anisotropy. Magn. Reson. Med. 36, 893–906 [DOI] [PubMed] [Google Scholar]
- 31. Basser P.J. and Jones D.K. (2002). Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed. 15, 456–467 [DOI] [PubMed] [Google Scholar]
- 32. Cutter G.R., Baier M.L., Rudick R.A., Cookfair D.L., Fischer J.S., Petkau J., Syndulko K., Weinshenker B.G., Antel J.P., Confavreux C., Ellison G.W., Lublin F., Miller A.E., Rao S.M., Reingold S., Thompson A., and Willoughby E. (1999). Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain 122, 871–882 [DOI] [PubMed] [Google Scholar]
- 33. Bergemann T.L., Bangirana P., Boivin M.J., Connett J.E., Giordani B.J., and John C.C. (2012). Statistical approaches to assess the effects of disease on neurocognitive function over time. J. Biom. Biostat. Suppl 7, 7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohamed M.A., Lentz M.R., Lee V., Halpern E.F., Sacktor N., Selnes O., Barker P.B., and Pomper M.G. (2010). Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology 254, 577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holshouser B., Pivonka-Jones J., Nichols J.G., Oyoyo U., Tong K., Ghosh N., and Ashwal S. (2019). Longitudinal metabolite changes after traumatic brain injury: a prospective pediatric magnetic resonance spectroscopic imaging study. J. Neurotrauma 36, 1352–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dennis E.L., Ellis M.U., Marion S.D., Jin Y., Moran L., Olsen A., Claudia Kernan C., Talin Babikian T., Richard Mink R., Christopher Babbitt C., Johnson J., Christopher C Giza C.C., Thompson P.M., and Asarnow R. (2015). Callosal function in pediatric traumatic brain injury linked to disrupted white matter integrity. J. Neurosci. 35, 10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wozniak J.R., Krach L., Ward E., Mueller B.A., Muetzel R., Schnoebelen S., Kiragu K., and Lim K.O. (2007). Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch. Clin. Neuropsychol. 22, 555–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yuh E.L., Hawryluk G.W., and Manley G.T. (2014). Imaging concussion: a review. Neurosurgery 75 Suppl 4, S50–S63 [DOI] [PubMed] [Google Scholar]
- 39. Mac Donald C.L., Dikranian K., Song S.K., Bayly P.V., Holtzman D.M., and Brody D.L. (2007). Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol. 205, 116–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Song S.K., Yoshino J., Le T.Q., Lin S.J., Sun S.W., Cross A.H., and Armstrong R.C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26, 132–140 [DOI] [PubMed] [Google Scholar]
- 41. Armstrong R.C., Mierzwa A.J., Marion C.M., and Sullivan G.M. (2016). White matter involvement after TBI: clues to axon and myelin repair capacity. Exp. Neurol. 275 Pt 3, 328–333 [DOI] [PubMed] [Google Scholar]
- 42. Perez A.M., Adler J., Kulkarni N., Strain J.F., Womack K.B., Diaz-Arrastia R., and Marquez de la Plata C.D. (2014). Longitudinal white matter changes after traumatic axonal injury. J. Neurotrauma 31, 1478–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bigler E.D. and Bazarian J.J. (2010). Diffusion tensor imaging: a biomarker for mild traumatic brain injury? Neurology 74, 626–627 [DOI] [PubMed] [Google Scholar]
- 44. Wilde E.A., Merkley T.L., Bigler E.D., Max J.E., Schmidt A.T., Ayoub K.W., McCauley S.R., Hunter J.V., Hanten G., Li X., Chu Z.D., and Levin H.S. (2012). Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 30, 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smith D.H. and Meaney D.F. (2000). Axonal damage in traumatic brain injury. Neuroscientist 6, 483–495 [Google Scholar]
- 46. Cattell R.B. and Horn J.L. (1978). A check on the theory of fluid and crystallized intelligence with description of new subtest designs. J. Educ. Meas. 15, 139–164 [Google Scholar]
- 47. Westerhausen R., Friesen C.M., Rohani D.A., Krogsrud S.K., Tamnes C.K., Skranes J.S., Håberg A.K., Fjell A.M., and Walhovd K.B. (2018). The corpus callosum as anatomical marker of intelligence? A critical examination in a large-scale developmental study. Brain Structure Funct. 223, 285–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoon Y.B., Shin W.G., Lee T.Y., Hur J.W., Cho K.I.K.C., Seunghyun Sohn W., Kim S.G., Lee K.H., and Kwon J.S. (2017). Brain structural networks associated with intelligence and visuomotor ability. Sci. Rep. 7, 2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colom R., Burgaleta M., Román F.J., Karama S., Alvarez-Linera J., Abad F.J., Martínez K., Ángeles Quiroga M., and Haier R.J. (2013). Neuroanatomic overlap between intelligence and cognitive factors: morphometry methods provide support for the key role of the frontal lobes. NeuroImage 72, 143–152 [DOI] [PubMed] [Google Scholar]
- 50. Colom R., Stein J.L., Rajagopalan P., Martínez K., Hermel D., Wang Y., Álvarez-Linera J., Burgaleta M., Ángeles Quiroga M., Chun Shih P., and Thompson P.M. (2013). Hippocampal structure and human cognition: key role of spatial processing and evidence supporting the efficiency hypothesis in females. Intelligence 41, 129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Soul J.S., Robertson R.L., Tzika A.A., du Plessis A.J., and Volpe J.J. (2001). Time course of changes in diffusion-weighted magnetic resonance imaging in a case of neonatal encephalopathy with defined onset and duration of hypoxic-ischemic insult. Pediatrics 108, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 52. Levin H.S., Hanten G., Chang C.C., Zhang L., Schachar R., Ewing-Cobbs L., and Max J.E. (2002). Working memory after traumatic brain injury in children. Ann. Neurol. 52, 82–88 [DOI] [PubMed] [Google Scholar]
- 53. Conklin H.M., Salorio C.F., and Slomine B.S. (2008). Working memory performance following paediatric traumatic brain injury. Brain Injury 22:847–857 [DOI] [PubMed] [Google Scholar]
- 54. Treble A., Hasan K.M., Iftikhar A., Stuebing K.K., Kramer L.A., Cox Jr C.S., Swank P.R., and Ewing-Cobbs L. (2013). Working memory and corpus callosum microstructural integrity after pediatric traumatic brain injury: a diffusion tensor tractography study. J. Neurotrauma 30, 1609–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Volpe D.S.J., Oliveira N.C.A.C., Santos A.C., Linhares M.B.M., and Carlotti A.P.C.P. (2017). Neuropsychological outcome of children with traumatic brain injury and its association with late magnetic resonance imaging findings: a cohort study. Brain Inj. 31, 1689–1694 [DOI] [PubMed] [Google Scholar]
- 56. Levin H., Hanten G., Max J., Li X., Swank P., Ewing-Cobbs L., Dennis M., Menefee D.S., and Schachar R. (2007). Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J. Dev. Behav. Pediatr. 28, 108–118 [DOI] [PubMed] [Google Scholar]
- 57. Max J.E., Lansing A.E., Koele S.L., Castillo C.S., Bokura H., Schachar R., Collings N., and Williams K.E. (2004). Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev. Neuropsychol. 25, 159–177 [DOI] [PubMed] [Google Scholar]
- 58. Rutgers D.R., Fillard P., Paradot G., Tadie M., Lasjaunias P., and Ducreux D. (2008). Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. A.J.N.R. Am. J. Neuroradiol. 29, 1730–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dyrby T.B., Lundell H., Burke M.W., Reislev N.L., Paulson O.B., Ptito M., and Siebner H.R. (2014). Interpolation of diffusion weighted imaging datasets. NeuroImage 103, 202–213 [DOI] [PubMed] [Google Scholar]
- 60. Smith S., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews. P.M., and Behrens T.E.J. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- 61. Zalesky A. (2011). Moderating registration misalignment in voxelwise comparisons of DTI data: a performance evaluation of skeleton projection. Magn. Reson. Imaging 29, 111–125 [DOI] [PubMed] [Google Scholar]
- 62. Bach M, Laun FB, Leemans A, Tax C.M.W. Biessels G.J. Stieltjes B., and Hein K.H. (2014). Methodological considerations on tract-based spatial statistics (TBSS). NeuroImage 100, 358–369 [DOI] [PubMed] [Google Scholar]